Abstract
Teaghrelins are unique acylated flavonoid tetraglycosides found in Chin-shin oolong tea, and have been demonstrated to be promising oral ghrelin analogues. The biosynthetic pathway of teaghrelins from quercetin-3-O-rutinoside (rutin) or kaempferol-3-O-rutinoside (nicotiflorin) was proposed to comprise three enzymatic steps according to the identification of putative intermediates in Chin-shin oolong tea. In addition to the two known teaghrelins in Chin-shin oolong tea, four teaghrelin-like compounds with different attachments of glycosides were identified in various oolong teas. Molecular modeling and docking were used to evaluate theoretically whether the putative biosynthetic intermediates of teaghrelins and the four teaghrelin-like compounds could be potential candidates of ghrelin analogues. The results showed that the attachment of a coumaroyl group was crucial for these tea compounds to bind to the ghrelin receptor. However, the additional attachment of a rhamnosyl glycoside to the flavonoid backbone of teaghrelin-like compounds at C-7 significantly reduced their binding affinity with the ghrelin receptor.
Keywords: ghrelin analogues, ghrelin receptor, molecular docking, oolong tea, teaghrelin
1. Introduction
Ghrelin is a peptide hormone consisting of 28 amino acids, in which the third serine residue is acylated with an n-octanoyl group essential for its biological functions [1–3]. It is colloquially called the “hunger hormone”, and its target receptor is a G protein-coupled receptor also known as the growth hormone secretagogue-1a receptor [1,4]. The remarkable physiological functions of ghrelin via activation of the ghrelin receptor are promotion of appetite by stimulation of hypothalamic arcuate nucleus and induction of growth hormone release from the anterior pituitary gland [5,6]. In addition, several other biological effects of ghrelin have been reported, including influence on the reproductive system, the gastrointestinal system, glucose metabolism, and cardiovascular functions [7–9]. On the basis of the multiple biological activities of ghrelin, both peptidyl and nonpeptidyl ghrelin analogues were synthesized and aimed to develop potential therapeutic applications of several diseases, e.g., gastrointestinal deficiency and anorexia [10–13]. Unfortunately, no synthetic oral ghrelin analogues were satisfactory and approved for clinical application thus far.
Tea is one of the most widely consumed beverages around the world [14], and its major ingredients, flavonols and polyphenols, have been demonstrated to provide a variety of health benefits [15–17]. According to the degree of fermentation in preparation, teas are mainly classified as green tea (unfermented), oolong tea (semifermented), and black tea (fully fermented), where the term fermentation refers to natural browning reactions induced by oxidative enzymes in the cells of tea leaves [18]. Over the past few decades, oolong tea has been the most favorite choice of Taiwanese because of its special taste and flavor [19,20].
Teaghrelins are unique acylated flavonoid tetraglycosides found in Chin-shin oolong tea, and have been demonstrated to be responsible for the hunger induction just as the endogenous hunger hormone, ghrelin [21,22]. Similar to ghrelin, teaghrelins are able to induce hunger sensation of rats as well as stimulate growth hormone secretion of rat primary anterior pituitary cells [21]. According to the observation in the aforementioned animal study and cell line assay, teaghrelins were proposed to be promising oral ghrelin analogues, provided they undergo necessary clinical trials.
In addition to teaghrelins, some flavonoid derivatives were also found in Chin-shin oolong tea [23]. Presumably, these flavonoid derivatives might serve as intermediates in the biosynthetic pathway of teaghrelins. Moreover, several teaghrelin-like compounds, acylated flavonoid tetraglycosides with different attachments of glycosides, were detected in various oolong teas [24]. In this study, we aimed to search for more potential oral ghrelin analogues by screening flavonoid derivatives in Taiwan oolong teas. We first identified the putative biosynthetic intermediates of teaghrelins in Chin-shin oolong tea and teaghrelin-like compounds in various oolong teas. To evaluate the possibility if the putative biosynthetic intermediates of teaghrelins and the teaghrelin-like compounds could be potential candidates of ghrelin analogues, molecular modeling of these compounds docking to the ghrelin receptor was exhibited.
2. Methods
2.1. Chemicals and materials
All chemicals were purchased from E. Merck Co. (Merck KGaA, Darmstadt, Germany) unless stated otherwise. High-performance liquid chromatography (HPLC) grade acetonitrile was purchased from Fisher Scientific (Fair Lawn, NJ, USA). Acetic acid (99.7%) was obtained from J.T. Baker (Mallinckrodt Baker, Inc., Phillipsburg, NJ, USA). Rutin was purchased from Sigma Co. (Sigma-Aldrich, St. Louis, MO, USA). Purified water was afforded by a Millipore clear water purification system (Direct-Q, Millipore, Billerica, MA, USA). Various oolong teas prepared from tea plants (Camellia sinensis L.), including Alishan Chin-shin, Dongding Chin-shin, Wuyi, Baihao, and Tieguanyin, were gifts or purchased from local tea producers. Teaghrelins were purified from Chin-shin oolong teas according to the protocol as described previously [22].
2.2. Preparation and HPLC analysis of tea infusions
Tea infusions were prepared by adding 20 mL of boiling water to 1 g of various oolong teas for 10 minutes. After cooling to room temperature, the brew was filtered through a 0.22 μm polyvinylidene difluoride (PVDF) membrane filter (Pall Corporation, Glen Cove, NY, USA) for the following analysis. Chemical constituents in oolong tea infusions were analyzed by HPLC system coupled to a 600E photodiode array detector (Waters Corporation, Milford, MA, USA), and separation was performed on the Syncronis C18 column (4.6 × 250 mm inner diameter, 5 μm, Thermo Scientific, Waltham, MA, USA). The separated condition of HPLC analysis was prepared according to Lo et al [21]. The mobile phase consisted of (A) water containing 0.5% acetic acid and (B) acetonitrile. The gradient was as follows: 0–60 minutes, linearly gradient from 10% to 30% B; 61–70 minutes 30% B; and 71–100 min, linearly gradient from 30% to 10% B. The column was maintained at room temperature and the injection volume was 10 μL at a flow rate of 1 mL/ min. The ultraviolet (UV) absorbance detection wavelength was set at 280 nm.
2.3. Mass spectrometric analysis
Mass spectrometric analysis was performed on a LTQ (linear trap quadrupole) tandem mass spectrometer (Thermo Electron, San Jose, CA, USA) equipped with an electrospray ionization (ESI) interface and connected to a Surveyor LC system (Thermo Electron, San Jose, CA, USA) with a 5 μL sample loop. The analytes were separated with the same condition of HPLC analysis. The flow rate was 1 mL/min. The mass spectra were obtained with negative ESI mode. The heated capillary temperature was 300°C, and the spray voltage was 4.5 kV. Flow rates of sheath gas, auxiliary gas, and sweep gas were 50, 13, and 3 arbitrary units, respectively. Data-dependent acquisition (DDA) was used to perform under automatic gain control conditions. The first scan was operated in full-scan mode with m/z values ranging from 150 to 1500. The other scans were set as the data-dependent MSn scan using the high-purity helium (>99.99%) as the collision gas and the relative collision energy of 33–35%. The flavonoid derivatives shown in the HPLC profiles were identified according to the same procedure as described previously [23].
2.4. Homology modeling of three-dimensional structure of human ghrelin receptor
The protein sequence of a human ghrelin receptor (growth hormone secretagogue receptor, Genbank accession number AAI13548) was uploaded to the SWISS-MODEL website (http://swissmodel.expasy.org/interactive#sequence) to search for proper templates for modeling [25]. Crystal structures of β1 and β2 adrenergic receptors (PDB 2YCY and 3PDS) with bound ligands, cyanopindolol, and FAUC50, were used to construct the human ghrelin receptor structure by homology modeling [26,27]. The two ligands were removed in the modeling structure first, and the loop region (H186-L210) was further refined by employing the Loop Refinement (MODELER) module under the disulfide bond set between C116 and C198 [28,29]. To assist the following ligand docking, the protrusive flexible loop (V184-N196) on the surface of the modeling ghrelin receptor structure was removed. The modeling structure with the lowest probability density function (PDF) total energy was selected for docking with a synthetic analogue of ghrelin, growth hormone-releasing hexapeptide-6 (GHRP-6), and the flavonoid derivatives identified in oolong teas. All the modeling processes were performed by using Discovery Studio 2.1 platform (http://accelrys.com/).
2.5. Molecular modeling and docking
The three-dimensional (3D) structures of GHRP-6 and rutin were downloaded from the PubChem compound database in the National Center for Biotechnology Information (NCBI) website (http://www.ncbi.nlm.nih.gov/). The 3D structures of the flavonoid derivatives identified in oolong teas were built based on the structure of rutin by using the Chem3D program (http://www.cambridgesoft.com/). The binding pocket for the ligand molecules was defined as the cavity among the extra-cellular loops linking transmembrane segments of III–VII in the modeling ghrelin receptor structure [30]. In the docking simulation, the ligand-binding site of the ghrelin receptor was defined as the spherical space with a 14 Å radius from the center of the binding pocket. Docking of GHRP-6 or tea flavonoid derivatives to the binding site of the ghrelin receptor was performed in silico by employing the LibDock module in the Discover Studio 2.1 package, and further minimized by smart minimize algorithm with CHARMm force field in the Discover Studio 2.1 package [31,32]. To compare the binding affinity between GHRP-6 and tea flavonoid derivatives, the intermolecular molecular interactions, H-bonding, charge-charge, cation-π interaction, and π-π interaction were further analyzed in the docking complex structures of the ghrelin receptor bound with ligands.
3. Results
3.1. Identification of flavonoid derivatives in Chin-shin oolong tea
To identify putative intermediates in the biosynthetic pathway of teaghrelins, flavonoid derivatives that were structurally related to teaghrelins were examined in Chin-shin oolong tea. The result showed that six candidate flavonoid derivatives (Compounds 1 to 6) were detected besides the two known teaghrelins (Compounds 7 and 8) (Figure 1). In comparison with three reference compounds (rutin and two teaghrelins) as well as the retention times of HPLC/UV profiles and Mass data published previously [22], the six candidate compounds were identified as quercetin-3-O-rutinoside (rutin), kaempferol-3-O-rutinoside (nicotiflorin), quercetin-3-O-glucosyl-rhamnosyl-glucoside, kaempferol-3-O-glucosyl-rhamnosyl-glucoside, quercetin-3-O-glucosyl-rhamnosyl-(p-coumaroyl) glucoside, and kaempferol-3-O-glucosyl-rhamnosyl-(p-coumaroyl) glucoside (Table 1).
Fig. 1.
High-performance liquid chromatography profiles of Chin-shin oolong tea infusion at 280 nm. Amplification of the 54–96-minute profile is shown on the inserted panel inside the diagram. The serial numbers of peaks corresponding to the identified compounds are shown in Table 1. Caffeine and the major catechin, epigallocatechin-3-gallate (EGCG), are labeled.
Table 1.
Putative biosynthetic intermediates of teaghrelins identified in Chin-shin oolong tea, and teaghrelin-like compounds identified in various oolong teas. The chromatographic profiles of these compounds are shown in Figure 1 and Figure 3.
| Peak no. | Rt (min) | [M–H]− (m/z) | MS/MS (m/z) | Compounds |
|---|---|---|---|---|
| 1 | 59.95 | 609 | 301 | Quercetin-3-O-rutinoside (Rutin) |
| 2 | 67.49 | 593 | 447, 285 | Kaempferol-3-O-rutinoside (nicotiflorin) |
| 3 | 56.63 | 771 | 609, 463, 301 | Quercetin-3-O-glucosyl-rhamnosyl-glucoside |
| 4 | 63.54 | 755 | 593, 447, 285 | Kaempferol-3-O-glucosyl-rhamnosyl-glucoside |
| 5 | 87.32 | 917 | 771, 753, 301 | Quercetin-3-O-glucosyl-rhamnosyl-(p-coumaroyl) glucoside |
| 6 | 93.40 | 901 | 755, 737, 285 | Kaempferol-3-O-glucosyl-rhamnosyl-(p-coumaroyl) glucoside |
| 7 | 83.95 | 1079 | 933, 915, 301 | Quercetin-3-O-glucosyl-rhamnosyl-(p-coumaroyl-glucosyl) glucose (Teaghrelin-1) |
| 8 | 89.14 | 1063 | 917, 899, 777, 285 | Kaempferol-3-O-glucosyl-rhamnosyl-(p-coumaroyl-glucoosyl) glucoside (Teaghrelin-2) |
| 9 | 87.87 | 1049 | 917, 903, 887, 885, 747 | Quercetin-3-O-glucosyl-rhamnosyl-(p-coumaroyl-arabinosyl) glucoside |
| 10 | 92.52 | 1033 | 901, 887, 871, 869, 747 | Kaempferol-3-O-glucosyl-rhamnosyl-(p-coumaroyl-arabinosyl) glucoside |
| 11 | 86.43 | 1063 | 917 | Quercetin-3-O-glucosyl-rhamnosyl-(p-coumaroyl) glucose-7-O-rhamnoside |
| 12 | 88.84 | 1047 | 901 | Kaempferol-3-O-glucosyl-rhamnosyl-(p-coumaroyl) glucose-7-O-rhamnoside |
3.2. Putative biosynthetic pathway of teaghrelins
On the basis of the presence of the six flavonoid derivatives in Chin-shin oolong tea, the biosynthetic pathway of teaghrelins was putatively proposed to comprise three enzymatic steps with rutin or nicotiflorin as the precursor molecule (Figure 2). In the first step, a glucose molecule was added to the rhamosyl group of rutin or nicotiflorin, and the elongation of rutinoside (diglycoside) led to the formation of flavonoid triglycoside (Compound 3 or 4). In the second step, p-coumaric acid was acylated with the initial glucose directly linked to the oxygen atom at C-3 position of flavonoid backbone, and the acylation led to the formation of acylated flavonoid triglycoside (Compound 5 or 6). In the third step, one more glucose molecule was added to the initial glucose after acylation with p-coumaric acid, and thus teaghrelin-1 and teaghrelin-2 (Compounds 7 and 8) were consequently formed from rutin and nicotiflorin, respectively.
Fig. 2.
Three enzymatic steps of the putative biosynthetic pathway of teaghrelins with rutin or nicotiflorin as the precursor molecule in Chin-shin oolong tea leaves.
3.3. Identification of teaghrelin-like compounds in various oolong teas
To search for more teaghrelin-like compounds, acylated flavonoid derivatives were screened in various Taiwan oolong teas. The results showed that in addition to the two known teaghrelins in Chin-shin oolong tea, four candidate teaghrelin-like compounds were identified in various oolong teas (Figure 3). Chin-shin oolong teas obtained from Alishan and Dongding contained the same two teaghrelins (Compounds 7 and 8), Wuyi oolong tea contained two teaghrelin-like compounds (Compounds 9 and 10), and both Baihao oolong tea and Tieguanyin contained another two teaghrelin-like compounds (Compounds 11 and 12). Detailed analyses by mass data (Table 1) showed that Compounds 9 and 10 differed from teaghrelins by replacing the last attachment of glucose (in the third enzymatic step of Figure 2) with arabinose (Figure 4). In contrast with teaghrelins, Compounds 11 and 12 did not contain the last attachment of glucose (R1 position in Figure 4); instead, they contained an extra attachment of rhamnose at C-7 of the flavonoid backbone (R2 position in Figure 4).
Fig. 3.
High-performance liquid chromatography profiles of (A) Alishan oolong, (B) Dongding oolong, (C) Wuyi oolong, (D) Tieguanyin, and (E) Baihao oolong tea infusions at 280 nm. Amplification of the 80–95-minute profile is shown on the inserted panel inside the diagram. The serial numbers of peaks corresponding to the identified compounds shown in Table 1.
Fig. 4.
Structures of teaghrelins and teaghrelin-like compounds identified in various oolong teas (Compounds 7–12).
3.4. Docking of GHRP-6 and teaghrelins into the ghrelin receptor
GHRP-6, a synthetic analogue of ghrelin, was selected as a positive control for the molecular modeling and docking to the binding pocket of the ghrelin receptor (Figures 5A–5D). As expected, GHRP-6 interacted strongly with the binding pocket of the ghrelin receptor, and three types of intermolecular interactions, including H-bonding, cation-π, and charge-charge interaction, were observed between GHRP-6 and the receptor (Figure 5D). Seven H-bonds were formed within the binding pocket; two between the ammonium group of GHRP-6 and E124 of the receptor, one between the ammonium group and Y313, one between amine group at 6-backbone and P177, one between carbonyl group at 6-backbone and Q120, one between carbonyl group at 4-backbone and S207, and one between amine group at 1-backbone and Q120. Two cation-π interactions were formed; one between the ammonium group of GHRP-6 and W276 of the receptor, and the other between phenylalanyl group of GHRP-6 and R283 of the receptor. One salt bridge was formed between nitrogen at 6-lysine of GHRP-6 and E124 of the receptor.
Fig. 5.
Modeling of growth hormone-releasing hexapeptide-6 (GHRP-6) (A–D) and teaghrelins (E–H) binding to the ghrelin receptor. (A) Modeling is displayed for the receptor (ribbon structure) with bound GHRP-6 (ball-stick structure). The binding pocket of receptor is shown as spherical surface representation. (B) Enlarged diagram is depicted without the membrane bilayer shown in the box of (A). (C, E, G) Amino acid residues around the binding pocket of the receptor are shown in stick structure, and ligand compounds (GHRP-6, teaghrelin-1, and teaghrelin-2) in ball-stick structure. (D, F, H) Detailed intermolecular interactions are illustrated between the receptor and ligands (GHRP-6, teaghrelin-1, and teaghrelin-2). The amino acids of the receptor involved in the formation of H-bonding, cation-π interaction and charge-charge interaction are shown in green, blue, and red squares, respectively. The H-bonding, cation-π interaction, and charge-charge interaction are shown by green dash, blue dot, and red line, respectively.
Similar to GHRP-6, both teaghrelin-1 (Compound 7) and teaghrelin-2 (Compound 8) were able to interact adequately with the binding pocket of the ghrelin receptor; however, only one type of intermolecular interaction, H-bonding, was observed in their interactions (Figures 5E–5H). Seven H-bonds were formed between teaghrelin-1 and the ghrelin receptor; four between glycosides of teaghrelin-1 and Q302/R107/W104 of the receptor, two between the C-4″ hydroxyl group and V212/R283, and one between C-7 hydroxyl group and E124 (Figure 5F). Comparably, there were also seven H-bonds formed between teaghrelin-2 and the ghrelin receptor; three between glycosides of teaghrelin-2 and E197/R199 of the receptor, two between the C-4″ hydroxyl group and V212/R283, one between C-7 hydroxyl group and E124, and one between C-4 carbonyl group and Q120 (Figure 5H).
4. Docking of biosynthetic intermediates of teaghrelins into the ghrelin receptor
Among the six putative biosynthetic intermediates of teaghrelins, Compounds 1–4 are only composed of flavonoid and glycoside moieties whereas Compounds 5 and 6 contained an extra coumaroyl group. Docking simulation showed that the hydrophilic glycoside moieties of Compounds 1–4 were close to five to 10 hydrophobic residues of the ghrelin receptor (distances < 5 Å) (Figures 6A–6D). Apparently, only one hydrophobic moiety, flavonoid, in the structures of Compounds 1–4 was not sufficient to interact with the hydrophobic residues in the binding pocket of the ghrelin receptor, and the hydrophilic-hydrophobic repulsions between glycosides of Compounds 1–4 and the hydrophobic binding pocket of the receptor significantly reduced the stability of the docking complexes.
Fig. 6.
Modeling of Compounds 1–6 (Tea 1 to Tea 6) docking to the ghrelin receptor. (A–D) Modeling is displayed for Compounds 1–4 (ball-stick structure) docking to the receptor (stick structure). The hydrophobic amino acids close to glycosides of Compounds 1–4 are shown in grey ball-stick style and indicated by grey boxes. (E, G) Amino acid residues around the binding pocket of the receptor are shown in stick structure, and ligands (Compounds 5 and 6) in ball-stick structure. (F, H) Detailed intermolecular interactions are illustrated between the receptor and ligands. The amino acids of the receptor involved in the formation of H-bonding and cation-π interaction are shown in green and blue squares, respectively. The H-bonding and cation-π interaction are shown by green dash and blue dot, respectively.
In contrast, flavonoid and the coumaroyl group of Compounds 5 and 6 could interact adequately with the hydrophobic binding pocket of the ghrelin receptor in a manner similar to teaghrelins (Figures 6E–6H). In addition to a cation-π interaction formed between the coumaroyl group of Compound 5 and R283 of the ghrelin receptor, five H-bonds were formed: three between the C-4′ hydroxyl group of Compound 5 and S123/Y313 of the receptor, one between the C-7 hydroxyl group and C198, and one between the C-4″ hydroxyl group and V212 (Figure 6F). Six H-bonds were formed between Compound 6 and the ghrelin receptor: four between glycosides of Compound 6 and E197/R199 of the receptor, one between the C-7 hydroxyl group and Y313, and one between the C-4″ hydroxyl group and R283 (Figure 6H).
4.1. Docking of teaghrelin-like compounds into the ghrelin receptor
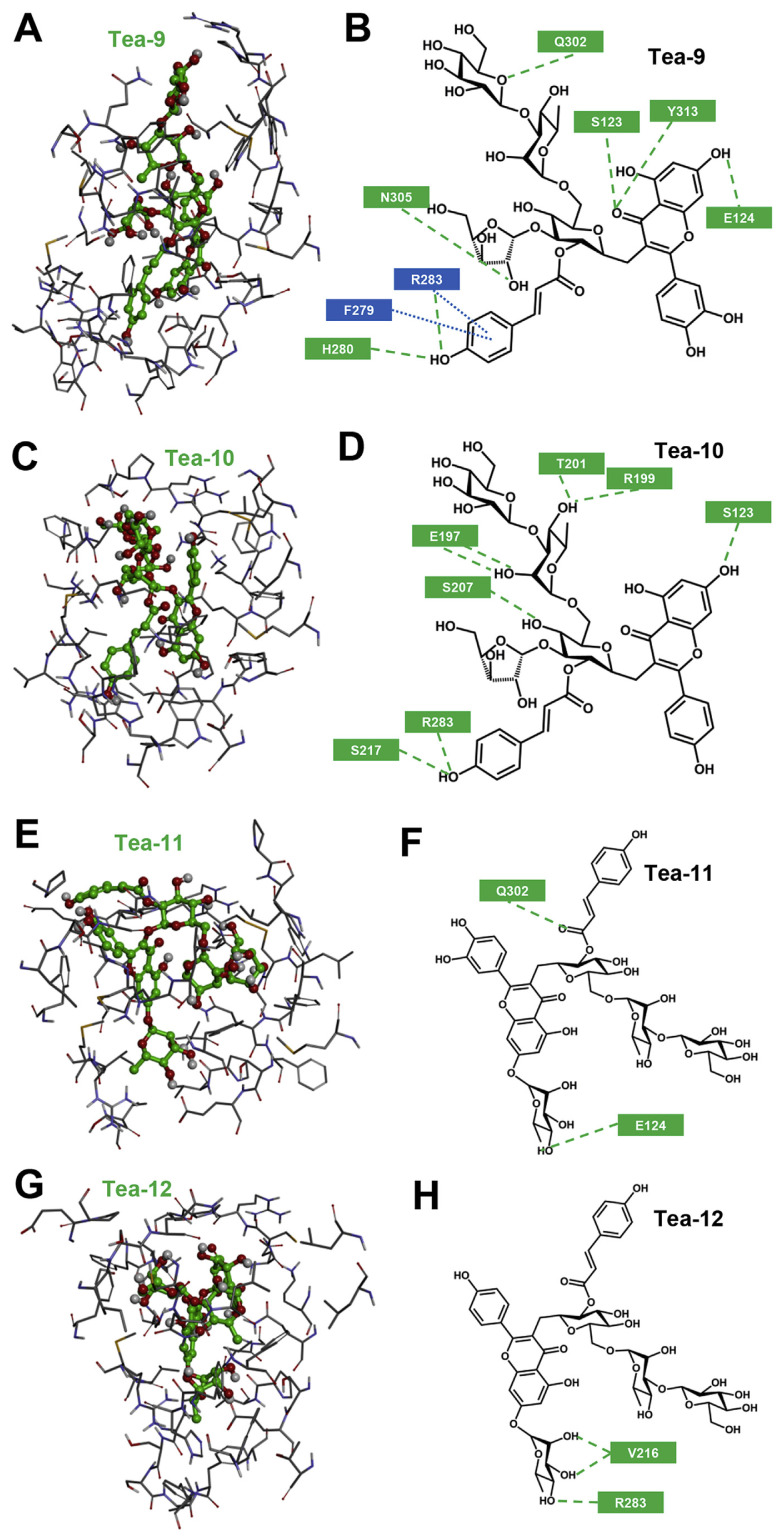
Structurally, Compounds 9 and 10 are nearly identical to teaghrelins except for the replacement of the last glucose with arabinose (R1 position in Figure 4). As expected, Compounds 9 and 10 interacted adequately with the binding pocket of the ghrelin receptor (Figures 7A–7D). In addition to cation-π and π-π interactions formed between the coumaroyl group of Compound 9 and R283/F279 of the ghrelin receptor, six H-bonds were formed: two between the C-4″ hydroxyl group of Compound 9 and H280/R283 of the receptor, two between the C-4 carbonyl group and S123/Y313, one between the C-7 hydroxyl group and E124, and two between glycosides and Q302/ N305 (Figure 7B). Eight H-bonds were formed between Compound 10 and the ghrelin receptor: five between glycosides of Compound 10 and E197/R199/T201/S207 of the receptor, two between the C-4″ hydroxyl group and S217/R283, and one between the C-7 carbonyl group and S123 (Figure 7D).
Fig. 7.
Modeling of Compounds 9–12 (Tea 9 to Tea 12) docking to the ghrelin receptor. (A, C, E, G) Amino acid residues around the binding pocket of the receptor are shown in stick structure, and ligands (Compounds 9–12) in ball-stick structure. (B, D, F, H) Detailed intermolecular interactions are illustrated between the receptor and ligands. The amino acids of the receptor involved in the formation of H-bonding and cation-π/π-π interactions are shown in green and blue squares, respectively. The H-bonding and cation-π/π-π interactions are shown by green dash and blue dot, respectively.
In contrast, Compounds 11 and 12 failed to interact properly with the ghrelin receptor as the attachment of C-7 rhamnose hindered the entrance of flavonoid and the coumaroyl group into the binding pocket of the ghrelin receptor (Figures 7E–7H). Only two or three H-bonds were observed between Compound 11 or 12 and the ghrelin receptor, and thus the additional attachment of a rhamnosyl glycoside to the flavonoid backbone of teaghrelin-like compounds at C-7 significantly reduced their binding affinity with the receptor.
5. Discussion
In this study, we proposed that teaghrelin was converted from rutin or nicotiflorin via three enzymatic steps according to the identification of putative biosynthetic intermediates in Chin-shin oolong tea. Molecular modeling and docking suggested that only Compounds 5 and 6 possessing an additional p-coumaric group were able to interact adequately with the binding pocket of the ghrelin receptor among the six putative biosynthetic intermediates. Moreover, four teaghrelin-like compounds were identified in various oolong teas. Molecular modeling and docking suggested that Compounds 9 and 10 possessing structures nearly identical to teaghrelins could also interact adequately with the binding pocket of the ghrelin receptor. In contrast, Compounds 11 and 12 possessing an extra rhamnose directly linked to the flavonoid backbone at C-7 failed to interact properly with the ghrelin receptor. Therefore, it was suggested that the attachment of a coumaroyl group was crucial for these tea compounds to bind to the ghrelin receptor, but the additional attachment of a rhamnosyl glycoside to the flavonoid backbone at C-7 significantly reduced their binding affinity with the ghrelin receptor. Our results suggest that Compounds 5, 6, 9, and 10 as well as teaghrelins are adequate ligands of the ghrelin receptor, and possess potential to be developed into oral ghrelin analogues. Moreover, it is reasonable to anticipate that different types of ghrelin analogues may be present in the rich sources of natural compounds from diverse herbal medicines. Indeed, emoghrelin found in Heshouwu was recently demonstrated to be a promising agonist of the ghrelin receptor [33]. It is expected that more candidate compounds for ghrelin analogues should be continually identified from natural sources.
Hydrophobic interaction between three nonpolar amino acids (two tryptophan and one phenylalanine residues) of GHRP-6 and the binding pocket of the ghrelin receptor plays an important role in the stabilization of the binding complex. Comparably, the nonpolar flavonoid and the coumaroyl group of teaghrelins provide adequate molecular interaction with the hydrophobic binding pocket of the ghrelin receptor. In addition to the hydrophobic interaction, the positively charged ammonium group of GHRP-6 provides a relatively strong charge-charge interaction with a negative charge residue (E124) in the binding pocket of the ghrelin receptor (Figure 5D). This strong charge-charge interaction (salt bridge) has been highlighted to play a critical role in the binding of GHRP-6 to the ghrelin receptor [30]. However, the salt bridge has no way to be formed in the docking simulation of teaghrelins within the binding pocket of the ghrelin receptor due to the lack of positively charged group in their structures. The lack of strong charge-charge interaction presumably explained why GHRP-6 possessed activity of growth hormone secretion approximately 1000 times higher than teaghrelins as observed previously [21].
Flavonoids, widely distributed in many plants, were found to have several pharmacological activities, e.g. antiallergic, anti-inflammatory, antioxidiant, antimicrobial activities, and glycosylation and acylation of flavonoids resulted in huge diversity of flavonoid derivatives [34]. Flavonoid glycosides acylated with certain hydroxycinnamic acid were recognized for their physicochemical property of UV absorbing, and thus accumulation of these flavonoid derivatives in plant tissues was regarded as a protective solution against the damage of UV radiation [35]. Possibly, the accumulation of teaghrelins in Chin-shin oolong tea leaves is also related to the defensive function of UV protection. It will be interesting to see if the teaghrelin contents in the leaves of Chin-shin oolong tea plants cultivated in various locations or harvested in different seasons are affected by the availability of sunlight (UV radiation) during their growth.
Acylation has been shown to modulate the physiological activity of flavonoids by improving stability, enhancing biological effects and conferring novel bioactivities [36,37]. Presumably, acylation with p-coumaric acid found in the structure of teaghrelins might play an important role in mimicking ghrelin activity as well as enhancing structural stability of these tea flavonoid derivatives during their metabolism in animals as observed in our pervious study [21]. Being a typical substrate constantly used for the study of flavonoid acylation in vitro, rutin has been found to be acylated at the 3-hydroxyl group of glucose and the 4-hydroxyl group of rhamnose by bacterial enzymes [38]. However, the acylation of p-coumaric acid in the structure of teaghrelins was unusually found at the 2-hydroxyl group of glucose on the rutin or nicotiflorin moiety. Whether this unusual acylation in teaghrelins is essential for their ghrelin-like activity and metabolic stability in animals seems to be an important task in the follow-up research.
Acknowledgments
The work was supported by grants to Jason TC Tzen of National Chung-Hsing University (NCHU-102S0503, NSC 100-3114-B-005-001 and MOST 104-2622-B-005-005).
Funding Statement
The work was supported by grants to Jason TC Tzen of National Chung-Hsing University (NCHU-102S0503, NSC 100-3114-B-005-001 and MOST 104-2622-B-005-005).
Footnotes
Conflicts of interest
All authors declare no conflicts of interest.
REFERENCES
- 1. Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature. 1999;402:656–60. doi: 10.1038/45230. [DOI] [PubMed] [Google Scholar]
- 2. Sakata I, Nakamura K, Yamazaki M, Matsubara M, Hayashi Y, Kangawa K, Sakai T. Ghrelin-producing cells exist as two types of cells, closed- and opened-type cells, in the rat gastrointestinal tract. Peptides. 2002;23:531–6. doi: 10.1016/s0196-9781(01)00633-7. [DOI] [PubMed] [Google Scholar]
- 3. Gutierrez JA, Solenberg PJ, Perkins DR, Willency JA, Knierman MD, Jin Z, Witcher DR, Luo S, Onyia JE, Hale JE. Ghrelin octanoylation mediated by an orphan lipid transferase. Proc Natl Acad Sci U S A. 2008;105:6320–5. doi: 10.1073/pnas.0800708105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Howard AD, Feighner SD, Cully DF, Arena JP, Liberator PA, Rosenblum CI, Hamelin M, Hreniuk DL, Palyha OC, Anderson J, Paress PS, Diaz C, Chou M, Liu KK, McKee KK, Pong SS, Chaung LY, Elbrecht A, Dashkevicz M, Heavens R, Rigby M, Sirinathsinghji DJ, Dean DC, Melillo DG, Patchett AA, Nargund R, Griffin PR, DeMartino JA, Gupta SK, Schaeffer JM, Smith RG, Van der Ploeg LH. A receptor in pituitary and hypothalamus that functions in growth hormone release. Science. 1996;273:974–7. doi: 10.1126/science.273.5277.974. [DOI] [PubMed] [Google Scholar]
- 5. Kojima M, Kangawa K. Structure and function of ghrelin. Results Probl Cell Differ. 2008;46:89–115. doi: 10.1007/400_2007_049. [DOI] [PubMed] [Google Scholar]
- 6. Castaneda TR, Tong J, Datta R, Culler M, Tschop MH. Ghrelin in the regulation of body weight and metabolism. Front Neuroendocrinol. 2010;31:44–60. doi: 10.1016/j.yfrne.2009.10.008. [DOI] [PubMed] [Google Scholar]
- 7. Kojima M, Kangawa K. Ghrelin: structure and function. Physiol Rev. 2005;85:495–522. doi: 10.1152/physrev.00012.2004. [DOI] [PubMed] [Google Scholar]
- 8. Broglio F, Gottero C, Benso A, Prodam F, Destefanis S, Gauna C, Maccario M, Deghenghi R, van der Lely AJ, Ghigo E. Effects of ghrelin on the insulin and glycemic responses to glucose, arginine, or free fatty acids load in humans. J Clin Endocrinol Metab. 2003;88:4268–72. doi: 10.1210/jc.2002-021940. [DOI] [PubMed] [Google Scholar]
- 9. Ghigo E, Broglio F, Arvat E, Maccario M, Papotti M, Muccioli G. Ghrelin: more than a natural GH secretagogue and/or an orexigenic factor. Clin Endocrinol. 2005;62:1–17. doi: 10.1111/j.1365-2265.2004.02160.x. [DOI] [PubMed] [Google Scholar]
- 10. Avau B, Carbone F, Tack J, Depoortere I. Ghrelin signaling in the gut, its physiological properties, and therapeutic potential. Neurogastroenterol Motil. 2013;25:720–32. doi: 10.1111/nmo.12193. [DOI] [PubMed] [Google Scholar]
- 11. Mequinion M, Langlet F, Zgheib S, Dickson S, Dehouck B, Chauveau C, Viltart O. Ghrelin: central and peripheral implications in anorexia nervosa. Front Endocrinol. 2013;4:15. doi: 10.3389/fendo.2013.00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Moulin A, Ryan J, Martinez J, Fehrentz JA. Recent developments in ghrelin receptor ligands. Chem Med Chem. 2007;2:1242–59. doi: 10.1002/cmdc.200700015. [DOI] [PubMed] [Google Scholar]
- 13. Smith RG. Development of growth hormone secretagogues. Endocr Rev. 2005;26:346–60. doi: 10.1210/er.2004-0019. [DOI] [PubMed] [Google Scholar]
- 14. Chen GH, Lin YL, Hsu WL, Hsieh SK, Tzen JTC. Significant elevation of antiviral activity of strictinin from Pu’er tea after thermal degradation to ellagic acid and gallic acid. J Food Drug Anal. 2015;23:116–23. doi: 10.1016/j.jfda.2014.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Yukihiko H. Elucidation of physiological functions of tea catechins and their practical applications. J Drug Food Anal. 2012;20:296–300. [Google Scholar]
- 16. Yang CS, Jin H, Guan F, Chen Y-K, Wang H. Cancer preventive activities of tea polyphenols. J Drug Food Anal. 2012;20:318–22. [Google Scholar]
- 17. Nakayama T, Ishii T, Uekusa Y, Kato K, Kumazawa S. Interaction of tea catechins with phospholipids-roles in their tastes and biological activities. J Drug Food Anal. 2012;20:305–8. [Google Scholar]
- 18. Haslam E. Thoughts on thearubigins. Phytochemistry. 2003;64:61–73. doi: 10.1016/s0031-9422(03)00355-8. [DOI] [PubMed] [Google Scholar]
- 19. Chen GH, Yang CY, Lee SJ, Wu CC, Tzen JTC. Catechin content and the degree of its galloylation in oolong tea were inversely correlated with cultivation altitude. J Food Drug Anal. 2014;22:303–9. doi: 10.1016/j.jfda.2013.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chung TY, Kuo PC, Liao ZH, Shih YE, Yang ML, Cheng ML, Wu CC, Tzen JTC. Analysis of lipophilic compounds of tea coated on the surface of clay teapots. J Food Drug Anal. 2015;23:71–81. doi: 10.1016/j.jfda.2014.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lo YH, Chen YJ, Chang CI, Lin YW, Chen CY, Lee MR, Lee VS, Tzen JTC. Teaghrelins, unique acylated flavonoid tetraglycosides in Chin-shin oolong tea, are putative oral agonists of the ghrelin receptor. J Agric Food Chem. 2014;62:5085–91. doi: 10.1021/jf501425m. [DOI] [PubMed] [Google Scholar]
- 22. Lee VS, Chen CR, Liao YW, Tzen JTC, Chang CI. Structural determination and DPPH radical-scavenging activity of two acylated flavonoid tetraglycosides in oolong tea (Camellia sinensis) Chem Pharm Bull (Tokyo) 2008;56:851–3. doi: 10.1248/cpb.56.851. [DOI] [PubMed] [Google Scholar]
- 23. Dou JP, Lee VSY, Tzen JTC, Lee MR. Identification and comparison of phenolic compounds in the preparation of oolong tea manufactured by semifermentation and drying processes. J Agric Food Chem. 2007;55:7462–8. doi: 10.1021/jf0718603. [DOI] [PubMed] [Google Scholar]
- 24. Dou J, Lee VSY, Tzen JTC, Lee MR. Rapid identification of acylated flavonol tetraglycosides in Oolong teas using HPLC-MSn. Phytochem Anal. 2008;19:251–7. doi: 10.1002/pca.1044. [DOI] [PubMed] [Google Scholar]
- 25. Biasini M, Bienert S, Waterhouse A, Arnold K, Studer G, Schmidt T, Kiefer F, Cassarino TG, Bertoni M, Bordoli L, Schwede T. SWISS-MODEL: modelling protein tertiary and quaternary structure using evolutionary information. Nucleic Acids Res. 2014;42:W252–8. doi: 10.1093/nar/gku340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Moukhametzianov R, Warne T, Edwards PC, Serrano-Vega MJ, Leslie AG, Tate CG, Schertler GF. Two distinct conformations of helix 6 observed in antagonist-bound structures of a beta1-adrenergic receptor. Proc Natl Acad Sci U S A. 2011;108:8228–32. doi: 10.1073/pnas.1100185108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rosenbaum DM, Zhang C, Lyons JA, Holl R, Aragao D, Arlow DH, Rasmussen SG, Choi HJ, Devree BT, Sunahara RK, Chae PS, Gellman SH, Dror RO, Shaw DE, Weis WI, Caffrey M, Gmeiner P, Kobilka BK. Structure and function of an irreversible agonist-beta(2) adrenoceptor complex. Nature. 2011;469:236–40. doi: 10.1038/nature09665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Fiser A, Do RKG, Sali A. Modeling of loops in protein structures. Protein Sci. 2000;9:1753–73. doi: 10.1110/ps.9.9.1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Shen MY, Sali A. Statistical potential for assessment and prediction of protein structures. Protein Sci. 2006;15:2507–24. doi: 10.1110/ps.062416606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Holst B, Lang MJ, Brandt E, Bach A, Howard A, Frimurer TM, Beck-Sickinger A, Schwartz TW. Ghrelin receptor inverse agonists: Identification of an active peptide core and its interaction epitopes on the receptor. Mol Pharmacol. 2006;70:936–46. doi: 10.1124/mol.106.024422. [DOI] [PubMed] [Google Scholar]
- 31. Brooks BR, Bruccoleri RE, Olafson BD, States DJ, Swaminathan S, Karplus M. CHARMM - a program for macromolecular energy, minimization, and dynamics calculations. J Comput Chem. 1983;4:187–217. [Google Scholar]
- 32. Dixon SL, Merz KM. One-dimensional molecular representations and similarity calculations: methodology and validation. J Med Chem. 2001;44:3795–809. doi: 10.1021/jm010137f. [DOI] [PubMed] [Google Scholar]
- 33. Lo YH, Chen YJ, Chung TY, Lin NH, Chen WY, Chen CY, Lee MR, Chou CC, Tzen JTC. Emoghrelin, a unique emodin derivative in Heshouwu, stimulates growth hormone secretion via activation of the ghrelin receptor. J Ethnopharmacol. 2015;159:1–8. doi: 10.1016/j.jep.2014.10.063. [DOI] [PubMed] [Google Scholar]
- 34. Schijlen EG, Ric de VC, van Tunen AJ, Bovy AG. Modification of flavonoid biosynthesis in crop plants. Phytochemistry. 2004;65:2631–48. doi: 10.1016/j.phytochem.2004.07.028. [DOI] [PubMed] [Google Scholar]
- 35. Ishihara K, Nakajima N. Structural aspects of acylated plant pigments: stabilization of flavonoid glucosides and interpretation of their functions. J Mol Catal B Enzym. 2003;23:411–7. [Google Scholar]
- 36. Danihelová M, Viskupičová J, Šturdík E. Lipophilization of flavonoids for their food, therapeutic and cosmetic applications. Acta Chimica Slovaca. 2012;5:59–69. [Google Scholar]
- 37. Viskupicová J, Ondrejovic M, Sturdík E. The potential and practical applications of acylated flavonoids. Pharmazie. 2009;64:355–60. [PubMed] [Google Scholar]
- 38. Mellou F, Loutrari H, Stamatis H, Roussos C, Kolisis FN. Enzymatic esterification of flavonoids with unsaturated fatty acids: effect of the novel esters on vascular endothelial growth factor release from K562 cells. Process Biochem. 2006;41:2029–34. [Google Scholar]