Abstract
Orthogonal experiment was applied to optimize the water extraction parameters of zinc from Flammulina velutipes, and then the extracts were separated by membrane filter (0.45 μm) and D101 macroporous resin. Six different species of Zn were obtained and the Zn content of various species were determined by flame atomic absorption spectrometry. The optimized conditions for the extraction of Zn were: ratio of dried material to water, 1:30; extraction temperature, 75°C; extraction time, 120 minutes. About 34.43 μg Zn was extracted from 1 g dried F. velutipes powder under the optimal conditions. The recovery value for Zn was 96.5% with a low relative standard deviation. In addition, the content of the organic state of Zn was more than that of the inorganic state, and most of the organic state Zn was found in the polysaccharide and protein fractions.
Keywords: flame atomic absorption spectrometry, Flammulina velutipes, separation, speciation analysis, zinc
1. Introduction
Flammulina velutipes is becoming a popular food for its immune-modulating, antioxidation and antitumor functional effects [1–4]. Along with the development of research, F. velutipes is well known for nutritional ingredients (e.g. polysaccharides and proteins) and health benefits [5]. It is also rich in mineral elements, especially the trace elements (e.g. zinc, chromium, and copper), which have strong physiological functions, and numerous medicinal and therapeutic effects of trace elements have been reported [6–8]. For example, Zn is essential for neurogenesis, synaptogenesis and neuronal growth [9], Cu is necessary for enzyme activation, while Cr plays an important role in the function of the pancreatic hormone insulin [10,11]. The physiological function is not only the focus of research, but also a basis of new product development. Accurate determinations of these elements are important for their study[12].
Zn, one of the essential trace elements in the human body, is helpful for health [13]. Zn benefits the nervous system: a deficiency in Zn has been associated with altered neuro-development and affects infant behavior, and cognitive and movement abilities [14,15]. F. velutipes is rich in Zn and approximately 35% of total Zn is combined with polysaccharides [16]. Therefore, it is important to study the chemical speciation of Zn in F. velutipes [17].
Some studies have been carried out on the enhancement of Zn concentration, and previous studies focused on the bio-accumulation capacity of elements [18–20]. Rabinovich et al studied the bioaccumulation capacity in mushrooms for the purpose of obtaining organisms enriched in certain essential minerals for human health [21]. Elless et al evaluated the possibility of mushrooms as a natural source of minerals supplements and the potential bioavailability of Zn was also researched [22,23]. However, only a few investigations on different species of Zn in F. velutipes have been reported. Specifically, there is a little information about the relationship between Zn speciation and its function. However, speciation information of Zn is very important since the biological role of any particular element greatly depends on its chemical form [24]. It can give useful explanation for the pharmacological mechanism to study Zn content, relative ratio of different species, and dissolution characteristics. Therefore, the objective of this study was to optimize the Zn extraction conditions for the water extraction procedure, including extraction time, temperature and solid–liquid ratio, and to separate and analyze its chemical speciation in F. velutipes. D101 macroporous resin was used as a retaining material in the column. The analytical parameters for the quantitative recoveries of analyte ions, such as pH, amounts of reagents, sample volume, were also investigated and six different species of the decoction were obtained. The trace element Zn in the species was determined by microwave digestion-flame atomic absorption spectrometry (FAAS) and the various forms of distribution and chemical speciation were studied [25].
2. Materials and methods
2.1. Material
F. velutipes was purchased from the local market (Nanjing, China). After drying in an oven (DHG-9030A, Shanghai, China) at 50°C for 1 day, F. velutipes was ground using a grinder (WFJ-15, Suzhou, China) to pass through a 200 mesh screen. The powder stored at 4°C until used.
2.2. Extraction of Zn
F. velutipes powder (2 g) was solubilized in distilled water at increasing dried material to water ratio (1:10–1:60). The extraction process was performed using a water bath set at increasing time periods (30–130 minutes) and at increasing temperature (within ±1.0°C, 50–100°C). On the basis of single-factor test results (not given in this study), an L9 (3 × 3) orthogonal test was used in this study, and the variables include extraction temperature, extraction time, and dried material to water ratio. The parameters of L9 (3 × 3) orthogonal test are shown in Table 1. The experiment was done in triplicate.
Table 1.
Orthogonal test results for Zn in Flammulina velutipes.
| Level | Temperature (°C) | Time (min) | Ratio of dried material to water (w/v) |
|---|---|---|---|
| 1 | 65 | 100 | 1:25 |
| 2 | 70 | 110 | 1:30 |
| 3 | 75 | 120 | 1:35 |
The extract was centrifuged (4480×g, 15 minutes), and the supernatant was filtered through a Whatman Nr.1 filter paper, then the filtrate was concentrated using a rotary evaporator at 50 °C under reduced pressure to obtain the extracting solution. The Zn extraction ratio (μg/g dried F. velutipes powder) was calculated as: Zn extraction ratio (μg/g) = M0/M, where M0 (μg) is the Zn content in different forms; M (g) is the weight of dried F. velutipes powder.
2.3. Separation of different speciation of Zn
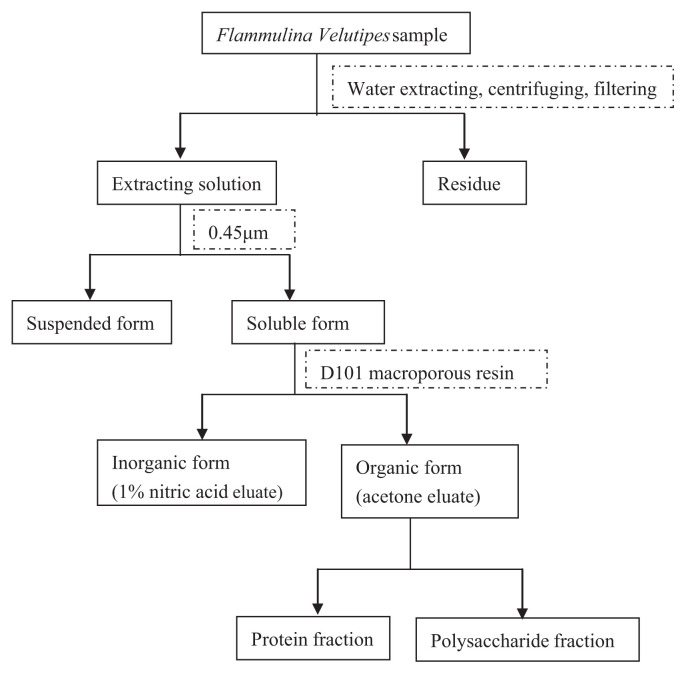
The analytical procedure for different fractions of the sample is presented in Fig. 1.
Fig. 1.
Analytical procedure for Zn speciation in samples.
2.3.1. Preparation of resin
D101 macroporous resins have found widespread application in the separation of organic and inorganic forms of trace metal ions [26]. With the macroreticular structures and high surface area, D101 macroporous has excellent physical, chemical, and thermal stability, and is good for the removal of a variety of metal ions [27]. In this study, D101 macroporous resin from Dingbei Bio-Tech Co. (Nanjing, China) was soaked in 95% aqueous ethanol for 24 hours, and washed thoroughly with deionized water for the next experiment.
2.3.2. Separation of the soluble and the suspended forms of Zn
Dried powder was mixed with water extracting solution for 120 minutes at 75°C and then solution was filtered with 0.45-μm filters, and soluble and suspended species were obtained in the solution and the precipitate separately.
2.3.3. Separation of the organic and the inorganic forms of Zn by D101 resin
Organic and inorganic forms of Zn were separated using D101 macroporous resin. Samples of D101 resins (150 g) were put in the chromatographic column (2.6 cm × 30 cm; Xuansheng Biochemistry Co., Shanghai, China). Samples of soluble Zn (3 mL) were prepared in duplicate, one of them was added to a 10 μg Zn standard sample. The pH of the soluble solution was adjusted between 2.0 and 8.0 with hydrochloric acid and aqueous ammonia solution, respectively. The samples passed through the resin column and Zn was adsorbed by the resin. Nitric acid (1%, 500 mL) solution was pumped at a flow rate of 1–3 mL/min through the column, and the collected eluent was the Zn inorganic forms. Then 500 mL of acetone, ethanol, methanol, nitric acid, or hydrochloric acid solution was pumped at a flow rate of 3 mL/min through the column. The collected eluent was the Zn organic forms. The eluents were then concentrated to 5 mL in the rotary evaporation apparatus and dried under vacuum before further analysis. The recovery rate of Zn was calculated as: Recovery rate of Zn (%) = (A1− A2)/A3 × 100, where A1 is the measurement of sample added Zn standard solution, A2 is the measurement of sample, A3 is amount of Zn standard sample.
Accuracy of the method was evaluated according to the recovery rate and relative standard deviation.
2.3.4. Separation of the protein fraction of Zn
The protein fraction of Zn was separated as described by Karadjova [19]. A 10-mL sample of organic solution of Zn was placed into a centrifuge tube and saturated with 50 mL ammonium sulfate. The mixture was shaken and then allowed to stand overnight at 4°C. The precipitate formed was centrifuged 15 minutes at 4480×g (TDL-5-A, Shanghai, China), and then dialyzed against running water and dissolved in 5 mL of 0.1M NaOH solution. The Zn content in the protein sample was determined by FAAS (Beijing Puxi Tongyong Ltd., Beijing, China) with microwave digestion.
2.3.5. Separation of the polysaccharide fraction of Zn
A 10-mL sample of organic solution of Zn was mixed with four-fold volume anhydrous ethanol (ethanol final concentration, 80%) and kept at 4°C for 24 hours. After centrifugation (TDL-5-A) at 4480×g for 15 minutes, the precipitate was washed twice with ethanol and dissolved in 5 mL double distilled water to obtain the polysaccharide sample. The Zn content was then determined by FAAS.
2.4. Determination of Zn in F. velutipes samples
A 5 mL sample of Zn solution or dried F. velutipes powder was put into polypropylene tubes, followed by the addition of 10 mL 65% nitric acid solution (digesting acid) and digestion in air/acetylene flame under optimal instrumental parameters. Blank tests were also performed at the same conditions. Samples were digested in microwave digestion instrument (CEM Company, Matthews, NC, USA), the digestion was conducted at 800 W with the following procedure: 120°C, ramp time 5 minutes, dwell time 0 minutes; 160°C, ramp time 5 minutes, dwell time 5 minutes; 180°C, ramp time 5 minutes, dwell time 10 minutes. Then the digested sample was made up to 50 mL using 0.5% nitric acid, and Zn content was determined by FAAS. The operating parameters for Zn were set as: wavelength, 213.9 nm; slit width, 0.4 nm; lamp current, 3 mA; acetylene flow rate, 1.8 L/min; gas height 6 mm.
2.5. Statistical analyses
The experimental data were analyzed using the SPSS 16.0. For each extraction procedure, a one-way ANOVA was conducted with subsequent means comparisons to identify differences among extraction methods. Values of p < 0.05 were considered to be statistically significant. All extractions and analysis were carried out in triplicate.
3. Results and discussion
3.1. Extraction of Zn
Based on the results of single factors experiments, the orthogonal table of L9 (3 × 3) was selected to optimize the extraction condition. The results of orthogonal test are shown in Table 2. The optimal extraction condition could not be chosen only based on these outcomes data. Thus, the K values (Ki is the sum of results according to level i in any column) and R values (the difference between maximum K and minimum K) were calculated and listed for further analysis. The influence to the extraction yield of Zn decreased in the order: A > B > C according to the R values. The optimized extraction conditions were: extraction temperature, 75°C; extraction time, 120 minutes; and ratio of raw material to liquid, 1:30. Under these conditions, the extraction yield of polysaccharides was 34.43 μg/g.
Table 2.
Orthogonal factor level of Zn in Flammulina velutipes.
| Experiment No. | Factors | Zn extraction ratio (μg/g) | |||
|---|---|---|---|---|---|
|
| |||||
| A Temperature (°C) | B Time (min) | C Ratio of material to water (g/mL) | Blank columns | ||
| 1 | 1 (65) | 1 (100) | 1 (1:25) | 1 | 17.26 |
| 2 | 1 | 2 (110) | 2 (1:30) | 2 | 23.72 |
| 3 | 1 | 3 (120) | 3 (1:35) | 3 | 25.25 |
| 4 | 2 (70) | 1 | 2 | 3 | 26.17 |
| 5 | 2 | 2 | 3 | 1 | 28.38 |
| 6 | 2 | 3 | 1 | 2 | 25.97 |
| 7 | 3 (75) | 1 | 3 | 2 | 29.50 |
| 8 | 3 | 2 | 1 | 3 | 32.10 |
| 9 | 3 | 3 | 2 | 1 | 34.43 |
| K1 | 22.077 | 24.310 | 25.110 | 26.690 | |
| K2 | 26.840 | 28.067 | 28.107 | 26.397 | |
| K3 | 32.010 | 28.550 | 27.710 | 27.840 | |
| R | 9.933 | 4.240 | 2.997 | 1.443 | |
In this study, ANOVA and F tests were performed to prove the significance of each factor. The analysis was carried out for a significance level of α = 0.05 (confidence level of 95%). F values of extraction temperature, extraction time, ratio of raw material to liquid were 42.408, 9.257, and 4.552, respectively. The F value of extraction temperature was larger than F critical value (F0.05 = 19.000), which indicated that extraction temperature significantly affected Zn yield. Therefore, extraction temperature was an important factor affecting the Zn yield, followed by extraction time and lastly the ratio of water to raw material according to the variance analysis.
3.2. Separation of different speciation of Zn
3.2.1. Effect of pH on the recovery of Zn for D101 macroporous resin column
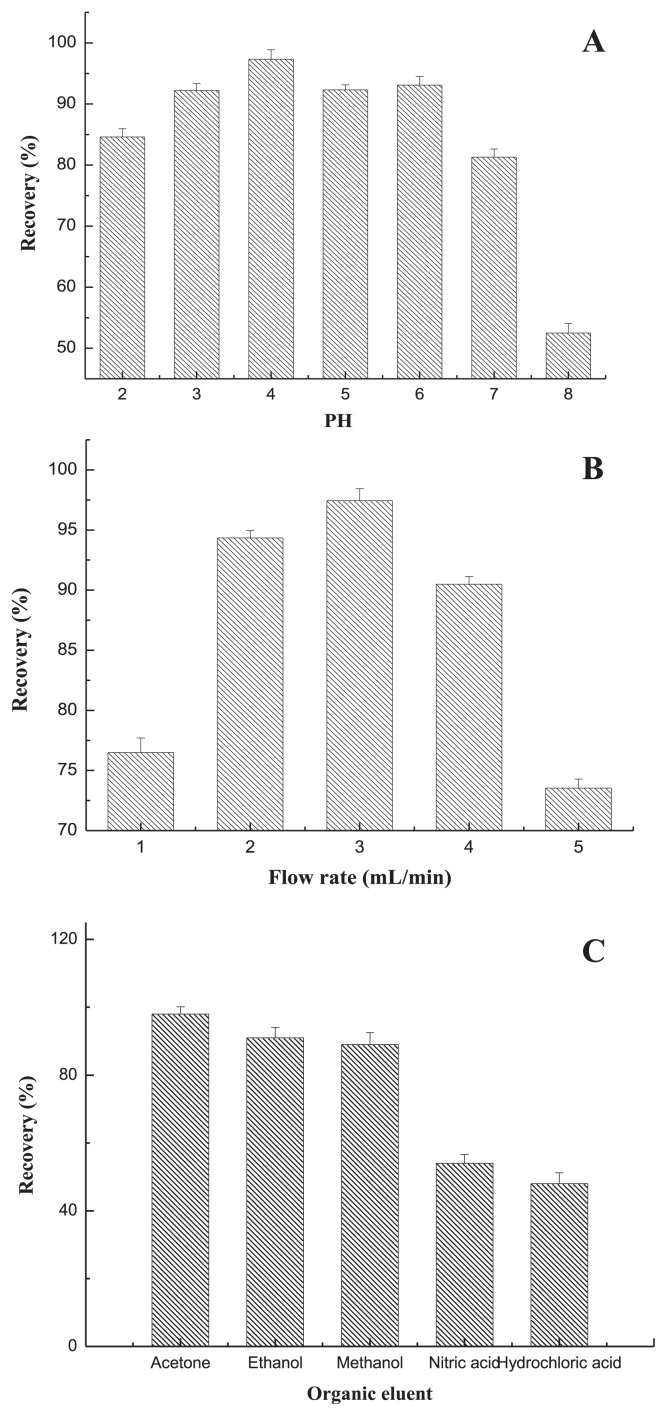
The initial pH of the Zn solution was an important factor, which controlled the sorption process, particularly was related to the sorption capacity. The formation of soluble metal complexes and their stabilities in aqueous solutions were strongly connected with the pH of the medium [28,29]. pH can change the electric charge of the sorbent surface, cause the ions present in the soluntion and the dissociation of functional groups on the active sites of the sorbent. The effect of pH on the Zn retention was investigated in the pH range 2.0–8.0 by adjusting the pH with different buffer solutions. Fig. 2A shows that the maximum recovery (97.8%) was obtained when the pH was adjusted to 4.0. Based on these results, pH 4.0 was recommended for subsequent experiments. This was consistent with reports from Tuzen et al [30].
Fig. 2.
Effect of (A) pH, (B) eluent flow rates, and (C) different eluent (C) on the Zn recovery for D101 macroporous resin column (n = 3).
3.2.2. Effect of eluent flow rates on the recovery of Zn for D101 macroporous resin column
Eluent flow rates were important parameters for quantitative retention and elution, respectively [28,31]. The influences of the flow rates were investigated in the range of 1–5 mL/min. Above 5.0 mL/min, the recoveries were not quantitative due to insufficient contact between analytes and adsorbent. As shown in Fig. 2B, the maximum recovery was 98.6% at the flow rate of 3 mL/min.
3.2.3. Effect of the organic eluent on the recovery of Zn for D101 macroporous resin column
To elute the adsorbed complex from the column, a variety of reagents were tested in order to choose the most effective eluent for quantitative recovery. Thus, acetone, ethanol, methanol, nitric acid and hydrochloric acid were studied under the optimal conditions. The results are given in Fig. 2C. It was found that acetone provided higher recovery (98%) efficiency compared to others, so acetone was selected in this study.
3.2.4. Accuracy of the method
In order to estimate the accuracy of the procedure, the proposed method was applied to study the separation and recovery of Zn in F. velutipes. Standard addition method was used to check reliabilities. Zn recovery value for the added standard was 96.5%. This indicated that this method was reliable and could be applied for the separation of Zn. The relative standard deviation value for the standard added sample was 1.67%. The limit of detection of the method for determination of Zn was studied by testing blank solutions in the same procedure under the optimal experimental conditions. The detection limit of analytical method was 1.6 μg/L.
3.3. Speciation of Zn in F. velutipes samples
Nine species, including raw material, extracting solution, residue, soluble form, suspension form, organic form, inorganic form, protein fraction, and polysaccharide fraction of Zn in F. velutipes, were analyzed and determined by FAAS. The results are shown in Table 3.
Table 3.
Analytical results of Zn speciation in Flammulina velutipes.
| Speciation | Extraction ratio of Zn (μg/g) | Percentage of Zn (%) |
|---|---|---|
| Raw material | 45.39 ± 1.34 | 100 |
| Extracting solution | 34.65 ± 1.02 | 76.34 |
| Residue | 10.21 ± 1.21 | 22.49 |
| Soluble form | 30.64 ± 0.36 | 67.50 |
| Suspension form | 3.72 ± 0.94 | 8.20 |
| Organic form | 19.68 ± 0.35 | 43.36 |
| Inorganic form | 10.85 ± 0.83 | 23.90 |
| Protein fraction | 4.83 ± 1.23 | 10.64 |
| Polysaccharide fraction | 6.74 ± 1.11 | 14.86 |
| Immerse–residue ratio | Not determined | 3.39 |
There was a high amount of Zn in F. velutipes and the extraction rate of Zn was 76.34%, which means that most Zn could be extracted efficiently from F. velutipes using water. The immerse-residue ratio of Zn was 3.39 indicating that Zn played an important role in the pharmacodynamics. In the soluble form, organic form of Zn and inorganic form was 43.36% and 23.90%, respectively. The content of organic form of Zn is almost two times of inorganic form. Furthermore, about 60% organic form of Zn was found in the polysaccharide and protein fractions. Metals and organic components existed in form of coordination compounds [8]. Organic Zn was a special group that could be used in medicine. For example, the conjugates of polysaccharides with Zn could be used as antidotes for heavy-metal poisoning [7]. Moreover, Zn also plays an important role in metabolism as an enzyme activator. It is a functional component of proteins, extensively involved in all aspects of life activities [9]. Combined with the organic compounds, Zn could be absorbed easily by our bodies and improve immune function.
4. Conclusions
Based on this study, F. velutipes is rich in trace element Zn. The optimal extraction conditions for Zn were: extraction temperature 75°C, extraction time 120 minutes and ratio of raw material to water 1:30 and extraction rate of 34.43 μg/g was obtained under these conditions. D101 macroporous resin was adapted to separate organic and inorganic forms of Zn when the sample pH was 4.0 and the elution flow rate was 3 mL/min. Six different species of Zn, namely the suspended, soluble, inorganic, organic species, protein, and polysaccharides fractions were obtained, and determined by FAAS. The soluble form of Zn was abundant, at about 67.50%. In soluble form, much of the Zn existed in organic form, about 43.36%. In addition, the content of the organic form of Zn was more than that of the inorganic form, and most of the organic Zn was found in the polysaccharide and protein fractions. Our research results are significant for medical and food applications. Speciation analysis of trace element Zn is helpful to elucidate its pharmacological mechanism.
Acknowledgments
This work was financially supported by the National Natural Science Foundation of China (No. 31101255) and the Fundamental Research Funds for the Central Universities (JKQ2011006).
Funding Statement
This work was financially supported by the National Natural Science Foundation of China (No. 31101255) and the Fundamental Research Funds for the Central Universities (JKQ2011006).
Footnotes
Conflicts of interest
All contributing authors declare no conflicts of interest.
REFERENCES
- 1. Wasser S. Medicinal mushrooms as a source of antitumor and immunomodulating polysaccharides. Appl Microbiol Biot. 2002;60:258–74. doi: 10.1007/s00253-002-1076-7. [DOI] [PubMed] [Google Scholar]
- 2. Park JP, Kim SW, Hwang HJ, Yun JW. Optimization of submerged culture conditions for the mycelial growth and exo-biopolymer production by Cordyceps militaris. Lett Appl Microbiol. 2002;33:76–81. doi: 10.1046/j.1472-765x.2001.00950.x. [DOI] [PubMed] [Google Scholar]
- 3. Yang W, Fang Y, Liang J, Hu Q. Optimization of ultrasonic extraction of Flammulina velutipes polysaccharides and evaluation of its acetylcholinesterase inhibitory activity. Food Res Int. 2002;44:1269–75. [Google Scholar]
- 4. Zhang R, Hu D, Zhang J, Zuo X, Zuo X, Jiang R, Wang H, Ng TB. Development and characterization of simple sequence repeat (SSR) markers for the mushroom Flammulina velutipes. J Biosci Bioeng. 2010;110:273–5. doi: 10.1016/j.jbiosc.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 5. Encarnacion AB, Fagutao F, Jintasataporn O, Worawattanamateekul W, Hirono I, Ohshima T. Application of ergothioneine-rich extract from an edible mushroom Flammulina velutipes for melanosis prevention in shrimp, Penaeus monodon and Litopenaeus vannamei. Food Res Int. 2012;45:232–7. [Google Scholar]
- 6. Weber G, Konieczyński P. Speciation of Mg, Mn and Zn in extracts of medicinal plants. Anal Bioanal Chem. 2003;375:1067–73. doi: 10.1007/s00216-002-1706-z. [DOI] [PubMed] [Google Scholar]
- 7. Sibikina O, Iozep A, Moskvin A. Polysaccharide complexes with metal cations: structure and application (a review) Pharm Chem J. 2009;43:341–5. [Google Scholar]
- 8. Debon SJJ, Tester RF. In vitro binding of calcium, iron and zinc by non-starch polysaccharides. Food Chem. 2001;73:401–10. [Google Scholar]
- 9. Maret W, Sandstead HH. Zinc requirements and the risks and benefits of zinc supplementation. J Trace Elem Med Biol. 2006;20:3–18. doi: 10.1016/j.jtemb.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 10. Yildiz O, Citak D, Tuzen M, Soylak M. Determination of copper, lead and iron in water and food samples after column solid phase extraction using 1-phenylthiosemicarbazide on Dowex Optipore L-493 resin. Food Chem Toxicol. 2011;49:458–63. doi: 10.1016/j.fct.2010.11.024. [DOI] [PubMed] [Google Scholar]
- 11. Divrikli U, Akdogan A, Soylak M, Elci L. Solid-phase extraction of Fe(III), Pb(II) and Cr(III) in environmental samples on amberlite XAD-7 and their determinations by flame atomic absorption spectrometry. J Hazard Mater. 2007;149:331–7. doi: 10.1016/j.jhazmat.2007.03.084. [DOI] [PubMed] [Google Scholar]
- 12. Wang Y, Bao L, Yang X, Li L, Li S, Gao H, Yao XS, Wen H, Liu HW. Bioactive sesquiterpenoids from the solid culture of the edible mushroom Flammulina velutipes growing on cooked rice. Food Chem. 2012;132:1346–53. doi: 10.1016/j.foodchem.2011.11.117. [DOI] [PubMed] [Google Scholar]
- 13. Wang X, Du Y, Liu H. Preparation, characterization and antimicrobial activity of chitosan–Zn complex. Carbohyd Polym. 2004;56:21–6. [Google Scholar]
- 14. Aimo L, Mackenzie GG, Keenan AH, Oteiza PI. Gestational zinc deficiency affects the regulation of transcription factors AP-1, NF-κB and NFAT in fetal brain. J Nutr Biochem. 2010;21:1069–75. doi: 10.1016/j.jnutbio.2009.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bertoni-Freddari C, Mocchegiani E, Malavolta M, Casoli T, Di Stefano G, Fattoretti P. Synaptic and mitochondrial physiopathologic changes in the aging nervous system and the role of zinc ion homeostasis. Mech Ageing Dev. 2006;127:590–6. doi: 10.1016/j.mad.2006.01.019. [DOI] [PubMed] [Google Scholar]
- 16. Matusiewicz H. Atom trapping and in situ preconcentration techniques for flame atomic absorption spectrometry. Spectrochim Acta B. 1997;52:1711–36. [Google Scholar]
- 17. Grindlay G, Mora J, Gras L, de Loos-Vollebregt MT. Atomic spectrometry methods for wine analysis: a critical evaluation and discussion of recent applications. Anal Chim Acta. 2011;691:18–32. doi: 10.1016/j.aca.2011.02.050. [DOI] [PubMed] [Google Scholar]
- 18. Li H, Zuo X. Speciation and size distribution of copper and zinc in urban road runoff. Bull Environ Contam Toxicol. 2013;90:471–6. doi: 10.1007/s00128-012-0953-8. [DOI] [PubMed] [Google Scholar]
- 19. Karadjova I, Izgi B, Gucer S. Fractionation and speciation of Cu, Zn and Fe in wine samples by atomic absorption spectrometry. Spectrochim Acta B. 2002;57:581–90. [Google Scholar]
- 20. Hosseini MS, Hosseini-Bandegharaei A, Raissi H, Belador F. Sorption of Cr(VI) by Amberlite XAD-7 resin impregnated with brilliant green and its determination by quercetin as a selective spectrophotometric reagent. J Hazard Mater. 2009;169:52–7. doi: 10.1016/j.jhazmat.2009.03.058. [DOI] [PubMed] [Google Scholar]
- 21. Rabinovich M, Figlas D, Delmastro S, Curvetto N. Copper- and Zinc-enriched mycelium of Agricus blazei Murrill: bioaccumulation and bioavailability. J Med Food. 2007;10:175–83. doi: 10.1089/jmf.2005.064. [DOI] [PubMed] [Google Scholar]
- 22. Elless M, Blaylock M, Huang J, Gussman CD. Plants as a natural source of concentrated mineral nutritional supplements. Food Chem. 2000;71:181–8. [Google Scholar]
- 23. Zuo X, Fu D, Li H. Speciation distribution and mass balance of copper and zinc in urban rain, sediments, and road runoff. Environ Sci Pollut R. 2012;19:4042–8. doi: 10.1007/s11356-012-0907-z. [DOI] [PubMed] [Google Scholar]
- 24. Narin I, Kars A, Soylak M. A novel solid phase extraction procedure on Amberlite XAD-1180 for speciation of Cr(III), Cr(VI) and total chromium in environmental and pharmaceutical samples. J Hazard Mater. 2008;150:453–8. doi: 10.1016/j.jhazmat.2007.04.125. [DOI] [PubMed] [Google Scholar]
- 25. Pancras P, Puri B. Column preconcentration and FAAS determination of copper, iron, nickel and zinc using 2-(5-bromo-2-pyridylazo)-5-diethylaminophenol–tetraphenyl borate–naphthalene adsorbent. Anal Bioanal Chem. 2002;374:1306–11. doi: 10.1007/s00216-002-1630-2. [DOI] [PubMed] [Google Scholar]
- 26. Yin L, Xu Y, Qi Y, Han X, Xu L, Peng J, Sun CK. A green and efficient protocol for industrial-scale preparation of dioscin from Dioscorea nipponica Makino by two-step macroporous resin column chromatography. Chem Eng J. 2010;165:281–9. [Google Scholar]
- 27. Bağ H, Türker AR, Coşkun R, Saşak M, Yiğitoğlu M. Determination of zinc, cadmium, cobalt and nikel by flame atomic absorption spectrometry after preconcentration by poly(ethylene terephthalate) fibers grafted with methacrylic acid. Spectrochim Acta B. 2000;55:1101–8. [Google Scholar]
- 28. Ghaedi M, Shokrollahi A, Kianfar AH, Mirsadeghi AS, Pourfarokhi A, Soylak M. The determination of some heavy metals in food samples by flame atomic absorption spectrometry after their separation-preconcentration on bis salicyl aldehyde, 1,3 propan diimine (BSPDI) loaded on activated carbon. J Hazard Mater. 2008;154:128–34. doi: 10.1016/j.jhazmat.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 29. Ghaedi M, Karami B, Ehsani S, Marahel F, Soylak M. Preconcentration–separation of Co2+, Ni2+, Cu2+ and Cd2+ in real samples by solid phase extraction of a calix resorcinarene modified Amberlite XAD-16 resin. J Hazard Mater. 2009;172:802–8. doi: 10.1016/j.jhazmat.2009.07.065. [DOI] [PubMed] [Google Scholar]
- 30. Tuzen M, Saygi KO, Soylak M. Novel solid phase extraction procedure for gold(III) on Dowex M 4195 prior to its flame atomic absorption spectrometric determination. J Hazard Mater. 2008;156:591–5. doi: 10.1016/j.jhazmat.2007.12.062. [DOI] [PubMed] [Google Scholar]
- 31. Soylak M, Unsal YE, Kizil N, Aydin A. Utilization of membrane filtration for preconcentration and determination of Cu(II) and Pb (II) in food, water and geological samples by atomic absorption spectrometry. Food Chem Toxicol. 2010;48:517–21. doi: 10.1016/j.fct.2009.11.005. [DOI] [PubMed] [Google Scholar]