Abstract
Xuefu Zhuyu decoction, a classic prescription in traditional Chinese medicine, has been widely used in the clinical treatment of cardiovascular and cerebrovascular diseases. In order to profile the chemical material basis of this formula, an ultra-performance liquid chromatography (UPLC) coupled with quadrupole time-of-flight mass spectrometry (Q/TOF MS) method has been established for rapid separation and structural characterization of compounds in the decoction. As a result, 103 compounds including phenolic acids, spermidines, C-glycosyl quinochalcones, terpenoids, flavonoids, saponins, and others were detected; 35 of them were unambiguously identified, and 68 were tentatively characterized by comparing the retention time, MS data, characteristic MS fragmentation pattern and retrieving the literature. In conclusion, the UPLC coupled with quadrupole time-of-flight mass spectrometry method developed in this work is an efficient approach to perform chemical material basis studies of traditional Chinese medicine formulae.
Keywords: chemical material basis, ultra-performance liquid chromatography, coupled with quadrupole, time-of-flight mass spectrometry, Xuefu Zhuyu decoction
1. Introduction
Cardiovascular and cerebrovascular diseases are common diseases of the elderly that have seriously threatened human health in recent years. Even when the most advanced and comprehensive treatment was applied, more than 50% of the survivors from cardiovascular and cerebrovascular incidents were still unable to provide for themselves completely. Every year around the world, as many as 15 million people die of cardiovascular and cerebrovascular diseases. They have become one of the primary causes of human death.
The traditional Chinese medicine formula Xuefu Zhuyu decoction (XFZYD) was first recorded in Yilin Gaicuo (Correction of Medical Errors, 1850) by Qingren Wang (1768–1831) [1]. The herbal combination is regarded as a modification of two famous classic prescriptions, Taohong Siwu decoction (Peach Seed and Safflower Decoction of Four Ingredients) and Sinisan (Powder for Regulating Liver and Spleen) [2], which comprises 11 herbs: Semen prunus (Taoren) 12 g, Radix Angelicae sinensis (Danggui) 9 g, Rhizoma chuanxiong (Chuanxiong) 4.5 g, Flos carthami (Honghua) 9 g, Radix Paeoniae rubra (Chishao) 6 g, Radix rehmanniae (Dihuang) 9 g, Fructus aurantii (Zhiqiao) 6 g, Radix Bupleuri (Chaihu) 3 g, Radix platycodonis (Jiegeng) 4.5 g, Radix Achyranthis bidentatae (Niuxi) 9 g, and Radix and Rhizoma glycyrrhizae (Gancao) 6 g [3,4]. XFZYD has been demonstrated to show definite protection in the cardiovascular and cerebrovascular system, and modern pharmacological studies have elucidated the protective mechanisms [5,6]. XYZFD could induce endothelial progenitor cell angiogenesis, hasten tube formation [7], and regulate blood lipid [8,9]. Satisfactory clinical efficiency has been achieved for cardiovascular and cerebrovascular diseases [10] such as atherosclerosis, hypertension, hyperlipidemia, thromboembolism, and angina pectoris.
It is well known that the therapeutic effects of herbal medicine are due to the synergistic contribution of multiple constituents [11]. Since XFZYD has centuries of clinical use and reliable curative efficacy, developing a feasible and rapid analytical method for characterizing the constituents in the decoction is valuable and vital to ensuring its reliability and safety in clinical therapy. Many researchers have made significant contributions to the studies of substance foundation in XFZYD. Zhang et al [12] and Liu et al [13] used high-performance liquid chromatography–mass spectrometry (HPLC-MS) methods to identify anti-atherogenic constituents of the decoction. Gao et al [14] introduced an HPLC–evaporative light scattering detector method to quantify chemical constituents in the XFZY capsule. In our previous study, an ultra-performance liquid chromatography (UPLC) coupled with diode array detector tandem MS method was undertaken to perform quantitative and qualitative analysis of the constituents in XFZYD products [15].
In order to deeply unveil the chemical compositions of XFZYD, a UPLC coupled with quadrupole time-of-flight (Q/TOF) MS method was introduced and established in this work. A total of 103 constituents were unambiguously identified or tentatively characterized. This also provides a valuable reference for further research and development of this formula and its related medicinal products.
2. Methods
2.1. Reagents and materials
HPLC grade acetonitrile and methanol were purchased from Merck (Merck, Darmstadt, Germany) and Sigma (Sigma–Aldrich, St Louis, MO, USA), respectively. Formic acid and dimethyl sulfoxide were obtained from Meridian Medical Technologies (Columbia, MD, USA). Water used in the experiment was purified by a Milli-Q water purification system (Millipore, Billerica, MI, USA).
Reference compounds (gallic acid, protocatechuic acid, p-hydroxybenzoic acid, chlorogenic acid, caffeic acid, hydroxysafflor yellow A, amygdalin, albiflorin, paeoniflorin, p-hydroxycinnamic acid, ferulic acid, schaftoside, 6-hydroxy kaempferol-3-O-glucoside, liquiritin, rutin, isoquercitrin, verbascoside, astragalin, narirutin, β-ecdysterone, naringin, rhoifolin, hesperidin, neohesperidin, liquiritigenin, naringenin, kaempferol, platycodin D, isoliquiritigenin, formononetin, ginsenoside-Ro, 18β-glycyrrhizic acid, chikusetsu saponin Iva, nobiletin, and saikosaponin A) were obtained from the National Institute for Food and Drug Control (Beijing, China), Tianjin ZhongXin Pharmaceutical Group Co., Ltd. (Tianjin, China), and Top High Bio Technology Co., Ltd. (Nanjing, China). The purities of standards were >98%.
2.2. Preparation of standard solutions
Reference compounds were accurately weighed and directly prepared in methanol and dimethyl sulfoxide as individual standard stock solutions; following this mixed standard stock solutions containing all 35 standards were prepared. A working standard solution was prepared by diluting the mixed stock solution with water (v/v, 1:3) to obtain a suitable concentration.
2.3. Plant material and sample preparation
The plant materials (Taoren, Danggui, Chuanxiong, Honghua, Chishao, Dihuang, Zhiqiao, Chaihu, Jiegeng, Niuxi, and Gancao) were purchased from Anguo (Hebei, China) and identified by Professor Tianxiang Li. All herbs were deposited in Tianjin State Key Laboratory of Modern Chinese Medicine.
According to the traditional formula, 11 plant materials (total weight of 78 g) were mixed and immersed in 600 mL deionized water for 1 hour at room temperature, and then refluxed for 2 hours twice. After filtration and concentration, aqueous extract was dried at 45°C in an oven under vacuum to give 30 g original extract powder. The yield of preparation was 38.5%. A 10 mL aliquot of methanol was added to 0.4 g of extract powder and sonicated for 30 min at room temperature. The solution was diluted with deionized water (v/v, 1:1) and then centrifuged at 17,968 g for 10 min. Finally, the supernatant was transferred to autosampler vials for UPLC-Q/TOF MS analysis.
2.4. UPLC-Q/TOF MS/MS analysis
Analysis was performed on a Waters ACQUITY UPLC system (Binary Solvent Manager, Sample Manger and thermostatically controlled column compartment; Waters Co., Parsippany, NJ, USA) coupled with Waters SYNAPT G2 high definition mass spectrometer (HDMS) with a LockSpray and an electrospray ionization (ESI) interface. The system was controlled under MassLynx V4.1 software (Waters).
Gradient elution was performed on an ACQUITY UPLC BEH C18 column (2.1 × 100 mm, 1.7 μm; Waters) at 50°C. The mobile phase was composed of 0.1% formic acid aqueous solution (A) and acetonitrile (B). The gradient program applied was as follows: 0–6.5 min, 3–11% B; 6.5–15 min, 11–20% B; 15–20 min, 20–36% B; 20–27 min, 36–48% B; 27–30 min, 48–55% B; 30–33 min, 55–74% B, and 33–35 min, 74–90% B. The flow rate was 0.3 mL/min and injection volume was 5 μL.
The analysis of mass spectra was performed in both positive and negative modes. And, the spectra were recorded in the range of m/z 100–1500 Da for full scan. The optimal MS parameters were: capillary voltage, −2.5 kV (negative ion mode), 3.0 kV (positive ion mode); capillary temperature, 120°C; desolvation temperature, 400°C; desolvation gas (N2) flow, 700 L/h; cone gas (N2) flow, 50 L/h; collision gas (Ar) flow rate, 0.20 mL/min; the MS and MS/MS acquisition rate was set at 0.2 s in centroid mode. Mass accuracy was maintained using a LockSpray with leucine–enkephalin for positive ion mode [(M+H)+ = 556.2771] and negative ion mode [(M–H)− = 554.2615] at a concentration of 200 pg/mL.
2.5. Data processing
MS and MS/MS data obtained from the robust UPLC-Q/TOF MS were performed using the aforementioned protocol. The chemical profiling study was based on retention time, precise molecular mass, isotopic pattern, MS/MS data and MS fragmentation behavior. The mass accuracy of MS and MS/MS data should be < 10.0 ppm.
3. Results and Discussion
3.1. UPLC-MS characterization of chemical constituents from XFZYD
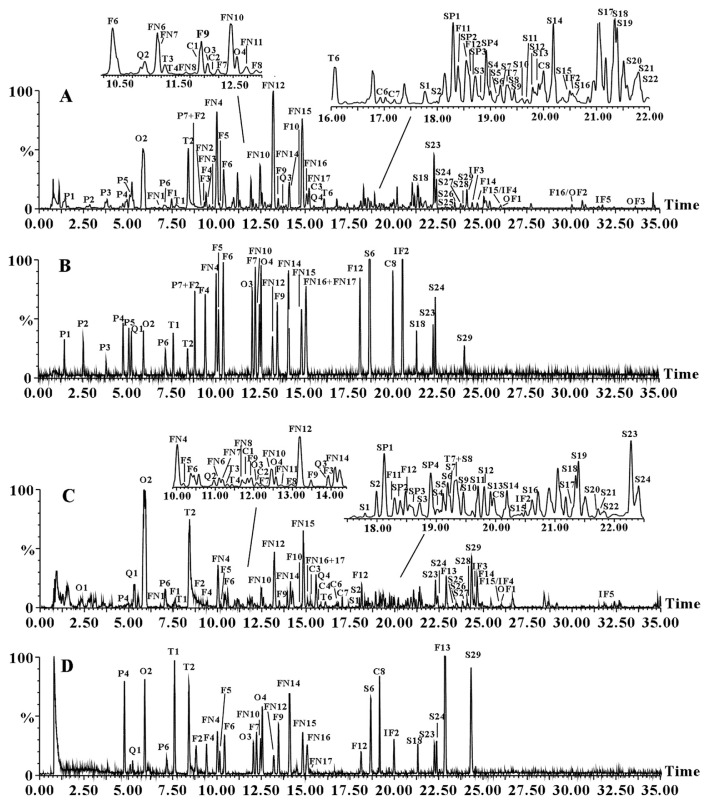
The representative base peak intensity chromatograms of XFZYD sample are presented in Fig. 1A and 1C corresponding to negative and positive ion mode, respectively. A total of 103 compounds (Fig. S1) including 7 phenolic acids, 4 spermidines, 7 terpenoids, 52 flavonoids, 29 saponins, and 4 others were characterized. Among them, 35 constituents were unambiguously identified by comparing retention time, MS, and MS/MS data with the base peak intensity chromatograms of authentic compounds (shown in Fig. 1B and 1D). A further 68 compounds were tentatively characterized by comparing accurate mass of quasimolecular and product ions, characteristic fragmentation patterns and related botanical biogenesis with the literature.
Fig. 1.
Base peak intensity chromatograms of Xuefu Zhuyu decoction extracts (A) in negative ion mode and (C) in positive ion mode, base peak intensity chromatograms of mixed standards (B) in negative ion mode and (D) in positive ion mode by ultra-performance liquid chromatography coupled with quadrupole time-of-flight mass spectrometry. C = chalcone; F = flavone; FN = flavanone; IF = isoflavone; O = others; OF = other flavonoid; Q = C-glycosyl quinochalcone; P = phenolic acid; S = saponin; SP = spermidine; T = terpenoid.
3.2. Fragmentation patterns study of XFZYD
The mass error for quasimolecular and product ions of all compounds identified by UPLC-Q/TOF MS was within ± 10 ppm. The exact identification of each group of components is outlined below.
3.3. Characterization of phenolic acids
Compounds P1, P2, P3, P4, P5, P6, and P7 were unequivocally identified as gallic acid, protocatechuic acid, p-hydroxybenzoic acid, chlorogenic acid, caffeic acid, hydroxycinnamic acid, and ferulic acid, respectively, by comparison with authentic compounds. Due to low ionization efficiency in positive ion mode, most phenolic acids were only presented in negative ion mode except chlorogenic acid. Compounds P1, P2, P3, P5, and P6 showed characteristic neutral loss of CO2. Compound P4 exhibited ions at m/z 353.0858 [M–H]− and 377.0849 [M+Na]+. The fragment ion at m/z 191.0533 [M–H–C9H6O3]− and 215.0596 [M+Na-C9H6O3]+ corresponded to the loss of caffeoyl residue. Compound P7 produced a dominant deprotonated ion at m/z 193.0499 [M–H]− with typical consecutive loss of CH3, CO, and CO2. The fragment ions of P7 at m/z 178.0242 [M–H–CH3]−, 149.0543 [M–H–CO2]−, and 134.0350 [M–H–CO2–CH3]− were shown in the negative MS/MS spectrum.
3.4. Characterization of flavonoids
Flavonoids exist in both free aglycones and glycoside forms which include several classes of compounds with similar structure having a common C6–C3–C6 flavone skeleton [16]. The cleavage of aglycones in flavonoids can be divided into two broad categories: one is the retro Diels–Alder reaction for C ring, the other is the loss of small molecular fragments such as CO, CH2O, CO2 and C2H2O. The flavonoid glycosides have many isomers with the same molecular weight but different aglycones and sugars conjugating at multiple linkage positions. Flavonoid glycosides exist in two main glycosylation modes: O-glycosylation formed by the linkage of a carbon–oxygen bond and C-glycosylation formed by the linkage of a carbon–carbon bond. These two flavonoid glycosides exhibit entirely different fragmentation behaviors. For the O-glycoside form, loss of sugar moiety is the characteristic fragmentation behavior. For the C-glycoside form, the stable carbon–carbon bond is resistant to cleavage, so cleavage of the sugar moiety is the typical MS fragmentation pattern.
3.4.1. Characterization of O-glycosylation flavonoids
The structures of flavonoids were identified at both positive and negative ion modes, and some structures were further validated with standards. Compounds FN12 and FN17 were identified as naringin and naringenin. These two compounds showed typical retro Diels–Alder reaction for C ring in both positive and negative ion modes. In positive ion spectra of compound FN12, the fragment ion at m/z 457.1137 [M+Na–C6H10O4]+ was observed, which was attributed to the loss of rhamnose. While the ion at m/z 271.0578 [M–H–C12H20O9]− in negative ion mode corresponded to the neutral loss of neohesperidose, the aglycone of compound FN12, compound FN17 showed fragment ions at m/z 147.0446 [M+H–C6H6O3]+ and 119.0508 [M+H–C6H6O3–CO]+ in positive ion mode, which were reasonably attributed to the loss of benzene-1,3,5-triol and CO. The ions at m/z 177.0160 [M–H–C6H6O]−, 151.0011 [M–H–C8H8O]−, and 107.0118 [M–H–C8H8O–CO2]− in negative ion mode corresponded to the loss of phenol, 4-vinylphenol, and CO2.
3.4.2. Characterization of C-glycosylation flavonoids
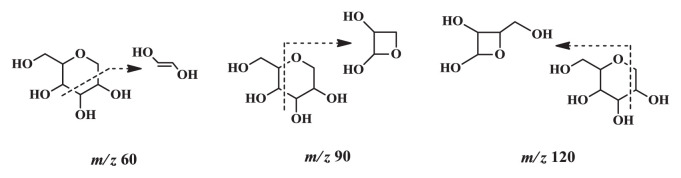
Compound F2 was identified as schaftoside. In positive ion mode, Compound F2 yielded a quasimolecular ion at m/z 565.1557 [M+H]+. The fragment ions at m/z 547.1499 [M+H–H2O]+, 529.1407 [M+H–2H2O]+, and 511.1284 [M+H–3H2O]+ were attributed to successive dehydration at sugar alcohol; the ion at m/z 445.1608 [M+H–C4H8O4]+ corresponded to sugar bond cleavage. In negative ion mode, compound F2 produced a quasimolecular ion at m/z 563.1393 [M–H]−. Subsequently, compound F2 yielded fragment ions at m/z 473.1020 [M–H–C3H6O3]−, 443.0919 [M–H–C4H8O4]−, 383.0731 [M–H–C4H8O4–C2H4O2]−, and 353.0613 [M–H–C3 H6O3–C4H8O4]−: typical fragment ions (m/z 120, 90, and 60) for cleavage of sugar bonds, which are presented in Fig. 2.
Fig. 2.
Fragmentation from glucose moieties of C-glycosyl flavonoids.
C-glycosyl quinochalcones are unique constituents in Flos carthami, which have the same quinochalcone skeleton with a hydroxyl and C-glucosyl group at the 5′-position [17]. C-glycosyl quinochalcones showed special fragmentation of glucose and branched groups in both positive and negative ion modes. These typical fragmentation pathways made it easy to characterize C-glycosyl quinochalcones in a complex sample. For example, compound Q1 showed characteristic internal cleavage of glucose, such as the loss of H2O, CH2O, C2H4O2, and C4H8O4. The fragmentation behaviors of compounds Q2–Q4 mainly occurred at branched chains. In positive ion spectra, compound Q4 yielded major fragment ions at m/z 517.0958 [M+Na–C8H8O]+, 473.1053 [M+Na–C9H8O3]+, 353.0487 [M+Na–C9H8O3–C8H8O]+, and 311.0524 [M+Na–C9H8O3–C8H8O–C2H2O]+, which was reasonably attributed to the loss of p-vinyl phenol, 3-(4-hydorxyphenyl)acrylic acid, and ethynol at side chain. Compounds Q2 and Q3 were C-glycosyl quinochalcones containing a nitrogen atom. Their fragmentations showed a special cleavage at the C–C bond except for the characteristic cleavage at branched chain. Homolysis (radical cleavage) of the C–C bond occurred, which yielded a glucose free radical. By comparison with the reported compounds and MS information, compounds Q1–Q4 were tentatively characterized as hydroxysafflor yellow A, hydroxycartormin, cartormin or isomer, and safflomin C or isomer.
3.5. Characterization of saponins
In negative ion mode, triterpene saponins yielded a dominant quasimolecular ion [M–H]− and typical fragment ions at m/z 351.0564 [glucuro-glucuronic acid-H]− and 193.0348 [glucuronic acid-H]−, or continuously lose sugars. In positive ion spectra, the characteristic MS fragmentation behavior of saponins is consecutive loss of sugars and H2O. In addition, acetylated saponins often lose an acetyl group before the behaviors mentioned above. All these features were highly characteristic for the identification of triterpene saponins. Finally, a total of 29 triterpene saponins (Tables 1 and S1) were identified in XFZYD and showed similar MS behavior [18]. For example, ions at m/z 823.4116 [M+H]+ and 821.3956 [M–H]− of compound S23 were found in positive and negative MS spectrum, respectively. The fragment ions at m/z 647.3810 [M+H–C6H8O6]+ and 453.3374 [M+H–2C6H8O6–H2O]+ were produced directly from the precursor ion, corresponding to the neutral loss of a glucuronic acid unit, a glucuroglucuronic acid unit, and H2O. In negative mode, the fragment ions at m/z 351.0544 [M–H–C30H46O4]− and 193.0343 [M–H–C30H46O4–C6H8O6]− corresponded to [glucuroglucuronic acid-H]− and [glucuronic acid-H]−, respectively. Based on the MS/MS data and retention time compared with a reference compound, compound S23 was identified as 18β-glycyrrhizic acid.
Table 1.
The retention time, mass spectrometry data and characterization of compounds of Xuefu Zhuyu decoction.
| Peak No. | Serial No. | tR (min) | Positive (+) ion mode | Negative (−) ion mode | Formula | Identification | ||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||||
| [M+H]+/[M+Na]+ | Theoretical [M+H]+/[M+Na]+ | Error (ppm) | [M–H]−/[M–H+HCOOH]− | Theoretical [M–H]−/[M–H+HCOOH]− | Error (ppm) | |||||
| * P1 | 1 | 1.485 | — | — | — | 169.0134 | 169.0137 | −1.8 | C7H6O5 | Gallic acid |
| O1 | 2 | 2.255 | 166.0865 | 166.0868 | −1.8 | 164.0693 | 164.0712 | −5.5 | C9H11NO2 | Phenylalanine |
| * P2 | 3 | 2.563 | — | — | — | 153.0485 | 153.0488 | −2.0 | C7H6O4 | Protocatechuic acid |
| * P3 | 4 | 3.829 | — | — | — | 137.0236 | 137.0239 | −2.2 | C7H6O3 | p-Hydroxybenzoic acid |
| * P4 | 5 | 4.790 | 377.0847 | 377.0849 | −0.5 | 353.0858 | 353.0873 | −4.2 | C16H18O9 | Chlorogenic acid |
| * P5 | 6 | 5.121 | — | — | — | 179.0339 | 179.0344 | −2.8 | C9H8O4 | Caffeic acid |
| * Q1 | 7 | 5.267 | 635.1578 | 635.1588 | −1.6 | 611.1619 | 611.1612 | 1.1 | C27H32O16 | Hydroxysafflor yellow A |
| * O2 | 8 | 5.864 | 480.1482 | 480.1482 | 0.0 | 456.1495 | 456.1506 | −2.4 | C20H27NO11 | Amygdalin |
| FN1 | 9 | 7.116 | 473.1028 | 473.1060 | −6.8 | 449.1076 | 449.1084 | −1.8 | C21H22O11 | Neocarthamin |
| * P6 | 10 | 7.182 | — | — | — | 163.0391 | 163.0395 | −2.5 | C9H8O3 | p-Hydroxycinnamic acid |
| F1 | 11 | 7.472 | 595.1670 | 595.1663 | 1.2 | 593.1503 | 593.1506 | −0.5 | C27H30O15 | Vicenin-2 |
| * T1 | 12 | 7.626 | 503.1540 | 503.1529 | 2.2 | 525.1608 | 525.1608 | 0.0 | C23H28O11 | Albiflorin |
| * T2 | 13 | 8.433 | 503.1525 | 503.1529 | −0.8 | 479.1541 | 479.1553 | −2.5 | C23H28O11 | Paeoniflorin |
| * P7 | 14 | 8.835 | — | — | — | 193.0499 | 193.0501 | −1.0 | C10H10O4 | Ferulic acid |
| * F2 | 15 | 8.835 | 565.1581 | 565.1557 | 4.2 | 563.1393 | 563.1401 | −1.4 | C26H28O14 | Schaftoside |
| FN2 | 16 | 9.290 | 765.2225 | 765.2218 | 0.9 | 741.2211 | 741.2242 | −4.2 | C33H42O19 | Naringinenin-4′-O-glucoside-7-O-rutinoside or Naringinenin-4′-O-glucoside-7-O-neoheperidoside |
| F3 | 17 | 9.309 | 633.1410 | 633.1432 | −3.5 | 609.1556 | 609.1534 | 3.6 | C27H30O16 | 6-Hydroxy kaempferol-3-O-rutinoside |
| * F4 | 18 | 9.429 | 487.0862 | 487.0852 | 2.1 | 463.0871 | 463.0877 | −1.3 | C21H20O12 | 6-Hydroxy kaempferol-3-O-glucoside |
| FN3 | 19 | 9.779 | 441.1137 | 441.1162 | −5.7 | 417.1181 | 417.1186 | −1.2 | C21H22O9 | Neoliquiritin |
| * FN4 | 20 | 10.045 | — | — | — | 417.1186 | 417.1186 | 0.0 | C21H22O9 | Liquiritin |
| * F5 | 21 | 10.176 | 633.1434 | 633.1432 | 0.3 | 609.1461 | 609.1456 | 0.8 | C27H30O16 | Rutin |
| FN5 | 22 | 10.414 | 573.1589 | 573.1584 | 0.9 | 549.1612 | 549.1608 | 0.7 | C26H30O13 | Liquiritin apioside |
| * F6 | 23 | 10.439 | — | — | — | 463.0835 | 463.0877 | −9.1 | C21H20O12 | Isoquercitrin |
| Q2 | 24 | 10.902 | 616.1622 | 616.1642 | −3.2 | 592.1658 | 592.1666 | −1.4 | C27H31NO14 | Hydroxycartormin |
| FN6 | 25 | 11.173 | 473.1031 | 473.1060 | −6.1 | 449.1083 | 449.1084 | −0.2 | C21H22O11 | 4′,5,6,7-Tetrahydroxyl flavanone-6-O-glucoside |
| FN7 | 26 | 11.207 | 619.1642 | 619.1639 | 0.5 | 595.1667 | 595.1663 | 0.7 | C27H32O15 | Neoeriocitrin |
| T3 | 27 | 11.320 | 655.1631 | 655.1639 | −1.2 | 631.1666 | 631.1663 | 0.5 | C30H32O15 | Galloyl-paeoniflorin or isomer |
| T4 | 28 | 11.541 | 655.1624 | 655.1580 | 6.7 | 631.1645 | 631.1663 | −2.9 | C30H32O15 | Galloyl-paeoniflorin or isomer |
| FN8 | 29 | 11.677 | 595.2019 | 595.2027 | −1.3 | 593.1890 | 593.1870 | 3.4 | C28H34O14 | Neoponcirin |
| C1 | 30 | 11.857 | 676.2595 | 676.2605 | −1.5 | 674.2450 | 674.2449 | 0.1 | C33H41NO14 | Isoliquiritigenin-4-O-apiosyl-(1→2)-[2-(2-piperidyl) acetyl]-glucoside or isomer |
| FN9 | 31 | 11.932 | 617.1864 | 617.1846 | 2.9 | 593.1907 | 593.1870 | 6.2 | C28H34O14 | Poncirin |
| * O3 | 32 | 12.069 | 647.1943 | 647.1952 | −1.4 | 623.1978 | 623.1976 | 0.3 | C29H36O15 | Verbascoside |
| C2 | 33 | 12.082 | 676.2595 | 676.2605 | −1.5 | 674.2424 | 674.2449 | −3.7 | C33H41NO14 | Isoliquiritigenin-4-O-apiosyl-(1→2)-[2-(2-piperidyl) acetyl]-glucoside or isomer |
| * F7 | 34 | 12.249 | 471.0902 | 471.0903 | −0.2 | 447.0915 | 447.0927 | −2.7 | C21H20O11 | Astragalin |
| * FN10 | 35 | 12.479 | 603.1680 | 603.1690 | −1.7 | 579.1716 | 579.1714 | 0.3 | C27H32O14 | Narirutin |
| * O4 | 36 | 12.573 | 503.2960 | 503.2985 | −5.0 | 479.2997 | 479.3009 | −2.5 | C27H44O7 | Ecdysterone |
| FN11 | 37 | 12.724 | 457.1096 | 457.1111 | −3.3 | 433.1130 | 433.1135 | −1.2 | C21H22O10 | Naringenin-7-O-glucoside |
| F8 | 38 | 12.867 | 649.1355 | 649.1381 | −4.0 | 625.1417 | 625.1405 | 1.9 | C27H30O17 | 6-Hydroxy kaempferol-di-O-glucoside |
| * FN12 | 39 | 13.215 | 603.1694 | 603.1690 | 0.7 | 579.1718 | 579.1714 | 0.7 | C27H32O14 | Naringin |
| * F9 | 40 | 13.485 | 579.1717 | 579.1714 | 0.5 | 577.1538 | 577.1557 | −3.3 | C27H30O14 | Rhoifolin |
| Q3 | 41 | 13.908 | 598.1504 | 598.1537 | −5.5 | 574.1539 | 574.1561 | −3.8 | C27H29NO13 | Cartormin |
| FN13 | 42 | 13.922 | 779.2367 | 779.2374 | −0.9 | 755.2375 | 755.2399 | −0.8 | C34H44O19 | Hesperetin-4′-O-rhamnoside-7-O-rutinoside |
| * FN14 | 43 | 14.106 | 611.2001 | 611.1976 | 4.1 | 609.1814 | 609.1819 | −1.8 | C28H34O15 | Hesperidin |
| F10 | 44 | 14.716 | 609.1842 | 609.1819 | 3.8 | 607.1652 | 607.1663 | 1.8 | C28H32O15 | Diosmin or Neodiosmin |
| * FN15 | 45 | 14.853 | 611.1969 | 611.1976 | −1.1 | 609.1830 | 609.1819 | −0.8 | C28H34O15 | Neohesperidin |
| * FN16 | 46 | 15.108 | 257.0808 | 257.0814 | −2.3 | 255.0650 | 255.0657 | −1.8 | C15H12O4 | Liquiritigenin |
| * FN17 | 47 | 15.154 | 273.0793 | 273.0763 | 11.0 | 271.0605 | 271.0606 | 1.8 | C15H12O5 | Naringenin |
| C3 | 48 | 15.249 | 419.1333 | 419.1342 | −2.1 | 417.1176 | 417.1186 | −2.7 | C21H22O9 | Isoliquiritin |
| Q4 | 49 | 15.512 | 637.1505 | 637.1533 | −4.4 | 613.1547 | 613.1557 | −0.4 | C30H30O14 | Safflomin C or isomer |
| C4 | 50 | 15.640 | 615.1701 | 615.1690 | 1.8 | 591.1727 | 591.1714 | −2.4 | C28H32O14 | Acetyl-isoliquiritin apioside |
| FN18 | 51 | 15.644 | 581.1885 | 581.1870 | 2.6 | 579.1666 | 579.1714 | −1.6 | C27H32O14 | Isonaringin |
| T5 | 52 | 15.772 | 547.1685 | 547.1639 | 8.4 | 523.1673 | 523.1663 | 2.2 | C21H32O15 | Rehmannioside A |
| IF1 | 53 | 15.813 | 269.0809 | 269.0814 | −1.9 | 267.0656 | 267.0657 | −8.3 | C16H12O4 | Pallidiflorin |
| C5 | 54 | 15.971 | 441.1192 | 441.1162 | 6.8 | 417.1155 | 417.1186 | 1.9 | C21H22O9 | Neoisoliquiritin |
| T6 | 55 | 16.294 | 623.1789 | 623.1741 | 7.7 | 599.1720 | 599.1765 | −0.4 | C30H32O13 | Benzoyl-hydroxyl-paeoniflorin |
| C6 | 56 | 16.944 | 719.3814 | 719.3892 | −10.8 | 695.3956 | 695.4007 | −7.4 | C35H36O15 | Licoriceglycoside B |
| C7 | 57 | 17.211 | 749.2064 | 749.2058 | 0.8 | 725.2033 | 725.2082 | −7.5 | C36H38O16 | Licoriceglycoside A |
| S1 | 58 | 17.792 | 1277.5834 | 1277.5778 | 4.4 | 1253.5830 | 1253.5803 | −7.3 | C58H94O29 | Deapioplatycodin D3 |
| S2 | 59 | 17.999 | 1409.6264 | 1409.6210 | 3.8 | 1385.6325 | 1385.6225 | −6.8 | C63H102O33 | Platycodin D2 or platycodin D3 |
| SP1 | 60 | 18.300 | 584.2766 | 584.2761 | 0.9 | 582.2606 | 582.2604 | 2.2 | C34H37N3O6 | Safflospermidine A or safflospermidine B or N1,N5,N10-(Z)-tri-p-coumaroylsperminine or N1,N5,N10-(E)-tri-p-coumaroylsperminine |
| F11 | 61 | 18.382 | 617.1455 | 617.1482 | −4.4 | 593.1478 | 593.1506 | 7.2 | C27H30O15 | Kaempferol-3-O-rutinoside |
| SP2 | 62 | 18.589 | 584.2751 | 584.2761 | −1.7 | 582.2601 | 582.2604 | 0.3 | C34H37N3O6 | Safflospermidine A or safflospermidine B or N1,N5,N10-(Z)-tri-p-coumaroylsperminine or N1,N5,N10-(E)-tri-p-coumaroylsperminine |
| * F12 | 63 | 18.691 | 287.0560 | 287.0556 | 1.4 | 285.0403 | 285.0399 | −4.7 | C15H10O6 | Kaempferol |
| SP3 | 64 | 18.716 | 584.2777 | 584.2761 | 2.7 | 582.2598 | 582.2604 | −0.5 | C34H37N3O6 | Safflospermidine A or safflospermidine B or N1,N5,N10-(Z)-tri-p-coumaroylsperminine or N1,N5,N10-(E)-tri-p-coumaroylsperminine |
| S3 | 65 | 18.735 | 825.4304 | 825.4273 | 3.8 | 823.4091 | 823.4116 | 1.4 | C42H64O16 | Uralsaponin C or isomer |
| SP4 | 66 | 18.915 | 584.2763 | 584.2761 | 0.3 | 582.2600 | 582.2604 | −1.0 | C34H37N3O6 | Safflospermidine A or safflospermidine B or N1,N5,N10-(Z)-tri-p-coumaroylsperminine or N1,N5,N10-(E)-tri-p-coumaroylsperminine |
| S4 | 67 | 19.017 | 1115.5288 | 1115.5250 | 3.4 | 1091.5365 | 1091.5274 | −3.0 | C52H84O24 | Deapioplatycodin D |
| S5 | 68 | 19.103 | 1409.6280 | 1409.6210 | 5.0 | 1385.6316 | 1385.6225 | −0.7 | C63H102O33 | Platycodin D2 or Platycodin D3 |
| * S6 | 69 | 19.195 | 1225.5851 | 1225.5853 | −0.2 | 1223.5737 | 1223.5697 | 8.3 | C57H92O28 | Platycodin D |
| S7 | 70 | 19.235 | 1451.6106 | 1451.6037 | 4.8 | 1427.6457 | 1427.6331 | 6.6 | C65H104O34 | 2″-O-Aceytlplatycodin D2 or 3″-O-aceytlplatycodin D2 or isomer |
| T7 | 71 | 19.307 | 607.1791 | 607.1791 | 0.0 | 583.1824 | 583.1816 | 3.3 | C30H32O12 | Benzoyl-paeoniflorin |
| S8 | 72 | 19.318 | 1289.5876 | 1289.5778 | 7.6 | 1265.5905 | 1265.5803 | 8.8 | C59H94O29 | Platycodin A or platycodin C |
| S9 | 73 | 19.389 | 897.4158 | 897.4120 | 4.2 | 895.4008 | 895.3964 | 1.4 | C44H64O19 | 22β-Acetoxy licorice saponin G2 |
| S10 | 74 | 19.491 | 845.3996 | 845.3936 | 7.1 | 821.2969 | 821.2960 | 8.1 | C42H62O16 | Uralsaponin B |
| S11 | 75 | 19.727 | 1451.6464 | 1451.6307 | 10.8 | 1427.6309 | 1427.6331 | 4.9 | C65H104O34 | 2″-O-Aceytlplatycodin D2 or 3″-O-aceytlplatycodin D2 or isomer |
| S12 | 76 | 19.817 | 1289.5904 | 1289.5778 | 9.8 | 1265.5800 | 1265.5803 | 1.1 | C59H94O29 | Platycodin A or platycodin C |
| S13 | 77 | 19.934 | 985.4635 | 985.4644 | −0.9 | 983.4548 | 983.4488 | −1.5 | C48H72O21 | Licoricesaponin A3 |
| * C8 | 78 | 20.010 | 257.0815 | 257.0814 | 0.4 | 255.0651 | 255.0657 | −0.2 | C15H12O4 | Isoliquiritigenin |
| S14 | 79 | 20.201 | 881.4156 | 881.4171 | −1.7 | 879.4031 | 879.4014 | 6.1 | C44H64O18 | 22β-Acetoxyl-glycyrrhizin or isomer |
| S15 | 80 | 20.509 | 839.4077 | 839.4065 | 1.4 | 837.3946 | 837.3909 | −2.4 | C42H62O17 | Yunganoside K2 |
| * IF2 | 81 | 20.550 | 269.0808 | 269.0814 | −2.2 | 267.0656 | 267.0657 | 1.9 | C16H12O4 | Formononetin |
| S16 | 82 | 20.603 | 705.3814 | 705.3826 | −1.7 | 681.3857 | 681.3850 | 4.4 | C36H58O12 | 3-O-Glucopyranosyl platycodigenin |
| S17 | 83 | 21.301 | 821.3887 | 821.3960 | −8.9 | 819.3806 | 819.3803 | −0.4 | C42H60O16 | Licorice saponin E2 |
| * S18 | 84 | 21.347 | 979.4777 | 979.4878 | −10.3 | 955.4912 | 955.4903 | 1.0 | C48H76O19 | Ginsenoside-Ro |
| S19 | 85 | 21.411 | 839.4058 | 839.4065 | −0.8 | 837.3914 | 837.3909 | 0.4 | C42H62O17 | Macedonoside A |
| S20 | 86 | 21.745 | 839.4077 | 839.4065 | 1.4 | 837.3898 | 837.3909 | 0.9 | C42H62O17 | Macedonoside B |
| S21 | 87 | 21.824 | 949.5778 | 949.5137 | 67.5 | 925.5179 | 925.5161 | 0.6 | C48H78O17 | Saikosaponin C |
| S22 | 88 | 21.854 | 887.4010 | 887.4041 | −3.5 | 863.4072 | 863.4065 | 0.0 | C44H64O17 | 22β-Acetoxyl-glycyrrhaldehyde |
| * S23 | 89 | 22.275 | 823.4107 | 823.4116 | −1.1 | 821.3956 | 821.3960 | 1.9 | C42H62O16 | 18β-Glycyrrhizic acid |
| * S24 | 90 | 22.399 | 817.4364 | 817.435 | 1.7 | 793.4322 | 793.4374 | 0.8 | C42H66O14 | Chikusetsusaponin Iva |
| * F13 | 91 | 22.887 | 403.1385 | 403.1393 | −2.0 | — | — | — C21H22O8 | Nobiletin | |
| S25 | 92 | 23.297 | 809.4266 | 809.4323 | −7.0 | 807.4196 | 807.4167 | 3.6 | C42H64O15 | 22-Dehydroxy-uralsaponin C[14] |
| S26 | 93 | 23.465 | 823.4104 | 823.4116 | −1.5 | 821.3963 | 821.3960 | 0.4 | C42H62O16 | Licorice saponin H2 |
| S27 | 94 | 23.803 | 979.4902 | 979.4878 | 2.5 | 955.4955 | 955.4903 | 5.4 | C48H76O19 | Yunganogenin A1/B1/C1 [24,26] |
| S28 | 95 | 23.822 | 823.4098 | 823.4116 | −2.2 | 821.4030 | 821.3960 | 8.5 | C42H62O16 | Licorice saponin K2 |
| * S29 | 96 | 24.033 | 803.4545 | 803.4558 | −1.6 | 779.4599 | 779.4582 | 2.2 | C42H68O13 | Saikosaponin A |
| IF3 | 97 | 24.134 | 369.1329 | 369.1338 | −2.4 | 367.1172 | 367.1182 | −2.7 | C21H20O6 | Glisoflavone |
| F14 | 98 | 24.731 | 355.1178 | 355.1182 | −1.1 | 353.1008 | 353.1025 | −4.8 | C20H18O6 | Uralenin |
| F15/IF4 | 99 | 26.001 | 339.1218 | 339.1232 | −4.1 | 337.1068 | 337.1076 | −2.4 | C20H18O5 | Eurycarpin A or glepidotin A [7] or 6-prenyl-5,7,4′-trihydroxyflavone or lupiwighteone |
| OF1 | 100 | 26.054 | 367.1131 | 367.1182 | −13.9 | 365.1020 | 365.1025 | −1.4 | C21H18O6 | Glycyrol or isoglycyrol or neoglycyrol |
| F16/OF2 | 101 | 30.672 | 425.2003 | 425.1964 | 9.2 | 423.1848 | 423.1808 | 9.5 | C25H28O6 | Gancaonin E or 3′-(γ,γ-dimethylallyl)-kievitone |
| IF5 | 102 | 31.780 | 423.1792 | 423.1808 | −3.8 | 421.1644 | 421.1651 | −1.7 | C25H26O6 | Angustone A or isomer |
| OF3 | 103 | 33.641 | 439.2529 | 439.2484 | 10.2 | 437.2294 | 437.2328 | −7.8 | C27H34O5 | Licorisoflavan A |
Compared with authentic compounds.
3.6. Characterization of terpenoids
Monoterpene glycosides are the major bioactive constituents in Radix Paeoniae Rubra. The chemical structure of their aglycones is generally a cage-like pinane skeleton, and glucose was the only hexose hitherto reported in monoterpene glycosides of paeonia species [19].Themonoterpene glycosides are usually esterified with an aromatic acid such as benzoic acid, p-hydroxybenzoic acid, or gallic acid. A total of five monoterpene glycosides were identified based on their mass spectra. For example, compound T2 was observed in high abundance and was confirmed as paeoniflorin by comparison with a reference standard. In positive ion spectra, the quasimolecular ion [M+H]+ was too weak to be observed. Instead, compound T2 yielded a prominent adduct ion at m/z 503.1525 [M+Na]+. In negative mode, a fragment ion at m/z 449.1378 [M–H–CH2O]− was observed, which was reasonably attributed to the loss of a HCHO (formaldehyde) and assigned as the 5′-hydroxymethyl radical of the glucose residue [20]. The ion at m/z 327.1041 [M–H–CH2O–C7H6O2]− originated from the loss of HCHO and benzoic acid. The product ion at m/z 121.0276 [M–H–CH2O–C15H20O8]− indicated the presence of a benzoyl group. Compound T1, an isomer of paeoniflorin, was unequivocally identified as albiflorin by comparison with reference standard. In positive ion mode, it showed a dominant adduct ion at m/z 503.1540 [M+Na]+. The fragment ions at m/z 341.0930 [M+Na–C6H10O5]+ and 219.0615 [M+Na–C6H10O5–C7H6O2]+ probably originated from the successive losses of a hexose residue and benzoic acid. The negative ion spectrum showed adduct ion at m/z 525.1608 [M–H+HCOOH]−. The product ion at m/z 121.0278 [M–H+HCOOH–HCOOH–C16H22O9]− indicated the presence of a benzoyl group. CompoundT3 and T4 were induced as galloylpaeoniflorin or isomer. The product ions at m/z 169.0145 [M–H–C23H26O10]− and 169.0191 [M–H–C23H26O10]− suggested the presence of galloyl radical; the productions at m/z 313.0562 [M–H–C17H18O6]− and m/z 313.0547 [M–H–C17H18O6]− corresponded to a [galloyl glucose residue-H]−.
3.7. Miscellaneous
Four isomers of spermidines (SP1–4) were detected and identified as N1,N5,N10-(E)-tri-p-coumaroylspermidine, N1,N5,N10-(Z)-tri-p-coumaroylspermidine, safflospermidine A, and safflospermidine B from F. carthami [21]. These four spermidines exhibited the same fragmentation pathways. For instance, compound SP1 yielded a quasimolecular ion at m/z 582.2606 [M–H]− in negative ion mode. The fragment ions at m/z 462.2029 [M–H–C8H8O]− and 342.1451 [M–H–2C8H8O]− originated from the loss of 4-vinylphenol. In positive ion mode, a quasimolecular ion at m/z 584.2761 [M+H]+ was observed. The product ions at m/z 438.2390 [M+H–C9H6O2]+, 420.2283 [M+H–C9H6O2–H2O]+, and 275.1764 [M+H–C9H6O2–C9H9NO2]+ originated from the successive losses of 4-(3-oxoallylidene)cyclohexa-2,5-dienone, H2O, and 3-(4-hydroxyphenyl)acrylamide.
In the positive ion spectra of compound O1, the fragment ion at m/z 120.0808 [M+H–CH2O2]+ was observed, corresponding to the neutral loss of a formic acid via a rearrangement specific to amino acids. By comparing the MS data, elemental composition and fragmentation patterns with the literature data [22], compound O1 was tentatively identified as phenylalanine. Compound O2 was definitely assigned to amygdalin by comparison with an authentic standard. The highest intensity product ions at m/z 323.0948 [M–H–C8H7NO]− and 347.0946 [M+Na–C8H7NO]+ were formed by the neutral loss of mandelonitrile from the parent ion. Compound O3 exhibited a quasimolecular ion at m/z 623.1978 [M–H]− in negative ion mode. The fragment ions at m/z 461.1594 [M–H–C9H6O3]− of MS/MS corresponded to a loss of 2-hydroxy-4-(3-oxoallylidene)cyclohexa-2,5-dienone. The fragment ion at m/z 161.0222 [M–H–C20H30O12]− also indicated the presence of 2-hydroxy-4-(3-oxoallylidene)cyclohexa-2,5-dienone. Compound O3 was identified as verbascoside by comparison with the authentic compound. In positive ion spectra, the adduction at m/z 647.1943 [M+Na]+ could further support identification. Compound O4 was identified as β-ecdysterone by comparison with authentic compound.
4. Conclusion
A rapid and effective method based on UPLC-Q/TOF MS has been developed for separation and characterization of chemical profiles of XFZYD. This method successfully detected 103 major compounds in XFZYD, including 7 phenolic acids, 4 spermidines, 7 terpenoids, 52 flavonoids, 29 saponins, and 4 other compounds. The results could pave the way for the further study of XFZYD in pharmacology and mechanism.
Acknowledgments
This work was supported by grants from the National Science Foundation of China (81202877) and National Major Scientific and Technological Special Project for Significant New Drugs Development (2015ZX09J15102-004-004).
Appendix A. Supplementary data
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.jfda.2015.06.004.
Funding Statement
This work was supported by grants from the National Science Foundation of China (81202877) and National Major Scientific and Technological Special Project for Significant New Drugs Development (2015ZX09J15102-004-004).
Footnotes
Conflicts of interest
The authors declare that they have no conflicts of interest.
REFERENCES
- 1. Shoja MM, Tubbs RS, Shokouhi G, Loukas M. Wang Qingren and the 19th century Chinese doctrine of the bloodless heart. Int J Cardiol. 2010;145:305–6. doi: 10.1016/j.ijcard.2009.10.042. [DOI] [PubMed] [Google Scholar]
- 2. Chen JB. Discussions of Xuefuzhuyu decoction in principle of composition and clinical validation formula. Acta Chin Med Pharmacol. 1989;(3):36–60. [Google Scholar]
- 3.National Commission of Chinese Pharmacopoeia. Pharmacopoeia of the People’s Republic of China. Vol. 1. Beijing: China Medical Science and Technology Press; 2010. p. 693. [Google Scholar]
- 4. Su B, Sun Q, Li XL. Pharmacological studies and clinical application of Xue Fu Zhu Yu decoction. Chin Tradit Patent Med. 2002;24:63–5. [Google Scholar]
- 5. Zhang YH, Hua HM. The Progress of the Xuefuzhuyu decoction in treating cardiovascular diseases. Info Tradit Chin Med. 2010;27:118–20. [Google Scholar]
- 6. Yu DY, Wei KB, Wo XD. Clinical and experimental study of xuefu zhuyu tang in treating qi stagnation and the blood stasis type of hyperlipidemia. Zhong Xi Yi Jie He Za Zhi. 1988;8:601–3582. [PubMed] [Google Scholar]
- 7. Gao D, Wu LY, Jiao YH, Chen WY, Chen Y, Kaptchuk TJ, et al. The effect of Xuefu Zhuyu decoction on in vitro endothelial progenitor cell tube formation. Chin J Integr Med. 2010;16:50–3. doi: 10.1007/s11655-010-0050-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lee JJ, Hsu WH, Yen TL, Chang NC, Luo YJ, Hsiao G, et al. Traditional Chinese medicine, Xue-Fu-Zhu-Yu decoction, potentiates tissue plasminogen activator against thromboembolic stroke in rats. J Ethnopharmacol. 2011;134:824–30. doi: 10.1016/j.jep.2011.01.033. [DOI] [PubMed] [Google Scholar]
- 9. Song X, Wang J, Wang P, Tian N, Yang M, Kong L. 1H NMR-based metabolomics approach to evaluate the effect of Xue-Fu-Zhu-Yu decoction on hyperlipidemia rats induced by high-fat diet. J Pharm Biomed Anal. 2013;78–79:202–10. doi: 10.1016/j.jpba.2013.02.014. [DOI] [PubMed] [Google Scholar]
- 10. Huang Q, Qiao X, Xu X. Potential synergism and inhibitors to multiple target enzymes of Xuefu Zhuyu decoction in cardiac disease therapeutics: a computational approach. Bioorg Med Chem Lett. 2007;17:1779–83. doi: 10.1016/j.bmcl.2006.12.078. [DOI] [PubMed] [Google Scholar]
- 11. Ren MT, Chen J, Song Y, Sheng LS, Li P, Qi LW. Identification and quantification of 32 bioactive compounds in Lonicera species by high performance liquid chromatography coupled with time-of-flight mass spectrometry. J Pharm Biomed Anal. 2008;48:1351–60. doi: 10.1016/j.jpba.2008.09.037. [DOI] [PubMed] [Google Scholar]
- 12. Zhang HJ, Cheng YY. An HPLC/MS method for identifying major constituents in the hypocholesterolemic extracts of Chinese medicine formula ‘Xue-Fu-Zhu-Yu decoction’. Biomed Chromatogr. 2006;20:821–6. doi: 10.1002/bmc.607. [DOI] [PubMed] [Google Scholar]
- 13. Liu L, Cheng Y, Zhang H. Phytochemical analysis of anti-atherogenic constituents of Xue-Fu-Zhu-Yu-Tang using HPLC-DAD-ESI-MS. Chem Pharm Bull (Tokyo) 2004;52:1295–301. doi: 10.1248/cpb.52.1295. [DOI] [PubMed] [Google Scholar]
- 14. Gao Y, Gao WY, Guo P, Li FG, Ma CY, Man SL, et al. Simultaneous determination of nine active components in traditional Chinese medicine ‘Xue-Fu-Zhu-Yu’ capsule by HPLC-ELSD. Latin Am J Pharm. 2011;30:281–8. [Google Scholar]
- 15. Zhang L, Zhu L, Wang Y, Jiang Z, Chai X, Zhu Y, et al. Characterization and quantification of major constituents of Xue Fu Zhu Yu by UPLC-DAD-MS/MS. J Pharm Biomed Anal. 2012;62:203–9. doi: 10.1016/j.jpba.2011.12.026. [DOI] [PubMed] [Google Scholar]
- 16. Jin Y, Xiao YS, Zhang FF, Xue XY, Xu Q, Liang XM. Systematic screening and characterization of flavonoid glycosides in Carthamus tinctorius L. by liquid chromatography/UV diode-array detection/electrospray ionization tandem mass spectrometry. J Pharm Biomed Anal. 2008;46:418–30. doi: 10.1016/j.jpba.2007.10.036. [DOI] [PubMed] [Google Scholar]
- 17. Jin Y, Zhang XL, Shi H, Xiao YS, Ke YX, Xue XY, et al. Characterization of C-glycosyl quinochalcones in Carthamus tinctorius L. by ultraperformance liquid chromatography coupled with quadrupole-time-of-flight mass spectrometry. Rapid Commun Mass Spectrom. 2008;22:1275–87. doi: 10.1002/rcm.3468. [DOI] [PubMed] [Google Scholar]
- 18. Zheng YF, Qi LW, Zhou JL, Li P. Structural characterization and identification of oleanane-type triterpene saponins in Glycyrrhiza uralensis Fischer by rapid-resolution liquid chromatography coupled with time-of-flight mass spectrometry. Rapid Commun Mass Spectrom. 2010;24:3261–70. doi: 10.1002/rcm.4768. [DOI] [PubMed] [Google Scholar]
- 19. Yin Q, Wang P, Zhang A, Sun H, Wu X, Wang X. Ultraperformance LC-ESI/quadrupole-TOF MS for rapid analysis of chemical constituents of Shaoyao-Gancao decoction. J Sep Sci. 2013;36:1238–46. doi: 10.1002/jssc.201201198. [DOI] [PubMed] [Google Scholar]
- 20. Huang H, Ji L, Song S, Wang J, Wei N, Jiang M, et al. Identification of the major constituents in Xuebijing injection by HPLC-ESI-MS. Phytochem Anal. 2011;22:330–8. doi: 10.1002/pca.1284. [DOI] [PubMed] [Google Scholar]
- 21. Jiang JS, Lu L, Yang YJ, Zhang JL, Zhang PC. New spermidines from the florets of Carthamus tinctorius. J Asian Nat Prod Res. 2008;10:447–51. doi: 10.1080/10286020801948540. [DOI] [PubMed] [Google Scholar]
- 22. Liu EH, Qi LW, Peng YB, Cheng XL, Wu Q, Li P, et al. Rapid separation and identification of 54 major constituents in Buyang Huanwu decoction by ultra-fast HPLC system coupled with DAD-TOF/MS. Biomed Chromatogr. 2009;23:828–42. doi: 10.1002/bmc.1193. [DOI] [PubMed] [Google Scholar]