Abstract
An increasing demand for natural additives has shifted the attention from synthetic to natural antioxidants and antifungal agents. This study was carried out to evaluate the antifungal and antioxidant activities of methanol, chloroform, and aqueous extracts of Annona squamosa Linn. leaves. The antifungal activities of all extracts of A. squamosa leaves against five different strains of fungi (Alternaria alternata, Candida albicans, Fusarium solani, Microsporum canis, and Aspergillus niger) were evaluated by the agar well diffusion method and the minimum inhibitory concentration of each extract was assessed by antifungal susceptibility using the broth microdilution method. The antioxidant potential of each extract was determined by free radicals (1,1-diphenyl-2-picrylhydrazyl, nitric oxide, and hydrogen peroxide) scavenging activity and reducing power property of A. squamosa leaves. Both organic and aqueous extracts were found to express dose-dependent inhibition against all tested fungi strains in both agar well diffusion and broth dilution methods. The free radical scavenging activity and reducing power property of all extracts were found to be concentration dependent, with the methanol extract exhibiting higher antioxidant activity than the chloroform extract, which was more effective than the aqueous extract of A. squamosa leaves. Results of phytochemical analysis of extracts showed the presence of glycosides, saponins, tannins, flavonoids, phenols, etc. The results obtained from in vitro studies of antifungal and antioxidant activities clearly suggest that the methanol, chloroform, and aqueous extracts of A. squamosa leaves possess antifungal and antioxidant activity.
Keywords: acetogenins, Annona, antifungal, antioxidant, extract
1. Introduction
In modern times, the use of herbal products has significantly increased in the developed countries as well as in several other countries. According to a World Health Organization estimate, 80% of the world’s population presently uses herbal medicine for some aspect of primary health care [1]. Many of the species are used in traditional medicines for the treatment of a variety of diseases [2]. During the past several years, there has been an increase in the incidence of fungal infections due to the rise in immunocompromised population (e.g., organ transplant recipients and patients with cancer or human immunodeficiency virus infection/acquired immune deficiency syndrome). This fact coupled with the resistance to antibiotics and with toxicity during prolonged treatment with several antifungal drugs has been the reason for an extended search for newer drugs to treat opportunistic fungal infections. Infectious diseases, particularly skin and mucosal infections, are common in most of the tribal inhabitants due to lack of sanitation, potable water, and awareness of hygienic food habits. An important group of these skin pathogens is the fungi, among which dermatophytes and Candida spp. are prominent. Antimicrobial properties of certain Indian medicinal plants were reported based on folklore information and few studies were carried out on the inhibitory activity of these plants against certain pathogenic fungi [3].
Annona is the second largest genus of flowering plants in the family Annonaceae after Guatteria. Annona squamosa Linn., one of the important medicinal plants, commonly called “custard apple,” is a well-known plant of this family. It has been reported to possess a wide variety of pharmacological activities and is used in traditional applications [4]. A. squamosa is cultivated throughout India, America, Brazil, Southern Florida, and West Indies mainly for its edible fruits [5]. The plant A. squamosa Linn. is commonly called custard apple (English), sharifa (Hindi), sitappalam (Tamil), sita phalamu (Telugu), and sitaphala (Kannada) [4]. A. squamosa is a small tree that grows up to 3–8 m, with broad, irregularly spreading branches of light brown bark having thin leaves that occur singly, measuring 5–17 cm in length and 2–6 cm in width. Flowering (greenish yellow flowers on a hairy, slender 2-cm long stalk) occurs during the period from spring to early summer and flowers are pollinated by nitidulid beetles. The round or heart-shaped greenish yellow, ripened aggregate fruit is pendulous on a thickened stalk. The pulp of the fruit is white-tinged yellow, edible, and sweetly aromatic. Each carpel contains an oblong, shiny and smooth, dark brown to black, 1.3–1.6-cm long seed (Fig. 1) [6]. Extensive biological research was carried out on this plant because of the presence of valuable annonaceous acetogenins in various parts of the plant, which are traditionally used for the treatment of many ailments [5]. So far, there are no systematic studies on the in vitro antifungal activity of the methanol, chloroform, and aqueous extracts as well as on the antioxidant effect of the chloroform extract of A. squamosa leaves. Therefore, this investigation was carried out to evaluate the antifungal and antioxidant potential of three different extracts of A. squamosa leaves.
Fig. 1.
Annona squamosa Linn. plant: (A) flowers; (B) fruits; (C) seeds [7–10].
2. Materials and methods
2.1. Chemicals and solvents
All the chemicals used in this study were of analytical reagent grade from SD Fine Chem Ltd. (Mumbai, Maharashtra, India), whereas, sabouraud 4% glucose agar and 1,1-diphenyl-2-pic-rylhydrazyl (DPPH) radical were from Sigma-Aldrich (Bangalore, Karnataka, India).
2.2. Collection of plant material
The leaves of A. squamosa were collected from fields of Dharwad, Karnataka, India, in February 2010. The leaves were identified and authenticated at the herbarium of the Botany Department of the Gulbarga University (voucher specimen HGUG No. 0019), Karnataka, India. After identification, the plant material was processed for extraction procedure.
2.3. Preparation of the plant extract
The leaves of A. squamosa were thoroughly cleaned with water to remove dust particles and shade-dried at room temperature and reduced to coarse powder using a mechanical mixer. The powder was subjected to extraction by maceration using water, chloroform, and methanol to obtain their respective extracts. To 75 g of the powdered drug, 750 mL of solvent (water or chloroform or methanol) was added and stirred occasionally. The mixture was filtered on the 8th day, and the solvent was evaporated at 40°C to obtain a solid mass [11]. The percentage yields of water, chloroform, and methanol extracts were found to be 8.7% w/w, 10.1% w/w, and 12.6% w/w, respectively, which are stored in refrigerator (−40°C) until further use.
2.4. Preliminary phytochemical screening
All extracts were subjected to preliminary qualitative phytochemical screening to detect the presence of glycosides, alkaloids, amino acids, flavonoids, carbohydrates, saponins, phenols, steroids, and tannins by standard methods [12,13].
2.5. Assessment of antifungal activity
Five pathogenic strains of fungi were used in the study, namely, Alternaria alternata, Candida albicans, Fusarium solani, Microsporum canis, and Aspergillus niger, which were collected from the Institute of Microbial Technology, Chandigarh, India. They were maintained in culture on Sabouraud glucose (4%) agar medium and used for antifungal assays as needed.
2.5.1. Zone of inhibition of fungal growth
The antifungal activity of the leaf extracts was evaluated by the agar well diffusion method. The fungal inoculates were aseptically swabbed on sterile and solidified Sabouraud dextrose agar plates. Then, aseptically, 7-mm diameter wells were bored in the inoculated plates and the extracts [1 mg/mL and 2 mg/mL of 10% dimethyl sulfoxide (DMSO)], standard (ketoconazole 100 μg/mL), and blank (10% DMSO) were added separately into the respective labeled wells. The plates were incubated at 35°C for 72 hours in an upright position and the zone of inhibition formed around the well was recorded. The experiment was carried out in triplicates to get the average reading [14]. The percentage inhibition of the methanol, chloroform, and aqueous extracts of A. squamosa leaves was calculated using the following formula: % inhibition = (diameter zone of sample/diameter zone of ketoconazole) × 100 [15].
2.5.2. Minimum inhibitory concentration against different fungal strains
The minimum inhibitory concentration (MIC) was determined using the broth dilution method. The fungal inoculum (105 dilution) was taken in test tubes with nutrient broth (1800 μL) supplemented with eight different concentrations (50 μg/mL, 100 μg/mL, 200 μg/mL, 300 μg/mL, 400 μg/mL, 600 μg/mL, 800 μg/mL, and 1000 μg/mL) of all leaf extracts separately. The results of the extracts were compared with a standard, positive control (100 μg/mL ketoconazole), and negative control (respective solvent of each extract). All the test tubes were incubated at 35°C for 24–48 hours. The tubes were examined for visual turbidity. The MIC values were taken as the lowest concentration that inhibited the visual growth of the tested organisms [14,16].
2.6. Assessment of antioxidant activity
2.6.1. Radical scavenging activity
The antioxidant activity was assessed through various in vitro assays. The free radical scavenging activities of the three extracts of A. squamosa leaves and l-ascorbic acid (vitamin C) were measured in terms of hydrogen donating or radical scavenging ability using the stable 1,1-diphenyl-2-picrylhydrazyl (DPPH) radicals. Nitric acid generated from sodium nitroprusside was measured by the Griess reaction. The antioxidant activity was further confirmed by hydrogen peroxide (H2O2) scavenging and reducing power properties of A. squamosa leaves extracts.
The percentage scavenging of free radical activity was calculated using the following formula:
| (1) |
The half-maximal inhibitory concentration (IC50) values were determined as the concentration of the test mixture that gave 50% reduction in absorbance from that of the control.
2.6.1.1. DPPH free radical scavenging activity
The DPPH assay was performed as described by Koleva et al [17]. Approximately 10 μL of different concentrations (10–500 μg/mL) of test sample solutions were added to 190 μL DPPH (150 μM) in ethanol solution. The solutions were later vortex mixed and incubated for 20 minutes at 37°C. The solvent alone was considered “blank.” The decrease in absorbance of test mixtures (due to quenching of DPPH free radicals) was determined at 517 nm; ascorbic acid (5–250 μg/mL) was used as the standard [18].
2.6.1.2. Hydrogen peroxide (H2O2) scavenging activity
To evaluate the hydrogen peroxide (H2O2) scavenging activity of the extracts, the H2O2 solution was prepared in phosphate buffer (pH 7.4) and then 0.6 mL of 40 mM H2O2 solution was mixed with 0.1 mL of different concentrations (10–500 μg/mL) of the extracts. Absorbance of hydrogen peroxide at 230 nm was determined after 10 minutes against a blank solution containing phosphate buffer without hydrogen peroxide. Ascorbic acid (5–250 μg/mL) was taken as the reference compound [19].
2.6.1.3. Nitric oxide radical scavenging activity
Nitric oxide (NO) generated from sodium nitroprusside was measured by the Griess reaction [20]. Sodium nitroprusside (5μM) in phosphate-buffered saline was mixed with 3 mL of different concentrations (10–500 μg/mL) of A. squamosa extracts and the mixture was incubated at 25°C for 150 minutes. The samples were then allowed to react with the Griess reagent (1% sulfanilamide and 0.1% naphthyl ethylenediamine dihydrochloride in 2% H3PO4). The absorbance of the chromophore during the diazotization of nitrite with sulfanilamide and subsequent coupling with naphthyl ethylenediamine was measured at 546 nm. A similar procedure was repeated with respective solvent instead of the extract, which served as the control; l-ascorbic acid (5–250 μg/mL) was used as the positive control [21]. All the tests were performed in triplicate. The percentage of scavenging activity was measured using the formula mentioned in the “Radical Scavenging Activity” section.
2.6.2. Reducing power property
The reducing power of extracts was determined according to the method suggested by Yen and Chen [22]. Different concentrations of each extract (25–500 μg/mL) were mixed with phosphate buffer (2.5 mL, 0.2M, pH 6.6) and potassium ferric cyanide [K3Fe(CN)6] (2.5 mL, 1%). The mixture was incubated at 50°C for 20 minutes. A portion (2.5 mL) of trichloroacetic acid (10%) was added to the mixture to stop the reaction, and the mixture was centrifuged at 3000 rpm for 10 minutes. The upper layer of solution (2.5 mL) was mixed with distilled water (2.5 mL) and ferric chloride (0.5 mL, 0.1%), and the absorbance was measured at 700 nm. A similar procedure was repeated with respective solvent instead of the extract, which served as the control. Ascorbic acid was used as the standard. All tests were performed in triplicate. Increased absorbance of the reaction mixture indicates the increased reducing power [23].
2.7. Statistical analysis
The experimental results pertaining to the antifungal activity were expressed as the mean ± standard error of the mean (SEM), and for antioxidant activity, the average values from three parallel measurements were depicted in Figs. 3 and 4. Linear regression analysis was used to calculate the IC50 values. Data were analyzed using one-way analysis of variance (ANOVA) followed by the Dunnett post hoc test using GraphPad Prism 5 (GraphPad Software, Inc., La Jolla, CA, USA).
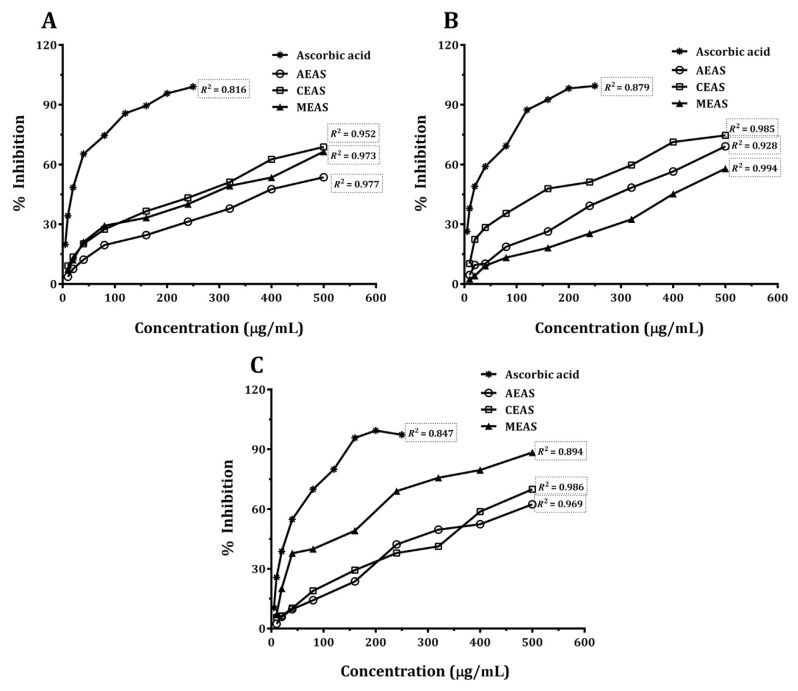
Fig. 3.
(A) 1,1-Diphenyl-2-picrylhydrazyl radical scavenging activity of Annona squamosa Linn. leaves. (B) H2O2 scavenging activity of A. squamosa leaves. (C) Nitric oxide radical scavenging activity of A. squamosa leaves. AEAS = aqueous extract; CEAS = chloroform extract; MEAS = methanol extract.
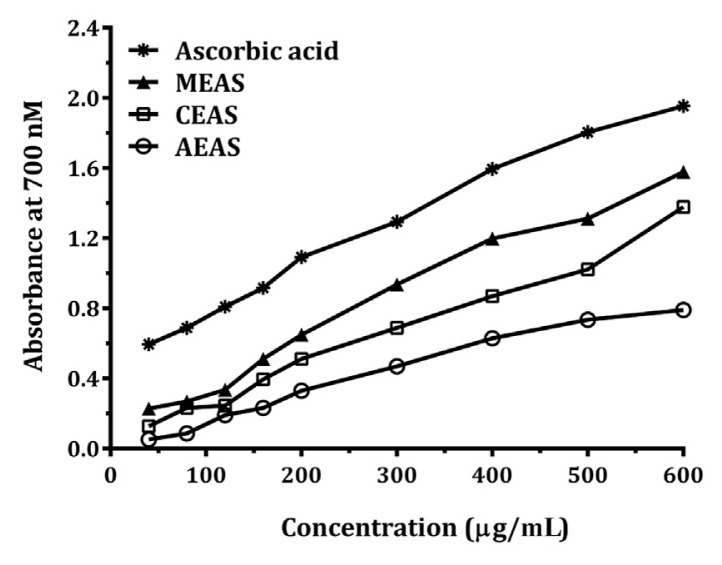
Fig. 4.
Reducing power property of Annona squamosa Linn. leavesextracts. AEAS =aqueous extract; CEAS =chloroform extract; MEAS = methanol extract.
3. Results
3.1. Phytochemical analysis
The phytochemical qualitative analysis revealed the presence of glycosides, flavonoids, phenols, tannins, saponins, alkaloids, carbohydrates, and steroids in different extracts as reported in Table 1.
Table 1.
Preliminary phytochemical analysis of the methanol, chloroform, and aqueous extracts of Annona squamosa Linn. leaves.
| Extract | Methanol extract | Chloroform extract | Aqueous extract |
|---|---|---|---|
| Alkaloids | + | − | − |
| Glycosides | + | + | + |
| Flavonoids | + | + | + |
| Tannins | + | + | − |
| Carbohydrates | + | − | + |
| Phenols | + | + | + |
| Saponins | + | − | + |
| Amino acids | − | − | + |
| Steroids | − | + | − |
+ = present; − = absent.
3.2. Antifungal assessment
3.2.1. Agar well diffusion method
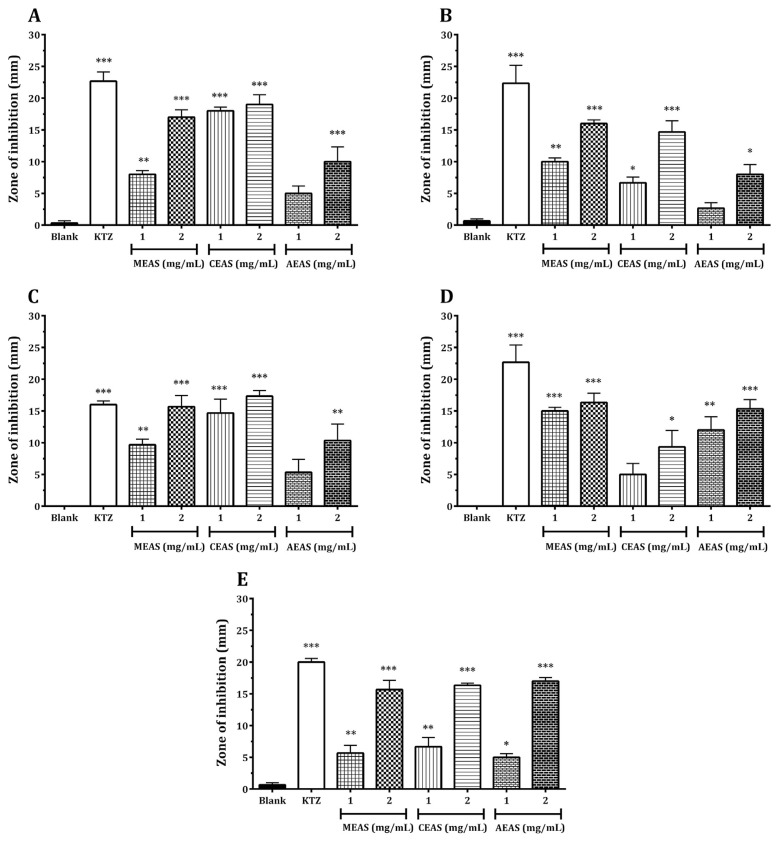
The results of this study revealed that the leaves of A. squamosa possess potential antifungal activity against all five pathogenic fungal strains studied. The antifungal activity was determined using the agar well diffusion method by measuring the diameter of zone of inhibition and by calculating the percentage inhibition. The antifungal activity of the tested extracts showed different selectivity for each micro-organism studied. Our analysis indicated that both chloroform and methanol extracts showed almost similar effect against A. alternata, C. albicans, and F. solani (Fig. 2A–2C, respectively) with percentage inhibition of 79.10%, 27.69%, and 91.67% at 1 mg/mL and 83.58%, 64.62%, and 108.33% at 2 mg/mL of the chloroform extract, respectively, and 34.33%, 43.08%, and 60.42% at 1 mg/mL and 74.63%, 70.77%, and 97.92% at 2 mg/mL of the methanol extract, respectively. However, the aqueous extract of A. squamosa showed relatively less activity against A. alternata and C. albicans (Fig. 2A and 2B, respectively), with percentage inhibition of 20.90% and 9.23% at 1 mg/mL and 43.28% and 33.85% at 2 mg/mL, respectively, whereas against F. solani, the aqueous extract exhibited intermediate effect (Fig. 2C) with percentage inhibition of 33.33% at 1 mg/mL and 64.58% at 2 mg/mL. Further, the methanolic and aqueous extracts showed maximum inhibitory activity against M. canis with percentage inhibition of 66.18% and 52.94% at 1 mg/mL and 72.06% and 67.65% at 2 mg/mL of the methanol and aqueous extracts, respectively, when compared with the chloroform extract (Fig. 2D) with percentage inhibition of 22.06% at 1 mg/mL and 41.18% at 2 mg/mL. All the three extracts exhibited similar effect against A. niger with percentage inhibitions of 31.08%, 25.86%, and 22.41% at 1 mg/mL and 81.03%, 77.59%, and 84.84% at 2 mg/mL of methanol, chloroform, and aqueous extracts, respectively, as shown in the Fig. 2E.
Fig. 2.
(A) Antifungal activity of Annona squamosa Linn. against Alternaria alternata. (B) Antifungal activity of A. squamosa against Candida albicans. (C) Antifungal activity of A. squamosa against Fusarium solani. (D) Antifungal activity of A. squamosa against Microsporum canis. (E) Antifungal activity of A. squamosa against Aspergillus niger. The values are expressed as mean ± standard error of the mean. * p < 0.05, ** p < 0.01, and *** p < 0.01 versus vehicle control (blank), calculated using one-way analysis of variance followed by Dunnett post hoc analysis (n = 3). AEAS = aqueous extract; CEAS = chloroform extract; KTZ = ketoconazole; MEAS = methanol extract.
3.2.2. Broth microdilution method
The MICs of methanol, chloroform, and aqueous extracts of A. squamosa leaves were estimated and presented in Table 2. The organic extracts exhibited significantly higher antifungal activity than the aqueous extract of A. squamosa leaves. The chloroform extract showed maximum potency among the three extracts tested against A. alternata and F. solani. The methanol extract exhibited the highest activity against M. canis and A. niger; however, against C. albicans both methanol and chloroform extracts showed the same potency. The aqueous extract showed relatively less activity when compared with the methanol and chloroform extracts against all tested fungal strains.
Table 2.
Minimum inhibitory concentrations of different extracts of Annona squamosa Linn. against various fungal strains.
| Fungus | Methanol extract (μg/mL) | Chloroform extract (μg/mL) | Aqueous extract (μg/mL) |
|---|---|---|---|
| Alternaria alternata | 800 | 200 | 1000 |
| Candia albicans | 600 | 600 | 800 |
| Fusarium solani | 600 | 300 | 800 |
| Microsporum canis | 400 | 800 | 600 |
| Aspergillus niger | 400 | 600 | 1000 |
3.3. Antioxidant assessment
3.3.1. Radical scavenging activity
The results pertaining to the DPPH free radical scavenging activity of the three different extracts of A. squamosa leaves, along with the standard reference, vitamin C, are shown in Fig. 3A. The potent free radical scavenging activity was exhibited by the chloroform extract (IC50 308.3 μg/mL), whereas the methanol extract (IC50 342.5 μg/mL) showed comparable scavenging activity and the aqueous extract (IC50 439.6 μg/mL) exhibited relatively less free radical scavenging activity. Ascorbic acid showed the highest DPPH radical scavenging activity (IC50 35.26 μg/mL).
Fig. 3B shows hydrogen peroxide scavenging activity of the methanol, chloroform, and aqueous extracts of A. squamosa leaves. All extracts caused a strong dose-dependent inhibition of hydrogen peroxide. The aqueous and chloroform extracts showed good H2O2 scavenging ability compared with the methanol extract. The IC50 values of the chloroform, aqueous, and methanol extracts were 242.7 μg/mL, 342.2 μg/mL, and 453.7 μg/mL, respectively; however, ascorbic acid showed maximum activity with IC50 of 32.73 μg/mL.
Results related to the NO radical scavenging activity of all the extracts and positive control showed significant NO radical scavenging activity in a concentration-dependent manner (Fig. 3C). In this assay, the methanol extract caused a potential inhibition when compared with the chloroform and aqueous extracts of A. squamosa leaves with IC50 values of 185.2 μg/mL, 345.8 μg/mL, and 366.3 μg/mL, respectively. The IC50 value of ascorbic acid was 58.7 μg/mL.
3.3.2. Reducing power assay
The reducing power property of the methanol, chloroform, and aqueous extracts is illustrated in Fig. 4. In the reductive ability measurement, Fe3+ to Fe2+ transformation in the presence of extract sample was investigated. In this study, the reductive capabilities of the chloroform, methanol, and aqueous extracts of A. squamosa leaves were increased with increase in their concentration.
4. Discussion
Antimicrobial activities of plant leaves, flowers, stems, roots, or fruits from various herbs and spices have been reported by many workers [24]. Successful prediction of botanical compounds from plant material is largely dependent on the type of solvent used in the extraction procedure. Results of the antifungal assay carried out with aqueous and organic extracts of A. squamosa leaves showed their inhibitory effect at both concentrations tested (1 mg/mL and 2 mg/mL) against the five pathogenic fungi in a dose-dependent manner. These pathogens included A. niger, M. canis, F. solani, A. alternata, and C. albicans, which are known to cause aspergillosis, dermatophytosis, Fusarium keratitis, respiratory tract infections, and candidiasis, respectively. The antifungal activity was higher for the methanol and chloroform extracts against A. alternata, C. albicans, and F. solani, whereas the methanol and aqueous extracts showed better effect than the chloroform extract against M. canis, which causes tinea corporis and tinea capitis in humans. However, all the tested extracts showed similar inhibitory activity against the fungus A. niger, which is a filamentous ascomycete that is ubiquitous in the environment and has been implicated in opportunistic infections of humans. It causes various diseases in plants and animals. In plants, it causes black mold and rot diseases and in human beings it causes aspergillosis, which leads to pulmonary allergy, bronchopulmonary aspergillosis, and pulmonary aspergilloma [16]. The presented result may further indicate that the antimicrobial properties/chemical constituents, which are either polar or nonpolar, might be effectively extracted through the organic solvent medium. Some of the earlier studies also reported the effective inhibitory activity of alcoholic solvents against the growth of pathogenic fungi. The aqueous (water) extract showed a much lesser inhibitory effect, which might be attributed to the extracting capacity of solvent and the concentration of the active ingredients in the extracts; in addition, most of the active ingredients dissolve better in alcoholic solvents than in water. Several researchers have reported that aqueous (water) extracts do not exhibit much activity against fungi [25]. Further, the evaluation of minimum inhibitory concentration of A. squamosa leaves extracts by the broth dilution method revealed the differential potency of each extract against the tested fungal strains. The most susceptible species for the chloroform extract of A. squamosa leaves were A. alternata and F. solani, whereas C. albicans, M. canis, and A. niger were found to be more susceptible to the methanol extract of A. squamosa The varying degree of sensitivity of tested fungi may be due to the intrinsic tolerance of microorganisms and the nature and combinations of phytocompounds present in the crude extracts. The bioactivity of plant extracts was attributed to the presence of phytochemical constituents; for example, plants rich in saponins have immune-boosting, anti-inflammatory [26], antiviral, and antibacterial properties [27]. Similarly, tannins have been reported to have antibacterial potential due to their basic character that allows them to react with proteins to form stable water-soluble compounds, thereby killing bacteria by directly damaging their cell membrane. Flavonoids are a major group of phenolic compounds reported for their antiviral, antimicrobial, and spasmolytic properties [26]. Alkaloids isolated from plants are commonly found to have antimicrobial properties [28]. With regard to natural products, it is generally accepted that phytochemicals are less potent anti-infectives than agents of microbial origin (i.e., antibiotics). However, new classes of antimicrobial drugs are urgently required and the flavonoids represent a novel set of leads. Future optimization of these compounds through structural alteration may allow the development of a pharmacologically acceptable antimicrobial agent or group of agents. The researchers reported that screening of these analogs might lead to the identification of compounds that are sufficiently potent to be useful as antifungal, antiviral or antibacterial chemotherapeutics [29]. The antifungal activity of the leaves extracts of A. squamosa as recorded in this study may be attributed to the presence of flavonoids; however, the higher degree of antifungal potency of the chloroform and methanol extracts recorded in this study might be due to the presence of tannins. Whereas the isolation and characterization of medicinally important bioactive phytoconstituents from the leaves of A. squamosa are required for the development of potential anti-fungal agents, our report provides a strong evidence of the ability of the methanolic and chloroform extracts of A. squamosa leaves against the fungal species tested.
The DPPH free radical scavenging model can be used to evaluate the antioxidant activity in a relatively short time. DPPH is a stable free radical and accepts either an electron or OH* to become a stable diamagnetic molecule [30]. The decreased absorbance results in a color change from purple to yellow, as radicals were scavenged by antioxidants through the donation of hydrogen to form the stable DPPH molecule [31]. Hydrogen peroxide is a weak oxidizing agent and can inactivate a few enzymes directly, usually by oxidation of the essential thiol groups. Hydrogen peroxide crosses cell membrane and reacts with ferric and copper ions, which show toxic effects [32]. Similarly, the reactive oxygen species NO is also implicated in inflammation, cancer, and other pathological conditions [33]. NO is a very unstable species under aerobic conditions [34]. The plant products have the property to counteract the effect of NO formation and, in turn, may be of considerable interest in relation to preventing the ill effects of excessive NO generation in the human body. Several studies have evaluated the relationship between the antioxidant activity of plant products and their phenolic content. Substances that are able to perform these reactions can be considered as antioxidants and radical scavengers [18]. Reducing power, which was used to measure the reductive ability of antioxidants, was evaluated by the transformation of Fe3+ to Fe2+ in the presence of the leaves extracts [35]. Antioxidants reduce the Fe3+/ferricyanide complex to the ferrous form by donating an electron. The color of the test solution then changes from yellow to different shades of green and blue [36]. The ability of these extracts to reduce Fe3+ may be attributed to the hydrogen donating effect of phenolic compounds [37]. It has been reported that phenolic compounds were the main antioxidant components, and their total contents were directly proportional to their antioxidant activity [38]. Therefore, in this study, the presence of the flavonoids and phenols in all the tested extracts of A. squamosa leaves might have contributed to the antioxidant activity.
5. Conclusion
From the aforementioned results, it can be concluded that the chloroform, methanol, and aqueous extracts of the leaves of A. squamosa have in vitro antifungal and antioxidant activities. Further studies are required to isolate the active components from the extracts and to elucidate the exact mechanism of action of the antifungal and antioxidant activities.
Footnotes
Conflicts of interest
The authors declare that there are no conflicts of interest.
REFERENCES
- 1. Mazid M, Khan TA, Mohammad F. Medicinal plants of rural India: a review of use by Indian folks. Indo Glob J Pharm Sci. 2012;2:286–304. [Google Scholar]
- 2. Bussmann RW, Glenn A, Meyer K, Kuhlman A, Townesmith A. Herbal mixtures in traditional medicine in Northern Peru. J Ethnobiol Ethnomed. 2010;6:10. doi: 10.1186/1746-4269-6-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Duraipandiyan V, Ignacimuthu S. Antifungal activity of traditional medicinal plants from Tamil Nadu, India. Asian Pac J Trop Biomed. 2011;1:S204–15. [Google Scholar]
- 4. Raj DS, Vennila JJ, Aiyavu C, Panneerselvam K. The hepatoprotective effect of alcoholic extract of Annona squamosa leaves on experimentally induced liver injury in Swiss albino mice. Int J Integr Biol. 2009;5:182–6. [Google Scholar]
- 5. Kaleem M, Medha P, Ahmed QU, Asif M, Bano B. Beneficial effects of Annona squamosa extract in streptozotocin-induced diabetic rats. Singapore Med J. 2008;49:800–4. [PubMed] [Google Scholar]
- 6. Pandey VK, Giri IC, Prakashdeep, Singh S, Srivastava A. Pharmacognostical and physiochemical study on the leaves of Annona squamosa Linn. Int J Res Pharm Sci. 2014;4:8–12. [Google Scholar]
- 7.Starr Environmental. Plants of Hawaii. [Last accessed: July 16, 2015]. Available from: http://www.starrenvironmental.com/images/species/?q=annona+squamosa&o=plants.
- 8.Wikimedia Commons. Annona squamosa. 2009. [Last accessed: July 16, 2015]. Available from: http://commons.wikimedia.org/wiki/File:Annona_squamosa01.JPG.
- 9.Flickr. Image by jayjayc. 2009. [Last accessed: July 16, 2015]. Available from: http://www.flickr.com/photos/jayjayc/292041435.
- 10.King Saud University. KSU faculty websites: image by Abdullah Alebidi. 2009. [Last accessed: July 16, 2015]. Available from: http://faculty.ksu.edu.sa/10439/Pictures%20Library/Forms/DispForm.aspx?ID=113.
- 11. Celeghini RMS, Vilegas JHY, Lanças FM. Extraction and quantitative HPLC analysis of coumarin in hydroalcoholic extracts of Mikania glomerata Spreng. (“guaco”) leaves. J Braz Chem Soc. 2001;12:706–9. [Google Scholar]
- 12.Trease G, Evans SM. Pharmacognosy. 15th ed. London: Bailer Tindal; 2002. pp. 23–67. [Google Scholar]
- 13.Harborne JB. Phytochemical methods: a guide to modern techniques of plant analysis. 2nd ed. London: Chapman and Hall; 1984. pp. 4–16. [Google Scholar]
- 14. Srinivasan S, Sarada DV. Antifungal activity of phenyl derivative of pyranocoumarin from Psoralea corylifolia L. seeds by inhibition of acetylation activity of trichothecene 3-o-acetyltransferase (Tri101) J Biomed Biotechnol. 2012;2012:310850. doi: 10.1155/2012/310850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lee CJ, Chen LW, Chen LG, Chang TL, Huang CW, Huang MC, Wanga CC. Correlations of the components of tea tree oil with its antibacterial effects and skin irritation. J Food Drug Anal. 2013;21:169–76. [Google Scholar]
- 16. Senguttuvan J, Paulsamy S, Krishnamoorthy K. In vitro antifungal activity of leaf and root extracts of the medicinal plant, Hypochaeris radicata L. Int J Pharm Pharm Sci. 2013;5:758–61. [Google Scholar]
- 17. Koleva II, van Beek TA, Linssen JP, de Groot A, Evstatieva LN. Screening of plant extracts for antioxidant activity: a comparative study on three testing methods. Phytochem Anal. 2002;13:8–17. doi: 10.1002/pca.611. [DOI] [PubMed] [Google Scholar]
- 18. Nandhakumar E, Indumathi P. In vitro antioxidant activities of methanol and aqueous extract of Annona squamosa (L.) fruit pulp. J Acupunct Meridian Stud. 2013;6:142–8. doi: 10.1016/j.jams.2012.09.002. [DOI] [PubMed] [Google Scholar]
- 19. Ahmad B, Khan MR, Shah NA, Khan RA. In vitro antioxidant potential of Dicliptera roxburghiana. BMC Complement Altern Med. 2013;13:140. doi: 10.1186/1472-6882-13-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Govindarajan R, Rastogi S, Vijayakumar M, Shirwaikar A, Rawat AK, Mehrotra S, Pushpangadan P. Studies on the antioxidant activities of Desmodium gangeticum. Biol Pharm Bull. 2003;26:1424–7. doi: 10.1248/bpb.26.1424. [DOI] [PubMed] [Google Scholar]
- 21. Mandal S, Rajani GP, Sharma RK, Gupta N. In vitro antioxidant and anti-inflammatory potential of Polyalthia longifolia in rats. Indian J Pharmacol. 2012;44:277–8. doi: 10.4103/0253-7613.93873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yen GC, Chen HY. Antioxidant activity of various tea extracts in relation to their antimutagenicity. J Agric Food Chem. 1995;43:27–32. [Google Scholar]
- 23. Ebrahimzadeh MA, Nabavi SM, Nabavi SF, Bahramian F, Bekhradnia AR. Antioxidant and free radical scavenging activity of H. officinalis L. var. angustifolius, V. odorata, B. hyrcana and C. speciosum. Pak J Pharm Sci. 2010;23:29–34. [PubMed] [Google Scholar]
- 24. Mau J, Chen CP, Hsieh PC. Antimicrobial effect of extracts from Chinese chive, cinnamon, and Corni fructus. J Agric Food Chem. 2001;49:183–8. doi: 10.1021/jf000263c. [DOI] [PubMed] [Google Scholar]
- 25. Krishnamoorthy K, Subramaniam P, Senguttuvan J. In vitro antifungal activity of various extracts of leaf and stem parts of Solena amplexicaulis (Lam.) Gandhi. Int J Pharm Pharm Sci. 2013;5:745–7. [Google Scholar]
- 26. David E, Elumalai EK, Sivakumar C, Therasa SV, Thirumalai T. Evaluation of antifungal activity and phytochemical screening of Solanum surattense seeds. J Pharm Res. 2010;3:684–7. [Google Scholar]
- 27. Lacaille-Dubois MA, Wagner H. A review of the biological and pharmacological activities of saponins. Phytomedicine. 1996;2:363–86. doi: 10.1016/S0944-7113(96)80081-X. [DOI] [PubMed] [Google Scholar]
- 28. Omulokoli E, Khan B, Chhabra S. Antiplasmodial activity of four Kenyan medicinal plants. J Ethnopharmacol. 1997;56:133–7. doi: 10.1016/s0378-8741(97)01521-3. [DOI] [PubMed] [Google Scholar]
- 29. Cushnie TP, Lamb AJ. Antimicrobial activity of flavonoids. Int J Antimicrob Agents. 2005;26:343–56. doi: 10.1016/j.ijantimicag.2005.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Yermilov V, Rubio J, Becchi M, Friesen MD, Pignatelli B, Ohshima H. Formation of 8-nitroguanine by the reaction of guanine with peroxynitrite in vitro. Carcinogenesis. 1995;16:2045–50. doi: 10.1093/carcin/16.9.2045. [DOI] [PubMed] [Google Scholar]
- 31. Dehpour AA, Ebrahimzadeh MA, Nabavi SF, Nabavi SM. Antioxidant activity of methanol extract of Ferula asafoetida and its essential oil composition. Grasas Aceites. 2009;60:405–12. [Google Scholar]
- 32. Wang KJ, Zhang YJ, Yang CR. Antioxidant phenolic compounds from rhizomes of Polygonum paleaceum. J Ethnopharmacol. 2005;96:483–7. doi: 10.1016/j.jep.2004.09.036. [DOI] [PubMed] [Google Scholar]
- 33. Moncada A, Palmer RM, Higgs EA. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991;43:109–42. [PubMed] [Google Scholar]
- 34. Shirwaikar A, Prabhu KS, Punitha ISR. In vitro antioxidant studies of Sphacranthus indicus (L) Indian J Exp Biol. 2006;44:993–6. [PubMed] [Google Scholar]
- 35. Gulcin I, Oktay M, Kirecci E, Kufrevioglu I. Screening of antioxidant and antimicrobial activities of anise (Pimpella anisum L.) seed extracts. Food Chem. 2003;83:371–82. [Google Scholar]
- 36. Chou ST, Chao WW, Chung YC. Antioxidative activity and safety of 50% ethanolic red bean extract (Phaseolus radiatus L. var. aurea) J Food Sci. 2003;68:21–5. [Google Scholar]
- 37. Shahidi F, Wanasundara PK. Phenolic antioxidants. Crit Rev Food Sci Nutr. 1992;32:67–103. doi: 10.1080/10408399209527581. [DOI] [PubMed] [Google Scholar]
- 38. Do QD, Angkawijaya AE, Tran-Nguyen PL, Huynh LH, Soetaredjo FE, Ismadji S, Ju Y. Effect of extraction solvent on total phenol content, total flavonoid content, and antioxidant activity of Limnophila aromatica. J Food Drug Anal. 2014;22:296–302. doi: 10.1016/j.jfda.2013.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]