Abstract
Gastrodia elata Blume is commonly used as a medical herb in China for ameliorating headaches, dizziness, and convulsions. In previous studies, water extracts of G. elata Bl. (WGE) have demonstrated potential to act as therapeutic agents to improve depression-like symptoms in rats. As gastrodin (GAS) is a major active compound in WGE, its quantitation in WGE is important for quality control. The objective of this study was to develop an optimized and validated reversed-phase high-performance liquid chromatography method for the analysis of GAS in different sources of WGE. We evaluated the GAS content in varieties of G. elata Bl. including G. elata Bl. f. glauca S. Chow and G. elata Bl. f. elata. We also evaluated the GAS content of the latter variety from two different origins, Yun-nan and Hu-nan. The results indicate that the amount of GAS analyzed in WGE from G. elata Bl. f. glauca S. Chow is five times higher than that of G. elata Bl. f. elata from Yun-nan and Hu-nan. A significant difference in GAS content was observed between varieties of G. elata Bl., although not between locations of origin.
Keywords: Gastrodia elata Blume, gastrodin, method validation, quality control
1. Introduction
Rhizoma Gastrodiae is the dried tuber of Gastrodia elata Blume (G. elata Bl., Orchidaceae), a prominent traditional Chinese medicinal herb. It is used to treat headaches, dizziness, convulsions, paralysis, rheumatism, and lumbago [1]. Five varieties of G. elata Bl. are cultivated in China, including G. elata f. elata, G. elata f. glauca S. Chow (GBY), G. elata f. viridis Makino, G. elata f. flavida S. Chow, and G. elata f. alba S. Chow [2]. Among these varieties, G. elata f. elata and GBY are the most widely cultivated [3,4].
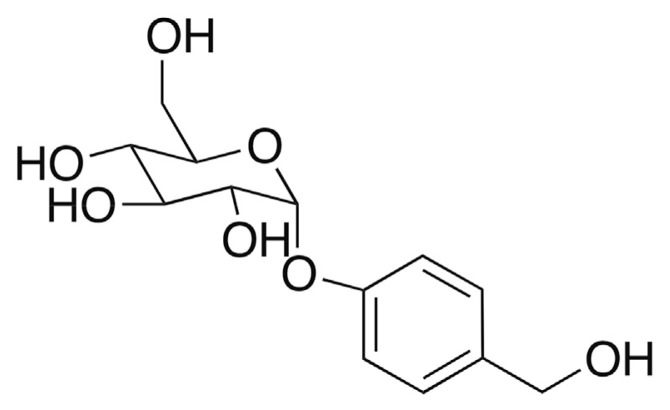
Previous reports have indicated that G. elata Bl. can serve as an anticonvulsant [5–7], antioxidant [8–10], antiepileptic [11], memory enhancer [12–14], smooth muscle relaxant [15], protectant against amyloid β peptide-induced cell death [16], and apoptosis inhibitor [7,11,17]. Our previous studies demonstrated the antidepressant properties of water extracts (WGE) in forced-swimming tests [18,19]. Five bioactive compounds—gastrodin (GAS), vanillyl alcohol, 4-hydroxy-3-methoxybenzaldehyde (vanillin), 4-hydroxybenzyl aldehyde (HB), and 4-hydroxybenzyl alcohol (HBA)—have been isolated from G. elata Bl. However, the antidepressant effect of the primary bioactive compounds in G. elata Bl. remains unknown. GAS is regarded as the major bioactive compound in G. elata Bl. [20]. GAS (the structure shown in Fig. 1) has also been found to serve as a memory enhancer [13,21], anticonvulsant [22], apoptosis inhibitor [23]. Thus, the quality of G. elata Bl. can be evaluated by the level of GAS.
Fig. 1.
Structure of gastrodin in Gastrodin elata Blume.
In the present study, we aimed to develop a simple, accurate, and selective method that is fully validated according to the ICH Q2A and Q2B guidelines [24,25], and which can be used to assay the active components of G. elata Bl. To determine the amount of GAS in different sources of WGE, we evaluated the GAS content in two varieties of G. elata Bl., including GBY and G. elata Bl. f. elata. We also evaluated two different origins of G. elata Bl. f. elata from Yun-nan (GRY) and from Hu-nan (GRH). Our study of the quantitation of GAS can provide useful information for selecting a source of G. elata Bl.
2. Experimental
2.1. Chemicals and reagents
G. elata Bl. f. elata (black G. elata Bl. from Yun-nan, GBY) and G. elata Bl. f glauca S. Chow (red G. elata Bl. from Yun-nan, GRY) were purchased from Chrysanthemum Village medicine market, a Chinese herb pharmacy in Kunming (Yun-nan, China). G. elata f. glauca S. Chow (red G. elata Bl. from Hu-nan, GRH) was purchased in Hu-nan, China. These G. elata Bl. varieties were identified by Professor Zhi-Hong Zhou in the Chinese Medicine Cultivation Center at Yun-nan University of Traditional Chinese Medicine (Yunnan, China). GAS (p-hydroxymethylphenyl-β-D-glucopyranoside) was obtained from Professor Chi-Tang Ho in the Department of Food Science at Rutgers University (New Brunswick, NJ, USA). HBA, HB, and vanillin were purchased from Sigma (Sigma–Aldrich, St. Louis, MO, USA). All standards used were of ≥ 99.5% purity.
2.2. Chromatography
2.2.1. Instrumentation and analytical conditions
The high-performance liquid chromatography (HPLC) system consisted of a PU-2089 Plus solvent delivery system (Jasco Corporation, Tokyo, Japan), an LC-NetII/ADC degasser (Jasco Corporation), and a UV-2075 Plus detector (Jasco Corporation). The data were acquired via Chrompass analytical software (version 1.7.403.1, Jasco). GAS was measured by the Jasco HPLC system with a phenomenex Luna C18 (250 mm × 4.6 mm i.d., 5 μm particle size; UV-2075 Plus detector, JASCO Corporation, Torrance, CA, USA) at 35 ± 1°C. The peaks of GAS were detected at 220 nm. The flow rate was set at 1.0 mL/min. The mobile phase was prepared freshly, filtered through a 0.22 μm polyethersulfone (PES) vacuum bottle top filter (Sigma–Aldrich) by an Aspirator AS-1 vacuum filtration apparatus (Fargo Instruments, Taipei, Taiwan) and degassed by Bransonic 5510R-DTH (Branson Ultrasonic Corporation, Danbury, CT, USA) for 30 minutes. The mobile phase consisted of a mixture of 0.02% phosphoric acid in water (solvent A) and acetonitrile (solvent B) pumped in the gradient mode as follows: 0–7 minutes, 5% B; 7–15 minutes, 5–20% B; 15–20 minutes, 20–25% B; 20–25 minutes, 25–95% B; 25–28 minutes, 95% B; 28–30 minutes, 95–5% B; and 30–35 minutes, 5% B.
The HPLC analysis method was fully validated according to the guidelines of ICH Q2A and Q2B [24,25] and the specifications of the Food Chemistry Analytical Method Validation by the Taiwan Food and Drug Administration [26]. The method was validated for linearity, precision (repeatability and intermediate precision), accuracy, specificity, detection limits, robustness (detection wavelength and the flow rate of mobile phase), and system suitability [27–30].
2.3. Preparation of G. elata Bl. extract
Extraction methods using ethanol and water were followed according to those in previous reports from the second edition of the Taiwan Herbal Pharmacopeia [31] and Teo et al [32] with slight modifications. For the ethanolic extraction, 1 g of slices/powder was immersed in 25 mL of 70% ethanol and refluxed in boiling water for 60 minutes. The supernatant was quickly cooled to 4°C and filtered through 0.45-μm nylon filters (Agela, Beijing, China). The ethanolic extracts (EGE) were concentrated by a rotary evaporator (Heidolph 4000; Heidolph Instruments, Schwabach, Germany) and lyophilized (SFD-25 model, Chang Juing Co., Kaohsiung, Taiwan) under high vacuum and re-dissolved with water prior to the HPLC assay. For the water extraction, 1 g of either slices or powder was immersed in 5 mL of deionized water and refluxed under boiling water for 60 minutes. Seven mL of deionized water was then added to and refluxed for another 50 minutes [33]. The WGE were collected as described above for the EGE, without the concentration step.
2.4. Preparation of stock and working solutions
The standard stock solution was prepared by dissolving GAS in water at a concentration of 1000 μg/mL and stored at 4°C. Working standard solutions were freshly prepared by diluting the stock solution in purified water to GAS concentrations of 10 μg/mL, 30 μg/mL, 50 μg/mL, 70 μg/mL, and 90 μg/mL, respectively.
2.5. Preparation of standard and quality control samples
We prepared nine quality control (QC) samples: three WGE solutions at concentrations of 30 μg/mL, 50 μg/mL, and 70 μg/mL; three standard solutions at concentrations of 30 μg/mL, 50 μg/mL, and 70 μg/mL; and three spiked solutions at concentrations of 30 μg/mL, 50 μg/mL, and 70 μg/mL. In order to extract GAS, the concentrations of the original WGE solutions were 1800 μg/mL, 3000 μg/mL, and 4200 μg/mL, which resulted in a GAS content of 30 μg/mL, 50 μg/mL, and 70 μg/mL, respectively. All of the QC sample solutions had concentrations between 30 μg/mL and 70 μg/mL. The concentrations of the calibration standards were within the range of 10–90 μg/mL. QC samples fell within the range of the calibration curve of GAS.
3. Results and discussion
3.1. Method development and optimization
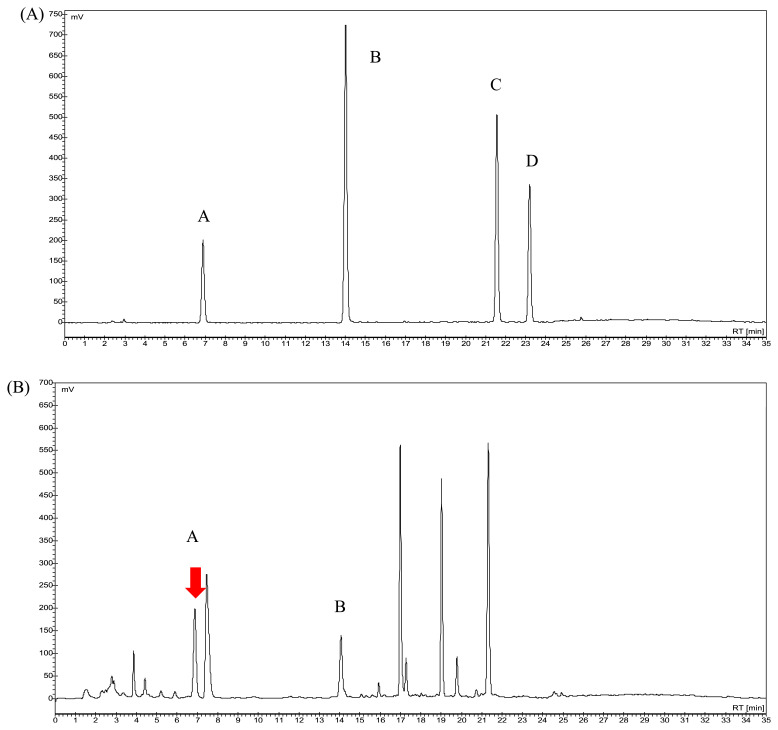
The preliminary experiments of the GAS assay were performed according to the HPLC method developed in our laboratory [34]. The HPLC chromatography was carried out by an isocratic elution of 10% acetonitrile (aqueous). A flow rate of 1 mL/min and a detection wavelength of 220 nm were used. GAS was identified according to the retention time of its peak, which was determined from a GAS standard with appropriate specificity and selectivity. However, the isocratic elution did not lead to satisfactory separation of all the peaks in a QC sample, which resulted in poor results from GAS quantification studies in our laboratory [34]. Thus, the chromatographic conditions were modified using a gradient elution method, according to Liu et al [35]. The gradient elution of a water and acetonitrile mixture resulted in lower resolution of peaks from a QC sample. Accordingly, peak resolution was improved by adding 0.2% phosphoric acid (aqueous) to the water of mobile phase. All peaks of the unknown components that were used to develop the method were well separated. GAS was identified by comparing the retention time of its peak with that of a commercial standard. HBA, an aglycone of GAS, was also identified in the HPLC chromatogram [36,37]. The results demonstrate the specificity of the developed method since none of the excipients interfered with target analytes (Fig. 2).
Fig. 2.
(A) Chromatogram of four standards of Gastrodia elata Blume by high-performance liquid chromatography; A = gastrodin, B = 4-hydroxybenzyl alcohol, C = 4-hydroxybenzyl aldehyde, D = vanillin (4- hydroxy-3-methoxybenzaldehyde). (B) Chromatogram of water extracts from G. elata Bl. A = gastrodin, B = 4-hydroxybenzyl aldehyde.
3.2. Validation of method
3.2.1. Linearity, limit of detection, and limit of quantitation
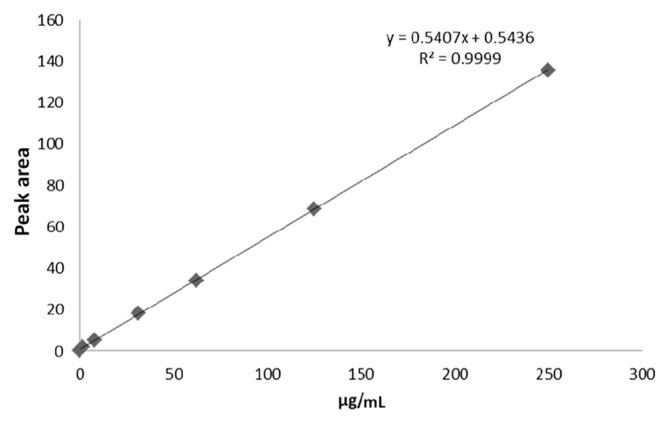
The calibration curve of GAS was linear within the concentration range of 10–90 μg/mL in water. The regression equation was y = 0.5407x + 0.5436 and the correlation coefficient (r2) was 0.9999 (Fig. 3). Limits of detection (LoD) and quantitation (LoQ) were expressed as LoD = 3.3σ/S and LoQ = 10σ/S (where σ = the standard deviation of y-intercepts of regression lines and S = the slope of the calibration curve). The LoD and LoQ were calculated to be 0.5 μg/mL and LoQ was 1.52 μg/mL, respectively.
Fig. 3.
Calibration curve obtained with gastrodin standard solution using the proposed high-performance liquid chromatography method.
3.2.2. Accuracy and precision
The relative standard deviation (RSD) of the overall intraday precisions for the peak area ratios obtained for GAS was 8.60% (n = 6; Table 1). This demonstrates that the equipment used for the study functions correctly for the developed analytical method, and the method can be repeated. The data for the accuracy measurements were expressed as percent of recovery of GAS in the real samples. The samples were spiked with GAS to obtain final concentrations of 60%, 100%, and 140% to the nominal analyte concentration of 50 μg/mL. Analyte samples at each of these concentrations were injected and analyzed in triplicate by three analysts. The mean recovery of GAS in real samples was within the range of 94.56–105.94% with 3.23% of mean RSD (Table 2). However, G. elata Bl. is a natural product and content of GAS varies by different variety, origin, and harvest season. Because G. elata Bl. is a traditional functional food, the analysis method should follow the criteria for the Food Chemistry Analytical Method Validation by Taiwan Food and Drug Administration, which mean recovery and RSD range were not more than 80.00–115.00% and 14.00%, respectively. As shown in Table 2, all recoveries were within the acceptable range.
Table 1.
System suitability results of the proposed method.
| Parameters | Results | Required limits |
|---|---|---|
| RSD | 8.60% | RSD < 10% |
| N | 2225 | N > 2000 |
| T | 1.12 | T < 1.5 |
| R | 1.4 | R > 1.0 |
| α | 1.15 | α > 1.05 |
| K′ | 3.41 | K′ > 3.0 |
Each value is the mean of six determinations.
α = selectivity factor; K′ = capacity factor; N = numbers of theoretical plate; R = resolution; RSD = relative standard deviation; T = tailing factor.
Table 2.
Intra- and interday accuracy and precision of high-performance liquid chromatography assay for gastrodin from water extracts of Gastrodia elata Blume.
| Analyst | Level (μg/mL) | Injection | Recovery | Recovery (%) | Average (n = 3) | RSD (%) | Repeatability | Intermediate | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
||||||||||
| Standarda | Sampleb (μg/mL) | Spikec | Average (n = 9) | RSD (%) | Average (n = 27) | RSD (%) | ||||||
| 1 | 30 | 1 | 30.75 | 27.04 | 29.08 | 101.20 | 102.67 | 1.28 | 100.81 | 2.77 | 100.22 | 3.23 |
| 2 | 29.64 | 27.78 | 29.27 | 103.75 | ||||||||
| 3 | 30.38 | 27.97 | 29.64 | 103.05 | ||||||||
| 50 | 1 | 49.27 | 48.72 | 50.20 | 104.89 | 101.88 | 2.53 | |||||
| 2 | 50.39 | 48.53 | 49.27 | 99.26 | ||||||||
| 3 | 49.46 | 46.87 | 48.53 | 101.50 | ||||||||
| 70 | 1 | 69.65 | 67.06 | 67.24 | 96.81 | 97.87 | 2.72 | |||||
| 2 | 69.28 | 68.73 | 68.73 | 99.20 | ||||||||
| 3 | 69.47 | 68.17 | 67.98 | 97.60 | ||||||||
| 2 | 30 | 1 | 29.45 | 29.27 | 29.08 | 98.11 | 96.84 | 1.45 | 97.98 | 2.34 | ||
| 2 | 29.64 | 29.08 | 28.71 | 95.62 | ||||||||
| 3 | 28.71 | 30.38 | 29.08 | 96.77 | ||||||||
| 50 | 1 | 48.90 | 48.53 | 47.79 | 96.21 | 99.62 | 1.46 | |||||
| 2 | 49.27 | 48.16 | 49.27 | 102.26 | ||||||||
| 3 | 47.61 | 48.90 | 48.35 | 100.39 | ||||||||
| 70 | 1 | 68.54 | 68.91 | 67.24 | 95.68 | 97.47 | 1.15 | |||||
| 2 | 68.17 | 68.54 | 67.43 | 97.28 | ||||||||
| 3 | 67.43 | 68.17 | 67.61 | 99.45 | ||||||||
| 3 | 30 | 1 | 31.81 | 31.81 | 31.98 | 101.08 | 99.10 | 3.97 | 101.87 | 3.38 | ||
| 2 | 31.63 | 33.01 | 31.46 | 94.56 | ||||||||
| 3 | 31.29 | 32.49 | 32.15 | 101.65 | ||||||||
| 50 | 1 | 52.10 | 49.69 | 52.44 | 105.94 | 104.64 | 1.90 | |||||
| 2 | 51.93 | 49.69 | 52.27 | 105.63 | ||||||||
| 3 | 51.24 | 50.38 | 51.41 | 102.35 | ||||||||
| 70 | 1 | 74.11 | 72.56 | 74.80 | 103.94 | 101.87 | 2.18 | |||||
| 2 | 73.94 | 71.19 | 72.39 | 99.53 | ||||||||
| 3 | 73.08 | 73.94 | 74.28 | 102.12 | ||||||||
RSD = relative standard deviation; Sample = determination of gastrodin content by injecting each sample preparation; Spike = determination of gastrodin content by injecting each spiked solution; Standard = determination of gastrodin content by injecting each standard solution.
Standard: determination of gastrodin content by injecting each standard solution.
Sample: determination of gastrodin content by injecting each sample preparation.
Spike: determination of gastrodin content by injecting each spiked solution.
3.2.3. Robustness
To show the reliability of the HPLC analysis in the presence of varied analytical parameters, we injected solutions under the following modified chromatographic conditions: wavelength at 219 nm and 221 nm or flow rate at 0.97 mL/min and 1.03 mL/min. As shown in Table 3, the numbers of theoretical plates (N), the tailing factor (T), resolution (R), and selectivity (α) of GAS were not significantly affected by these slight changes. These results indicate the robustness of HPLC analysis despite variation of the parameters, including detection wavelength and flow rate.
Table 3.
Results of robustness study of high-performance liquid chromatography assay for gastrodin from water extracts of Gastrodia elata Blume.
| Wavelength (nm) | Flow rate (mL/min) | |||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| 219 | 220 | 221 | 0.97 | 1.00 | 1.03 | |
| N | 2218 | 2270 | 2322 | 2241 | 2270 | 2289 |
| T | 1.14 | 1.13 | 1.13 | 1.10 | 1.13 | 1.18 |
| R | 1.40 | 1.44 | 1.39 | 1.39 | 1.44 | 1.40 |
| α | 1.21 | 1.22 | 1.23 | 1.22 | 1.22 | 1.21 |
α = selectivity factor; N = number of theoretical plate; R = resolution; T = tailing factor.
3.2.4. System suitability
To confirm system suitability, the peak GAS values of 4200 μg/mL WGE were analyzed six times consecutively for RSD, N, T, R, α, and capacity factor (K′). The results of this analysis are listed in Table 1. The RSD was 8.60%, which fell within the criterion of ≤ 10.00% described in the Food Chemistry Analytical Method Validation guidelines [26]. This indicates that our results met the criteria and ours is a suitable method of analysis of the GAS content in WGE. Our analysis method is fully in compliance with the Food Chemistry Analytical Method Validation Guidelines.
3.2.5. Comparison between WGE and EGE from different sources of G. elata Bl
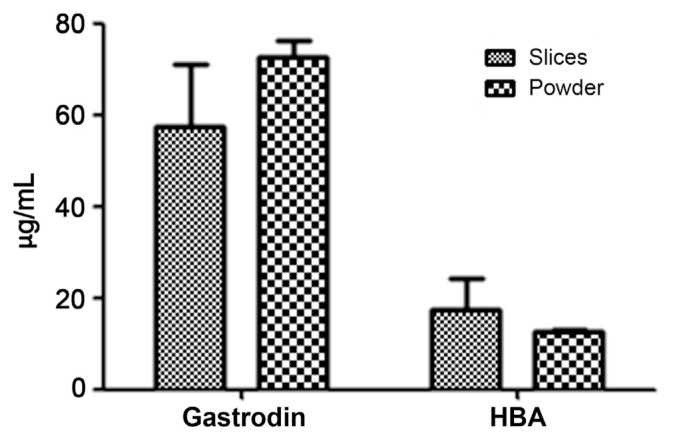
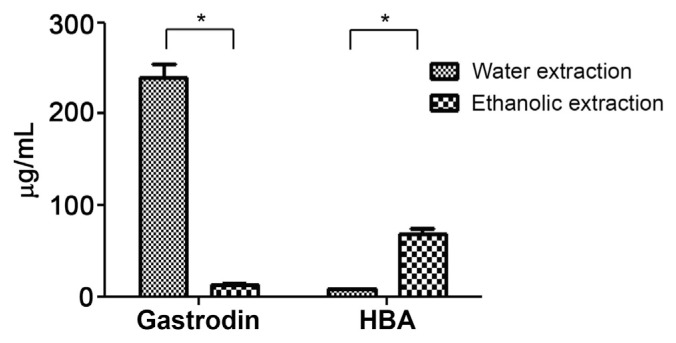
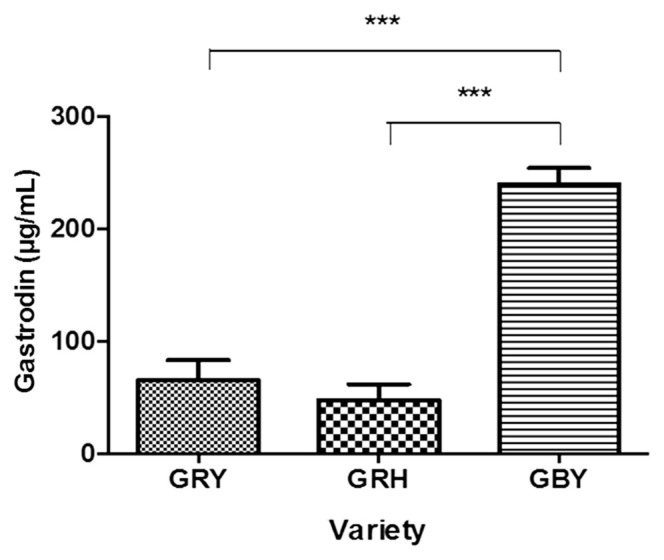
In our pilot study, powder of G. elata Bl. was gelatinized during water extraction due to the presence of high levels of starch. The suspension from the extracts was difficult to obtain by centrifugation and filtration through 0.45 μm nylon filters. Therefore, the powder was deemed unsuitable for GAS preparation. The HPLC chromatogram indicated that the quantities of GAS and HBA were not significantly different between slices and powder (Fig. 4). Thus, the slice from G. elata Bl. could be preferable over the powder for use in subsequent studies. As shown in Fig. 5, the extraction solvent affected the quantity of GAS. The amount of GAS in WGE was significantly higher than that in EGE. Since GAS is a highly water-soluble compound, its extraction efficiency was higher in water than in methanol as the extraction solvent [38,39]. Therefore, this method of water extraction can be applied to further analyses of G. elata Bl. According to the Chinese medicinal dictionary report, the quality of G. elata Bl. harvested during winter is superior to that harvested during summer [40,41]. The G. elata Bl. samples used in the present study were harvested during winter. To verify the quantities of GAS from different sources of G. elata Bl., all samples were initially steamed for 30 minutes. Once cooled, the samples were sliced, frozen, and lyophilized. The chromatography results demonstrated that the yield of GAS from GRY and GRH did not differ significantly (Fig. 6). However, we found that the amount of GAS from GBY was five times higher than the amount from GRY and GRH. These results indicate that GBY provides a better source for GAS than GRY and GRH under water extraction.
Fig. 4.
The analysis of gastrodin and 4-hydroxybenzyl alcohol (HBA) quantities from slices and powder of Gastrodia elata Blume under water extraction by high performance liquid chromatography. Each value represents mean ± standard deviation (n = 3). Compared with water and ethanolic extraction, the data were not significantly different (p > 0.05) according to ANOVA.
Fig. 5.
The analysis of gastrodin and 4-hydroxybenzyl alcohol (HBA) quantities under water and ethanolic extraction by high-performance liquid chromatography. Each value represents mean ± standard deviation (n = 3). * Indicates p < 0.05 (Student t test) compared with water and ethanolic extraction.
Fig. 6.
The analysis of gastrodin quantity from different variety sources under water extraction by high-performance liquid chromatography. Each value represents mean ± standard deviation (n = 3). The overall p value shown was obtained by analysis of variance Tukey’s test for multiple comparisons and indicated a significant difference (***p < 0.001) between Gastrodia elata Blume f. elata from Yun-nan (GRY) and G. elata Bl. f. glauca S. Chow from Yun-nan (GBY), and also between G. elata Bl. f. elata. from Hu-nan (GRH) and GBY.
4. Conclusion
To summarize, a specific HPLC-UV method to measure the amount of GAS in water and ethanolic extracts from different sources of G. elata Bl. was developed and validated. By optimizing the chromatographic conditions, we discovered that water extraction was more efficient than ethanolic extraction. Our results indicate that GAS levels vary significantly between the different varieties of G. elata Bl., although not between the different origins. Therefore, this validated HPLC method can be effectively used for the quality control of WGE in the development of health products in the future. Further studies on the determinations of GAS contents from all varieties and sources of G. elata Bl. will be worthwhile in establishing a quality control database.
Acknowledgments
We thank Professor Zhi-Hong Zhou for identifying the varieties of G. elata Bl. We also thank Tong-Rong Chen, Shiun-Ling Yu, Ching-Yi Weng, and Meaghan Tobin for technical support. This study was supported in part by grants from the National Science Council (NSC 100-2321-B-002-004; NSC 100-2313-B-002-036; NSC 101-2313-B-002-061-MY2; and NSC 102-2628-B-002-010-MY2) and National Taiwan University (Aim for Top University Program 102R-7620), Taipei, Taiwan.
Funding Statement
This study was supported in part by grants from the National Science Council (NSC 100-2321-B-002-004; NSC 100-2313-B-002-036; NSC 101-2313-B-002-061-MY2; and NSC 102-2628-B-002-010-MY2) and National Taiwan University (Aim for Top University Program 102R-7620), Taipei, Taiwan.
Footnotes
Conflicts of interest
All authors declare no conflicts of interest.
REFERENCES
- 1. Chen PJ, Sheen LY. Gastrodiae Rhizoma (天麻tiän má): a review of biological activity and antidepressant mechanisms. J Tradit Complement Med. 2011;1:31–40. doi: 10.1016/s2225-4110(16)30054-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Xu J, Guo S. Retrospect on the research of the cultivation of Gastrodia elata Bl., a rare traditional Chinese medicine. Chin Med J (Engl) 2000;113:686–92. [PubMed] [Google Scholar]
- 3.Editorial committee of Flora of China. Flora Reipublicae Popularis Sinicae. 18. Vol. 31. Beijing: Science Press; 1999. [In Chinese]. [Google Scholar]
- 4.Zhou X, Yang X, Lian XH, Liu CY. Tianma morphological. Beijing: Science Press; 1987. pp. 5–10. [In Chinese]. [Google Scholar]
- 5. Hsieh CL, Chiang SY, Cheng KS, Lin YH, Tang NY, Lee CJ, Pon CZ, Hsieh CT. Anticonvulsive and free radical scavenging activities of Gastrodia elata Bl. in kainic acid-treated rats. Am J Chin Med. 2001;29:331–41. doi: 10.1142/S0192415X01000356. [DOI] [PubMed] [Google Scholar]
- 6. Hsieh CL, Tang NY, Chiang SY, Hsieh CT, Lin JG. Anticonvulsive and free radical scavenging actions of two herbs, Uncaria rhynchophylla (MIQ) Jack and Gastrodia elata Bl., in kainic acid-treated rats. Life Sci. 1999;65:2071–82. doi: 10.1016/s0024-3205(99)00473-7. [DOI] [PubMed] [Google Scholar]
- 7. Kim HJ, Moon KD, Oh SY, Lee SP, Lee SR. Ether fraction of methanol extracts of Gastrodia elata, a traditional medicinal herb, protects against kainic acid-induced neuronal damage in the mouse hippocampus. Neurosci Lett. 2001;314:65–8. doi: 10.1016/s0304-3940(01)02296-0. [DOI] [PubMed] [Google Scholar]
- 8. Hsieh CL, Chang CH, Chiang SY, Li TC, Tang NY, Pon CZ, Hsieh CT, Lin JG. Anticonvulsive and free radical scavenging activities of vanillyl alcohol in ferric chloride-induced epileptic seizures in Sprague–Dawley rats. Life Sci. 2000;67:1185–95. doi: 10.1016/s0024-3205(00)00706-2. [DOI] [PubMed] [Google Scholar]
- 9. Lee JY, Jang YW, Kang HS, Moon H, Sim SS, Kim CJ. Anti-inflammatory action of phenolic compounds from Gastrodia elata root. Arch Pharm Res. 2006;29:849–58. doi: 10.1007/BF02973905. [DOI] [PubMed] [Google Scholar]
- 10. Liu J, Mori A. Antioxidant and pro-oxidant activities of p-hydroxybenzyl alcohol and vanillin: effects on free radicals, brain peroxidation and degradation of benzoate, deoxyribose, amino acids and DNA. Neuropharmacology. 1993;32:659–69. doi: 10.1016/0028-3908(93)90079-i. [DOI] [PubMed] [Google Scholar]
- 11. Hsieh CL, Lin JJ, Chiang SY, Su SY, Tang NY, Lin GG, Lin IH, Liu CH, Hsiang CY, Chen JC, Ho TY. Gastrodia elata modulated activator protein 1 via c-Jun N-terminal kinase signaling pathway in kainic acid-induced epilepsy in rats. J Ethnopharmacol. 2007;109:241–7. doi: 10.1016/j.jep.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 12. Hsieh MT, Peng WH, Wu CR, Wang WH. The ameliorating effects of the cognitive-enhancing Chinese herbs on scopolamine-induced amnesia in rats. Phytother Res. 2000;14:375–7. doi: 10.1002/1099-1573(200008)14:5<375::aid-ptr593>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 13. Hsieh MT, Wu CR, Chen CF. Gastrodin and p-hydroxybenzyl alcohol facilitate memory consolidation and retrieval, but not acquisition, on the passive avoidance task in rats. J Ethnopharmacol. 1997;56:45–54. doi: 10.1016/s0378-8741(96)01501-2. [DOI] [PubMed] [Google Scholar]
- 14. Shuchang H, Qiao N, Piye N, Mingwei H, Xiaoshu S, Feng S, Sheng W, Opler M. Protective effects of Gastrodia elata on aluminium-chloride-induced learning impairments and alterations of amino acid neurotransmitter release in adult rats. Restor Neurol Neurosci. 2008;26:467–73. [PMC free article] [PubMed] [Google Scholar]
- 15. Hayashi J, Sekine T, Deguchi S, Lin Q, Horie S, Tsuchiya S, Yano S, Watanabe K, Ikegami F. Phenolic compounds from Gastrodia rhizome and relaxant effects of related compounds on isolated smooth muscle preparation. Phytochemistry. 2002;59:513–9. doi: 10.1016/s0031-9422(02)00008-0. [DOI] [PubMed] [Google Scholar]
- 16. Kim HJ, Moon KD, Lee DS, Lee SH. Ethyl ether fraction of Gastrodia elata Blume protects amyloid β peptide-induced cell death. J Ethnopharmacol. 2003;84:95–8. doi: 10.1016/s0378-8741(02)00290-8. [DOI] [PubMed] [Google Scholar]
- 17. Huang NK, Lin YL, Cheng JJ, Lai WL. Gastrodia elata prevents rat pheochromocytoma cells from serum-deprived apoptosis: the role of the MAPK family. Life Sci. 2004;75:1649–57. doi: 10.1016/j.lfs.2004.05.008. [DOI] [PubMed] [Google Scholar]
- 18. Chen PJ, Hsieh CL, Su KP, Hou YC, Chiang HM, Lin IH, Sheen LY. The antidepressant effect of Gastrodia elata Bl. on the forced-swimming test in rats. Am J Chin Med. 2008;36:95–106. doi: 10.1142/S0192415X08005618. [DOI] [PubMed] [Google Scholar]
- 19. Chen PJ, Hsieh CL, Su KP, Hou YC, Chiang HM, Sheen LY. Rhizomes of Gastrodia elata Bl. possess antidepressant-like effect via monoamine modulation in subchronic animal model. Am J Chin Med. 2009;37:1113–24. doi: 10.1142/S0192415X09007533. [DOI] [PubMed] [Google Scholar]
- 20. Feng HC. Studies on the constituents of Gastrodia elata. Acta Chim Sin. 1979;37:175. [Google Scholar]
- 21. Wu CR, Hsieh MT, Huang SC, Peng WH, Chang YS, Chen CF. Effects of Gastrodia elata and its active constituents on scopolamine-induced amnesia in rats. Planta Med. 1996;62:317–21. doi: 10.1055/s-2006-957892. [DOI] [PubMed] [Google Scholar]
- 22. An SJ, Park SK, Hwang IK, Choi SY, Kim SK, Kwon OS, Jung SJ, Baek NI, Lee HY, Won MH, Kang TC. Gastrodin decreases immunoreactivities of γ-aminobutyric acid shunt enzymes in the hippocampus of seizure-sensitive gerbils. J Neurosci Res. 2003;71:534–43. doi: 10.1002/jnr.10502. [DOI] [PubMed] [Google Scholar]
- 23. Kumar H, Kim IS, More SV, Kim BW, Bahk YY, Choi DK. Gastrodin protects apoptotic dopaminergic neurons in a toxin-induced Parkinson’s disease model. Evid Based Complement Alternat Med. 2013;2013:1–10. doi: 10.1155/2013/514095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. International Conference on Harmonisation. Guideline on “Text on Validation of Analytical Procedures”. Federal Register. 1995;60:11260–3. [Google Scholar]
- 25. International Conference on Harmonisation. Guideline on “Validation of Analytical Procedures: Methodology”. Federal Register. 1997;62:27463–7. [Google Scholar]
- 26.Department of Health Food and Drug Administration. Validation of Analytical Specification in Food Chemistry. Taiwan Ministry of Health and Welfare;; 2012. pp. 1–3. [In Chinese]. [Google Scholar]
- 27. Al-Rimawi F. Development and validation of a simple reversed-phase HPLC-UV method for determination of oleuropein in olive leaves. J Food Drug Anal. 2014;22:285–9. doi: 10.1016/j.jfda.2013.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Shabir GA. Validation of high-performance liquid chromatography methods for pharmaceutical analysis-Understanding the differences and similarities between validation requirements of the US Food and Drug Administration, the US Pharmacopeia and the International Conference on Harmonization. J Chromatogr A. 2003;987:57–66. doi: 10.1016/s0021-9673(02)01536-4. [DOI] [PubMed] [Google Scholar]
- 29. Lu KH, Chen CY, Shih RL, Chin FS, Chien CS. Method development and validation for the GC-FID assay of ethanol in reservoir-type fentanyl transdermal patches. J Food Drug Anal. 2008;16:1–7. [Google Scholar]
- 30. Karakuş S, Küçükgüzel I, Küçükgüzel SG. Development and validation of a rapid RP-HPLC method for the determination of cetirizine or fexofenadine with pseudoephedrine in binary pharmaceutical dosage forms. J Pharm Biomed Anal. 2008;46:295–302. doi: 10.1016/j.jpba.2007.10.018. [DOI] [PubMed] [Google Scholar]
- 31.Editorial team of China Pharmacopoeia Medicine Committee. Taiwan Herbal Pharmacopeia. 2nd ed. Taipei: Ministry of Health and Welfare; 2013. pp. 39–40. [In Chinese]. [Google Scholar]
- 32. Teo CC, Tan SN, Yong JWH, Hew CS, Ong ES. Evaluation of the extraction efficiency of thermally labile bioactive compounds in Gastrodia elata Blume by pressurized hot water extraction and microwave-assisted extraction. J Chromatogr A. 2008;1182:34–40. doi: 10.1016/j.chroma.2008.01.011. [DOI] [PubMed] [Google Scholar]
- 33. Chen PJ, Liang KJ, Lin HC, Hsieh CL, Su KP, Hung MC, Sheen LY. Gastrodia elata Bl. attenuated learning deficits induced by forced-swimming stress in the inhibitory avoidance task and Morris water maze. J Med Food. 2011;14:610–7. doi: 10.1089/jmf.2010.1209. [DOI] [PubMed] [Google Scholar]
- 34. Lin SH, Chen WC, Lu KH, Chen PJ, Hsieh SC, Pan TM, Chen ST, Sheen LY. Down-regulation of Slit-Robo pathway mediating neuronal cytoskeletal remodeling processes facilitates the antidepressive-like activity of Gastrodia elata Blume. J Agric Food Chem. 2014;62:10493–503. doi: 10.1021/jf503132c. [DOI] [PubMed] [Google Scholar]
- 35. Liu CL, Liu MC, Zhu PL. Determination of gastrodin, p-hydroxybenzyl alcohol, vanillyl alcohol, p-hydroxylbenzaldehyde and vanillin in tall gastrodia tuber by high-performance liquid chromatography. Chromatographia. 2002;55:317–20. [Google Scholar]
- 36. Wu T, Wang N, Zhang Y, Xu XB. Advances in the study on microbial fermentation and transformation of traditional Chinese medicine. Afr J Microbiol Res. 2013;7:1644–50. [Google Scholar]
- 37. Kim JA, Park MS, Kang SA, Geun EJ. Production of γ-aminobutyric acid during fermentation of Gastrodia elata Bl. by co-culture of Lactobacillus brevis GABA 100 with Bifidobacterium bifidum BGN4. Food Sci Biotechnol. 2014;23:459–66. [Google Scholar]
- 38. Cai Z, Huang J, Luo H, Lei X, Yang Z, Mai Y, Liu Z. Role of glucose transporters in the intestinal absorption of gastrodin, a highly water-soluble drug with good oral bioavailability. J Drug Deliv. 2013;21:574–80. doi: 10.3109/1061186X.2013.778263. [DOI] [PubMed] [Google Scholar]
- 39. Ong ES, Heng MY, Tan SN, Hong Yong JW, Koh H, Teo CC, Hew CS. Determination of gastrodin and vanillyl alcohol in Gastrodia elata Blume by pressurized liquid extraction at room temperature. J Sep Sci. 2007;30:2130–7. doi: 10.1002/jssc.200700002. [DOI] [PubMed] [Google Scholar]
- 40. Chiu MJ. The Efficacy & Safety of Tian-ma on Dementia of Alzheimer’s type - A Clinic and PET study. Yearbook Chin Med Pharm. 2004;22:167–72. [In Chinese, English abstract] [Google Scholar]
- 41.Hus GJ, Ho HX, Hus LS, Jin RY. Chinese pharmacognosy. Beijing: China Medical Science Press; 1996. p. 696. [In Chinese]. [Google Scholar]