Abstract
Iron deficiency is one of the most concerning deficiency problems in the world. It may generate several adverse effects such as iron deficiency anemia (IDA) and reduced physical and intellectual working capacity. The aim of this study is to evaluate the Fe(II)-binding activity of collagen peptides from fishery by-products. Lates calcarifer, Mugil cephalus, Chanos chanos, and Oreochromis spp are four major cultivated fishes in Taiwan; thousands of scales of these fish are wasted without valuable utilization. In this study, scales of these fish were hydrolyzed by papain plus flavourzyme. Collagen peptides were obtained and compared for their Fe(II)-binding activity. Collagen peptides from Chanos chanos showed the highest Fe(II)-binding activity, followed by those from Lates calcarifer and Mugil cephalus; that from Oreochromis spp exhibited the lowest one. Fe(II)-binding activity of collagen peptides from fish scales was also confirmed with a dialysis method. Molecular weight (MW) distributions of the collagen peptides from scales of four fish are all < 10 kDa, and averaged 1.3 kDa. Hydrolysates of fish scales were further partially purified with ion exchange chromatography. Fractions having Fe(II)-binding activity were obtained and their activity compared. Data obtained showed that collagen peptides from fish scales did have Fe(II)-binding activity. This is the first observation elucidating fish scale collagen possessing this functionality. The results from this study also indicated that collagen peptides from fish scales could be applied in industry as a bioresource.
Keywords: collagen peptide, enzymatic hydrolysates, fish scale, iron-binding activity
1. Introduction
Iron is recognized as a trace nutrient for plants, animals, and humans. It plays an important role in the constituents of cytochromes, hemoglobin, myoglobin, and some enzymes [1]. According to the study from the World Health Organization, iron deficiency was one of the most concerning deficiency problems in the world [2]. Iron deficiency may generate several adverse effects such as iron deficiency anemia (IDA) and reduced physical and intellectual working capacity [3]. Iron deficiency is generally induced by insufficient intake, such as a plant-based diet or low bioavailability of iron [4]. Iron can be supplied in salts, elemental iron, metal chelates, and iron-binding proteins or peptides [1]. Among these, iron salts may be the popular way for iron supplementation. However, they have low bioavailability in the body because of their reactivity with other food components, such as phytic acid, polyphenols, and certain fibers [1]. Caseinophosphopeptides (CPPs), a group of milk-derived phosphorylated peptides, are now used widely as a mineral supplement to promote iron absorption [5]. However, some people may suffer an allergy problem when using milk products. In certain cases, the use of milk-derived products is not allowed due to religious reasons. By contrast, recent studies have investigated other sources of iron binding peptides, such as those from vegetative protein, meat, or fish [6].
Iron binding activity was also found in the hydrolysates of whey protein [7], porcine blood plasma protein [6], anchovy muscle protein [8], and shrimp processing by-products [9], but not found in that of fish processing by-products. In general, round fish contain about 20–25% edible meat and 75–80% wastes, predominately viscera, heads, bone, skin, and scale. Most of these wastes are discarded without valuable utilization. For example, numerous fish scales are produced from the processing of fish fillets. The surface of a fish scale is an osseous layer consisting of randomly oriented collagen fibrils with many hydroxyapatite [Ca10(O4)6(OH)2] crystals. Thin layers of the oriented collagen fibrils are piled up to form the fibrillary plate under the osseous layer. The direction of the fibrils in each thin layer differs from each other. This plywood-like structure provides the high mechanical strength of scales, and is probably the reason why it was discarded before. However, nowadays we pay attention to the recovery of collagen from fish scales. The collagen obtained can be used in cosmetics, biomedical materials, and as functional food [10].
The collagen of fish scale is used predominantly in the hydrolysate form, i.e., collagen peptide, probably due to its physical properties, tight structure, and thermal stability [11]. Collagen peptide of fish scales was reported previously as having physiological functions, such as antioxidative activity [12], antihypertensive activity [13], and an ability to proliferate human keratinocytes [14]. In the present work, we found that collagen peptides from fish scales were possessed of Fe(II)-binding activity. This is the first investigation demonstrating fish scale collagen to have this functionality. The aim of this study is to evaluate the Fe(II)-binding activity of collagen peptides from fish scales of Lates calcarifer, Mugil cephalus, Chanos chanos, and Oreochromis spp, which are four major cultivated fishes in Taiwan, and to find possible applications for fish scales as a bioresource [15].
2. Methods
2.1. Materials
The fish scales were all obtained from the fishery factory in Southern Taiwan. Fresh fish scales were kept in iced water and transported to the laboratory immediately (Fig. 1). Fish scales were washed and dried at 100°C for 2 hours. The dried fish scales were then milled into powder [16] and stored at room temperature until use. Aprotinin, vitamin B12, glutathione, and tyrosine were purchased from Sigma-Aldrich (St. Louis, MO, USA), and all other reagents if not declared were purchased from Sigma-Aldrich.
Fig. 1.
Photographs of fish scales from: (A) Lates calcarifer; (B) Mugil cephalus; (C) Chanos chanos; and (D) Oreochromis spp.
2.2. Preparation of enzymatic hydrolysates
A reaction mixture consisting of dried fish scale powder (1 g), papain, and flavourzyme (enzyme activity, 3000 U) in 100 mL PBS (bisodium phosphate) buffer (pH 7.0) was incubated at 50°C with constant agitation (200 rpm) for 2 hours. Reactions were terminated by heating the mixture in boiling water for 10 minutes. After cooling to room temperature, the hydrolysates were filtrated using Toyo No. 5A filter paper. The enzymatic hydrolysates were then demineralized by 1% Chelex 100 resin (Bio-rad, Hercules, CA, USA) for 1 hour and filtrated again using Toyo No. 3 filter paper.
2.3. Molecular weight distributions of fish scale hydrolysates
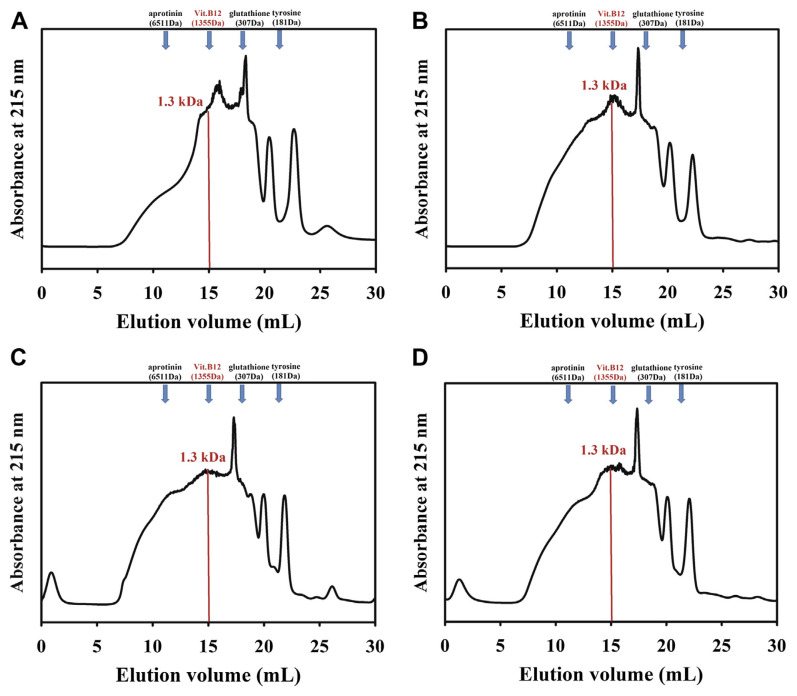
Molecular weight (MW) distributions of hydrolysates from fish scales were determined by gel filtration chromatography on a Superdex Peptide column (10 mm × 300 mm, GE Healthcare (Uppsala, Sweden), USA) using a fast protein liquid chromatography system (FPLC, AKTA purifier, GE Healthcare). The mobile phase used was de-ion water. The flow rate was 0.4 mL/minute. Absorbance was monitored at 215 nm. The Superdex Peptide column showed excellent efficiency on separation of the peptides within MW 0.1 kDa to 7 kDa as described by the supplier. The calibration standards for MW contain aprotinin (MW 6511), vitamin B12 (MW 1355), glutathione (MW 307), and tyrosine (MW 181).
2.4. Determination of Fe(II)-binding activity
2.4.1. Phosphate precipitation method [8]
To the hydrolysates mixed with equal volumes of 20 mM phosphate buffer (pH 7.0) was added 5 mM FeCl2, and the mixture was stirred at room temperature for 1 hour. The reaction mixture was then centrifuged at 3500 × g for 20 minutes to remove precipitates. The iron concentration in the supernatant assayed with a colorimetric method by using orthophenanthroline reagent [17] was used as an indicator of Fe(II)-binding activity.
2.4.2. Dialysis method
Fe(II)-binding activity was also determined according to a procedure by Bao et al [18] with some modification. Samples together with ferrous chloride (5 mM) were added into a dialysis bag (24 mm, MW cut off 500 Da, Merck, Darmstadt, Germany), and dialyzed at 4°C in 500 volumes of tris buffer (0.02 M, pH 7.0) to remove ferrous chloride. Tris buffer was changed every 4 hours and a sample was collected every 12 hours for iron determination. The concentration of ferrous ion which remained in the dialysis bag was used as an indicator for Fe(II)-binding activity.
2.5. Determination of peptide concentration
The peptide concentration was analyzed with the orthophtaldialdehyde method [19,20] using a spectrophotometer (Amersham Pharmacia U-2000, Uppsala, Sweden) at 340 nm, and DL-serine was used as the standard.
2.6. Separation of iron-binding peptides
The hydrolysates were fractionated by a HiTrap Q HP column (1.6 cm × 2.5 cm, GE Healthcare) using a liquid chromatography system (AKTA prime plus, GE Healthcare). The column was equilibrated with 20 mM Tris buffer (pH 7.5), and eluted with a linear gradient of 0–0.3 M NaCl at a flow rate of 2 mL/ minute. The fraction of 2 mL was collected and assayed for iron-binding activity [17].
3. Results
3.1. Iron-binding activity and peptide concentration of fish scale collagen hydrolysates
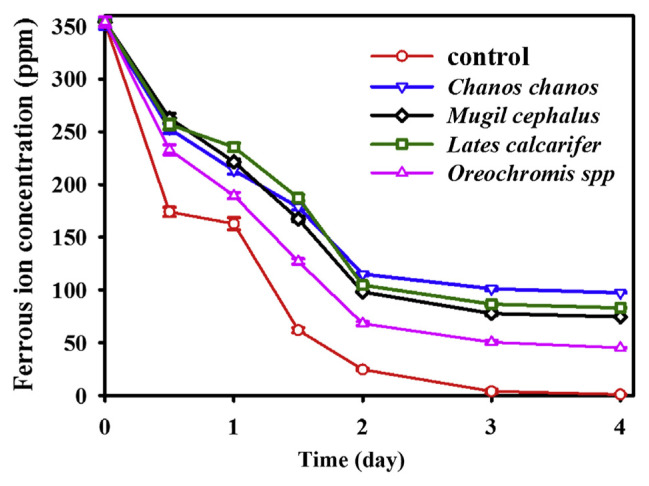
Collagen peptides obtained from different fish scales treated with papain plus flavourzyme were assayed for their Fe(II)-binding activity using the phosphate precipitation method, and listed in Table 1. Collagen peptides from four different fish scales were all found to have Fe(II)-binding activity. This is the first observation describing fish scale collagen possessing this functionality. Based on Fe(II)-binding activity/peptide concentration, that from Chanos chanos (Fe/peptide: 35.3) showed the highest one, followed by those from Lates calcarifer (Fe/peptide: 30.5) and Mugil cephalus (Fe/peptide: 24.4), and that from Oreochromis spp (Fe/peptide: 5.1) exhibited the lowest one (Table 1). Fe(II)-binding activity was also examined with a dialysis method. Ferrous ion together with collagen peptides from four fish scales were placed in a dialysis bag (MW Cut off 500 Da), and dialyzed for 4 days. Concentrations of residue of ferrous ion were determined during different dialysis periods and are shown in Fig. 2. A concentration of ferrous ion in the absence of scale collagen peptide (control) was not detected after dialysis for more than 3 days. The remaining concentration of ferrous ion in the presence of scale collagen peptide from Chanos chanos was detected to be 101.3 ppm and 97.4 ppm after dialysis for 3 and 4 days, respectively; 77.8 ppm and 74.6 ppm, Mugil cephalus; 86.6 ppm and 83.1 ppm, Lates calcarifer; and 50.5 ppm and 45.3 ppm, Oreochromis spp. Data obtained confirmed that collagen peptide from fish scales did have Fe(II)-binding activity.
Table 1.
Iron-binding activity and peptide concentration of collagen peptides from four fish scales.
| Fish scales | Fe binding (ppm) | Peptide concentration (mg/ml) | Fe/peptide (ppm/mg) |
|---|---|---|---|
| Lates calcarifer | 375.1 ± 2.68a | 12.3 | 30.5 |
| Mugil cephalus | 302.7 ± 0.98 | 12.4 | 24.4 |
| Chanos chanos | 321.6 ± 3.53 | 9.1 | 35.3 |
| Oreochromis spp | 53.7 ± 0.84 | 10.5 | 5.1 |
Mean ± standard deviation, n = 3.
Fig. 2.
Changes in ferrous ion concentration in the dialysis bag (MW Cut off 500 Da) containing ferrous ion in the absence (control) or presence of hydrolysates from four fish scales during different dialysis periods.
3.2. MW distributions of collagen peptides from four fish scales
Collagen peptides from the scales of four fish, hydrolyzed with papain plus flavourzyme, were subjected to a Superdex Peptide column, and the MW distributions are shown in Fig. 3. The MWs of collagen peptides from four fish scale were all < 10 kDa, and averaged 1.3 kDa.
Fig. 3.
Gel filtration profiles on Superdex Peptide column for collagen peptides from scale of: (A) Lates calcarifer; (B) Mugil cephalus; (C) Chanos chanos; and (D) Oreochromis spp. The calibration standards for molecular weight (MW) contain aprotinin (MW 6511), Vit B12 (MW 1355), glutathione (MW 307), and tyrosine (MW 181).
3.3. Separation of iron-binding peptides
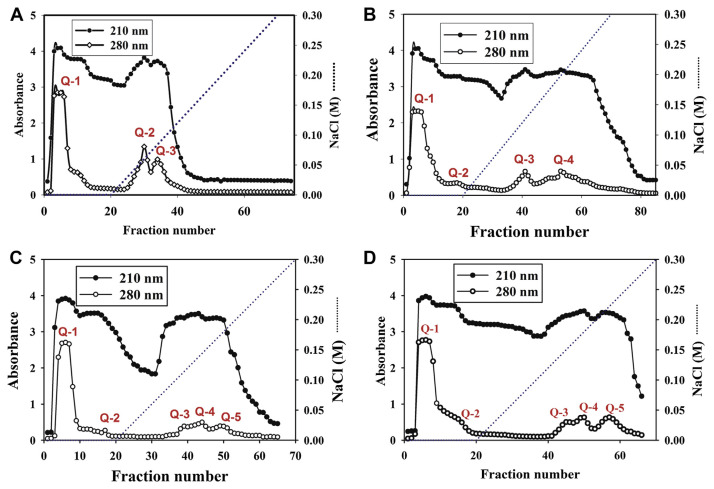
It is suggested that an iron-binding peptide is related to its net charge for binding [21]. To separate the iron-binding peptides, collagen peptides from four fish scales were applied to a HiTrap Q ion exchange column. Different fractions were collected and assayed for their Fe(II)-binding activity (Fig. 4). Three peaks were obtained from collagen peptides of Lates calcarifer and named Q-1, Q-2, and Q-3, respectively. Four major peaks (Q-1, Q-2, Q-3, Q-4) were obtained from those of Mugil cephalus, five major peaks (Q-1, Q-2, Q-3, Q-4, Q-5) from Chanos chanos and five major peaks (Q-1, Q-2, Q-3, Q-4, Q-5) from Oreochromis spp. All of the first fraction (Q-1) from those of four fish collagen peptides did not have Fe(II) binding activity. The second fraction (Q-2) from collagen peptides of Lates cal-carifer; Q-3, Q-4 from Mugil cephalus; Q-3, Q-4, Q-5 from Chanos chanos; and Q-4 from Oreochromis spp were found to have higher iron-binding capacity based on Fe(II) binding activity/ peptide concentration (Table 2). Among these fractions, the third fraction (Q-3) from Chanos chanos was found to possess the highest iron-binding capacity, approximately 22.1 based on Fe(II) binding activity/peptide concentration (Table 2).
Fig. 4.
Column chromatograms on HiTrap Q ion exchange for collagen peptides obtained from fish scales of: (A) Lates calcarifer; (B) Mugil cephalus; (C) Chanos chanos; and (D) Oreochromis spp.
Table 2.
Fe(II)-binding activity and peptide concentration of different fractions obtained from collagen peptides of four fish scales fractionated by HiTrap Q column.
| Fish scales | Fe binding (ppm) | Peptide concentration (mg/mL) | Fe/peptide (ppm/mg) |
|---|---|---|---|
| Lates calcarifer | |||
| Q-1 | 0 | ND | ND |
| Q-2 | 10.5 ± 0.21a | 0.8 | 14.0 |
| Q-3 | 5.5 ± 0.14 | 1.2 | 4.5 |
| Mugil cephalus | |||
| Q-1 | 0 | ND | ND |
| Q-2 | 11.3 ± 0.14 | 7.8 | 1.4 |
| Q-3 | 10.8 ± 1.39 | 0.9 | 11.7 |
| Q-4 | 13.8 ± 0.45 | 1.0 | 13.8 |
| Chanos chanos | |||
| Q-1 | 0 | ND | ND |
| Q-2 | 15.9 ± 0.61 | 4.5 | 3.5 |
| Q-3 | 12.2 ± 0.83 | 0.6 | 22.1 |
| Q-4 | 15.9 ± 1.27 | 0.9 | 17.6 |
| Q-5 | 14.0 ± 0.85 | 1.0 | 14.4 |
| Oreochromis spp | |||
| Q-1 | 0 | ND | ND |
| Q-2 | 12.9 ± 0.56 | 5.2 | 2.3 |
| Q-3 | 6.6 ± 0.42 | 0.8 | 8.3 |
| Q-4 | 13.2 ± 1.18 | 0.9 | 14.3 |
| Q-5 | 4.2 ± 0.25 | 0.9 | 4.9 |
Mean ± standard deviation, n = 3.
4. Discussion
There are two major components in fish scales; one is collagen and the other is hydroxyapatite. In native fish scales, collagen was bound with hydroxyapatite tightly and made collagen unextractable by water or other solvents [13]. Three methods could be used to extract collagen from fish scales: (1) a chemical method: extracting collagen by using acidic or alkaline reagent; (2) an enzymatic method: using protease to hydrolyze fish scales to obtain soluble collagen peptide [13]; and (3) a microbial method: using microorganisms as source of enzyme to obtain collagen peptide. Among them, enzymatic hydrolysis seems to be the best way to hydrolyze scale protein without losing nutritional value. Hydrolysis of food proteins is performed not only to improve their functional properties like solubility, digestibility, gelation, emulsifying, foaming properties, and mineral-binding activity, but also to reduce allergenic problems [22–24]. The current study used protease to hydrolyze collagen from fish scales, and the hydrolysates (collagen peptide) were obtained to evaluate their Fe(II)-binding activity. In a preliminary experiment, protamex, alcalase, trypsin, bromelain, papain, flavourzyme, protease N, and pepsin were used to hydrolyze collagen from fish scales. Collagen peptides were obtained and compared for their Fe(II)-binding activity. Collagen peptides from fish scales treated with papain plus flavourzyme were found to have higher Fe(II)-binding activity [25]. Thus, papain plus flavourzyme were selected and used in this study. Previously, collagen peptide derived from fish scales was reported to have anti-oxidative activity [12], antihypertensive activity [13], and the ability to proliferate human keratinocytes [14]. In the present work, we detect Fe(II)-binding activity in the collagen peptides of fish scales treated with papain plus flavourzyme (Table 1). This is the first observation elucidating collagen peptide from fish scales to be possessed of this new functionality. In order to make a confirmation, scale collagen peptides and ferrous ion were placed in a dialysis bag (MW Cut off 500 Da). The remaining concentration of ferrous ion inside the dialysis bag could be used as an indicator for Fe(II)-binding activity of collagen peptides. Bao et al [18] used the same technique to prove that the soybean protein hydrolysate possessed calcium binding activity. The concentration of ferrous ion in the dialysis bag was not detected in the absence of scale collagen peptide (control) after dialysis for more than 3 days (Fig. 2). This result agreed with the finding of Bao et al [18]. The remaining concentration of ferrous ion in the dialysis bag was detected to be 97.4 ppm in the presence of scale collagen peptide from Chanos chanos after dialysis for 4 days; 74.6 ppm, Mugil cephalus; 83.1 ppm, Lates calcarifer; and 45.3 ppm, Oreochromis spp (Fig. 2). Although the values of Fe(II)-binding activity were different from those assayed with the phosphate precipitation method (Table 1), both methods exhibited the same trend in comparison of Fe(II)-binding activity from different fish scales. Data obtained indicated that collagen peptide from fish scales did have Fe(II)-binding activity.
Papain used in this study is a cysteine protease [26], and also known as papaya proteinase I present in papaya (Carica papaya) and mountain papaya (Vasconcellea cundinamarcensis). Hydrolysates from wheat gluten [27], peanut meal [28] treated with papain, were all found to improve their digestibility or other functional properties when compared with native protein. Hydrolysates of bovine whey protein concentrate treated with papain revealed low immunoreactivity and could be used in hypoallergenic dairy products [29]. Hydrolysates of byproducts from Atlantic cod, rawhide, skate (Raja clavata) cartilage, and tuna backbone protein were found to have new functionalities after papain digestion [30–33]. Flavourzyme, a fungal endo-and exoprotease/peptidase complex, was produced by submerged fermentation of Aspergillus oryzae. Byproducts of salmon and cod (Gadus morhua), tuna liver, yellow stripe trevally (Selaroides leptolepis), Atlantic salmon (Salmo salar) muscle, and whey protein treated with flavourzyme were all found to improve their functionalities [34–39]. In this study, papain plus flavourzyme with endo- and exoprotease activity was used to hydrolyze fish scales, and the collagen peptides obtained demonstrated Fe(II)-binding activity as shown in Table 1 and Fig. 2. Fe(II)-binding peptide in anchovy muscle protein was identified as Ser-(Gly)7-Leu-Gly-Ser-(Gly)2-Ser-Ile-Arg and Ile-(Glu)2-Leu-(Glu)3-Ile-Glu-Ala-Glu-Arg [8]; that in hydrolysates of porcine blood plasma protein was identified as Asp–Leu–Gly–Glu–Gln–Tyr–Phe–Lys–Gly [6]. Exposure of glycine residue seems to be important for ferrous ions and peptides forming table complexes [6,8]. Since collagen from fish scales is rich in glycine [13], Fe(II)-binding activity is probably derived from the exposure of glycine during hydrolysis of scale collagen. However, the actual mechanism needs further study.
The MW distributions of commercial collagen peptides from fish scales are mostly between 2 kDa and 20 kDa, and averaged 2–3 kDa [10]. The MWs of collagen peptides from the present study are all < 10 kDa, and averaged 1.3 kDa (Fig. 3). Our method seems to be more efficient for hydrolyzing fish scale collagen into smaller molecules. The smaller molecules of collagen peptide possess many physiological functions [12–14], and are valuable for industries as cosmetics, biomedical materials, and functional food. The current findings provide important information for fish scales to be used in industries.
In order to remove the interfering substances, hydrolysates from fish scales were further partially purified with ion exchange chromatography (Fig. 4). Two fractions with Fe(II)-binding activity were obtained from hydrolysates of Lates calcarifer; three fractions from that of Mugil cephalus; and four fractions from both Chanos chanos and Oreochromis spp (Table 2). Data obtained indicate that many Fe(II)-binding peptides are present in the hydrolysates of fish scales. Further study will be focused on identification and sequence analysis of the purified Fe(II)-binding peptide.
5. Conclusion
Collagen peptides from the scales of four fish all demonstrated Fe(II)-binding activity. This is the first investigation elucidating fish scale collagen to possess this functionality. Collagen peptides from scales of Chanos chanos had the highest Fe(II)-binding activity, followed by those from Lates calcarifer and Mugil cephalus, and that from Oreochromis spp exhibited the lowest one. The MWs of collagen peptides obtained were all < 10 kDa, and averaged 1.3 kDa. The current method is efficient to hydrolyze fish scale collagen into smaller molecules. Collagen peptides were further fractionated into different fractions by ion exchange chromatography. Two fractions with Fe(II)-binding activity were partially purified from hydrolysates of Lates calcarifer; three fractions from that of Mugil cephalus; and four fractions from both Chanos chanos and Oreochromis spp. These findings provide important information for fish scales to be used as a bioresource in industries. Identification and sequence analysis of the purified Fe(II)-binding peptide need further study.
Acknowledgments
This work was supported by the National Science Council of Republic of China with grant number NSC100-2221-E-022-008.
Funding Statement
This work was supported by the National Science Council of Republic of China with grant number NSC100-2221-E-022-008.
Footnotes
Conflicts of interest
All authors declare no conflicts of interest.
REFERENCES
- 1. Gaucheron F. Iron fortification in dairy industry. Trends Food Sci Tech. 2000;11:403–9. [Google Scholar]
- 2. Guilbert JJ. The world health report 2002: reducing risks, promoting healthy life. Educ Health (Abingdon) 2003;16:230. doi: 10.1080/1357628031000116808. [DOI] [PubMed] [Google Scholar]
- 3. Schümann K, Ettle T, Szegner B, Elsenhans B, Solomons NW. On risks and benefits of iron supplementation recommendations for iron intake revisited. J Trace Elem Med Bio. 2007;21:147–68. doi: 10.1016/j.jtemb.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 4. Chiplonkar SA, Tarwadi KV, Kavedia RB, Mengale SS, Paknikar KM, Agte VV. Fortification of vegetarian diets for increasing bioavailable iron density using green leafy vegetables. Food Res Int. 1999;32:169–74. [Google Scholar]
- 5. Ait-Oukhatar N, Peres JM, Bouhallab S, Neuville D, Bureau F, Bouvard G, Arhan P, Bougle D. Bioavailability of caseinophosphopeptide-bound iron. J Lab Clin Med. 2002;140:290–4. doi: 10.1067/mlc.2002.128146. [DOI] [PubMed] [Google Scholar]
- 6. Lee SH, Song KB. Purification of an iron-binding nonapeptide from hydrolysates of porcine blood plasma protein. Process Biochem. 2009;44:378–81. [Google Scholar]
- 7. Kim SB, Seo IS, Khan MA, Ki KS, Lee WS, Lee HJ, Shin HS, Kim HS. Enzymatic hydrolysis of heated whey: Iron-binding ability of peptides and antigenic protein fractions. J Dairy Sci. 2007;90:4033–42. doi: 10.3168/jds.2007-0228. [DOI] [PubMed] [Google Scholar]
- 8. Wu HH, Liu ZY, Zhao YH, Zeng M. Enzymatic preparation and characterization of iron-chelating peptides from anchovy (Engraulis japonicus) muscle protein. Food Res Int. 2012;48:435–41. [Google Scholar]
- 9. Huang GR, Ren L, Jiang JX. Purification of a histidine-containing peptide with calcium binding activity from shrimp processing byproducts hydrolysate. Eur Food Res Technol. 2011;232:281–7. [Google Scholar]
- 10. Pan MH, Tsai ML, Chen WM, Hwang A, Sun Pan B, Hwang YR, Kuo JM. Purification and characterization of a fish scale-degrading enzyme from a newly identified Vogesella sp. J Agric Food Chem. 2010;58:12541–6. doi: 10.1021/jf1034042. [DOI] [PubMed] [Google Scholar]
- 11. Ikoma T, Kobayashi H, Tanaka J, Walsh D, Mann S. Microstructure, mechanical, and biomimetic properties of fish scales from Pagrus major. J Struct Biol. 2003;142:327–33. doi: 10.1016/s1047-8477(03)00053-4. [DOI] [PubMed] [Google Scholar]
- 12. Kuo JM, Lee GC, Liang WS, Yang JI. Process optimization for production of antioxidant gelatin hydrolysates from tilapia skin. J Fish Soc Taiwan. 2009;36:15–28. [Google Scholar]
- 13. Fahmi A, Morimura S, Guo HC, Shigematsua T, Kidaa K, Uemura Y. Production of angiotensin I converting enzyme inhibitory peptides from sea bream scales. Process Biochem. 2004;39:1195–200. [Google Scholar]
- 14.Lai CH. Masters Thesis. Keelung, Taiwan: National Taiwan Ocean University; 2006. Study on the extraction of collagen of fish scale and antioxidant and keratinocyte proliferation of their hydrolysates. [Google Scholar]
- 15. Mori H, Tone Y, Shimizu K, Zikihara K, Tokutomi S, Ida T, Ihara H, Hara M. Studies on fish scale collagen of Pacific saury (Cololabis saira) Mater Sci Eng C. 2013;33:174–81. doi: 10.1016/j.msec.2012.08.025. [DOI] [PubMed] [Google Scholar]
- 16. Kuo JM, Hwang A. A new process for the preparation of collagen peptide from fish scale. Patent of Taiwan. 2012;(I):362393. [Google Scholar]
- 17. Huang GR, Ren ZY, Jiang JX. Separation of iron-binding peptides from shrimp processing by-products hydrolysates. Food Bioprocess Tech. 2011;4:1527–32. [Google Scholar]
- 18. Bao XL, Lv Y, Yang BC, Ren CG, Guo ST. A study of the soluble complexes formed during calcium binding by soybean protein hydrolysates. J Food Sci. 2008;73:C117–21. doi: 10.1111/j.1750-3841.2008.00673.x. [DOI] [PubMed] [Google Scholar]
- 19. Kuo JM, Yang JI, Chen WM, Pan MH, Tsai ML, Lai YJ, Hwang A, Pand BS, Lin CY. Purification and characterization of a thermostable keratinase from Meiothermus sp. I40. Int Biodeter Biodegr. 2012;70:111–6. [Google Scholar]
- 20. Silvestre M. Review of methods for the analysis of protein hydrolysates. Food Chem. 1997;60:263–71. [Google Scholar]
- 21. Chaud MV, Izumi C, Nahaal Z, Shuhama T, Bianchi Mde L, de Freitas O. Iron derivatives from casein hydrolysates as a potential source in the treatment of iron deficiency. J Agr Food Chem. 2002;50:871–7. doi: 10.1021/jf0111312. [DOI] [PubMed] [Google Scholar]
- 22. Hrckova M, Rusnakova M, Zemanovic J. Enzymatic hydrolysis of defatted soy flour by three different proteases and their effect on the functional properties of resulting protein hydrolysates. Czech J Food Sci. 2002;20:7–14. [Google Scholar]
- 23. Kristinsson HG, Rasco BA. Fish protein hydrolysates: Production, biochemical, and functional properties. Crit Rev Food Sci Nutr. 2000;40:43–81. doi: 10.1080/10408690091189266. [DOI] [PubMed] [Google Scholar]
- 24. Kristinsson HG, Rasco BA. Biochemical and functional properties of Atlantic salmon (Salmo salar) muscle proteins hydrolyzed with various alkaline proteases. J Agr Food Chem. 2000;48:657–66. doi: 10.1021/jf990447v. [DOI] [PubMed] [Google Scholar]
- 25.Li YH. Master Thesis. Kaohsiung, Taiwan: National Kaohsiung Marine University; 2012. Minerals binding activity of collagen peptide from fish scale. [Google Scholar]
- 26. Katunuma N, Kominami E. Structure, properties, mechanisms, and assays of cysteine protease inhibitors: cystatins and e-64 derivatives. Method Enzymol. 1995;251:382–97. doi: 10.1016/0076-6879(95)51142-3. [DOI] [PubMed] [Google Scholar]
- 27. Wang JS, Wei ZY, Li L, Bian K, Zhao MM. Characteristics of enzymatic hydrolysis of thermal-treated wheat gluten. J Cereal Sci. 2009;50:205–9. [Google Scholar]
- 28. Su GW, Ren JY, Yang B, Cui C, Zhao M. Comparison of hydrolysis characteristics on defatted peanut meal proteins between a protease extract from Aspergillus oryzae and commercial proteases. Food Chem. 2011;126:1306–11. [Google Scholar]
- 29. Izquierdo FJ, Peñas E, Baeza ML, Gomez R. Effects of combined microwave and enzymatic treatments on the hydrolysis and immunoreactivity of dairy whey proteins. Int Dairy J. 2008;18:918–22. [Google Scholar]
- 30. Aspmo SI, Horn SJ, Eijsink HVG. Enzymatic hydrolysis of Atlantic cod (Gadus morhua L.) viscera. Process Biochem. 2005;40:1957–66. [Google Scholar]
- 31. Damrongsakkul S, Ratanathammapan K, Komolpis K, Tanthapanichakoon W. Enzymatic hydrolysis of rawhide using papain and neutrase. J Ind Eng Chem. 2008;14:202–6. [Google Scholar]
- 32. Murado MA, Fraguas J, Montemayor MI, Vázqueza JA, Gonzáleza P. Preparation of highly purified chondroitin sulphate from skate (Raja clavata) cartilage by-products. Process optimization including a new procedure of alkaline hydroalcoholic hydrolysis. Biochem Eng J. 2010;49:126–32. [Google Scholar]
- 33. Je JY, Qian ZJ, Byun HG, Kim SK. Purification and characterization of an antioxidant peptide obtained from tuna backbone protein by enzymatic hydrolysis. Process Biochem. 2007;42:840–6. [Google Scholar]
- 34. Ahn CB, Jeon YJ, Kim YT, Je JY. Angiotensin I converting enzyme (ACE) inhibitory peptides from salmon byproduct protein hydrolysate by alcalase hydrolysis. Process Biochem. 2012;47:2240–5. [Google Scholar]
- 35. Je JY, Lee KH, Lee MH, Ahn CB. Antioxidant and antihypertensive protein hydrolysates produced from tuna liver by enzymatic hydrolysis. Food Res Int. 2009;42:1266–72. [Google Scholar]
- 36. Klompong V, Benjakul S, Kantachote D, Shahidi F. Antioxidative activity and functional properties of protein hydrolysate of yellow stripe trevally (Selaroides leptolepis) as influenced by the degree of hydrolysis and enzyme type. Food Chem. 2007;102:1317–27. [Google Scholar]
- 37. Daukšas E, Falch E, Šližytė R, Rustad T. Composition of fatty acids and lipid classes in bulk products generated during enzymic hydrolysis of cod (Gadus morhua) by-products. Process Biochem. 2005;40:2659–70. [Google Scholar]
- 38. Kristinsson HG, Rasco BA. Kinetics of the hydrolysis of Atlantic salmon (Salmo salar) muscle proteins by alkaline proteases and a visceral serine protease mixture. Process Biochem. 2000;36:131–9. [Google Scholar]
- 39. Cheison SC, Wang Z, Xu SY. Multivariate strategy in screening of enzymes to be used for whey protein hydrolysis in an enzymatic membrane reactor. Int Dairy J. 2007;17:393–402. [Google Scholar]