Abstract
Caffeoylquinic acid (CQA) derivatives are known to possess antioxidative potential and have many beneficial effects on human health. The present study compared the CQA contents and antioxidant activities of aerial parts of sweet potato plants. The effects of drying methods (freeze drying, and drying at 30°C, 70°C, and 100°C) on these two parameters of the first fully expanded leaves were also assessed. The results indicated that the CQA derivatives were detectable in leaves, stem, and flowers of sweet potato plants (varied from 39.34 mg/g dry weight to 154.05 mg/g dry weight), with the leaves (particularly expanding and first fully expanded leaves) containing more CQA derivatives than other aerial plant parts. The expanding and first fully expanded leaves also exhibited greater antioxidant activities than other aerial plant parts, possibly due to their higher contents of CQA derivatives. Drying method significantly affected the content of CQA derivatives in dried sweet potato leaf tissues. Drying treatments at both 70°C and 100°C significantly reduced the CQA derivative content and antioxidant activity in the first fully expanded leaves. Among the tested drying methods, the freeze-drying method demonstrated the preservation of the highest amount of CQA derivatives (147.84 mg/g) and antioxidant property. However, 30°C cool air drying was also a desirable choice (total CQA derivative content was reduced to only 129.52 mg/g), compared to 70°C and 100°C hot air drying, for commercial-scale processing of sweet potato leaves, if the higher operation cost of freeze drying was a major concern.
Keywords: antioxidants, caffeoylquinic acid derivatives, cool air drying, freeze drying, hot air drying, sweet potato
1. Introduction
Sweet potato (Ipomoea batatas L.) is a tuberous root plant originated from South America but is now widely cultivated in many parts of the world. It is mainly planted for its tubers, but the young leaves of sweet potato plants can also be consumed as leafy vegetables [1,2]. Studies have reported that the leaves of sweet potato plants are a rich source of caffeoylquinic acid (CQA) derivatives (esters of caffeic acid and quinic acid) [3], which are known to exhibit antioxidative potential and have many beneficial effects on human health [4–8]. Among these CQA derivatives, chlorogenic acid (ChA) is commercially available for application in medicine and cosmetics [9,10]. Thus, sweet potato leaves are a potential source of natural CQA derivatives. However, in plants, polyphenols are not evenly distributed, and the content of a specific phenolic compound can vary greatly among different plant tissues. Therefore, in order to obtain an end product with a very high content of CQA derivatives, selection of appropriate tissues from sweet potato plants for commercial-scale extraction is necessary.
Similar to other leafy vegetables, fresh sweet potato leaves are perishable under ambient conditions, which may lead to significant changes in the content of phenolic compounds that are suitable for commercial-scale processing. Hence, they have to be processed to extend their shelf-life for commercial-scale processing. Drying is a process that extends the shelf-life of the final product by reducing moisture content, inhibiting enzymatic degradation, and limiting microbial growth. However, drying also affects the quality of plant tissues in many ways, and various drying techniques are developed for dehydration of plants [11]. Among these techniques, hot air drying is frequently used to dry plant materials because of its lower cost and less drying time [12]. However, it has inevitable disadvantages such as low energy efficiency and loss of quality with respect to color and nutritive values [13,14]. Lowering of the drying temperature has been reported to be helpful in retaining the quality, with respect to both color and phytochemical content, of dried leafy vegetables to some extent [15]. Freeze drying is generally better in preserving the quality of plant tissues during drying. It is often used for drying medicinal and aromatic herbs, as it minimizes the loss of bioactivity and flavor [16]. However, the cost of freeze drying is considerably higher than that of hot air drying [17].
Little information is available in the literature regarding the level of CQAs in sweet potato aerial tissues after drying. Drying of sweet potato leaf tissues at a high temperature may result in significant degradation of CQA derivatives and decrease of antioxidant capacity. Therefore, development of an optimal drying technique is warranted in order to preserve these valuable bioactive substances in sweet potato tissues. The objectives of this study were to assess the differences in CQA derivative contents and antioxidant activities among various aerial tissues of sweet potato plants. The effects of drying treatment on the contents of CQA derivatives of dried sweet potato leaves were also evaluated. Knowledge of these variations should provide useful information on the commercial potential of using sweet potato to produce high-value nutraceuticals.
2. Materials and methods
2.1. Plant materials and chemicals
Aerial parts of sweet potato (I. batatas L.) variety SM-2 were obtained from the Agriculture Research Institute (Wufeng, Taichung City, Taiwan). The institute is located at 120°43′ N, 24°02′ E. Harvested plant tissues were hand-separated into expanding leaves; first, second, third, and fourth fully expanded leaves; stems; and flowers. The leaf samples were further separated into leaf blades and petioles. All the samples were freeze dried, ground to powders, and then kept in a deep freezer at −20°C for antioxidants analyses. The first fully expanded leaves (including leaf blades and petioles) were also sampled and used for testing the effects of drying treatment.
Chemicals including 1,1-diphenyl-2-picrylhydrazyl (DPPH), 2,2′-azinobis-(3-ethylbenzothiazoline-6-sulfonate) radical anion (ABTS−), 2,2′-azobis-(2-methypropionamidine) dihydrochloride, neochlorogenic acid, caffeic acid, ChA, and 4-O-caffeoylquinic acid were purchased from Sigma Chemical Co. (St Louis, MO, USA). Compounds including as 4,5-di-O-caffeoylquinic acid (4,5-di-CQA), 3,5-di-O-caffeoylquinic acid (3,5-di-CQA), 3,4-di-O-caffeoylquinic acid (3,4-di-CQA), and 3,4,5-tri-O-caffeoylquinic acid (3,4,5-tri-CQA) were kindly provided by Dr Rie Kurata of National Agriculture and Food Organization (Koshi, Okinawa, Japan) (NARO) Kyushu Okinawa Agricultural Research Center. All the other chemicals were purchased from Merck (Darmstadt, Germany).
2.2. Drying treatments
For the drying test, 20 randomly selected first fully expanded leaves were subjected to oven drying at different temperatures (30°C, 70°C, and 100°C; air flow rate: 1.5 m/second) or to freeze-drying treatments until the samples contained approximately 10% moisture. Drying times for treatments at 30°C, 70°C, and 100°C were 32 hours, 8 hours, and 5 hours, respectively. Dried samples were stored in a deep freezer at −20°C for further analyses.
2.3. High-performance liquid chromatography analysis
For the preparation of polyphenol extracts, 1 g of ground materials was mixed with 20 mL of 80% methanol. The mixture was shaken for 24 hours at room temperature, filtered through a Whatman number 42 filter paper, freeze dried at −40°C until use. The polyphenol profile of tested extract was determined according to the method of Kurata et al [18]. The polyphenol extract was filtered through a cellulose acetate membrane (0.20 μm; Advantec, Tokyo, Japan), and 10 μL of the filtrate was injected into a high-performance liquid chromatography (HPLC) system (Waters 2996; Newark, Delaware, USA) using an ODS column (ODS-AM 301 column, 4.6 mm × 150 mm, 5 μm particles; YMC, Kyoto, Japan). The temperature was set at 40°C. The mobile phase consisted of water containing 0.2% (v/v) formic acid (A) and methanol (B). Elution was performed with the linear gradient as follows: 2% B from 0 minutes to 15 minutes, 2%–45% B from 15 minutes to 50 minutes, and 45% B from 50 minutes to 65 minutes. The flow rate was 1 mL/minute. Polyphenolic compounds were then compared with the obtained standards.
2.4. Determination of DPPH radical-scavenging activity
DPPH radical-scavenging activity was assayed according to the method of Kim et al [19]. One gram of freeze-dried polyphenolic extract was diluted to 1% in dimethyl sulfoxide. DPPH reagent contained 30 mg of DPPH dissolved in 200 mL of pure ethanol and 200 mL of distilled water. For the DPPH test, 2.5 mL of the DPPH reagent was incubated with 250 μL sample for 1 minute, and the mixture was monitored at 517 nm, and trolox was used for the construction of the standard curve. The DPPH radical-scavenging activity was expressed as trolox equivalent per unit dry weight (DW).
2.5. Determination of ferric reducing antioxidant power
The ferric reducing antioxidant power (FRAP) of tested samples was determined according to the method of Mau et al [20], with some modifications. The freeze-dried extract (0.5 mg) was mixed with 2.5 mL of 66.7 mM sodium phosphate buffer (pH 6.6) containing 0.33% K3Fe(CN)6, and the mixture was incubated at 50°C for 20 minutes. After the addition of 0.5 mL of 10% trichloroacetic acid (w/v), the mixture was centrifuged at 200 × g for 10 minutes. The upper layer (0.25 mL) was mixed with 4.15 mL of deionized water containing 0.1% ferric chloride, and the absorbance was monitored at 700 nm, and trolox was used for the construction of the standard curve. The reducing power was expressed as trolox equivalent per unit DW.
2.6. Determination of trolox equivalent antioxidant capacity
A solution of ABTS− was prepared in 0.1 M phosphate buffer saline (pH 7.4, 0.15 M sodium chloride) by mixing 2.5 mM 2,2′-azobis-(2-methypropionamidine) dihydrochloride with 2.0 mM ABTS2−. The solution was heated for 16 minutes at 60°C, protected from light, and stored at an ambient temperature until use. The seed extract was dissolved in phosphate buffer saline at a concentration of 0.17 mg/mL. For measuring the trolox equivalent antioxidant capacity (TEAC), 40 mg of the sample was mixed with 2 mL of ABTS– solution. Absorbance of the above solution was monitored at 734 nm, and trolox was used for the construction of the standard curve.
2.7. Statistical analysis
The results of antioxidant characteristics were the average of four replicates. Analyses of variance for the antioxidants data were performed with SPSS version 10.0 (SPSS Inc., Chicago, IL, USA). Differences among means were evaluated using the Duncan’s multiple-range test. Correlation analysis was also used to characterize the relationships between CQA derivatives and DPPH radical-scavenging activity, FRAP, and TEAC.
3. Results and discussion
3.1. CQA derivative contents of various parts of sweet potato plant tissues
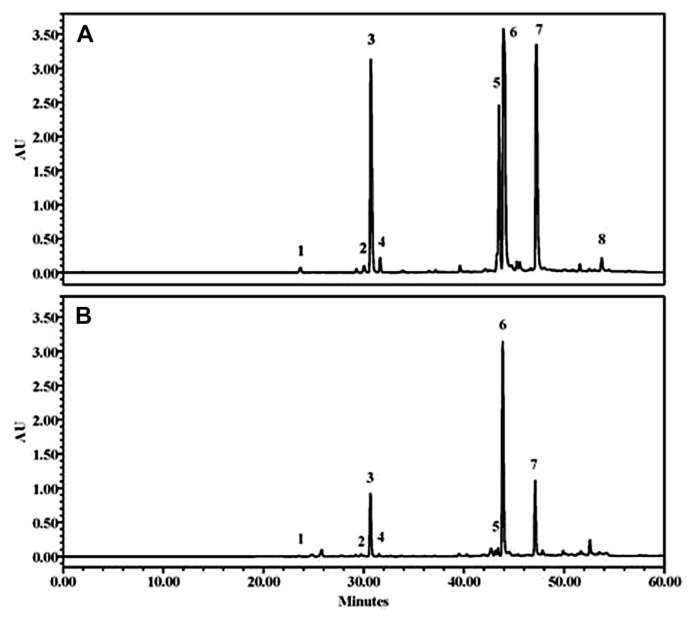
CQA derivatives are nonflavonoid compounds distributed in many fruits and vegetables [21]. Many reports have indicated that CQA derivatives have potent antioxidant activities and many beneficial effects on human health [4–6]. Cui et al [22] further demonstrated the therapeutic use of CQA derivatives in the management of diabetes-related complications. Leaves of sweet potato plants also contain a substantial level of CQA derivatives [3]. In this study, the HPLC chromatograms of phenolic compounds extracted from sweet potato plant tissues showed that eight major peaks were separated with elution times of 24–51 minutes (Fig. 1; only the results of first fully expanded leaves and flowers were presented). These compounds were identified as neochlorogenic acid (peak 1), caffeic acid (peak 2), ChA (peak 3), 4-O-caffeoylquinic acid (peak 4), 4,5-di-CQA (peak 5), 3,5-di-CQA (peak 6), 3,4-di-CQA (peak 7), and 3,4,5-tri-CQA (peak 8). Profiles of the obtained CQA derivatives differed slightly from those reported by Truong et al [7] and Yoshimoto et al [8]. Truong et al [7] found caffeic acid, ChA, 4,5-di-CQA, 3,5-di-CQA, and 3,4-di-CQA in the leaf extracts of three commercial sweet potato cultivars in the United States, whereas Yoshimoto et al [8] isolated ChA, 3,4-di-CQA, 3,5-di-CQA, 4,5-di-CQA, and 3,4,5-tri-CQA from the leaves of sweet potato cultivar K66Mu72-2 grown in Japan.
Fig. 1.
HPLC chromatograms of caffeoylquinic acid derivatives detected from (A) first fully expanded leaf and (B) flower of sweet potato plant tissues. Peak 1: neochlorogenic acid, peak 2: caffeic acid, peak 3: chlorogenic acid, peak 4: 4-O-caffeoylquinic acid, peak 5: 4,5-di-O-caffeoylquinic acid, peak 6: 3,5-di-O-caffeoylquinic acid, peak 7: 3,4-di-O-caffeoylquinic acid, and peak 8: 3,4,5-tri-O-caffeoylquinic acid. HPLC = high performance liquid chromatography.
The amounts of CQA derivatives accumulated in the aerial parts of sweet potato tissue are shown in Table 1. They varied from 39.34 mg/g DW to 154.05 mg/g DW. These values were significantly higher than the value (18.6 mg/g DW) reported by Yoshimoto et al [8]. All the examined sweet potato tissues accumulated substantial levels of total CQA derivatives, with the leaves containing more total CQA derivatives than stems and flowers, and the leaf blades containing more total CQA derivatives than their respective petioles. The total amount of CQA derivatives in the examined plant tissues decreased in the following order: expanding leaf > first fully expanded leaf > second fully expanded leaf > third fully expanded leaf > fourth fully expanded leaf > stem > flower. The total amounts of CQA derivatives obtained from expanding, first fully expanded, and second fully expanded leaves were significantly higher than the values reported by Truong et al [7].
Table 1.
Amounts (mg/g freeze-dried tissue dry weight) of CQA derivatives accumulated in different aerial parts of sweet potato plants.*
| 5-CQA | CA | ChA | 4-CQA | 4,5-di-CQA | 3,5-di-CQA | 3,4-di-CQA | 3,4,5-tri-CQA | Total CQA derivatives | |
|---|---|---|---|---|---|---|---|---|---|
| Leaf blade | |||||||||
| Expanding leaf | 0.91 ± 0.12a,b | 4.92 ± 0.53c | 22.95 ± 2.65a | 1.93 ± 0.22c | 9.60 ± 0.72d | 63.21 ± 7.54c | 49.13 ± 5.70c | 1.55 ± 0.12a | 154.05 ± 12.84c |
| First fully expanded leaf | 0.83 ± 0.02b | 0.32 ± 0.01a | 31.13 ± 0.50c | 1.61 ± 0.11a | 21.14 ± 1.50a,c | 47.13 ± 3.78a | 31.33 ± 0.82a | 2.12 ± 0.30c | 135.43 ± 1.99a |
| Second fully expanded leaf | 1.01 ± 0.11a | 0.13 ± 0.01b | 27.31 ± 0.62a | 2.07 ± 0.13c | 23.07 ± 2.61c | 31.23 ± 1.03b | 14.90 ± 0.87e | 1.56 ± 0.08a | 99.92 ± 2.96b |
| Third fully expanded leaf | 1.23 ± 0.02c | 0.22 ± 0.10a | 24.54 ± 1.90a | 2.16 ± 0.21c | 23.72 ± 1.33c | 25.25 ± 1.49e | 9.23 ± 0.96d | 1.12 ± 0.11b,e | 87.23 ± 5.40e |
| Fourth fully expanded leaf | 1.33 ± 0.12c | 0.14 ± 0.02b | 21.45 ± 1.61a | 2.22 ± 0.14c | 20.13 ± 0.79a | 19.44 ± 1.37f | 4.82 ± 0.55f | 0.53 ± 0.02d,f | 69.82 ± 4.17d |
| Leaf petiole | |||||||||
| Expanding leaf | 0.53 ± 0.02e | 0.31 ± 0.02a | 26.23 ± 0.12a | 1.23 ± 0.11b | 9.83 ± 0.16d | 52.73 ± 1.36a | 45.54 ± 4.11c | 1.53 ± 0.14a | 137.72 ± 5.55a |
| First fully expanded leaf | 0.44 ± 0.03d | 0.14 ± 0.01b | 21.47 ± 0.50a | 1.46 ± 0.13a,b | 15.41 ± 0.26b,e | 30.21 ± 0.46b | 34.17 ± 1.23a | 1.41 ± 0.03a,b | 104.41 ± 2.79b |
| Second fully expanded leaf | 0.40 ± 0.01d | 0.11 ± 0.01b | 17.81 ± 0.91a | 1.62 ± 0.67a | 18.70 ± 0.75a,b | 23.96 ± 0.91e | 25.24 ± 0.7b | 1.32 ± 0.03b | 88.90 ± 1.23e |
| Third fully expanded leaf | 0.43 ± 0.02d | 0.12 ± 0.01b | 14.25 ± 0.32e | 1.51 ± 0.58a | 17.53 ± 0.43b | 17.62 ± 0.65f | 15.7 ± 0.45e | 1.06 ± 0.09e | 68.02 ± 1.57d |
| Fourth fully expanded leaf | 0.41 ± 0.02d | 0.14 ± 0.01b | 10.75 ± 0.16d | 1.32 ± 0.20b | 13.50 ± 0.18e | 12.26 ± 0.86g | 7.63 ± 0.21d | 0.63 ± 0.04d | 46.45 ± 0.92g |
| Stem | 0.54 ± 0.02e | 0.10 ± 0.01b | 14.90 ± 0.51e | 0.91 ± 0.02e | 9.35 ± 0.38d | 21.07 ± 0.64d | 7.22 ± 0.61d | 0.42 ± 0.03f | 54.31 ± 1.36f |
| Flower | 0.16 ± 0.01f | 0.12 ± 0.01b | 7.43 ± 0.14f | 0.31 ± 0.01d | 0.91 ± 0.06f | 23.55 ± 0.59d,e | 7.07 ± 0.33d | 0 | 39.34 ± 2.09h |
Results are presented as means of four determinations ± standard deviation.
CA = caffeic acid; ChA = chlorogenic acid; CQA = caffeorylquinic acid; 4-CQA = 4-O-caffeoylquinic acid; 5-CQA = neochlorogenic acid; 3,4-di-CQA = 3,4-di-O-caffeoylquinic acid; 3,5-di-CQA = 3,5-di-O-caffeoylquinic acid; 4,5-di-CQA = 4,5-di-O-caffeoylquinic acid; 3,4,5-tri-CQA = 3,4,5-tri-O-caffeoylquinic acid.
Values with the same superscript letters within rows are not significantly different at p < 0.05.
As shown in Table 1, all of the eight CQA derivatives were detectable in the expanding and the fully expanded leaves and the stem, but no 3,4,5-tri-CQA was assessable in the flower tissue (Fig. 1). In general, 3,5 di-CQA, 3,4-di-CQA, ChA, and 4,5-di-CQA were the main compounds (representing about 95% of total CQA derivatives) in the aerial tissues of sweet potato plants (Table 1). The profiles of detected CQA derivatives also differed among the examined tissue samples, with the blades and petioles of expanding leaves accumulating approximately 41% of 3,5-di-CQA and 32% of 3,4-di-CQA, and the flowers containing 59% of 3,5-di-CQA and 18% of 3,4-di-CQA (Table 2). By contrast, stems accumulated 39% of 3,5-di-CQA, 27% of ChA, and 17% of 4,5-di-CQA. In all these cases, 3,5-di-CQA was the main compound (range, 12.26–63.21 mg/g DW) in the aerial parts of sweet potato plants. Islam et al [23] quantified ChA, 3,5-di-CQA, 4,5-di-CQA, 3,4-di-CQA, and 3,4,5-tri-CQA in the leaves of 1389 sweet potato genotypes, and also concluded that 3,5-di-CQA (range, 9.53–35.03 mg/g DW) was the main component of CQA derivatives.
Table 2.
Antioxidant activities (mg trolox equivalent/g freeze-dried tissue dry weight) of different aerial parts of sweet potato plants.*
| Antioxidant activity | |||
|---|---|---|---|
|
| |||
| DPPH | FRAP | TEAC | |
| Leaf blade | |||
| Expanding leaf | 237.90 ± 15.63a | 885.64 ± 88.08a | 108.94 ± 2.27a |
| First fully expanded leaf | 150.68 ± 3.37b | 445.69 ± 68.95b | 99.96 ± 2.85a |
| Second fully expanded leaf | 90.58 ± 5.88c | 237.31 ± 16.32c | 89.85 ± 9.04d |
| Third fully expanded leaf | 76.64 ± 3.28e | 203.25 ± 12.71c,e | 80.91 ± 1.19d |
| Fourth fully expanded leaf | 64.76 ± 3.78f | 155.82 ± 9.05f | 73.75 ± 0.68b |
| Leaf petiole | |||
| Expanding leaf | 176.02 ± 2.75d | 530.27 ± 304.87d | 103.28 ± 4.61a |
| First fully expanded leaf | 96.45 ± 2.57c | 227.21 ± 30.89c | 70.64 ± 12.99b |
| Second fully expanded leaf | 65.74 ± 2.05f | 224.90 ± 16.69c | 71.78 ± 5.14b |
| Third fully expanded leaf | 48.64 ± 0.79g | 187.02 ± 16.61e | 58.15 ± 4.64c |
| Fourth fully expanded leaf | 9.20 ± 1.25i | 117.51 ± 9.93g | 33.56 ± 0.55f |
| Stem | 37.53 ± 0.34h | 122.99 ± 16.11g | 35.81 ± 1.54f |
| Flower | 44.60 ± 0.59g | 148.88 ± 11.83f | 49.33 ± 7.51e |
Results are presented as means of four determinations ± standard deviation.
DPPH = 1,1-diphenyl-2-picrylhydrazyl; FRAP = ferric reducing antioxidant power; TEAC = trolox equivalent antioxidant capacity.
Values with same superscript letters within rows are not significantly different at p < 0.05.
3.2. Antioxidant activity in various parts of sweet potato tissues
Several methods have been developed to assay the antioxidant activity of plant extracts. The DPPH test is designed to measure the ability of the extract to donate hydrogen atoms or electrons to the stable radical DPPH formed in the solution [24]. As found by this method, the DPPH radical-scavenging activity values ranged from 9.20 (petioles of the 4th fully expanded leaves) to 237.90 mg trolox equivalent/g tissue DW (blades of expanding leaves; Table 2). Leaf blades generally had greater DPPH radical-scavenging activity than their respective petioles. The measured DPPH values were higher than the DPPH values of most of the selected herbs reported by Wojdylo et al [25].
The reducing power, which can be measured by the direct reduction of Fe[(CN)6]3 to Fe[(CN)6]2, reflects the electron-donating capacity of bioactive compounds and may serve as an indicator of their potential antioxidant activity. In this study, large variations were also observed in the FRAP values among the examined plant tissues, ranging from 122.99 mg trolox/g DW to 885.64 mg trolox/g DW (Table 2). The FRAP values obtained for the expanding and first fully expanded leaves were higher than those for the medicinal herbs reported by Wojdylo et al [25]. The TEAC assay, which is based on the scavenging of ABTS−, is also a common method for measuring the antioxidant activity of bioactive compounds [26]. Large variations were also observed in the TEAC values among the examined sweet potato tissues, ranging from 33.56 mg trolox/g DW to 108.94 mg trolox/g DW (Table 2). These TEAC values were higher than those of common vegetables and fruits, but were lower than some of the traditional Chinese medicinal plants reported by Cai et al [26].
The ranking in FRAP and TEAC among the tissues generally paralleled that of DPPH scavenging activities. The potent antioxidant activities of tested sweet potato tissues possibly result from their high levels of 3,5-di-CQA, 3,4-di-CQA, and ChA (Table 1). These CQA derivatives are reported to possess many phenolic hydroxyl groups having strong radical scavenging capacity [10]. Results of the correlation analyses between the tested antioxidant activities and the levels of 3,5-di-CQA, 3,4-di-CQA, and ChA, across all the plant tissues, support these findings (Table 3). Nakajima et al [27] also reported that 3,5-di-CQA, 3,4-di-CQA, and ChA in the water extract of propolis showed neuroprotective effects through inhibition of lipid peroxidation in mouse forebrain homogenates. However, negative and statistically insignificant correlations were found between 4,5-di-CQA, DPPH, and TEAC, and a positive but insignificant correlation (at p < 0.05) between 4,5-di-CQA and FRAP (Table 3). These results may be attributed to the extremely low levels of 4,5-di-CQA found in the blade and petiole portions of expanding leaves (about 6% and 7% of total CQA derivatives, respectively) compared to other fully expanded leaves (about 16–29%; Table 1), although expanding leaf tissues exhibited the highest antioxidant abilities (Table 2). Why expanding sweet potato leaves accumulate less 4,5-di-CQA than other CQA derivatives is currently unknown and should be ascertained through metabolomic examination in the future.
Table 3.
Correlation coefficients between antioxidant activities and four major caffeorylquinic acid derivatives of different aerial parts of sweet potato plants.
| Antioxidant activity | Chlorogenic acid | 4,5-Di-o-caffeoylquinic acid | 3,5-Di-o-caffeoylquinic acid | 3,4-Di-o-caffeoylquinic acid |
|---|---|---|---|---|
| DPPH | 0.6329* | −0.0633 | 0.9683* | 0.8865* |
| TEAC | 0.4794* | −0.1501 | 0.9035* | 0.8334* |
| FRAP | 0.8315* | 0.3064 | 0.8336* | 0.7288* |
DPPH = 1,1-diphenyl-2-picrylhydrazyl; FRAP = ferric reducing antioxidant power; TEAC = trolox equivalent antioxidant capacity.
Significant at p < 0.01.
3.3. Drying effects on CQA contents and antioxidant activity
The fresh sweet potato leaves contain many bioactive compounds, but their high moisture content renders them perishable and limits the availability of these compounds. Therefore, there is a need to preserve these phytochemicals through proper processing and safe storage of sweet potato leaves prior to using them for producing functional products. However, no study has previously been conducted on sweet potato leaves focusing on this topic. As shown in Tables 1 and 2, both expanding and first fully expanded leaves contained substantially higher levels of CQA derivatives than other aerial plant parts. Therefore, the first fully expanded leaves were further used to examine the effect of drying methods on the possible changes in CQA derivative contents. Table 4 shows the amounts of CQA derivatives for the first fully expanded leaves of sweet potato plants dried using the methods tested in this study. Among the different drying methods, freeze drying was found to retain the maximum total CQA derivatives (147.84 mg/g) in the test leaf tissues, followed by 30°C cool air drying (129.52 mg/g) and 70°C hot air drying (58.26 mg/g), whereas leaf tissues undergoing 100°C hot air drying retained the least amount of total CQA derivatives (20.53 mg/g). However, freeze-dried leaf tissues contained slightly less CQA derivatives than nondried fresh tissues (Table 1). This result was not unexpected because both leaf blades and petioles were harvested and used for drying treatments. It appears that the CQA derivatives are sensitive to heat treatment, and an increase in the drying temperature will result in a significant loss of CQA derivatives in sweet potato leaf tissues. Similar decreases in caffeic acid derivatives in 70°C hot-air-dried Echinacea purpurea were also reported by Lin et al [28].
Table 4.
Effects of drying methods on the amounts (mg/g freeze-dried tissue dry weight) of CQA derivatives in the first fully expanded leaves of sweet potato plants.*
| Treatment | 5-CQA | CA | ChA | 4-CQA | 4,5-di-CQA | 3,5-di-CQA | 3,4-di-CQA | 3,4,5-di-CQA | Total CQA derivatives |
|---|---|---|---|---|---|---|---|---|---|
| 100°C drying | 1.02 ± 0.04a | 1.44 ± 0.10b | 4.54 ± 0.11c | 0.75 ± 0.03c | 1.84 ± 0.16d | 8.13 ± 0.34d | 2.40 ± 0.11d | 0.60 ± 0.01c | 20.53 ± 0.71d |
| 70°C drying | 1.04 ± 0.04a | 2.64 ± 0.12a | 6.02 ± 0.25c | 1.34 ± 0.07a | 9.53 ± 0.45c | 26.74 ± 0.85c | 9.78 ± 0.4c | 1.18 ± 0.05b | 58.26 ± 2.15c |
| 30°C drying | 0.85 ± 0.04b | 1.16 ± 0.08c | 12.23 ± 0.69b | 1.26 ± 0.21a,b | 23.70 ± 0.24a | 65.19 ± 0.34b | 19.24 ± 0.42b | 5.44 ± 0.13a | 129.52 ± 5.08b |
| Freeze drying | 0.62 ± 0.02c | 1.03 ± 0.02c | 28.54 ± 1.04a | 1.14 ± 0.09b | 11.63 ± 0.45b | 72.31 ± 3.65a | 31.70 ± 0.84a | 1.22 ± 0.1b | 147.84 ± 6.67a |
Results are presented as means of four determinations ± standard deviation.
CA = caffeic acid; ChA = chlorogenic acid; CQA = caffeorylquinic acid; 4-CQA = 4-O-caffeoylquinic acid; 5-CQA = neochlorogenic acid; 3,4-di-CQA = 3,4-di-O-caffeoylquinic acid; 3,5-di-CQA = 3,5-di-O-caffeoylquinic acid; 4,5-di-CQA = 4,5-di-O-caffeoylquinic acid; 3,4,5-tri-CQA = 3,4,5-tri-O-caffeoylquinic acid.
Values with same superscript letters within rows are not significantly different at p < 0.05.
Comparisons were also made, based on the quotation provided by a food machinery company, between the construction costs of a commercial-scale (2 m × 2 m) freeze dryer and a cool air dryer with similar space (data not shown). The results indicated that the construction cost required for building a freeze dryer was approximately six-fold higher than that for a cool air dryer. Additionally, cost of the power needed for operating the constructed freeze dryer was 17-fold more than that needed for operating the cool air dryer. Based on these results, it can be suggested that 30°C cool air drying is an acceptable method for retaining the CQA derivatives (the derivative content being reduced by only 12% compared to freeze drying) present in sweet potato leaves, if the high cost of freeze drying is a major concern.
Similar to that of nondried leaf tissues (Table 1), HPLC analyses of dried leaf tissues revealed eight different CQA derivatives (data not presented). The obtained results reconfirmed that 3,5-di-CQA, 3,4-di-CQA, ChA, and 4,5-di-CQA represented the main compounds (Table 4). Quantitatively, the levels of detected CQA derivatives were significantly affected by the method of drying (p < 0.05). For instance, the four major CQA derivatives obtained from freeze-dried leaf tissues were in the following decreasing order: 3,5-di-CQA > 3,4-di-CQA > ChA >4,5-di-CQA; the major CQA derivatives obtained from 100°C hot-air-dried leaf tissues were in the following decreasing order: 3,5-di-CQA > ChA > neochlorogenic acid >3,4-di-CQA (Table 4).
As shown in Table 5, all the sweet potato leaf samples retained antioxidant activities (DPPH, FRAP, and TEAC) but to different extents, depending on the drying method applied. All the tested antioxidant activities (DPPH, FRAP, and TEAC) exhibited similar decreasing order: freeze drying > 30°C cool air drying > 70°C hot air drying > 100°C hot air drying. Leaf samples subjected to freeze drying had the highest antioxidant capacity, which was in agreement with their highest CQA derivative contents (Table 4).
Table 5.
Effects of drying methods on the antioxidant activities (mg trolox equivalent/g freeze-dried tissue dry weight) of the first fully expanded leaves of sweet potato plants.*
| Antioxidant activity | |||
|---|---|---|---|
|
| |||
| DPPH | FRAP | TEAC | |
| 100°C drying | 37.93 ± 1.19a | 103.64 ± 5.90b | 56.83 ± 0.89b |
| 70°C drying | 62.24 ± 0.78b | 190.63 ± 16.40c | 72.89 ± 4.05c |
| 30°C drying | 137.73 ± 0.74c | 398.75 ± 47.08d | 86.722 ± 2.98d |
| Freeze drying | 142.81 ± 1.70d | 422.95 ± 28.83d | 92.01 ± 6.55d |
Results are presented as means of four determinations ± standard deviation.
DPPH = 1,1-diphenyl-2-picrylhydrazyl; FRAP = ferric reducing antioxidant power; TEAC = trolox equivalent antioxidant capacity.
Values with same superscript letters within rows are not significantly different at p < 0.05.
4. Conclusion
The present study demonstrated that the expanding and first fully expanded leaves of sweet potato plants contained higher amounts of CQA derivatives than other plant parts. These leaf tissues also showed higher antioxidant activity than other aerial tissues, possibly due to their high levels of 3,5-di-CQA, 3,4-di-CQA, and ChA. Therefore, these two types of leaves are economically suitable for producing high-quality CQA derivatives nutraceuticals. Our study also reconfirmed that the drying method affected the contents of CQA derivatives in dried sweet potato leaf tissues, with freeze drying retaining the highest amount of total CQA derivatives (147.84 mg/g) in the first fully expanded leaves, followed by 30°C cool air drying (129.52 mg/g). The lowest amount of total CQA derivatives (20.53 mg/g) were obtained from the leaves subjected to 100°C hot air drying. Leaf tissues treated by both freeze drying and 30°C cool air drying also maintained relatively higher antioxidant activity than those treated with hot air drying. However, the major disadvantage of freeze drying is its high operational cost. Therefore, large-scale drying may be carried out using 30°C cool air drying even though it tended to slightly reduce the total CQA derivatives (reduced by 12% compared to freeze drying) retained in treated leaf tissues.
Acknowledgments
The authors thank Dr Rie Kurata of NARO Kyushu Okinawa Agricultural Research Center for kindly providing the CQA derivative standards.
Footnotes
Conflicts of interest
There is no conflict of interest.
REFERENCES
- 1. Mosha TC, Gaga HE. Nutritive value and effect of blanching on the trypsin and chymotrypsin inhibitor activities of selected leafy vegetables. Plant Food Hum Nutr. 1999;54:271–83. doi: 10.1023/a:1008157508445. [DOI] [PubMed] [Google Scholar]
- 2. Nagai M, Tani M, Kishimoto Y, Lizuka M, Saita E, Toyozaki M, Kamiya T, Ikeguchi M, Kondo K. Sweet potato (Ipomoea batatas L.) leaves suppressed oxidation of low density lipoprotein (LDL) in vitro and in human subjects. J Clin Biochem Nutr. 2011;48:203–8. doi: 10.3164/jcbn.10-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zheng W, Clifford MN. Profiling the chlorogenic acids of sweet potato (Ipomoea batatas) from China. Food Chem. 2008;106:147–52. [Google Scholar]
- 4. Han J, Lv Q-Y, Jin S-Y, Zhang T-T, Jin S-X, Li X-Y, Yuan H-L. Comparison of anti-bacterial activity of three types of di-O-caffeoylquinic acids in Lonicera japonica flowers based on microcalorimetry. Chin J Nat Med. 2004;12:108–13. doi: 10.1016/S1875-5364(14)60017-0. [DOI] [PubMed] [Google Scholar]
- 5. Van der Werf R, Marcic C, Khalil A, Sigrist S. ABTS radical scavenging capacity in green and roasted coffee extracts. LWT Food Sci Technol. 2014;58:77–85. [Google Scholar]
- 6. Scalbert A, Manach C, Morand C, Rémésy C, Jiménez L, et al. Dietary polyphenols and the prevention of diseases. Crit Rev Food Sci Nutr. 2005;45:287–306. doi: 10.1080/1040869059096. [DOI] [PubMed] [Google Scholar]
- 7. Truong V-D, McFeeters RF, Thompson RT, Dean LL, Shofran B. Phenolic acid content and composition in leaves and roots of common commercial sweet potato (Ipomea batatas L.) cultivars in the United States. J Food Sci. 2007;72:C343–9. doi: 10.1111/j.1750-3841.2007.00415.x. [DOI] [PubMed] [Google Scholar]
- 8. Yoshimoto M, Yahara S, Okuno S, Islam S, Ishiguro K, Yamakawa O. Antimutagenicity of mono-, di-, and tricaffeoylquinic acid derivatives isolated from sweetpotato (Ipomoea batatas L.) leaf. Biosci Biotech Biochem. 2002;66:2336–41. doi: 10.1271/bbb.66.2336. [DOI] [PubMed] [Google Scholar]
- 9. Clifford MN. Chlorogenic acids and other cinnamates—nature, occurrence, dietary burden, absorption and metabolism. J Sci Food Agric. 2000;80:1033–43. [Google Scholar]
- 10. Xiang Z, Ning Z. Scavenging and antioxidant properties of compound derived from chlorogenic acid in South-China honeysuckle. LWT Food Sci Technol. 2008;41:1189–203. [Google Scholar]
- 11. Lewicki PP. Design of hot air drying for better foods. Trends Food Sci Technol. 2006;17:153–63. [Google Scholar]
- 12. Vashisth T, Singh RK, Pegg RB. Effects of drying on the phenolics content and antioxidant activity of muscadine pomace. LWT Food Sci Technol. 2011;44:1649–57. [Google Scholar]
- 13. Capecka E, Mareczeek A, Leja M. Antioxidant activity of fresh and dry herbs of some Lamiaciae species. Food Chem. 2005;93:223–6. [Google Scholar]
- 14. Leonid AB, Vladimir PG, Andrew VB, Alexander ML, Valeriy L, Vladimir AK. The investigation of low temperature vacuum drying processes of agricultural materials. J Food Eng. 2006;74:410–5. [Google Scholar]
- 15. Nagaya K, Li Y, Jin Z, Fukumuro M, Ando Y, Akaishi A. Low-temperature desiccant-based food drying system with airflow and temperature control. J Food Eng. 2006;75:71–7. [Google Scholar]
- 16. Rawson A, Tiwari BK, Tuohy MG, O’Donnell CP, Brunton N. Effect of ultrasound and blanching pretreatments on polyacetylene and carotenoid content of hot air and freeze dried carrot discs. Ultrason Sonochem. 2011;18:1172–9. doi: 10.1016/j.ultsonch.2011.03.009. [DOI] [PubMed] [Google Scholar]
- 17. Chan EWC, Lim YY, Wong SK, Lim KK, Tan SP, Lianto FS, Yong MY. Effects of different drying methods on the antioxidant properties of leaves and tea of ginger species. Food Chem. 2009;113:166–72. [Google Scholar]
- 18. Kurata R, Yahara S, Yamakawa O, Yoshimoto M. Simple high-yield purification of 3,4,5-tri-O-caffeoylquinin acid from sweetpotato (Ipomoea batatas L.) leaf and its inhibitory effects on aldose reductase. Food Sci Technol Res. 2011;17:87–92. [Google Scholar]
- 19. Kim JA, Jung WS, Chun SC, Yu CY, Ma KH, Gwag JG, Chung IM, et al. A correlation between the level of phenolic compounds and the antioxidant capacity in cooked-with-rice and vegetable soybean (Glycine max L.) varieties. Eur Food Res Technol. 2006;224:259–70. [Google Scholar]
- 20. Mau J-L, Chang C-N, Huang S-J, Chen C-C. Antioxidant properties of methanolic extracts from Grif frondosa. Morchella esculenta and Termitomyces albuminosus mycelia. Food Chem. 2004;87:111–8. [Google Scholar]
- 21. Jurgoński A, Juśkiewicz J, Zduńczyk Z, Król B. Caffeoylquinic acid-rich extract from chicory seeds improves glycemia, atherogenic index, and antioxidant status in rats. Nutrition. 2012;28:300–6. doi: 10.1016/j.nut.2011.06.010. [DOI] [PubMed] [Google Scholar]
- 22. Cui C-B, Jeong SK, Lee YS, Lee SO, Kang HI, Lim SS. Inhibitory activity of caffeoylquinic acids from the aerial parts of Artemisia princeps on rat lens aldose reductase and on the formation of advanced glycation end products. J Kor Soc Appl Biol Chem. 2009;52:655–62. [Google Scholar]
- 23. Islam MS, Yoshimoto M, Yahara S, Okuno S, Ishiguro K, Yamakawa O. Identification and characterization of foliar polyphenolic composition in sweetpotato (Ipomoea batatas L.) genotypes. J Agric Food Chem. 2002;50:3718–22. doi: 10.1021/jf020120l. [DOI] [PubMed] [Google Scholar]
- 24. Tepe B, Daferera D, Sokmen A, Sokmen M, Polissiou M. Antimicrobial and antioxidant activities of the essential oil and various extracts of Salvia tomentosa Miller (Lamiaceae) Food Chem. 2005;90:333–40. [Google Scholar]
- 25. Wojdylo A, Oszmiański J, Czemerys R. Antioxidant activity and phenolic compounds in 32 selected herbs. Food Chem. 2007;105:940–9. [Google Scholar]
- 26. Cai Y, Luo Q, Sun M, Corke H. Antioxidant activity and phenolic compounds of 112 traditional Chinese medicinal plants associated with anticancer. Life Sci. 2004;74:2157–84. doi: 10.1016/j.lfs.2003.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Nakajima Y, Shimazawa M, Mishima S, Hara H. Water extract of propolis and its main constituents, caffeoylquinic acid derivatives, exert neuroprotective effects via antioxidant actions. Life Sci. 2007;80:370–7. doi: 10.1016/j.lfs.2006.09.017. [DOI] [PubMed] [Google Scholar]
- 28. Lin S-D, Sung J-M, Chen C-L. Effect of drying and storage conditions on caffeic acid derivatives and total phenolics of Echinacea purpurea grown in Taiwan. Food Chem. 2011;125:226–31. [Google Scholar]