Abstract
Chitosan is a promising biopolymer for drug delivery systems. Because of its beneficial properties, chitosan is widely used in biomedical and pharmaceutical fields. In this review, we summarize the physicochemical and drug delivery properties of chitosan, selected studies on utilization of chitosan and chitosan-based nanoparticle composites in various drug delivery systems, and selected studies on the application of chitosan films in both drug delivery and wound healing. Chitosan is considered the most important polysaccharide for various drug delivery purposes because of its cationic character and primary amino groups, which are responsible for its many properties such as mucoadhesion, controlled drug release, transfection, in situ gelation, and efflux pump inhibitory properties and permeation enhancement. This review can enhance our understanding of drug delivery systems particularly in cases where chitosan drug-loaded nanoparticles are applied.
Keywords: chitosan, drug delivery system, nanoparticle composite, wound healing
1. Introduction
Chitosan is a natural polysaccharide and is considered the largest biomaterial after cellulose in terms of utilization and distribution [1]. It is produced from chitin—the structural element found in the exoskeleton of crustaceans such as shrimps, lobsters, and crabs. The shells of these crustaceans are first removed and then ground into powder, which is further processed to produce chitosan. Chitosan also occurs naturally in some microorganisms such as fungi and yeast [2]. Although chitosan is structurally similar to cellulose, it contains, in addition to hydroxyl groups, acetylamine or free amino groups, which display very different properties from those of cellulose [3]. Chitosan has attracted attention because of its biological properties and effective uses in the medical field, food industries, and agricultural sector [4]. It shows a variety of biological activities such as phytoalexin elicitor activity, activation of immune response, cholesterol lowering activity, and antihypertension activity [5,6]. Similarly, mesoporous silica nanoparticles (NPs) have the ability to efficiently entrap cargo molecules because of their unique characteristic of having a huge pore size. They have already been recognized as a promising drug carrier and have recently become a new area of interest in the field of biomedical applications [7]. For instance, Zhu et al [7] focused on the stimuli-responsive controlled-release systems that responded to tumor cell environmental changes, such as pH, glucose, adenosine-50-triphosphate, glutathione, and H2O2.
Chitosan’s therapeutic properties have also been reported by other researchers, such as inhibition of growth of micro-organisms and pain alleviation [8,9] and promotion of hemostasis and epidermal cell growth [10]. However, some researchers are interested in the potential applications of chitosan for medical and pharmaceutical purposes. The increased interest in chitosan, particularly its use in the pharmaceutical field, is attributed to its favorable properties such as biocompatibility, ability to bind some organic compounds, susceptibility to enzymatic hydrolysis, and intrinsic physiological activity combined with nontoxicity and heavy metal ions [11–13]. These properties are particularly amenable to a wide variety of biomedical applications in drug delivery and targeting, wound healing, and tissue engineering, as well as in the area of nanobiotechnology. Chitosan has attracted attention as a material for drug delivery biomedical applications in the past few years because of its biological and physicochemical properties, leading to the recognition of chitosan as a drug delivery element and a promising material specifically for the delivery of macromolecules [14–16]. In this regard, chitosan-based delivery systems range from micro-particles to NP composites and films. However, there are several drawbacks in the use of chitosan for drug delivery systems. The main drawback is its poor solubility at physiological pH owing to the partial protonation of the amino groups, thereby causing presystemic metabolism of drugs in intestinal and gastric fluids in the presence of proteolytic enzymes. To overcome these inherent drawbacks, various derivatives of chitosan such as carboxylated, different conjugates, thiolated, and acylated chitosan have been used in drug delivery systems [17,18]. Researchers reported on the goals of using chitosan as an excipient for drug delivery systems [19–23]. Therefore, the main objective of this review is to highlight and investigate the application of chitosan and chitosan-based NP composites in drug delivery systems and to provide some insight for its future potential.
2. Preparation and physicochemical properties of chitosan
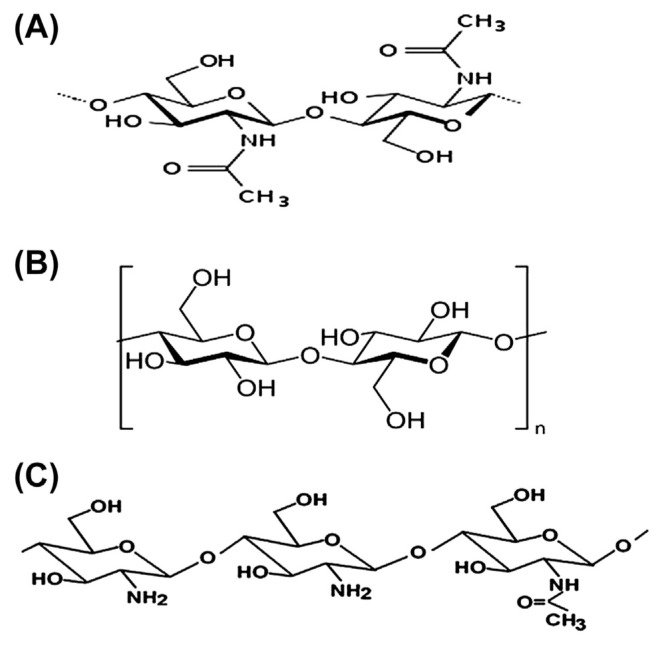
Fig. 1 shows the structures of chitin, cellulose, and chitosan. Chitosan is recognized as a linear binary heteropolysaccharide composed of β-1,4-linked glucosamine with various degrees of N-acetylation of glucosamine residues [24,25]. It is prepared from chitin by alkaline N-deacetylation [24,26] using concentrated sodium hydroxide (NaOH) solutions at high temperatures for a long period. Another method for the production of chitosan is N-deacetylation using enzymes under relatively mild conditions [27]. The commercially available chitosan is mostly derived from chitin of crustaceans by alkaline N-deacetylation because it is easily obtainable [28]. The production of chitosan involves a two-step process. The first step is extraction of chitin [a linear chain consisting of N-acetyl-d-glucosamine (2-acetamido-2-deoxy-β-d-gluconopyr-anose) joined together by β (1→ 4) linkage] and removal of calcium carbonate (CaCO3) from crustaceans’ shells using dilute hydrochloric acid and deproteination with dilute aqueous NaOH. In the second step, 40–50% aqueous NaOH at 110–115°C is used for deacetylation of chitin for several hours without oxygen. When the degree of deacetylation exceeds 50%, then chitosan is produced [29]. Chitin with a degree of deacetylation of ≥ 75% is also recognized as chitosan [28].
Fig. 1.
Structures of (A) chitin, (B) cellulose, and (C) chitosan.
The degree of deacetylation and molecular weight are the two fundamental parameters that can affect the properties and functionality of chitosan [26,30]. These properties include solubility, viscosity, reactivity of proteinaceous material coagulation, and heavy metal ion chelation [31–33], and physical properties of films formulated using chitosan such as tensile strength, elasticity, elongation, and moisture absorption [34]. Chitosan is soluble in aqueous acidic solutions, but insoluble in both water and alkaline solutions [25]. The majority of polysaccharides are usually found neutral or negatively charged in an acidic environment. When dissolved, the amino groups (−NH2) of the glucosamine are protonated to [35], and the cationic polyelectrolyte readily forms electrostatic interactions with other anionic groups [36]. Therefore, the cationic chitosan molecule interacts with negatively charged surfaces that modify its physicochemical characteristics [2,37]. These modifications of chitosan molecules are the source of its unique functional properties.
3. Drug delivery properties of chitosan
3.1. Anionic drug delivery properties
When a technique of drug discharge cannot be achieved by using a simple drug dissolution process such as diffusion, membrane layer handle along with erosion as well as osmotic systems, retardation mediated by ionic relationships is often used. The latter technique can be carried out with regard to cationic drugs by using anionic polymeric excipients such as polyacrylates, alginate, or carboxymethylcellulose salt. However, in anionic drug delivery systems, chitosan is the sole selection. Chitosan was used as a medication provider matrix to investigate medication release devices for the anionic medication naproxen [38]. It was found that the interactions between chitosan and the therapeutic agent was more evident, and stable complexes can also be formed from which this medicine can be produced, actually spanning a more extended period counted on an ionic cross-linking. For example, the delivery systems of enoxaparin/chitosan nano-particulate provided more stable complexes and resulted in significantly improved drug uptake [39]. Some anionic polymeric excipients such as carrageenan, pectin, alginate, and polyacrylates can be homogenized with chitosan, leading to high-density, relatively stable complexes. However, a similar result can be achieved by homogenizing chitosan with an alternative to multivalent anionic and inorganic polymer anions such as sulfate or tripolyphosphate (TPP) [40].
3.2. Mucoadhesive properties
The mucoadhesive properties of chitosan are probably attributable to its cationic character. Furthermore, hydrophobic interactions may help with the mucoadhesive components. The mucoadhesive properties of chitosan are weak as compared with various anionic polymeric excipients such as hyaluronic acid, polycarbophil, and carbomer [41]. In order to attain substantial mucoadhesive attributes, a polymer should have high cohesive properties because adhesive bond normally fails within the mucoadhesive polymer as opposed to involving the polymer along with the mucus gel layer. Regarding chitosans, these cohesive properties tend to be comparatively weak. It may be improved by the formation of complexes with multivalent anionic drug treatments, multivalent anionic polymeric excipients, and also multivalent inorganic anions. This strategy is effective to a very limited extent, as the cationic substructures of chitosan being accountable for mucoadhesion via ionic interactions while using the mucus are blocked in such cases. Lueβen et al [42] demonstrated a significantly improved oral bioavailability involving buserelin when being administered in rats with mucoadhesive polymers, for instance, chitosan and carbomer. However, this particular effect could not be attained anymore when chitosan was mixed with polyanionic carbomer in the same formulation. More cationic character of the polymer is provided by the trimethylation of the primary amino group of chitosan. It was found that when trimethylated chitosan is added to PEGylated, its mucoadhesive properties were improved up to 3.4-fold [43]. The mucoadhesive properties of chitosan can be substantially improved as a result of the immobilization of thiol groups on it. It was reported that chitosan is able to form disulfide bonds with mucus glycoproteins when found with the mucus gel layer, and this phenomenon makes it the most mucoadhesive polymer [44].
3.3. Gelling properties
As hydrogels form, one advantage of in situ gelling properties can be achieved when the pH-dependent hydrostability of chitosan is properly addressed. Gupta and Vyas [45] improved an in situ gelling delivery system by using a mixture of polyacrylic acid and chitosan. They observed that the resulting formulation was in a liquid state at pH 6.0 even though the same formulation underwent a rapid transition to viscous gel phase at pH 7.4. Further improvements through thiolation may also enhance the in situ gelling characteristics of chitosan. As a result of the access of oxygen on mucosal surfaces, for instance, nasal mucosa or ocular surfaces, immediately after the mixture is applied in liquid form using oxygen-free single unit forms, a cross-linking process via disulfide bond formation takes place, causing a significant increase in viscosity.
Based on the cross-linking properties, the viscosity increased 16,500-fold in a period of 20 minutes using aqueous 1% (m/v) of chitosan–thioglycolic acid conjugate [46].
3.4. Gene expression properties
Chitosan was also modified to improve its properties for gene expression purposes. For instance, the self-branching of chitosans was used as a strategy to improve its gene transfer properties, and this can be carried out without compromising the safety profile [47]. In this respect, self-branched trisac-charide-substituted chitosans, in addition to a self-branched molecular mass of 11–71 kDa, were synthesized, characterized, and also compared in contrast to their own linear counterparts with respect to transfection efficiency.
The results revealed that self-branched chitosans could yield gene expression levels two as well as five times greater than that of Lipofectamine and Exgen, respectively. In another investigation, thiolated chitosan forming intrachain bonds of disulfide was used as a good strategy to stabilize the chitosan/plasmid NP complex, resulting in higher stability properties toward nucleases [48]. In addition, owing to the reducing conditions of the cytoplasma, the plasmid was mainly released in the target cells because the disulfide bonds werelargely cleaved there, resulting in the release of the plasmid at the target site. The transfection rate of the thiolated chitosan/plasmid NP complex was found to be five times higher compared with that of the unmodified chitosan/pDNA NP complex. Owing to the trimethylation of the remaining primary amino groups, this strategy was further improved by raising the cationic character of thiolated chitosan [49]. Furthermore, chitosan/cyclodextrin and PEGylated chitosan NPs were identified as promising tools for DNA-based drug delivery [50,51].
In contrast to small molecules, where a controlled release of anionic drugs can be achieved, stable complexes with chitosan can be formed using comparatively large polyanionic molecules such as small interfering RNA and DNA-based drugs. If the ratio of the cationic polymer is sufficiently high in the complex, NPs exhibiting a positive zeta potential can be formed. Because of the small size of these particles and the net positive charge, endocytosis was achieved particularly when the sizes of the particles were smaller than 100 nm [52]. From a toxicology viewpoint, chitosan is comparatively recognized as a less toxic polymer than other cationic polymers such as polyarginine, polylysine, and polyethyleneimine [53]. This property makes chitosan a promising excipient for nonviral gene delivery systems. It was reported that the bioavailability of DNA-based drugs delivered into the body can be improved if chitosan–DNA-based drug complexes are protected to some extent toward degradation by DNAses [54].
3.5. Permeation enhancing properties
Based on the positive charges of chitosan, it was found that these charges are responsible for the mechanism of permeation enhancement, which can interact with the cell membrane of chitosan, resulting in a structural reorganization of tight junction-associated proteins [55]. A primary amino group that led to a more pronounced cationic character using the trimethylation strategy did not lead to further improvements of permeation enhancing properties. It was demonstrated that the permeation enhancing properties and toxicity to a large extent were attributable to the structural properties of chitosan including the degree of deacetylation and molecular mass [56]. Chitosans with high molecular mass and high degree of deacetylation exhibited a comparatively higher increase in epithelial permeability, which could be due to molecular mass and other permeation enhancing polymers such as polyacrylates [57]. Various in vivo studies can be used to confirm this permeation enhancing effect. A 2-fold improvement of the oral bioavailability of ganciclovir was demonstrated owing to the coadministration of chitosan [58]. Chitosan can be combined with other permeation enhancers because it acts in a completely different manner from these enhancers, leading to an additive or even a synergistic effect. Using this strategy, the oral bioavailability of ganciclovir could even be improved by 4-fold, using a combination of sodium dodecyl sulfate and chitosan compared with just a 2-fold improvement with sodium dodecyl sulfate alone. Recently, it was reported that chitosan NPs exhibit only in the first segment of the duodenum a permeation enhancing effect for small peptides. The permeation enhancing effect was enlarged over the entire duodenum owing to the addition of cyclodextrin [59]. However, >30-fold further improvement in the permeation enhancing properties of chitosan on certain mucosal membranes can be achieved because of thiolation [60].
4. Selected studies on utilization of chitosan composites for drug delivery systems
Many studies have been conducted recently using chitosan as a drug delivery biomaterial to treat diseases such as cancer [61], optical diseases [62], and colon diseases [63]. Table 1 shows a selection of studies on the use of chitosan composites for drug delivery applications. A systematic series of N-trimethyl chitosan chloride polymer synthesized from different chitosans based on molecular weight (low, medium, and high molecular weight) have been coformulated into a hydrogel with polyethylene glycol (PEG) and glycerophosphate and investigated for nasal drug delivery [64]. The authors found that hydrogels derived from N-trimethyl chitosan with high or medium average molecular weight exhibit relatively short sol–gel transition times at physiologically relevant temperatures. The same hydrogels display good water-holding capacity and strong mucoadhesive potential. They revealed that an aqueous hydrogel formulation, which was derived from N-trimethyl chitosan of medium average molecular weight, appears particularly promising because it exhibited the most favorable rheological and mucoadhesive behaviors and a sol–gel transition that occurs at 32.5°C within 7 minutes.
Table 1.
Selected studies on utilization of chitosan composites for drug delivery applications.
| Name of chitosan composites | Purpose of utilization | Findings |
|---|---|---|
| Chitosan-based responsive hybrid nanogels | Integration of optical pH-sensing | Chitosan-based responsive hybrid nanogels exhibit a nonreversible pH-sensitive property and a significant cytotoxicity after 24 h treatment. It is critical to construct highly stable biopolymer-hybrid nanogel quantum dots [43]. |
| Chitosan–zinc–pectin composite | Delivery of resveratrol to the colon | Formulation prepared at pH 1.5, 1% chitosan, 120 min cross-linking time, and pectin/drug ratio of 3:1 demonstrated the best colon-specific drug release [79]. |
| Chitosan oligomer–zidovudine composite | Prevents disappearance of Zidovudine in human plasma and prolong its shelf life | The study indicated longer mean retention time for chitosan oligomer–zidovudine composite with values of about 1.5 h vs. 0.59 h for zidovudine alone. Chitosan oligomer–zidovudine composite was had a shelf life of 12 h [66]. |
| Chitosan–sodium alginate tablet | Vaginal delivery of chlorhexidine digluconate | Tablet containing 6% chitosan, 24% sodium alginate gave the best result of drug release [70]. |
| Chitosan–cyclosporin A | Management of extraocular diseases | Enhancement of therapeutic index of clinically challenging drugs with potential application at extraocular level and achievement of fast drug release and therapeutic concentrations in external ocular tissues during a period of 24 h [122]. |
| Chitosan–polyelectrolyte films | Delivery of drug for skin | The films gave significantly different drug release and drug permeation through the skin [123]. |
Chitosan was also investigated as an injectable vehicle for drug delivery in the presence of sodium bicarbonate (NaHCO3) [65]. The hydrogels of chitosan/NaHCO3 system showed porous morphologies with some diversification depending on the NaHCO3 concentration, which affected their erosion and drug release rate behaviors. An in vivo gelation test was performed via a dorsal subcutaneous injection of chitosan/NaHCO3 solution in adult Sprague–Dawley rats. Exactly 2% (w/v) of chitosan solution without NaHCO3 was also administered as a control. Sterile solutions were prepared via UV sterilization of solid chitosan powder, 0.22 μm filtration of 1% acetate acid solution and NaHCO3 solutions, and sterilized chitosan solution and chitosan/NaHCO3 mixtures. An aqueous urethane solution was injected intraperitoneally to anesthetize the rats. Each injection was 0.4 mL in volume and performed subcutaneously through a syringe equipped with a G2 gauge needle. The formation of in situ gels suggested that such systems have promising applications in injectable drug delivery. The drug delivery system prepared from chitosan oligomer–zidovudine composites for the in vitro release of zidovudine was investigated [66]. A conjugate study was confirmed in mice plasma and renal homogenate. The pharmacokinetics study indicated a longer mean retention time for the chitosan oligomer–zidovudine conjugate with values of about 1.5 hours compared with 0.59 hour for zidovudine alone. The chitosan oligomer–zidovudine conjugates were found to accumulate (aside from the heart and the liver) in the lung, spleen, brain, and kidney after their in vivo administration. The study concluded that chitosan oligomer–zidovudine conjugates have the potential to be developed into a renal-targeting drug delivery system.
5. Selected studies on chitosan-based NPs for drug delivery systems
Nowadays, it is considered that nanomedicine will lead breakthroughs for the detection, diagnosis, and treatment of cancer [67]. Chitosan NPs are a drug carrier with the advantage of slow or controlled drug release, which improves drug solubility and stability, enhances efficacy, and reduces toxicity. In vitro and in vivo studies have also shown that chitosan has antitumor effects, leading to good prospects for their application as a supplementary antitumor drug and drug carrier [68]. Chitosan-based nanostructures predominantly work on the involved chemical cross-linking within the polymer chain. Earlier chitosan/silica nanocomposites were formed using the reaction of hydroxyl groups on chitosan monomers with tet-ramethoxysilane. The first data presented involved chitosan nanospheres for drug delivery applications [69]. The authors used the water-in-oil (w/o) emulsion method, which was followed by glutaraldehyde cross-linking of the chitosan amino groups. They produced nanospheres loaded by 5-fluorouracil, an anticancer drug. These studies further revealed the feasibility of reproducible synthesizing stable nanosized chitosan particles, which can entrap and deliver drugs [70]. One of chitosan’s properties is its ability to form gel upon contact with special polyanions, a process referred to as “ionotropic gelation,” which occurs as a result of the formation of intra and inter cross-linkages within/between polymer chains mediated by the polyanions.
Based on ionotropic gelation of TPP with chitosan, chitosan NPs have been developed for drug encapsulation [71,72]. This simple technique involves mixing of the acidic phase (pH 4–6) containing chitosan with an alkaline phase (pH 7–9) containing TPP. NPs were immediately formed based on the mixing of these two phases through intra- and intermolecular linkages created between chitosan amino groups and TPP phosphates. Insulin-loaded chitosan NPs have also been successfully prepared using a TPP solution mixed with insulin and then adding the mixture to chitosan solution under constant stirring [73]. In brief, various concentrations of chitosan and TPP were dissolved in acetic acid (pH 4) and purified water, respectively. Different volumes of the TPP solution was mixed with 4 mL of the chitosan solution through a syringe needle under magnetic stirring at room temperature, and chitosan NPs were present in the suspension. Insulin-loaded chitosan NPs were formed spontaneously upon the incorporation of the TPP aqueous solution containing insulin to the chitosan acetic acid solution. The size of chitosan NPs were 300–400 nm with a surface positive charge ranging from +54 to +25 mV. In this study, the ability of chitosan NPs to enhance both relative bioavailability and intestinal absorption of insulin was investigated by monitoring the glucose level of plasma in alloxan-induced diabetic Wistar male rats. Various doses of insulin-loaded chitosan NPs were orally administrated. The stable positively charged chitosan NPs showed particle sizes within the range of 250–400 nm, and an insulin association ratio of up to 80% was used. The in vitro release investigations indicated an initial burst phase that was pH-sensitive. The intestinal absorption of insulin was enhanced by chitosan NPs to a greater extent than the aqueous solution of chitosan in vivo. It was noticed that hypoglycemia was prolonged over 15 hours after the administration of 21.1 IU/kg insulin loaded in the chitosan NPs. However, the average bioavailability relative to the subcutaneous injection of free insulin solution showed up to 14.9%. In another study, different formulations of chitosan NPs produced by the ionic gelation of TPP and chitosan were investigated [74]. Drug delivery systems prepared using low molecular weight (LMW) chitosan NPs and monodisperse using the ionic gelation technique were also investigated [75]. The results showed that LMW chitosan NPs has good compatibility with erythrocytes, and they can be easily attached to the erythrocyte membrane surface. This indicates that the erythrocyte load of LMW chitosan can be used as a potential vascular drug delivery system.
The complex coacervation technique was previously used to prepare chitosan–DNA NPs [76,77]. The phosphate and amino groups were used in a ratio between 8 and 3, respectively, in the presence of chitosan. This particle size was optimized to 100–250 nm range using a narrow distribution. It is possible that chitosan–DNA NPs could partially protect the encapsulated plasmid DNA via the degradation of nuclease. Coalescence and emulsion–droplet coalescence methods were reported by Tokumitsu et al [78]. They used the principles of both emulsion cross-linking and precipitation. With this method, instead of cross-linking in the stable droplets, precipitation is elicited by allowing the coalescence of small chitosan droplets with NaOH droplets. A stable emulsion containing an aqueous solution of chitosan combined with the drug to be loaded is stated in liquid paraffin. At that time, another stable emulsion containing aqueous chitosan mixed with NaOH is produced in a similar manner. When both emulsions are combined under high-speed stirring, droplets of each and every emulsion would randomly collide [79].
The preparation of ultrafine polymeric NPs with a narrow size distribution may be achieved using a reverse micellar medium. Such particles can be prepared using the aqueous core of the reverse micellar droplets as a nanoreactor. The size of these very narrow and monodispersed reverse micellar tiny droplets normally lies between 1 nm and 10 nm [80], which turns them into potential and promising NPs in drug delivery investigations. A method to encapsulate doxorubicin–dextran conjugates in chitosan NPs was used by Mitra et al [81]. In this method, an organic solvent was applied to dissolve the surfactant for preparing reverse micelles. Several studies have been done on the self-assembly of chemically modified chitosan into NPs with an eye toward delivering macromolecules [82–84]. Fractional conjugation connected with PEG at a basic pH was proven to yield self-aggregation via an amide linkage to soluble chitosan [84]. After incubation in phosphate buffer saline, these kinds of aggregates could trap insulin because electrostatic interactions were developed between the un-conjugated chitosan monomers and the anionic residues of protein. Table 2 shows a selection of studies on the utilization of chitosan NP composites for drug delivery systems.
Table 2.
Selected studies on utilization of chitosan NP composites for drug delivery systems.
| Name of chitosan NP composites | Purpose of utilization | Findings |
|---|---|---|
| Chitosan nanospheres loaded by 5-fluorouracil | Delivery of 5-fluorouracil for cancer treatment. | These stable nanosized chitosan particles can entrap and deliver drugs in tumor cells [79]. |
| Chitosan–TPP NPs loaded with insulin | Delivery of insulin for diabetics | Chitosan NPs enhanced both the relative bioavailability and intestinal absorption of insulin, resulting in lower blood glucose level in rats [73]. |
| Chitosan–DNA NPs | Delivery of encapsulated of plasmid DNA | Chitosan–DNA NPs protect the encapsulated plasmid DNA from nuclease degradation [77]. |
| Chitosan NPs conjugated with doxorubicin–dextran complex | Delivery of encapsulated dextran–doxorubicin conjugate | These NPs target tumor cells with good efficiency [79]. |
| Chitosan NPs labeled with fluorescein isothiocyanate–bovine serum albumin | Delivery of fluorescein | These NPs shows potentiality for drug delivery on the epithelial cells of ocular mucosa [124]. |
NP = nanoparticle; TPP = tripolyphosphate.
6. Selected studies on chitosan films for drug delivery systems
Chitosan was also used in the preparation of films for drug delivery systems [85–87]. Films prepared using chitosan have been utilized for oral delivery of many drugs such as chlorhexidine digluconate [88], 5-fluorouracil [89], mitoxantrone [90], cytarabine [91], and paclitaxel [92]. The characteristics of chitosan including the drug delivery behavior of nanocomposite films prepared from mixtures of chitosan and organic rectorite (OREC), which is a type of layered silicate, were investigated [93]. The films of chitosan and chitosan–OREC nanocomposite were prepared with different chitosan/OREC mass ratios (2:1, 6:1, 12:1, 20:1, 50:1) and dissolved in a 2% (w/v) aqueous acetic acid to obtain 2% (w/v) chitosan and chitosan/OREC nanocomposite films. The films exhibited the strongest antibacterial behaviors. It was observed that all films showed equivalent drug release in the initial stages, but after several hours the release became slower compared to films prepared using pure chitosan. Chitosan and gelatin solutions were mixed together to obtain two final polymeric concentrations, F1 (1% w/v) and F2 (2% w/v), and the films prepared from the mixture were investigated for drug delivery [94]. The results showed that only the film based on gelatin alone provided complete drug release owing to its dissolution. In 30 minutes, films with an excess of chitosan showed a higher release of drug—up to 83% as compared with 48% of the drug for films containing greater amounts of gelatin.
Films of chitosan were prepared for dexamethasone delivery [95]. Dexamethasone was loaded in chitosan films at a percentage of 1.5 (wt.%). Later, the films were dried in a glass Petri dish at room temperature for 1–3 days until monolayer films were obtained. An analog procedure was performed to achieve a bilayer film formation with dexamethasone. Release tests suggest that the dexamethasone–chitosan films are potential sustained-release carriers for dexamethasone. It was also found that the release time of the films was longer than that of conventional ocular topical delivery dosage forms. Moreover, a second layer of chitosan film significantly modified the drug release profile. Therefore, the monolayer dexamethasone–chitosan film might be considered a promising ocular delivery carrier for dexamethasone in hours and bilayer dexamethasone–chitosan film in weeks.
Films prepared from chitosan and PEG with ciprofloxacin hydrochloride as the model drug incorporated at different concentrations were studied [96]. PEG was used in concentrations of 2.0 wt.%, 3.5 wt.%, 5.5 wt.%, and 8.0 wt.% of total films. Ciprofloxacin hydrochloride (0.1 g and 0.3 g) was loaded in the films. From the controlled release tests, it was found that the release of ciprofloxacin hydrochloride increased with PEG and decreased with the increase in the amount of drug loaded in the film. However, the cumulative release amount of the drug increased significantly. The chitosan–PEG films were also found to be sensitive to pH and ionic strength. In simulated intestinal fluid, a reduction of the ciprofloxacin hydrochloride concentration from 100% to 71% with an increase in thickness of the film from 35 μm to 85 μm was observed.
7. Selected studies on wound healing based on chitosan
Chitosan is used as a wound healing accelerator in many studies [97–108]. It enhances the functions of inflammatory cells such as macrophages and polymorphonuclear leukocytes, as well as the production of osteopontin and leukotriene B4, transforming growth factor β1, and platelet-derived growth factor and fibroblasts [97]. Chitosan also possesses other biological activities and affects the macrophage function that favors faster wound healing [109]. Moreover, it has histoarchitectural tissue organization and displays an aptitude to stimulate cell proliferation [110]. The biological properties, especially bacteriostatic and fungistatic properties, are useful for wound treatment [111]. Films with flexible, thin, transparent properties prepared from a composite of chitosan–alginate polyelectrolyte complex caused acceleration in healing of incision wounds in the rat model compared with conventional gauze dressing. It was observed that the closure rate and appearance of polyelectrolyte complex-treated wounds were comparable with Opsite1-treated wounds [112]. An application of cross-linkable chitosan hydrogel on full-thickness skin incisions made on the backs of mice significantly induced wound contraction and resulted in a substantial acceleration of wound closure and healing compared with the untreated controls [98]. In another research, an early return to normal skin color in chitosan-treated areas was observed [113]. Treatment with chitosan demonstrated a substantial decrease in treatment time with minimum scar formation in various animals. The biochemistry and histology of chitosan in wound healing have also been investigated [114]. It was found that silver sulfadiazine incorporated with bilayer chitosan wound dressing exhibited tremendous oxygen permeability, water uptake capability, and controlled water vapor transmission rate. The dressing showed excellent antibacterial activity when in vitro culture was performed for 1 week [115]. Chitosan has been studied widely as a wound dressing material. Acetate bandage for wound healing dressing as a topical antimicrobial dressing in mice was investigated by Burkatovskaya et al [116]. It was found that the bandage provided important benefits by reducing the number of inflammatory cells in the wound at Day 2 and Day 4 and by healing the wound especially during the early period where its antimicrobial effect is most important.
8. Safety assessment of biomedical application of chitosan and its NP composite
Although nanotechnology is a promising technology offering great benefits in the biomedical field, current knowledge on the safety of various NPs in biomedical application is not sufficient. Generally, chitosan has been considered comparatively safe because of its biodegradable and biocompatible properties. LMW chitosan is excreted through the kidney, whereas the excessive molecular weight can be degraded into fragments suitable for renal clearance [117]. However, the use of chitosan in unmodified forms is restricted because they are water-insoluble and highly viscous and have the tendency to coagulate with proteins at high pH values [118]. Chitosan NPs exhibit toxic properties, which make chitosan NPs applicable for cancer treatment. Some studies have reported the cytotoxicity effects of chitosan NPs in vitro [119,120]. A few research studies have been performed on genotoxicity effects and skin irritation. An in vivo study also reported that chitosan NPs affected the mice’s survival rate [121]. However, despite several drawbacks, chitosan is considered a promising agent for drug delivery systems.
9. Conclusion
This review summarizes the biomedical application of chitosan and chitosan-based NP composites with emphasis on drug delivery systems. Chitosan is an important and amazing material that has so many applications in various fields of drug delivery systems. It is biodegradable and biocompatible, and can be found in abundance in nature from renewable sources. Recently, nanochitosan composites have acquired a remarkable advantage over their conventional counterparts owing to the presence of a huge surface area, which gives them additional properties, particularly in terms of biomedical applications. Further study on the drug delivery properties of chitosan and its NP composites may lead to the realization of more effective drug delivery systems.
Acknowledgments
This paper is a part of research funded by the Department of Pharmacology and Chemistry, Faculty of Pharmacy, Universiti Technologi MARA, Selangor, Malaysia.
Funding Statement
This paper is a part of research funded by the Department of Pharmacology and Chemistry, Faculty of Pharmacy, Universiti Technologi MARA, Selangor, Malaysia.
Footnotes
Conflicts of interest
All authors declare no conflicts of interest.
REFERENCES
- 1. Mincea M, Negrulescu A, Ostafe V. Preparation, modification, and applications of chitin nanowhiskers: a review. Rev Adv Mater Sci. 2012;30:225–42. [Google Scholar]
- 2. Illum L, Jabbal-Gill I, Hinchcliffe M, Fisher AN, Davis SS. Chitosan as a novel nasal delivery system for vaccines. Adv Drug Deliv Rev. 2001;51:81–96. doi: 10.1016/s0169-409x(01)00171-5. [DOI] [PubMed] [Google Scholar]
- 3. Hwang JK, Shin HH. Rheological properties of chitosan solutions. Korea-Aust Rheol J. 2000;12:175–9. [Google Scholar]
- 4.Li Q, Dunn ET, Grandmaison EW. Applications and properties of chitosan. In: Goosen MFA, editor. Applications of chitin and chitosan. Lancaster: Technomic Publishing Co.; 1997. pp. 3–29. [Google Scholar]
- 5. Nishimura K, Nishimura S, Nishi N. Immunological activity of chitin and its derivatives. Vaccine. 1984;2:93–9. doi: 10.1016/s0264-410x(98)90039-1. [DOI] [PubMed] [Google Scholar]
- 6.Allan GG, Altman LC, Bensinger RE, Ghosh DK, Hirabayashi Y, Neogi AN, Neogi S. Biomedical applications of chitin and chitosan. In: Zikakis JP, editor. Chitin, chitosan and related enzymes. New York: Academic Press; 1984. pp. 119–33. [Google Scholar]
- 7. Zhu CL, Wang XW, Lin ZZ, Xie ZH, Wang XR. Cell microenvironment stimuli-responsive controlled-release delivery systems based on mesoporous silica nanoparticles. J Food Drug Anal. 2014;22:18–28. doi: 10.1016/j.jfda.2014.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Badawy MEI, Rabea EI, Rogge TM, Stevens CV, Smagghe G, Steurbaut W, Höfte M. Synthesis and fungicidal activity of new N,O-acyl chitosan derivatives. Biomacromolecules. 2004;5:589–95. doi: 10.1021/bm0344295. [DOI] [PubMed] [Google Scholar]
- 9. Balakrishnan B, Mohanty M, Umashankar PR, Jayakrishnan A. Evaluation of an in situ forming hydrogel wound dressing based on oxidized alginate and gelatin. Biomaterials. 2005;26:6335–42. doi: 10.1016/j.biomaterials.2005.04.012. [DOI] [PubMed] [Google Scholar]
- 10. Howling GI, Dettmar PW, Goddard PA, Hampson FC, Dornish M, Wood EJ. The effect of chitin and chitosan on the proliferation of human skin fibroblasts and keratinocytes in vitro. Biomaterials. 2001;22:2959–66. doi: 10.1016/s0142-9612(01)00042-4. [DOI] [PubMed] [Google Scholar]
- 11. Li Q, Dunn ET, Grandmaison EW, Goosen MFA. Applications and properties of chitosan. J Biol Compat Polym. 1992;7:370–97. [Google Scholar]
- 12. Ravi Kumar MNV. A review of chitin and chitosan applications. React Functional Polym. 2000;46:1–27. [Google Scholar]
- 13. Wang XH, Cui FZ, Zhang YH. Preparation and characterization of collagen/chitosan matrices as potential biomaterials. J Bioactive Compatible Polym. 2003;18:453–67. [Google Scholar]
- 14. Patel M, Shah T, Amin A. Therapeutic opportunities in colon specific drug delivery system. Crit Rev Ther Drug Carrier Syst. 2007;24:147–202. doi: 10.1615/critrevtherdrugcarriersyst.v24.i2.20. [DOI] [PubMed] [Google Scholar]
- 15. George M, Abraham TE. Polyionic hydrocolloids for the intestinal delivery of protein drugs: alginate and chitosan —a review. J Control Release. 2006;114:1–14. doi: 10.1016/j.jconrel.2006.04.017. [DOI] [PubMed] [Google Scholar]
- 16. Bernkop-Schnürch A, Walker G. Multifunctional matrices for oral peptide delivery. Crit Rev Ther Drug Carr Syst. 2001;18:459–501. [PubMed] [Google Scholar]
- 17. Chopra S, Mahdi S, Kaur J, Iqbal Z, Talegaonkar S, Ahmad FJ. Advances and potential applications of chitosan derivatives as mucoadhesive biomaterials in modern drug delivery. J Pharm Pharmacol. 2006;58:1021–32. doi: 10.1211/jpp.58.8.0002. [DOI] [PubMed] [Google Scholar]
- 18. Riva R, Ragelle H, Rieux A, Duhem N, Jérôme C, Préat V. Chitosan and chitosan derivatives in drug delivery and tissue engineering. Adv Polym Sci. 2011;244:19–44. [Google Scholar]
- 19. Anitha A, Maya S, Deepa N, Chennazhi KP, Nair SV, Tamura H, Jayakumar R. Efficient water soluble O-carboxymethyl chitosan nanocarrier for the delivery of curcumin to cancer cells. Carbohydr Polym. 2011;83:452–61. [Google Scholar]
- 20. Chen M, Liu Y, Yang W, Li X, Liu L, Zhou Z, Wang Y, Li R, Zhang Q. Preparation and characterization of self-assembled nanoparticles of 6-O-cholesterol-modified chitosan for drug delivery. Carbohydr Polym. 2011;84:1244–51. [Google Scholar]
- 21. Ferrari PC, Souzab FM, Giorgettib L, Oliveiraa GF, Chaud MV, Ferraz HG, Evangelista RC. In vitro drug permeation from chitosan pellets. Carbohydr Polym. 2012;87:2526–31. doi: 10.1016/j.carbpol.2012.08.044. [DOI] [PubMed] [Google Scholar]
- 22. Pawar H, Douroumis D, Boateng J. Preparation and optimization of PMAA–chitosan–PEG nanoparticles for oral drug delivery. Colloids Surf B Biointerfaces. 2012;90:102–8. doi: 10.1016/j.colsurfb.2011.10.005. [DOI] [PubMed] [Google Scholar]
- 23. Termsarasab U, Cho HJ, Kim DH, Chong S, Chung SJ, Shim CK, Moon HT, Kim DD. Chitosan oligosaccharide–arachidic acid-based nanoparticles for anti-cancer drug delivery. Int J Pharm. 2013;441:373–80. doi: 10.1016/j.ijpharm.2012.11.018. [DOI] [PubMed] [Google Scholar]
- 24. Kittur FS, Vishu Kumar AB, Tharanathan RN. Low molecular weight chitosans— preparation by depolymerization with Aspergillus niger pectinase and characterization. Carbohydr Res. 2003;338:1283–90. doi: 10.1016/s0008-6215(03)00175-7. [DOI] [PubMed] [Google Scholar]
- 25. Krajewska B. Membrane-based processes performed with use of chitin/chitosan materials. Sep Purif Technol. 2005;41:305–12. [Google Scholar]
- 26. Berger J, Reist M, Mayer JM, Felt O, Gurny R. Structure and interactions in chitosan hydrogels formed by complexation or aggregation for biomedical applications. Eur J Pharm Biopharm. 2004;57:35–52. doi: 10.1016/s0939-6411(03)00160-7. [DOI] [PubMed] [Google Scholar]
- 27. Prashanth KVH. Solid state structure of chitosan prepared under different N-deacetylation conditions. Carbohydr Polym. 2002;50:27–33. [Google Scholar]
- 28. Cervera MF, Heinamaki J, Rasanen M, Maunu SL, Karjalainen M, Nieto Acosta OM, Iraizoz Colarte A, Yliruusi J. Solid-state characterization of chitosans derived from lobster chitin. Carbohydr Polym. 2004;58:401–8. [Google Scholar]
- 29. Steenkamp GC, Keizer K, Neomagus HWJP, Krieg HM. Copper (II) Removal from polluted water with alumina/chitosan composite membranes. J Membr Sci. 2002;197:147–56. [Google Scholar]
- 30. Cho J, Heuzey MC, Begin A, Carreau PJ. Viscoelastic properties of chitosan solutions: effect of concentration and ionic strength. J Food Eng. 2006;74:500–15. [Google Scholar]
- 31. Rege PR, Block LH. Chitosan processing: influence of process parameters during acidic and alkaline hydrolysis and effect of the processing sequence on the resultant chitosans properties. Carbohydr Res. 1999;321:235–45. [Google Scholar]
- 32. Duarte ML, Ferreira MC, Marvao MR, Rochac J. An optimised method to determine the degree of acetylation of chitin and chitosan by FTIRspectroscopy. IntJ BiolMacromol. 2002;31:1–8. doi: 10.1016/s0141-8130(02)00039-9. [DOI] [PubMed] [Google Scholar]
- 33. Gamage A, Shahidi F. Use of chitosan for the removal of metal ion contaminants and proteins from water. Food Chem. 2007;104:989–96. [Google Scholar]
- 34. Nunthanid J, Puttipipatkhachorn S, Yamamoto K, Peck GE. Physical properties and molecular behavior of chitosan films. Drug Dev Ind Pharm. 2001;27:143–57. doi: 10.1081/ddc-100000481. [DOI] [PubMed] [Google Scholar]
- 35. Wang B, Wang K, Dan W, Zhang T, Ye Y. Konjac glucomannan–collagen–chitosan blend films. J Biomed Eng. 2006;23:102–6. [PubMed] [Google Scholar]
- 36. Fee M, Errington N, Jumel K, Illum L, Smith A, Harding SE. Correlation of SEC/MALLS with ultracentrifuge and viscometric data for chitosans. Eur Biophys J. 2003;32:457–64. doi: 10.1007/s00249-003-0317-8. [DOI] [PubMed] [Google Scholar]
- 37. Xu YX, Kim KM, Hanna MA, Nag D. Chitosan–starch composite film: preparation and characterization. Ind Crops Prod. 2005;21:185–92. [Google Scholar]
- 38. Bhise KS, Dhumal RS, Paradkar AR, Kadam SS. Effect of drying methods on swelling, erosion and drug release from chitosan–naproxen sodium complexes. AAPS Pharmscitech. 2008;9:1–12. doi: 10.1208/s12249-007-9001-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sun W, Mao S, Wang Y, Junyaprasert VB, Zhang T, Na L, Wang J. Bioadhesion and oral absorption of enoxaparin nanocomplexes. Int J Pharm. 2010;386:275–81. doi: 10.1016/j.ijpharm.2009.11.025. [DOI] [PubMed] [Google Scholar]
- 40. Shavi G, Nayak U, Reddy M, Karthik A, Deshpande PB, Kumar AR, Udupa N. Sustained release optimized formulation of anastrozole-loaded chitosan microspheres: in vitro and in vivo evaluation. Mater Sci Mater Med. 2011;22:865–78. doi: 10.1007/s10856-011-4274-y. [DOI] [PubMed] [Google Scholar]
- 41. Grabovac V, Guggi D, Bernkop-Schnürch A. Comparison of the mucoadhesive properties of various polymers. Adv Drug Deliv Rev. 2005;57:1713–23. doi: 10.1016/j.addr.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 42. Lueßen HL, de Leeuw BJ, Langemeÿer MWE, de Boer AG, Verhoef JC, Junginger HE. Mucoadhesive polymers in peroral peptide drug delivery: VI. Carbomer and chitosan improve the intestinal absorption of the peptide drug buserelin in vivo. Pharm Res. 1996;13:1668–72. doi: 10.1023/a:1016488623022. [DOI] [PubMed] [Google Scholar]
- 43. Jintapattanakit A, Junyaprasert VB, Kissel TJ. The role of mucoadhesion of trimethyl chitosan and PEGylated trimethyl chitosan nanocomplexes in insulin uptake. Pharm Sci. 2009;98:4818–30. doi: 10.1002/jps.21783. [DOI] [PubMed] [Google Scholar]
- 44. Werle M, Bernkop-Schnürch A. Thiolated chitosans: useful excipients for oral drug delivery. J Pharm Pharmacol. 2008;60:273–81. doi: 10.1211/jpp.60.3.3001. [DOI] [PubMed] [Google Scholar]
- 45. Gupta S, Vyas SP. Carbopol/chitosan based pH triggered in situ gelling system for ocular delivery of timolol maleate. Sci Pharm. 2010;78:959–76. doi: 10.3797/scipharm.1001-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Sakloetsakun D, Hombach J, Bernkop-Schnürch A. In situ gelling properties of chitosan–thioglycolic acid conjugate in the presence of oxidizing agents. Biomaterials. 2009;30:6151–7. doi: 10.1016/j.biomaterials.2009.07.060. [DOI] [PubMed] [Google Scholar]
- 47. Malmo J, Vrum KM, Strand SP. Effect of chitosan chain architecture on gene delivery:comparison of self-branched and linear chitosans. Biomacromolecules. 2011;12:721–9. doi: 10.1021/bm1013525. [DOI] [PubMed] [Google Scholar]
- 48. Martien R, Loretz B, Thaler M, Majzoob S, Bernkop-Schnürch A. Chitosan–thioglycolic acid conjugate: an alternative carrier for oral nonviral gene delivery? J Biomed Mater Res A. 2007;82:1–9. doi: 10.1002/jbm.a.31135. [DOI] [PubMed] [Google Scholar]
- 49. Varkouhi AK, Verheul RJ, Schiffelers RM, Lammers T, Storm G, Hennink WE. Gene silencing activity of siRNA polyplexes based on thiolated N,N,N-trimethylated chitosan. Bioconjugate Chem. 2010;21:2339–46. doi: 10.1021/bc1003789. [DOI] [PubMed] [Google Scholar]
- 50. Teijeiro-Osorio D, Remuñan-López C, Alonso MJ. Chitosan/cyclodextrin nanoparticles can efficiently transfect the airway epithelium in vitro. Eur J Pharm Biopharm. 2009;71:257–63. doi: 10.1016/j.ejpb.2008.09.020. [DOI] [PubMed] [Google Scholar]
- 51. Malhotra M, Lane C, Tomaro-Duchesneau C, Saha S, Prakash S. A novel scheme for synthesis of PEG-grafted-chitosan polymer for preparation of nanoparticles and other applications. Int J Nanomed. 2011;6:485–94. doi: 10.2147/IJN.S17190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Mao S, Sun W, Kissel T. Chitosan-based formulations for delivery of DNA and siRNA. Adv Drug Deliv Rev. 2010;62:12–27. doi: 10.1016/j.addr.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 53. Yu H, Chen X, Lu T, Sun J, Tian H, Hu J, Wang Y, Zhang P, Jing X. Poly(l-lysine)-graft–chitosan copolymers: synthesis, characterization, and gene transfection effect. Biomacromolecules. 2007;8:1425–35. doi: 10.1021/bm060910u. [DOI] [PubMed] [Google Scholar]
- 54.Lee D, Mohapatra SS. Chitosan nanoparticle-mediated gene transfer. New York: Humana Press; 2008. [DOI] [PubMed] [Google Scholar]
- 55. Schipper NGM, Olsson S, Hoogstraate JA, deBoer AG, Vårum KM, Artursson P. Chitosans as absorption enhancer for poorly absorbable drugs: 2. Mechanism of absorption enhancement. Pharm Res. 1997;14:923–9. doi: 10.1023/a:1012160102740. [DOI] [PubMed] [Google Scholar]
- 56. Schipper NGM, Varum KM, Artursson P. Chitosans as absorption enhancers for poorly absorbable drugs. 1. Influence of molecular weight and degree of deacetylation on drug transport across human intestinal epithelial (Caco-2) cells. Pharm Res. 1996;13:1686–92. doi: 10.1023/a:1016444808000. [DOI] [PubMed] [Google Scholar]
- 57. Kast CE, Bernkop-Schnurch A. Influence of the molecular mass on the permeation enhancing effect of different poly(acrylates) STP Pharm Sci. 2002;6:351–6. [Google Scholar]
- 58. Shah P, Jogani V, Mishra P, Mishra AK, Bagchi T, Misra A. Modulation of ganciclovir intestinal absorption in presence of absorption enhancers. J Pharm Sci. 2007;96:2710–22. doi: 10.1002/jps.20888. [DOI] [PubMed] [Google Scholar]
- 59. Trapani A, Lopedota A, Franco M, Cioffi N, Ieva E, Garcia-Fuentes M, Alonso MJ. A comparative study of chitosan and chitosan/cyclodextrin nanoparticles as potential carriers for the oral delivery of small peptides. Eur J Pharm Biopharm. 2010;75:26–32. doi: 10.1016/j.ejpb.2010.01.010. [DOI] [PubMed] [Google Scholar]
- 60. Langoth N, Kahlbacher H, Schöffmann G, Schmerold I, Schuh M, Franz S, Kurka P, Bernkop-Schnürch A. Thiolated chitosans: design and in vivo evaluation of a mucoadhesive buccal peptide drug delivery system. Pharm Res. 2006;23:573–9. doi: 10.1007/s11095-005-9533-5. [DOI] [PubMed] [Google Scholar]
- 61. Kim JH, Kim YS, Park K, Kang E, Lee S, Nam HY, Kim K, Park JH, Chi DY, Park RW, Kim IS, Choi K, Chan Kwon I. Self-assembled glycol chitosan nanoparticles for the sustained and prolonged delivery of antiangiogenic small peptide drugs in cancer therapy. Biomaterials. 2008;29:1920–30. doi: 10.1016/j.biomaterials.2007.12.038. [DOI] [PubMed] [Google Scholar]
- 62. Wu W, Shen J, Banerjee P, Zhou S. Chitosan-based responsive hybrid nanogels for integration of optical pH-sensing, tumor cell imaging and controlled drug delivery. Biomaterials. 2010;31:8371–81. doi: 10.1016/j.biomaterials.2010.07.061. [DOI] [PubMed] [Google Scholar]
- 63. Saboktakin MR, Tabatabaie RM, Maharramov A, Ramazanov MA. Synthesis and in vitro evaluation of carboxymethyl starch–chitosan nanoparticles as drug delivery system to the colon. Int J Biol Macromol. 2011;48:381–5. doi: 10.1016/j.ijbiomac.2010.10.005. [DOI] [PubMed] [Google Scholar]
- 64. Nazar H, Fatouros DG, van der Merwe SM, Bouropoulos N, Avgouropoulos G, Tsibouklis J, Roldo M. Thermosensitive hydrogels for nasal drug delivery: the formulation and characterisation of systems based on N-trimethyl chitosan chloride. Eur J Pharm Biopharm. 2011;77:225–32. doi: 10.1016/j.ejpb.2010.11.022. [DOI] [PubMed] [Google Scholar]
- 65. Liu L, Tang X, Wang Y, Guo S. Smart gelation of chitosan solution in the presence of NaHCO3 for injectable drug delivery system. Int J Pharm. 2011;414:6–15. doi: 10.1016/j.ijpharm.2011.04.052. [DOI] [PubMed] [Google Scholar]
- 66. Liang Z, Gong T, Sun X, Tang JZ, Zhang Z. Chitosan oligomers as drug carriers for renal delivery of zidovudine. Carbohydr Polym. 2012;87:2284–90. [Google Scholar]
- 67. Fan Z, Fu PP, Yu H, Raya PC. Theranostic nanomedicine for cancer detection and treatment. J Food Drug Anal. 2014;22:3–17. doi: 10.1016/j.jfda.2014.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Wang JJ, Zeng ZW, Xiao RZ, Xie T, Zhou GL, Zhan XR, Wang SL. Recent advances of chitosan nanoparticles as drug carriers. Int J Nanomed. 2011;6:765–74. doi: 10.2147/IJN.S17296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Ohya Y, Shiratani M, Kobayashi H, Ouchi T. Release behavior of 5-fluorouracil from chitosan-gel nano-spheres immobilizing 5-fluorouracil coated with polysaccharides and their cell specific cytotoxicity. J Macromol Sci Pure Appl Chem. 1994;31:629–42. [Google Scholar]
- 70. Janes KA, Calvo P, Alonso MJ. Polysaccharide colloidal particles as delivery systems for macromolecules. Adv Drug Del Rev. 2001;47:83–97. doi: 10.1016/s0169-409x(00)00123-x. [DOI] [PubMed] [Google Scholar]
- 71. Shirashi S, Imai T, Otagiri M. Controlled release of indomethacin by chitosan–polyelectrolyte complex: optimization and in vivo/in vitro evaluation. J Control Release. 1993;25:217–25. [Google Scholar]
- 72. Gan Q, Wang T, Cochrane C, McCarron P. Modulation of surface charge, particle size and morphological properties of chitosan–TPP nanoparticles intended for gene delivery. Colloids Surf B Biointerfaces. 2005;44:65–73. doi: 10.1016/j.colsurfb.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 73. Pan Y, Li Y, Zhao H, Zheng JM, Xu H, Wei G, Hao JS, Cui FD. Bioadhesive polysaccharide in protein delivery system: chitosan nanoparticles improve the intestinal absorption of insulin in vivo. Int J Pharm. 2002;249:139–47. doi: 10.1016/s0378-5173(02)00486-6. [DOI] [PubMed] [Google Scholar]
- 74. Xu Y, Du Y. Effect of molecular structure of chitosan on protein delivery properties of chitosan nanoparticles. Int J Pharm. 2003;250:215–26. doi: 10.1016/s0378-5173(02)00548-3. [DOI] [PubMed] [Google Scholar]
- 75. Fan W, Yan W, Xu Z, Ni H. Formation mechanism of monodisperse, low molecular weight chitosan nanoparticles by ionic gelation technique. Colloids Surf B Biointerfaces. 2012;90:21–7. doi: 10.1016/j.colsurfb.2011.09.042. [DOI] [PubMed] [Google Scholar]
- 76. Dodane V, Vilivalam D. Pharmaceutical application of chitosan. Pharm Sci Technol Today. 1998;1:246–53. [Google Scholar]
- 77. Ray K, Mao HQ, Lin KY, Huang SK, Leong KW. Oral immunization with DNA chitosan nanoparticles. Proc Int Symp Control Release Mater. 1999;26:348–9. [Google Scholar]
- 78. Tokumitsu H, Ichikawa H, Fukumori Y. Chitosan–gadopentetic acid complex nanoparticles for gadolinium neutron-capture therapy of cancer: preparation by novel emulsion–droplet coalescence technique and characterization. Pharm Res. 1999;16:1830–5. doi: 10.1023/a:1018995124527. [DOI] [PubMed] [Google Scholar]
- 79. Hamidi M, Azadi A, Rafiei P. Hydrogel nanoparticles in drug delivery. Adv Drug Deliv Rev. 2008;60:1638–49. doi: 10.1016/j.addr.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 80. Maitra A. Determination of size parameters of water aerosol OT-oil reverse micelles from their nuclear magnetic resonance data. J Phys Chem. 1984;88:5122–5. [Google Scholar]
- 81. Mitra S, Gaur U, Ghosh PC, Maitra AN. Tumour targeted delivery of encapsulated dextran–doxorubicin conjugate using chitosan nanoparticles as carrier. J Control Release. 2001;74:317–23. doi: 10.1016/s0168-3659(01)00342-x. [DOI] [PubMed] [Google Scholar]
- 82. Yu S, Hu J, Pan X, Yao P, Jiang M. Stable and pH-sensitive nanogels prepared by self-assembly of chitosan and ovalbumin. Langmuir. 2006;22:2754–9. doi: 10.1021/la053158b. [DOI] [PubMed] [Google Scholar]
- 83. Ichikawa S, Iwamoto S, Watanabe J. Formation of biocompatible nanoparticles by self-assembly of enzymatic hydrolysates of chitosan and carboxymethyl cellulose. Biosci Biotechnol Biochem. 2005;69:1637–42. doi: 10.1271/bbb.69.1637. [DOI] [PubMed] [Google Scholar]
- 84. Ohya Y, Cai R, Nishizawa H, Hara K, Ouchi T. Preparation of PEG-grafted chitosan nano-particle for peptide drug carrier. Proc Int Symp Control Release Bioact Mater. 1999;26:655–6. [Google Scholar]
- 85. Macleod GS, Collett JH, Fell JT. The potential use of mixed films of pectin, chitosan and HPMC for bimodal drug release. J Control Release. 1999;58:303–10. doi: 10.1016/s0168-3659(98)00168-0. [DOI] [PubMed] [Google Scholar]
- 86. Shu XZ, Zhu KJ. The influence of multivalent phosphate structure on the properties of ionically cross-linked chitosan films for controlled drug release. Eur J Pharm Biopharm. 2002;54:235–43. doi: 10.1016/s0939-6411(02)00052-8. [DOI] [PubMed] [Google Scholar]
- 87. Perugini P, Genta I, Conti B, Modena T, Pavanetto F. Periodontal delivery of ipriflavone: new chitosan/PLGA film delivery system for a lipophilic drug. Int J Pharm. 2003;252:1–9. doi: 10.1016/s0378-5173(02)00602-6. [DOI] [PubMed] [Google Scholar]
- 88. Senel S, Ikinci G, Kas S, Yousefi-Rad A, Sargon MF, Hincal AA. Chitosan films and hydrogels of cholhexidine gluconate for oral mucosal delivery. Int J Pharm. 2000;193:197–203. doi: 10.1016/s0378-5173(99)00334-8. [DOI] [PubMed] [Google Scholar]
- 89. Ouchi T, Banba T, Fujimoto M, Hamamoto S. Synthesis and antitumor activity of chitosan carrying 5-fluorouracil. Makromol Chem Physics. 1989;190:1817–25. [Google Scholar]
- 90. Jameela SR, Jayakrisnan A. Glutaraldehyde cross-linked chitosan microspheres as a long acting biodegradable drug delivery vehicle: studies on the in vitro release of mitoxantrone and in vivo degradation of microspheres in rat muscle. Biomaterials. 1995;16:769–75. doi: 10.1016/0142-9612(95)99639-4. [DOI] [PubMed] [Google Scholar]
- 91. Blanco MD, Gomez C, Olmo R, Muñiz E, Teijón JM. Chitosan microspheres in PLG films as devices for cytarabine release. Int J Pharm. 2000;202:29–39. doi: 10.1016/s0378-5173(00)00408-7. [DOI] [PubMed] [Google Scholar]
- 92. Miwa A, Ishibe A, Nakano M, Yamahira T, Itai S, Jinno S, Kawahara H. Development of novel chitosan derivatives as micellar carriers of taxol. Pharm Res. 1998;15:1844–50. doi: 10.1023/a:1011901921995. [DOI] [PubMed] [Google Scholar]
- 93. Wang X, Du Y, Luo J, Kennedy JF. Chitosan/organic rectorite nanocomposite films: structure, characteristic and drug delivery behaviour. Carbohydr Polym. 2007;69:41–9. [Google Scholar]
- 94. Abruzzo A, Bigucci F, Cerchiara T, Cruciani F, Vitali B, Luppi B. Mucoadhesive chitosan/gelatin films for buccal delivery of propranolol hydrochloride. Carbohydr Polym. 2012;87:581–8. doi: 10.1016/j.carbpol.2011.08.024. [DOI] [PubMed] [Google Scholar]
- 95. Rodrigues LB, Leite HF, Yoshida MI, Saliba JB, Cunha AS, Jr, Faraco AA. In vitro release and characterization of chitosan films as dexamethasone carrier. Int J Pharm. 2009;368:1–6. doi: 10.1016/j.ijpharm.2008.09.047. [DOI] [PubMed] [Google Scholar]
- 96. Wang Q, Dong Z, Du Y, Kennedy JF. Controlled release of ciprofloxacin hydrochloride from chitosan/polyethylene glycol blend films. Carbohydr Polym. 2007;69:336–43. [Google Scholar]
- 97. Ueno H, Mori T, Fujinaga T. Topical formulations and wound healing applications of chitosan. Adv Drug Deliv Rev. 2001;52:105–15. doi: 10.1016/s0169-409x(01)00189-2. [DOI] [PubMed] [Google Scholar]
- 98. Ishihara M, Nakanishi K, Ono K, Sato M, Kikuchi M, Saito Y, Yura H, Matsui T, Hattori H, Uenoyama M, Kurita A. Photocrosslinkable chitosan as a dressing for wound occlusion and accelerator in healing process. Biomaterials. 2002;23:833–40. doi: 10.1016/s0142-9612(01)00189-2. [DOI] [PubMed] [Google Scholar]
- 99. Kweon DK, Song SB, Park YY. Preparation of water-soluble chitosan/heparin complex and its application as wound healing accelerator. Biomaterials. 2003;24:1595–601. doi: 10.1016/s0142-9612(02)00566-5. [DOI] [PubMed] [Google Scholar]
- 100. Alemdaroğlu C, Değim Z, Çelebi N, Zor F, Oztürk S, Erdoğan D. An investigation on burn wound healing in rats with chitosan gel formulation containing epidermal growth factor. Burns. 2006;32:319–27. doi: 10.1016/j.burns.2005.10.015. [DOI] [PubMed] [Google Scholar]
- 101. Minagawa T, Okamura Y, Shigemasa Y, Minami S, Okamoto Y. Effects of molecular weight and deacetylation degree of chitin/chitosan on wound healing. Carbohydr Polym. 2007;67:640–4. [Google Scholar]
- 102. Hong HJ, Jin SE, Park JS, Ahn WS, Kim CK. Accelerated wound healing by smad3 antisense oligonucleotides-impregnated chitosan/alginate polyelectrolyte complex. Biomaterials. 2008;29:4831–7. doi: 10.1016/j.biomaterials.2008.08.023. [DOI] [PubMed] [Google Scholar]
- 103. Bae JW, Lee JH, Choi WS, Lee DS, Bae EH, Park KD. EPDIM peptide-immobilized porous chitosan beads for enhanced wound healing: preparation, characterizations and in vitro evaluation. Mater Sci Eng. 2009;29:697–701. [Google Scholar]
- 104. Sung JH, Hwang MR, Kim JO, Lee JH, Kim YI, Kim JH, Chang SW, Jin SG, Kim JA, Lyoo WS, Han SS, Ku SK, Yong CS, Choi HG. Gel characterisation and in vivo evaluation of minocycline-loaded wound dressing with enhanced wound healing using polyvinyl alcohol and chitosan. Int J Pharm. 2010;392:232–40. doi: 10.1016/j.ijpharm.2010.03.024. [DOI] [PubMed] [Google Scholar]
- 105. Yang C, Xu L, Zhou Y, Zhang X, Huang X, Wang M, Han Y, Zhai M, Wei S, Li J. A green fabrication approach of gelatin/CM-chitosan hybrid hydrogel for wound healing. Carbohydr Polym. 2010;82:1297–305. [Google Scholar]
- 106. Li X, Chen S, Zhang B, Li M, Diao K, Zhang Z, Li J, Xu Y, Wang X, Chen H. In situ injectable nano-composite hydrogel composed of curcumin, N,O-carboxymethyl chitosan and oxidized alginate for wound healing application. Int J Pharm. 2012;437:110–9. doi: 10.1016/j.ijpharm.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 107. Li X, Nan K, Li L, Zhang Z, Chen H. In vivo evaluation of curcumin nanoformulation loaded methoxy poly(ethylene glycol)-graft-chitosan composite film for wound healing application. Carbohydr Polym. 2012;88:84–90. [Google Scholar]
- 108. Wijekoon A, Fountas-Davis N, Leipzig ND. Fluorinated methacrylamide chitosan hydrogel systems as adaptable oxygen carriers for wound healing. Acta Biomater. 2013;9:5653–64. doi: 10.1016/j.actbio.2012.10.034. [DOI] [PubMed] [Google Scholar]
- 109.Balassa LL, Prudden JF. Application of chitin and chitosan in wound healing acceleration. In: Zikakis JP, editor. Chitin, chitosan and related enzymes. San Diego, CA: Academic Press; 1984. pp. 296–305. [Google Scholar]
- 110.Muzzarelli RAA. In: Amphoteric derivatives of chitosan and their biological significance in chitin and chitosan. Skjak-Braek G, Anthonsen T, Sandford P, editors. London: Elsevier Applied Science; 1989. pp. 87–99. [Google Scholar]
- 111.Minami S, Okamoto Y, Matsuhashi A. Application of chitin and chitosan in large animal practice. In: Brine CJ, Sandford PA, Zikakis JP, editors. Advances in chitin and chitosan. New York: Elsevier; 1992. pp. 61–9. [Google Scholar]
- 112. Wang LS, Khor E, Wee A, Lim LY. Chitosan–alginate PEC membrane as a wound dressing: assessment of incisional wound healing. J Biomed Mater Res. 2002;63:610–8. doi: 10.1002/jbm.10382. [DOI] [PubMed] [Google Scholar]
- 113. Minami S, Okamoto Y, Hamada K, Fukumoto Y, Shigemasa Y. Veterinary practice with chitin and chitosan. EXS. 1999;87:265–77. doi: 10.1007/978-3-0348-8757-1_19. [DOI] [PubMed] [Google Scholar]
- 114.Muzzarelli RA, Mattioli-Belmonte M, Pugnaloni A. Biochemistry histology and clinical uses of chitins and chitosans in wound healing. In: Jolles P, Muzzarelli RAA, editors. Chitin and chitinases. Basel: Birkhauser; 1999. pp. 251–64. [DOI] [PubMed] [Google Scholar]
- 115. Mi FL, Wu YB, Shyu SS, Schoung JY, Huang YB, Tsai YH, Hao JY. Control of wound infections using a bilayer chitosan wound dressing with sustainable antibiotic delivery. J Biomed Mater Res. 2002;59:438–49. doi: 10.1002/jbm.1260. [DOI] [PubMed] [Google Scholar]
- 116. Burkatovskaya M, Castano AP, Demidova-Rice TN, Tegos GP, Hamblin MR. Effect of chitosan acetate bandage on wound healing in infected and noninfected wounds in mice. Wound Repair Regen. 2008;16:425–31. doi: 10.1111/j.1524-475X.2008.00382.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Kean T, Thanou M. Biodegradation, biodistribution and toxicity of chitosan. Adv Drug Deliv Rev. 2010;62:3–11. doi: 10.1016/j.addr.2009.09.004. [DOI] [PubMed] [Google Scholar]
- 118.Halim AS, Lim CK. Biomedical-grade chitosan in wound management and its biocompatibility in vitro. In: Elnashar M, editor. Biopolymers. New York: Sciyo Publisher; 2010. pp. 19–36. [Google Scholar]
- 119. Qi L, Xu Z. In vivo antitumor activity of chitosan nanoparticles. Bioorg Med Chem Lett. 2006;16:4243–5. doi: 10.1016/j.bmcl.2006.05.078. [DOI] [PubMed] [Google Scholar]
- 120. Hu YL, Qi W, Han F. Toxicity evaluation of biodegradable chitosan nanoparticles using a zebrafish embryo model. Int J Nanomed. 2011;6:3351–9. doi: 10.2147/IJN.S25853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Alnasser Y. MSc thesis. MI, USA: Albany College of Pharmacy and Health Sciences, ProQuest LLC; 2013. Safety assessment of commonly used nanoparticles in biomedical application: impact on inflammatory processes. [Google Scholar]
- 122. Miyazaki S, Yamaguchi H, Takada M. Pharmaceutical application of biomedical polymers: XXIX. Preliminary study on film dosage form prepared from chitosan for oral drug delivery. Acta Pharm Nord. 1990;2:401–6. [PubMed] [Google Scholar]
- 123. Chandy T, Sharma CP. Biodegradable chitosan matrix for the controlled release of steroids. Biomater Artif Cells Immobilization Biotechnol. 1991;19:745–60. doi: 10.3109/10731199109117852. [DOI] [PubMed] [Google Scholar]
- 124. de Salamanca AE, Diebold Y, Calonge M, García-Vazquez C, Callejo S, Vila A, Alonso MJ. Chitosan nanoparticles as a potential drug delivery system for the ocular surface: toxicity, uptake mechanism and in vivo tolerance. Invest Ophthalmol Vis Sci. 2006;47:1416–25. doi: 10.1167/iovs.05-0495. [DOI] [PubMed] [Google Scholar]