Abstract
The cell walls of a number of filamentous, gliding cyanobacteria of the genus Oscillatoria were examined by transmission electron microscopy of ultrathin sections, of freeze-etched replicas, and of whole cells crushed between glass slides and negatively stained. All three techniques revealed the presence of a highly ordered array of parallel fibrils, seen in transverse sections to be situated between the peptidoglycan and the outer membrane. Approximately 200 individual fibrils, each 25 to 30 nm in width, form a parallel, helical array that completely surrounds each cyanobacterial filament, running at an angle of 25 to 30° to its long axis. This highly regular arrangement of the fibrillar layer may imply some underlying symmetry responsible for its organization. A possible source of such symmetry would be the peptidoglycan, and some form of interaction between this layer and the fibrils might provide the necessary scaffolding for the fibrillar array. In crushed, negatively stained samples of fresh cells, individual fibrils were seen outside the filament, released from the cell wall. These released fibrils were of the same width as those observed in situ but were in short lengths, mostly of 100 to 200 nm, and were invariably bent, sometimes even into U shapes, implying great flexibility. Negative staining of released fibrils showed no evidence that they were hollow tubes but did give some indication of a substructure, implying that they were composed of many subunits. The function of this fibrillar array is unknown, although its position in the cell wall, as well as the correspondence between the angle of the fibrils with respect to the long axis of the filament and the rotation of the filament during gliding, may imply an involvement in gliding motility.
There are two main forms of locomotion in bacteria. The first, swimming by means of flagella, is well characterized. The second is gliding, in which active translocation of cells requires contact with a solid or semisolid substrate. In contrast to swimming, little is known about the mechanism of gliding. Despite extensive ultrastructural studies of a wide range of bacteria, including cyanobacteria, which are capable of gliding motility, no conclusive evidence of motor structures has been obtained, although many such structures have been hypothesized (4).
Cyanobacteria are a large group of phototrophic prokaryotes of various morphologies, from simple unicellular organisms to complex filamentous forms capable of cellular differentiation (1, 2, 12). With one possible exception (15), cyanobacteria cannot swim, but many of the filamentous forms, such as members of the family Oscillatoriaceae, are capable of gliding motility (4). In most cases, forward movement is accompanied by revolution of the filament about its long axis, the direction of revolution being species specific (5). Following the performance of electron microscopy studies, Halfen and Castenholz (7) proposed a model for gliding that was based on the theories of Jarosch (13). They speculated that contractile fibrils in the cell wall, possibly consisting of protein, provided the motor for gliding in the Oscillatoriaceae, with the rotation of the filaments resulting from the helical arrangement of the fibrils. However, during a recent ultrastructural examination of the cell walls of four gliding filamentous cyanobacteria, Hoiczyk and Baumeister (9) were unable to identify any fibrillar structures, of the type described by Halfen and Castenholz (5, 7), beneath the outer membrane. However, they did observe a complex layer, external to the outer membrane, consisting of an array of helically arranged surface fibrils, with a regular spacing of 14 nm, positioned above a tetragonal S layer anchored to the outer membrane. An additional sheath layer, consisting of carbohydrate fibrils with a high degree of crystallinity, was also observed in old, immotile filaments (8). Hoiczyk and Baumeister concluded that the helical surface fibrils were unlikely to provide the motor for gliding but rather served as a screw thread, guiding the rotation of the trichome, with the power being derived from extrusion of slime from the junctional pores that form a ring around the filament at each cell septum (9, 11). They have since shown that the fibrils consist of a single calcium-binding protein that they call oscillin (10). Spontaneous, nonmotile mutants of Phormidium uncinatum fail to produce extracellular slime and lack the S layer and oscillin fibrils, implying that one or more of these components are essential for motility.
In the present article, we describe a highly regular array of parallel fibrils situated between the peptidoglycan and the outer membrane of several motile filamentous cyanobacteria. The individual fibrils of the array have a far larger diameter than any previously reported, leading us to conclude that they have not been previously observed. The correspondence between the angle of the fibrils with respect to the long axis of the filament, and the rotation of the filament during gliding, may imply an involvement of the fibrils in gliding motility.
MATERIALS AND METHODS
Cyanobacterial strains and culture conditions.
The Oscillatoria strains (as defined in reference 12) were isolated by the authors from fish tanks, but only Oscillatoria sp. strain A2 was grown in culture, the others being used immediately after sampling. The strains were assigned to the genus Oscillatoria rather than Phormidium (Lyngbya) because they did not produce an obvious sheath (12). Strain A2 was maintained on agar plates of BG11 medium (14) containing 17.6 mM NaNO3, solidified with 1.5% (wt/vol) purified agar (Oxoid). Plate cultures were grown at 30°C, at an incident light irradiance of 28 μmol m−2 s−1, and subsequently maintained at room temperature (approximately 20°C) at a low incident-light irradiance of approximately 3 μmol m−2 s−1. The experimental organism was derived from a single filament picked from an agar plate, but it was not freed of bacterial contamination. Indeed, individual filaments freed of bacteria failed to survive.
Preparation of samples for electron microscopy.
Samples were fixed in 3% (vol/vol) glutaraldehyde in 0.02 M KH2PO4 buffer for a minimum of 2 h. Fixed cells were washed three times for 10 min each in 0.02 M KH2PO4 buffer, postfixed for 1 h in 1% (wt/vol) osmium tetroxide in the same phosphate buffer, and then washed twice for 10 min each in distilled water. Samples were incubated in 2% aqueous uranyl acetate for 1 h and rinsed in distilled water. They were then dehydrated in a graded series of ethanol, resuspended three times for 20 min each in propylene oxide, and left overnight in 3:1 propylene oxide-TAAB embedding resin (medium mix; TAAB Laboratories Equipment Ltd., Aldermaston, United Kingdom); this was followed by incubation for 4 h in 2:1 propylene oxide-resin, 3 h in 1:1 propylene oxide-resin, and 3 h in neat resin. Samples were finally transferred to fresh resin in capsules and polymerized for 24 h at 60°C. Thin sections were mounted on Formvar-coated 200-mesh copper grids and then poststained for 2 h with 5% (wt/vol) uranyl acetate and for 7 min with Reynold’s lead citrate. For electron microscopy, a Philips model CM10 transmission electron microscope was used at 200 kV.
Negative staining.
Actively motile samples were crushed between two glass slides before being transferred to carbon-collodion-coated copper grids and stained with 4% (wt/vol) aqueous uranyl acetate for from 30 s to 2 min.
Freeze-etching.
Samples were fixed in 3% (vol/vol) glutaraldehyde in 0.02 M KH2PO4 buffer for a minimum of 2 h. Fixed cells were washed three times for 10 min each in 0.02 M KH2PO4 buffer and cryoprotected with glycerol in 0.02 M KH2PO4 by gradually increasing the concentration of glycerol to 30% (wt/vol) over a 5-h period. Samples were left overnight in 30% glycerol and resuspended in fresh 30% glycerol before being frozen in liquid nitrogen slush and stored in liquid nitrogen. Frozen samples were fractured and etched for 2 min at 173 K, and then a standard 2-nm platinum–20-nm carbon replica was laid down. The shadowing angle was 45°. Replicas were floated off on 30% glycerol in 0.02 M KH2PO4 buffer and cleaned with 40% (vol/vol) bleach overnight, followed by a 3-h incubation in neat bleach, two 10-min rinses in double-distilled water, a 3-h incubation in 30% chromic acid, and a final five washes in double-distilled water. The cleaned replicas were picked up on Formvar-coated 200-mesh copper grids and viewed in a Philips model CM10 electron microscope at 200 kV.
RESULTS
Transmission electron microscopy of thin sections.
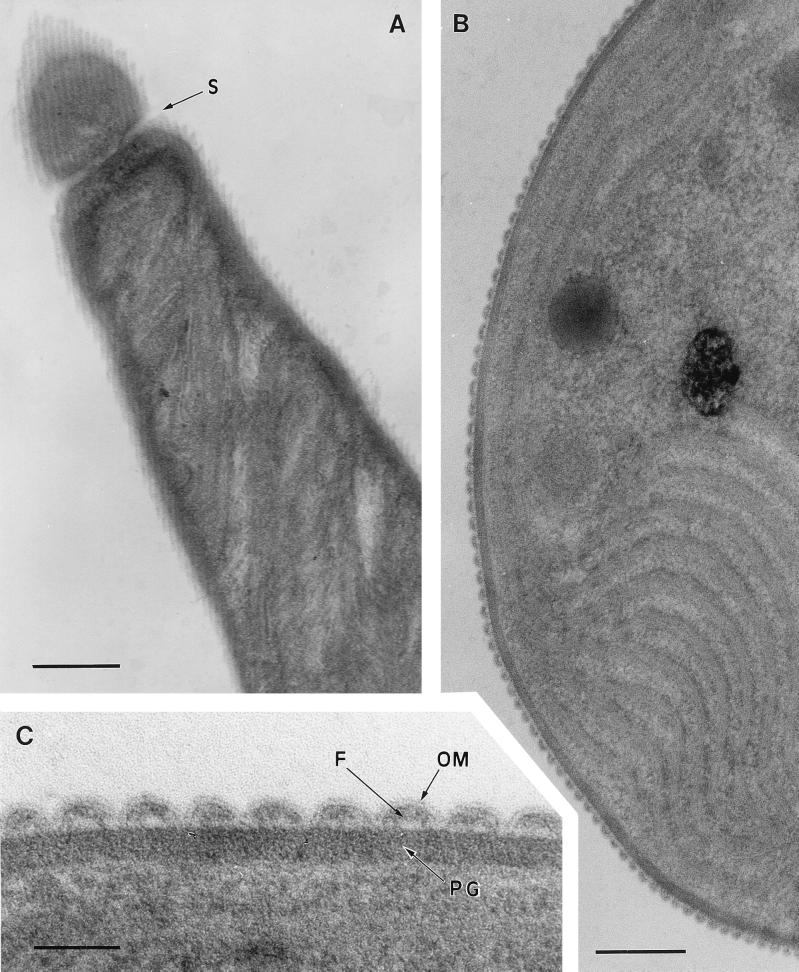
Where longitudinal thin sections grazed the surface layers of Oscillatoria sp. strain FT2 filaments, an array of parallel fibrils could be seen (Fig. 1A). Each fibril was approximately 25 to 30 nm wide and was separated from neighboring fibrils by an electron-transparent space of approximately 8 nm. The fibrils formed a parallel array at an angle of 25 to 30° to the long axis of the filament (Fig. 1A). Transverse thin sections confirmed the presence and size of these fibrils and revealed that they were sandwiched between a peptidoglycan layer with a thickness of approximately 30 nm and the outer membrane (approximately 5 nm thick) (Fig. 1B and C). The outer membrane formed invaginations between fibrils, leaving little if any gap between itself and the peptidoglycan (Fig. 1C). Approximately 200 fibrils surrounded the circumference of each cyanobacterial filament. The space occupied by the fibrils, between the outer membrane and the peptidoglycan, was not of uniform electron density (Fig. 1C).
FIG. 1.
Transmission electron micrographs of thin sections of Oscillatoria sp. strain FT2 filaments. (A) Longitudinal section. At the top of the figure, beyond a cell septum (S), the section has grazed the surface of the filament, revealing an array of parallel fibrils running at an angle of approximately 25 to 30° to the filament’s long axis. Bar, 400 nm. (B) Part of a transverse section, showing an end view of the fibrillar array in the cell wall. Bar, 200 nm. (C) Enlarged view of part of panel B, showing the double line of the outer membrane (OM) covering the fibrillar array and dipping between adjacent fibrils (F) to contact the electron-dense peptidoglycan layer (PG). Bar, 50 nm.
Transmission electron microscopy of negatively stained whole cells.
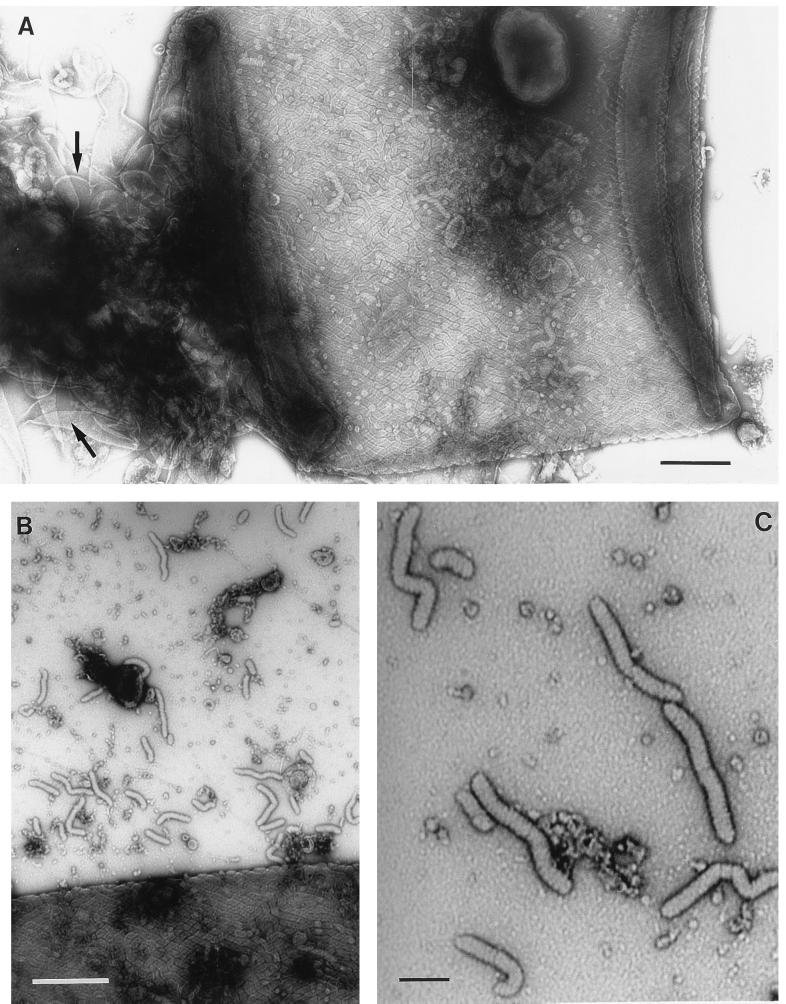
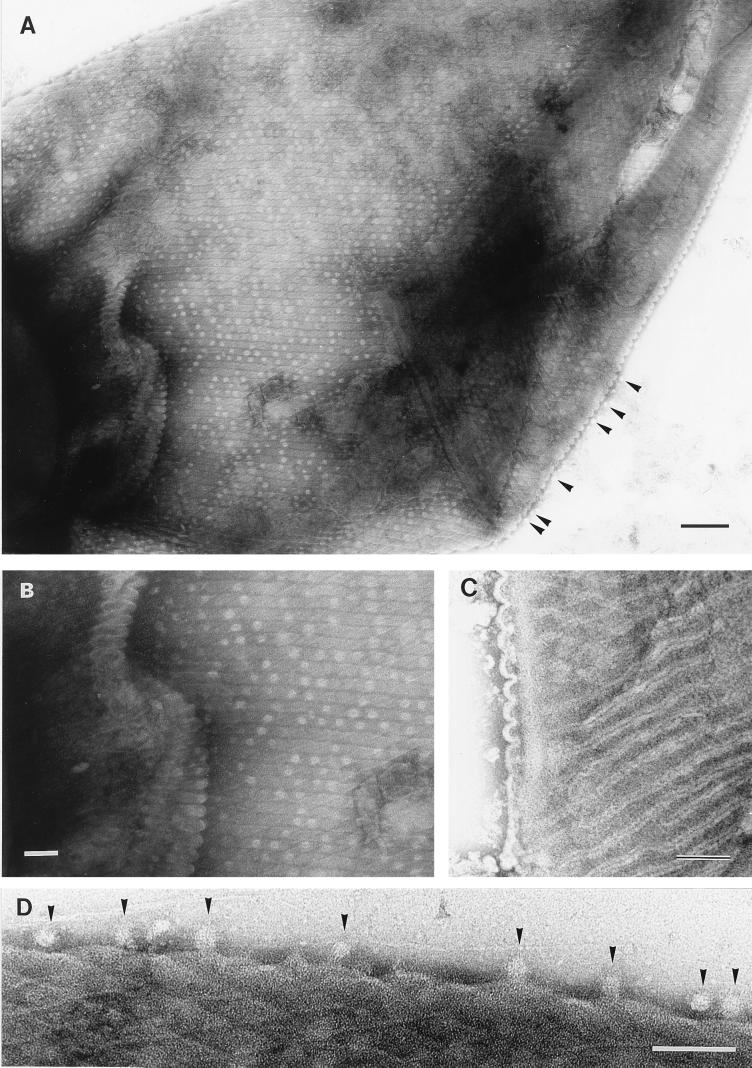
To avoid potential artifacts caused by the preparation of samples for thin sectioning, negative staining of whole cells was also used. The fibrils formed an array covering the entire filament, with the individual fibrils running continuously in a helix around the filament (Fig. 2). As a result of this helical arrangement, fibrils at opposite sides of each cell ran at opposing angles to the longitudinal axis of the cell, producing a criss-cross effect when the cell was flattened (Fig. 2, 3A, and 4A and B). The fibrils were separated from each other by an electron-dense line (Fig. 2 and 4A and B) that was sometimes bordered by electron-transparent lines (Fig. 4C). Where the fibrils encountered a cell septum, they appeared to be overlaid by a narrow (approximately 40-nm-wide) band (Fig. 2 and 5) but apparently continued, uninterrupted, on the other side of the septum (Fig. 5). In cells seriously damaged by the crushing process, fibril-free cell wall could be seen, together with many liberated fibrils (Fig. 3). The latter were of the same width as those present in situ in the cell wall but were mostly in short lengths of approximately 100 to 200 nm. In some cases, there was evidence of a substructure to the individual, free fibrils, in the form of striations at approximately right angles to the long axis of the fibril (Fig. 3C). In samples of Oscillatoria sp. strain FT1, the cell walls were covered with regularly arranged circular structures, approximately 20 nm in diameter, that were unstained except at the center (Fig. 4A, B, and D). These structures, located between the electron-dense lines that separated the fibrils, had a center-to-center spacing of approximately 45 nm and appeared to be external to the outer membrane. They are unlikely to be pores because they could be lost from the surface, leaving a gap where they would have been (Fig. 4A and B), and structures identical in size and appearance could be found free, external to the cell (Fig. 4A and D).
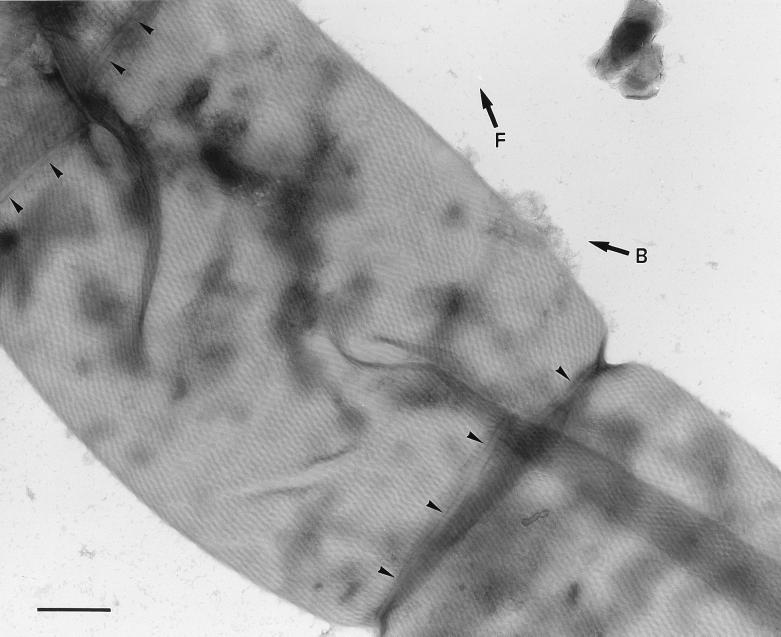
FIG. 2.
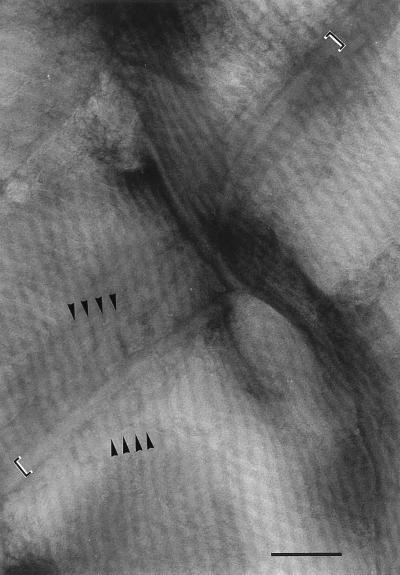
Transmission electron micrograph of part of a filament of Oscillatoria sp. strain FT3. An actively motile sample was crushed between glass slides and negatively stained. The micrograph shows several cells from which the contents have been extruded and the cell wall has been flattened, bringing the fibrils at the front and back of the filament into close contact and thus allowing them to be viewed simultaneously. The fibrils run helically around the entire surface of the filament, producing the observed criss-cross effect because those in the wall in the foreground run in the direction shown by the arrow marked F and those in the wall in the background run in the direction of the arrow marked B. The fibrils appear to cross the cell septa (at the top left and bottom right of the photo), although they are overlayed by a band with a width of approximately 40 nm (arrowheads). Bar, 500 nm.
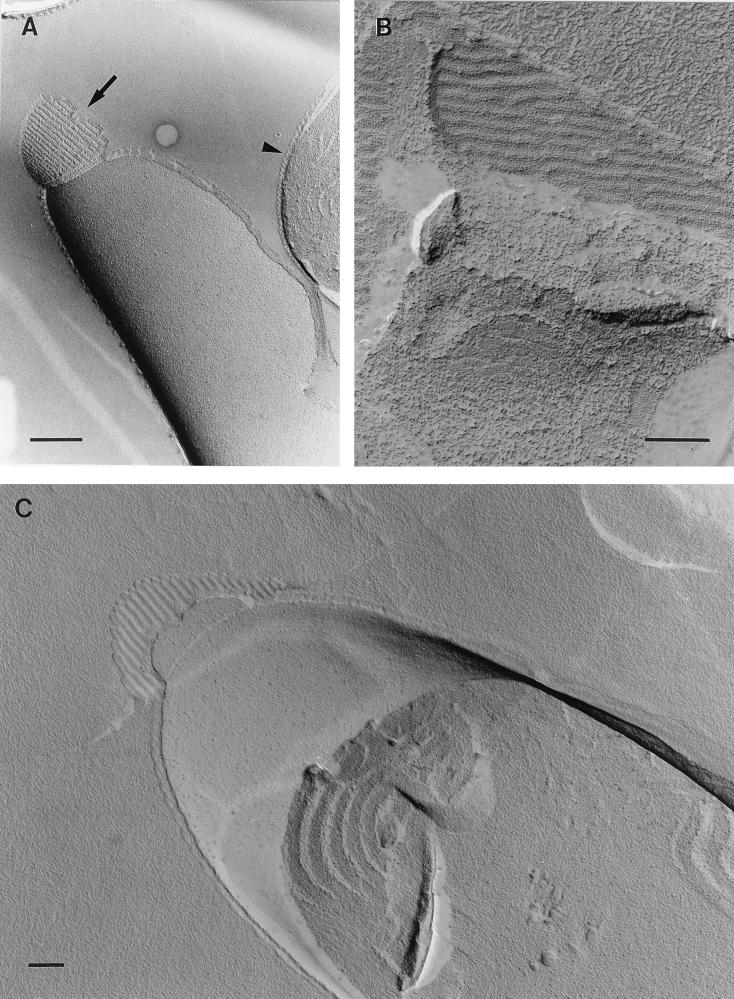
FIG. 3.
(A and B) Transmission electron micrographs of Oscillatoria sp. strain A2. Actively motile samples were crushed between glass slides and negatively stained. (A) Although an intact fibrillar array covering the cell can be seen, the crushing process has caused damage to the cell wall, leaving some areas (arrows) completely free of fibrils. Some of the fibrils released in this process can be seen in panel B, and some of them show possible evidence of a substructure (C). Bars, 400 nm (A and B) and 100 nm (C).
FIG. 4.
Transmission electron micrographs of Oscillatoria sp. strain FT1. Actively motile samples were crushed between glass slides and negatively stained. (A) The cell walls are covered with regularly arranged circular structures approximately 20 nm in diameter, which are unstained except at the center. These structures are located between the electron-dense lines that separate the fibrils and appear not to be pores, because they can be lost from the surface and seen free, external to the cell (arrowheads here and in panel D). Bar, 200 nm. (B) Shown is an enlarged view of part of panel A, revealing the circular structures that follow the line of each fibril. Bar, 100 nm. (C) The fibrils are separated by an electron-dense line bounded on each side by electron-transparent regions. The former is caused by the accumulation of stain in the cleft in the outer membrane, where it dips between each row of fibrils, and the latter is the unstained membrane itself, on either side of the cleft. These corrugations in the outer membrane can be seen to the left of the micrograph (and are illustrated in Fig. 7). Bar, 100 nm. (D) Enlarged view of part of the edge of the cell from the top left corner of panel A, showing the unstained, 20-nm-diameter circular structures (arrowheads) external to the cell. Bar, 100 nm.
FIG. 5.
Enlarged view of the top left corner of Fig. 2. The cell septum runs from the bottom left to the top right of the photograph. Each fibril is seen as a pale line bounded by two parallel dark lines, and at the point at which they cross the cell septum they appear to pass beneath a narrow band (indicated by the square brackets) that follows the line of the septum. The continuation of three of the fibrils, on either side of the septum, is shown by the arrowheads indicating the dark lines between the fibrils. Bar, 200 nm.
Freeze-etching.
Indentations in the exoplasmic fracture face of the outer membrane, caused by the fibrillar layer, were clearly visible in freeze-etched replicas (Fig. 6A and C). In some samples were seen what appeared to be undulations in the fibrillar array (Fig. 6B).
FIG. 6.
Transmission electron micrographs of freeze-etched replicas of Oscillatoria sp. strain A2. (A) Corrugations in the outer membrane, caused by the fibrillar array, can be seen in a transverse view (arrowhead) and in a longitudinal view in the exoplasmic fracture face of the membrane (arrow). (B) Apparent undulations in the fibrillar array are evident. (C) Indentations in the outer membrane, caused by the fibrillar array, can be seen in the exoplasmic fracture face of the membrane, confirming that the fibrillar array itself is external to the cytoplasmic membrane and peptidoglycan. Bars, 400 nm (A) and 200 nm (B and C).
DISCUSSION
Since the work of Halfen and Castenholz (5–7), little progress has been made in elucidating the mechanism of cyanobacterial gliding motility. In a recent ultrastructural examination of four gliding filamentous cyanobacteria, Hoiczyk and Baumeister (9) failed to identify any fibrillar structures, of the type described by Halfen and Castenholz, beneath the outer membrane. In contrast, the work presented here provides very clear ultrastructural evidence for a complex array of fibrils being located between the peptidoglycan layer and the outer membrane of several Oscillatoria spp. Negative staining of whole fresh cells, crushed between glass slides to extrude the cell contents, confirmed that the fibrils surround the entire filament (Fig. 2 to 4). The fibrils described by Halfen and Castenholz (7) were thought to be located on the surface of the peptidoglycan layer, in the same position as those reported here. However, there is a considerable difference in the widths of the respective fibrils, with those of Halfen and Castenholz being 5 to 8 nm wide (6) and those in the present study being 25 to 30 nm wide. It is clear, also, that the fibrils reported here are much larger than the 8- to 12-nm-wide surface fibrils reported by Hoiczyk and Baumeister (9, 10) and are in a completely different location. These observations led us to conclude that the fibrils described here have never been reported before. Nevertheless, the angle between the fibrils and the long axis of the filament determined by Halfen and Castenholz (7) (30°) and by Hoiczyk and Baumeister (9) (25°) is very similar to that measured in the present study (25 to 30°). It is known that cyanobacteria such as those used in these three studies rotate about their long axes as they glide, so that a point on the surface traces a helical path (5). This correspondence between the angle of the fibrils and the rotation of the filament has been taken as evidence that the fibrils are the motor for gliding, although they may simply play a passive role, guiding the movement of the filament. The regular arrangement of the fibrillar layer observed here and its consistent angle with the filament’s long axis may imply some underlying symmetry responsible for its organization. One possible source of such symmetry is the peptidoglycan itself, and some form of interaction between this layer and the fibrils might provide the necessary scaffolding for the fibrillar array.
The proposal by Halfen and Castenholz (7), that contractile-protein fibrils in the cell wall provide the motor for gliding could be applied to the fibrillar array described here, although there is presently no direct evidence for such a role. An alternative proposition, that the fibrillar layer provides additional strengthening for the cell wall, seems unlikely because these cyanobacteria already have peptidoglycan layers that are commonly 30 nm thick (Fig. 1C) and can have a thickness of over 500 nm in Oscillatoria princeps, which has filaments up to 100 μm in diameter. In addition, fibrils released from freshly crushed cells show great flexibility, being invariably bent, sometimes even into U shapes (Fig. 3). Negative staining of released fibrils showed no evidence of a hollow center but did give some indication of a substructure, implying that they may be composed of many subunits. Although fibrils seen in situ (e.g., Fig. 4A) appeared to be continuous, they fragmented into short lengths when released from the cell wall. Many of these fragments were of similar lengths (e.g., Fig. 3), possibly implying that breakage occurs at natural weak points or junctions.
An alternative to the contractile-fibril model of force generation for gliding is slime extrusion from junctional pores (9, 11). In such a model, the reversal of direction that commonly occurs when cyanobacteria glide could be explained by a change in the extrusion of slime from one set of junctional pores, pointing in one direction, to the set on the opposite side of the septum, pointing in the other direction. However, a potential problem with this model is the motility of Spirulina spp. These cyanobacteria have tightly coiled spiral filaments, and the junctional pores cover only part of the filament circumference in the concave region of the peptidoglycan, in the center of the spiral (3). It is difficult to envisage how slime extruded at the inner surface of a tight spiral could interact with the substratum in contact with the outside of the spiral.
In transverse thin sections of the Oscillatoria spp. examined here, the outer membrane appeared to dip between each row of fibrils, leaving little or no space between it and the peptidoglycan (Fig. 1C). This is unlikely to be an artifact caused by shrinkage during fixation and dehydration, because the same undulating outer membrane could be seen when live filaments were crushed and negatively stained without dehydration (Fig. 3A and 4). This was particularly apparent when areas of the cell wall of Oscillatoria sp. strain FT1 became folded (Fig. 4A and B). The circular, electron-transparent structures seen in these same samples (Fig. 4A, B, and D) may be proteins on the surface of the outer membrane, and the staining pattern would imply a ring-like structure with a hollow center. These are very unlikely to be S layer proteins, because of their wide spacing; nor do they seem to be pores, because of the apparent ease with which the individual structures could be lost, leaving a gap in the regular arrangement along the line of a fibril (Fig. 4A and B). Similar structures were seen in samples of Oscillatoria sp. strain A2 (Fig. 3A), but not in other cyanobacteria prepared in the same way, although they may be lost more easily from some strains, and no attempt was made to enhance release of the structures by, for example, sonication. Their significance is unknown, yet their symmetrical and spaced positioning with respect to the fibrils may imply some relationship or interaction with the fibrillar layer beneath the outer membrane.
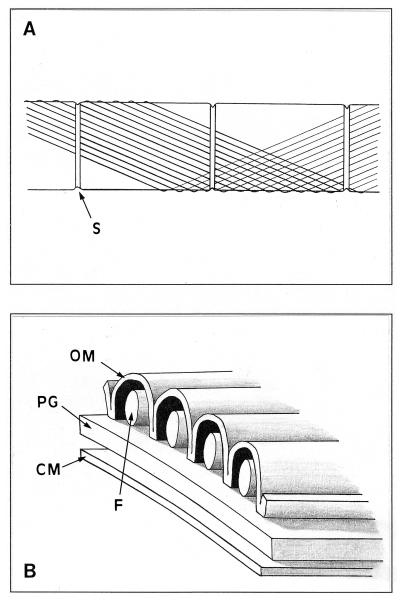
Our interpretation of the ultrastructural data presented here is given in Fig. 7. Although transmission electron micrographs of transverse thin sections of Oscillatoria strains were unable to reveal significant detail of the fibrils, the space between the invaginations of the outer membrane (Fig. 1B and C) was of the same dimensions as the fibrils seen in longitudinal sections (Fig. 1A) and in the crushed, negatively stained cells (Fig. 2 to 5). This led us to hypothesize that the fibrils are located between the outer membrane and the peptidoglycan layer. This conclusion is supported by the freeze-etched micrographs (Fig. 6). The fibrils have been drawn with a circular cross-section (Fig. 7B), although the resolution of the transverse thin sections (Fig. 1) is too poor to give a clear idea of their true shape. The outer membrane makes contact with the peptidoglycan between each fibril, and although no attempt has been made to draw any additional layers above the outer membrane, both an S layer and a fibrillar layer may be present (9), although they were not observed in the strains examined here.
FIG. 7.
Schematic diagrams showing the fibrillar array in the cell wall of Oscillatoria spp. (A) Part of a filament showing three cell septa (S). Each fibril follows a helical path at 25 to 30° to the long axis of the filament. For clarity, only a small number of fibrils and only one turn of the helix are shown here. At the cell septa, the fibrils pass beneath a band that encircles the septum. (B) Cross section of the cell wall, showing the arrangement of the fibrils (F) in relation to the outer membrane (OM), the cytoplasmic membrane (CM), and the peptidoglycan layer (PG).
It appears that the fibrils described here are continuous along the length of the filament, crossing cell septa (Fig. 2 and 5), implying that there is a continuous space between the peptidoglycan and the outer membrane along the entire filament. This seems at odds with the conclusion by Hoiczyk and Baumeister (9) that an invagination of the outer membrane (the circumferential junction) brings it into contact with the underlying peptidoglycan at the cell septa, thus separating the periplasmic space (between the peptidoglycan and outer membrane) of each cell from that of its neighbor. An additional problem is the presence of the junctional pores on either side of the cell septa. In the four motile cyanobacteria examined by Hoiczyk and Baumeister (9), these pores were approximately 14 to 16 nm in diameter and were separated by approximately 15 nm. Junctional pores were not evident in the electron micrographs presented here, but it seems highly likely that they are present in strain A2 and the other Oscillatoria spp. examined, and if the spacing between pores is also 15 nm, this does not leave sufficient space for 25- to 30-nm-wide fibrils to pass through as they traverse the septum. A more detailed understanding of the ultrastructure of the cell walls of these organisms is needed to resolve these problems. In addition, clarification of the role of the fibrillar array, whether in gliding motility or some other cell function, will require the isolation and chemical analysis of its individual components. This work is in progress.
ACKNOWLEDGMENT
We thank Paul McPhie for help with the negative staining of crushed filaments.
REFERENCES
- 1.Adams D G. Multicellularity in cyanobacteria. In: Mohan S, Dow C, Cole J A, editors. Prokaryotic structure and function: a new perspective. Cambridge, United Kingdom: Cambridge University Press; 1992. pp. 341–384. [Google Scholar]
- 2.Adams D G. Cyanobacteria. In: Shapiro J A, Dworkin M, editors. Bacteria as multicellular organisms. Oxford, United Kingdom: Oxford University Press; 1997. pp. 109–148. [Google Scholar]
- 3.Guglielmi G, Cohen-Bazire G. Structure et distribution des pores et des perforations de l’enveloppe de peptidoglycane chez quelques cyanobactéries. Protistologica. 1982;18:151–165. [Google Scholar]
- 4.Häder D-P, Hoiczyk E. Gliding motility. In: Melkonian M, editor. Algal cell motility. New York, N.Y: Chapman and Hall; 1992. pp. 1–38. [Google Scholar]
- 5.Halfen L N. Gliding motility in Oscillatoria: ultrastructural and chemical characterization of the fibrillar layer. J Phycol. 1973;9:248–253. [Google Scholar]
- 6.Halfen L N. Gliding movements. In: Haupt W, Feinleib M E, editors. Encyclopedia of plant physiology, new series. Vol. 7. Berlin, Germany: Springer-Verlag; 1979. pp. 250–269. [Google Scholar]
- 7.Halfen L N, Castenholz R W. Gliding in a blue-green alga, Oscillatoria princeps. J Phycol. 1971;7:133–145. [Google Scholar]
- 8.Hoiczyk E. Structural and biochemical analysis of the sheath of Phormidium uncinatum. J Bacteriol. 1998;180:3923–3932. doi: 10.1128/jb.180.15.3923-3932.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hoiczyk E, Baumeister W. Envelope structure of four gliding filamentous cyanobacteria. J Bacteriol. 1995;177:2387–2395. doi: 10.1128/jb.177.9.2387-2395.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hoiczyk E, Baumeister W. Oscillin, an extracellular, Ca2+-binding glycoprotein essential for the gliding motility of cyanobacteria. Mol Microbiol. 1997;26:699–708. doi: 10.1046/j.1365-2958.1997.5971972.x. [DOI] [PubMed] [Google Scholar]
- 11.Hoiczyk E, Baumeister W. The junctional pore complex, a prokaryotic secretion organelle, is the molecular motor underlying gliding motility in cyanobacteria. Curr Biol. 1998;8:1161–1168. doi: 10.1016/s0960-9822(07)00487-3. [DOI] [PubMed] [Google Scholar]
- 12.Holt J G, Krieg N R, Sneath P H, Staley J T, Williams S T, editors. Bergey’s manual of determinative bacteriology. 9th ed. Baltimore, Md: Williams and Wilkins; 1994. pp. 377–425. [Google Scholar]
- 13.Jarosch R. Grundlagen einer Schraubenmechanik des Protoplasmas. Protoplasma. 1963;57:448–500. [Google Scholar]
- 14.Rippka R, Deruelles J, Waterbury J B, Herdman M, Stanier R Y. Generic assignments, strain histories and properties of pure cultures of cyanobacteria. J Gen Microbiol. 1979;111:1–61. [Google Scholar]
- 15.Waterbury J B, Willey J M, Franks D G, Valois F W, Watson S W. A cyanobacterium capable of swimming motility. Science. 1985;230:74–76. doi: 10.1126/science.230.4721.74. [DOI] [PubMed] [Google Scholar]