OBJECTIVES:
To compare the clinical, laboratory, and hemodynamic parameters during hospitalization for patients with multisystem inflammatory syndrome in children (MIS-C), across the Original/Alpha and the Delta variants of severe acute respiratory syndrome coronavirus 2 infection.
DESIGN:
Retrospective cohort study.
SETTING:
Single-center quaternary children’s hospital.
PATIENTS:
Children with MIS-C admitted from May 2020 to February 2021(Original and Alpha variant cohort) and August 2021 to November 2021 (Delta variant cohort).
MEASUREMENTS AND MAIN RESULTS:
Continuous vital sign measurements, laboratory results, medications data, and hospital outcomes from all subjects were evaluated. Of the 134 patients (102 with Original/Alpha and 32 with Delta), median age was 9 years, 75 (56%) were male, and 61 (46%) were Hispanics. The cohort with Original/Alpha variant had more males (61% vs 41%; p = 0.036) and more respiratory/musculoskeletal symptoms on presentation compared with the Delta variant (p < 0.05). More patients in the Original/Alpha variant cohort received mechanical ventilation (16 vs 0; p = 0.009). Median hospital length of stay (LOS) was 7 days, and ICU LOS was 3 days for the entire cohort. ICU LOS was shorter in cohort with the Delta variant compared with the Original/Alpha variant (4 vs 2 d; p = 0.001). Only one patient had cardiac arrest, two needed extracorporeal membrane oxygenation, and two needed left ventricular assist device (Impella, Danvers, MA), all in the Original/Alpha variant cohort; no mortality occurred in the entire cohort. MIS-C cohort associated with the Delta variant had lower INR, prothrombin time, WBCs, sodium, phosphorus, and potassium median values (p < 0.05) during hospitalization compared with the Original/Alpha variants. Hemodynamic assessment showed significant tachycardia in the Original/Alpha variants cohort compared with the Delta variant cohort (p < 0.05).
INTERVENTIONS:
None.
CONCLUSIONS:
Patients with MIS-C associated with the Delta variants had lower severity during hospitalization compared with the Original/Alpha variant. Analysis of distinct trends in clinical and laboratory parameters with future variants of concerns will allow for potential modification of treatment protocol.
Keywords: critical illness, hemodynamic profile, laboratory markers, multisystem inflammatory syndrome in children, time series data
AT THE BEDSIDE
Severe multiple organ involvement in MIS-C is associated with rapid improvement over 3–5 days following early and aggressive multimodal anti-inflammatory therapies.
Clinical manifestation and outcomes for MIS-C are similar between patients affected with Original/Alpha and Delta viruses.
Few patients have persistent immune dysregulation despite targeted therapies, with associated prolonged hospitalization and need for extracorporeal therapies.
Multisystem inflammatory syndrome in children (MIS-C) is a life-threatening hyperinflammatory condition associated with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) (1). Since the first described case series in the United Kingdom, the condition has progressively been diagnosed in children across continents (2, 3). The severity of clinical presentation varies widely with many aspects of disease mimicking other inflammatory conditions, including Kawasaki disease (KD), KD shock syndrome, toxic shock syndrome, sepsis, macrophage activation syndrome, and murine typhus (2, 4, 5).
Although the clinical characteristics of patients with MIS-C upon admission and outcomes are now well defined, their day-to-day clinical course following admission has not been extensively described (6, 7). Additionally, data comparing clinical characteristics and outcomes in patients with MIS-C associated with the Original or Alpha variants with the Delta variant are lacking. The severe COVID-19 and MIS-C surge in the southern United States has allowed us to compare MIS-C data with the initial surges (Original/Alpha variants; first and second surges) and the third surge (Delta). Understanding disease evolution is vital to the clinical community caring for this relatively novel pathophysiologic disease state. We present and compare the time series data of pharmacological, laboratory, and hemodynamic profiles of children admitted with the diagnosis of MIS-C affected with Original/Alpha and Delta variants at our tertiary center.
MATERIALS AND METHODS
This retrospective cohort study examines all children less than 21 years admitted to Texas Children’s Hospital, Houston, TX, with a diagnosis of MIS-C, between May 2020 and February 2021 affected by the Original or Alpha variant, and between August and September of 2021 affected by the Delta variant. The association with SARS-CoV-2 variants and MIS-C cohorts is assumed based on local sequencing data, representing the predominant strain of SARS-CoV-2 across the two time periods (5/20–2/21 period, 20G was 50–66%; 8/21–9/21 period, Delta was approximately 100%). We used the U.S. Centers for Disease Control and Prevention (CDC) definition for the diagnosis of MIS-C to identify our cohort (7). The Baylor College of Medicine Institutional Review Board (protocol H-48161) approved the study with a waiver of consent.
The primary aim of this study was to compare the clinical, laboratory, and hemodynamic characteristics of patients with MIS-C associated with the Original/Alpha variant and the Delta variant. Our secondary aim was to compare and describe the clinical course of two subgroups of hospitalized patients—including patients admitted to a PICU during their hospitalization and those not requiring ICU admission (non-ICU) during their hospitalization. The criteria for admission to ICU include: 1) a need for escalation of care including the need for vasoactive medication, noninvasive, or invasive respiratory support, 2) concern for poor cardiac function on presentation, and 3) concern for worsening clinical condition requiring intensive monitoring.
The Sickbay platform (Medical Informatics, Houston, TX) obtains continuous vital sign measurements, laboratory results, and medication administration records from all subjects in intensive care and acute care beds. The vital signs obtained include the heart rate (HR), respiratory rate (RR), Spo2, invasive and noninvasive systolic, diastolic blood pressure (dBP), and mean blood pressure (mBP) sampled at 0.5 Hz. The Sickbay system facilitates the data storage and analysis using the MATLAB statistical and signal processing toolboxes (The MathWorks, Natick, MA). For vital sign analysis, the patient serves as their own control by establishing a referent value, computed as the average of the individual vital signs over a window of 6 hours immediately before the discontinuation of monitoring and computing the percent deviation from the referent at any given time during the hospitalization.
We evaluated serial laboratory and hemodynamic data over a 2-week period after admission to assess the trend and identify a time period to recovery. The laboratory data included in the analysis were grouped to represent system involvement:
Activation of the innate immune system (WBC, neutrophils, lymphocytes, and procalcitonin)
Gastrointestinal/liver dysfunction (albumin, aspartate aminotransferase, alanine transaminase, gamma glutamyl transferase, and alkaline phosphatase)
Cardiovascular (brain natriuretic peptide [BNP], troponin, and lactate), renal (blood urea nitrogen [BUN], creatinine, and electrolytes)
Coagulation (platelets, international normalized ratio [INR], partial thromboplastin time [PTT], prothrombin time [PT], thrombin time, fibrinogen, and d-dimer)
Inflammatory (ferritin, fibrinogen, and C-reactive protein [CRP])
Hematologic (WBC, hemoglobin, hematocrit, platelets, and mean corpuscular hemoglobin [MCH])
Laboratory data used represent the percentage of subgroup individuals with abnormal values on the day of testing. An internal clinical care algorithm was standardized by a taskforce that included rheumatology, cardiology, critical care medicine, and hematology early during the first MIS-C surge and has been consistently used throughout subsequent surges. This algorithm incorporated aspects of the American College of Rheumatology treatment recommendations yet followed a “step-down approach” in which early multimodal immunomodulation and targeted biologic therapy (anakinra) were initiated for all patients with shock or significant cardiac dysfunction (8).
To test the statistical significance of differences in laboratory values between MIS-C due to the Alpha and the Delta variants, we used the nonparametric two-sided Wilcoxon rank-sum test for nonnormally distributed data including the first set of laboratory results and the vital signs at admission. Holm-Bonferroni method was used to account for multiple outcomes measures to ensure that the familywise type I error rate is less than 0.05. Fisher exact test was used to compare the prevalence of coronary dilation between the subgroups (z score > 2). The body mass index (BMI) of the patients was transformed to age- and gender-adjusted percentiles using the growth chart from the CDC.
We also assessed the effect of ICU care and virus variant on the time-dependent trajectory of physiologic variables: laboratory results, ejection fraction (EF), and vital signs. For this purpose, we used the covariance of the data, estimated from the time series of residuals obtained after removing a 1-day rolling window average of the data, as a nonparametric representation of the mean response over time. Again, Holm-Bonferroni method was used to account for multiple outcomes measures to ensure that the familywise type I error rate is less than 0.05. Utilizing mixed-effects regression models of the residuals for each physiologic variable to account for the repetition of measurements over time for each patient to determine group differences (9). All analyses were performed using the MATLAB environment (The MathWorks).
RESULTS
Clinical Data
Patient demographics, shown in Table 1, demonstrate our cohort included 134 patients: 102 (76%) infected with the Original or Alpha variant and 32 (24%) infected with the Delta variants of the SARS-CoV-2 virus. The median age was 9 years (interquartile range [IQR], 5–13 yr) with 56% male. Most patients enjoyed previous health, and MIS-C symptoms were the primary reason for hospitalization for all the patients in the cohort. Fifty-seven percent of the cohort had a positive SARS-CoV-2 polymerase chain reaction tests within 30 days prior to admission. All patients had positive SARS-CoV-2 antibody tests at admission. The age- and gender-adjusted median BMIs were the 71st percentile (IQR, 42nd–94th percentile).
TABLE 1.
Summary of Demographics, COVID-19 Status, Clinical Presentation, and Hospitalization
| Cohort Size | All, n = 134 | Original/Alpha, n = 102 | Delta, n = 32 | p |
|---|---|---|---|---|
| Demographics | ||||
| Age (yr), median (IQR) | 9 (5–13) | 9 (5–13) | 10 (6–13) | 0.448 |
| Body mass index (Centers for Disease Control and Prevention percentile), median (IQR) | 71 (42–94) | 73 (47–95) | 58 (30–86) | 0.152 |
| Gender (male), n (%) | 75 (56) | 62 (61) | 13 (41) | 0.036a |
| COVID status | ||||
| Positive COVID-19 polymerase chain reaction test within prior 30 d, n (%) | 76 (57) | 59 (58) | 17 (53) | 0.394 |
| COVID-19 antibody test (reactive), n (%) | 134 (100) | 102 (100) | 32 (100) | 1.000 |
| Contact with COVID-19 positive person, n (%) | 106 (79) | 74 (73) | 32 (100) | < 0.001a |
| Clinical presentation | ||||
| Gastrointestinal, n (%) | 119 (89) | 93 (91) | 26 (81) | 0.112 |
| Mucocutaneous, n (%) | 104 (78) | 81 (79) | 23 (72) | 0.254 |
| Hematologic, n (%) | 97 (72) | 74 (73) | 23 (72) | 0.553 |
| Cardiac, n (%) | 96 (72) | 72 (71) | 24 (75) | 0.405 |
| Renal/electrolyte abnormalities, n (%) | 85 (63) | 60 (59) | 25 (78) | 0.036a |
| Musculoskeletal, n (%) | 64 (48) | 55 (54) | 9 (28) | 0.009a |
| Neurologic, n (%) | 55 (41) | 39 (38) | 16 (50) | 0.165 |
| Respiratory, n (%) | 49 (37) | 45 (44) | 4 (13) | < 0.001a |
| Shock, n (%) | 37 (28) | 26 (25) | 11 (34) | 0.223 |
| Suspected Kawasaki-like disease, n (%) | 31 (23) | 30 (29) | 1 (3) | < 0.001a |
| Suspected lower respiratory tract infection, n (%) | 11 (8) | 9 (9) | 2 (6) | 0.486 |
| Suspected central nervous infection, n (%) | 6 (4) | 5 (5) | 1 (3) | 0.559 |
| Hospitalization | ||||
| Transferred to ICU, n (%) | 93 (69) | 70 (69) | 23 (72) | 0.455 |
| Intubation, n (%) | 16 (12) | 16 (16) | 0 (0) | 0.009a |
| Entire cohort LOS (d), median (IQR) | 7 (5–8) | 7 (5–9) | 6 (5–7) | 0.064 |
| Non-ICU subgroup LOS (d), median (IQR) | 5 (4–6) | 5 (4–7) | 5 (4–6) | 0.603 |
| ICU subgroup LOS (d), median (IQR) | 8 (5–9) | 8 (6–10) | 6 (5–8) | 0.015a |
| ICU subgroup LOS in ICU (d), median (IQR) | 3 (2–5) | 4 (3–6) | 2 (2–3) | 0.001a |
IQR = interquartile range, LOS = length of stay.
The adjusted statistical significance according to the Holm-Bonferroni method to account for multiple outcomes measures to ensure that the familywise type I error rate is < 0.05.
Gastrointestinal symptoms (nausea, vomiting, diarrhea, and abdominal pain).
Mucocutaneous symptoms (conjunctivitis or eye pain, skin rash, erythema or edema of extremities, lip swelling or redness, and swollen lymph nodes).
Respiratory (cough, sputum production, hemoptysis, shortness of breath, wheezing, sore throat, rhinorrhea, tachypnea, and retractions).
Neurologic symptoms (headache, altered mental status, and seizure).
Shock (need for fluid or vasoactive resuscitation).
p values correspond to comparison between the Alpha and Delta variant using the nonparametric Wilcoxon rank-sum test for continuous variables and Fisher exact test for categorical variables.
Comparing MIS-C patients between the Original/Alpha variant and the Delta variant, there were more males (61% vs 41%; p = 0.036). Cohort with the Original/Alpha variant presented with more musculoskeletal (54% vs 28%; p = 0.009) and respiratory (44% vs 13%; p < 0.001) symptoms, and was more likely to have suspicion of Kawasaki-like disease (29% vs 3%; p < 0.001). All patients in the Delta variant cohort had contact with person with COVID-19 (100% vs 73%; p < 0.001).
Comparison of median hospital length of stay (LOS) showed a trend toward shorter stay in the Delta cohort (6 d [IQR, 5–7 d] vs 7 d [IQR, 5–9 d]; p = 0.06). Although there was no difference between the cohort for ICU admissions (69% vs 72%; p = 0.45), cohort with the Delta variant had shorter median ICU LOS (2 d [IQR, 2–3 d] vs 4 d [IQR, 3–6 d]; p = 0.001). Sixteen patients in the Original/Alpha variant cohort received intubation compared with none in the Delta variant cohort (p = 0.009).
Evaluation of longitudinal data shows that on hospital day 1, 59% of the patients resided in the ICU and 41% in non-ICU settings. By day 7, 17% of the entire cohort remained in the ICU, 40% remained hospitalized not in the ICU, and 43% were discharged home. By day 14, 3% of the cohort remained in the ICU, 7% in non-ICU, and 90% discharged home. Of those patients admitted to the ICU, 12% received intubation, and cardiac arrest occurred in 1%. In the ICU patients, 2% received extracorporeal membrane oxygenation (ECMO), 2% received an Impella left ventricular assist device (one as stand-alone left ventricular mechanical circulatory support device and one for left ventricular decompression), and 2% received continuous renal replacement therapy (dialysis). All patients were discharged alive.
Laboratory Data
Supplemental Table 1 (http://links.lww.com/PCC/C76) displays the effects of virus variant (original or Alpha vs Delta) and physical unit of care (non-ICU vs ICU) on laboratory values in relation to the mean trajectory of these values for the entire cohort over time. The reference variables were the Original or Alpha variant and the non-ICU level of care. The intercept estimates the deviation from the mean response for this reference group. The ICU and Delta variables estimate the association in comparison with the reference group.
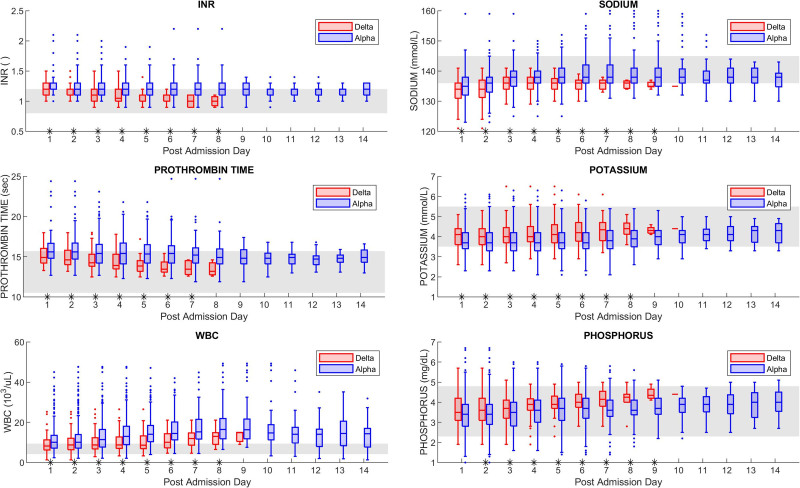
We observe, when controlling for level of care, the Delta variant cohort shows lower values for the INR, PT, WBC, sodium, and phosphorus levels, and higher values for the potassium levels (all p < 0.05). Conversely, when controlling for the virus variant, patients admitted to the ICU exhibit (in comparison to the non-ICU group) higher values for the INR, PT, CRP, BNP, troponin I, WBC, procalcitonin, neutrophils, BUN, MCH, and mean corpuscular volume labs, and lower values for the albumin, phosphorus, and RBC (all p < 0.05). The time series evolution of significant laboratory values between the Original/Alpha variant cohort and the Delta variant cohort during the hospital stay is shown in Figure 1.
Figure 1.
Day-by-day significant laboratory values for the Original/Alpha and Delta subgroups. The gray band denotes the reference range for each laboratory. Asterisks on the time axis denote a statistically significant difference between the Alpha and Delta groups according to the nonparametric two-sided Wilcoxon rank-sum test (p < 0.05). INR = international normalized ratio.
The trend of abnormal data during the hospital stays for the entire cohort, with comparison between ICU and non-ICU groups, is shown in Supplemental Figure 1 (http://links.lww.com/PCC/C77; legend, http://links.lww.com/PCC/C89) and Supplemental Figure 2 (http://links.lww.com/PCC/C78; legend, http://links.lww.com/PCC/C89). Supplemental Table 2 (http://links.lww.com/PCC/C79) compares the initial laboratory values between the ICU and non-ICU subgroups. Supplemental Figure 3 (http://links.lww.com/PCC/C80; legend, http://links.lww.com/PCC/C89), Supplemental Figure 4 (http://links.lww.com/PCC/C81 legend, http://links.lww.com/PCC/C89), Supplemental Figure 5 (http://links.lww.com/PCC/C82; legend, http://links.lww.com/PCC/C89), Supplemental Figure 6 (http://links.lww.com/PCC/C83; legend, http://links.lww.com/PCC/C89), and Supplemental Figure 7 (http://links.lww.com/PCC/C84; legend, http://links.lww.com/PCC/C89) compare the day-to-day laboratory values across different organ systems between the ICU and non-ICU subgroups.
Fibrinogen and ferritin were the first markers to show rapid resolution in patients with clinical improvement. CRP and ferritin remained elevated for longer duration, particularly in patients with prolonged hospitalization. Analyses of innate and adaptive immune activation laboratory markers reveal an elevated WBC, neutrophilia, lymphopenia, and procalcitonin on presentation, with rapid improvement for the non-ICU group. However, these markers, particularly WBC, remained elevated for a longer period in the ICU group. WBC increased up to hospitalization day 5 with subsequent resolution.
Coagulation abnormalities were initially present in 90% of the cohort with elevated d-dimer, hyperfibrinogenemia and thrombocytopenia being the most prevalent laboratory findings. Fibrinogen levels improved by days 4 and 5 in most patients. The ICU group had more persistent abnormalities in the PTT, INR, PT, and thrombin time, reflecting the persistent coagulation abnormalities in this high-risk subgroup.
Markers of cardiac injury and inflammation (BNP and troponin) remained elevated for up to 4 days before improving in the majority. Elevated cardiac markers were twice as prevalent in the ICU group compared with the non-ICU group upon admission, with statistically higher levels in the ICU group for up to 7 days of hospitalization. Echocardiographic assessment shows that 21% of the ICU and 9% of the non-ICU patients had low EFs (<55%), with cardiac function returning to normal by day 8 for most of the ICU group and by day 6 for the non-ICU group. Coronary artery dilation (z score >2) was present in 34% of patients at some point during their hospitalization.
Overall, there was a trend toward improvement in abnormal laboratory markers by days 3–5 after initiation of protocolized targeted immune-modulator therapies for the entire cohort.
Hemodynamic Data
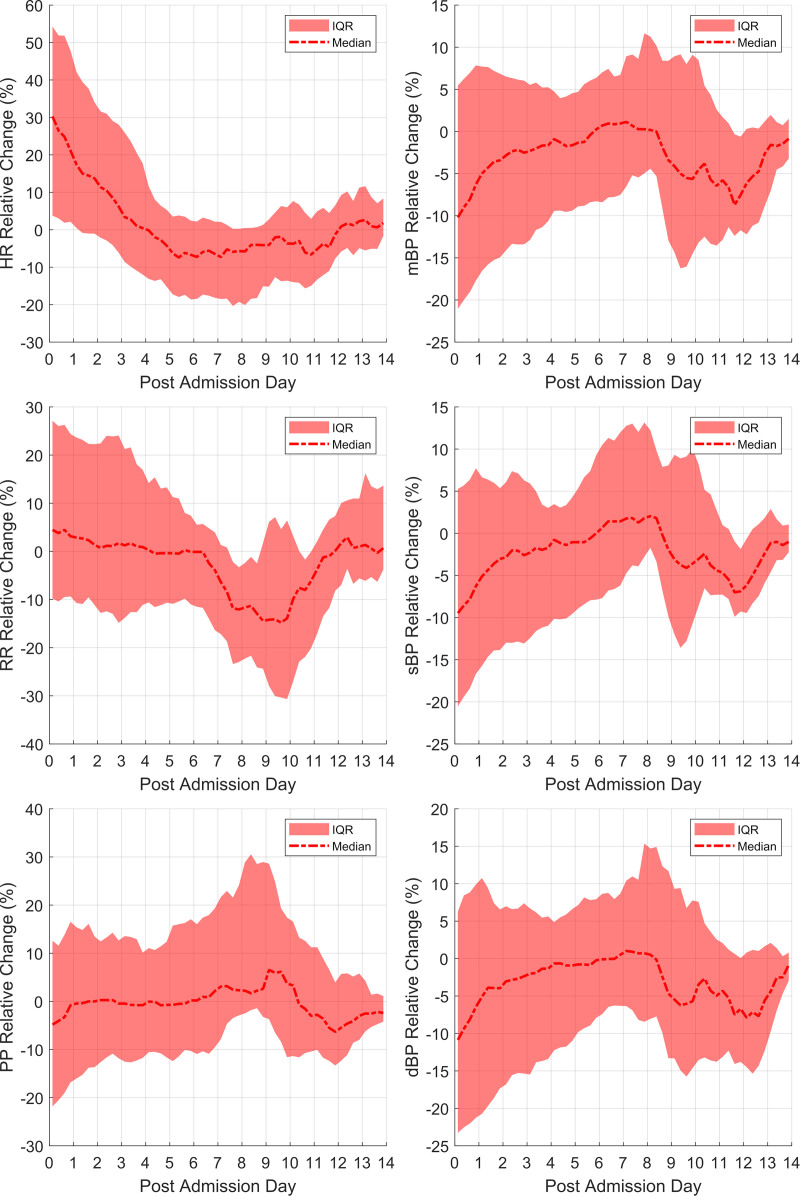
Analysis of available hemodynamic data for the cohort is shown in Figure 2. The mean and sd of the raw vital signs at admission and discharge for entire cohort are summarized and compared using paired t tests in Supplemental Table 3 (http://links.lww.com/PCC/C85). Patients with the Original/Alpha variant cohort show elevated HR compared with patients with the Delta variant (p = 0.032) (Supplemental Table 1, http://links.lww.com/PCC/C85). There were no differences in other hemodynamic parameters between the two variant cohorts.
Figure 2.
Change in vital signs relative to the values at discharge from hospital. These plots were generated from the vital sign data produced by all patients in the study. dBP = diastolic blood pressure, HR = heart rate, IQR = interquartile range, mBP = mean blood pressure, PP = pulse pressure, RR = respiratory rate, sBP = systolic blood pressure.
Evaluation of hemodynamic data for the entire cohort reveals that HR is statistically higher at admission (p < 0.001), with a median HR 30% higher than discharge values. Tachycardia improved by day 4 to predischarge referent values for the cohort.
The blood pressure (BP) values, mBP and dBP, are significantly lower at admission than discharge referent (p < 0.05). Half of the cohort presented with BP at least 10% below referent, with 26% of the cohort having severe hypotension (at least 20% below referent). Median BP values tend to normalize by day 6 before showing another fall by day 10 of admission for patients with a longer hospital stay. The second fall in BP was not associated with significant HR changes.
Tachypnea existed in most of the cohort at admission, with many of the patients presenting an RR elevated by 10% from referent. This improved by day 6 to predischarge values for the cohort before showing a drop for some patients around day 10 of admission in patients who required a longer hospital stay.
Pharmacological Interventions
Corticosteroids (predominantly methylprednisolone) were the primary anti-inflammatory agent prescribed to 87% of the ICU group and 53% of the non-ICU group during the first day of admission (Supplemental Fig. 8, http://links.lww.com/PCC/C86; legend, http://links.lww.com/PCC/C89). All ICU patients and 88% of non-ICU patients received corticosteroids, with gradual taper, at some point during their hospitalization.
Ninety-three percent of the ICU patients and 66% of the non-ICU patients were administered one or more immunomodulators at some point during their hospitalization. Anakinra was predominantly used in the ICU patients with severe clinical presentation (67% in ICU group and 9% in non-ICU group), whereas IV immunoglobulin (IVIG) was used in 73% within the ICU group and 66% in the non-ICU group (Supplemental Table 4, http://links.lww.com/PCC/C87). was used in 73% within the ICU group and 66% in the non-ICU group. Over the entire cohort, 92% of the patients received prophylactic anticoagulation, given the coagulopathy risk associated with COVID-19.
On day 1, all patients required volume expansion (normal saline, lactated ringer, or 5% albumin) in the ICU group and 97% in the non-ICU group. Volume expansion was gradually discontinued over the 14 days. Fifty-seven percent of ICU patients were administered vasoactive-inotropic agents (epinephrine, norepinephrine, milrinone, vasopressin, dopamine, or dobutamine) on day 1. Forty percent of ICU patients and 13% in non-ICU patients required diuretics (loop diuretics or thiazides) during hospitalization. Details of anti-infective agents are detailed in Supplemental Table 5 (http://links.lww.com/PCC/C88).
DISCUSSION
We compare the clinical, laboratory, and hemodynamic time series data from 134 patients diagnosed with MIS-C at a single tertiary center in the United States, associated with three distinct waves of COVID-19 infection. Although the acuity of initial clinical presentation is high, with greater than 50% requiring admission to ICU, the severity of organ involvement and clinical outcomes are similar between the patients affected by the Original/Alpha and the Delta variants of SARS-CoV-2 infection. Consistent with previously published reports, our cohort predominantly included previously healthy, young children (6, 10).
Our study shows that there are differences in organ involvement at presentation between the cohorts affected by two SARS-CoV-2 variants, including laboratory and hemodynamic parameters. However, there is rapid improvement in laboratory and hemodynamic parameters by days 3–5 of hospital admission with corticosteroid, IVIG, and targeted biologic therapy in majority of patients. Though targeted immunomodulator therapies are the mainstays for treatment of postinfectious MIS-C, it is imperative for clinicians to treat infectious sepsis.
Patients demonstrate similar characteristics regardless of the associated SARS-CoV-2 variant driving the pathophysiology. This is the first study that describes the time series data depicting evolution of disease pathophysiology in patients with MIS-C associated with three different variants of SARS-CoV-2 affecting children in the Southern United States. Despite profound critical illness in some patients, including the need for extracorporeal therapies, there were no inhospital deaths in our cohort.
The Delta variant of SARS-CoV-2 was found to be more transmissible compared with the Alpha variant across the population, including children, though the degree of virulence and associated variance in clinical manifestation remain unknown (20). Our data show that although there are few statistically significant laboratory values associated with Delta variant, overall clinical and hemodynamic variables are similar between the two variants. The shorter LOS and lack of need for mechanical or ECMO support in the Delta cohort were unexpected and not well understood. We do not believe these differences are related to treatment regimens, as these were standardized early in the pandemic. In a recently published data showing increased severity of clinical symptoms in MISC patients affected with the Alpha variant of SARS-CoV-2 in comparison with the original variant, our study shows that MIS-C patients with the Delta variant had lower disease severity (21). With anticipated emerging SARS-CoV-2 variants of concerns on the horizon, future studies comparing the clinical and laboratory values between variants of concern are warranted.
The pathophysiological presentation of severe cases of MIS-C has been that of distributive shock with multiple organ failure (6, 10, 11). Children in our cohort experienced cardiac involvement, including elevated troponin, depressed cardiac function, and occasionally dilated coronary arteries. Children in our cohort demonstrated improvement in cardiac function following anti-inflammatory therapies, suggesting immunologic/inflammatory insult resulting in cardiac involvement, similar to that reported in previously published case series (12–14). This trend to resolution over 3–5 days from presentation, which is similar between different epochs, could help clinicians anticipate a timeline for recovery, allowing them to identify patients with a tendency for prolonged critical illness based on a lack of rapid recovery of hemodynamic and laboratory parameters. Future studies are needed to determine the correlation between the rate of change of laboratory and hemodynamic markers of cardiac injury and time to myocardial recovery.
Although the exact pathophysiology is still unknown, MIS-C is likely to be a consequence of postinfectious immune dysregulation (15–17). Our study found significantly higher initial levels of ferritin, CRP, and procalcitonin associated with patients requiring ICU admission on presentation. The ensuing systemic hyperinflammatory state, along with the clinical manifestation of associated hemodynamic instability, has some similarities to KD shock syndrome. Yet, it also resembles the more innate immunity-driven process that approximates toxic shock syndrome (6, 18, 19). All clinical parameters improved with medical support and immunomodulatory therapies, though a few patients required mechanical circulatory support despite immunomodulator therapies. It is imperative to note that patients with slow resolution of inflammatory markers represent the most critically ill subgroup, with high likelihood of requiring hemodynamic support in the form of vasoactive and/or mechanical circulatory support.
Our study has some limitations. This is a single-center cohort study, and our results may not be generalizable to other centers. The sequencing data for SARS-CoV-2 were not available for all patients. Not all laboratory data were collected for all patients exhibiting clinical recovery, consistent with the standard of care. Although continuous hemodynamic data were available within the ICU, the frequency of monitoring was lower on the floor/acute care units. In addition, high troponin levels were no longer the reason for ICU admission between the three surges of SARS-CoV-2, representing a change in admission criteria to ICU in patients affected with Delta variants. In the absence of a baseline at a prehospitalization healthy state, predischarge hemodynamic values representing the least critical state for these patients were used as referent values. Our center’s MIS-C task force operationalized a standardized “step down” regimen that initiated biologic therapy earlier than other published guidelines (at presentation often and prior to failing corticosteroids and/or IVIG). This approach may not be used at many centers. We cannot rule out the possibility of prior infection with SARS-CoV-2, providing some degree of protection from severe disease. Finally, it is important to acknowledge the overlapping and undifferentiating clinical presentation with MIS-C, acute COVID, and other infectious illness. We followed the current CDC guidelines for identifying our cohorts with MIS-C. Despite these limitations, this study includes clinical and hemodynamic parameters over successive days of admission on the largest cohort of MIS-C patients at a tertiary center to date. Presented data may guide clinicians regarding the anticipated course of illness, the need for support of organ dysfunction, and time to recovery. Data analytics may allow for future research efforts and quality improvement initiatives.
CONCLUSIONS
Our study shows lower disease severity in MIS-C patients with Delta variant of SARS-CoV-2 compared with Original or Alpha variant. Although there is high acuity with initial clinical manifestations in patients with MIS-C, clinicians should expect rapid recovery in clinical, hemodynamic, and laboratory parameters over 3–5 days following initiation of targeted and multimodal therapies. Future studies comparing timing of various immunomodulator therapies and associated clinical course will help identify the optimal timing of medication administration.
RESEARCH IN CONTEXT
The comparison of clinical course and outcomes in patients with MIS-C associated with evolving SARS-CoV-2 strains is lacking.
A recent report showed increased severity of illness in patients with MIS-C across first two waves of SARS-CoV-2 variants.
Patients with MIS-C associated with the Delta variant of SARS-CoV-2 showed lower disease severity compared with the Original/Alpha variant.
Supplementary Material
Footnotes
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (http://journals.lww.com/pccmjournal).
Supported, in part, by the National Institute of Health - National Institute for Child Health & Development Award number: 1R61HD105593. The National Institutes of Health had no role in the study design, collection/analysis/interpretation of the data, writing of this manuscript, and the decision to submit the article for publication. All authors had full access to the data presented in this study and accept responsibility to submit it for publication.
Dr. Jain’s institution received funding from the National Institute for Child Health and Human Development (1R61HD105593). Drs. Jain, Annapragada, Muscal, Vogel, and Rusin received support for article research from the National Institutes of Health (NIH). Dr. Annapragada’s institution received funding from the NIH (R61HD105593); he received funding from Alzeca and Sensulin; he disclosed that he is the inventor on numerous patents that are either licensed to or assigned to his employer, past employers, or other entities. Drs. Muscal and Vogel disclosed the off-label product use of Anakinra. Dr. Vogel’s institution received funding from the NIH; she disclosed the off-label product use of IV immunoglobulin for multisystem inflammatory syndrome in children. Dr. Rusin received funding from Medical Informatics Corp; he disclosed that he is the cofounder and Chief Technology Officer of Medical Informatics, and he disclosed that his wife is the cofounder and Chief Executive Officer of Medical Informatics. The remaining authors have disclosed that they do not have any potential conflicts of interest.
REFERENCES
- 1.Ahmed M, Advani S, Moreira A, et al. : Multisystem inflammatory syndrome in children: A systematic review. EClinicalMedicine 2020; 26:100527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Whittaker E, Bamford A, Kenny J, et al. ; PIMS-TS Study Group and EUCLIDS and PERFORM Consortia: Clinical characteristics of 58 children with a pediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2. JAMA 2020; 324:259–269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jiang L, Tang K, Levin M, et al. COVID-19 and multisystem inflammatory syndrome in children and adolescents. Lancet Infect Dis. 2020; 20:e276–e288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Harwood R, Allin B, Jones CE, et al. ; PIMS-TS National Consensus Management Study Group: A national consensus management pathway for paediatric inflammatory multisystem syndrome temporally associated with COVID-19 (PIMS-TS): Results of a national Delphi process. Lancet Child Adolesc Health 2021; 5:133–141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Greene AG, Saleh M, Roseman E, et al. : Toxic shock-like syndrome and COVID-19: Multisystem inflammatory syndrome in children (MIS-C). Am J Emerg Med 2020; 38:2492.e5–2492.e6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Feldstein LR, Rose EB, Randolph AG: Multisystem inflammatory syndrome in children in the United States. Reply. N Engl J Med 2020; 383:1794–1795 [DOI] [PubMed] [Google Scholar]
- 7.Centers for Disease Control and Prevention: Information for Healthcare Providers About Multisystem Inflammatory Syndrome in Children (MIS-C). Available at: https://www.cdc.gov/mis-c/hcp/. Accessed April 26, 2022
- 8.Henderson LA, Canna SW, Friedman KG, et al. : American College of Rheumatology clinical guidance for multisystem inflammatory syndrome in children associated with SARS-CoV-2 and hyperinflammation in pediatric COVID-19: Version 1. Arthritis Rheumatol 2020; 72:1791–1805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Diggle P, Diggle PJ, Heagerty P, et al. Analysis of Longitudinal Data. Oxford, Oxford University Press, 2002 [Google Scholar]
- 10.Godfred-Cato S, Bryant B, Leung J, et al. ; California MIS-C Response Team: COVID-19-associated multisystem inflammatory syndrome in children - United States, March-July 2020. MMWR Morb Mortal Wkly Rep 2020; 69:1074–1080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kaushik S, Aydin SI, Derespina KR, et al. Multisystem inflammatory syndrome in children associated with severe acute respiratory syndrome coronavirus 2 infection (MIS-C): A multi-institutional study from New York city. J Pediatr 2020; 224:24–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Most ZM, Hendren N, Drazner MH, et al. : Striking similarities of multisystem inflammatory syndrome in children and a myocarditis-like syndrome in adults: Overlapping manifestations of COVID-19. Circulation 2021; 143:4–6 [DOI] [PubMed] [Google Scholar]
- 13.Guo T, Fan Y, Chen M, et al. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19). JAMA Cardiol 2020; 5:811–818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Belhadjer Z, Méot M, Bajolle F, et al. : Acute heart failure in multisystem inflammatory syndrome in children in the context of global SARS-CoV-2 pandemic. Circulation 2020; 142:429–436 [DOI] [PubMed] [Google Scholar]
- 15.Dasgupta K, De P, Finch SE. The present state of understanding of multisystem inflammatory syndrome in children (MIS-C) associated with SARS-CoV-2 infection–a comprehensive review of the current literature. South Dakota Med 2020; 73:510–519 [PubMed] [Google Scholar]
- 16.Nakra NA, Blumberg DA, Herrera-Guerra A, et al. : Multi-system inflammatory syndrome in children (MIS-C) following SARS-CoV-2 infection: Review of clinical presentation, hypothetical pathogenesis, and proposed management. Children (Basel) 2020; 7:E69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sood M, Sharma S, Sood I, et al. Emerging evidence on multisystem inflammatory syndrome in children associated with SARS-CoV-2 infection: A systematic review with meta-analysis. SN Compr Clin Med 2021 Jan 7. [online ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cattalini M, Della Paolera S, Zunica F, et al. ; Rheumatology Study Group of the Italian Pediatric Society: Defining Kawasaki disease and pediatric inflammatory multisystem syndrome-temporally associated to SARS-CoV-2 infection during SARS-CoV-2 epidemic in Italy: Results from a national, multicenter survey. Pediatr Rheumatol Online J 2021; 19:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Consiglio CR, Cotugno N, Sardh F, et al. ; CACTUS Study Team: The immunology of multisystem inflammatory syndrome in children with COVID-19. Cell 2020; 183:968–981.e7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Siegel DA, Reses HE, Cool AJ, et al. Trends in COVID-19 cases, emergency department visits, and hospital admissions among children and adolescents aged 0-17 years — United States, August 2020-August 2021. MMWR Recomm Reports 2021; 70:1249–1254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harahsheh AS, Sharron MP, Bost JE, et al. ; Children’s National Hospital MIS-C Taskforce: Comparison of first and second wave cohorts of multisystem inflammatory disease syndrome in children. Pediatr Infect Dis J 2022; 41:e21–e25 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.