Abstract
A foodborne Escherichia coli O157:H7 outbreak in December 2006 included 77 illnesses reported in Iowa and Minnesota. Epidemiologic investigations by health departments in those states and the U.S. Centers for Disease Control and Prevention (CDC) identified shredded iceberg lettuce (Lactuca sativa L.) as the vehicle of transmission. The U.S. Food and Drug Administration (FDA) and Minnesota and California public health agencies traced the lettuce to several growing regions in California based on information from a lettuce processor in Minnesota.
Samples from an environmental investigation initiated by the California Food Emergency Response Team (CalFERT) revealed a genetic match between the outbreak strain and environmental samples from a single farm, leading to an in-depth systems-based analysis of the irrigation water system on that farm. This paper presents findings from that systems-based analysis, which assessed conditions on the farm potentially contributing to contamination of the lettuce. The farm had three sources of irrigation water: groundwater from onsite wells, surface water delivered by a water management agency and effluent from wastewater lagoons on nearby dairy farms. Wastewater effluent was blended with the other sources and used only to irrigate animal feed crops. However, water management on the farm, including control of wastewater blending, appeared to create potential for cross-contamination. Pressure gradients and lack of backflow measures in the irrigation system might have created conditions for cross-contamination of water used to irrigate lettuce. The irrigation network on the farm had evolved over time to meet various needs, without an overall analysis of how that evolution potentially created vulnerabilities to contamination of irrigation water. The type of systems analysis described here is one method for helping to ensure that such vulnerabilities are identified and addressed. A preventive, risk-based management approach, such as the Water Safety Plan process for drinking water, may also be useful in managing irrigation water quality.
3 Introduction
On December 14, 2006 the U.S. Centers for Disease Control and Prevention (CDC) issued a health alert regarding a multi-state foodborne Escherichia coli O157:H7 outbreak that was linked to two restaurants of the same chain located in Iowa and Minnesota. CDC reported the numbers of associated illnesses in Iowa and Minnesota to be 50 and 27, respectively (CDC, 2006). Isolates of E. coli O157:H7 in both states presented indistinguishable patterns that confirmed the two clusters were linked. Epidemiologic and traceback investigations by the affected states and the Food and Drug Administration (FDA) in collaboration with CDC took place for this outbreak (MDH, 2006; CalFERT, 2008). Those investigations identified shredded iceberg lettuce as the vehicle of transmission.
Investigators from FDA and Minnesota and California public health agencies traced the lettuce to growing regions in California’s Central Coast and Central Valley based on information from a lettuce processor in Minnesota. The California Food Emergency Response Team (CalFERT) started an environmental investigation on December 15, 2006. The environmental investigation was conducted by a multi-agency and multidisciplinary team of epidemiologists, environmental health specialists, and laboratory personnel. A detailed description on how the environmental investigation was conducted, the overall findings, and discussion of the findings have been reported by CalFERT (CalFERT, 2008). Samples from the initial environmental investigation revealed a genetic match between the E. coli O157:H7 outbreak strain and environmental samples (two swabs, four water, three water and sediment, and one soil) from a single farm in the Buttonwillow area of the Central Valley hereafter referred to as Farm A, and two adjacent dairy farms. Farm A’s irrigation system and the dairies’ wastewater effluent conveyance system were combined into a complex piping network. Concerns about potential cross-contamination between irrigation systems for lettuce fields on Farm A and the nearby dairies emphasized the need to conduct additional environmental sampling and a detailed systems-based assessment of the farm’s irrigation water management system. These results led to a more in-depth systems-based environmental assessment at Farm A. The objective of this paper is to report the results of that assessment.
Irrigation water can become a source of pathogenic microorganisms due to its interaction with complex upstream environmental factors such as watershed hydrology, hydrogeological conditions, adjacent land uses, waste disposal, climatic events, etc., as well as integrity of on-farm irrigation water management and practices (Suslow et al., 2003). Contact with contaminated irrigation water can thus potentially contaminate fresh produce with pathogenic microorganisms.
Recent studies reported that leafy greens have been associated with a significant number of fresh produce outbreaks for decades in the United States. Among 10,421 foodborne outbreaks reported during 1973–2006, 502 (4.8%) outbreaks, 18,242 (6.5%) illnesses, and 15 (4.0%) deaths were associated with leafy greens (Herman et al., 2008). The proportion of foodborne illness outbreaks due to leafy greens has increased, and cannot be accounted for completely by an increase in leafy green consumption (Palumbo et al., 2007; Shelton et al., 2011). Gelting et al. (2011) reported that environmental factors such as land use, hydrology, and irrigation practices may also contribute to this increase as a result of the environmental investigation of an E. coli O157:H7 outbreak in September 2006. Findings of the investigation identified tainted prepackaged spinach (Spinacia oleracea L.) as the vehicle of the outbreak that resulted in five deaths and 205 illnesses (Gelting et al., 2011).
Lettuce is the most frequent vehicle in leafy greens associated outbreaks. During the decade 1990–1999 there were 10 outbreaks linked to contaminated lettuce with a total of 479 reported cases, from which 264 (55%) were cases from six outbreaks related to E. coli O157:H7 and 113 (24%) cases from three outbreaks that were linked to both iceberg lettuce and E. coli O157:H7 (CSPI, 1999). The number of outbreaks linked to consumption of contaminated fresh produce, especially lettuce, appears to have increased since the year 2000. During the period 1998 to 2007, 12 outbreaks were linked to both contaminated lettuce and E. coli with a total of 361 reported cases (CSPI, 2009).
Mechanisms of lettuce contamination are still not fully understood. Research on the subject has focused on the farm environment (Natvig et al., 2002; Johannessen et al., 2004; Franz et., al 2007), microbial growth and survival in the environment (Wang et al., 1996; Kudva et al., 1998; Ibekwe et al., 2004; Koseki and Isobe, 2005; Ibekwe et al., 2007), plant pathogen interactions (Takeuchi et al., 2001; Duffy et al., 2005; Ells et al., 2006) and treatment interventions and technologies (Zhang and Farber, 1996; Seo and Frank, 1999; Kondo et al., 2006, Ajlouni et al., 2006). Lettuce may become contaminated if improperly treated manure is used as fertilizer in the growing fields (Beuchat, 1999; Solomon et al., 2002; Islam et al., 2004) since research has demonstrated the long-term survival of E. coli O157:H7 in manure under a variety of conditions (Wang et al., 1996, Kudva et al., 1998). Other prior work found that E. coli O157:H7 contaminated lettuce via flood irrigation using water contaminated with cattle feces or by contact with contaminated surface runoff (Ackers et al., 1998; Hilborn et al., 1999). Findings on the ability of the pathogen to survive in water for extended periods of time (Wang et al., 1998; Chalmers et al., 2000) and its association to outbreaks caused by consumption of contaminated water or ice (CDC, 1999) led some researchers to study the mechanisms of lettuce and other vegetable contamination with E. coli O157:H7 via contaminated irrigation water (Wachtel et al., 2002; Islam et al., 2004; Steele and Odumeru, 2004; Gelting et al., 2011).
4 Methods
The initial outbreak investigation identified fields on Farm A as the likely source of the contaminated lettuce involved in this outbreak (CalFERT, 2008). Furthermore, the field environmental assessment pointed to the irrigation system on the farm that was potentially microbiologically compromised. Understanding the factors associated with the microbial quality of irrigation water requires a thorough assessment and analysis of the source, conveyance and on-farm distribution of water based on a systems approach (Stine et al., 2005). A systems-based approach is used to study and analyze the entirety of a problem, considering the underlying interactions and interrelationships among all the constituting elements (Gelting et al., 2005). Such a systems approach was used to assess the irrigation water system on Farm A and to analyze the conditions on the farm that might have led to contamination of the lettuce and consequently to the 2006 E. coli O157:H7 outbreak linked to chain restaurants in Iowa and Minnesota.
An irrigation system is characterized by sources of irrigation water, and the conveyance and distribution networks on the farm (Suslow, 2010). The quantity and quality of water delivered to fields by a system are often highly dynamic. This is reflected in the diverse and variable concentrations of microorganisms, including pathogens, present in irrigation water. Data on flow and water quality are instrumental in assessing microbial loads delivered to irrigated fields and the risk of produce contamination. Using a systems-based approach, we evaluated and analyzed the information collected during the field environmental assessment to identify potential risk factors for produce contamination arising from contaminated irrigation water or a compromised irrigation system. This approach encompassed the following elements:
Characterization of the irrigation system
Identification of contamination sources
Identification of contamination pathways
Specific conditions adding to the vulnerability of the overall system
Major components of the irrigation water management system on Farm A were identified. Component interactions in terms of inputs and outputs and their relationship to the overall system behavior were determined. Sources and pathways of potential contamination were identified and conditions pertaining to hydrology and hydraulics of the irrigation system were assessed. The above elements served to identify critical events in space and time that may have compromised the microbial quality of the irrigation water used on lettuce crops. Analytical results of environmental samples collected from all the components of the system supported the assessments. Similarly, data relevant to hydrology and hydraulics were analyzed to assess conditions potentially leading to contamination of irrigation water.
5 Results and Discussion
5.1 Preliminary Environmental Sampling
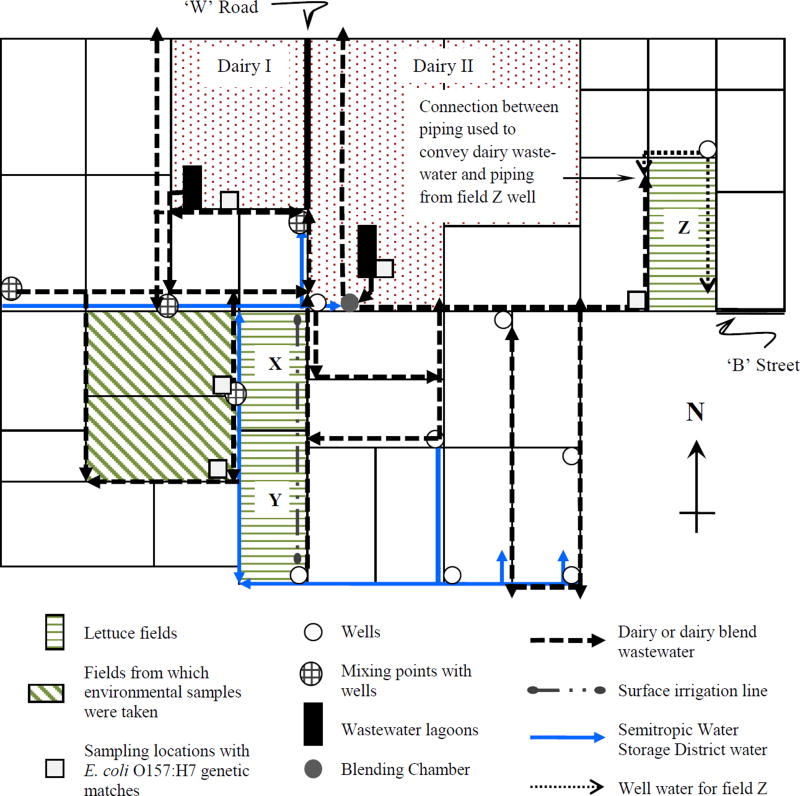
Field visits were conducted as part of the initial environmental investigation to determine the root causes and environmental contributing factors to this E. coli O157:H7 outbreak. As part of the investigation, a total of 251 environmental samples (water, soil, sediment from irrigation lines and ditches, swabs [wipe-type] from surfaces such as irrigation lines or farm equipment, animal fecal matter, and product specimens) and eight controls were collected from suspect lettuce growing fields and dairy farms by CalFERT investigators according to an environmental sampling plan. Investigators took samples from suspect growing fields and dairies in two areas of California, Buttowillow in Kern County (194 samples), and Santa Maria in Santa Barbara County (57 samples). All positive E. coli O157:H7 samples (32 out of 251 or ~13%) came from Farm A, located in the Buttonwillow area, and 10 of the 32 positive specimens (~31%) were determined to genetically match the outbreak strain using pulsed field gel electrophoresis (PFGE) (CalFERT, 2008). Of these 10 matching samples, six were collected from a field to the west of suspect lettuce growing fields X and Y (Fig. 1). Matching samples were one swab from a water-dairy wastewater blend line, one water-dairy wastewater blend, three water and sediment from water-dairy wastewater blend lines, and one soil from the bottom corner of the field to the west of lettuce growing field Y. The remaining four were collected at the two dairies near Farm A in Fig. 1. Matching samples were one water-dairy wastewater blend from Dairy I, one wastewater lagoon of Dairy II, and two (one swab and one water) from water-dairy wastewater blend line of Dairy II adjacent to lettuce growing field Z. The sampling locations where the ten different matching samples were collected can be seen in Fig. 1 (more than one sample was taken at some locations).
Figure 1.
Schematic of the piping layout for the irrigation system in Farm A (non-scale). Arrows indicate direction of flow and irrigation.
5.2 Description of Farm A
Farm A is located in the California’s Central Valley, adjacent to two large dairy farms. In addition to the three lettuce growing fields, Farm A was also growing animal feed crops at the time of the investigation. The location of the three lettuce growing fields (X, Y, and Z) and the two dairies (I and II) are shown in Fig. 1 in a schematic plan of Farm A. Fields X (28.5 ha) and Y (28.9 ha) were located on the farm grounds, south of Dairy I and west of ‘W’ road; field Z (29.7 ha) was located on the property of Dairy II, north of ‘B’ street, but managed by Farm A. Farmers used fields X and Y to grow lettuce and tomatoes (Lycopersicon esculentum Mill.) in separate parcels of the fields. They used field Z to grow lettuce and cucumbers (Cucumis sativus L.) also in separate parcels of the field. Interviewees at the farm and dairies reported that all the land and wells were previously owned and maintained by Farm A. In 2003, parcels of land were sold to the dairies, but the farm maintained control over the irrigation system.
5.2.1 Water Sources
Three sources of water were used for the irrigation system at Farm A: water from Semitropic Water Storage District (SWSD), effluent from the wastewater lagoon systems of the dairies, and groundwater withdrawals from wells. The type of irrigation water used on a field depended on the types of plants grown in that field. For example, a blend of district water and effluent from the wastewater lagoons was applied to fields only growing animal feed crops. District water or well water was applied to fields where crops for human consumption were grown.
5.2.1.1 Semitropic Water Storage District
The SWSD is one of eight water storage districts in California that manage and deliver water for irrigation (SWSD, 2009). In 1995, SWSD started a water banking project that involved delivery of surface water to reduce groundwater pumping (SWSD, 2007a). The managers of Farm A reported that SWSD water was used to irrigate all fields where crops were growing, including two of the three lettuce fields implicated in the outbreak (fields X and Y). The water distributed by SWSD came from the California Aqueduct, which delivers water diverted from the Sacramento River Delta to farms in the Central Valley.
5.2.1.2 Effluent from Dairies Wastewater Lagoon Systems
At the time of the environmental investigation, each dairy had a lagoon system for wastewater treatment that consisted of settling ponds followed by storage lagoons. Effluent from the storage lagoons was blended with SWSD water and used as a source of irrigation water although levels of treatment achieved by the lagoon systems were unknown due to lack of monitoring data. Farm A managers reported that blended water was used only to irrigate animal feed crops, while lettuce and other crops for human consumption were only irrigated with water from SWSD and wells.
5.2.1.3 Groundwater Wells
Farm A used well water to irrigate one of the fields where the lettuce implicated in the outbreak was grown (field Z). This well was located on the northeast corner of the field, in the vicinity of the dairy farms and their wastewater treatment lagoons. This created conditions for increased risk of well water contamination from lagoon seepage. A detailed discussion is provided in the following sections.
5.2.1.4 Irrigation Water Piping/Distribution Network
As previously discussed, effluent from the dairy storage lagoons was pumped into the farm irrigation system. It was reported that:
The effluent from the storage lagoon of Dairy I was mixed with SWSD water and water from wells at designated mixing points in the irrigation piping network (see dairy or dairy blend wastewater lines of this dairy in Fig. 1).
Effluent from the storage lagoon of Dairy II was mixed with SWSD water and/or well water in a blending chamber equipped with an air gap in close proximity to the Dairy II lagoon (see dairy or dairy blend wastewater lines of this dairy in Fig. 1). Blended water was then distributed through the farm irrigation system by a complex piping network.
5.3 Potential Sources of Contamination
The location of two dairy farms and their wastewater treatment lagoons in close proximity to Farm A and the use of blended dairy wastewater for irrigation raise serious concerns about the safety of ready-to-eat type foods or foods that may be eaten raw with no kill step (e.g., fresh produce). The prevalence and survival of pathogenic E. coli including E. coli O157:H7 has been researched extensively in dairy wastewater. Ravva et al. (2006) investigated the proliferation of E. coli O157:H7 in dairy wastewater from on-site holding lagoons and determined that E. coli O157:H7 declined rapidly and disappeared in less than four weeks. However, Ibekwe et al. (2007) have shown that if introduced into the physical environment, E. coli O157:H7 can survive for a long period of time. These authors showed that the pathogen can survive in soil for more than 90 days even under harsh conditions of post fumigation. Earlier, Ibekwe et al. (2004) found that the persistence of E. coli O157:H7 in the phyllosphere (leaf surface of plants), rhizosphere (soil tightly held by plant roots) and non-rhizosphere soils over 45 days may play a significant role in contaminating produce in the environment. These studies demonstrate that E. coli O157:H7 can survive for long periods of time in soil and other media, and can threaten food safety if introduced to fields where products such as leafy green produce is grown. Therefore, the two dairy wastewater holding lagoons adjacent to Farm A, and the usage of lagoon water for irrigation purposes, warrant consideration in an investigation of potential sources of contamination for this outbreak.
5.4 Potential Contamination Pathways
As discussed above, the irrigation system on Farm A consisted of a complex piping network that integrated elements from both the farm and the two dairies. Understanding how that network functioned was challenging, but was important for assessing the possible pathways of water and lettuce contamination at the farm. Two major considerations arose from that assessment: cross-contamination of irrigation water with blended wastewater, and groundwater contamination due to seepage from the wastewater lagoons. These are discussed in the following sections.
5.4.1 Cross Contamination of Irrigation Water with Wastewater Lagoon Effluent
As described above, effluent from the dairy wastewater lagoons was mixed with SWSD and/or groundwater at mixing points in the piping network as well as in a blending chamber for the irrigation of animal feed crops on Farm A. This blended water was conveyed in piping networks that had direct connections to the SWSD system and wells used for irrigation. The investigation team analyzed the piping network to ascertain reliability of cross contamination prevention mechanisms. A non-scale schematic of the irrigation system, the lettuce fields (X, Y and Z), and the dairies (I and II) is provided in Fig. 1.
5.4.1.1 Backflow Prevention
In February 2007, the California Regional Water Quality Control Board (CRWQCB) served notices of violation to both Dairies I and II after an inspection of the dairy facilities ascertained inadequate backflow prevention and lack of inspection ports. These notices of violation stated: “The wastewater distribution system could allow mixed dairy wastewater and freshwater to enter the Semitropic WSD supply system and agricultural supply wells, causing or threatening to cause a condition of pollution.” (CalFERT, 2008). In addition, blending of water from various sources (SWSD water, groundwater pumped from onsite wells, and dairy lagoon wastewater) was controlled by manipulating valves in the pipe network and monitoring flow meters to achieve the desired percentages of water from those sources that followed no standard guidelines, but Farm A management’s own established practices. Depending on the crop, water from any specific source constituted up to 100% of the blend. For example, during the environmental assessment, irrigation water applied to a field southwest of and adjacent to field X consisted of 2.5’×10−2 cubic meters per second (m3/s) dairy lagoon water and 0.1 m3/s well water.
The valves used for controlling this blending were gate valves and butterfly valves, neither of which were designed for this purpose. Gate valves are not designed to regulate flow, but rather to be used as shutoff valves to be either completely open or closed. If used to regulate flow, they can be subject to premature wear and/or failure that can cause leakage. Butterfly valves can be used to regulate flow, but are designed for use with clean water. Using these valves in wastewater applications, as in this case, can lead to the valves not seating properly upon closure and consequently to leakage. If leakage through either of these types of valves had occurred when the valves were thought to be closed, contamination of water from SWSD or from the wells by dairy lagoon wastewater could have occurred.
5.4.1.2 Conditions in the Irrigation Piping Network on Farm A
Field Z (see Fig. 1) was at least 6 feet higher in elevation than the adjacent field directly to the west. Farm managers indicated that because the farm irrigation system was unable to pump water up to Field Z, this field was irrigated with water from a well at the northeast corner of the field and blended wastewater never reached it. However, blended dairy wastewater was used to irrigate part of the field adjacent to the west of field Z, which was at a lower elevation (CalFERT, 2008).
During the environmental assessment under this investigation, blended wastewater was being applied to a portion of the field west of field Z. In order to irrigate as much of this field as possible, the wastewater blending chamber was bypassed at times to maintain higher head in the irrigation piping network. Although the irrigation system at Farm A could not pump water up to field Z, the piping used to convey blended wastewater was directly connected to piping from the well at the northeast corner of Field Z. There was no shutoff valve between the pipeline on the north side of field Z that carried well water and the pipeline on the east side of the adjacent field that carried blended wastewater. Thus water being pumped from the field Z well could be carried down into the rest of the farm irrigation system.
Field observations indicated that while the Field Z well was being pumped, a pressure gauge at the well head showed an operating pressure of 1.9×105 Pa. The elevation difference between this well and a turnout1 of the SWSD distribution subsystem located on ‘B’ street, near the intersection with ‘W’ road, was estimated at 6.4 m, equivalent to 6.2×104 Pa. A static head of approximately 2.6×105 Pa was therefore possible at this turnout. According to the SWSD Subsystem Design Information (SWDS, 2007b), the design operating head at this turnout was 6.6 m or 6.5×104 Pa. If the blending chamber was bypassed at a time when the Field Z well was pumping, it appears possible to create enough static head at the turnout to overcome the pressure in the SWSD system and force water from the farm irrigation system into the SWSD system if the gate valve at the turnout was open or not functioning properly. Also, no check valve was observed at this turnout to prevent backflow. It is possible that any water forced back into the SWSD system carried residual wastewater along with it because portions of the Farm A pipeline, between the turnout and the Field Z well, were used to convey blended wastewater. This might have occurred if the blending chamber was bypassed and valves at the Field Z well were open, allowing water to flow back down into the farm irrigation system.
Low pressure in the SWSD system due to low demand would have favored the possibility of cross contamination because there would not be sufficient pressure in the SWSD system to prevent water from the Farm A irrigation system from flowing into the SWSD system. There were several days of low or no demand recorded in the SWSD distribution subsystem supplying Farm A during the period of time when the lettuce crop of interest was in the field. For example, on September 6 and 29, the afternoon of September 30, and on October 7 and 12 of 2006, the demand was zero in this SWSD subsystem. Very low demands in the subsystem were also registered on November 7 and 19, 2006 based on SWDS Turnout Delivery Data. According to SWSD officials, low demands corresponded with relatively low pressures in the distribution system, as operators managed water levels in the storage tanks to correspond to demands. However, no records of pressures in the SDWS system were available to verify if low pressures indeed occurred during these days of low or no demand.
5.4.2 Contamination of Groundwater due to Lagoon Wastewater Seepage
Dairy wastewater lagoons are designed and used for biological treatment and long term storage of dairy waste. Lagoons are usually lined with clay, concrete or a synthetic liner; however, some lagoons may be entirely unlined. Dairy wastewater lagoons present a growing concern due to their impact on the environment, specifically contamination of groundwater even at depths greater than 30.5 m. Contaminants of concern are microbial pathogens and chemicals such as nutrients (nitrates, and phosphorus and potassium compounds), ammonia, heavy metals and organic chemicals like hormones and antibiotics. Parker et al. (1999) reviewed the results of field seepage measurements and groundwater and soil sampling studies and concluded that seepage can occur from animal wastewater holding earthen ponds and lagoons. Arnold and Meister (1999) collected samples from 26 monitoring wells around seven wastewater lagoons on seven dairies over a six year period and found that mean contaminant concentrations exceeded groundwater quality standards for nitrate, ammonia, chloride and total dissolved solids at all dairies and all wells. Harter et al. (2002) determined higher levels of total nitrogen concentrations and electric conductivities in groundwater in the proximity of dairies compared to monitoring wells immediately up gradient of these dairies. Campagnolo et al. (2002) detected multiple classes of antimicrobial compounds in groundwater samples collected in the proximity of swine and poultry farms. Arnon et al. (2008) detected testosterone and estrogen in sediments of monitoring wells, as deep as 32 and 45 m respectively, in a monitoring well adjacent to a dairy farm wastewater lagoon. Considering the potential for groundwater contamination from wastewater lagoon seepage, the risk of contamination of groundwater drawn from wells located on the farm cannot be ruled out as a factor contributing to the contamination of produce. This was noted in reports from inspections of the dairies by CRWQCB, which notes the shallow depth to groundwater in the area, ranging from 8.2 to 12 m on Dairy I, and 9 to 16.5 m on Dairy II. Possible sources of groundwater contamination mentioned in CRWQCB’s Notices of Violation to both dairies include ponding of dairy lagoon wastewater in fields (CRWQCB, 2007a; CRWQCB, 2007b). Reports from CRWQCB inspections of Dairy II also indicated “the facility has the potential to discharge dairy waste constituents to groundwater.” (CRWQCB, 2007b). Results from prior sampling in 2006 by the California Department of Food and Agriculture showed some samples from wells located on Dairy II were positive for total coliforms (CalFERT, 2008). Although total coliforms do not necessarily point to fecal contamination, those positive results imply that microbial contamination reached the groundwater in the Dairy II wells. Potential sources and routes of groundwater contamination were also noted during the environmental assessment; they included unprotected vents on wells at this dairy and cross-connections between irrigation wells and effluent from dairy wastewater treatment lagoons (CalFERT, 2008).
5.5 Additional Sampling of Irrigation Water and Spectral Analysis
During the in-depth environmental assessment at Farm A, additional samples beyond the 251 environmental samples and eight controls previously mentioned were taken from various sources of irrigation water for fluorescence characterization to assess the potential for using natural and artificial tracers to detect possible cross-connections in the irrigation system. Different analytical techniques have been developed for detection of bacterial proteins in complex matrices like environmental samples (Wang et al., 1998; Rizzo et al., 2004). Spectral analysis was performed using techniques elaborated in Baker (2002) and Hudson et al. (2007). Analytical results are presented in Table 1 below.
Table 1.
Fluorescence characterization in water samples taken in Farm A
| Sample # | Sample Description | Spectral Signature |
|---|---|---|
| 1 | SWSD turnout located in the southeastern corner of field Y | Tyrosine-like and Tryptophan-like |
| 2 | Blended water* applied to the field adjacent to the southwest corner of field X | Tyrosine-like |
| 3 | Well at the field adjacent to the west of field X | Tryptophan-like |
| 4 | SWSD turnout located on ‘B’ street near the intersection with ‘W’ road | Humic substances |
| 5 | Blended water* applied to the field adjacent to the west of field Z | Tyrosine-like |
| 6 | Well at Field Z | Tryptophan-like |
The blended mix included wastewater from dairy farms
The two blended water samples (#2 and 5) had similar spectral protein signatures (labeled Tyrosine-like) to sample #1, which was taken from the SWSD turnout located in the southeastern corner of field Y. The presence of protein-like fluorophores indicate the production of bacterially-generated amino acids, and often represent contamination by wastewater or runoff from storm water and agricultural areas (Baker and Inverarity, 2004; Hur et al., 2008). Finding similar protein signatures in the two blended water samples was not surprising since the blended mix included wastewater from the dairy farms. However, a spectral signature in water from the above-referenced SWSD turnout matched that from the two blended water samples, and suggested that a cross-connection could exist that caused local contamination of the SWDS system . Sample #4 (SWSD turnout located on ‘B’ street near the intersection with ‘W’ road), in contrast, showed a humic spectral signature typical of surface waters, which would be expected for water supplied by SWSD, which was drawn from surface water sources. No groundwater was pumped into the SWSD system in 2006 because no additional water was required to fulfill water delivery demands. The two well water samples showed similar protein signatures (labeled Tryptophan-like) which were different protein signatures from those found in the blended wastewater samples. Since protein signatures in water can indicate contamination from wastewater or runoff, potential groundwater contamination, as mentioned in CRWQCB reports, may have occurred.
Results from the fluorescence characterization indicated that, not only was background fluorescence a useful environmental tracer in this case, but also that the protein-like and humic-like spectral signatures were distinct from certain fluorescent tracer dyes. Tracer testing using sub-visual dye concentrations could therefore potentially be used for further analysis of cross-connections or other contamination at this site. This investigation showed that fluorescence screening is a rapid technique that can be adapted for use in the field, and which may be useful in conducting environmental assessments of other produce-related outbreaks.
6 Conclusions
The iceberg lettuce implicated in this outbreak came from a farm that used multiple sources of water for irrigation. Those sources included groundwater pumped from onsite wells, surface water delivered from a local water management agency, and effluent from dairy wastewater lagoons. Water from these sources was delivered to fields through a complex piping network on the farm. Different sources of water were blended in that network depending on irrigation needs, but blended wastewater was only directly applied to fields dedicated to animal feed crops and not to ready-to-eat crops for human consumption, according to farm managers. Nonetheless, water management on the farm, including control of the wastewater blending process, created potential vulnerabilities to cross-contamination.
As is common in agriculture, the irrigation system on the farm had evolved over time to attempt to meet current needs at various points in time. After the farm had sold the land that was developed into the dairies, dairy wastewater effluent was incorporated into the irrigation system. Because the farm was located in an arid area, dairy wastewater effluent was logically viewed as a resource for blending with other sources to irrigate animal feed crops, especially as it contained nutrients that could also lower the need for chemical fertilizers. These incremental changes were undertaken without an overall analysis of how they created possibilities for contamination of irrigation water. In this case, backflow prevention between piping networks used to convey blended wastewater and water from the other two sources was insufficient. The hydraulics in the combined piping networks were such that pressure differentials could also create potential for cross-contamination.
In addition, the dairy wastewater lagoons created possibilities for contamination of local groundwater resources that were also used for irrigation on the farm. Irrigation systems like the one on Farm A evolve over time to meet varying needs, potentially leading to unintended vulnerabilities if the effects of incremental changes on the entire irrigation system are not assessed. When risk factors such as the use of blended wastewater effluent for irrigation are evident, a systems analysis of the entire irrigation system may be necessary to identify potential vulnerabilities created by incremental changes and help prevent contamination. This paper described an example of such a detailed systems analysis of an entire irrigation system and the potential vulnerabilities to irrigation water quality within that system.
A preventive approach such as that contained within the World Health Organization’s (WHO) Water Safety Plan (WSP) process, a risk-based management approach for drinking water, may be useful in managing irrigation water quality. Water Safety Plans represent “a comprehensive risk assessment and risk management approach that encompasses all steps in water supply from catchment to consumer” (WHO, 2011). In other words, the WSP is a systematic methodology to identify and address risks to water quality at each step along the entire drinking water supply chain. Principles and concepts of the Hazard Analysis Critical Control Points used in the food industry were applied to drinking water systems during the development of the WSP methodology (WHO, 2005 and 2011). The WSP process incorporates the type of systems analysis discussed above in that it includes a systems assessment and risk analysis of drinking water systems. The process also takes a further step, looking at factors in the broader environment that may influence drinking water quality.
Such a preventive approach for irrigation would include a systematic identification of risks to irrigation water quality both within an irrigation system, as described above for Farm A, as well as in the broader environment. For example, seasonal variation in the quality of surface waters used for irrigation would need to be taken into account, as well as the multiple pathways of contamination potentially contributing to surface water quality. The timing and magnitude of precipitation and flood events are other factors that may need to be included. Factors such as well construction and protection, the presence of abandoned wells and groundwater/surface water interactions would need to be analyzed for groundwater sources. Other factors such as the type of irrigation used (spray, flood, drip, etc) can also be included in this type of comprehensive assessment of risks to irrigation water quality. In the drinking water arena, the preventive principles of WSPs have been applied in both non-regulatory and regulatory ways, and the same could apply for a preventive process for managing irrigation water.
Identifying the exact sources of pathogen contamination of produce is challenging, and, in the case of this particular outbreak, was not definitively accomplished. However, implementing the methods described here, including a systems analysis of entire irrigation systems and preventive approaches modeled after the WSP process, can help to identify actual or potential sources of irrigation water contamination and also help to prevent that contamination.
HIGHLIGHTS FOR REVIEW.
We critically assessed all reviewers’ comments.
We addressed all reviewers’ comments in the Response to Reviewers Comments document.
We revised our responses before re-submitting the manuscript.
We thoroughly revised the edited manuscript before re-submitting it.
Acknowledgments
The initial environmental investigation for this outbreak was conducted by the California Food Emergency Response Team, which includes personnel from FDA and the Food and Drug Branch, California Department of Public Health. The more detailed environmental assessment reported here would not have been possible without the results and efforts from that initial investigation. The California Department of Water Resources and California Department of Food and Agriculture also contributed to that original investigation. Larry MacKay from the University of Tennessee provided valuable input to the manuscript. Gabriella Lockhart and Matthew Murphy from CDC and Mark Starr from the California Department of Public Health reviewed the manuscript and made helpful comments.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
1 Disclaimer Notice
"The findings and conclusions in this article are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention, the California Department of Public Health and/or the Food and Drug Administration."
A turnout is a termination point of the SDWS system, and constitutes a connection between the SWDS system and the farm irrigation system.
References
- Ackers ML, Mahon BE, Leahy E, Goode B, Damrow T, Hayes PS, Bibb WF, Rice DH, Barrett TJ, Hutwagner L, Griffin PM, Slutsker L. An outbreak of Escherichia coli O157:H7 infections associated with leaf lettuce consumption. J. Infect. Dis. 1998;177:1588–1593. doi: 10.1086/515323. [DOI] [PubMed] [Google Scholar]
- Ajlouni S, Sibrani H, Premier R, Tomkins B. Untrasonication and fresh Produce (Cos lettuce) preservation. J. Food Sci. 2006;71(2):M62–M68. [Google Scholar]
- Arnold SD, Meister EA. Dairy feedlot contributions to groundwater contamination: A preliminary study in New Mexico. J. Environ. Health. 1999;62:16–19. [Google Scholar]
- Arnon S, Dahan O, Elhanany S, Cohen K, Pankratov I, Gross A, Ronen Z, Baram S, Shore LS. Transport of testosterone and estrogen from dairy - farm waste lagoons to groundwater. Environ. Sci. Technol. 2008;42:5521–5526. doi: 10.1021/es800784m. [DOI] [PubMed] [Google Scholar]
- Baker A. Fluorescence properties of some farm wastes: Implications for water quality monitoring. Water Res. 2002;36:189–195. doi: 10.1016/s0043-1354(01)00210-x. [DOI] [PubMed] [Google Scholar]
- Baker A, Inverarity R. Protein-like fluorescence intensity as a possible tool for determining river water quality. Hydrol. Proc. 2004;18:2927–2945. [Google Scholar]
- Beuchat LR. Survival of enterohemorrhagic Escherichia coli O157:H7 in bovine feces applied to lettuce and the effectiveness of chlorinated water as a disinfectant. J. Food Prot. 1999;62(8):845–849. doi: 10.4315/0362-028x-62.8.845. [DOI] [PubMed] [Google Scholar]
- CalFERT, California Food Emergency Response Team. Investigation of the Taco John’s Escherichia coli O157:H7 Associated with Iceberg Lettuce – Final Report. California Department of Public Health, Food and Drug Branch Sacramento, CA. 2008:95899–7435. [Google Scholar]
- Campagnolo ER, Johnson KR, Karpati A, Rubin CS, Kolpin DW, Meyer MT, Esteban JE, Currier RW, Smith K, Thug KM, McGeehin M. Antimicrobial residues in animal waste and water resources proximal to large-scale swine and poultry feeding operations. Sci. Total Environ. 2002;299:89–95. doi: 10.1016/s0048-9697(02)00233-4. [DOI] [PubMed] [Google Scholar]
- CDC, Centers for Disease Control and Prevention. Outbreak of Escherichia coli O157:H7 and Campylobacter among attendees of the Washington County Fair – New York, 1999. Morb. Mortal. Wkly. Rep. 1999;48:803–804. [PubMed] [Google Scholar]
- CDC, Centers for Disease Control and Prevention. Multi-state outbreak of E.coli O157 Infections. 2006. November – December, 2006. [Last accessed March 3, 2009]. Available at: http://www2a.cdc.gov/HAN/ArchiveSys/ViewMsgV.asp?AlertNum=00255.
- Chalmers RM, Aird H, Bolton FJ. Waterborne Escherichia coli O157. Journal of Applied Microbiology Symposium Supplement. 2000;88:124S–132S. doi: 10.1111/j.1365-2672.2000.tb05340.x. [DOI] [PubMed] [Google Scholar]
- CRWQCB, California Regional Water Quality Control Board. Notice of violation dated February 27, 2007 2007a [Google Scholar]
- CRWQCB, California Regional Water Quality Control Board. Notice of violation dated February 27, 2007 2007b [Google Scholar]
- CSPI, Center for Science in the Public Interest. Produce-related outbreaks 1990–1999. 1999. [Last accessed March 5, 2009]. Available at: http://www.cspinet.org/new/prodhark.html.
- CSPI, Center for Science in the Public Interest. Outbreak alert! 2009: Analyzing foodborne outbreaks, 1998 to 2007. 2009. [Last accessed April 29, 2014]. Available at: http://cspinet.org/new/pdf/outbreakalertreport09.pdf.
- Duffy EA, Lucia LM, Kells JM, Castillo A, Pillai SD, Acuff GR. Concentration of Escherichia coli and genetic diversity and antibiotic resistance profiling of Salmonella isolated from irrigation water, packing shed equipment, and fresh produce in Texas. J Food Prot. 2005;68:70–79. doi: 10.4315/0362-028x-68.1.70. [DOI] [PubMed] [Google Scholar]
- Ells TC, Hansen LT. Strain and growth temperature influence Listeria spp. attachment to intact and cut cabbage. International Journal of Food Microbiology. 2006;111:34–42. doi: 10.1016/j.ijfoodmicro.2006.04.033. [DOI] [PubMed] [Google Scholar]
- Franz E, Visser AA, van Diepeningen AD, Klerks MM, Termorshuizen AJ, van Bruggen AHC. Quantification of contamination of lettuce by GFP-expressing Escherichia coli O157:H7 and Salmonella enterica serovar Typhimurium. Food Microbiol. 2007;24:106–112. doi: 10.1016/j.fm.2006.03.002. [DOI] [PubMed] [Google Scholar]
- Gelting R, Sarisky J, Selman C, Otto C, Higgins C, Bohan PO, Buchanan SB, Meehan PJ. Use of a systems-based approach to an environmental health assessment for a waterborne disease outbreak investigation at a snowmobile lodge in Wyoming. Int J. Hyg. Envir. Heal. 2005;208:67–73. doi: 10.1016/j.ijheh.2005.01.009. [DOI] [PubMed] [Google Scholar]
- Gelting RJ, Baloch MA, Zarate-Bermudez MA, Selman C. Irrigation water issues potentially related to the 2006 multistate E. coli O157:H7 outbreak associated with spinach. Agricultural Water Management. 2011;98:1395–1402. [Google Scholar]
- Harter T, Meyer RD, Mathews MC. Nonpoint source pollution from animal farming in semi-arid regions: Spatio-temporal variability and groundwater monitoring strategies. In: Ribeiro L, editor. Future Groundwater Resources at Risk; Proceedings of the 3rd International Conference; Lisbon, Portugal. June, 2001; 2002. pp. 363–372. [Google Scholar]
- Herman KM, Ayers TL, Lynch MF. Foodborne Disease Outbreaks Associated with Leafy Greens, 1973–2006; International Conference on Emerging Infectious Diseases; March 16–19; Centers for Disease Control and Prevention, Atlanta, GA. 2008. [Google Scholar]
- Hilborn ED, Mermim JH, Msha PA, Hadler JL, Voetsch A, Wojtkunski C, Swartz M, Mshar R, Lambert-Fair MA, Farrar JA, Glynn MK, Slutsker L. A multistate outbreak of Escherichia coli O157:H7 infections associated with consumption of mesclun lettuce. Arch. Intern. Med. 1999;159:1758–1764. doi: 10.1001/archinte.159.15.1758. [DOI] [PubMed] [Google Scholar]
- Hudson NJ, Baker A, Reynolds D. Fluorescence analysis of dissolved organic matter in natural, waste and polluted waters - a review. River Res. Appl. 2007;23(6):631–649. [Google Scholar]
- Hur J, Hwang SJ, Shin JK. Using synchronous fluorescence technique as a water quality monitoring tool for an urban river. Water, Air, and Soil Pollution. 2008;191(1–4):231–243. [Google Scholar]
- Ibekwe AM, Watt PM, Shouse PJ, Grieve CM. Fate of Escherichia coli O157:H7 in irrigation water on soils and plants as validated by culture method and real-time PCR. Canadian Journal of Microbiology. 2004;50:1007–1014. doi: 10.1139/w04-097. [DOI] [PubMed] [Google Scholar]
- Ibekwe AM, Grieve CM, Yang C. Survival of Escherichia coli O157:H7 in soil and on lettuce after fumigation. Canadian Journal of Microbiology. 2007;53:623–635. doi: 10.1139/W07-003. [DOI] [PubMed] [Google Scholar]
- Islam M, Doyle MP, Phatak SC, Millner P, Jiang X. Persistence of enterohemorrhagic Escherichia coli O157:H7 in soil and on leaf lettuce and parsley grown in fields treated with contaminated manure composts or irrigation water. J. Food Prot. 2004;67(7):1365–1370. doi: 10.4315/0362-028x-67.7.1365. [DOI] [PubMed] [Google Scholar]
- Johannessen GS, Froseth RB, Solemdal L, Jarp J, Wasteson Y, Rorvik LM. Influence of bovine manure as fertilizer on the bacteriological quality of organic iceberg lettuce. J Appl. Microbiol. 2004;96:787–94. doi: 10.1111/j.1365-2672.2004.02208.x. [DOI] [PubMed] [Google Scholar]
- Koseki S, Isobe S. Prediction of pathogen growth on iceberg lettuce under real temperature history during distribution from farm to table. International Journal of Food Microbiology. 2005;104:239–248. doi: 10.1016/j.ijfoodmicro.2005.02.012. [DOI] [PubMed] [Google Scholar]
- Kondo N, Murata M, Isshike K. Efficiency of sodium hypo-chlorite, fumaric acid, and mild heat in killing native microflora and Escherichia coli O157:H7, Salmonella Typhimurium DT104, and Staphylococcus aureus attached to fresh-cut lettuce. J. Food Prot. 2006;69:323–329. doi: 10.4315/0362-028x-69.2.323. [DOI] [PubMed] [Google Scholar]
- Kudva IT, Blanch K, Hovde CJ. Analysis of Escherichia coli O157:H7 survival in ovine or bovine manure and manure slurry. Appl. Environ. Microbiol. 1998;64(9):3166–3174. doi: 10.1128/aem.64.9.3166-3174.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MDH, Minnesota Department of Health. E. coli Outbreak Alert. 2006. [Last accessed February 27, 2012]. Available at: http://www.health.state.mn.us/foodsafety/alert/ecoli1206.html.
- Natvig EE, Ingham SC, Ingham BH, Cooperband LR, Roper TR. Salmonella enterica serovar Typhimurium and Escherichia coli contamination of root and leaf vegetables grown in soils with incorporated bovine manure. Applied and Environmental Microbiology. 2002;68(6):2737–2744. doi: 10.1128/AEM.68.6.2737-2744.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palumbo MS, Gorny JR, Gombas DE, Beuchat LR, Bruhn CM, Cassens B, DelaquIs P, Farber JM, Harris LJ, Ito K, Osterholm MT, Smith M, Swanson KMJ. Recommendations for handling fresh-cut leafy green salads by consumers and retail foodservice operators. Food Prot. Trends. 2007;27:892–898. [Google Scholar]
- Parker DB, Schulte DD, Eisenhauer DE. Seepage from earthen animal waste ponds and lagoons: An overview of research results and state regulations. Trans. ASAE. 1999;42(2):485–493. [Google Scholar]
- Ravva SV, Sarreal CZ, Duffy B, Stanker LH. Survival of Escherichia coli O157:H7 in wastewater from dairy lagoons. Journal of Applied Microbiology. 2006;101:891–902. doi: 10.1111/j.1365-2672.2006.02956.x. [DOI] [PubMed] [Google Scholar]
- Rizzo MA, Springer GH, Granada B, Pinston DW. An improved cyan fluorescent protein variant useful for FRET. Nature Biotechnology. 2004;22(4):445–449. doi: 10.1038/nbt945. [DOI] [PubMed] [Google Scholar]
- Seo KH, Frank JF. Attachment of Escherichia coli O157:H7 to lettuce leaf surface and bacterial viability in response to chlorine treatment as demonstrated by using confocal scanning laser microscopy. Journal of Food Protection. 1999;62:3–9. doi: 10.4315/0362-028x-62.1.3. [DOI] [PubMed] [Google Scholar]
- Shelton DR, Karns JS, Coppock C, Patel J, Sharma M, Pachepsky YA. Relationship between eae and stx virulence genes and Escherichia coli in an agricultural watershed: implications for irrigation water standards and leafy green commodities. J Food Prot. 2011;74:18–23. doi: 10.4315/0362-028X.JFP-10-241. [DOI] [PubMed] [Google Scholar]
- Solomon EB, Yaron S, Matthews KR. Transmission of Escherichia coli O157:H7 from contaminated manure and irrigation water to lettuce plant tissue and its subsequent internalization. Appl. Environ. Microbiol. 2002;68(1):397–400. doi: 10.1128/AEM.68.1.397-400.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steele M, Odumeru J. Irrigation water as source of foodborne pathogens on fruit and vegetables. J. Food Prot. 2004;67(12):2839–2849. doi: 10.4315/0362-028x-67.12.2839. [DOI] [PubMed] [Google Scholar]
- Stine SW, Song I, Choi CY, Gerba CP. Application of microbial risk assessment to the development of standards for enteric pathogens in water used to irrigate fresh produce. J. Food Prot. 2005;68:913–918. doi: 10.4315/0362-028x-68.5.913. [DOI] [PubMed] [Google Scholar]
- Suslow TV, Oria MP, Beuchat LR, Garrett EH, Parish ME, Harris LJ, Farber JN, Busta FF. Production Practices as Risk Factors in Microbial Food Safety of Fresh and Fresh-Cut Produce, Chapter 2 in Analysis and Evaluation of Preventive Control Measures for the Control and Reduction/Elimination of Microbial Hazards on Fresh and Fresh-cut Produce. Comprehensive Reviews in Food Science and Food Safety. 2003;2(Supplement) [Google Scholar]
- Suslow TV. Standards for irrigation and foliar contact water: produce safety project issue brief. 2010. [Last accessed February 27, 2012]. Available at: http://www.producesafetyproject.org/admin/assets/files/Water-Suslow-1.pdf.
- SWSD, Semitropic Water Storage District. Biennial groundwater monitoring report for the Semitropic Water Storage District Water Banking Project (2003–2004) Report prepared by K.D. Smith and Associates; Bakersfield, CA: 2007a. May 2007: 47 pp plus appendices. [Google Scholar]
- SWSD, Semitropic Water Storage District. B369 System Design Information Sheet 2007b [Google Scholar]
- SWSD, Semitropic Water Storage District. Semitropic Water Storage District – About Us webpage. 2009. [Last accessed March 24, 2009]. Available at: http://www.semitropic.com/AboutUs.htm.
- Takeuchi K, Hassan AN, Frank JF. Penetration of Escherichia coli O157:H7 into lettuce as influenced by modified atmosphere and temperature. J. Food Prot. 2001;64:1820–1823. doi: 10.4315/0362-028x-64.11.1820. [DOI] [PubMed] [Google Scholar]
- Wang G, Zhao T, Doyle MP. Fate of enterohemorrhagic Escherichia coli O157:H7 in bovine feces. Appl. Environ. Microbiol. 1996;62(7):2567–2570. doi: 10.1128/aem.62.7.2567-2570.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Russon L, Li L, Roser DC, Long SR. Investigation of spectral reproducibility in direct analysis of bacteria proteins by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry. Rapid Commun. Mass Spectrom. 1998;12:456–464. doi: 10.1002/(SICI)1097-0231(19980430)12:8<456::AID-RCM177>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- Wachtel MR, Whitehand LC, Mandrell RE. Association of Escherichia coli O157:H7 in water. J Food Prot. 2002;65:18–25. doi: 10.4315/0362-028x-65.1.18. [DOI] [PubMed] [Google Scholar]
- WHO, World Health Organization. Water Safety Plans, Managing drinking-water quality from catchment to consumer. World Health Organization; Geneva: 2005. http://www.who.int/water_sanitation_health/dwq/wsp170805.pdf . [Google Scholar]
- WHO, World Health Organization. Guidelines for drinking-water quality. 4. World Health Organization; Geneva: 2011. [Google Scholar]
- Zhang S, Farber JM. The effects of various disinfectants against Listeria monocytogenes on fresh-cut vegetables. Food Microbiol. 1996;13:311–321. [Google Scholar]