Abstract
The icd gene of Escherichia coli, encoding isocitrate dehydrogenase, was shown to be expressed from two different promoters: the previously identified icd P1 and a newly detected second promoter, icd P2, whose expression is positively regulated by the catabolite repressor-activator protein Cra, formerly called FruR. In each case, we determined the mRNA start site by primer extension analysis of in vivo transcripts and examined the interaction of the icd control region with either RNA polymerase or Cra. We observed that (i) the Cra factor binds to and activates transcription from a site centered at position −76.5 within the icd P2 promoter region and (ii) three particular mutations in the C-terminal end of the α subunit of RNA polymerase (L262A, R265A, and N268A) considerably diminish transcription initiating from the icd P2 promoter, as shown by in vitro experiments performed in the presence of mutant RNA polymerases carrying Ala substitutions.
In microorganisms growing on acetate as the sole carbon source, isocitrate can be catabolized either by the Krebs cycle or by the glyoxylate bypass (18). In Escherichia coli, this branch point is regulated by the reversible phosphorylation and the concomitant inactivation of the NADP+-dependent isocitrate dehydrogenase (IDH; EC 1.1.1.42) (9, 19, 26). Such a phosphorylation or dephosphorylation process plays a pivotal role in cell survival by connecting, via the glyoxylate pathway, the metabolism of two-carbon compounds with gluconeogenesis and by allowing bacteria to draw energy through the oxidative decarboxylation steps of the Krebs cycle.
In addition to posttranslational control, IDH is also regulated at the level of expression of its gene (icd). It has been demonstrated that under anaerobic cell growth conditions, the icd gene is negatively controlled by both the ArcAB system and the Fnr factor (15). Recently, Chao et al. (5) provided direct evidence of the negative role played by ArcA in icd gene regulation by demonstrating in vitro binding of this repressor at a target operator site overlapping the icd promoter. Also, previous work has shown that the Cra protein (initially called FruR) exerts positive control over a number of genes and operons encoding biosynthetic and oxidative enzymes, including icd (reviewed by Saier and Chin [30]). In the latter case, a single Cra-binding site was detected by electrophoretic mobility shift analysis (EMSA) in the DNA fragment encompassing the regulatory region of the icd gene (28).
This paper focuses on the role of protein Cra in transcription of the icd gene. Using an in vitro transcription approach, we found that in addition to its main promoter (P1), the icd control region contains a second promoter whose activation is dependent on the action of Cra (P2). The start points relative to these promoter sites were mapped by primer extension analysis, and then the precise contacts between RNA polymerase (RNAP) and its promoters and between Cra and its DNA operator were analyzed by the base removal method and the DNase I footprinting technique, respectively. Finally, testing of specific point mutations in the α subunit of RNAP revealed that a number of RNAP-DNA contacts play a key role in transcription of the icd gene.
MATERIALS AND METHODS
Proteins.
Recombinant active Cra protein with a His6 tag at its C-terminal end was prepared from E. coli BL21(DE3) cells (33) harboring the overproducing plasmid pJCD2 (6). Wild-type and mutant α subunits of RNAP were reconstituted in vitro from separately purified subunits as described previously (14). The specific activity of wild-type and mutant α-subunit holoenzymes was determined by measuring the level of poly(dA-dT)-dependent poly(AU) synthesis.
Plasmids. (i) Plasmid pJFC2.
The icd promoter region (EMBL accession no. J02799) (34) was generated by PCR amplification from E. coli K-12 chromosomal DNA. The synthetic oligonucleotide primers used in this reaction were Picd-EcoRI (5′-TATGAATTCAGGTTTACGCC-3′) and Picd-PstI (5′-TATCTGCAGGGTGATCTT-3′). Following PCR, the fragment was restricted with EcoRI-PstI and cloned in fusion with the lacZ gene into the compatible sites of the vector pNM481 (21) to create plasmid pJFC2. Plasmid pJFC2 was also used to generate radioactively labeled DNA fragments for gel retardation, base removal interference, and DNase I footprinting studies. In all cases, after restriction by EcoRI-PstI, the DNA fragment carrying the icd promoter fragment was end labeled at the EcoRI site by [α-32P]dATP (3,000 Ci/mmol) (bottom strand), using the Klenow fragment of DNA polymerase.
(ii) Plasmid pJFC1.
pJFC1, derived from the pUC19 vector (GenBank accession no. X02514), was used as the template for in vitro transcription studies. It contained the EcoRI-PstI icd promoter-bearing DNA fragment inserted into the corresponding sites of pUC19, upstream of the transcriptional termination signal T1T2 of the rrnB operon (1).
Primer extension analysis.
RNA was isolated from E. coli [pJFC2] cells essentially as described by Reddy et al. (29). The oligonucleotide primer 5′-TGCATATGCGTTTGCGTCCTGCGATACGGA-3′ (250 pmol) was end labeled according to the standard procedure (31), using 30 μCi of [γ-32P]ATP (3,000 Ci/mmol) and T4 polynucleotide kinase (Promega Corp.). Primer extension reaction was performed as described elsewhere (25), using 45 μg of total cellular RNA. Extension products were resolved by electrophoresis in a 6% (wt/vol) polyacrylamide–7 M urea gel and visualized by autoradiography.
In vitro transcription assay.
Single-round in vitro transcription experiments were performed with template plasmid pJFC1 as follows. Five picomoles of supercoiled plasmid pJFC1 was preincubated for 25 min at 30°C with 1 pmol of either wild-type or mutant α-subunit RNAPs in a 20-μl assay mixture containing 50 mM Tris-acetate (pH 8.0), 100 mM potassium acetate, 8% (vol/vol) glycerol, 0.1 mM EDTA, 8 mM magnesium acetate, 0.1 mM dithiothreitol, and 500 U of RNAsin per ml. When required, 25 pmol of active Cra protein was added. Transcription reactions were initiated by the addition of 0.2 mM each ATP, GTP, and CTP, 0.01 mM UTP, 2 μCi of [α-32P]UTP (800 Ci/mmol), and 100 μg of heparin per ml. After 10 min of incubation at 37°C, the reactions were blocked with 1 volume of gel loading buffer, the mixtures were heated at 65°C for 3 min and analyzed in a 6% polyacrylamide–7 M urea gels. Quantification of bands on the gel was achieved by densitometric analysis of the autoradiograms.
EMSA.
Gel shift assays were performed as previously described (10). Typically, a 5′-end-labeled DNA fragment (105 cpm) was incubated with either RNAP (100 nM) or active Cra protein (50 nM) for 10 min at 25°C in 20 μl of DNA binding buffer [12 mM HEPES-NaOH (pH 7.9), 4 mM Tris-HCl (pH 7.9), 95 mM KCl, 1 mM EDTA, 1 mM dithiothreitol, 9% [vol/vol] glycerol, 0.02% [vol/vol] Nonidet P-40, 2 μg of poly(dI-dC) · poly(dI-dC), 10 μg of bovine serum albumin per ml]. Complexes were resolved from free DNA on a 4% nondenaturing polyacrylamide gel and electrophoresed in Tris-glycine buffer (50 mM Tris, 384 mM glycine, 2.1 mM EDTA) at 200 V for 1 to 2 h at 25°C. Radioactive bands were visualized by autoradiography.
DNase I footprinting.
DNase I footprinting was performed in 100 μl of DNA binding buffer (see above) with purified Cra protein (10 or 50 nM) and the 5′-end-labeled DNA fragment (105 cpm) that contained the icd promoter. Following incubation of the reaction mixture for 10 min at 25°C, 1.6 U of DNase I (Stratagene) in the presence of 2 mM CaCl2 and 6 mM MgCl2 was added; digestion was allowed to proceed for 50 s and was stopped by the addition of 10 mM EDTA. After precipitation with ethanol, nucleic acids were dissolved in formamide dye mix and heated at 80°C for 3 min prior to electrophoresis in a 6% acrylamide–7 M urea sequencing gel.
Base removal experiments.
Base removal experiments were performed by the procedure described by Brunelle and Schleif (2) as modified by Nègre et al. (23).
RESULTS AND DISCUSSION
In vitro transcription of the icd gene.
An in vitro transcription assay was carried out with the supercoiled plasmid pJFC1, which contains the icd regulatory region (Fig. 1A) cloned upstream of the strong rrnB T1 and T2 terminators (24). The products obtained from single-round transcription experiments were analyzed by electrophoresis in a 6% sequencing gel and detected by autoradiography.
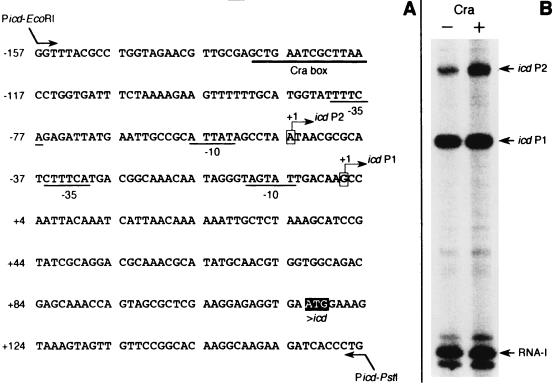
FIG. 1.
(A) Nucleotide sequence of the icd regulatory region (from EMBL accession no. J02799). The −10 and −35 regions of the icd P1 and icd P2 promoters are underlined; locations of the transcription initiation sites of the two promoters are indicated by arrows. The high-affinity Cra-binding site (Cra box) is centered at position −76.5 relative to the P2 transcription initiation site. (B) In vitro transcription from icd promoters with wild-type RNAP. Transcription assays were performed with plasmid pJFC1 as the template. The positions of the RNA-I (nt 107 to 108), icd P1 (nt 225), and icd P2 (nt 272) transcripts are shown by arrows.
When RNAP alone was present in the reaction mixture, plasmid pJFC1 produced two major transcripts (Fig. 1B); one corresponded to RNA-I, and the other represented an RNA product comparable in length (225 bp) to the predicted transcript from the relevant P1 promoter (5). Interestingly, a third transcript (Fig. 1B, icd P2) about 272 bp in length could be detected as a faint band on the autoradiogram. Addition of the Cra protein resulted in an about 3.5-fold stimulation of P2 transcript synthesis, whereas no Cra-mediated stimulation of transcription was observed at the P1 promoter under the same conditions of incubation.
Together, these data showed that besides the previously identified P1 promoter, the E. coli icd gene was expressed from an additional promoter, P2, whose expression was subject to Cra control.
Location of the icd mRNA start sites.
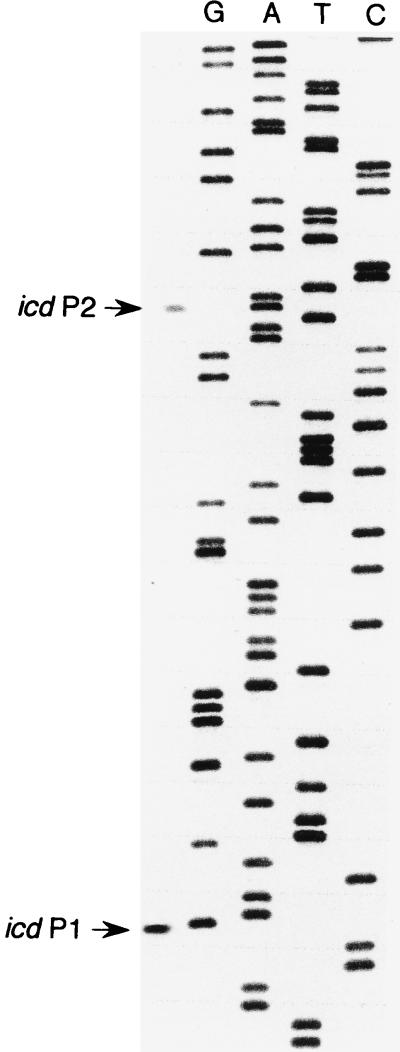
Primer extension analysis was performed to identify the transcriptional start points of in vivo transcription from icd P1 and icd P2. Total cellular RNA was primed with a radiolabeled oligonucleotide complementary to the icd regulatory sequence, and this primer was extended by using reverse transcriptase. The primer used for cDNA synthesis was also used in a DNA sequencing reaction to determine the length of each extension DNA product.
To identify the initiation sites of the plasmid-encoded icd gene promoters, RNA was isolated from E. coli JM109[pJFC2] and mapped by as the primer a 30-mer synthetic oligodeoxyribonucleotide complementary to nucleotides (nt) +40 to +69 of the icd gene 5′ flanking sequence (Fig. 1A). In agreement with the in vitro transcription data described above, the results presented in Fig. 2 indicate that in vivo transcription of the icd gene was initiated at two sites, even though the corresponding extension products differed greatly in intensity. This analysis thus confirmed both the position of the start site at P1 (designated icd P1 in Fig. 1A) that had been recently reported (5) and the occurrence of a new initiation site at P2 (designated icd P2 in Fig. 1A) which could be precisely mapped at an adenosine residue located 162 bp upstream from the translation initiation codon.
FIG. 2.
Mapping of the icd transcriptional start sites by primer extension analysis. A 32P-end-labeled oligonucleotide primer (106 cpm) was annealed to 45 μg of total RNA isolated from E. coli JM109[pJCD2] cells and extended by using deoxyribonucleoside triphosphates and avian myeloblastosis virus reverse transcriptase. The cDNA products were separated by electrophoresis on a 6% polyacrylamide–7 M urea gel. The mobility of cDNA products was compared to a DNA sequencing ladder (GATC) generated with the same primer as that used for the primer extension reactions. Arrows indicate the nucleotides corresponding to the lengths of the extension products.
Interaction of RNAP with the icd promoter region.
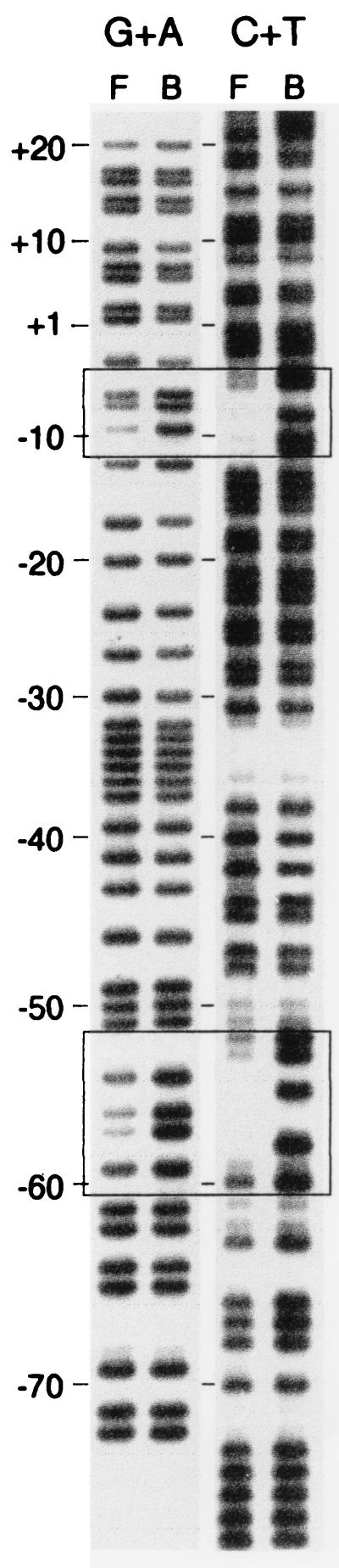
In bacteria, the transcription process initiates through the formation of a closed complex between RNAP and the promoter site, followed by the isomerization to a transcription-competent open structure in which the DNA encompassing residues from approximately −12 to +2 (with respect to the transcription start site at +1) represents the melting domain. To assess the role of individual base residues in the specific interaction between RNAP and the promoter, the base removal interference technique (2) has been found to be a powerful approach (17, 23, 36).
The EcoRI-PstI end-labeled DNA fragment (328 bp) containing the icd promoter region (Fig. 1A) was subjected to partial base-specific cleavage by the method of Maxam and Gilbert (20) and incubated with ς70-saturated RNAP holoenzyme (24). After separation by gel shift electrophoresis in a preparative 4% nondenaturing polyacrylamide gel and piperidine treatment, free and complexed DNA molecules were analyzed in a 6% sequencing gel. The corresponding electrophoretic pattern (Fig. 3) demonstrates that RNAP binding was greatly enhanced when gaps were generated in the icd regulatory sequence between nt −4 and −11 and between −52 and −60. The particular areas thus revealed by the base removal technique could be defined as the melted domains that are integral parts of the open complexes formed upon initiation of transcription at P1 and P2 (3, 32). Taken together, our data showed that the matches to the consensus sequences in the −10 (TATAAT) and −35 (TTGACA) regions (12, 13) of the two promoters were four of six (TAGTAT) and three of six (CTTTCA) for icd P1, as already reported (5), and four of six for the −10 sequence (CATTAT) and three of six for the putative −35 hexamer (CTTTCA) in the icd P2 region (Fig. 1A). The in vitro transcription assays performed in the absence of the Cra protein demonstrated that icd P1 is stronger than icd P2, which suggests that the way in which RNAP recognizes icd P2 differs from that used for icd P1 (Fig. 1B). Moreover, it appeared that the formation of the initiation complex at icd P2 was activated in the presence of Cra, whose main function would be to bend the DNA region of the promoter (25) (see below).
FIG. 3.
Identification of RNAP-icd DNA contacts by base removal footprinting. The 328-bp icd regulatory region, 32P-labeled at the EcoRI site (Fig. 1A), was treated with either formic acid to remove G+A or hydrazine to remove C+T and then incubated with RNAP (approximately 10 nM active protein) prior electrophoresis on a 4% preparative polyacrylamide gel at high ionic strength. Free and complexed DNA molecules were then subjected to piperidine cleavage and analyzed on a 6% sequencing gel to reveal the positions of the interfering modifications. Lanes: B, modified DNA isolated from complexes; F, DNA that had dissociated or was free of complex.
Cra binding to the icd promoter region.
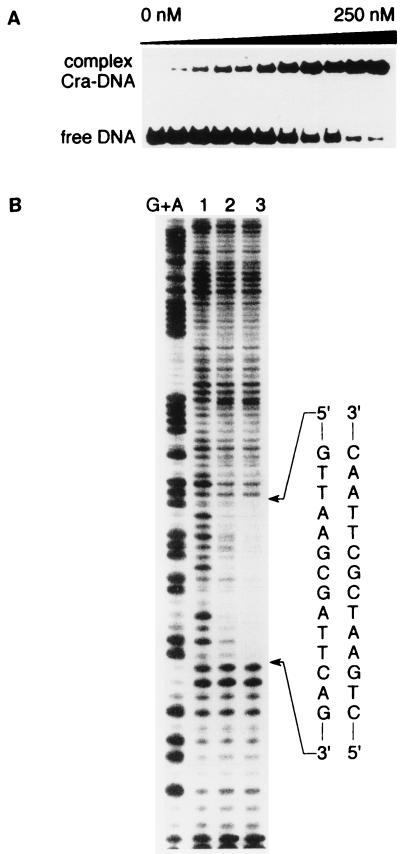
The binding affinity of the active Cra factor for the icd promoter region was measured by the EMSA (10). The radiolabeled 328-bp DNA fragment carrying both icd P1 and icd P2 was incubated with increasing concentrations of Cra, and the corresponding complexes were resolved from free DNA by gel electrophoresis and quantified after autoradiography. At the concentrations of Cra used in these experiments, single stable complexes could be resolved by EMSA (Fig. 4A), which indicated that Cra could interact in a specific manner with one operator site in the icd regulatory region. The bandshift assay was performed at a low concentration of DNA (4 pM) such that the approximate free protein concentration equaled the total protein concentration; thus, at half-maximal saturation, there was >10-fold molar excess of protein relative to nucleic acid (4). The equilibrium binding constant, Kd, could therefore be derived as the total protein concentration at half-maximal saturation. The Kd value for Cra binding to the icd regulatory region at pH 7.9 and 25°C was about 10 nM, which indicated a relatively high binding affinity of Cra for this particular region of DNA.
FIG. 4.
Characterization of the Cra-icd regulatory region complex. (A) Relative strength of binding of Cra to the icd regulatory region. The 328-bp 32P-labeled icd DNA (4 pM) was incubated at 25°C with increasing (from 0 to 250 nM) concentrations of Cra protein and analyzed by EMSA. (B) Cra-mediated protection of the icd regulatory region against digestion by DNase I. Target DNA was incubated in the absence (lane 1) or in the presence of 10 and 50 nM Cra protein (lanes 2 and 3, respectively). Samples were separated on a 6% polyacrylamide sequencing gel. G+A shows the Maxam-Gilbert sequencing ladder of the probe; arrows delineate the footprint produced by Cra.
To characterize further the zone of the icd P2 promoter that specifically interacts with Cra, the nucleotide sequence protected from DNase I digestion after binding of the protein was analyzed. The results presented in Fig. 4B show that upon addition of Cra, a protected region centered at position −76.5 with respect to the transcription start site of the downstream P2 promoter could be identified. This protected region contained the sequence 5′-CTGAATC/GCTTAAC-3′, which is displaced by one base pair compared to the previously determined (23, 27, 28) consensus Cra binding site sequence, 5′-GCTGAATC/GCT-3′. This finding suggested that similarly to the ppsA promoter (25, 27), transcription is activated only when Cra and RNAP bind to opposite faces of the DNA helix. In this regard, previous reports on the regulation of different transcription systems have demonstrated that when a correct helical phasing with RNAP is achieved, the cyclic AMP receptor protein, for instance, is able to activate transcription at a varying distance upstream from the transcription start site of the promoter, even though the extent of such activation varies with the length of the spacer (7, 11, 35, 37). It has been proposed that upon binding to DNA, Cra, like the cyclic AMP receptor protein, helps to recruit RNAP by bending the proximal DNA region located upstream of the promoter, thereby favoring tight contacts between DNA and the C-terminal domain of the α subunit (αCTD) of RNAP (7, 25).
Effects of Ala substitutions in the αCTD of RNAP on icd transcriptional activation.
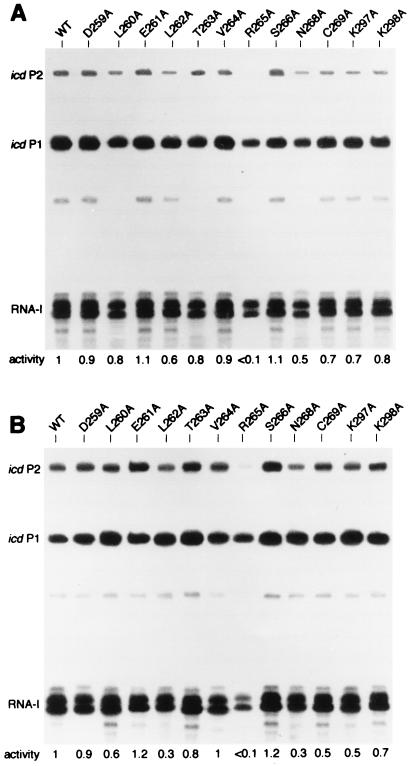
To determine the importance of the αCTD in icd P2 transcription, we treated a series of alanine mutant RNAPs containing point mutations in this region (22) for transcription in the presence of plasmid pJFC1. The results (Fig. 5) showed that each mutant RNAP tested could initiate transcription from the icd P1 promoter at nearly the same rate as the wild-type enzyme. On the other hand, the transcription initiated from the icd P2 promoter, with or without Cra, was considerably reduced when incubation was carried out with mutant RNAPs containing substitution L262A, R265A, and N268A (α[L262A], α[R265A], and α[N268A]), whereas a less drastic effect was observed with mutant RNAPs α[L260A], α[C269A], and α[K297A]. Moreover, comparison of the icd P2/icd P1 transcription ratio, in both the presence and absence of Cra, showed that both basal transcription (Fig. 5A) and the Cra-dependent activation of transcription (Fig. 5B) were affected concomitantly by these mutations. This result strongly supports the hypothesis that during formation of the closed complex, basal transcription initiation as well as Cra-dependent activation of transcription strictly rely on contacts established between the α subunit and the proximal DNA region upstream from the promoter.
FIG. 5.
In vitro transcription by wild-type (WT) and mutant RNAPs. Reconstituted RNAP holoenzymes containing Ala substitutions in the αCTD were assayed for transcription in the presence (A) or absence (B) of Cra. Single-round transcriptions were performed by using supercoiled plasmid pJFC1 as the template in the presence of mutant RNAP and protein Cra in a molar ration of 1:1. Transcripts were separated in a 6% sequencing gel and detected by autoradiography. The ratio of the transcriptional activity at icd P2 relative to that at icd P1 is indicated below each gel.
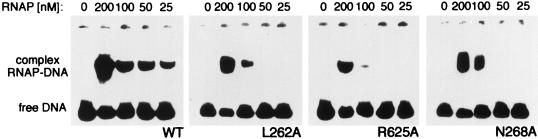
We assessed the DNA-binding capacity of each of the three mutants (α[L262A], α[R265A], and α[N268A]) that exhibited the highest deficiency in transcription initiation. In each case, EMSA in the presence of the radiolabeled icd P2 promoter was performed. The results (Fig. 6) showed that DNA binding was strongly impaired in all three mutant enzymes compared to the wild-type RNAP. It therefore appears that any substitution of an amino acid residue either located on the DNA contact surface of the α subunit (Arg-265 and Asn-268) or required for maintaining the correct conformation of this DNA-binding surface (Leu-262) (16) would result in the destabilization of the α-subunit-mediated anchoring of the RNAP on the icd P2 promoter.
FIG. 6.
Characterization of the complexes between wild-type (WT) or mutant RNAPs and the icd P2 promoter. A 120-bp 32P-labeled DNA carrying the icd P2 promoter region was incubated at 25°C with a decreasing concentration of RNAP and analyzed by EMSA. Positions of the protein-DNA complex and free DNA are indicated.
Concerning the suggested role of Cra in the activation of transcription at icd P2, the DNA bending caused by this protein (25) at its binding site around position −76.5 would modify the structure of the DNA region upstream of the promoter so as to facilitate its interaction with RNAP and thereby activate formation of the closed complex. Cra would thus compensate for the absence of an UP (upstream) element in icd P2 (8) by changing the structure of proximal DNA so as to make it operate like an UP module (22).
ACKNOWLEDGMENTS
This work was supported by the CNRS (UPR 412), Université de Lyon, Institut Universitaire de France, and grants from the Ministry of Education, Science and Culture of Japan.
We thank Antony W. Coleman for reading the manuscript. We also thank Christian Van Herrewege and Alain Bosch for help in iconography.
REFERENCES
- 1.Brownlee G G, Sanger F, Barrell B G. Nucleotide sequence of 5S ribosomal RNA from Escherichia coli. Nature. 1967;215:735–736. doi: 10.1038/215735a0. [DOI] [PubMed] [Google Scholar]
- 2.Brunelle A, Schleif R F. Missing contact probing of DNA-protein interactions. Proc Natl Acad Sci USA. 1987;84:6673–6676. doi: 10.1073/pnas.84.19.6673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buc H, McClure W R. Kinetics of open complex formation between Escherichia coli RNA polymerase and the lac UV5 promoter. Evidence for a sequential mechanism involving three steps. Biochemistry. 1985;24:2712–2723. doi: 10.1021/bi00332a018. [DOI] [PubMed] [Google Scholar]
- 4.Carey J. Gel retardation at low pH resolves trp repressor-DNA complexes for quantitative study. Proc Natl Acad Sci USA. 1988;85:975–979. doi: 10.1073/pnas.85.4.975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chao G C, Shen J, Tseng C P, Park S J, Gunsalus R P. Aerobic regulation of isocitrate dehydrogenase gene (icd) expression in Escherichia coli by the arcA and fnr gene products. J Bacteriol. 1997;179:4299–4304. doi: 10.1128/jb.179.13.4299-4304.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cortay J C, Nègre D, Scarabel M, Ramseier T M, Vartak N B, Reizer J, Cozzone A J. In vitro asymmetric binding of the pleiotropic regulatory protein, FruR, to the ace operator controlling glyoxylate shunt enzyme synthesis. J Biol Chem. 1994;269:14885–14891. [PubMed] [Google Scholar]
- 7.Dethiollaz S, Eichenberger P, Geiselmann J. Influence of DNA geometry on transcriptional activation in Escherichia coli. EMBO J. 1996;15:5449–5458. [PMC free article] [PubMed] [Google Scholar]
- 8.Estrem S T, Gaal T, Ross W, Gourse R L. Identification of an UP element consensus sequence for bacterial promoters. Proc Natl Acad Sci USA. 1998;95:9761–9766. doi: 10.1073/pnas.95.17.9761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Garnak M, Reeves H C. Purification and properties of phosphorylated isocitrate dehydrogenase of Escherichia coli. J Biol Chem. 1979;254:7915–7920. [PubMed] [Google Scholar]
- 10.Garner M M, Revzin A. A gel electrophoresis method for quantifying the binding of proteins to specific DNA regions: application to components of the Escherichia coli lactose operon regulatory system. Nucleic Acids Res. 1981;9:3047–3060. doi: 10.1093/nar/9.13.3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gaston K, Bell A, Kolb A, Buc H, Busby S. Stringent spacing requirements for transcription activation by CRP. Cell. 1990;62:733–743. doi: 10.1016/0092-8674(90)90118-x. [DOI] [PubMed] [Google Scholar]
- 12.Harley C B, Reynolds R P. Analysis of E. coli promoter sequences. Nucleic Acids Res. 1987;15:2343–2346. doi: 10.1093/nar/15.5.2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hawley D K, McClure W R. In vitro comparison of initiation properties of bacteriophage lambda wild-type PR and ×3 mutant promoters. Proc Natl Acad Sci USA. 1980;77:6381–6385. doi: 10.1073/pnas.77.11.6381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Igarashi K, Ishihama A. Bipartite functional map of the E. coli RNA polymerase alpha subunit: involvement of the C-terminal region in transcription activation by cAMP-CRP. Cell. 1991;32:319–325. doi: 10.1016/0092-8674(91)90553-b. [DOI] [PubMed] [Google Scholar]
- 15.Iuchi S, Weiner L. Cellular and molecular physiology of Escherichia coli in the adaptation to aerobic environments. J Biochem. 1996;120:1055–1063. doi: 10.1093/oxfordjournals.jbchem.a021519. [DOI] [PubMed] [Google Scholar]
- 16.Jeon Y H, Negishi T, Shirakawa M, Yamazaki T, Fujita N, Ishihama A, Kyogoku Y. Solution structure of the activator contact domain of the RNA polymerase α subunit. Science. 1995;270:1495–1497. doi: 10.1126/science.270.5241.1495. [DOI] [PubMed] [Google Scholar]
- 17.Jorgensen E D, Durbin R K, Risman S S, McAllister W T. Specific contacts between the bacteriophage T3, T7 and SP6 RNA polymerases and their promoters. J Biol Chem. 1991;266:645–651. [PubMed] [Google Scholar]
- 18.Kornberg H L. The role and control of the glyoxylate shunt in Escherichia coli. Biochem J. 1966;99:1–11. doi: 10.1042/bj0990001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.LaPorte D C, Koshland D E., Jr A protein with kinase and phosphatase activities involved in regulation of tricarboxylic acid cycle. Nature. 1982;300:458–460. doi: 10.1038/300458a0. [DOI] [PubMed] [Google Scholar]
- 20.Maxam A M, Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65:499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- 21.Minton N P. Improved plasmid vectors for the isolation of translational lac gene fusions. Gene. 1984;31:269–273. doi: 10.1016/0378-1119(84)90220-8. [DOI] [PubMed] [Google Scholar]
- 22.Murakami K, Fujita N, Ishihama A. Transcription factor recognition surface on the RNA polymerase alpha subunit is involved in contact with the DNA enhancer element. EMBO J. 1996;15:4358–4367. [PMC free article] [PubMed] [Google Scholar]
- 23.Nègre D, Bonod-Bidaud C, Geourjon C, Deléage G, Cozzone A J, Cortay J C. Definition of a consensus DNA-binding site for the Escherichia coli pleiotropic regulatory protein, FruR. Mol Microbiol. 1996;21:257–266. doi: 10.1046/j.1365-2958.1996.6341350.x. [DOI] [PubMed] [Google Scholar]
- 24.Nègre D, Bonod-Bidaud C, Oudot C, Prost J F, Kolb A, Ishihama A, Cozzone A J, Cortay J C. DNA flexibility of the UP element is a major determinant for transcriptional activation at the Escherichia coli acetate promoter. Nucleic Acids Res. 1997;25:713–718. doi: 10.1093/nar/25.4.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nègre D, Oudot C, Prost J F, Murakami K, Ishihama A, Cozzone A J, Cortay J C. FruR-mediated transcriptional activation at the ppsA promoter of Escherichia coli. J Mol Biol. 1998;276:355–365. doi: 10.1006/jmbi.1997.1548. [DOI] [PubMed] [Google Scholar]
- 26.Nimmo G A, Nimmo H G. The regulatory properties of isocitrate dehydrogenase kinase and isocitrate dehydrogenase phosphatase from Escherichia coli and the role of these activities in the control of isocitrate dehydrogenase. Eur J Biochem. 1984;141:409–414. doi: 10.1111/j.1432-1033.1984.tb08206.x. [DOI] [PubMed] [Google Scholar]
- 27.Ramseier T M, Bledig S, Michotey V, Feghali R, Saier M H., Jr The global regulatory protein FruR modulates the direction of carbon flow in Escherichia coli. Mol Microbiol. 1995;16:1157–1169. doi: 10.1111/j.1365-2958.1995.tb02339.x. [DOI] [PubMed] [Google Scholar]
- 28.Ramseier T M, Nègre D, Cortay J C, Scarabel M, Cozzone A J, Saier M H., Jr In vitro binding of the pleiotropic transcriptional regulatory protein, FruR, to the fru, pps, ace, pts and icd operons of Escherichia coli and Salmonella typhimurium. J Mol Biol. 1993;234:28–44. doi: 10.1006/jmbi.1993.1561. [DOI] [PubMed] [Google Scholar]
- 29.Reddy K J, Webb R, Sherman L A. Bacterial RNA isolation with one-hour centrifugation in a table-top ultracentrifuge. BioTechniques. 1990;8:250–251. [PubMed] [Google Scholar]
- 30.Saier M H, Jr, Chin A M. Energetics of the bacterial phosphotransferase system in sugar transport and the regulation of carbon metabolism. In: Krulwich T A, editor. Bacterial energetics. New York, N.Y: Academic Press, Inc.; 1990. pp. 273–299. [Google Scholar]
- 31.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- 32.Sasse-Dwight S, Gralla J. KMnO4 as a probe for lac promoter DNA melting and mechanism in vivo. J Biol Chem. 1989;264:8074–8081. [PubMed] [Google Scholar]
- 33.Studier W F, Rosenberg A H, Dunn J J, Dubendorff J W. Use of T7 RNA polymerase to direct expression of cloned genes. Methods Enzymol. 1990;185:60–89. doi: 10.1016/0076-6879(90)85008-c. [DOI] [PubMed] [Google Scholar]
- 34.Thorsness P E, Koshland D E., Jr Inactivation of isocitrate dehydrogenase by phosphorylation is mediated by the negative charge of the phosphate. J Biol Chem. 1987;262:10422–10425. [PubMed] [Google Scholar]
- 35.Ushida C, Aiba H. Helical phase dependent action of CRP: effect of the distance between the CRP site and the −35 region on promoter activity. Nucleic Acids Res. 1990;18:6325–6330. doi: 10.1093/nar/18.21.6325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Werel W, Schickor P, Heumann H. Flexibility of the DNA enhances promoter affinity of Escherichia coli RNA polymerase. EMBO J. 1991;10:2589–2594. doi: 10.1002/j.1460-2075.1991.tb07800.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wing H, Williams S, Busby S. Spacing requirements for transcription activation by Escherichia coli FNR protein. J Bacteriol. 1995;177:6704–6710. doi: 10.1128/jb.177.23.6704-6710.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]