Abstract
Wearable grip sensing shows potential for hand rehabilitation, but few studies have studied feasibility early after stroke. Here, we studied a wearable grip sensor integrated with a musical computer game (MusicGlove). Among the stroke patients admitted to a hospital without limiting complications, 13% had adequate hand function for system use. Eleven subjects used MusicGlove at home over three weeks with a goal of nine hours of use. On average they achieved 4.1 +/− 3.2 (SD) hours of use and completed 8627 +/− 7500 grips, an amount comparable to users in the chronic phase of stroke measured in a previous study. The rank-order usage data were well fit by distributions that arise in machine failure theory. Users operated the game at high success levels, achieving note-hitting success >75% for 84% of the 1061 songs played. They changed game parameters infrequently (31% of songs), but in a way that logically modulated challenge, consistent with the Challenge Point Hypothesis from motor learning. Thus, a therapy based on wearable grip sensing was feasible for home rehabilitation, but only for a fraction of subacute stroke subjects. Subjects made usage decisions consistent with theoretical models of machine failure and motor learning.
Keywords: Wearable sensing, stroke, rehabilitation, hand movement, home therapy, music therapy
I. INTRODUCTION
UPPER limb sensorimotor function is severely impacted after stroke with about 80% of patients experiencing deficits early after symptom onset. Additionally, upper limb impairment persists in about 60% of patients 6 months post-stroke [1]. Intensive movement practice can help reduce hand impairment after stroke [2]–[7], but cost and accessibility limit an individual’s ability to reach the high number of task repetitions thought necessary to improve recovery [8]–[10].
Home-based rehabilitation programs have been prescribed after stroke with the intent to increase the amount of rehabilitation exercise individuals can perform. The most common approach to home-based therapy is following a printed handout of exercises prescribed by a therapist. But, compliance with performing a list of exercises prescribed for in-home rehabilitation therapy is poor across a wide range of exercise types [11]–[15]. Thus, a critical outstanding question is how to motivate stroke patients to exercise in the home setting.
Several studies in the chronic phase (>6 months post stroke) after stroke [15]–[19] have examined different strategies for in-home hand rehabilitation with mixed results. Modified constraint-induced movement therapy performed under the supervision of a nonprofessional coach in the home setting produced similar benefits compared to a program performed with a trained therapist in a clinical setting [16]. Greater self-reported use of the impaired limb in comparison to conventional therapy. [17] was also observed. Another approach is tele-rehabilitation, which enables a therapist to guide training remotely. A systematic review of 10 trials with 933 total subjects found insufficient evidence to reach any substantial conclusions about the effectiveness of tele-rehabilitation after stroke, and most of these studies were applied in the chronic phase of stroke [20]. However, a recent study suggested that home-based telerehabilitation with a sensor-based system [21] that encouraged upper extremity movement practice following subacute stroke was not inferior to in-clinic training [19]. Other approaches to home-based hand rehabilitation include functional electrical stimulation [22], computer gaming with custom devices [23]–[25], and music-based therapy [26].
Despite the variety of options, it is still unclear which methods are the most viable for providing hand rehabilitation training at home, particularly early after a stroke (defined here as the first six months post stroke). Previous studies have shown that wearable movement sensors coupled with computer games can be motivating for rehabilitation [24], [27]–[29]. We explored this concept further by developing the MusicGlove device, an instrumented glove with sensors on each of the fingertips and the lateral aspect of the index finger (Fig. 1) [30], [31]. Home-based training by persons in the chronic phase of stroke led to significantly greater improvements in self-reported functional use of the impaired hand [32].
Fig. 1.
MusicGlove device used in study. Users viewed a musical computer game that visually cued them using scrolling notes to make specific gripping movements in time with the notes. The device detects the movements using conductive finger pads. For the present study, the game was played on a 9 in. tablet computer.
The present study sought to evaluate the feasibility of the MusicGlove as a home-based rehabilitation tool for individuals in the subacute period following stroke. Using such a wearable sensor soon after stroke at home raises several questions. First, as with many wearable sensors for hand rehabilitation, users need a moderate level of preserved hand function to effectively operate the MusicGlove. Users must be able to self-don it at home and complete the required gripping movements to play the associated computer game. Hence, the first feasibility goal of this study was to determine the fraction of individuals in the subacute phase of stroke who had adequate hand function to use such a wearable grip sensing approach.
Second, individuals in the subacute phase of stroke have just experienced a major life-changing event and are typically receiving standard-of-care rehabilitation therapy. They often have more medical appointments than people in the chronic phase after stroke, which might influence motivation to participate in additional therapies. A second feasibility goal was to determine if individuals in this population would use the MusicGlove as much as people in the chronic phase, as measured in an identical study protocol [32].
Third, a concern about self-administered care in the home setting is whether patients will appropriately challenge themselves. We therefore sought to characterize how users chose the game parameters that determined the challenge they experienced as they played.
Finally, we sought to establish a preliminary estimate of the effect of MusicGlove use on hand function in subacute stroke.
II. METHODS
A. Study Design, Recruitment, and Inclusion Criteria
The University of California, Irvine (UCI) Institutional Review Board approved this randomized, controlled single-blind cross-over study, and all subjects provided informed consent prior to enrollment in the study. The study was designed to compare self-guided exercise with the MusicGlove to self-guided conventional hand therapy, both performed in the participant’s home. The study was registered at ClinicalTrials.gov (NCT02410629). We included a control group because the original intent was to determine the therapeutic effect of MusicGlove. However, budgetary constraints and slow recruitment limited sample size, causing us to focus in this paper on feasibility rather than therapeutic results. Subjects were recruited by fliers distributed to local rehabilitation programs and by screening all new stroke subjects admitted at the UCI Medical Center. The inclusion criteria for the study are shown in Table I. Note that Table I contains more detail about the final cutoffs used for various impairments in comparison to the table presented on ClincialTrials.gov. Potential subjects who did not qualify for the study were re-assessed after a few weeks to determine if their hand recovery progressed to a level that would allow them to participate.
TABLE I.
INCLUSION CRITERIA
| 18 to 80 years of age |
| History of stroke affecting the hand |
| Between 1–10 weeks post-stroke |
| Upper extremity weakness, defined as score of 15–62 (out of 66) on the Upper Extremity Fugl-Meyer Test |
| Able to perform at least 3 blocks on the Box and Blocks Test (BBT) but not greater than 80% of the score of the non-affected hand on the BBT |
| No other active major neurological disease other than stroke |
| Absence of severe pain in the stroke-affected upper extremity - score ≤ 3 on Visual Analog Pain scale |
| Absence of severe spasticity or contractures at the affected upper extremity (score <4 on the Modified Ashworth Scale) |
| Absence of severe aphasia |
| Absence of severe reduction in level of consciousness |
| Absence of severe sensory / proprioception deficit at the affected upper extremity (score of 0 in all categories of the Fugl-Meyer Sensory Examination) |
| Not currently pregnant |
| No active major psychiatric problems, or neurological/orthopedic problems affecting the stroke affected upper extremity |
| No difficulty in understanding or complying with instructions given by the experimenter |
| Able to perform the experimental task |
B. Group Assignment and Intervention
In this cross-over design, a total of 11 subjects were randomized to receive either MusicGlove therapy first (MG 1st group) or conventional therapy (MG 2nd group) (Fig. 2). To ensure matched levels of impairment between groups, subjects were first stratified by their Box and Blocks Test (BBT) baseline score (3–30 or 30–60) and then assigned to a group by adaptive randomization [33]. The BBT is an established clinical measure of hand function that measures the number of blocks an individual can pick up and move over a divider in one minute; a normal score is about 60 blocks/min [34]. The MG 2nd group was trained to follow a booklet of conventional hand exercises [32] while the MG 1st group was trained to use a MusicGlove and tablet computer (Fig. 1) as their first intervention; training took about 30 minutes.
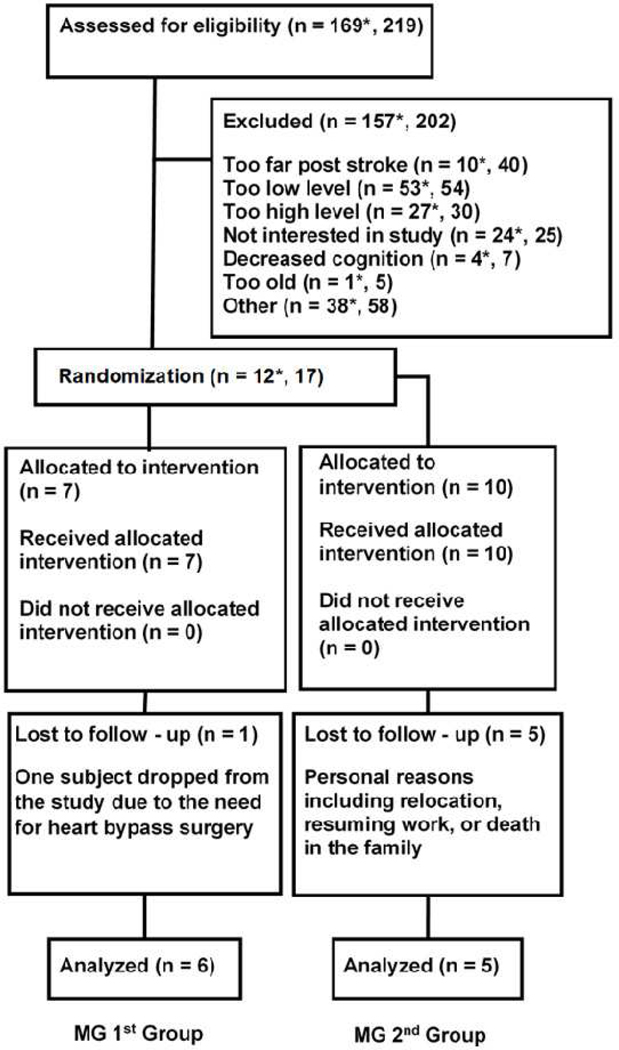
Fig. 2.
Consolidated Standards of Reporting Trials Diagram. * denotes numbers from consecutively enrolled patients to a single hospital; the total number from all recruitment sources is shown as well.
In the initial training session, the project therapist showed subjects how to play the game, including changing game difficulty parameters and how changing the parameters affected the game. The therapist also instructed subjects that they were free to change the difficulty of the games as they wished. When the subjects took the MusicGlove home, they started at whatever difficulty setting they chose. Subjects were asked to perform at least three hours of their intervention per week for three weeks.
Subjects were free to modulate the difficulty of their MusicGlove training by changing the number of grip types (1–5: lateral pinch, index-thumb, middle-thumb, ring-thumb, pinky-thumb grips) needed to play, and/or by selecting songs at three different difficulty levels, where difficulty was determined by the number of target notes per minute of song. Subjects were also free to choose whether to play the game in “Song Mode” or “Session Mode”. In “Session Mode”, several songs at the same difficulty level are played in series and subjects can make changes to the game parameters after the series of songs has ended. Subjects could select series of 15, 30, 45, or 60 minutes in length. In “Song Mode” subjects could modify game parameters after each individually-selected song.
After the 3-week exercise period, the participants returned for a post therapy assessment, at which they returned the MusicGlove device or booklet of hand exercises. Then, after another 3-week period, they returned for the 3-wk follow-up assessment, followed by an assessment when they were 16-weeks post-stroke. At the 16-wk follow-up, individuals in the MG 2nd group were given the MusicGlove to use while individuals in the MG 1st group were given a booklet of hand therapy exercises. Each group matched the previous protocol, used the given intervention for three weeks, ceased activity for 3 weeks, and then returned for their follow-up at 6 months post stroke. During this study subjects received simultaneous rehabilitation therapy as part of their standard-of-care treatment. We did not control for the amount or content of this treatment as we deemed it both impractical and unethical.
C. Outcome Measurements
An experienced, blinded rehabilitation therapist performed a set of clinical assessments at baseline and at each additional time point during the study. We choose the follow-up periods (16-wk post-stroke, 6 months post-stroke) with respect to the onset of stroke as opposed to start of intervention in order to minimize the variance caused by spontaneous recovery, since the rate of spontaneous recovery varies depending on the time post stroke. The BBT score evaluated at the 3-wk post-intervention follow-up was preregistered on clinicaltrials.gov as the primary outcome measure. This paper focuses on this clinical measure of hand function only.
D. Data Analysis
We analyzed the data from periods of use of the MusicGlove device for usership metrics for each subject including: the success rate (# of notes completed / # of notes presented), amount of practice (as measured by the # of grips presented and the total usage time), and the types of in-game adjustments (i.e. changing song difficulty or grip types used). We assessed the distribution of the amount of grip practice by rank-ordering subjects, a common approach in non-parametric statistics. We used the R package fitdistrplus [35] to fit probability distributions to the data, and used Akaike Information Criterion (AIC) to evaluate goodness of fit.
We tested whether the probability of making a parameter change on the next song depended on the level of success achieved with the previous song using linear regression. For this analysis, we considered only songs that were not already at the lowest or highest difficulty levels. If the user increased the difficulty of one or both game parameters, we classified that as increasing game difficulty, and vice versa. Instances in which users increased one parameter and decreased the other were treated as no change in difficulty. The probability of changing the difficulty of the game was calculated for ranges of success using a sliding window of 10 jumping by 2 (i.e. success of previous song was between 0–10, then 2–12, etc.). Usership analyses were first applied to individual subjects, then averaged across all subjects.
III. RESULTS
A. Fraction of Subacute Stroke Patients Suitable for Device
A total of 219 potential subjects were screened; 169 of these were stroke patients at a single university hospital and were available to enroll in the study (Fig. 2). Considering the consecutively screened stroke patients only, 92 met all other inclusion/exclusion criteria (Table I) before considering level of hand impairment. However, when considering hand impairment, 58% (53) of the consecutively screened potential subjects had too little hand function, 29% (27) had too high hand function, and 13% (12) had an appropriate level of hand function and enrolled in the study. Five subjects referred from other hospitals also enrolled, for a total of 17.
Five subjects withdrew from the MG 1st group due to personal reasons including moving to a different country, resuming work, or a death in the family. One more subject withdrew from the MG 2nd group due to the need to undergo heart surgery. Thus, there were a total of six subjects in the MG 1st group and five in the MG 2nd group who completed all research procedures.
B. Usage Patterns: Amount of Use
The MusicGlove computer logs revealed that the 11 subjects used the device on average 4.1 (+/− 3.2 SD) hours, which was 46% of the recommended 9 hours, and completed on average a total of 8627 (+/− 7500 SD) grips (Figure 3). This number of grips was comparable to the amount in the previous study of individuals in the chronic phase after stroke (mean 6953 +/− 6546 SD, t-test, p = 0.8) (Figure 3C) [30]. In this previous study, subjects followed an identical protocol. In the present study, the MG 1st group had an initial BBT score of 21 +/− 14 (compared to 33.0 +/− 10.6 in the prior study), while the MG 2nd group had an initial BBT score of 33 +/− 15 (compared to 32.6 +/− 10.6 in the prior study).
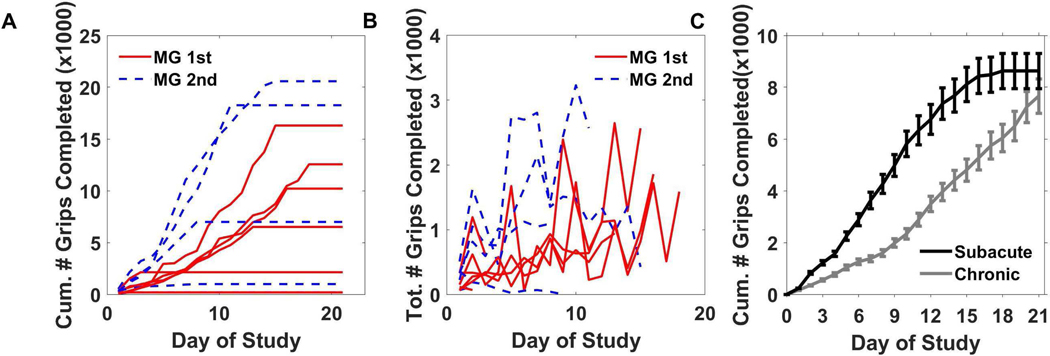
Fig. 3.
Summary of usership of the MusicGlove device. A) The cumulative number of grips completed by each subject in the group that received the MusicGlove first (MG 1st), and the group that used the MusicGlove second, after three weeks of conventional home therapy (MG 2nd). B) The total number of grips completed each day by each subject for both groups. C) The average cumulative number of grips completed by the subjects from the current study compared to number completed by chronic stroke survivors from a previous study [30]. Bars show ±1 SE.
We compared this level of compliance in total use time to other studies of technologies for home rehabilitation of the upper extremity that report individual usage data (Fig 4B) [36], [37]. Both studies were conducted with chronic stroke survivors with the time-after-stroke being 32.8 +/− 12.0 and 91.3-weeks post stroke respectively. However, in [36] the intervention was a virtual reality glove while in [37] the intervention was a hand orthosis combined with an arm support system. In terms of the level of impairment subjects in [36] had an average Wolf Motor Function Test score of 3.8 +/− 3.9. While subjects in [37] had an average Fugl-Meyer Assessment score of 37.0. Note that in [37] only averages were given, and standard deviations were not reported. For comparison the average Fugl-Meyer Assessment score was 44.3 +/− 12.6 for the MG 1st group, and 42.4 +/− 8.7 for the MG 2nd group. In these studies, the average compliance was 58% and 46%. Additionally, when program compliance was plotted against the subject normalized rank for each study, they both followed a similar pattern that decreased continuously (i.e. subjects could not be classified easily as high and low users).
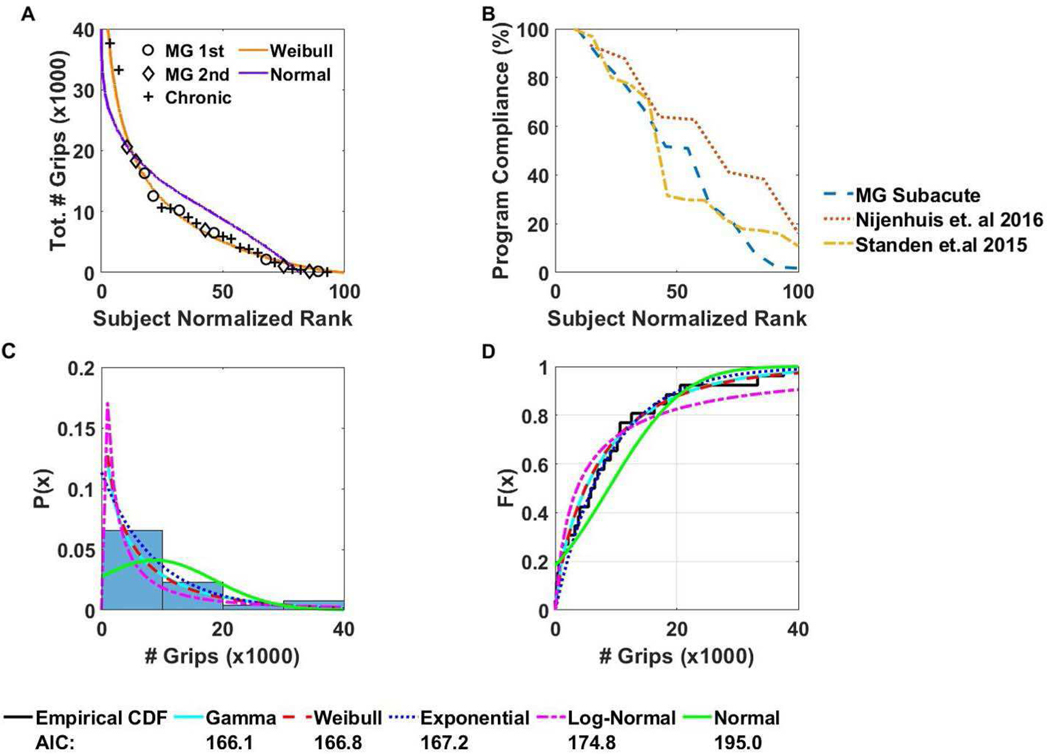
Fig. 4.
Analysis of underlying distribution of grip data, and user program compliance. A) The total number of grips completed versus the subject normalized rank (subject number / total number of subjects ∗100). Data from present and previous study with chronic users are combined. A Weibull distribution (shape parameter λ = 9400, scale parameter k = 0.96) fit the data well, better than a normal distribution B) Program compliance (# of hours device used / recommended hours of use) versus the subject normalized rank. Each line represents a different study which utilized a different home rehabilitation technology for the upper extremity C) Histogram of the total number of grips completed by all subjects against the probability distribution function estimate. Each distribution’s probability distribution is also plotted over the histogram. D) Empirical cumulative distribution function plotted against the theoretical cumulative distribution function of various distributions.
The difference between subacute and chronic study populations in cumulative amount of practice at each day during the study was not statistically significant (Fig. 3C). However, the subacute user group in the present study did, on average, significantly decrease the number of grips during week 3 compared to week 1 (paired t-test, p = .05), a pattern different from the chronic users, who significantly increased the number of grips during week 3 compared to week 1 (p = 0.008).
When we rank-ordered the users in terms of number of grips (Fig. 4A), we found that subjects again could not be grouped easily into clusters of high and low users. Rather, the rank-order distribution decreased smoothly, similar to the distribution from the previous chronic study. This led us to consider what type of probability distribution can generate this data. We combined data from the subacute and chronic studies for this analysis since they were not significantly different at any day (Fig. 4C). We found that the Gamma, Weibull, and Exponential fit the data well (Fig. 4D). These are related distributions that arise due to failure dynamics of machines, a connection we will return to in the Discussion.
C. Usage Patterns: Challenge Selection
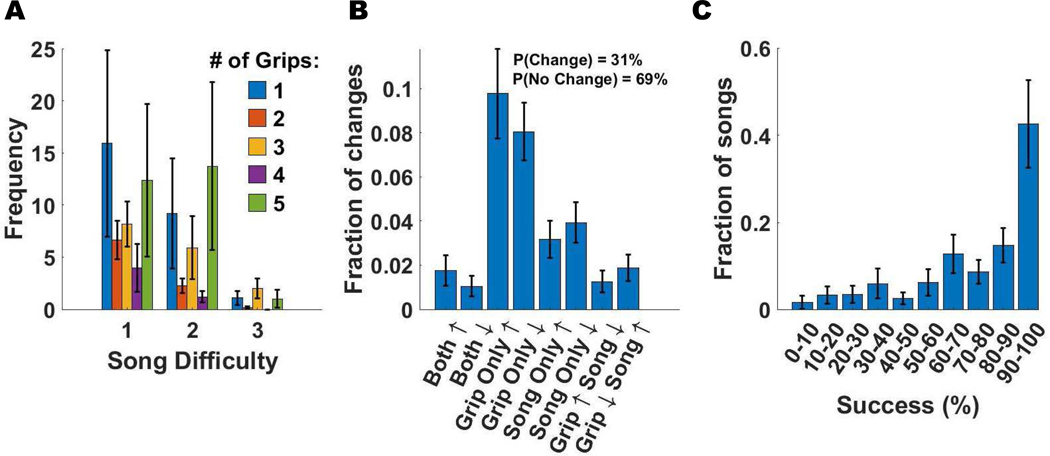
Subjects predominately played the game at song difficulty levels 1 and 2 and rarely at the most difficult level 3 (Fig. 5A). They most frequently used 1 or 5 grip types (Fig. 5A). Subjects changed parameters after 31% of the songs, favoring changing the number of grips over song difficulty (Fig. 5B). They achieved note-hitting success of greater than 75% for 84% of the 1061 songs played (Fig. 5C).
Fig. 5.
Analysis of adjustments made to game parameters across 1061 songs, as well as analysis of success levels. Each color on the plots A, B, D, E represents a different subject while each dot represents one song. A) Scatter plot of the song difficulty (1: easiest, 3: hardest) versus the number of grip types used B) Fraction of different types of parameter changes. The percent of games were a parameter was not changed was 69%. C) Fraction of songs played at different success levels.
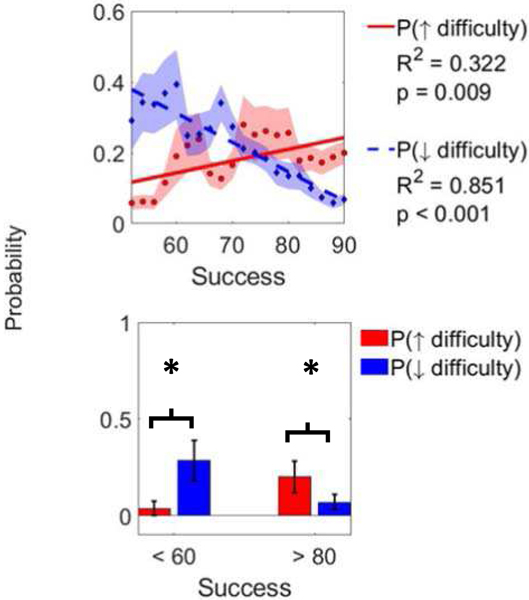
The probability of subjects increasing difficulty of gameplay increased with success (linear regression, R2 = 0.32, p = 0.009), and the probability of decreasing difficulty decreased with success (R2 = 0.85, p < 0.001) (Fig. 6). The success level at which the probability of increasing and decreasing difficulty were equal was 74%. Even though the probability of increasing difficulty increased with higher success, note that there was still a finite chance that subjects decreased difficulty (∼6% chance of decreasing difficulty at 90% success). The same pattern of randomness was true at low success levels (Fig. 6). When success was lower than 60% subjects were more likely to decrease the game difficulty (p =.05, two-tailed, paired t-test) while when success was higher than 80% subjects were more likely to increase game difficulty (p = .02).
Fig. 6.
Top. Probability of increasing (solid line) or decreasing (dashed line) game difficulty (via song difficulty or number of grips) as a function of success on previous song. Each point is a probability calculated based on all songs played within 10 points of success level of that point. We required at least 100 songs to plot a point. Since subjects rarely played at low success levels, no points below 65% success were included. Bottom. Comparison of probabilities of increasing or decreasing game difficulty at low and high success. ∗ denotes p < 0.05.
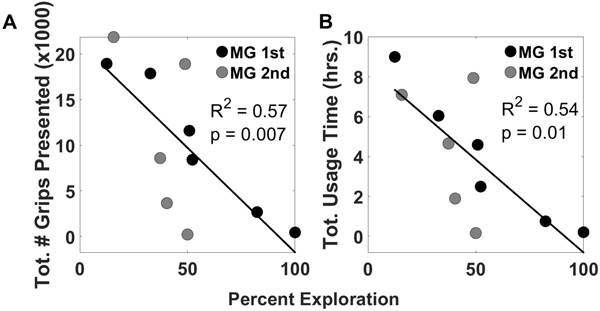
The amount of practice (measured by either the number of grips presented or total usage time) was not correlated with the average level of success experienced or initial impairment level, measured with the BBT. However, the amount of practice (measured as total # of grips presented Fig. 7A or total usage time, Fig 7B) was inversely correlated with the amount of parameter exploration (defined as the total number of parameter adjustments/total number of songs played).
Fig. 7.
Amount of practice of each subject from both groups represented as both the total number of grips presented and the total usage time versus the percent exploration. Percent exploration is defined as the total number of song parameter adjustments / total number of sessions played.
D. Preliminary Estimate of the Effect of MusicGlove on Hand Function
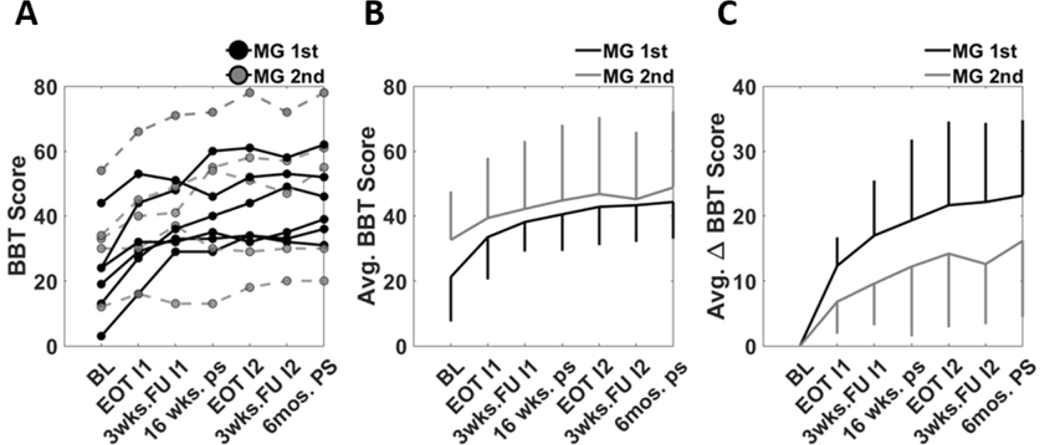
The average baseline BBT score prior to any intervention was 21 +/− 14 for the MG 1st group, and 33 +/− 15 for MG 2nd group (Fig. 8). The BBT score increased throughout the study. The MG 1st group had a greater average change in BBT score as compared to the MG 2nd group at all evaluations (e.g. 12 +/− 4 for MG 1st group vs 7 +/− 5 MG 2nd group at the end of the first phase of therapy). We did not perform a statistical analysis comparing groups because of the small sample size.
Fig. 8.
A) Individual trajectories of BBT score throughout the study. B) The average BBT scores for the two groups. Vertical lines represent one SD. C) The average change in BBT score relative to the baseline evaluation.
IV. DISCUSSION
A. Feasibility of Using Wearable Sensing for Finger Rehabilitation at Home Early After Stroke
Like many wearable movement sensors for hand rehabilitation, the MusicGlove requires a moderate level of hand function to be used effectively as the user must engage the sensor for it to register that a movement has occurred. From our previous work we determined that individuals with a score of at least three on the BBT can reliably operate the device [31]. Here, we found that only ~13% of individuals 1–10 weeks post-stroke who met all other inclusion criteria also met this hand function criterion. Conversely, nearly 60% had too poor of hand function to participate. This observation indicates the importance of continuing to develop alternative hand training technologies, especially for people early after stroke when the brain is considered to be more receptive to rehabilitation.
One possible solution is to design sensors that allow more subtle movements to be detected. MusicGlove is limited by the use of contact sensing pads that require specific movements to be completed, and thus the features of “acceptable movements” cannot be varied. MusicGlove also only provides information about movement completion rather than real-time position data. A recent study that examined the percentage of subacute stroke subjects able to control mobile gaming technologies found that about 60% could use the paretic hand to control a cursor with a tablet or smartphone with swiping motions, and 93% could control a cursor with isometric grip force control [38]. Such interfaces, coupled with games, could help make home-based hand training more accessible to more people with stroke.
Another possible solution is to add actuation to the device to physically assist the user in making gripping movements. However, adding actuation doesn’t solve the problem that the user must still generate hand-related control signals to activate the assistance. Robotic assistance applied to a passive user has little therapeutic benefit [39]. Detecting movement-related signals at the level of the brain [40], [41] or muscle [42], rather than relying on the resulting movement itself, is a possible solution, but increases complexity for home use because of the requirement to apply electrodes.
B. Usership of MusicGlove: Amount of Use
For subacute subjects with adequate hand function, using the MusicGlove was feasible. On average, the subjects in the present study utilized the system to achieve a number of grips slightly greater than the number that was completed by chronic stroke survivors in the previous study. Thus, the life circumstances associated with the subacute phase of stroke did not limit engagement with this technology. However, on average, the subacute users significantly decreased their usage over time, a pattern different from chronic users. Perhaps their ongoing spontaneous hand recovery contributed to more rapid abandonment. Alternately, they may have had relatively more untried therapy options available compared to chronic users, and abandoned MusicGlove in favor of exploring those options. One other possible explanation could that these are receiving standard-of care rehabilitation therapy and thus have an increased amount of medical appointments. This increased busy-ness could interfere with research procedures [43].
Despite achieving a relatively large number of grips on average (>8000), user compliance was moderate (46%) in completing the requested hours of use. Few studies exist that were conducted in the home setting with subjects from the sub-acute stroke population that report individual usage data, making direct comparisons difficult. Although the other home-based studies compared in this manuscript (Fig. 4B) were conducted with subjects from the chronic stroke population, they provide a start for understanding compliance with upper extremity rehabilitation devices in the home setting. In the current study moderate user compliance may have arisen in part due to poorer motivation to use the device amongst users with higher hand function, although amount of practice was not correlated with initial or final BBT score. Continuing to understand the factors influencing compliance is an important direction for future work.
Some insight might be gained by considering the distribution of amount of practice. We found that the Gamma and Weibull distributions fit the data well in comparison to a Normal distribution. These related distributions are commonly used to model machine failure. For example, a Gamma distribution arises as a time-to-first-fail distribution for a redundant system. If there are n-1 backup units and all backup units have exponential lifetimes, then the total lifetime has a Gamma distribution [44]. The Weibull distribution characterizes the time to failure for many machines [45]. This is because machines are typically made of many parts, each of which can cause the machine to fail. When each part lasts a minimum time, but then fails probabilistically, Extreme Value Theory can be used to show that the Weibull distribution arises for weak conditions on the part failure probability distributions [45]. It may be possible to draw an analogy to understand usership patterns of home rehabilitation technology. For example, there are dozens of probabilistic factors (e.g. psychological, technological, sociological, cultural, neurologic) that can cause a person to stop practicing with a home-based rehabilitation technology, and each likely has a minimum time to “activate”. Thus, one would expect usage to follow a Weibull distribution. The fact that a Weibull distribution fit the data well then suggests usership may rely on a large number of subject specific factors. Exploring the use of machine failure theory and reliability analysis to gain insight into home usership is an interesting future research direction.
C. Usership of MusicGlove: Challenge Selection
The Challenge Point Hypothesis (CPH) from the motor learning literature posits that there is an optimal task difficulty for promoting skill development [46]. The CPH has been proposed to apply to rehabilitation as well [47]. In the context of movement recovery, rehabilitation therapists normally select an appropriate challenge level for each patient for each therapy task, consist with the CPH. A concern about self-administered care in the home setting is whether patients will challenge themselves enough during therapy. In the present study, we allowed the user to modify at will two parameters that affected the challenge of training. A key question was whether they would use this ability in a way consistent with the CPH.
We observed that the subjects tended to leave the parameters at a level that allowed them to play the game at high success levels (>75% success for 84% of songs), infrequently making changes to the parameters (on only 31% of songs), though higher difficulty settings were available. When they adjusted parameters, they did so in a way consistent with the CPH - tending to increase difficulty if their success at the last song was high, or decrease difficulty if success was low. The magnitude of these changes was low (14% increase across a change in success of 40%). These findings illustrates that 1) users tended to not make changes to difficulty; 2) when users did make a change they tended to do the logical thing (increasing difficulty when success is high, and decreasing difficulty when success is low); 3) user behavior was stochastic or explorative, as there was still a finite probability users did the “illogical” thing (increase difficulty when success was low).
Within the stroke rehabilitation technology literature there exist many examples of adaptive algorithms for adjusting task difficulty based on movement performance [48]. These algorithms often adapt task parameters to modulate the level of challenge experienced by the user after each sensed movement attempt. These types of algorithms are thought to be advantageous as they can be tuned to provide assistance matching an individual’s changing needs. However, we observed in the current study that people infrequently made changes to the game parameters. Further, subjects who exhibited less exploration (defined as total number of game adjustments / total number of songs played) used the system more. This suggests that if we are to make algorithms that more closely align with desirable human usership behavior, a less aggressive (i.e. not adapting as often) and more stochastic approach (i.e. sometimes adapting in the “wrong” direction) may be warranted.
We also recently found in a study of robotic finger training that training with a higher success level (80% - generated by robotic assistance) resulted in higher motivation and better long-term retention, particularly for more-impaired users [49]. The fact that the subjects in the present study preferred similarly high success levels, coupled with their CPH-consistent parameter adjustment behavior, suggests that persons with a stroke indeed have intuition about how best to practice.
An interesting possibility is to more rigorously characterize each home user as a stochastic decision process and analyze whether subject-specific decision rules predict greater usage or better therapeutic results. Such analyses will require larger data sets, which hopefully will become available with the growth of home-based commercial rehabilitation technologies.
D. Limitations and Directions for Future Research
Budgetary constraints coupled with the small percentage of people who could qualify for the study hindered our ability to recruit the planned number of subjects for the study (N = 20 for each group). The small sample size, plus the fact the MG 2nd group had a higher baseline BBT score, made it unfeasible to directly compare the therapeutic effect of MG 1st to MG 2nd in the first training phase. However, the data provide an initial estimate of effect size, which can be useful for planning future studies. The data were also suggestive that earlier access to the MG produced a larger change in BBT score. These findings support conducting larger efficacy studies to test whether MusicGlove or other movement sensors for hand training can facilitate quicker or larger recovery of fine motor function.
We asked subjects to log their conventional hand training, but they did not consistently do so. Thus, we could not make comparisons in compliance or analyze possible dose effects of the conventional training approach. The amount of difficulty adjustment we observed may have been influenced by the instructions and by how subjects interpreted them. The influence of pre-training on the way users use home rehabilitation technology is an interesting topic for future research. We pooled subjects from the MG 1st and MG 2nd groups for analysis. There may have been order effects, such as that subjects in the MG 2nd had a lower level of hand impairment when they started using the MusicGlove because of ongoing recovery. However, we found significant effects for the combined group even with this possible source of increased variance.
V. CONCLUSION
Only a small fraction of consecutively enrolled stroke patients could qualify for this study, having the appropriate level of hand function for using a wearable movement sensor-based rehabilitation approach, and meeting the other inclusion criteria. This suggests that further research needs to be done to develop devices that can help a larger proportion of people who have a severe hand impairment early after stroke. Among the population with the required amount of hand function, the sensor and musical game presented in this study were feasible for autonomous home use and caused no adverse effects. We found a possible connection between machine failure theory and usership via the form of the distribution of amount of use. We also observed that subjects played mostly at high success levels, infrequently making parameter changes when playing the game. When they did make changes, they did so in a way consistent with the Challenge Point Hypothesis, but with an element of randomness suggestive of exploration. These analyses point to the need to analyze “in-the-wild” user decisions in larger populations to understand how usage patterns might be associated with longer and/or more effective use of rehabilitation devices.
Acknowledgments
This work was supported by grant 2R44HD074331-02 from the National Center for Medical Rehabilitation Research at the National Institute of Health.
Contributor Information
Quentin Sanders, Department of Mechanical and Aerospace Engineering, University of California, Irvine, CA, 92697 USA.
Vicky Chan, Rehabilitation Services, University of California at Irvine Medical Center, Irvine, CA, 92697 USA.
Renee Augsburger, Rehabilitation Services, University of California at Irvine Medical Center, Irvine, CA, 92697 USA.
Steven C. Cramer, Departments of Neurology, Anatomy and Neurobiology, and Physical Medicine and Rehabilitation at the University of California at Irvine, Irvine, CA, 92697 USA Department of Neurology at the University of California Irvine, Irvine, CA, 92697 USA.
David J Reinkensmeyer, Department of Mechanical and Aerospace Engineering, University of California, Irvine, CA, 92697 USA; department of Biomedical Engineering at the University of California Irvine; Departments of Neurology, Anatomy and Neurobiology, and Physical Medicine and Rehabilitation at the University of California at Irvine, Irvine, CA, 92697 USA.
An H. Do, Department of Neurology at the University of California Irvine, Irvine, CA, 92697 USA
REFERENCES
- [1].Schwarz A, Kanzler CM, Lambercy O, Luft AR, and Veerbeek JM, “Systematic review on kinematic assessments of upper limb movements after stroke,” Stroke, vol. 50, no. 3, pp. 718–727, 2019. [DOI] [PubMed] [Google Scholar]
- [2].Cramer SC, “The EXCITE trial: A major step forward for restorative therapies in stroke,” Stroke, vol. 38, no. 7, pp. 2204–2205, 2007. [DOI] [PubMed] [Google Scholar]
- [3].Sawaki L, “Use-dependent plasticity of the human motor cortex in health and disease,” IEEE Eng Med Biol Mag, vol. 24, no. 1, pp. 36–9., 2005. [DOI] [PubMed] [Google Scholar]
- [4].Ada L, Dorsch S, and Canning CG, “Strengthening interventions increase strength and improve activity after stroke: a systematic review.,” Aust. J. Physiother, vol. 52, no. 4, pp. 241–8, 2006. [DOI] [PubMed] [Google Scholar]
- [5].van der LJH, IA S, Beckerman H, GJ L, RC W, and LM B, “Exercise therapy for arm function in stroke patients: a systematic review of randomized controlled trials.,” Clin Rehabil, vol. 15(1), no. 01, pp. 20–31, 2001. [DOI] [PubMed] [Google Scholar]
- [6].Kloosterman MG, Snoek GJ, and Jannink MJ, “Systematic review of the effects of exercise therapy on the upper extremity of patients with spinal-cord injury,” Spinal cord Off. J. Int. Med. Soc. Paraplegia, 2008. [DOI] [PubMed] [Google Scholar]
- [7].Nudo RJ, “Postinfarct cortical plasticity and behavioral recovery,” Stroke, vol. 38, no. 2 PART 2, pp. 840–845, 2007. [DOI] [PubMed] [Google Scholar]
- [8].Lang CE et al. , “Observation of Amounts of Movement Practice Provided During Stroke Rehabilitation,” Arch. Phys. Med. Rehabil, vol. 90, no. 10, pp. 1692–1698, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Byl NN, a Pitsch E, and Abrams GM, “Functional outcomes can vary by dose: learning-based sensorimotor training for patients stable poststroke.,” Neurorehabil. Neural Repair, vol. 22, no. 5, pp. 494–504, 2008. [DOI] [PubMed] [Google Scholar]
- [10].Jeffers MS et al. , “Does Stroke Rehabilitation Really Matter? Part B: An Algorithm for Prescribing an Effective Intensity of Rehabilitation,” Neurorehabil. Neural Repair, vol. 32, no. 1, pp. 73–83, 2018. [DOI] [PubMed] [Google Scholar]
- [11].Cox KL, Burke V, Gorely TJ, Beilin LJ, and Puddey IB, “Controlled comparison of retention and adherence in home- vs center-initiated exercise interventions in women ages 40–65 years: The S.W.E.A.T. Study (Sedentary Women Exercise Adherence Trial).,” Prev Med, vol. 36, pp. 17–29, 2003. [DOI] [PubMed] [Google Scholar]
- [12].Rejeski WJ, Brawley LR, Ettinger W, Morgan T, and Thompson C, “Compliance to exercise therapy in older participants with knee osteoarthritis: implications for treating disability.,” Med Sci Sport. Exerc, vol. 29, pp. 977–985, 1997. [DOI] [PubMed] [Google Scholar]
- [13].Sluijs EM, Kok GJ, and Van Der Zee J, “Correlates of Exerice Compliance in Physical Therapy,” Phys. Ther, vol. 73, no. 1, pp. 771–782, 1993. [DOI] [PubMed] [Google Scholar]
- [14].Turton A.and Fraser C, “The use of home therapy programmes for improving recovery of the upper limb following stroke,” Br. J. Occup. Ther 1990. Nov;53(11)457–462, vol. 53, no. November, pp. 457–462, 1990. [Google Scholar]
- [15].Jurkiewicz MT, Marzolini S, and Oh P, “Adherence to a home-based exercise program for individuals after stroke.,” Top. Stroke Rehabil, vol. 18, no. 3, pp. 277–284, 2011. [DOI] [PubMed] [Google Scholar]
- [16].Barzel A.et al. , “Comparison of two types of Constraint-Induced Movement Therapy in chronic stroke patients: A pilot study,” Restor. Neurol. Neurosci, vol. 27, no. 6, pp. 673–680, 2009. [DOI] [PubMed] [Google Scholar]
- [17].Barzel A.et al. , “Enhancing activities of daily living of chronic stroke patients in primary health care by modified constraint-induced movement therapy (HOMECIMT): study protocol for a cluster randomized controlled trial.,” Trials, vol. 14, p. 334–2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Dodakian L.et al. , “A Home-Based Telerehabilitation Program for Patients With Stroke,” Neurorehabil. Neural Repair, vol. 31, no. 10–11, pp. 923–933, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Cramer SC et al. , “Efficacy of Home-Based Telerehabilitation vs InClinic Therapy for Adults after Stroke: A Randomized Clinical Trial,” JAMA Neurol., 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Laver KE, Schoene D, Crotty M, George S, Lannin NA, and Sherrington C, “Telerehabilitation services for stroke,” Cochrane Database Syst. Rev, vol. 2013, no. 12, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Dodakian L.et al. , “A home-based telerehabilitation program for patients with stroke,” Neurorehabil. Neural Repair, vol. 31, no. 10–11, pp. 923–933, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Hara Y, Ogawa S, Tsujiuchi K, and Muraoka Y, “A home-based rehabilitation program for the hemiplegic upper extremity by power-assisted functional electrical stimulation.,” Disabil. Rehabil, vol. 30, no. 4, pp. 296–304, 2008. [DOI] [PubMed] [Google Scholar]
- [23].Donoso Brown EV, McCoy SW, Fechko AS, Price R, Gilbertson T, and Moritz CT, “Preliminary investigation of an electromyography-controlled video game as a home program for persons in the chronic phase of stroke recovery,” Arch. Phys. Med. Rehabil, vol. 95, no. 8, pp. 1461–1469, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].King M, Hijmans J, Sampson M, Satherley J, and Hale L, “Homebased stroke rehabilitation using computer gaming,” New Zeal. J. Physiother, vol. 40, no. 3, pp. 128–134, 2012. [Google Scholar]
- [25].Slijper A, Svensson KE, Backlund P, Engström H, and Sunnerhagen KS, “Computer game-based upper extremity training in the home environment in stroke persons: a single subject design.,” J. Neuroeng. Rehabil, vol. 11, p. 35–2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Villeneuve M.and Lamontagne A, “Playing piano can improve upper extremity function after stroke: Case studies,” Stroke Res. Treat, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Hijmans JM, Hale L. a., Satherley J. a., McMillan NJ, and King MJ, “Bilateral upper-limb rehabilitation after stroke using a movement-based game controller,” J. Rehabil. Res. Dev, vol. 48, no. 8, p. 1005, 2011. [DOI] [PubMed] [Google Scholar]
- [28].Yoo J, “The Role of Therapeutic Instrumental Music Performance in Hemiparetic Arm Rehabilitation,” Music Ther. Perspect, vol. 27, no. 1, pp. 16–24, 2009. [Google Scholar]
- [29].Rinne P.et al. , “Democratizing neurorehabilitation:how accessible are low-cost mobile-gaming technologies for self-rehabilitation of arm disability in stroke?,” PLoS One, vol. 11, no. 10, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Friedman N, Chan V, Zondervan D, Bachman M, and Reinkensmeyer DJ, “MusicGlove: Motivating and quantifying hand movement rehabilitation by using functional grips to play music,” in Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology Society, EMBS, 2011. [DOI] [PubMed] [Google Scholar]
- [31].Friedman N.et al. , “Retraining and assessing hand movement after stroke using the MusicGlove: comparison with conventional hand therapy and isometric grip training,” J. Neuroeng. Rehabil, vol. 11, no. 1, p. 76, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Zondervan DK et al. , “Home-based hand rehabilitation after chronic stroke: Randomized, controlled single-blind trial comparing the MusicGlove with a conventional exercise program,” J. Rehabil. Res. Dev, vol. 53, no. 4, pp. 457–472, 2016. [DOI] [PubMed] [Google Scholar]
- [33].Meinert C.and Tonascia S, Clinical trials: Design, conduct, and analysis. New York: Oxford University Press, 1986. [Google Scholar]
- [34].Mathiowetz V, Volland G, Kashman N, and Weber K, “Adult norms for the Box and Block Test of manual dexterity.,” Am. J. Occup. Ther. Off. Publ. Am. Occup. Ther. Assoc, vol. 39, no. 6, pp. 386–391, 1985. [DOI] [PubMed] [Google Scholar]
- [35].Delignette-Muller ML and Dutang C, “fitdistrplus: An R package for fitting distributions,” J. Stat. Softw, 2015. [Google Scholar]
- [36].Standen PJ et al. , “Innovative Technologies Special Series,” vol. 95, no. 3, 2015. [Google Scholar]
- [37].Nijenhuis S, Prange G, Amirabdollahian F, Infarinato F, Buurke J, and Reitman J, “Feasibility of a second iteration wrist and hand supported training system for self-administered training at home in chronic stroke,” eighth Int. Conf. eHealth, telemedicine, Soc. Med., 2016. [Google Scholar]
- [38].Rinne P.et al. , “Democratizing neurorehabilitation:how accessible are low-cost mobile-gaming technologies for self-rehabilitation of arm disability in stroke?,” PLoS One, vol. 11, no. 10, pp. 1–12, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Hu XL, Tong K-Y, Song R, Zheng XJ, and Leung WWF, “A comparison between electromyography-driven robot and passive motion device on wrist rehabilitation for chronic stroke.,” Neurorehabil. Neural Repair, vol. 23, no. 8, pp. 837–46, Oct. 2009. [DOI] [PubMed] [Google Scholar]
- [40].McCrimmon CM, King CE, Wang PT, Cramer SC, Nenadic Z, and Do AH, “Brain-controlled functional electrical stimulation therapy for gait rehabilitation after stroke: a safety study.,” J. Neuroeng. Rehabil, vol. 12, p. 57, Jul. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Norman SL et al. , “Controlling pre-movement sensorimotor rhythm can improve finger extension after stroke,” J. Neural Eng, vol. 15, no. 5, Aug. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Bos RA et al. , “A structured overview of trends and technologies used in dynamic hand orthoses,” Journal of NeuroEngineering and Rehabilitation, vol. 13, no. 1. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Cramer SC et al. , “Stroke Recovery and Rehabilitation Research: Issues, Opportunities, and the National Institutes of Health StrokeNet,” Stroke, vol. 48, no. 3, pp. 813–819, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Inglis J, Meeker WQ, and Escobar LA, “Statistical Methods for Reliability Data,” J. Am. Stat. Assoc, 2000. [Google Scholar]
- [45].Castillo E, Extreme value theory in engineering. Academic Press, 1988. [Google Scholar]
- [46].Guadagnoll MA and Lee TD, “Challenge Point: A Framework for Conceptualizing the Effects of Various Practice Conditions in Motor Learning,” Journal of Motor Behavior, vol. 36, no. 2. pp. 212–224, Jun2004. [DOI] [PubMed] [Google Scholar]
- [47].Brown DA, Lee TD, Reinkensmeyer DJ, and Duarte JE, Designing robots that challenge to optimize motor learning. 2016. [Google Scholar]
- [48].Marchal-Crespo L.and Reinkensmeyer DJ, “Review of control strategies for robotic movement training after neurologic injury,” J. Neuroeng. Rehabil, vol. 6, no. 1, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Rowe JB, Chan V, Ingemanson ML, Cramer SC, Wolbrecht ET, and Reinkensmeyer DJ, “Robotic Assistance for Training Finger Movement Using a Hebbian Model: A Randomized Controlled Trial,” Neurorehabil. Neural Repair, vol. 31, no. 8, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]