OBJECTIVE:
To summarize knowledge and identify gaps in evidence about the relationship between social determinants of health (SDH) and postsepsis outcomes.
DATA SOURCES:
We conducted a comprehensive search of PubMed/Medical Literature Analysis and Retrieval System Online, Excerpta Medica database, and the Cochrane Library.
STUDY SELECTION:
We identified articles that evaluated SDH as risk factors for mortality or readmission after sepsis hospitalization. Two authors independently screened and selected articles for inclusion.
DATA EXTRACTION:
We dual-extracted study characteristics with specific focus on measurement, reporting, and interpretation of SDH variables.
DATA SYNTHESIS:
Of 2,077 articles screened, 103 articles assessed risk factors for postsepsis mortality or readmission. Of these, 28 (27%) included at least one SDH variable. Inclusion of SDH in studies assessing postsepsis adverse outcomes increased over time. The most common SDH evaluated was race/ethnicity (n = 21, 75%), followed by payer type (n = 10, 36%), and income/wealth (n = 9, 32%). Of the studies including race/ethnicity, nine (32%) evaluated no other SDH. Only one study including race/ethnicity discussed the use of this variable as a surrogate for social disadvantage, and none specifically discussed structural racism. None of the studies specifically addressed methods to validate the accuracy of SDH or handling of missing data. Eight (29%) studies included a general statement that missing data were infrequent. Several studies reported independent associations between SDH and outcomes after sepsis discharge; however, these findings were mixed across studies.
CONCLUSIONS:
Our review suggests that SDH data are underutilized and of uncertain quality in studies evaluating postsepsis adverse events. Transparent and explicit ontogenesis and data models for SDH data are urgently needed to support research and clinical applications with specific attention to advancing our understanding of the role racism and racial health inequities in postsepsis outcomes.
Keywords: mortality, readmission, sepsis, social determinants
Sepsis, life-threatening organ dysfunction caused by a dysregulated host response to infection, is responsible for significant acute and chronic morbidity and mortality (1–4). Two commonly studied adverse outcomes for sepsis survivors are: 1) rehospitalization and 2) long-term mortality (5–7). Existing reports suggest that sepsis survivors are at increased risk for both adverse outcomes, including an estimated 40% rate of hospital readmission rate at 90 days and 28–44% mortality rate at 1 year after sepsis discharge (6–9). Research developing risk models and identifying predictors of adverse outcomes among sepsis survivors has proliferated in the last decade (6, 10–14).
Despite the increased attention to recovery after sepsis, an important topic that remains understudied is the association of social determinants of health (SDH) with adverse outcomes. The clinical significance of SDH has been demonstrated in numerous other settings (15–19), and SDH may be a particularly salient contributor to the risk of hospital readmission (20, 21). Sepsis has specifically been identified as a condition that is affected by a combination of medical and social forces, implying that identification and mitigation of social factors is necessary to improve outcomes (22).
Although past attempts to summarize the literature on predictors of adverse events after sepsis have been made (12), we found no systematic exploration of the relationship between SDH and risk for rehospitalization and mortality after sepsis in adults. Understanding the social determinants that impact adverse outcomes after sepsis is paramount to inform interventions that adequately address the whole-person needs of sepsis survivors. The purpose of this review is to summarize knowledge and identify gaps in evidence about the relationship between social determinants and postsepsis outcomes.
MATERIALS AND METHODS
This study conformed to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines for scoping reviews (23) (Supplemental Digital Content, http://links.lww.com/CCX/B19). The study protocol is available in Supplemental Digital Content, http://links.lww.com/CCX/B19.
Inclusion Criteria
We searched for observational studies and randomized clinical trials published from 1992 (the year of the first consensus sepsis definition) to the date of the literature search (May 31, 2021) in Medical Literature Analysis and Retrieval System Online, Cochrane Library and its associated databases, and EMBASE. With the guidance of a medical librarian, we developed a search strategy using controlled vocabulary terms and text words for sepsis and postsepsis mortality or hospital readmission, and the search set was limited to humans and English language. The full electronic search strategy for Medical Literature Analysis and Retrieval System Online is available in Online Supplement Table 1 (http://links.lww.com/CCX/B19) and modified for other databases. We updated the search on February 16, 2022, to identify additional studies published since the first search date.
Study Selection
Two reviewers (R.S.H., S.T.) independently screened citations for those evaluating a cohort of sepsis patients and reporting either of the primary outcomes after index sepsis discharge: 1) all-cause mortality or 2) hospital readmission in the title or abstract. The full text of any citation considered potentially relevant by either reviewer was retrieved. Eligible studies: 1) had a cohort, case-control, or randomized controlled trial design, 2) enrolled survivors of a hospital admission for sepsis, and 3) reported all-cause readmission or postdischarge mortality as a primary outcome. For inclusion into the review, sepsis was defined as infection-related organ dysfunction managed in hospital setting including studies using terminology of sepsis, severe sepsis, and septic shock. To maximize the generalizability of the results, we excluded studies restricted to children and to special populations such as those with HIV, cancer, and other immunocompromised states. We also excluded studies enrolling survivors of uncomplicated infections, such as pneumonia, without referring to organ dysfunction or to International Classification of Diseases codes for sepsis, severe sepsis, or septic shock in their index sepsis case definitions. We screened reference lists of included studies, related review articles, and editorials.
Data Collection and Validity Assessment
Four authors (R.S.H., K.H., M.S., S.T.) extracted data from the included studies, and issues of uncertainty were resolved by consensus. We included full articles and conference abstracts for assessing the count of studies assessing social determinants but only the full manuscripts for assessing social determinants as independent risk factors. We defined social determinants as factors belonging to the five key domains for SDH as defined by Healthy People 2020 (neighborhood and built environment, economic stability, education, health and healthcare, and social and community context). Uncertainty about whether a measure should be included as SDH was resolved by discussion of all authors. From each of the included studies, we extracted data on study design, number of patients, duration of follow-up, description of index sepsis admission, rehospitalization events, mortality events, and social determinants assessed as independent risk factors for rehospitalization or mortality.
Assessment of Methodological Quality
For studies reported as full-text articles, we determined cohort data source and duration of follow-up for outcome. We assessed the following characteristics and quality of SDH data: 1) type of SDH, 2) source of SDH data, 3) reporting and handling of SDH data missingness, 3) validity checks of SDH data (e.g., cross-checking multiple data sources or use of a validated data quality assessment software tool) (24), and 4) level of SDH assessment (e.g., individual, neighborhood, and county). The SDH reported as independent risk factors for mortality and rehospitalization were identified only from studies that used methods to account for confounders.
Data Analysis
Study characteristics are reported as number (%). We described the characteristics of social determinants included among studies and report those social determinants identified as increasing the risk of rehospitalization or mortality in sepsis survivors between studies.
RESULTS
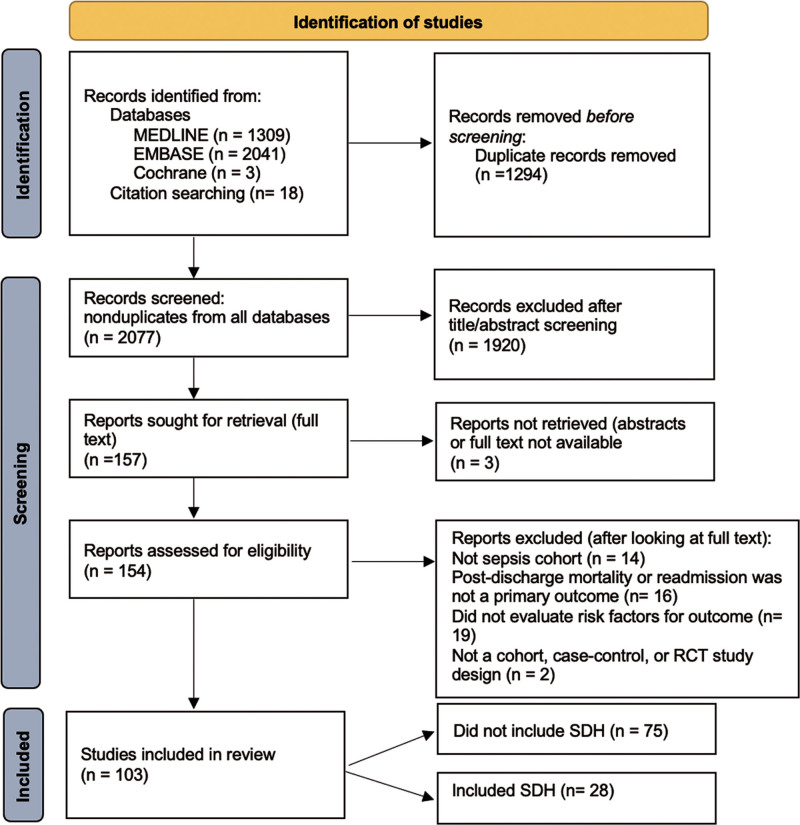
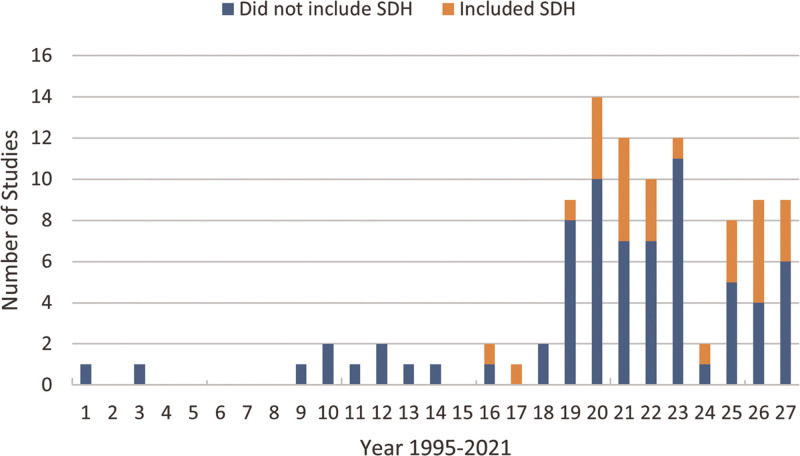
The initial bibliographical database search identified 3,371 records (Fig. 1). After exclusion of duplicates, we screened 2,077 records. Following screening, 154 records were sought for retrieval and were accessible. Based on the initial full-text evaluation, 99 records met our specified inclusion criteria (Online Supplement Table 2, http://links.lww.com/CCX/B19). The second search revealed six additional eligible articles for a total of 105 records meeting eligibility. Of these, 28 (27%) of studies evaluated one or more SDH as a risk factor for postsepsis adverse events (23 full articles and five conference abstracts) (6, 9, 11, 25–49). There were no major differences in study characteristics between studies that included SDH and those that did not (Online Supplement Table 3 and 4, http://links.lww.com/CCX/B19). The proportion of studies including SDH increased over time (Fig. 2).
Figure 1.
PRISMA diagram showing study selection for inclusion in the review. EMBASE = Excerpta Medica database, MEDLINE = Medical Literature Analysis and Retrieval System Online, PRISMA = Preferred Reporting Items for Systematic Reviews and Meta-Analyses, RCT = randomized controlled trial, SDH = social determinants of health.
Figure 2.
Trend in proportion of studies evaluating postsepsis adverse events thvat included social determinants of health (SDH) over time.
Study Characteristics
Table 1 shows the characteristics of the eligible studies grouped by their inclusion of SDH data. Of the 28 articles that included one or more SDH, 12 (43%) were published between 2010 and 2015 (6, 27, 28, 31, 33, 35, 37, 39, 41, 42, 44, and 46), 13 (46%) were published between 2016 and 2020 (29, 30, 32, 34, 36, 38, 40, 43, 45, 47, 48, 50, and 51), and three (11%) were published after 2020 (25, 26, and 49). Cohort size varied from eight studies (29%) of 100–1,000 patients (27, 32, 37, 38, 40, 41, 43, 45) to 14 (50%) with more than 10,000 patients (6, 9, 11, 25, 28, 29, 34, 36, 39, 42, 47–49). Nineteen (68%) included patients from multiple centers (6, 9, 11, 25, 28, 29, 31, 34, 36, 38–40, 42–44, 46–49). The primary outcome was postdischarge mortality in 14 (50%) (9, 25, 26, 32–38, 40, 46–48) and readmission in 18 (64%) studies (6, 11, 25–32, 39, 41–45, 47, 49); four (14%) studies evaluated both mortality and readmission (25, 26, 32, 47). For timing of follow-up, 15 (53%) studies evaluated the primary outcome at 7–30 day postdischarge (6, 11, 27–29, 31, 35, 39–45, 49), seven (25%) evaluated outcomes at 90 days to 1-year postdischarge (9, 27, 32, 34, 36, 46, 47), and 10 (36%) evaluated outcomes at greater than 1-year postdischarge (25, 26, 33, 34, 36–38, 40, 46, 48).
Table 1.
Characteristics of Studies Including Greater Than 1 Social Determinants of Health in Evaluation of Postsepsis Adverse Outcomes (n = 28)
| Variable | n (%) |
|---|---|
| Year | |
| 2010–2015 | 12 (43) |
| 2016–2020 | 13 (46) |
| 2020 to present | 3 (11) |
| Cohort size | 14 (50) |
| < 100 | 0 (0) |
| 100–1,000 | 8 (29) |
| 1,000–10,000 | 6 (21) |
| > 10,000 | 19 (68) |
| Multicenter | |
| Postdischarge outcome evaluated | |
| Mortality | 14 (50) |
| Readmission | 18 (64) |
| Outcome follow-up | |
| 7–30 d | 15 (53) |
| 30–90 d | 0 (0) |
| 90 d to 1 yr | 7 (25) |
| >1 yr | 10 (36) |
Methodologic Results
Table 2 shows the characteristics of SDH evaluation and reporting among included studies. The sources of SDH data were documented as coming from the electronic health record (EHR) in six studies (21%) (25–27, 30, 40, 41) and from linkage to administrative dataset such as census or database in 20 studies (71%) (6, 9, 11, 25, 26, 28, 29, 31, 32, 34, 36, 37, 39, 42, 44–49) with four studies (14%) not reporting the source of SDH data (33, 35, 38, 43). None of the studies reported any validity checks on SDH data. Eight (29%) reported missingness of SDH data as low (9, 11, 25, 32, 34, 40, 41, 49), but none of the studies discussed methods for handling missing data.
Table 2.
Characteristics of Included Social Determinants of Health
| SDH feature (n = 28) | n (% Hilton) |
|---|---|
| Type of SDH | |
| Race/ethnicity | 21 (75) |
| Economic stability | |
| Income/wealth | 8 (29) |
| Education | |
| Education level attained | 1 (4) |
| Healthcare access/quality | |
| Payer type | 10 (36) |
| Neighborhood and built environment | |
| Population setting (rural/urban) | 6 (21) |
| Neighborhood socioeconomic status | 6 (21) |
| Preillness living situation | 1 (4) |
| Social community | |
| Marital status | 5 (18) |
| SDH level | |
| Individual | 28 (100) |
| Neighborhood | 10 (36) |
| SDH sourcing | |
| Electronic health record | 6 (21) |
| Linkage to other administrative dataset | 20 (71) |
| Patient-reported | 0 (0) |
| Not reported | 4 (14) |
| Handling of SDH missing data | |
| Reported as “low” | 8 (29) |
| Not reported | 20 (71) |
SDH = social determinants of health.
The most common SDH evaluated was race/ethnicity (n = 21, 75%) (6, 9, 11, 25–27, 30, 31, 33–35, 37, 39–47), followed by payer type (n = 10, 36%) (6, 26, 29, 31, 39, 42–45, 49), and income/wealth (n = 8, 29%) (9, 25, 26, 31, 32, 36, 46, 48). Of those studies evaluating race/ethnicity, nine (32%) evaluated no other SDH (11, 27, 30, 33–35, 37, 40, 41). One study including race/ethnicity discussed the use of this variable as a surrogate for social disadvantage (26), and none specifically discussed systemic or structural racism (50). Education (32) and preillness living situation (e.g., homelessness) (28) were both evaluated by one article (4%) each.
All articles included at least one SDH measured at the individual level, and 10 (36%) included determinants measured at both the aggregate and individual levels (25, 26, 29, 36, 39, 42, 45, 47–49). Among the articles that included aggregated measures, geographic level included three out of 10 (30%) using areas smaller than a ZIP code (e.g., a census tract) (39, 45, 47) and seven out of 10 (70%) using ZIP code–level measures (25, 26, 29, 36, 42, 48, 49).
Several studies reported independent associations between SDH and outcomes after sepsis discharge; however, these findings were mixed across studies (Table 3). Five studies reported an independent association between race or ethnicity and readmission after sepsis discharge, of which three report that Black race had a higher risk of readmission (6, 26, 42), and two reported that Indigenous (including Native American and Australian Aboriginal) ethnicity had a higher risk of readmission (33, 42). Payer type was also reported to be independently associated with postsepsis readmission although in inconsistent directions: one study reporting higher readmission rate among Medicaid beneficiaries (6), one study reporting decreased readmission in uninsured patients (26), and two studies reporting decreased readmission for private insurance, self-pay, and “other” (39, 49). One study reported lower income and metropolitan residence as factors increasing the risk of postsepsis readmission (42). Three studies reported that lower neighborhood socioeconomic status was associated with increased risk for postsepsis readmission (45, 47, 49). Studies reporting independent associations between SDH and mortality following sepsis discharge were more varied, with marital status reported as a protective factor (38), and rural residence (48), low neighborhood socioeconomic status (47), low income (32), and Asian or “other” race reported as increasing risk for mortality (34).
Table 3.
Full-Text Studies Reporting Independent Associations Between Social Determinants of Health and Outcomes After Sepsis Discharge
| Study | Study Cohort | Outcome Assessment | Social Determinants Evaluated | Risk Association Between Social Determinant and Study Outcome |
|---|---|---|---|---|
| Lopes et al (37) | Centro Hospitalar Lisboa Norte, Infectious Disease ICU, 2002–2007; n = 234 | 2-yr mortality | Race | No association reported |
| Davis et al (35) | Royal Darwin Hospital, Tiwi, Darwin, NT, 2007–2008; n = 1090 | 28-d mortality | Race | No association reported |
| Lemay et al (46) | Department of Veterans’ Affairs Healthcare databases, 2001–2007; n = 2,727 | 90–365-d mortality | Race, marital status, and income | No association reported |
| > 365-d mortality | ||||
| Davis et al (33) | Royal Darwin Hospital, Tiwi, Darwin, 2007–2008; n = 1028 | 5-yr mortality | Race | Increased risk for Indigenous [Aboriginal Australian] race |
| Ortego et al (41) | Hospital of the University of Pennsylvania, Dec 2007 to Jan 2010; n = 997 | 30-d readmission | Race | No association reported |
| Chang et al (42) | HCUP—California State Inpatient Database, 2009–2011; n = 240,198 | 30-d readmission | Race, income, urbanicity, and payer | Increased risk for Black and Native American race, lower income, and metropolitan residence |
| Jones et al (44) | University of Pennsylvania Health System, 2010–2012; n = 3,620 | 30-d readmission | Race, marital status, and payer | No association reported |
| Goodwin et al (6) | HCUP State Inpatient Databases (CA, FL, and NY), 2011; n = 43,452 | 30-d readmission | Race and payer | Increased risk for Black race, Medicare, and/or Medicaid insurance |
| Donnelly et al (39) | United Healthcare clinical database; n = 345,657 | 30-d readmission | Race, payer, population setting, and census region | Decreased risk for private insurance, self-pay, and “other” with Medicare as reference |
| Sun et al (43) | University of Pennsylvania Health System, 2012; n = 444 | 30-d readmission | Race, marital status, and payer | No association reported |
| Chao et al (36) | Taiwan’s National Health Insurance Research Database, 1995–2011; n = 272,879 | 1-yr morality | Income and urbanization | No association reported |
| 2-yr morality | ||||
| 5-yr morality | ||||
| Schnegelsberg et al (32) | Aarhus University Hospital, Denmark, 2008–2010; n = 387 | 180-d readmission | Education level, income, and cohabitation status | Increased risk of 30-d mortality for low income |
| 30-d mortality | ||||
| 180-d mortality | ||||
| Abu-Kaf et al (38) | Israeli Sepsis Group database, 2003–2011; n = 409 | 2-yr mortality | Marital status | Increased risk for married marital status |
| Gadre et al (49) | Healthcare Cost and Utilization Project National Readmission Data, 2013–2014; n = 1,030,335 | 30-d readmission | Payer and income | Decreased risk for private insurance/self-pay, and higher neighborhood SES |
| Shankar-Hari et al (34) | ICNARC Case Mix Programme, 2009–2014; n = 94,748 | Up to 6-yr mortality | Race | Decreased risk for Asian race and “other” race |
| Bowles et al (11) | Medicare beneficiaries national dataset, 2013–2014; n = 165,228 | 30-d readmission | Race | No association reported |
| Courtright et al (9) | Medicare Beneficiaries, 2013–2014; n = 87,581 | 1-yr mortality | Race and Medicaid eligibility | No association reported |
| Gameiro et al (40) | Division of Intensive Medicine of the Centro Hospitalar Universitário Lisboa Norte, 2008–2014; n = 256 | 30-d mortality | Race | No association reported |
| 5-yr mortality | ||||
| Shankar-Hari et al (47) | ICNARC Case Mix Programme database & Hospital Episode Statistics database and Office for National Statistics death registrations, 2009–2014; n = 94,748 | 1-yr readmission | Race and neighborhood socioeconomic status | Increased risk for lower neighborhood SES for both readmission and mortality |
| 1-yr mortality | ||||
| Galiatsatos et al (45) | Johns Hopkins Bayview Medical Center, 2017; n = 647 | 30-d readmission | Race, payer, and area deprivation index | Increased risk for higher neighborhood disadvantage |
| Lizza et al (26) | Barnes Jewish Hospital, 2010–2017; n = 3390 | 1-yr readmission | Race, insurance, and income | Increased risk for Black race and decreased risk for uninsured |
| Oh et al (48) | National Health Insurance database, South Korea, 2011–2014; n = 45,826 | 5-yr mortality | Population setting and income | Increased risk for residence in metropolitan city other than Seoul, and “other area” in South Korea |
| Farrah et al (25) | National dataset in Canada; n = 196,922 | > 1 yr readmission | Urbanicity, income, and Ontario marginalization index | No associations reported |
| >1 yr mortality |
HCUP = Healthcare Costs and Utilization Project, ICNARC = Intensive Care National Audit & Research Center, SES = socioeconomic status.
DISCUSSION
SDH are increasingly recognized as critical upstream drivers of poor outcomes and higher costs (51–53). However, our review of studies assessing risk factors for adverse outcomes following sepsis indicates that SDH are infrequently evaluated and, even when evaluated, vulnerable to measurement error and interpretation challenges.
Low Utilization of SDH in Studies of Postsepsis Adverse Events
Our review found that only one-quarter of studies evaluating postsepsis events included SDH data in their analysis. Notably, 35% of these studies were classified as evaluating SDH based only on their inclusion of race or ethnicity. How race is defined and used is a critically important question for medical research (54–56). “Race” is widely acknowledged to be an indistinct, nonbiologic construct that is weakly measured, poorly analyzed, and inadequately reported (54, 57–59). We chose to include race and ethnicity as SDH variables to explore how this construct is used in research on postsepsis adverse events.
Measurement Error
Significant progress has been made in the recognition of SDH as key influencers of health, and a long list of variables have emerged, which are proposed to capture socioeconomic aspects such as health insurance, access to care, deprivation, geography, education, social support, financial mobility, and health behaviors. Unfortunately, these variables remain nonstandardized and are often inaccurately measured. Many SDH data included in the studies were obtained through EHRs. Despite widespread acknowledgement that SDH data are frequently missing or inaccurate in EHRs and large datasets (60), SDH data quality, including characteristics of completeness, correctness, and consistency, was seldom addressed in the studies included in this review—three out of four studies failed to report handling of missing data, and no study reported applying any validation method for SDH data. Measurement error may explain the mixed findings of independent associations between some SDH and postsepsis outcomes reported among our studies. Because data related to SDH are often not accessible through structured data fields, but embedded in free-text fields (61), continued development and application of novel clinical natural language processing (NLP) methods are needed to harness valuable SDH data from unstructured EHR data (62, 63).
However, even with improved extraction techniques such as NLP, EHR-derived SDH data are not likely sufficient to constitute a complete and accurate set of SDH domains, as many social and behavioral determinants that may influence health and mortality such as living arrangement and economic stability are not reliably captured and recorded (64). Researchers and practitioners face multiple challenges related to documenting SDH, including a lack of standardization, knowledge and buy-in from providers and staff members, and time to discuss SDH (65–68). Further, many SDH are time-varying, meaning that this information must be regularly updated to effectively inform research on outcomes and interventions to improve care (54). In our review, SDH data were often organized at city or national level rather than individual or neighborhood level, indicating the challenges in obtaining person-level SDH data.
Handling of “Race” as a Social Determinant of Health
The quality and validity of race as an included SDH in risk factor studies deserve special attention. Although race was the most commonly included SDH, its inclusion may not be informative. Misclassification of race and ethnicity variables in administrative sources has been identified as a limitation in health disparities research (69). Importantly, none of the studies identified in our review clearly defined race or justified its inclusion as a surrogate for sociologic constructs, for example, racism. This is consistent with other reports that racism is rarely explicitly named in published articles (54, 70, 71). The use of race as a variable in medical research is evolving, with the goal of ensuring that its use does not perpetuate inequities but rather intentionally seeks to resolve them. Specifically, journals have called for authors to clearly define race and justify inclusion in analyses (54, 72, 73), explicitly name racism and explore the contributions of racism and other race-related social constructs to study findings. Of note, efforts to control for other SDH may obscure rather than reveal the impact of race on health outcomes (55). If naming racism as a determinant of health is necessary for racial health equity, our review suggests that additional work is needed in the field of postsepsis research.
Associations Between Social Determinants and Postsepsis Outcomes
Due to heterogeneous definitions and study procedures, most associations between SDH factor and postsepsis outcomes were mixed across studies. The results for payer type were particularly mixed, possibly due to different healthcare payer models (e.g., single payer vs for-profit healthcare systems) in different geographic regions. Overall, neighborhood disadvantage was one factor that was consistently associated with increased readmission after sepsis and should be the focus of additional research.
Our review has important limitations. Although we employed an exhaustive search strategy, some relevant articles may have been missed. Additionally, data extraction was complicated by variability in reporting among studies. For example, SDH validity checks may have been done in studies but not reported in the article. Finally, the heterogeneity of SDH data prevented a meaningful statistical synthesis of the results. Nonetheless, to the best of our knowledge, this is the first in-depth review describing utilization and quality of SDH data in understanding risk of adverse outcomes after sepsis.
CONCLUSIONS
The discovery of which social determinants affect recovery after sepsis is pivotal to advancing knowledge to further guide support and management approaches in this vulnerable population. Although our review suggests that more recent studies of postsepsis adverse events are more likely to include social risk factors than older studies, SDH remain underutilized in risk models, and SDH data are often of uncertain quality. Transparent and explicit ontogenesis and data models for SDH data are urgently needed to support research and clinical applications with specific attention to advancing our understanding of the role racism and racial health inequities in postsepsis outcomes.
ACKNOWLEDGMENTS
We thank Laura Leach, MLIS, for assistance developing the search strategy for this study.
Supplementary Material
Footnotes
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (http://journals.lww.com/ccejournal).
Research reported in this publication was supported, in part, by the National Library Of Medicine of the National Institutes of Health under Award Number R21LM013373.
Dr. Hauschildt is employed by the Veteran’s Administration, and this work does not represent the official position of the United States Government or the Department of Veterans Affairs. This material is the result of work supported with resources and use of facilities at the Ann Arbor VA Medical Center. The remaining authors have disclosed that they do not have any potential conflicts of interest.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
REFERENCES
- 1.Fleischmann C, Scherag A, Adhikari NK, et al. ; International Forum of Acute Care Trialists: Assessment of global incidence and mortality of hospital-treated sepsis. Current estimates and limitations. Am J Respir Crit Care Med 2016; 193:259–272 [DOI] [PubMed] [Google Scholar]
- 2.Prescott HC, Angus DC: Enhancing recovery from sepsis: A review. JAMA 2018; 319:62–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yende S, Austin S, Rhodes A, et al. : Long-Term quality of life among survivors of severe sepsis: Analyses of two international trials. Crit Care Med 2016; 44:1461–1467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Iwashyna TJ, Cooke CR, Wunsch H, et al. : Population burden of long-term survivorship after severe sepsis in older Americans. J Am Geriatr Soc 2012; 60:1070–1077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mayr FB, Talisa VB, Balakumar V, et al. : Proportion and cost of unplanned 30-day readmissions after sepsis compared with other medical conditions. JAMA 2017; 317:530–531 [DOI] [PubMed] [Google Scholar]
- 6.Goodwin AJ, Rice DA, Simpson KN, et al. : Frequency, cost, and risk factors of readmissions among severe sepsis survivors. Crit Care Med 2015; 43:738–746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Prescott HC, Langa KM, Liu V, et al. : Increased 1-year healthcare use in survivors of severe sepsis. Am J Respir Crit Care Med 2014; 190:62–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yende S, Kellum JA, Talisa VB, et al. : Long-term host immune response trajectories among hospitalized patients with sepsis. JAMA Netw Open 2019; 2:e198686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Courtright KR, Jordan L, Murtaugh CM, et al. : Risk factors for long-term mortality and patterns of end-of-life care among Medicare sepsis survivors discharged to home health care. JAMA Netw Open 2020; 3:e200038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weinreich MA, Styrvoky K, Chang S, et al. : Sepsis at a safety net hospital: Risk factors associated with 30-day readmission. J Intensive Care Med 2019; 34:1017–1022 [DOI] [PubMed] [Google Scholar]
- 11.Bowles KH, Murtaugh CM, Jordan L, et al. : Sepsis survivors transitioned to home health care: Characteristics and early readmission risk factors. J Am Med Dir Assoc 2020; 21:84–90.e2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shankar-Hari M, Saha R, Wilson J, et al. : Rate and risk factors for rehospitalisation in sepsis survivors: Systematic review and meta-analysis. Intensive Care Med 2020; 46:619–636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goodwin AJ, Ford DW: Readmissions among sepsis survivors: Risk factors and prevention. Clin Pulm Med 2018; 25:79–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Norman BC, Cooke CR, Ely EW, et al. : Sepsis-associated 30-day risk-standardized readmissions: Analysis of a nationwide Medicare sample. Crit Care Med 2017; 45:1130–1137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Braveman P, Gottlieb L: The social determinants of health: It’s time to consider the causes of the causes. Public Health Rep 2014; 129(Suppl 2):19–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Galea S, Tracy M, Hoggatt KJ, et al. : Estimated deaths attributable to social factors in the United States. Am J Public Health 2011; 101:1456–1465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Phelan JC, Link BG, Tehranifar P: Social conditions as fundamental causes of health inequalities: Theory, evidence, and policy implications. J Health Soc Behav 2010; 51(Suppl):S28–S40 [DOI] [PubMed] [Google Scholar]
- 18.Marmot M: Social determinants of health inequalities. Lancet 2005; 365:1099–1104 [DOI] [PubMed] [Google Scholar]
- 19.Office of Disease Prevention and Health Promotion: Social Determinants of Health. Department of Health and Human Services. Available at: https://www.healthypeople.gov/2020/topics-objectives/topic/social-determinants-of-health#five. Accessed February 15, 2022
- 20.Baker MC, Alberti PM, Tsao TY, et al. : Social determinants matter for hospital readmission policy: Insights from New York City. Health Aff (Millwood) 2021; 40:645–654 [DOI] [PubMed] [Google Scholar]
- 21.Meddings J, Reichert H, Smith SN, et al. : The impact of disability and social determinants of health on condition-specific readmissions beyond Medicare risk adjustments: A cohort study. J Gen Intern Med 2017; 32:71–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rudd KE, Mair CF, Angus DC: Applying syndemic theory to acute illness. JAMA 2022; 327:33–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Page MJ, McKenzie JE, Bossuyt PM, et al. : The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021; 372:n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nasir A, Liu X, Gurupur V, et al. : Disparities in patient record completeness with respect to the health care utilization project. Health Informatics J 2019; 25:401–416 [DOI] [PubMed] [Google Scholar]
- 25.Farrah K, McIntyre L, Doig CJ, et al. : Sepsis-associated mortality, resource use, and healthcare costs: A propensity-matched cohort study. Crit Care Med 2021; 49:215–227 [DOI] [PubMed] [Google Scholar]
- 26.Lizza BD, Betthauser KD, Juang PH, et al. : Racial disparities in readmissions following initial hospitalization for sepsis. Crit Care Med 2021; 49:e258–e268 [DOI] [PubMed] [Google Scholar]
- 27.Leu M, Gonzalez C, Rico-Crescencio JC, et al. : Survivors of severe sepsis/septic shock - a need to focus on healthcare utilization post-discharge among inner-city minority New-Yorkers. In: D22. Advancing Critical Care Through New Approaches and Paradigms. American Thoracic Society International Conference Abstracts. American Thoracic Society, Philadelphia, PA, 2013, pp A5306–A5306 [Google Scholar]
- 28.Ahmad S, Baig S, Taneja A, et al. : The outcomes of severe sepsis in homeless. Chest 2014; 146:230A [Google Scholar]
- 29.Bath AS, Farishta M, Deepak V: Trends and 30-day readmission rate for patients discharged with septicemia: Analysis of 3,082,888 admissions. In: B49. Critical Care: East of Eden - Predicting and Measuring Outcomes. American Thoracic Society International Conference Abstracts. American Thoracic Society. Dallas, TX, 2019, pp A3457–A3457 [Google Scholar]
- 30.Kaplan M, Baker Chowdhury MA, Hurwitz J, et al. : 150 sepsis recidivism: Return visits and recurrence (S3R Analysis). Ann Emerg Med 2017; 70:S60 [Google Scholar]
- 31.Rice D, Goodwin AJ, Simpson K, et al. : Risk factors for 30-day hospital readmissions in sepsis. In: B105. Sepsis: Care Models and Outcomes. American Thoracic Society International Conference Abstracts. American Thoracic Society, San Diego, CA, 2014, pp A3784–A3784 [Google Scholar]
- 32.Schnegelsberg A, Mackenhauer J, Nibro HL, et al. : Impact of socioeconomic status on mortality and unplanned readmission in septic intensive care unit patients. Acta Anaesthesiol Scand 2016; 60:465–475 [DOI] [PubMed] [Google Scholar]
- 33.Davis JS, He V, Anstey NM, et al. : Long term outcomes following hospital admission for sepsis using relative survival analysis: A prospective cohort study of 1,092 patients with 5 year follow up. PLoS One 2014; 9:e112224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shankar-Hari M, Harrison DA, Ferrando-Vivas P, et al. : Risk factors at index hospitalization associated with longer-term mortality in adult sepsis survivors. JAMA Netw Open 2019; 2:e194900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Davis JS, Cheng AC, McMillan M, et al. : Sepsis in the tropical top end of Australia’s Northern Territory: Disease burden and impact on Indigenous Australians. Med J Aust 2011; 194:519–524 [DOI] [PubMed] [Google Scholar]
- 36.Chao PW, Chu H, Chen YT, et al. : Long-term outcomes in critically ill septic patients who survived cardiopulmonary resuscitation. Crit Care Med 2016; 44:1067–1074 [DOI] [PubMed] [Google Scholar]
- 37.Lopes JA, Fernandes P, Jorge S, et al. : Long-term risk of mortality after acute kidney injury in patients with sepsis: A contemporary analysis. BMC Nephrol 2010; 11:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Abu-Kaf H, Mizrakli Y, Novack V, et al. : Long-term survival of young patients surviving ICU admission with severe sepsis. Crit Care Med 2018; 46:1269–1275 [DOI] [PubMed] [Google Scholar]
- 39.Donnelly JP, Hohmann SF, Wang HE: Unplanned readmissions after hospitalization for severe sepsis at academic medical center-affiliated hospitals. Crit Care Med 2015; 43:1916–1927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gameiro J, Carreiro C, Fonseca JA, et al. : Acute kidney disease and long-term outcomes in critically ill acute kidney injury patients with sepsis: A cohort analysis. Clin Kidney J 2021; 14:1379–1387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ortego A, Gaieski DF, Fuchs BD, et al. : Hospital-based acute care use in survivors of septic shock. Crit Care Med 2015; 43:729–737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chang DW, Tseng CH, Shapiro MF: Rehospitalizations following sepsis: Common and costly. Crit Care Med 2015; 43:2085–2093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sun A, Netzer G, Small DS, et al. : Association between index hospitalization and hospital readmission in sepsis survivors. Crit Care Med 2016; 44:478–487 [DOI] [PubMed] [Google Scholar]
- 44.Jones TK, Fuchs BD, Small DS, et al. : Post-acute care use and hospital readmission after sepsis. Ann Am Thorac Soc 2015; 12:904–913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Galiatsatos P, Follin A, Alghanim F, et al. : The association between neighborhood socioeconomic disadvantage and readmissions for patients hospitalized with sepsis. Crit Care Med 2020; 48:808–814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lemay AC, Anzueto A, Restrepo MI, et al. : Predictors of long-term mortality after severe sepsis in the elderly. Am J Med Sci 2014; 347:282–288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shankar-Hari M, Rubenfeld GD, Ferrando-Vivas P, et al. : Development, validation, and clinical utility assessment of a prognostic score for 1-year unplanned rehospitalization or death of adult sepsis survivors. JAMA Netw Open 2020; 3:e2013580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Oh TK, Park HY, Song IA: Depression and long-term survival among South Korean sepsis survivors: A nationwide cohort study from 2011 to 2014. Crit Care Med 2021; 49:1470–1480 [DOI] [PubMed] [Google Scholar]
- 49.Gadre SK, Shah M, Mireles-Cabodevila E, et al. : Epidemiology and predictors of 30-day readmission in patients with sepsis. Chest 2019; 155:483–490 [DOI] [PubMed] [Google Scholar]
- 50.Bonilla-Silva E: Rethinking racism: Toward a structural interpretation. Am Sociol Rev 1997; 62:465–480 [Google Scholar]
- 51.World Health Organization: Closing the Gap in a Generation: Health Equity Through Action on the Social Determinants of Health: Commission on Social Determinants of Health FinalReport. 2008. Available at: https://www-who-int.libproxy.lib.unc.edu/publications/i/item/WHO-IER-CSDH-08.1. Accessed February 15, 2022 [DOI] [PubMed]
- 52.Marmot M, Friel S, Bell R, et al. ; Commission on Social Determinants of Health: Closing the gap in a generation: Health equity through action on the social determinants of health. Lancet 2008; 372:1661–166 [DOI] [PubMed] [Google Scholar]
- 53.Daniel H, Bornstein SS, Kane GC, et al. ; Health and public policy committee of the American College of Physicians: Addressing social determinants to improve patient care and promote health equity: An American College of Physicians Position Paper. Ann Intern Med 2018; 168:577–578 [DOI] [PubMed] [Google Scholar]
- 54.Lett E, Asabor E, Beltrán S, et al. : Conceptualizing, contextualizing, and operationalizing race in quantitative health sciences research. Ann Fam Med 2022; 20:157–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Graetz N, Boen CE, Esposito MH: Structural racism and quantitative causal inference: A life course mediation framework for decomposing racial health disparities. J Health Soc Behav 2022; 63:232–249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zuberi TBSE: White Logic, White Methods: Racism and Methodology Rowman & Littlefield; Lanham, MD. 2008 [Google Scholar]
- 57.Lin SS, Kelsey JL: Use of race and ethnicity in epidemiologic research: Concepts, methodological issues, and suggestions for research. Epidemiol Rev 2000; 22:187–202 [DOI] [PubMed] [Google Scholar]
- 58.Fullilove MT: Comment: Abandoning “race” as a variable in public health research–an idea whose time has come. Am J Public Health 1998; 88:1297–1298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bhopal R, Donaldson L: White, European, Western, Caucasian, or what? Inappropriate labeling in research on race, ethnicity, and health. Am J Public Health 1998; 88:1303–1307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cook LA, Sachs J, Weiskopf NG: The quality of social determinants data in the electronic health record: A systematic review. J Am Med Inform Assoc 2021; 29:187–196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hatef E, Weiner JP, Kharrazi H: A public health perspective on using electronic health records to address social determinants of health: The potential for a national system of local community health records in the United States. Int J Med Inform 2019; 124:86–89 [DOI] [PubMed] [Google Scholar]
- 62.Reeves RM, Christensen L, Brown JR, et al. : Adaptation of an NLP system to a new healthcare environment to identify social determinants of health. J Biomed Inform 2021; 120:103851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hatef E, Singh Deol G, Rouhizadeh M, et al. : Measuring the value of a practical text mining approach to identify patients with housing issues in the free-text notes in electronic health record: Findings of a retrospective cohort study. Front Public Health 2021; 9:697501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Truong HP, Luke AA, Hammond G, et al. : Utilization of social determinants of health ICD-10 Z-codes among hospitalized patients in the United States, 2016-2017. Med Care 2020; 58:1037–1043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Adler NE, Stead WW: Patients in context–EHR capture of social and behavioral determinants of health. N Engl J Med 2015; 372:698–701 [DOI] [PubMed] [Google Scholar]
- 66.Gottlieb L, Sandel M, Adler NE: Collecting and applying data on social determinants of health in health care settings. JAMA Intern Med 2013; 173:1017–1020 [DOI] [PubMed] [Google Scholar]
- 67.Cantor MN, Thorpe L: Integrating data on social determinants of health into electronic health records. Health Aff (Millwood) 2018; 37:585–590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Moscrop A, Ziebland S, Bloch G, et al. : If social determinants of health are so important, shouldn’t we ask patients about them? BMJ 2020; 371:m4150. [DOI] [PubMed] [Google Scholar]
- 69.Jarrín OF, Nyandege AN, Grafova IB, et al. : Validity of race and ethnicity codes in Medicare administrative data compared with gold-standard self-reported race collected during routine home health care visits. Med Care 2020; 58:e1–e8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hardeman RR, Murphy KA, Karbeah J, et al. : Naming institutionalized racism in the public health literature: A systematic literature review. Public Health Rep 2018; 133:240–249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bailey ZD, Krieger N, Agénor M, et al. : Structural racism and health inequities in the USA: Evidence and interventions. Lancet 2017; 389:1453–1463 [DOI] [PubMed] [Google Scholar]
- 72.Boyd RW, Lindo EG, Weeks LD, et al. : On Racism: A New Standard for Publishing on Racial Health Inequities. Health Affairs Blog. Washington, DC. 2020 [Google Scholar]
- 73.Andrews AL, Unaka N, Shah SS: New author guidelines for addressing race and racism in the Journal of Hospital Medicine. J Hosp Med 2021; 16:197. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.