Abstract
Beta-thalassemia and particularly its transfusion-dependent form (TDT) is a demanding clinical condition, requiring life-long care and follow-up, ideally in specialized centers and by multidisciplinary teams of experts. Despite the significant progress in TDT diagnosis and treatment over the past decades that has dramatically improved patients’ prognosis, its management remains challenging. On one hand, diagnostic and therapeutic advances are not equally applied to all patients across the world, particularly in several high-prevalence eastern regions. On the other, healthcare systems in low-prevalence western countries that have recently received large numbers of migrant thalassemia patients, were not ready to address patients’ special needs. Thalassaemia International Federation (TIF), a global patient-driven umbrella federation with 232 member-associations in 62 countries, strives for equal access to quality care for all patients suffering from thalassemia or other hemoglobinopathies in every part of the world by promoting education, research, awareness, and advocacy. One of TIF’s main actions is the development and dissemination of clinical practice guidelines for the management of these patients. In 2021, the fourth edition of TIF’s guidelines for the management of TDT was published. The full text provides detailed information on the management of TDT patients and the clinical presentation, pathophysiology, diagnostic approach, and treatment of disease complications or other clinical entities that may occur in these patients, while also covering relevant psychosocial and organizational issues. The present document is a summary of the 2021 TIF guidelines for TDT that focuses mainly on clinical practice issues and recommendations.
INTRODUCTION
The hemoglobinopathies constitute the most common monogenic disorders in humans.1 Among them, thalassemia results from defective globin chain synthesis leading to chronic hemolytic anemia.2 Although highly prevalent and once confined to certain geographic areas, including the Mediterranean Basin, Middle East and Southeast Asia, thalassemia now has a global distribution owing to the migration of populations from high-prevalent regions to the Western World, a phenomenon that has lately been intensified due to the massive migration movements from Middle East and Asia to Europe. Thalassemia and particularly transfusion-dependent thalassemia (TDT) is a demanding clinical condition, requiring life-long care and follow-up, ideally in specialized centers and by multidisciplinary teams of experts, and results in huge healthcare expenditures. Although advances in diagnosis and treatment have dramatically improved prognosis and survival of patients with thalassemia, offering them the possibility of a nearly normal lifespan with high quality, these advances have not been equally adopted and applied to all patient populations and this is particularly true in some highly prevalent areas.3 In addition, the healthcare systems in western countries, where the disease has always been extremely rare, are not ready to address the special needs of migrant thalassemia populations. Therefore, the management of patients with thalassemia remains challenging.
Thalassaemia International Federation (TIF) is a global patient-driven umbrella Federation with 232 member-associations in 62 countries across the world, operating in official relations with the World Health Organization, the United Nations Economic and Social Council and the European Commission. TIF aims at ensuring equal access to quality care for all thalassemia and other hemoglobinopathy patients in every part of the world by promoting education, research, awareness, and advocacy. One of the main actions of TIF is the development and dissemination of clinical practice guidelines for the management of thalassemia and other hemoglobinopathies. TIF guideline documents are drafted and reviewed by world experts in the field and are periodically updated to incorporate the continuous advances in the diagnosis and management of patients with hemoglobinopathies.
In 2021, the fourth edition of the guidelines for the management of TDT were published by TIF. The full version of the guidelines provides detailed information on the management of patients with TDT and the clinical presentation, pathophysiology, diagnostic approach, and treatment of disease complications or other clinical entities that may occur in these patients, while also covering relevant psychosocial issues.4 The present document is a summary of the 2021 TIF guidelines that focuses mainly on clinical practice issues and recommendations. Where appropriate, the level of evidence supporting certain recommendations is provided and classified as (A) data derived from multiple randomized clinical trials or meta-analyses; (B) data derived from a single-randomized clinical trial or large nonrandomized studies; (C) expert consensus or opinion and small studies, retrospective studies, registries.
DIAGNOSIS OF THALASSEMIA
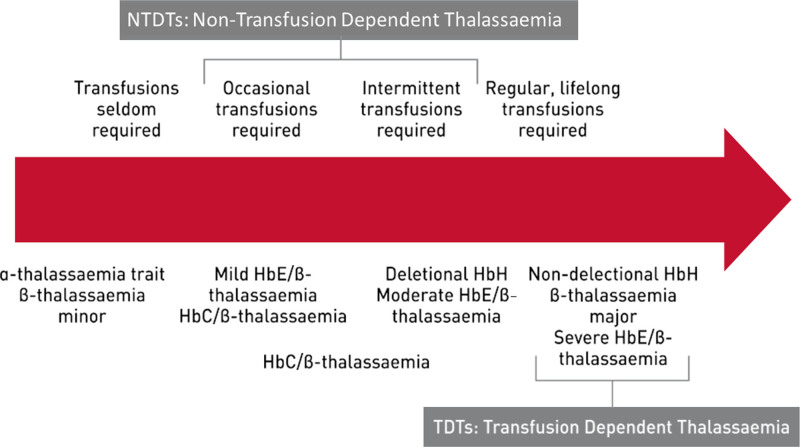
The term “thalassemia” refers to a group of blood diseases characterized by decreased or absent synthesis of one or more of the normal globin chains.2 According to the chain whose synthesis is impaired, the thalassemias are called α, β, γ, δ, δβ, or εγδβ thalassemias. Most thalassemias are inherited as recessive traits. The most clinically relevant types are α and β thalassemias, resulting from the decrease of one of the two types of polypeptide chains (α or β) that form the normal adult human hemoglobin molecule (Hb A, α2β2). Based on their clinical severity and transfusion requirement, thalassemia syndromes can be classified phenotypically into transfusion-dependent thalassemias (TDTs) and nontransfusion-dependent thalassemias (NTDTs) as shown in Figure 1.
Figure 1.
Phenotypic classification of thalassemia syndromes based on clinical severity and transfusion requirement.
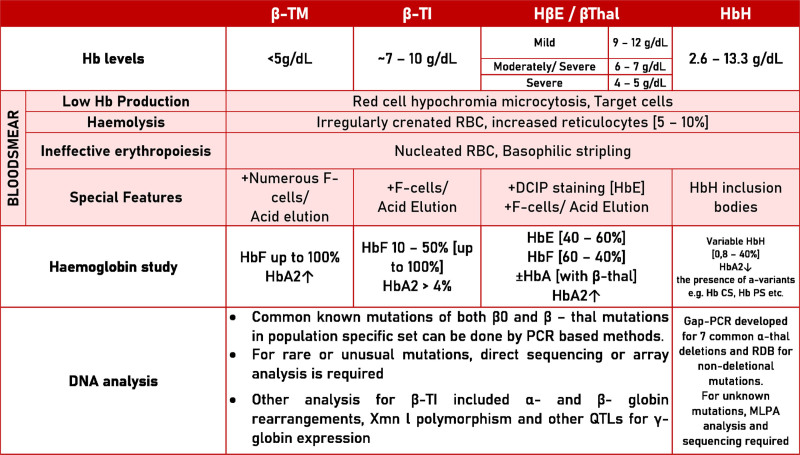
The diagnosis of thalassemias relies on measuring red blood cell indices and hemoglobin analysis and assessing the clinical severity of anemia. Molecular genetic testing may be useful for predicting the clinical phenotype and enabling presymptomatic diagnosis of at-risk family members and prenatal diagnosis. The diagnostic criteria for thalassemia and hemoglobinopathies are summarized in Figure 2.
Figure 2.
Summary of diagnostic methods for thalassemia and hemoglobinopathies. DCIP = dichlorophenolindophenol; Hb = hemoglobin; MLPA = multiplex ligation-dependent probe amplification. QTL = quantitative locus; PRC = polymerase chain reaction; RBC = red blood cells; RDB = reverse dot blot; TI = thalassemia intermedia; TM = thalassemia major.
Key points and recommendations
Diagnosis of thalassemia should be considered in all those who have hypochromic microcytic anemia (A).
In the diagnostic work-up for hypochromic microcytosis, iron deficiency anemia should always be excluded (A).
Molecular analysis is not required to confirm the diagnosis of a β carrier, but it is necessary to confirm the α thalassemia carrier status (A).
An α gene triplication or quadruplication should be taken into consideration in heterozygous β thalassemia subjects with a thalassemia intermedia phenotype (A).
The hematological parameters including red-cell indices and morphology, followed by separation and measurement of Hb fractions are the basis for the identification of β thalassemia carriers (A).
Detection of Hb H as Hb H inclusion body in a peripheral blood film using supravital staining (brilliant cresyl blue) is the hallmark of this condition (A).
β globin gene sequence analysis may be considered first if the affected individual is not of an ancestry at high risk or if targeted analysis reveals only one or no pathogenic variant (B).
α thalassemia are mainly due to deletions of different length and they can be detected with methods such reverse dot blot (RDP), Gap-polymerase chain reaction (PCR), or multiplex ligation-dependent probe amplification (MLPA) (B).
Methods that may be used to detect rare or unknown deletions include quantitative PCR, long-range PCR and, above all, MLPA (B).
TREATMENT OF THALASSEMIA
Blood transfusions
The aim of blood transfusion in thalassemia is to deliver an effective and safe transfusion regimen while minimizing the burden of transfusion therapy on everyday life.
An effective transfusion regimen will result in (i) good growth and development; (ii) good energy levels, (iii) sufficient suppression of intra and extramedullary hematopoiesis.5,6
A safe transfusion regimen will (i) use a product that is collected, tested, selected, issued, and administered adherent to established quality and safety regulations and guidance; (ii) be administered by staff trained in blood transfusion; (iii) involve informed patient consent; (iv) be performed in a system with a good hemovigilance structure.7–13
Blood transfusion therapy is decided upon the following criteria:
Confirmed diagnosis of thalassemia.
- Laboratory criteria:
- o Hemoglobin level (Hb) <70 g/L on 2 occasions, > 2 weeks apart (excluding all other contributory causes such as infections) or
- Clinical criteria irrespective of hemoglobin level:
- o Hemoglobin > 70 g/L with any of the following:
Significant symptoms of anemia
Poor growth/failure to thrive
Complications from excessive intramedullary hematopoiesis such as pathological fractures and facial changes
Clinically significant extramedullary hematopoiesis
Key points and recommendations
The diagnosis of thalassaemia should be confirmed with appropriate clinical and laboratory methods before the onset of transfusions (A).
An informed consent should be obtained for blood transfusion therapy.
Hemovigilance and adverse events reporting should be applied, as they key to the safety of blood transfusions.
Careful donor selection and screening should be used, favoring voluntary, regular, nonremunerated blood donors (A).
Before first transfusion, extended red-cell antigen typing of patients at least for D, C, c, E, e and Kell (A) and if available a full red-cell pheno/genotype should be performed.
At each transfusion, ABO, Rh(D) compatible blood should be administered. Choosing units compatible for ABO, C, c, E, e, and Kell antigens is highly recommended (A).
Before each transfusion, screening for new antibodies and an IAT cross-match should be performed, or in centers that meet regulatory requirements, an electronic cross-match should be performed where allowed (A).
Leucodepleted packed red cells should be used where available. Prestorage filtration is strongly recommended, but blood bank pretransfusion filtration is acceptable. Bedside filtration is only acceptable if there is no capacity for prestorage filtration or blood bank pretransfusion filtration (A).
Washed red cells should be used for patients who have severe allergic reactions (A).
Red cells should be stored in CPD-A within 1 week of collection and in additive solutions for less than 2 weeks of collection where available (A).
Transfusions should be performed every 2–4 weeks, maintaining pretransfusion hemoglobin above 90–105 g/L or up to 110–120 g/L for patients with cardiac complications (A).
A record of red-cell antibodies, transfusion reactions and annual transfusion requirements should be kept for each patient (A).
The post-transfusion hemoglobin should be kept below 140–150 g/L (A).
Iron overload and iron chelation
Iron overload occurs when iron intake is increased over a sustained period of time, either as a result of red blood cell transfusions or increased absorption of iron through the gastrointestinal (GI) tract.14 Both of these occur in thalassemias, with blood transfusion therapy being the major cause of iron overload in TDT and increased GI absorption being more important in NTDT. When TDT patients receive regular blood transfusion, iron overload is inevitable because the human body lacks a mechanism to excrete excess iron. Iron accumulation is toxic to many tissues, causing heart failure, cirrhosis, liver cancer, growth retardation, and multiple endocrine abnormalities.15
Diagnosis and monitoring of iron overload
The diagnosis and monitoring of iron overload are based on the complementary use of the following parameters:
Serum ferritin
Serum ferritin (SF) concentration is measured at least every 3 months (1–3 months). Target value is currently between 500 and 1000 μg/L. Measuring the trends in SF is a more reliable indicator for adjusting therapy than the use of single values.
Liver iron concentration
Liver iron concentration (LIC) is measured by magnetic resonance imaging (MRI)-based methods.16,17 LIC should be assessed using an externally validated and standardized MRI technique. Ideally, the same technique, if possible, should be used for each individual patient. Normal LIC values are up to 1.8 mg/g dry weight (wt). The risk of side effects is much lower even below 3–5 mg/g dry wt, while with levels of up to 7 mg/g dry wt are seen in some nonthalassaemic populations without apparent adverse effects. Sustained high LIC above 15 mg/g dry wt have been linked to worsening prognosis, liver fibrosis progression, or liver function abnormalities.18 LIC can also be used to calculate total body iron using the formula: total body iron (in mg/kg body wr) = 10.6 × LIC (in mg/g dry wt).19
The frequency of LIC assessment should be guided by its level and its rate of change:
Stable levels in the range 3–7 mg/g dry weight (dw): Every 1 or 2 years
Levels >7 mg/g dw: yearly
Levels falling rapidly or <3 mg/g dw: 6–12 monthly
Myocardial iron
Myocardial iron is assessed by T2* cardiac MRI, using an externally validated protocol and software, which should undergo periodic external calibration.20 The frequency of cardiac MRI scan should be guided by myocardial iron level, for example:
Stable T2* >20 ms: 2 yearly
T2* 10–19 ms: yearly
T2* <10 ms: 6 monthly
It is particularly important to measure cardiac function when cardiac iron is high (eg, T2* <10 ms), as this is associated with a high risk of deteriorating function and heart failure and requires urgent intensification of chelation.
Target organ function and other parameters
In addition to measuring SF, LIC and cardiac T2*, target organ function, including cardiac, hepatic, and endocrine function, should monitored and patients should be regularly screened for iron-mediated damage including cardiac dysfunction, diabetes, hypothyroidism, hypoparathyroidism, and hypogonadotropic hypogonadism.21
Additional parameters of iron overload include:
24 hours urinary iron estimation, not widely used in routine monitoring;
plasma nontransferrin bound iron and labile plasma iron, the value of which as a guide to routine treatment or prognosis has not yet been established.
Monitoring of iron overload is essential in establishing effective iron chelation regimes, tailored to individuals’ specific needs. In addition to SF, LIC and myocardial T2*, to guide chelation therapy it is essential to:
document the age of onset of regular transfusions and iron chelation therapy for each patient;
maintain an annual record of blood usage (ml/kg packed red cells) and daily iron loading (mg/kg/day) for each patient.
Iron chelation therapy
The aims of iron chelation therapy are as follows:
Prevention therapy: to maintain safe levels of body iron at all times, by balancing iron intake from blood transfusion with iron excretion by chelation (iron balance).
Rescue therapy: to remove excess iron stored in the body.
Emergency therapy: to urgently intensify iron chelation in case of iron-induced heart failure.
Dose adjustment of therapy: to adjust dosing and treatment regimens to changing circumstances identified by careful monitoring of body iron and its distribution; monitoring is important to avoid: (a) under-chelation with increased iron toxicity; or (b) over-chelation and increased chelator toxicity.
Adherence to therapy: to adhere to prescribed regular regimen; intermittent high-dose chelation can induce negative iron balance but does not provide continuous protection from labile iron and also risks increased toxicity from the iron chelator.
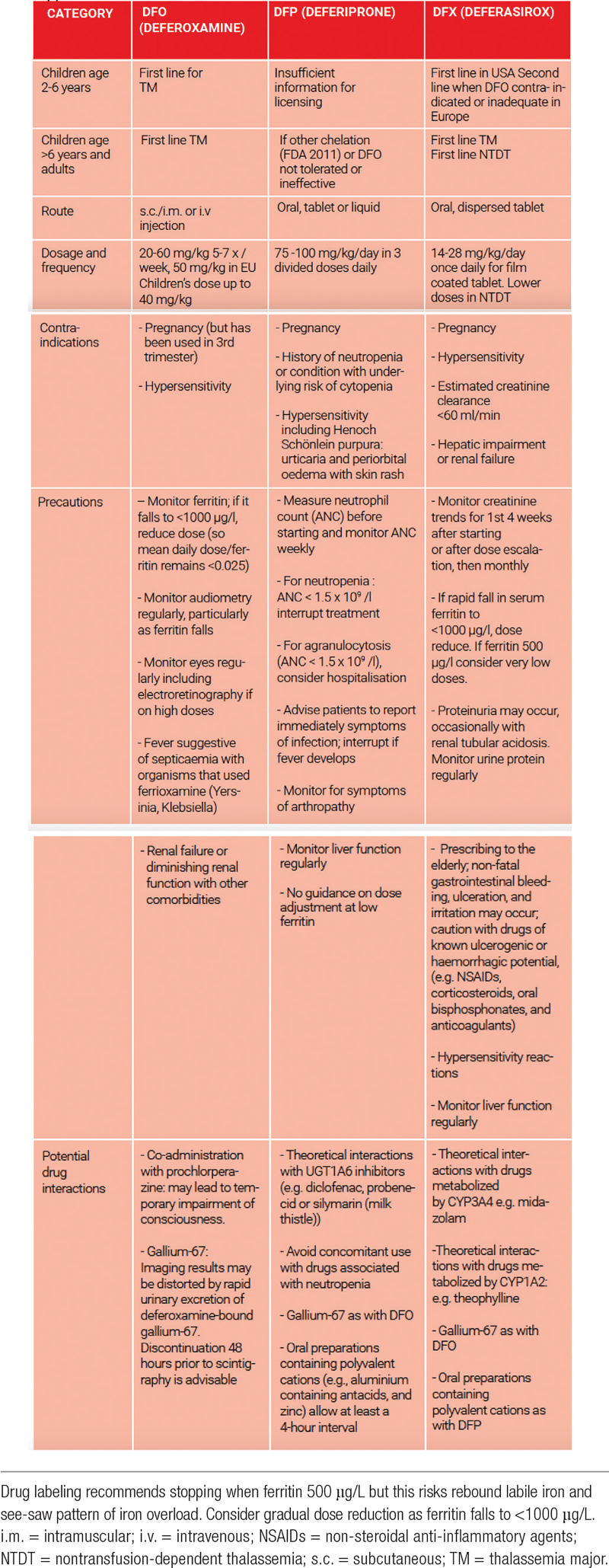
Three iron chelators are currently licensed for clinical use, deferoxamine, deferiprone, and deferasirox;22–24 their properties and indicated doses are reviewed in Table 1.
Table 1.
Licensed indications and precautions for chelation therapy in thalassemia
Key points and recommendations
Uncontrolled transfusional iron overload increases the risks of heart failure, endocrine damage, liver cirrhosis, and hepatocellular carcinoma (B).
Liver iron concentration can be used to calculate total body and serum ferritin is an approximate marker of LIC (B).
MRI-based measurements should be performed by standardized and externally validated techniques, ideally in validated centers (C).
Chelation therapy is an effective treatment modality in improving survival, decreasing the risk of heart failure, and decreasing morbidities from transfusion-induced iron overload (A).
Chelation therapy at the correct doses and frequency can balance iron excretion with iron accumulation from transfusion (A).
Absolute change in total body iron in response to chelation can be calculated from change in LIC (B).
Direction of change in body iron in response to transfusion and chelation can usually but not always be estimated from the trend in serum ferritin (B).
Prevention of iron accumulation using chelation therapy is preferable to rescue treatment because iron-mediated damage is often irreversible, and removal of storage iron by chelation is slow—particularly after it has escaped the liver (B).
Response to chelation is dependent on the dose applied and the duration of exposure (A).
Response to chelation is affected by the rate of blood transfusion (B).
Cardiac iron accumulates later than liver iron, and is rare before the age of 8 years, affecting a subset of patients (B).
Chelation of storage iron from the liver tends to be faster than from myocardium (B).
Cardiac storage iron concentration is directly related to the risk of heart failure, which can be reliably estimated by MRI (eg, cardiac T2*), provided the center performing the measurement uses a validated method that has been independently calibrated (B).
Chelation can reverse iron-mediated cardiac dysfunction rapidly (within weeks) by rapid chelation of labile iron, if 24 hours chelation cover is achieved (A).
Chelation therapy removes myocardial storage iron slowly (months or years) (A).
Over-chelation increases side effects from chelation therapy, and doses should therefore be decreased as serum ferritin or liver iron levels fall (demonstrated most clearly with DFO) (B).
The optimal chelation regime must be tailored for the individual and will vary with their current clinical situation.
Chelation therapy will not be effective if it is not taken regularly—a key aspect of chelation management is to work with patients to optimize adherence (B).
Novel therapies
An improved understanding of the pathophysiology of β thalassemia has paved the way for the development of novel therapies. These can be classified into two major categories based on the underlying pathophysiology that they address:
correction of the α/β globin chain imbalance by hematopoietic stem cell transplantation or gene therapy;
targeting ineffective erythropoiesis or iron dysregulation by novel agents such as luspatercept.
Hematopoietic stem cell transplantation
Up to date, allogeneic hematopoietic stem cell transplantation (HSCT) is the only and curative treatment option for thalassemia major, with more than 3000 HSCTs performed worldwide.25–27 In a large EBMT survey of 1061 cases of MSD HSCT performed between 2000 and 2010 in 132 centers in 28 countries with a median patient age of 7 years, long-term overall survival (OS) and thalassemia-free survival (TFS) were 91% and 83%, respectively.28 In recent years, a number of factors including improved conditioning regimen, improved prevention of graft versus host disease (GvHD) and more effective antibacterial, antiviral, and antifungal treatment resulted in a significant improvement of outcomes for HSCT with a cure of thalassemia achieved in 80% to 90% of patients today.
Key points and recommendations
HSCT should be offered to thalassemia patients and their parents at an early age, before complications due to iron overload have developed, if an HLA identical sibling is available.
Either bone marrow or cord blood from an HLA identical sibling can be used.
A matched unrelated donor can be used, provided that high compatibility criteria for both HLA class I and II loci are present.
Haploidentical HSCT in thalassemia can be considered in experienced HSCT centers in the context of well-designed clinical trials.
Myeloablative conditioning regimens should always be used for standard transplantation.
Post-transplant care should include all transplant and thalassemia related complications.
In thalassemia patients, HSCT is cost-effective when compared to life-long supportive therapy.
Gene therapy
For years, the only curative treatment has been allogeneic bone marrow transplantation, limited however by its availability only to young patients with a well-matched donor and the requirement for long-term immunosuppression to prevent or treat transplant-related immunological complications such as GvHD and rejection, carrying a nonnegligible risk of mortality.29 Gene therapy aspires to provide cure for thalassemia through the manipulation of the genome of hematopoietic stem cells, thus compensating for the inadequate or faulty function of mutated genes. This can be achieved by30,31:
gene addition via a semirandom insertion of a healthy copy of the therapeutic gene into the cells using viral vectors or
gene editing via a precisely directed mutation that repairs the gene in situ or induces a disease-modifying effect (ie, reactivation of Hb F synthesis) using site-specific nucleases.
Among the novel gene therapies, lentiviral vector gene therapy is the most mature intervention, shown to provide clinical efficacy and safety as a one-off, life-changing treatment.32 Nevertheless, the long-term safety and sustainability of the response needs also to be demonstrated; thus treated patients are followed in follow-up trials lasting overall 15 years. Precision medicine with genome editing tools has enabled overcoming of some of the obstacles associated with gene addition gene therapy; however, clinical experience with gene editing is currently limited and more clinical data and large-scale trials are needed to demonstrate that gene editing is a potential safe and curative treatment for hemoglobinopathies. The recent clinical data of patients with β0 genotypes create optimism for Zynteglo’s LentiGlobin vector approach.33,34
Key points and recommendations
Although waiting for the long-term clinical data on gene therapy for β-thalassemia, currently and on the basis of existing indications, patients with β-thalassemia major have potentially the following options for treatment:
allogeneic hematopoietic stem cell (HSC) transplantation: young patients (≤17-year-old) with a β+ or β0 genotype having an HLA-compatible sibling or a 10/10 matched volunteer donor.
gene therapy with Zynteglo: young patients in the 12- to 17-year-old age group with a β+ genotype who do not have an HLA-compatible sibling donor.
gene therapy with Zynteglo: patients in the 17- to 55-year-old age group with a β+ genotype who do not have severe comorbidities and are at-risk or ineligible to undergo an allo-HSC transplant but can otherwise undergo an autologous gene therapy procedure with an acceptable risk.
Targeting ineffective erythropoiesis—luspatercept
Luspatercept (ACE-536) is a recombinant fusion protein that binds to specific ligands of the TGF-β superfamily and enhances erythroid maturation. It is the most recently approved therapy [Food and Drug Administration (FDA) and European Medicines Agency (EMA)] for the management of TDT. The approval of luspatercept was based on the results of the phase 3 BELIEVE trial, which showed that subcutaneous luspatercept led to a reduction in transfusion burden of at least 33% from baseline during weeks 13 through 24 plus a reduction of at least 2 red-cell units over this 12-week interval in significant more patients than placebo (21.4% versus 4.5%); the percentage of patients with a reduction in transfusion burden of at least 33% during any 12-week interval was also greater with luspatercept (70.5% versus 29.5%).35 Parallel reductions in SF levels were further observed. Adverse events more common with luspatercept compared with placebo and included bone pain, arthralgia, dizziness, hypertension, and hyperuricemia. Hypertension developed in 10.7% and thromboembolic events in 3.6% of patients. Data on the long-term use of luspatercept, its real-life application and its use in the pediatric population are awaited. More details on this drug can be found in the full text of these guidelines.
Key points and recommendations
- Luspatercept can be considered for:
-
oPatients who require regular red blood cell transfusions,
-
o≥18 years of age.
-
o
The recommended starting dose of luspatercept is 1 mg/kg once every 3 weeks by subcutaneous injection.
If the predose hemoglobin level is ≥115 g/L and is not influenced by recent transfusion, consider delaying dosing of luspatercept until the level is ≤110 g/L.
Before administration of luspatercept, hemoglobin level, and liver function tests including alanine transferase and aspartate transferase levels should be monitored to ensure proper dosing and metabolism of the medication.
If a TDT patient does not achieve a reduction in red-cell transfusion burden after at least 2 consecutive doses (6 weeks) at the 1 mg/kg starting dose, increase the luspatercept dose to 1.25 mg.
If a patient experienced a response followed by a lack of or lost response to luspatercept, consider initiating a search for causative factors.
Luspatercept should be discontinued if a patient does not experience a decrease in transfusion burden after 9 weeks of treatment (administration of 3 doses) at the maximum dose level or if unacceptable toxicity occurs at any time.
It is important to monitor any TDT patient receiving luspatercept for signs and symptoms of thromboembolic events and initiate treatment accordingly.
Blood pressure should be monitored before each administration of luspatercept.
As no data are currently available on luspatercept use in pregnant women, all pregnant women should be advised of the potential risk to a fetus.
Safety and efficacy of luspatercept in pediatric patients has not yet been established and its use in pediatric patients in therefore not currently recommended.
DIAGNOSIS AND MANAGEMENT OF THALASSEMIA-RELATED COMPLICATIONS
Cardiovascular disease
Cardiovascular (CV) complications represent the leading cause of mortality in patients with thalassemia, including TDT and NTDT.22 In contemporary cohort studies, however, CV mortality has significantly declined, reflecting the overall mortality reduction observed in the same cohorts achieved by the effective implementation of modern diagnostic and therapeutic modalities, including magnetic resonance imaging (MRI)-guided chelation therapy with the use of the T2* technique.36,37 This progress is not, however, the case in thalassemia populations with limited access to modern therapy and therefore the global burden of CV disease in thalassemia remains high.38
The pathophysiology and phenotypes of CV disease depend on the interaction between the main disease and the applied therapy.39 A wide range of CV abnormalities are seen in patients with thalassemia. The spectrum of cardiac disease includes left or right ventricular dysfunction, with or without heart failure, pulmonary hypertension, tachyarrhythmias such as atrial fibrillation, bradyarrhythmias such as atrioventricular block, valvular disease, pericarditis, and myocarditis.39,40 Further CV disorders include, thromboembolic events, resulting from either venous or arterial thrombosis, cerebrovascular disease, manifested as either ischemic or hemorrhagic stroke, and vascular abnormalities, including endothelial dysfunction and increased arterial stiffness.41–43 Among the above disorders, iron overload cardiomyopathy has long been the main form of heart disease in thalassemia patients treated with transfusions but is now being effectively prevented and managed with MRI-guided contemporary chelation therapy.15,44
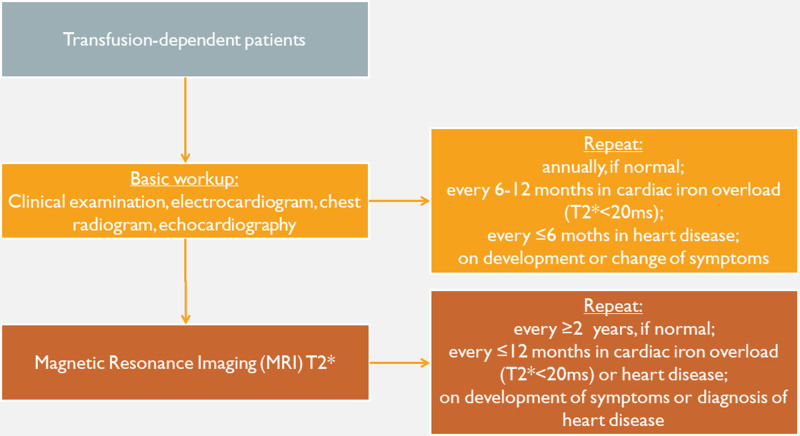
Regular CV assessment should be part of a multidisciplinary monitoring programme and should ideally be performed by or in consultation with clinics or physicians with experience in CV disease in hemoglobinopathies and in close collaboration with the attending thalassemia physician. Regular CV monitoring should be performed on an annual basis in all thalassemia patients, regardless of the presence of history or symptoms of CV disease. In the presence of CV disease, the frequency and content of CV assessment should be tailored to meet each patient’s needs and shorter intervals (eg, 3 or 6 monthly) may be applied according to severity (Figure 3).39
Figure 3.
Basic algorithm for the cardiac evaluation of patients with thalassemia. BMI = body mass index.39
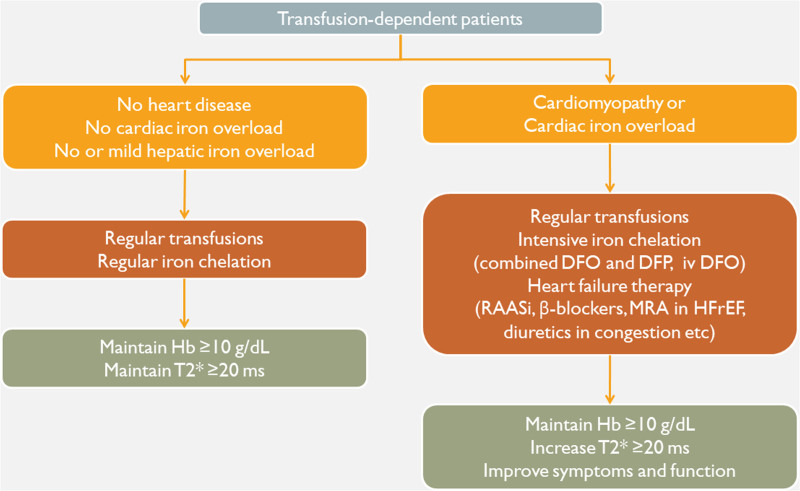
The prevention and treatment of CV disease in thalassemia consists of two pillars: the optimization of disease-specific therapy (blood transfusions, iron chelation, multidisciplinary care) and cardioactive therapy in the presence of CV disease (Figure 4).39 A lifestyle that promotes CV health is an important part of CV prevention, whereas the particularities of CV disease in thalassemia should be taken under consideration in the management of patients. Most importantly, cardiac dysfunction and heart failure may be reversible by timely therapy.
Figure 4.
A basic therapeutic algorithm for thalassemia patients on regular blood transfusions. DFO = deferoxamine; DFP: deferiprone; Hb = hemoglobin concentration; HFrEF = heart failure with reduced left ventricular ejection fraction; MRA = mineralocorticoid receptor antagonists; RAASi = renin-angiotensin-aldosterone system inhibitors.39
Key points and recommendations
Regular CV assessment should be part of a patient’s multidisciplinary monitoring programme (C).
CV assessment and management should ideally be performed by or in consultation with clinics or physicians with experience in CV disease in hemoglobinopathies and in close collaboration with the attending thalassemia physician (C).
MRI T2* guided chelation therapy represents the best available approach to prevent cardiac dysfunction related to iron overload (B).
In places lacking CMR T2* assessments, worsening of LV function in serial echocardiograms in transfusion-dependent patients may be used as a red flag for iron toxicity and should prompt aggressive and sustained escalation of chelation therapy (B).
Echocardiographic screening for pulmonary hypertension should be part of annual CV assessment. Patients having a TRV greater than 3 m/s should undergo cardiac catheterization to confirm the diagnosis of pulmonary hypertension if a proximate cause cannot be identified and corrected (B).
Intensified chelation therapy, preferably with two-drug combinations, is required for thalassemia major patients with cardiac iron overload, with or without overt cardiac dysfunction or heart failure (B).
Diagnosis of CV disease should prompt optimization of disease-specific therapy in addition to cardioactive treatment (C).
Screening and treatment of endocrine and metabolic comorbidities is crucial for the prevention and management of CV disease (C).
Management of cardioactive therapies must account for a patient’s unique physiology compared with the general population (C).
Cardiac abnormalities, including ventricular dysfunction, heart failure, and arrhythmias, are often reversible following intensification of disease-specific therapy, albeit after several weeks or months (C).
Lifestyle choices that promote CV health (absence of smoking, physical exercise, weight control, healthy diet) should be vigorously promoted in thalassemia patients (C).
Liver disease
Iron overload and viral hepatitis are the two main causes of liver disease in patients with thalassemia leading to chronic inflammation, fibrosis and ultimately to cirrhosis. In iron overload states, unbound iron accumulates in the hepatocytes and leads to severe oxidative stress with overproduction of toxic ROS, which lead to severe hepatic inflammation and fibrosis.45 LIC estimated by MRI R2 or R2* is currently the method of choice to monitor iron overload specifically in this organ.46 Current iron chelators reduce significantly LIC and slow down liver fibrosis.22,24,47
Hepatitis C virus (HCV) or hepatitis B virus (HBV) may further accelerate liver fibrosis in thalassaemic patients. Adequate blood donor checking, widespread vaccination for HBV and access to antiviral treatments are mandatory to minimize this risk.48,49 More specifically, a new generation of highly effective and safe direct-acting antiviral agents (DAA) have currently replaced interferon-based regimens in the HCV treatment landscape and have simplified treatment approaches for thalassaemic patients with chronic HCV infection.50
The achievement of prolonged survival of thalassaemic patients in recent years is associated with effective iron overload treatments and lower risk of heart disease. However, there are several reports of a recent increase in hepatocellular carcinoma (HCC) incidence.48 Based on this observation, biannual screening for HCC with ultrasound for all thalassaemic patients is strongly recommended.
Key points and recommendations
MRI R2 or R2* is the method of choice to assess liver iron concentration (LIC) and monitor chelation therapy effectiveness (A).
Deferoxamine, deferiprone, and deferasirox are effective in decreasing total body iron burden as well as LIC (A).
Screening for HCV and HBV-chronic infection is recommended in thalassaemic patients (A).
Vaccination against hepatitis B is recommended in all patients with thalassemia who are seronegative for HBV markers (A)
If anti-HCV antibodies are detected, the presence of HCV-RNA in serum or plasma should be determined to identify patients with chronic infection (A).
The new IFN-free, ribavirin-free direct-acting antiviral drugs for hepatitis C are effective and safe in patients with thalassemia (B).
Serum transaminases, HBV DNA levels and liver fibrosis assessment by transient elastography are the main tools to guide treatment decision in HBV-chronic hepatitis (A).
Oral nucleoside and nucleotide analogues are well tolerated and effective drugs for HBV-chronic hepatitis, although loss of HbsAg remains a rare event (A).
Biannual ultrasound screening for hepatocellular carcinoma should be performed in all thalassaemic patients (B).
Growth abnormalities
Growth failure in TDT has persisted despite major therapeutic advances.51 In the past, prevalence of growth failure and short stature in children with thalassemia varied from 30% to 60%. In the current era, the adherence to modern transfusion and iron chelation protocols and avoidance of iron chelator overdosage has clearly reduced the risk of short stature and may have potentially enhanced endocrine development in children with TDT.
The pathogenesis of growth failure is multifactorial. Key contributing factors to stunted growth in patients with TM include chronic anemia, transfusion-related iron overload, and chelation toxicity. Other contributing factors include hypothyroidism, hypogonadism, GH deficiency/insufficiency, zinc deficiency, chronic liver disease, under-nutrition, and psychosocial stress.52
Patients’ growth should be assessed every 6 months by accurate measurement of standing and sitting height and weight. Diagnosis requires careful clinical evaluation to establish:
Short stature—height below the third centile for sex and age (based on national growth charts), or
Slow growth rates—growth velocity expressed in cm/year, below 1 SD for age and sex (based on growth velocity charts), or
Signs of other pituitary hormone deficiencies (eg, gonadotrophins, growth hormone, central hypothyroidism)
Signs of other possible causes of retarded growth (nutritional deficiencies, chronic hepatic disease, chronic heart failure).
Management consists of optimizing blood transfusion; improving nutrition with high caloric balanced diet. Optimizing iron chelation can be achieved by using the new oral chelators or intensive and combined chelation therapy in patients with severe iron overload. Also, an early diagnosis and treatment of associated endocrinopathies are important. Recombinant GH (rhGH) treatment is not always as effective as expected in nonthalassaemic children with GHD.
Key points and recommendations
The adherence to modern transfusion and iron chelation protocols and avoidance of iron chelator overdosage is crucial for the prevention of short stature and the endocrine development of children with TDT (B).
Although the cause of short stature in children with TM is not well understood, it is believed to be multifactorial and multiple parameters should be evaluated during patients’ assessment including treatment of the main disease, endocrine disorders, nutritional deficiencies, and comorbid conditions (A).
The hormonal cause of growth retardation in TDT is complex. Besides hypothyroidism and hypogonadism, growth hormone (GH) deficiency should also be considered (A).
Excessive iron deposition in the pituitary and liver appears to be the major etiology for GH or IGF-I deficiency (A).
The efficacy of rhGH treatment in the management of children with TDT with growth failure secondary to GH deficiency has been a matter of debate (A). The linear growth velocity attained after exogenous GH administration in children with thalassemia is reported to be lower than that seen in children with primary GH deficiency, possibly due to GH insensitivity (B).
Essentially there is no pathognomonic clinical feature to lead to suspicion of GH deficiency in adults (B).
Endocrine complications
Endocrine abnormalities are the most common complications of TDT. Prevalence varies because of the different levels of treatment followed by centers across the world, particularly the severity of the defective genetic background, the levels of hemoglobin and the level of iron load in various patient groups. Another contributing factor is that of the increased survival to adulthood.51,53
Endocrine-related complications include the following:
Delayed puberty and hypogonadism: Hypogonadotropic hypogonadism ranges from 50% to 100%. Adult-onset hypogonadism (AOH) in TDT patients ranges between 8.3% and 12%. These complications result mainly from iron overload (A).
Hypothyroidism: It varies from 6% to 35%.
Impaired glucose tolerance and insulin-dependent diabetes mellitus (IDDM): It increases with age and varies from 10% to 24%. The etiology of IDDM is multifactorial (genetic factors, insulin deficiency, insulin resistance, and liver dysfunction secondary to viral hepatitis).
Hypoparathyroidism: It varies from 1% to 19%. Most patients show a mild form of the disease.
Adrenal insufficiency: “Biochemical adrenal insufficiency” varies up to 45%, but clinical adrenal insufficiency is rare.
Key points and recommendations
Endocrine complications are very common in multitransfused TM patients (A) and periodic evaluation for endocrine complications should be carried out in TDT patients with iron overload, particularly after the age of 11 years (B).
Sub-clinical hypothyroidism (basal TSH 5–8 mUI/ml) requires regular follow-up and optimizing chelation therapy (B).
Normalization of total body iron load with intensive combined chelation (desferrioxamine plus deferiprone) can reverse endocrine complications of TDT (B).
Monitoring of growth, pubertal development, reproductive ability, and endocrine functions in general are essential to achieve a good quality of life in TDT (B).
Infectious disease
Infections and their complications were previously the second commonest cause of death in TDT,22 but are currently becoming the leading cause of mortality in western countries due, in part, to a significant reduction in the deaths from iron-induced cardiac disease.54 There is a lack of properly controlled studies evaluating infections in thalassemia. The knowledge of infections depends more on anecdotal reports and experimental studies. The mechanisms of susceptibility to infections in thalassemia have yet to be clarified. The better understanding of underlying mechanisms and their impact on evolving infections, regional and community-based differences in infectious risks and preventative measures may contribute to a reduction in infection-related mortality in thalassemia.
Key points and recommendations
Physicians must be aware of the potential life-threatening infections in thalassemia and patients should be educated to seek early care when fever develops.
Control of iron homeostasis may have therapeutic benefit against infections.
Temporary discontinuation of deferoxamine and prompt initiation of antibiotics are strongly advised; whereas, iron-loaded patients can continue to use synthetic oral iron chelators such as deferiprone and deferasirox during febrile episodes.
Transfusion of prestorage leucodepleted red cells that have been stored <14 days may have therapeutic benefit against infections.
Quality assurance guidelines and strict regulatory standards should be established for enhancing transfusion safety.
Splenectomy indications and preventive measures for postsplenectomy risk of sepsis should be revisited.
Bone disease
Bone abnormalities are often seen in thalassemia patients and have become a major cause of skeletal complications. These skeletal unusual changes include decreased bone mineral density (BMD), spontaneous fractures, spinal deformities with compression of the vertebrae and nerves often causing severe pain and discomfort. Males with TDT seem to be more frequently and severely affected from bone disease, suggesting a possible gender variation.
Osteoporosis is a prominent cause of morbidity in patients with thalassemia major; it is present in approximately 40%–50% of patients.55 The pathogenesis includes genetic factors as well as endocrine complications (mainly hypogonadism), iron overload, bone marrow expansion, vitamin deficiencies, and lack of physical activity.56 These factors can lead to bone destruction through the increase of osteoclast function and/ or the reduction of osteoblast activity.
Management of thalassemia-associated osteoporosis consists of adequate calcium and vitamin D intake, sufficient iron chelation, hormone replacement, and inhibition of the osteoclast function mainly by bisphosphonates.56–58 Intravenous administration of pamidronate or zoledronic acid seems to be more efficient than oral bisphosphonates. Other novel agents such as the novel osteoclast inhibitor, denosumab, teriparatide, and the activin antagonist, sotatercept, are under investigation but their effects in TM-induced osteoporosis remains to be proven.56,59
Key points and recommendations
Annual checking of BMD starting in adolescence is considered indispensable (C).
Biochemical markers of bone metabolism that can be done every year: N-telopeptide of type I collagen (NTX), C-telopeptide of type I collagen (CTX), bone-specific alkaline phosphatase (bALP) (C).
Physical activity must always be encouraged (C).
Smoking should be discouraged (C).
Adequate calcium intake during skeleton development can increase bone mass in adult life and in combination with administration of low doses of vitamin D may prevent bone loss and fractures (C).
Early diagnosis and treatment of diabetes mellitus (C).
Adequate iron chelation may prevent iron toxicity in the bone and sufficient blood transfusions may inhibit uncontrolled bone marrow expansion (A).
Hormonal replacement where it is needed (A).
Bisphosphonates should begiven concomitantly with calcium and vitamin D and not for longer than 2 years (A).
Splenomegaly and splenectomy
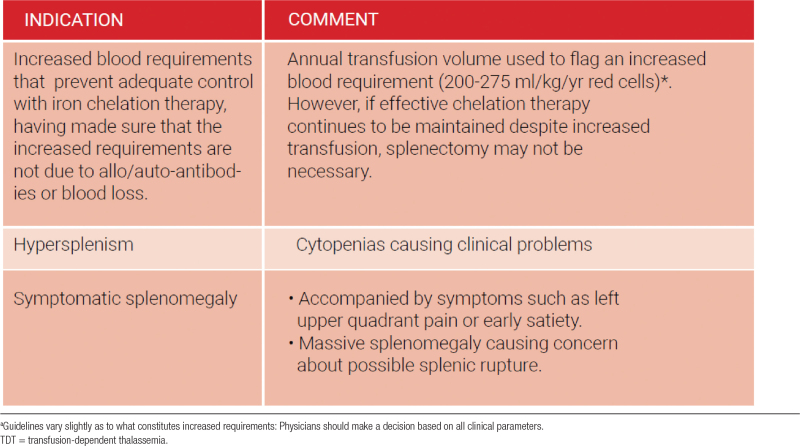
Increased destruction of red blood cells by the reticuloendothelial system, in particular the spleen together with extramedullary hemopoiesis result in splenic enlargement (splenomegaly).60 The main therapeutic rationale for splenectomy in transfusion-dependent patients with TDT has been to decrease blood consumption and transfusion requirements with the ultimate goal of reducing iron overload.61 However, current transfusion guidelines setting more adequate pretransfusional hemoglobin levels (90–105 g/L) have considerably reduced the incidence of splenomegaly and the need for splenectomy in TDT patients.62 Splenectomy should be restricted to certain indications in view of the increased risk of venous thrombosis and pulmonary hypertension, alongside overwhelming infections after splenectomy (Table 2).63–66 Splenectomy should be avoided in children less than 5 years of age because of a considerably greater risk of fulminant postsplenectomy sepsis.
Table 2.
Indications for splenectomy in TDT
Key points and recommendations
The size of the spleen should be carefully monitored and recorded on physical examination and, as needed, by ultrasonography.
Splenectomy is the recommended intervention to reduce excessive blood consumption and consequent severe iron overload. However, physicians should keep a guarded approach towards splenectomy because of the high disease burden associated with splenectomy. Current optimal transfusion regimens and iron chelation have considerably reduced the incidence of splenomegaly and iron overload in transfusion-dependent thalassemia patients.
At the current time, we do not recommend splenectomy as a standard procedure in patients with thalassemia (C). There is large amount of evidence that links splenectomy to a variety of complications such as pulmonary hypertension, silent brain infarcts, venous thrombosis, and sepsis to name a few. Splenectomy should be considered in patients with thalassemia in three clinical scenarios: increased blood requirement that prevents adequate control with iron chelation therapy, hypersplenism, and symptomatic splenomegaly (C).
When performing splenectomy, the laparoscopic approach seems to be the most favorable (B).
The most frequent pathogens that cause infections in splenectomised patients are Streptococcus pneumoniae, Haemophilus influenzae type B, and Neisseria meningitidis; therefore, immune prophylaxis is recommended against these agents at least 2 weeks before the operation and repeated postoperatively as recommended. Additionally, an annual influenza vaccination is encouraged.
Chemoprophylaxis with oral penicillin depends on the age of the individual and the treating physician’s opinion (C). Life-long prophylactic antibiotics with oral penicillins or macrolides should be offered to patients considered at constant high risk of pneumococcal infection, including those who have previously experienced an episode of sepsis or those suffering from concomitant hematological diseases or other immune system impairment.
In current practice, due to strict transfusion and chelation protocols, the disease is very well controlled and we are seeing fewer splenectomies than before. Nevertheless, a large percentage of the thalassemia population (particularly in the older age group), is already splenectomised. These patients are at an increased risk of many disease-related morbidities and should be monitored closely.
Adhere to current transfusion guidelines to prevent or delay splenomegaly.
If splenectomy cannot be avoided, make sure immunization protocols are followed.
Be aware of the dangers of thrombosis in the peri-and post-operative period.
Discuss postsplenectomy chemoprophylaxis with patient/family and make sure they are aware of the dangers of postsplenectomy sepsis.
Start appropriate parenteral antibiotics in case of suspected sepsis while awaiting culture results.
Dental care
Thalassemia patients show a tendency for higher caries, plaque rates, gingivitis, and periodontitis scores than control subjects.67 Those may be attributed to poor oral hygiene, improper dietary habits, lack of dental awareness, reduced salivary flow rate, and neglected dental care. As patients with TDT develop certain disease-related orofacial changes, awareness of these changes is important for the proper dental care.68
Key points and recommendations
Dentists need to be aware of the orofacial manifestations of thalassemia so that they can be identified early and appropriately managed.
Patients with TDT are at increased risk of developing dental caries and periodontal disease.
Patients should be maintained closely on a preventive programme with regular follow-up. Oral hygiene instructions, dietary advice, and preventive measures including prophylaxis, fluoride application, and fissure sealants should be implemented to minimize the need for invasive dental procedures.
Close liaison with the hematology team is required to determine the potential complications when delivering invasive dental treatment and measures put in place to reduce risk.
Predisposing factors for infections in thalassaemic patients include severe anemia, iron overload, splenectomy, and a range of immune abnormalities. Guidelines regarding antibiotic prophylaxis vary from country to country with some recommending prophylaxis similar to that used for the prevention of bacterial endocarditis.
Correction of drifted maxillary anterior teeth and increased overjet should be undertaken to improve aesthetics, reduce susceptibility to trauma, avoid gingival inflammation, and improve functional ability. It is recommended that orthodontic treatment be initiated as early as possible, concentrating on preventive and interceptive approaches.
All patients should ideally have a comprehensive dental assessment with their local dentist prior to the commencement of bisphosphonate therapy, to ensure that they are as dentally fit as feasible and be aware of bisphosphonate-related complication (bisphosphonate-related osteonecrosis of the jaw). Preventive dental advice should be given, emphasizing the importance of reporting any symptoms such as loose teeth, pain, or swelling, as soon as possible.
PSYCHOSOCIAL ISSUES AND ORGANIZATION OF CARE
Fertility and pregnancy
The expectation of having a family—a key aspect of quality of life—is an important aspiration for patients with TDT. Although spontaneous fertility can occur in well-transfused and well-chelated patients with spontaneous puberty and normal menstrual function, the majority are subfertile mainly due to hypogonadotrophic hypogonadism as a consequence of transfusional hemosiderosis.69 Those who fail to achieve pregnancy spontaneously require assisted reproductive techniques (ART).
Planned pregnancy is essential both in spontaneous and ART conceptions, since pregnancies in patients with TM are high risk for both the mother and the baby.70 However, these risks can be minimized through pre-pregnancy counseling involving the various members of the multidisciplinary team: the hematologist, the reproductive medicine specialist, the cardiologist, and the obstetrician, in conjunction with the specialist nurse.71
Key points and recommendations
Iron overload in the pituitary is the main cause of infertility in females.
Successful pregnancy can be achieved in thalassemia major though ovulation induction because ovarian function is usually preserved.
Ovulation in females and spermatogenesis in males can be induced by exogenous gonadotrophin therapy.
Management of infertility requires careful planning and preparation.
Induction of ovulation should only be undertaken by a specialist reproductive team.
Several factors must be taken into consideration before encouraging women with thalassemia major to embark on pregnancy. These include the degree of pre-existing cardiac impairment and of liver dysfunction, as well as the possibility of vertical transmission of viruses.
Pregnancy per se does not alter the natural history of thalassemia—it is safe, provided they have started early on proper treatment and have normal resting cardiac function. If cardiac function deteriorates during pregnancy, deferoxamine may be used cautiously after the first trimester.
Lifestyle and quality of life
Although TDT is burdensome conditions, requiring life-long treatment and close follow-up and can often be complicated by complications arising from many different systems, patients with optimally treated thalassemia can now enjoy a near-normal life and lifestyle, and experience full physical and emotional development from childhood to adulthood.
Key points and recommendations
A holistic approach to patient care includes recognition of the need for social integration and a “normal” life.
The treating physician should be able to provide advice on lifestyle issues.
Physical activity should be encouraged but the condition of each individual patient should be recognized and comorbidities identified. Cardiovascular assessment and ergometry if available should be undertaken before recommending exercise and activity.
Clinic and transfusion times should be flexible and take into consideration the patient’s commitments, such as their education and work. Availability of some evening and weekend clinic and transfusion sessions is highly recommended.
Liaison with teachers and employers to provide understanding of the condition and its management may be necessary.
Routine monitoring of growth is necessary, and nutritional factors such as caloric intake and micronutrient deficiencies should be considered in instances of poor growth. The services of a dietician are helpful in this respect.
Zinc supplements may be given in cases of deficiency, poor growth and reduced bone mass, but are not recommended as routine for all patients.
Dietary iron restriction is recommended in patients on low transfusion regimens with low pretransfusion hemoglobins.
Calcium and vitamin D supplements are recommended for all patients at a dose of 2000 IU per day, along with measurements of vitamin D levels every 6 months. A diet high in calcium is also recommended (through milk, fish, cheese, etc.).
Folic acid supplements of up to 1 mg/day are needed for all patients with low hemoglobin levels. Supplementation for all patients may be considered, since the risk of thrombosis may be reduced and toxicity is low.
A diet rich in foods with high vitamin E content, such as eggs and vegetable oils is recommended. Prolonged use of supplements requires further research.
Vitamin C supplements are recommended, in conjunction with deferoxamine infusions at a dose of 2–3 mg/kg/day, or if deficiency is proven.
L-carnitine may be beneficial at a dose of 50 mg/kg/day, although caution should be exercised in patients with thyroid dysfunction.
There is insufficient evidence on any long-term benefits from wheat grass.
Silymarin at a dose of 140 mg three times daily is recommended if liver involvement is detected, and on consultation with a hepatologist.
Consumption of alcohol and tobacco and substance abuse should be avoided.
A quality of life assessment should form part of the regular evaluation of each patient’s progress, and may usefully also highlight issues in service delivery.
Psychological support
Overall, despite a general lack of large-scale, randomized, controlled trial evidence conducted with patients with thalassemia, there are innumerable cohorts of case controlled analytical studies to suggest that psychological well-being has an impact on adherence to treatment for chronic disease in general (B).72–78 In thalassemia, the published reports to demonstrate this linkage are mainly descriptive studies (C). A meta-analysis would suggest that more recent efforts are more towards B grade investigations (usually ancillary studies attached to robust controlled trials in other clinical areas). However, the lack of uniform instruments and standardized measurements weakens this assessment. The findings to date suggest that:
Psychological well-being impacts on adherence to chelation treatment in TDT and hence on survival (C).
Patients with thalassemia are vulnerable to experiencing psychological challenges (C).
Patient-reported health outcomes show that oral chelation therapy has a beneficial impact, relative to parenteral chelation (B).
Neuropsychological investigation of cognitive deficits shows that there are clear intellectual and psychopathological problems in a very limited number of thalassemia patients (B).
- Benefits of psychological support have been suggested using a variety of approaches (C) which include:
- o targeting changes in institutional organization practices
- o patient group sessions
- o family therapy
- o patient chelation camps
In all chronic illness, continuity of comprehensive care across the lifespan is essential for long-term, beneficial health outcome (A). Institutional organizational support for multidisciplinary teams is essential (A). There is a growing body of evidence that highlight the problems associated with transition from pediatric care to adult internal medicine in inherited chronic disease (B). Rare and neglected diseases complicate resource allocation models and lead to notable health disparities (A). In thalassemia, these problems are known and reports from expert committees recommend addressing them, but there are no formal studies of the problems, much less any standardized evidence.
While A and B grade evidence for psychological support in thalassemia is scarce, experience in several large thalassemia centers strongly suggests that psychological well-being is key to adherence and to outcome.
Expert psychological support has to be available at all centers specializing in thalassemia care (C).
- Additional psychosocial support provided by trained specialists (eg, social workers or family or child health specialists) should be tailored to the patient’s age
- o Children (in general, A, thalassemia C)
- o Adolescents – transition (in general, B, thalassemia C)
- o Older adults –pain issues (in general, A, thalassemia C)
Funding for clinical psychological support services could be more widely achieved if well-designed, multicenter, interventional studies using common standardized instruments were undertaken to evaluate the benefit of psychological and psychosocial support to treatment adherence. The use of established behavioral and social science approaches in such studies need to identify the active components of “psychological support” that are most applicable to patients with thalassemia.
Multidisciplinary care
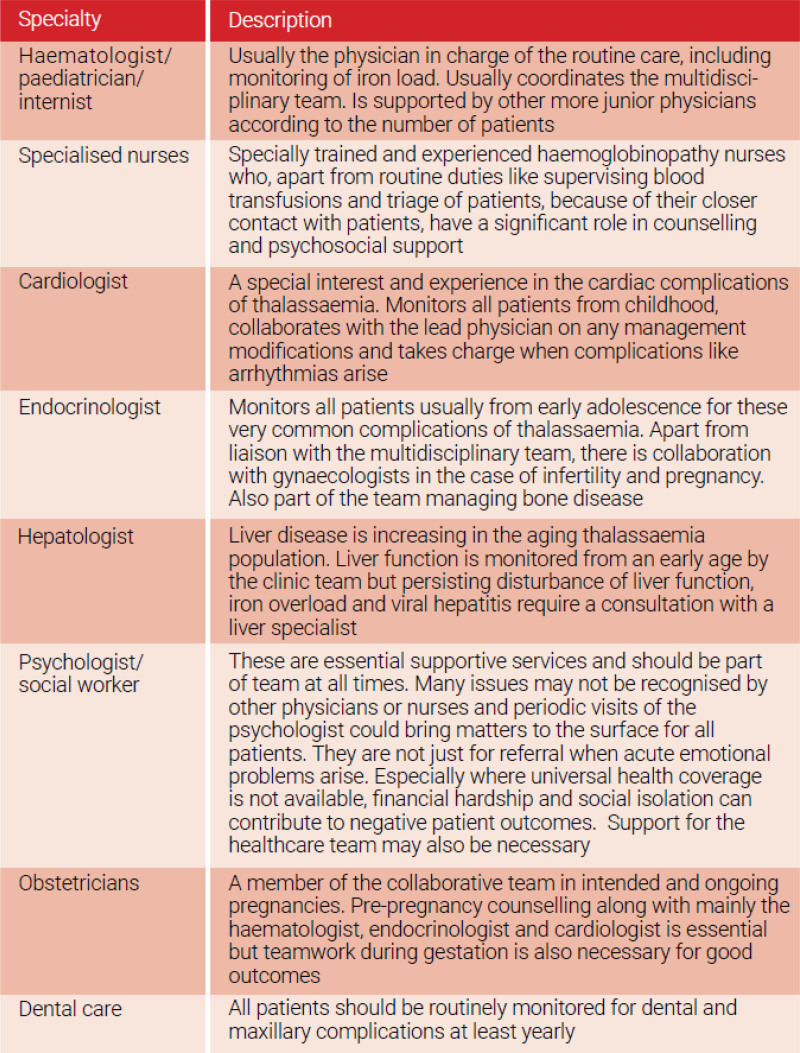
The life-long and multiorgan nature of TDT imposes a multidisciplinary approach to patient’s care. As patients advance in years, the basic needs in blood transfusion and iron chelation, even if and when provided appropriately and in accordance to international standards, gradually become inadequate to sustain life, maintain well-being, and achieve social integration. For this reason, specialists in several medical disciplines including but not confined to heart, liver, and endocrine, are called upon to contribute by monitoring and offering proactive management of iron toxicity and organ dysfunction in their field of expertise.79 An example of the structure of interdisciplinary team for the care of hemoglobin disorders as extracted from some well-established European Reference Centres with successful patient outcomes is presented in the Table 3.80 A suggested timetable for the multidisciplinary clinical and laboratory investigation and follow-up of patients with TDT is provided in Table 4.
Table 3.
Interdisciplinary team for the care of hemoglobin disorders
Table 4.
General Timetable for Clinical and Laboratory Investigation.
AUTHOR CONTRIBUTIONS / 2021 TIF GUIDELINES TASKFORCE MEMBERS
Editors: John Porter, Ali Taher, Maria Domenica Cappellini, and Dimitrios Farmakis.
Authors and Reviewers: Ali Alassaf, Michael Angastiniotis, Emanuele Angelucci, Yesim Aydinok, Rayan Bou-Fakhredin Rayan, Loris Brunetta, Maria Domenica Cappellini, George Constantinou, Shahina Daar, Vincenzo De Sanctis, Geoffrey Dusheiko, Riyad Elbard, Androulla Eleftheriou, Perla Eleftheriou, Panos Englezos, Dimitrios Farmakis, Dru Haines, Faiez N Hattab, George Kaltsounis, Antonios Kattamis, John Koskinas, Navdeep Kumar, Andreas Kulozik, Andreas Kyriakou, Aurelio Maggio, Roanna Maharai, Lauren Mednick, Eleni Michalaki, Wendy Murphy, Lena Oevermann, Raffaella Origa, Penelope-Georgia Papayanni, Constantina Politis, John Porter, Farukh Shah, Anton Skafi, Nikos Skordis, Pietro Sodani, Ashraf Soliman, Seni Subair, Ali Taher, Maria Tampaki, Sara Trompeter, Shobha Tuli, Malcolm Walker, Robert Yamashita, Evangelia Yannaki, and Anne Yardumian.
Special Contribution: Barbara Bain (language editing) and Androulla Eleftheriou (coordination)..
DISCLOSURES
AT reports consultancy fess from Novartis Pharmaceuticals, Bristol-Myers Squibb (Celgene), Ionis Pharmaceuticals, Vifor and Agios; research funding from Novartis Pharmaceuticals, Bristol-Myers Squibb (Celgene), Ionis Pharmaceuticals and Vifor; speaker honoraria from Novartis Pharmaceuticals. EY reports advisory board fees from BlueBirdBio and Vertex/CrispR Therapeutics and speaker honoraria from Pfizer. The remaining authors have no conflicts of interest to disclose.
Footnotes
The 2021 TIF Guidelines Taskforce members are listed at the end of this article.
Contributor Information
Collaborators: John Porter, Ali Taher, Maria Domenica Cappellini, Dimitrios Farmakis, Ali Alassaf, Michael Angastiniotis, Emanuele Angelucci, Yesim Aydinok, Rayan Bou-Fakhredin Rayan, Loris Brunetta, Maria Domenica Cappellini, George Constantinou, Shahina Daar, Vincenzo De Sanctis, Geoffrey Dusheiko, Riyad Elbard, Androulla Eleftheriou, Perla Eleftheriou, Panos Englezos, Dimitrios Farmakis, Dru Haines, Faiez N Hattab, George Kaltsounis, Antonios Kattamis, John Koskinas, Navdeep Kumar, Andreas Kulozik, Andreas Kyriakou, Aurelio Maggio, Roanna Maharai, Lauren Mednick, Eleni Michalaki, Wendy Murphy, Lena Oevermann, Raffaella Origa, Penelope-Georgia Papayanni, Constantina Politis, John Porter, Farukh Shah, Anton Skafi, Nikos Skordis, Pietro Sodani, Ashraf Soliman, Seni Subair, Ali Taher, Maria Tampaki, Sara Trompeter, Shobha Tuli, Malcolm Walker, Robert Yamashita, Evangelia Yannaki, and Anne Yardumian
REFERENCES
- 1.Williams TN, Weatherall DJ. World distribution, population genetics, and health burden of the hemoglobinopathies. Cold Spring Harb Perspect Med. 2012;2:a011692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Taher AT, Musallam KM, Cappellini MD. β-Thalassemias. N Engl J Med. 2021;384:727–743. [DOI] [PubMed] [Google Scholar]
- 3.Farmakis D, Giakoumis A, Angastiniotis M, et al. The changing epidemiology of the ageing thalassaemia populations: A position statement of the Thalassaemia International Federation. Eur J Haematol. 2020;105:16–23. [DOI] [PubMed] [Google Scholar]
- 4.Guidelines for the Management of Transfusion. Dependent Thalassaemia (4th Edition—2021) by Thalassaemia International Federation (TIF). Available at: Issuuhttps://issuu.com/internationalthalassaemiafederation/docs/final_guideline_4th. Accessed January 18, 2022. [PubMed]
- 5.Cazzola M, Borgna-Pignatti C, Locatelli F, et al. A moderate transfusion regimen may reduce iron loading in beta-thalassemia major without producing excessive expansion of erythropoiesis. Transfusion. 1997;37:135–140. [DOI] [PubMed] [Google Scholar]
- 6.Cazzola M, De Stefano P, Ponchio L, et al. Relationship between transfusion regimen and suppression of erythropoiesis in beta-thalassaemia major. Br J Haematol. 1995;89:473–478. [DOI] [PubMed] [Google Scholar]
- 7.EUR-Lex. 32004L0033—EN—EUR-Lex. Available at: https://eur-lex.europa.eu/eli/dir/2004/33/oj/eng. Accessed January 18, 2022.
- 8.EUR-Lex. 32005L0061—EN—EUR-Lex. Available at: https://eur-lex.europa.eu/eli/dir/2005/61/oj/eng. Accessed January 18, 2022.
- 9.EUR-Lex. 32005L0062—EN—EUR-Lex. Available at: https://eur-lex.europa.eu/eli/dir/2005/62/oj/eng. Accessed January 18, 2022.
- 10.EUR-Lex. 32009L0135—EN—EUR-Lex. Available at: https://eur-lex.europa.eu/eli/dir/2009/135/oj/eng. Accessed January 18, 2022.
- 11.EUR-Lex. 32011L0038—EN—EUR-Lex. Available at: https://eur-lex.europa.eu/eli/dir_impl/2011/38/oj/eng. Accessed January 18, 2022.
- 12.EUR-Lex. 32014L0110—EN—EUR-Lex. Available at: https://eur-lex.europa.eu/eli/dir/2014/110/oj/eng. Accessed January 18, 2022.
- 13.EUR-Lex. 32016L1214—EN—EUR-Lex. Available at: https://eur-lex.europa.eu/eli/dir/2016/1214/oj/eng. Accessed January 18, 2022.
- 14.Porter JB, Garbowski M. The pathophysiology of transfusional iron overload. Hematol Oncol Clin North Am. 2014;28:683–701, vi. [DOI] [PubMed] [Google Scholar]
- 15.Kremastinos DT, Farmakis D. Iron overload cardiomyopathy in clinical practice. Circulation. 2011;124:2253–2263. [DOI] [PubMed] [Google Scholar]
- 16.St Pierre TG, Clark PR, Chua-anusorn W, et al. Noninvasive measurement and imaging of liver iron concentrations using proton magnetic resonance. Blood. 2005;105:855–861. [DOI] [PubMed] [Google Scholar]
- 17.Garbowski MW, Carpenter JP, Smith G, et al. Biopsy-based calibration of T2* magnetic resonance for estimation of liver iron concentration and comparison with R2 Ferriscan. J Cardiovasc Magn Reson. 2014;16:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Angelucci E, Giovagnoni A, Valeri G, et al. Limitations of magnetic resonance imaging in measurement of hepatic iron. Blood. 1997;90:4736–4742. [PubMed] [Google Scholar]
- 19.Angelucci E, Brittenham GM, McLaren CE, et al. Hepatic iron concentration and total body iron stores in thalassemia major. N Engl J Med. 2000;343:327–331. [DOI] [PubMed] [Google Scholar]
- 20.Anderson LJ, Holden S, Davis B, et al. Cardiovascular T2-star (T2*) magnetic resonance for the early diagnosis of myocardial iron overload. Eur Heart J. 2001;22:2171–2179. [DOI] [PubMed] [Google Scholar]
- 21.Davis BA, O’Sullivan C, Jarritt PH, et al. Value of sequential monitoring of left ventricular ejection fraction in the management of thalassemia major. Blood. 2004;104:263–269. [DOI] [PubMed] [Google Scholar]
- 22.Borgna-Pignatti C, Rugolotto S, De Stefano P, et al. Survival and complications in patients with thalassemia major treated with transfusion and deferoxamine. Haematologica. 2004;89:1187–1193. [PubMed] [Google Scholar]
- 23.Agarwal MB, Gupte SS, Viswanathan C, et al. Long-term assessment of efficacy and safety of L1, an oral iron chelator, in transfusion dependent thalassaemia: Indian trial. Br J Haematol. 1992;82:460–466. [DOI] [PubMed] [Google Scholar]
- 24.Cappellini MD, Cohen A, Piga A, et al. A phase 3 study of deferasirox (ICL670), a once-daily oral iron chelator, in patients with beta-thalassemia. Blood. 2006;107:3455–3462. [DOI] [PubMed] [Google Scholar]
- 25.Angelucci E. Hematopoietic stem cell transplantation in thalassemia. Hematology Am Soc Hematol Educ Program. 2010;2010:456–462. [DOI] [PubMed] [Google Scholar]
- 26.Lucarelli G, Gaziev J. Advances in the allogeneic transplantation for thalassemia. Blood Rev. 2008;22:53–63. [DOI] [PubMed] [Google Scholar]
- 27.Isgrò A, Gaziev J, Sodani P, et al. Progress in hematopoietic stem cell transplantation as allogeneic cellular gene therapy in thalassemia. Ann N Y Acad Sci. 2010;1202:149–154. [DOI] [PubMed] [Google Scholar]
- 28.Baronciani D, Angelucci E, Potschger U, et al. Hemopoietic stem cell transplantation in thalassemia: a report from the European Society for Blood and Bone Marrow Transplantation Hemoglobinopathy Registry, 2000-2010. Bone Marrow Transplant. 2016;51:536–541. [DOI] [PubMed] [Google Scholar]
- 29.Lucarelli G, Andreani M, Angelucci E. The cure of thalassemia by bone marrow transplantation. Blood Rev. 2002;16:81–85. [DOI] [PubMed] [Google Scholar]
- 30.Antoniani C, Meneghini V, Lattanzi A, et al. Induction of fetal hemoglobin synthesis by CRISPR/Cas9-mediated editing of the human β-globin locus. Blood. 2018;131:1960–1973. [DOI] [PubMed] [Google Scholar]
- 31.Boulad F, Wang X, Qu J, et al. Safe mobilization of CD34+ cells in adults with β-thalassemia and validation of effective globin gene transfer for clinical investigation. Blood. 2014;123:1483–1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Psatha N, Papayanni PG, Yannaki E. A new era for hemoglobinopathies: more than one curative option. Curr Gene Ther. 2017;17:364–378. [DOI] [PubMed] [Google Scholar]
- 33.Thompson AA, Walters MC, Kwiatkowski J, et al. Gene therapy in patients with transfusion-dependent β-Thalassemia. N Engl J Med. 2018;378:1479–1493. [DOI] [PubMed] [Google Scholar]
- 34.Lal A, Locatelli F, Kwiatkowski JL, et al. Northstar-3: Interim results from a phase 3 study evaluating lentiglobin gene therapy in patients with transfusion-dependent β-thalassemia and either a β0 or IVS-I-110 mutation at both alleles of the HBB gene. Blood 2019;134:815. [Google Scholar]
- 35.Cappellini MD, Viprakasit V, Taher AT, et al. A phase 3 trial of luspatercept in patients with transfusion-dependent β-thalassemia. N Engl J Med. 2020;382:1219–1231. [DOI] [PubMed] [Google Scholar]
- 36.Voskaridou E, Ladis V, Kattamis A, et al. A national registry of haemoglobinopathies in Greece: deducted demographics, trends in mortality and affected births. Ann Hematol. 2012;91:1451–1458. [DOI] [PubMed] [Google Scholar]
- 37.Modell B, Khan M, Darlison M, Westwood MA, Ingram D, Pennell DJ. Improved survival of thalassaemia major in the UK and relation to T2* cardiovascular magnetic resonance. J Cardiovasc Magn Reson Off J Soc Cardiovasc Magn Reson 2008;10:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Koohi F, Kazemi T, Miri-Moghaddam E. Cardiac complications and iron overload in beta thalassemia major patients-a systematic review and meta-analysis. Ann Hematol. 2019;98:1323–1331. [DOI] [PubMed] [Google Scholar]
- 39.Farmakis D, Triposkiadis F, Lekakis J, et al. Heart failure in haemoglobinopathies: pathophysiology, clinical phenotypes, and management. Eur J Heart Fail. 2017;19:479–489. [DOI] [PubMed] [Google Scholar]
- 40.Farmakis D, Aessopos A. Pulmonary hypertension associated with hemoglobinopathies: prevalent but overlooked. Circulation. 2011;123:1227–1232. [DOI] [PubMed] [Google Scholar]
- 41.Taher A, Isma’eel H, Mehio G, et al. Prevalence of thromboembolic events among 8,860 patients with thalassaemia major and intermedia in the Mediterranean area and Iran. Thromb Haemost. 2006;96:488–491. [PubMed] [Google Scholar]
- 42.Aessopos A, Farmakis D, Karagiorga M, et al. Pseudoxanthoma elasticum lesions and cardiac complications as contributing factors for strokes in beta-thalassemia patients. Stroke. 1997;28:2421–2424. [DOI] [PubMed] [Google Scholar]
- 43.Cheung YF, Chan GC, Ha SY. Arterial stiffness and endothelial function in patients with beta-thalassemia major. Circulation. 2002;106:2561–2566. [DOI] [PubMed] [Google Scholar]
- 44.Kremastinos DT, Farmakis D, Aessopos A, et al. Beta-thalassemia cardiomyopathy: history, present considerations, and future perspectives. Circ Heart Fail. 2010;3:451–458. [DOI] [PubMed] [Google Scholar]
- 45.Sikorska K, Bernat A, Wroblewska A. Molecular pathogenesis and clinical consequences of iron overload in liver cirrhosis. Hepatobiliary Pancreat Dis Int. 2016;15:461–479. [DOI] [PubMed] [Google Scholar]
- 46.Brittenham GM. Iron-chelating therapy for transfusional iron overload. N Engl J Med. 2011;364:146–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Maggio A, Filosa A, Vitrano A, et al. Iron chelation therapy in thalassemia major: a systematic review with meta-analyses of 1520 patients included on randomized clinical trials. Blood Cells Mol Dis. 2011;47:166–175. [DOI] [PubMed] [Google Scholar]
- 48.Borgna-Pignatti C, Garani MC, Forni GL, et al. Hepatocellular carcinoma in thalassaemia: an update of the Italian Registry. Br J Haematol. 2014;167:121–126. [DOI] [PubMed] [Google Scholar]
- 49.Di Marco V, Capra M, Angelucci E, et al. ; Italian Society for the Study of Thalassemia and Haemoglobinopathies; Italian Association for the Study of the Liver. Management of chronic viral hepatitis in patients with thalassemia: recommendations from an international panel. Blood. 2010;116:2875–2883. [DOI] [PubMed] [Google Scholar]
- 50.European Association for the Study of the Liver. Electronic address: easloffice@easloffice.eu, Clinical Practice Guidelines Panel: Chair, EASL Governing Board representative, Panel members: EASL recommendations on treatment of hepatitis C: Final update of the series. J Hepatol 2020;73:1170–1218. [DOI] [PubMed] [Google Scholar]
- 51.De Sanctis V, Soliman AT, Elsedfy H, et al. Growth and endocrine disorders in thalassemia: The international network on endocrine complications in thalassemia (I-CET) position statement and guidelines. Indian J Endocrinol Metab. 2013;17:8–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Skordis N, Kyriakou A. The multifactorial origin of growth failure in thalassaemia. Pediatr Endocrinol Rev. 2011;8(Suppl 2):271–277. [PubMed] [Google Scholar]
- 53.De Sanctis V, Elsedfy H, Soliman AT, et al. Endocrine profile of β-thalassemia major patients followed from childhood to advanced adulthood in a tertiary care center. Indian J Endocrinol Metab. 2016;20:451–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wanachiwanawin W. Infections in E-beta thalassemia. J Pediatr Hematol Oncol. 2000;22:581–587. [DOI] [PubMed] [Google Scholar]
- 55.De Sanctis V, Soliman AT, Elsedfy H, et al. ; I-CET (International Network on Growth Disorders and Endocrine Complications in Thalassemia). Osteoporosis in thalassemia major: an update and the I-CET 2013 recommendations for surveillance and treatment. Pediatr Endocrinol Rev. 2013;11:167–180. [PubMed] [Google Scholar]
- 56.Voskaridou E, Terpos E. Pathogenesis and management of osteoporosis in thalassemia. Pediatr Endocrinol Rev. 2008;6(Suppl 1):86–93. [PubMed] [Google Scholar]
- 57.Giusti A. Bisphosphonates in the management of thalassemia-associated osteoporosis: a systematic review of randomised controlled trials. J Bone Miner Metab. 2014;32:606–615. [DOI] [PubMed] [Google Scholar]
- 58.Bhardwaj A, Swe KM, Sinha NK, et al. Treatment for osteoporosis in people with ß-thalassaemia. Cochrane Database Syst Rev. 2016;3:CD010429. [DOI] [PubMed] [Google Scholar]
- 59.Voskaridou E, Ntanasis-Stathopoulos I, Papaefstathiou A, et al. Denosumab in transfusion-dependent thalassemia osteoporosis: a randomized, placebo-controlled, double-blind phase 2b clinical trial. Blood Adv. 2018;2:2837–2847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cappellini MD, Porter JB, Viprakasit V, et al. A paradigm shift on beta-thalassaemia treatment: How will we manage this old disease with new therapies? Blood Rev. 2018;32:300–311. [DOI] [PubMed] [Google Scholar]
- 61.Rachmilewitz EA, Giardina PJ. How I treat thalassemia. Blood. 2011;118:3479–3488. [DOI] [PubMed] [Google Scholar]
- 62.Piga A, Serra M, Longo F, et al. Changing patterns of splenectomy in transfusion-dependent thalassemia patients. Am J Hematol. 2011;86:808–810. [DOI] [PubMed] [Google Scholar]
- 63.Standard of Care Guidelines for Thalassaemia 2012. Available at: https://thalassemia.com/documents/SOCGuidelines2012.pdf. Accessed July 1, 2022.
- 64.Guidelines for the Clinical Care of Patients with Thalassemia in Canada. Available at: http://www.thalassemia.ca/wp-content/uploads/Thalassemia-Guidelines_LR.pdf. Accessed July 1, 2022.
- 65.Taher AT, Weatherall DJ, Cappellini MD. Thalassaemia. Lancet. 2018;391:155–167. [DOI] [PubMed] [Google Scholar]
- 66.Aessopos A, Farmakis D, Deftereos S, et al. Cardiovascular effects of splenomegaly and splenectomy in beta-thalassemia. Ann Hematol. 2005;84:353–357. [DOI] [PubMed] [Google Scholar]
- 67.Duggal MS, Bedi R, Kinsey SE, et al. The dental management of children with sickle cell disease and beta-thalassaemia: a review. Int J Paediatr Dent. 1996;6:227–234. [DOI] [PubMed] [Google Scholar]
- 68.Hattab FN. Periodontal condition and orofacial changes in patients with thalassemia major: a clinical and radiographic overview. J Clin Pediatr Dent. 2012;36:301–307. [PubMed] [Google Scholar]
- 69.Skordis N, Christou S, Koliou M, et al. Fertility in female patients with thalassemia. J Pediatr Endocrinol Metab. 1998;11(Suppl 3):935–943. [PubMed] [Google Scholar]
- 70.Aessopos A, Karabatsos F, Farmakis D, et al. Pregnancy in patients with well-treated beta-thalassemia: outcome for mothers and newborn infants. Am J Obstet Gynecol. 1999;180(2 Pt 1):360–365. [DOI] [PubMed] [Google Scholar]
- 71.Skordis N, Petrikkos L, Toumba M, et al. Update on fertility in thalassaemia major. Pediatr Endocrinol Rev. 2004;2(Suppl 2):296–302. [PubMed] [Google Scholar]
- 72.Anie KA, Massaglia P. Psychological therapies for thalassaemia. Cochrane Database Syst Rev 2001;(3):CD002890. [DOI] [PubMed] [Google Scholar]
- 73.Aydinok Y, Erermis S, Bukusoglu N, et al. Psychosocial implications of Thalassemia Major. Pediatr Int. 2005;47:84–89. [DOI] [PubMed] [Google Scholar]
- 74.Cakaloz B, Cakaloz I, Polat A, et al. Psychopathology in thalassemia major. Pediatr Int. 2009;51:825–828. [DOI] [PubMed] [Google Scholar]
- 75.Mazzone L, Battaglia L, Andreozzi F, et al. Emotional impact in beta-thalassaemia major children following cognitive-behavioural family therapy and quality of life of caregiving mothers. Clin Pract Epidemiol Ment Health. 2009;5:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mednick L, Yu S, Trachtenberg F, et al. ; Thalassemia Clinical Research Network. Symptoms of depression and anxiety in patients with thalassemia: prevalence and correlates in the thalassemia longitudinal cohort. Am J Hematol. 2010;85:802–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Saini A, Chandra J, Goswami U, et al. Case control study of psychosocial morbidity in beta thalassemia major. J Pediatr. 2007;150:516–520. [DOI] [PubMed] [Google Scholar]
- 78.Tsiantis J, Dragonas T, Richardson C, et al. Psychosocial problems and adjustment of children with beta-thalassemia and their families. Eur Child Adolesc Psychiatry. 1996;5:193–203. [DOI] [PubMed] [Google Scholar]
- 79.Angastiniotis M, Eleftheriou A. Requirements for a reference or expert thalassemia center: the structure/model for centers dealing with chronic/hereditary blood disorders. Hemoglobin. 2009;33 Suppl 1:S204–S210. [DOI] [PubMed] [Google Scholar]
- 80.European Commission - European Reference Networks Overview. Available at: https://ec.europa.eu/health/european-reference-networks/overview_en. Accessed January 20, 2022.