Introduction:
Our emergency department updated our care algorithm to provide evidence-based, standardized care to 0- to 60-day-old febrile neonates. Specifically, we wanted to increase the proportion of visits for which algorithm-adherent care was provided from 90% to 95% for infants 0–28 days, and from 67% to 95% for infants 29–60 days, by June 30, 2020.
Methods:
Our emergency medicine team outlined our theory for improvement and used multiple plan-do-study-act cycles to test interventions aimed at key drivers. Interventions included constructing an updated care algorithm, clinician, and nurse education, integrating an updated opt-out order set, and streamlined discharge instructions. Our primary outcome was the proportion of patient encounters in which clinicians ordered algorithm-adherent care. In addition, our quality improvement team manually reviewed all failures to determine the reasons for failure and inform further interventions.
Results:
We evaluated 2,248 visits between January 2018 and October 2021. Algorithm-adherent care for 29- to 60-day-old infants improved from 67% to 92%. Algorithm-adherent care for 0- to 28-day infants improved from 90% to 96%. We sustained these improvements for 22 months. Failure to adhere to the algorithm in the 29- to 60-day-old infant group was primarily due to clinicians not ordering procalcitonin.
Conclusions:
Using quality improvement methods, we successfully increased algorithm-adherent evaluation of febrile neonates 0–60 days old in our pediatric emergency departments. Education and opt-out order sets were keys to implementing our new algorithm.
INTRODUCTION
Almost 500,000 infants present to emergency departments (EDs) in the United States for evaluation of fever annually.1,2 Although most of these infants have viruses, 8%–12.5% of infants 0–60 days of age and up to 20% of those younger than 28 days have serious or invasive bacterial infections without specific history or examination findings.3,4 Serious bacterial infections (SBIs) include bacteremia, bacterial gastroenteritis, cellulitis, osteomyelitis, septic arthritis, meningitis, pneumonia, and urinary tract infection. Invasive bacterial infections (IBIs) include bacteremia and acute bacterial meningitis. Accurate identification of SBIs and IBIs in infants has remained a clinical challenge. Multiple clinical prediction models have been proposed, such as the Rochester, Philadelphia, and Boston criteria,5–7 which combine history, physical examination findings, and laboratory data to stratify the risk of SBI/IBI in young infants. Different clinical prediction models have contributed to significant variation in the evaluation and management of febrile infants under 60 days of age.8,9 Standardizing management of febrile infants can decrease hospitalization rates and encourage judicious antimicrobial stewardship for infants identified as low risk for SBI.10 Most recently, the AAP published a clinical practice guideline (2021) for managing well-appearing febrile neonates, which seeks to further expand upon previous guidelines.11 We believe updated systematic evaluation and management of fever of uncertain source (FUS) in this age group will provide standardized, evidence-based care in the ED.
Local Problem
Our primary quaternary medical center ED and our community hospital ED evaluate febrile neonates. Before any intervention, there was variability in the laboratory evaluation performed on these infants. Despite changes in the epidemiology of bacterial pathogens12,13 and the introduction of biomarkers such as procalcitonin and c-reactive protein (CRP),14–16 and changing national standards of care for this patient population, the hospital guideline for FUS in infants at our institution had not been updated since 2010. A 2019 PECARN study included derivation and validation of a rule with a sensitivity of 97.7% to detect SBIs and IBIs while decreasing the rate of lumbar punctures, antimicrobial therapy, and hospitalizations, especially in infants 29–60 days of age.17 To update care in light of current evidence, physician leaders from the Divisions of Emergency Medicine, Infectious Disease, Community Pediatrics, and Hospital Medicine, as well as representatives from Pharmacy and Clinical Laboratory, formed a multidisciplinary committee to review relevant existing literature and guidelines, resulting in an updated hospital-wide evidence-based care guideline for the evaluation and management of infants 0–60 days who presented with FUS.
The purpose of this Quality Improvement (QI) initiative was to provide reliable, algorithm-adherent care for infants 0–60 days of age with fever (historical or documented rectal temperature ≥ 38 °C) presenting to one of our institution’s pediatric EDs (PED) based on an updated institutional care algorithm constructed by a cross-divisional, multidisciplinary group. Therefore, we wanted to increase the proportion of visits for which algorithm-adherent care was provided from 90% to 95% for infants 0–28 days, and from 67% to 95% for infants 29–60 days, by June 30, 2020.
METHODS
Setting and Context
We conducted this study at a quaternary care children’s hospital with two PEDs (an urban academic center and a satellite community setting). These PEDs have a combined annual volume of ~100,000 visits. The parent pediatric institution is a level I trauma center responsible for 85% to 90% of pediatric admissions from a population base of two million people. The study was an institutional QI project and nonhuman subjects research, exempt from Institutional Review Board approval.
Pediatric emergency medicine (PEM) faculty, general pediatricians, advanced practice registered nurses (APRN), and resident physicians staff the PEDs. Our institution uses a large commercial EMR system (EPIC Systems Corporation, Verona, Wis.) that allows PED care algorithms to be uploaded to a repository-specific to the ED context. PED order sets are suggested based on a patient’s chief complaints. Otherwise, clinicians must search for an order set by name if the EMR does not suggest it.
Our team sought to improve the reliability of algorithm-adherent care for infants 0–60 days of age with any temperature ≥38º C without an apparent source after a thorough history and physical examination. Our institutional guideline defined an apparent source like skin or soft-tissue infection, specifically cellulitis, omphalitis, or mastitis. We did not consider congestion, rhinorrhea, cough, and acute otitis media as apparent sources of infection because viral infections may not preclude bacterial infection. In addition, we excluded infants currently receiving antibiotics, immunized within 48 hours, or presenting with isolated hypothermia.
Interventions
Key Driver and Global SMART Aim Development
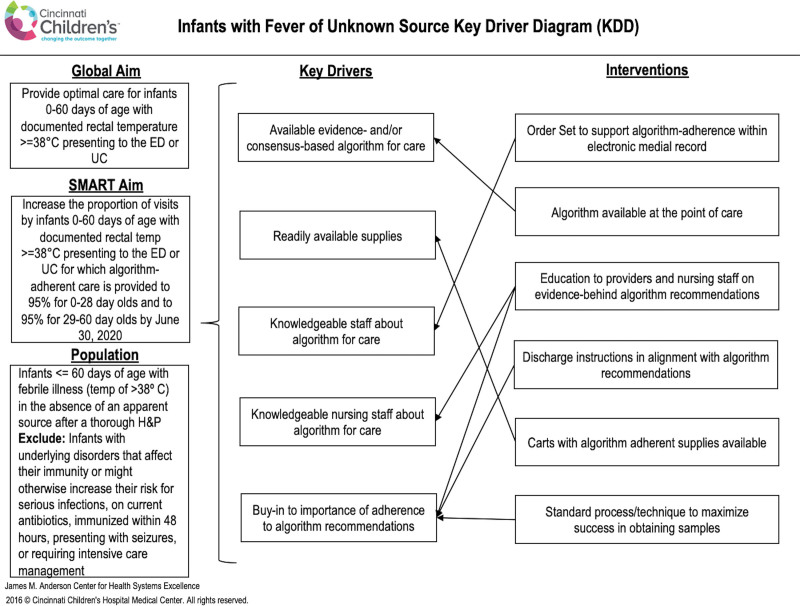
A multidisciplinary QI team of key stakeholders was assembled: ED physicians, pediatric resident physicians, nurses, and pharmacists. The team first defined their global and smart aim and developed a key driver diagram using the Model for Improvement.18 We linked interventions to each driver (Fig. 1) and tested these potential interventions using Plan-Do-Study-Act cycles. Finally, we adopted and incorporated effective interventions into the clinical process.
Fig. 1.
Febrile neonate evaluation and treatment key driver diagram. ED, emergency department; SMART, specific, measurable, attainable, realistic, timely; UC, urgent care.
Algorithm Development
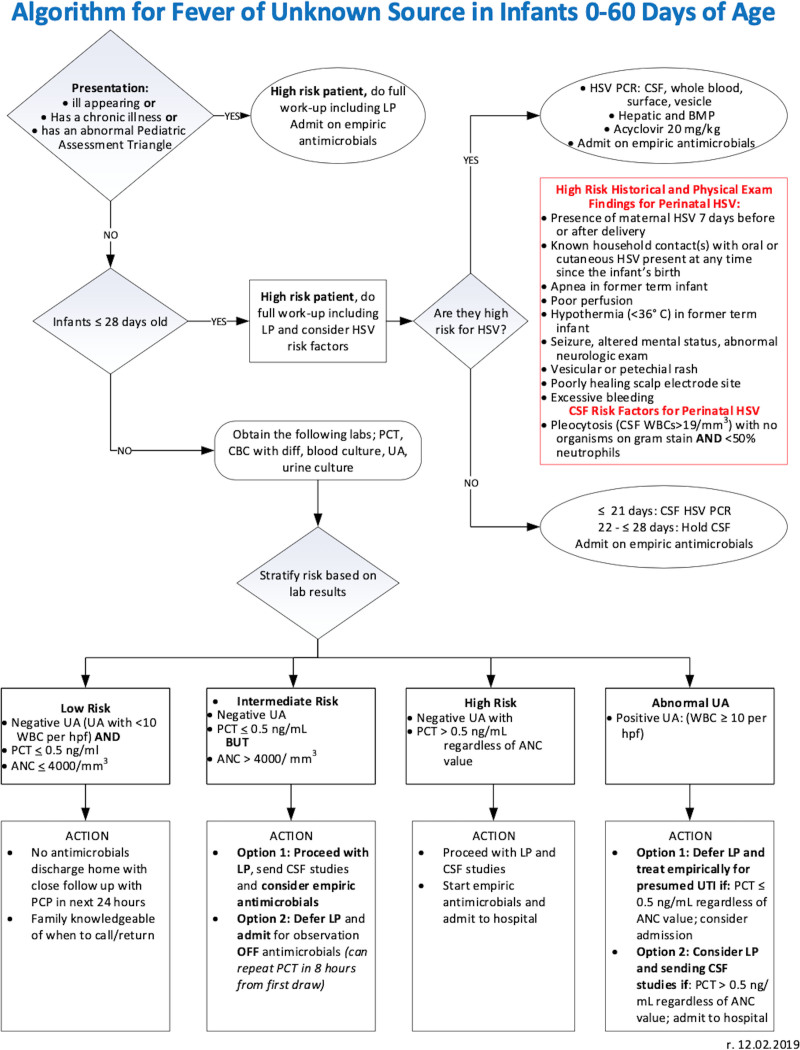
An interdisciplinary team of physicians, nurse practitioners, and nurses adapted the updated hospital febrile neonate guideline for the PED to generate our divisional clinical care algorithm (Fig. 2), which was linked to the electronic medical record (EMR) of any infant between 0 and 60 days who presented for possible infection starting in October 2019.
Fig. 2.
Febrile neonatal fever care algorithm for Cincinnati Children’s Pediatric Emergency Department.
The algorithm includes laboratory, imaging, and treatment recommendations for FUS in infants 0–60 days based on assessing risk (high, intermediate, or low) of IBI and suspicion of urinary tract infection. First, the algorithm identifies any ill-appearing infant who has an abnormal Pediatric Assessment Triangle,19 or has a chronic illness, and all infants 0–28 days old as high risk with a recommendation to send blood, urine, and cerebrospinal fluid (CSF) cultures and administer empiric antibiotics in the PED. Additionally, infants 0–28 days of age who meet high-risk criteria for herpes simplex virus (HSV)20 (identified by the algorithm) should have CSF, blood, and surface HSV PCR. In addition, they should receive additional laboratories, including a hepatic panel and a basic metabolic panel, and be treated with empiric acyclovir.
Blood and urine cultures are recommended for all clinically well-appearing, previously healthy infants 29–60 days. These infants are then risk-stratified into low-, intermediate-, or high-risk groups based on the results of three recommended laboratories: complete blood count (CBC), procalcitonin, and urinalysis (UA) and dispositioned accordingly (Fig. 2).
Education
Education of PEM providers occurred at our monthly division staff meeting beginning in January 2019 with the introduction of the hospital algorithm and periodically after that as we developed the divisional algorithm. Additionally, we separately educated the staff pediatricians and APRNs specifically about the divisional algorithm at a clinical staff meeting in October 2019. The education consisted of a slide presentation outlining the evidence for the recommendations and a detailed introduction to the algorithm. In addition, the hospital medicine team educated pediatric residents in collaboration with emergency medicine as part of the inpatient rotation. We reinforced classroom education with just-in-time education about the order set during the ED rotation when residents cared for an infant with a fever. We also provided education to the PED nurses at their monthly meeting beginning in September 2019 and periodically after that. We reinforced algorithm education using video monitor education in common areas of the PED during February 2020. Finally, the Division of Hospital Medicine conducted community outreach to inform local pediatricians of the new clinical care guideline.
EMR-based Interventions
We developed a point-of-care order set and integrated it with a previously existing PED neonatal HSV algorithm, which was available in a protocol repository linked to the EMR. We divided the order sets into categories based on the two age groups: 0–28 and 29–60 days, with algorithm-adherent orders based on age preselected to facilitate ease of ordering. We based the opt-out design of the order set on previous work to identify the optimal setup for order set use within our institution. The order set became available on the EMR in December 2019.
Discharge Instructions
To streamline and standardize the discharge of patients 29–60 days of age who qualified for discharge per low-risk criteria, the QI team developed discharge instructions with detailed, extensive return precautions. This document was uploaded to the EMR in March 2020 to facilitate a standard discharge process.
Study of the Interventions
We compiled data between January 2018 and October 2021 from the EMR to identify PED encounters for febrile infants. We initially abstracted all patients aged 0-60 days at the time of visit who had a temperature ≥38 °C at the visit OR had a blood culture ordered at the visit OR had a urine culture ordered at the visit from the EMR. The team then performed a standard chart review of any visit that was a “failure” for algorithm adherence to determine if the visit met inclusion criteria. We excluded the following groups by chart review: evaluation performed for reasons other than fever (including hypothermia), fever within 48 hours of vaccines, and a follow-up visit to ED during the same febrile illness. Additionally, we excluded infants with skin and soft tissue infections or surgical site infections, regardless of fever.
Measures
Our primary process measures were the proportions of patient visits in which clinicians provided algorithm-adherent care for the evaluation and treatment of (1) patients 0–28 days of age and (2) patients 29–60 days of age who presented to our PEDs with FUS. For patients 0–28 days of age, algorithm-adherent care was defined as blood, urine, and CSF cultures, and cephalosporin or gentamicin was ordered before hospital admission. The denominator for this measure was all encounters for infants 0–28 days undergoing evaluation for FUS. We defined algorithm-adherent ordered care for infants 29-60 days as CBC, blood culture, procalcitonin, UA, and urine culture. The denominator for this measure was all encounters for infants 29–60 days undergoing evaluation for FUS. We also tracked a secondary process measure of the proportion of visits in which the new order set was used. The proportion of PED visits resulting in admissions over time was tracked. No special cause variation was related to our interventions in the admission rate in either the 0- to 28-day age group or the 29- to 60-day age group.
Analysis
For each age group, we constructed a P-chart to demonstrate the proportion of eligible patient encounters that were algorithm adherent. We tracked the process measures over time using a statistical process control chart to evaluate the impact of the described interventions. We analyzed the p-charts per the rules for Shewhart chart interpretation to identify special cause.21
RESULTS
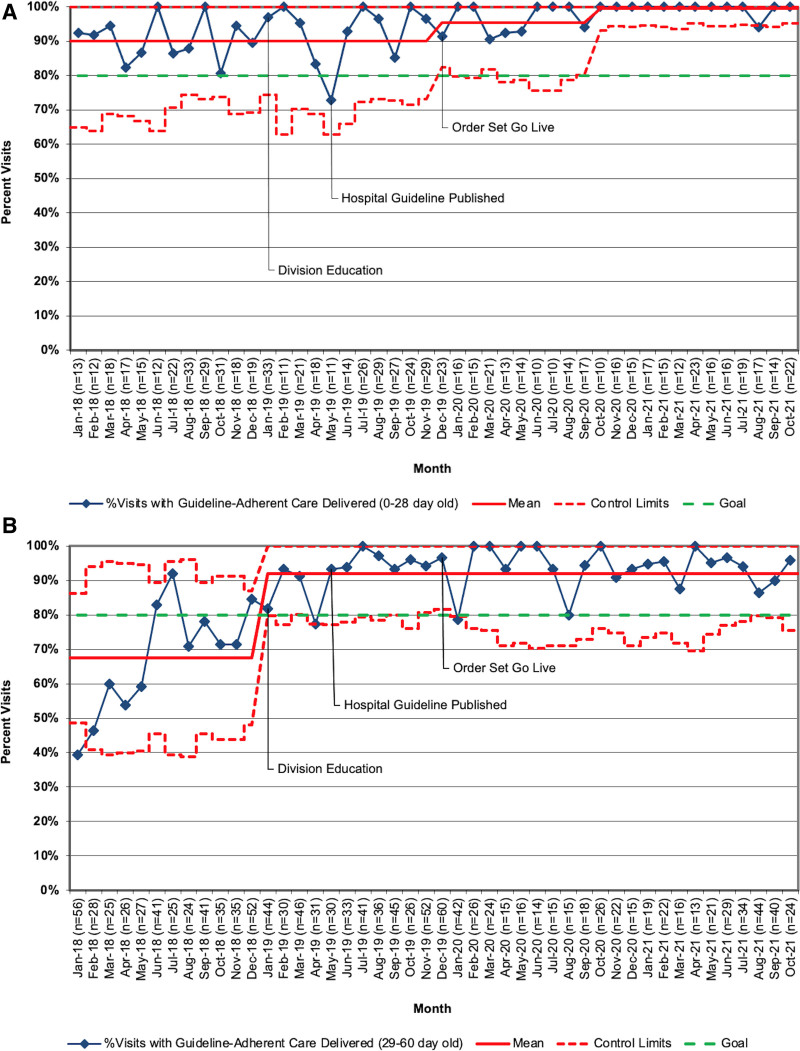
We evaluated a total of 2,248 encounters between January 2018 and October 2021. Of these, 849 encounters were with neonates 0–28 days, and 1,399 were with neonates 29–60 days. During the baseline period (January 2018–December 2018), there were 654 patient encounters. During the implementation and sustainment periods (January 2019–October 2021), there were 1594 patient encounters. Algorithm-adherent care for 0–28 day infants improved from 90% to >96% (Fig. 3A). Special cause variation was demonstrated in December 2019 for this group and again in October 2020. We successfully sustained this performance at this new baseline through October 2021. Algorithm-adherent care for 29–60 day infants improved from 67% to 92% (Fig. 3B). The team identified special cause variation in January 2019 in the older age group and sustained performance at this new baseline through October 2021. However, this falls short of the 95% goal set in the specific aim.
Fig. 3.
A, Proportion of encounters for febrile neonates 0–28 days of age with algorithm-adherent care, January 2019–October 2021; (B) Proportion of encounters for febrile neonates 29–60 days of age with algorithm-adherent care, January 2019–October 2021.
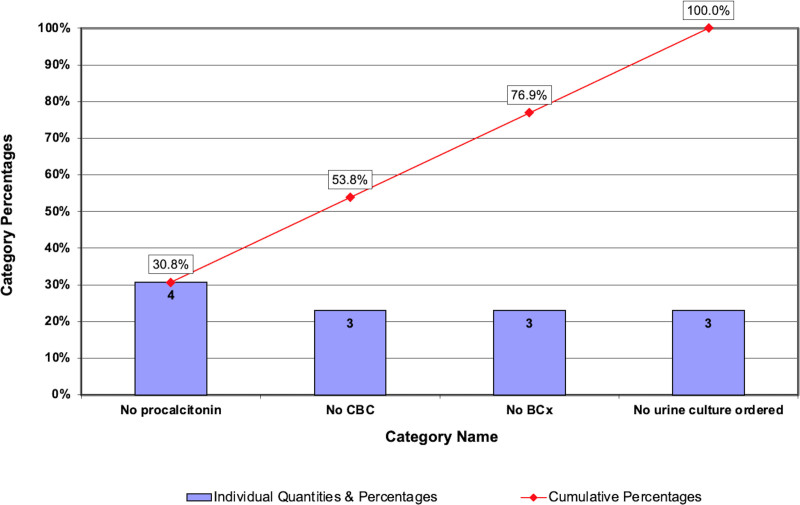
From July 2020 to December 2020, after we demonstrated special cause and shifted the centerline, we analyzed the reasons for algorithm nonadherence using a Pareto chart to understand how to target future interventions. There was a single failure in the 0- to 28-day age group due to no antibiotics given after a failed LP attempt. There were eight failures in the 29- to 60-day age group, with the most common reasons for algorithm nonadherence noted as no procalcitonin or no blood culture ordered. A Pareto chart highlights results for the older age group (Fig. 4). Additionally, when manual chart reviews were performed to determine algorithm eligibility from January to October 2021, we noted an additional two failures in the 0- to 28-day age group and 15 failures in the 29- to 60-day age group. In 2021, both failures in the younger age group were due to the provider’s decision not to perform an LP. The most common reasons for algorithm nonadherence in infants 29–60 days of age were: eight visits with no urine culture ordered, three visits with no procalcitonin ordered, two visits with no blood culture ordered, and two visits with no bloodwork ordered at all.
Fig. 4.
Postintervention Pareto chart of reasons for nonadherent care for 29- to 60-day-old infants (July–December 2020, n = 8). BCx, blood culture.
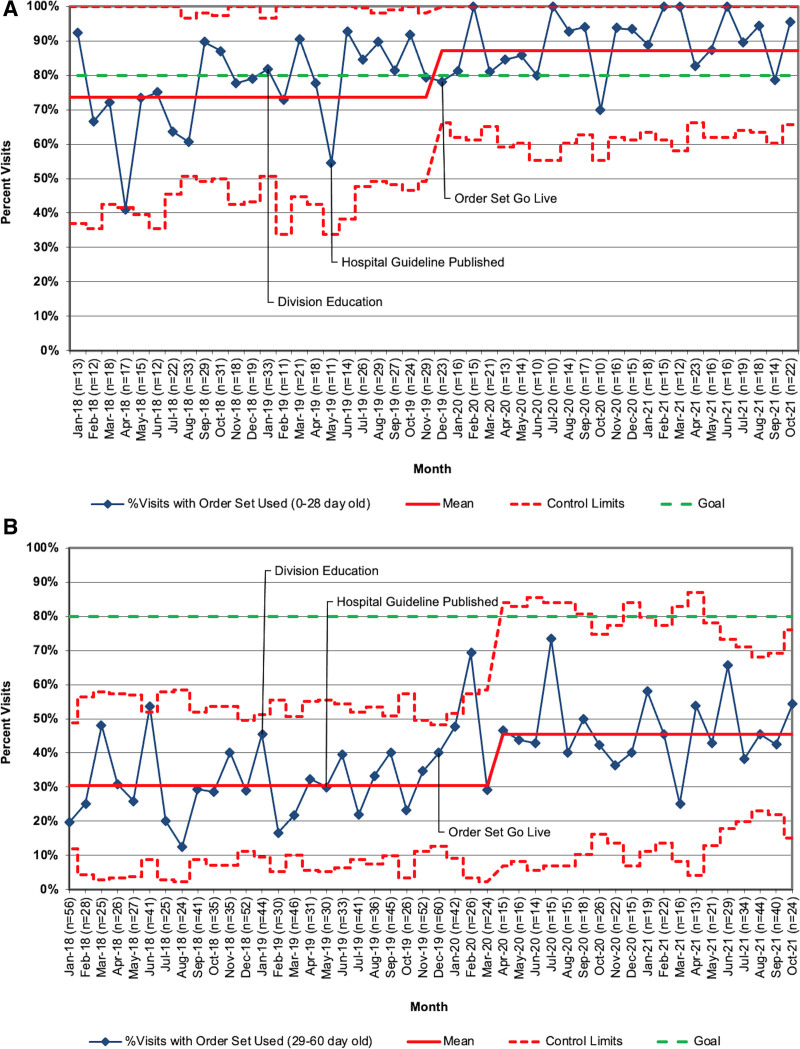
Order set use improved in both groups (Fig. 5A and B). Order set use increased from 74% to 87% for visits in infants 0–28 days with special cause variation in December 2019 (month of updated order set Go Live). Order set use increased from 30% to 46% of visits in infants 29–60 days. These changes were sustained through October 2021.
Fig. 5.
A, Proportion of encounters for febrile neonates 0–28 days of age with the order set use, January 2019–October 2021; (B) Proportion of encounters for febrile neonates 29–60 days of age with the order set use, January 2019–October 2021.
DISCUSSION
Summary and Interpretation
Historically, there has been variation in the management of febrile neonates across the United States and our institution, as physicians follow different clinical guidelines. With the recent publication of the derivation and validation of a new rule based on a large cohort of febrile neonates from the PECARN database,17 we aimed to rapidly update our hospital febrile neonate guideline, derive an ED-specific algorithm, and standardize adherence to the algorithm. Our team achieved our goal of increasing adherence to the FUS algorithm using interventions including education of patient-facing stakeholders, EMR-based interventions and care adherent discharge instructions.
Our institution’s strong culture of QI bolstered these interventions, including using order sets and algorithms for care. Order sets in our ED have traditionally been an opt-out model with preselected algorithm-adherent orders. For this project, we had the support of our admitting service, the Division of Hospital Medicine, and the pediatric residency program. As key stakeholders, they aided in the successful and rapid implementation of a change in practice. For example, we used the pediatric residency program education conferences to disseminate evidence-based practices. In turn, these resident physicians staff the ED and inpatient services. In addition, this patient population is particularly suitable for clear guidelines given the rare but high-risk nature of IBI.
We found greater variation in the management of older febrile neonates, as demonstrated by lower baseline and postintervention algorithm adherence in the 29- to 60-day age group. This variation may be partially attributable to the historical use of varying clinical prediction models in infants over 28 days. Most guidelines recommend a more conservative universal approach to IBI evaluation in infants from 0 to 28 days. However, the step-by-step model lowers that threshold to 0–21 days,22 and the recent AAP guideline suggests room for even more clinical variation by age.11 Our efforts to standardize the care for febrile neonates pre-dates the most recent AAP guidelines. Therefore, we have made a deliberate decision at our institution to be more conservative and not to incorporate these newest guidelines into our standardized practice at this time. A case-by-case chart review of the failures revealed that most failures seemed to fall into three general categories: issues around patient age, identification of fever, and missed laboratory orders. For example, in the 29- to 60-day group, one failure was an age miscalculation, and another was a well-appearing 59-day-old to whom the provider chose not to apply the algorithm. Other failures reflect uncertainty around the identification of fever, especially when obtained at home.
Additionally, providers may be less concerned about fever in older, well-appearing infants despite the guideline. Two failures in the 29- to 60-day group included well-appearing infants presenting to the urgent care with a temperature of 38 °C, one with viral respiratory symptoms, and one with a diaper rash. However, the primary reason for failure was that ordering providers deviated from algorithm-recommended care. Initially, procalcitonin as a marker of potential bacterial infection was only recently introduced at our institution; failure to order procalcitonin became less common as a point of failure over the years of this improvement. When clinicians did not order urine cultures, it was due to providers not using the order set and instead utilizing a different order option which only reflexively sent a culture based on laboratory-set criteria, which did not completely align with the algorithm.
Order set use was also quite different between the age groups, with the usage of 87% in the 0- to 28-day group and 46% in the 29- to 60-day group. We suspect that the greatest reason for this difference is that the order set facilitates ordering tests and medications for younger infants who “need everything” from the initial assessment, including weight-based antibiotics. Particularly, ensuring that all the labs required to assess for meningitis are ordered can be time-consuming and confusing, and using the order set eliminates this uncertainty. Conversely, evaluating older infants who do not automatically require lumbar puncture requires the entry of fewer orders. This behavior may be unlikely to change as providers become even more familiar with the recommended orders but is less important than outright adherence to the algorithm. However, it is important to note that algorithm-adherent care was provided more often for infants 29–60 days old when the order set was used, showing that there is still a role for increased order set use among providers.
Limitations
Our work has several limitations. First, we performed it at a single institution, which may limit generalizability. Our institutional culture and resources, particularly the residency program education and the support of our admitting service, the Division of Hospital Medicine, aided in the successful and rapid implementation of a change in practice, which may present a challenge in other settings. Additionally, we have access to timely procalcitonin results for clinical decision-making at both clinical sites, a resource not available at all institutions. Finally, although we have demonstrated at least 22 months of postimplementation data about algorithm adherence, some of that time was affected by the COVID-19 pandemic. Our PED patient volumes decreased due to the COVID-19 pandemic in March 2020. Compared to previous years, we experienced only ~60% of historical febrile neonate patient volumes in 2020. However, these numbers started to rebound in July 2021 and reached (at times exceeding) historical norms by October 2021. Therefore, sustained adherence to the guideline recommendations could be related to lower patient volumes during that time, allowing more time for careful consideration of patient evaluation and management to facilitate guideline adherence. However, we are hopeful as we have seen continued success during patient surges.
CONCLUSIONS
Implementing a QI initiative has resulted in increased adherence to an updated evidence-based care algorithm for the care of febrile neonates 0–28 and 29–60 days of age. Multiple key drivers have been used to achieve and sustain this improvement, including the availability of a PED care algorithm, providers and nursing staff education, and adding an algorithm-adherent order set into the EMR. We will continue to monitor adherence to febrile neonate guidelines and examine failures as we move this QI process into operations.
This report employed the Standards for QI Reporting Excellence 2.0 (SQUIRE 2.0) publication guidelines for reporting healthcare QI research.23
Footnotes
Published online August 1, 2022
To Cite: Yu L, Bensman RS,Hariharan SL, McAneney CM, Ovalle VW, Kurowski EM. Improving the Evidence-based Care of Febrile Neonates: A Quality Improvement Initiative. Pediatr Qual Saf 2022;7:e583.
REFERENCES
- 1.McCaig LF, Nawar EW. National hospital ambulatory medical care survey: 2004 emergency department summary. Adv Data. 2006; 373:1–29.. [PubMed] [Google Scholar]
- 2.Woll C, Neuman MI, Aronson PL. Management of the febrile young infant: update for the 21st century. Pediatr Emerg Care. 2017;33:748–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huppler AR, Eickhoff JC, Wald ER. Performance of low-risk criteria in the evaluation of young infants with fever: review of the literature. Pediatrics. 2010;125:228–233. [DOI] [PubMed] [Google Scholar]
- 4.Schwartz S, Raveh D, Toker O, et al. A week-by-week analysis of the low-risk criteria for serious bacterial infection in febrile neonates. Arch Dis Child. 2009;94:287–292. [DOI] [PubMed] [Google Scholar]
- 5.Jaskiewicz JA, McCarthy CA, Richardson AC, et al. Febrile infants at low risk for serious bacterial infection–an appraisal of the Rochester criteria and implications for management. Febrile Infant Collaborative Study Group. Pediatrics. 1994;94:390–396. [PubMed] [Google Scholar]
- 6.Baker MD, Bell LM, Avner JR. Outpatient management without antibiotics of fever in selected infants. N Engl J Med. 1993;329:1437–1441. [DOI] [PubMed] [Google Scholar]
- 7.Baskin MN, O’Rourke EJ, Fleisher GR. Outpatient treatment of febrile infants 28 to 89 days of age with intramuscular administration of ceftriaxone. J Pediatr. 1992;120:22–27. [DOI] [PubMed] [Google Scholar]
- 8.Aronson PL, Thurm C, Alpern ER, et al. ; Febrile Young Infant Research Collaborative. Variation in care of the febrile young infant <90 days in US pediatric emergency departments. Pediatrics. 2014;134:667–677. [DOI] [PubMed] [Google Scholar]
- 9.Jain S, Cheng J, Alpern ER, et al. Management of febrile neonates in US pediatric emergency departments. Pediatrics. 2014;133:187–195. [DOI] [PubMed] [Google Scholar]
- 10.Byington CL, Reynolds CC, Korgenski K, et al. Costs and infant outcomes after implementation of a care process model for febrile infants. Pediatrics. 2012;130:e16–e24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pantell RH, Roberts KB, Adams WG, et al. ; Subcommittee on Febrile Infants. Evaluation and management of well-appearing febrile infants 8 to 60 days old. Pediatrics. 2021;148:e2021052228. [DOI] [PubMed] [Google Scholar]
- 12.Biondi E, Evans R, Mischler M, et al. Epidemiology of bacteremia in febrile infants in the United States. Pediatrics. 2013;132:990–996. [DOI] [PubMed] [Google Scholar]
- 13.Greenhow TL, Hung YY, Herz AM, et al. The changing epidemiology of serious bacterial infections in young infants. Pediatr Infect Dis J. 2014;33:595–599. [DOI] [PubMed] [Google Scholar]
- 14.Gomez B, Bressan S, Mintegi S, et al. Diagnostic value of procalcitonin in well-appearing young febrile infants. Pediatrics. 2012;130:815–822. [DOI] [PubMed] [Google Scholar]
- 15.Milcent K, Faesch S, Gras-Le Guen C, et al. Use of procalcitonin assays to predict serious bacterial infection in young febrile infants. JAMA Pediatr. 2016;170:62–69. [DOI] [PubMed] [Google Scholar]
- 16.Woelker JU, Sinha M, Christopher NC, et al. Serum procalcitonin concentration in the evaluation of febrile infants 2 to 60 days of age. Pediatr Emerg Care. 2012;28:410–415. [DOI] [PubMed] [Google Scholar]
- 17.Kuppermann N, Dayan PS, Levine DA, et al. ; Febrile Infant Working Group of the Pediatric Emergency Care Applied Research Network (PECARN). A clinical prediction rule to identify febrile infants 60 days and younger at low risk for serious bacterial infections. JAMA Pediatr. 2019;173:342–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Berwick DM. A primer on leading the improvement of systems. BMJ. 1996;312:619–622.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dieckmann RA, Brownstein D, Gausche-Hill M. The pediatric assessment triangle: a novel approach for the rapid evaluation of children. Pediatr Emerg Care. 2010;26:312–315. [DOI] [PubMed] [Google Scholar]
- 20.Brower LH, Wilson PM, Murtagh Kurowski E, et al. Using quality improvement to implement a standardized approach to neonatal herpes simplex virus. Pediatrics. 2019;144:e20180262. [DOI] [PubMed] [Google Scholar]
- 21.Provost LP, Murray SK. The Health Care Data Guide: Learning from Data for Improvement. 1st ed. Jossey-Bass; 2011:xxviii, p. 445. [Google Scholar]
- 22.Gomez B, Mintegi S, Bressan S, et al. ; European Group for Validation of the Step-by-Step Approach. Validation of the “Step-by-Step” approach in the management of young febrile infants. Pediatrics. 2016;138:e20154381. [DOI] [PubMed] [Google Scholar]
- 23.Ogrinc G, Davies L, Goodman D, et al. SQUIRE 2.0 (Standards for QUality Improvement Reporting Excellence): revised publication guidelines from a detailed consensus process. BMJ Qual Saf. 2016;25:986–992. [DOI] [PMC free article] [PubMed] [Google Scholar]