Abstract
COVID-19 vaccination leads to a less intense humoral response in patients with multiple myeloma (MM) compared with healthy individuals, whereas the SARS-CoV-2-specific immunity fades over time. The purpose of this study was to explore the kinetics of SARS-CoV-2 neutralizing antibodies (NAbs) in patients with MM after vaccination with the BNT162b2 mRNA vaccine, focusing on their response before (B4D) and at 1 month after the fourth vaccination (M1P4D). Overall, 201 patients with a median age of 67 years were included, whereas 114 (56.7%) were men. The median NAbs levels B4D were 80.0% (±3.5%) and at M1P4D they increased to a median value of 96.1% (±3.7%). The NAb values at M1P4D were similar to those at 1 month post the third dose and superior to all previous timepoints. At M1P4D, the NAbs levels of all the treatment groups increased, apart from the anti-BCMA group. A significant increase in median NAbs values was observed for those receiving CD38-based treatment (n = 43, from 71.0% B4D to 96.0% at M1P4D) and those who did not receive CD38- or BCMA-targeted therapy (n = 137, from 89.6% B4D to 96.3% at M1P4D). Regarding the patients under BCMA-based therapy (n = 21), there was no remarkable increase in NAbs values following the second booster shot (from 3.0% B4D to 4.0% at M1P4D). In conclusion, booster vaccination with the BNT162b2 results in a substantially improved humoral response against SARS-CoV-2 in patients with MM. Anti-BCMA treatment remains an adverse predictive factor for NAbs response; thus, tailored prevention measures should be considered for this patient subgroup.
INTRODUCTION
The emergence and prevalence of new SARS-CoV-2 variants along with the declining immune protection following vaccination has necessitated the implementation of booster vaccine doses.1,2 However, COVID-19 vaccination leads to a less intense humoral response in individuals with immune cell dysfunction including patients with multiple myeloma (MM) compared with healthy individuals.3–6 Among these patients, the SARS-CoV-2-specific immunity is anticipated to fade quickly over time.7 Patients with hematologic malignancies are at high risk of developing breakthrough COVID-19 infections, as well.8 Interestingly, the booster-induced reduction in the viral load of breakthrough infections declines over time and becomes rather negligeable at 4 months following the third BNT162b2 shot.9 Patients with MM and COVID-19 present with a high rate of moderate and serious disease course along with high mortality that reaches almost one-fifth of the cases.10,11 In this context, 2 booster doses have been recommended to maintain an adequate antibody response in this patient population.12 Therefore, the aim of this study was to investigate the levels of SARS-CoV-2 neutralizing antibodies (NAbs) in patients with multiple myeloma (MM) up to 1 month after their fourth (second booster) BNT162b2 (Pfizer-BioNTech) mRNA vaccination.
METHODS
Patients
This prospective study enrolled consecutive patients with MM who were vaccinated against SARS-CoV-2 in a single institution (ClinicalTrials.gov number: NCT04743388). Adult patients with MM had to be eligible for COVID-19 vaccination under the national immunization program and provide written informed consent to be included. Patients with end-stage renal disease were excluded from the trial. The data of the participants were kept private in compliance with the General Data Protection Regulation. All patients’ identities were kept fully confidential, and names were deidentified using pseudoanonymization methods immediately after sample collection. Age, gender, body mass index (BMI), type of therapy, concomitant diseases, and staging scores at diagnosis were among the relevant variables gathered from the medical records. The study was approved by the Institutional Ethics Committee (Ref. No. 15/23 December 2020, General Hospital Alexandra, Athens, Greece), and it was conducted in accordance with the Declaration of Helsinki and the International Conference on Harmonization for Good Clinical Practice.
Neutralizing antibody measurement
Measurements of NAbs took place at 1 month (M1P2D), 3 months (M3P2D), 6 months (M6P2D) after the second vaccination, as well as before the first booster dose (B3D), at 1 (M1P3D) and at 3 (M3P3D) months after the third vaccination. NAbs were also evaluated before the fourth dose (B4D) and at 1 month after the fourth dose (M1P4D). The second booster shot was provided at 6 months following the first booster vaccination.
Serum was extracted and stored at –80°C until the day of measurement within 4 hours of blood collection. Stored samples from different time points of the same donor were evaluated in parallel experiments. SARS-CoV-2 neutralizing antibodies were measured using an FDA-approved technique. To detect possible SARS-CoV-2 Nabs in blood, the cPass SARS-CoV-2 Nabs Detection Kit (GenScript, Piscataway, NJ) was utilized. This approach is being used to explore antibody-mediated reduction of SARS-CoV-2 RBD binding to the human host receptor angiotensin converting enzyme type 2.
Statistical analysis
The demographic data, concomitant diseases, and prescriptions of the patients were collected through personal contact with them. BMI was calculated for each subject based on their weight and height. Statistical analysis began with the calculation of descriptive criteria such as mean, median, quartiles, and estimation of dispersion metrics like interquartile range (IQR) and standard error (expressed as ± next to the estimates).
Patients were classified into 3 categories based on their treatment: (a) those receiving treatment based on anti-CD38 monoclonal antibodies, (b) those receiving treatment based on monoclonal antibodies targeting the B-cell maturation antigen (BCMA), and (c) patients receiving any other treatment, including lenalidomide, bortezomib, ixazomib, carfilzomib, and their combinations with dexamethasone. Subjects were also classified into 2 groups based on their BMI using a cutoff value of 25; therefore, underweight and normal weight patients constituted the first group, while over-weight and obese patients were included into the second group. For age, the cutoff value of 67 years, which refers to the median of the study patients, was used to define the 2 groups.
A normality test was performed before any statistical comparison between 2 or more groups. The Shapiro-Wilk procedure was used to determine the normality of the data distribution. If the nominal normality hypothesis was rejected, it was assumed that the data did not conform to the normal distribution. In this study, demographic factors like age and BMI followed a normal distribution, so parametric methods were used to analyze them. NAbs levels on all occasions (except M1P2D and M3P2D) deviated from normality and therefore nonparametric methods have been applied. The Mann-Whitney U test was used for comparisons between 2 independent groups, such as examining gender effects on Nabs. For the simultaneous comparison of more than 2 groups, such as when comparing the 3 treatment methods, the Kruskal-Wallis methods was utilized. The Wilcoxon signed-rank test was used for pairwise group comparisons, for example, comparing neutralizing antibody levels between 2 occasions, while the Friedman’s test was used for comparisons of NAbs across multiple time points. The significance level was set at 5% in all situations, and a result was considered significant if the estimated P value was less than the significance level. The entire statistical analysis was implemented in IBM SPSS Statistics (version 26).
RESULTS
Baseline characteristics
The results of the primary and the first booster dose have been previously published.5,7,13 The present analysis included N = 201 patients with MM who received the fourth dose of the BNT162b2 vaccine at a median of 6 months (range 5–7) after the third vaccine shot. Table 1 summarizes the demographic data gathered from study participants. Overall, 43 patients were receiving treatment based on anti-CD38 monoclonal antibodies, 21 patients were receiving treatment based on anti-BCMA antibodies, whereas 137 patients were receiving combinations based on proteasome inhibitors (PIs) and immunomodulatory drugs (IMiDs).
Table 1.
Demographic Characteristics of the Study Participants
| Characteristic | Patients | |||
|---|---|---|---|---|
| Entire patient group | CD38-based | BCMA-based | Other | |
| Sample size | 201 | 43 | 21 | 137 |
| Men (n, %) | 114 (56.7%) | 25 (58.1%) | 13 (61.9%) | 76 (55.5%) |
| Women (n, %) | 87 (43.3%) | 18 (41.9%) | 8 (38.1%) | 61 (44.5%) |
| Age (median, IQR) | 67 (15) | 66.5 (17.5) | 67.1 (16) | 67 (14) |
| BMI (median, IQR) | 25.8 (5.1) | 25.2 (3.8) | 25.3 (4.2) | 26 (5.7) |
Based on the administered medication, 3 groups of patients with multiple myeloma were included in the study: (a) those receiving treatment based on anti-CD38 agents (N = 43), (b) those receiving treatment based on monoclonal antibodies targeting the B-cell maturation antigen (BCMA) (N = 21), and (c) patients (N = 137) receiving any other treatment, including lenalidomide, bortezomib, ixazomib, carfilzomib, and their combinations with dexamethasone.
n= number of patients; IQR = interquartile range.
The demographics of the 3 treatment subgroups appear to be quite similar, in line with the baseline characteristics of the entire cohort (Table 1). Overall, 114 (56.7%) patients were males, whereas the median age was 67 years and the median BMI was 25.8 kg/m2. Statistical comparisons among the 3 subgroups (CD38-based, BCMA-based, and “Other” treatment) revealed no significant differences in any of the abovementioned characteristic; the ANOVA P values for age and BMI were 0.672 and 0.588, respectively, indicating that patients in the 3 groups share similar characteristics that can be investigated further.
Furthermore, between the third and fourth doses, a subset of patients (N = 34, or 16.9%) were found to be COVID-19 positive. The demographics and NAbs levels of COVID-19 positive and negative patients were compared on all occasions. There was no significant difference in patient demographics (e.g., age, BMI, gender participation) or antibody levels. The non-COVID group had a median age of 67 years, while the COVID group had a median age of 65.5 years (Table 2). In terms of gender distribution, 56.9% and 55.9% were men as well as 43.1% and 44.1% were women in the non-COVID and COVID groups, respectively. Similarly, the distribution of ISS and RISS stage was quite similar in the COVID and non-COVID groups, respectively (Table 2). Finally, no significant differences regarding the distribution of treatment type between the 2 groups emerged. Overall, the COVID-19 positive patients had similar characteristics (as expected due to the randomness of COVID-19 infection), and there was no difference in response after the fourth vaccination.
Table 2.
Patient Characteristics Based on History of Prior COVID-19 Infection Between the Third and Fourth Vaccine Dose
| Characteristic | COVID-19 positive between third and fourth dose | |
|---|---|---|
| No | Yes | |
| Sample size (n, %) | 167 (83.1%) | 34 (16.9%) |
| Men (n, %) | 95 (56.9%) | 19 (55.9%) |
| Women (n, %) | 72 (43.1%) | 15 (44.1%) |
| Age (median, IQR, y) | 67 (14.5) | 65.5 (21.5) |
| BMI (median, IQR, kg/m2) | 26 (5) | 25.5 (5.5) |
| ISS at diagnosis (n, %) | ||
| Stage 1 or 2 | 126 (75.4%) | 24 (70.6%) |
| Stage 3 | 41 (24.6%) | 10 (29.4%) |
| RISS at diagnosis (n, %) | ||
| Stage 1 or 2 | 144 (86.2%) | 31 (91.2%) |
| Stage 3 | 23 (13.8%) | 3 (8.8%) |
| Treatment: | ||
| CD38 (n, %) | 38 (22.8%) | 5 (14.7%) |
| BCMA (n, %) | 19 (11.4%) | 2 (5.88%) |
| Other (n, %) | 110 (65.9%) | 27 (79.4%) |
n = number of patients; IQR = interquartile range; ISS = International Staging System; RISS = Revised ISS.
Neutralizing antibody levels
The effect of a second booster shot on NAb levels
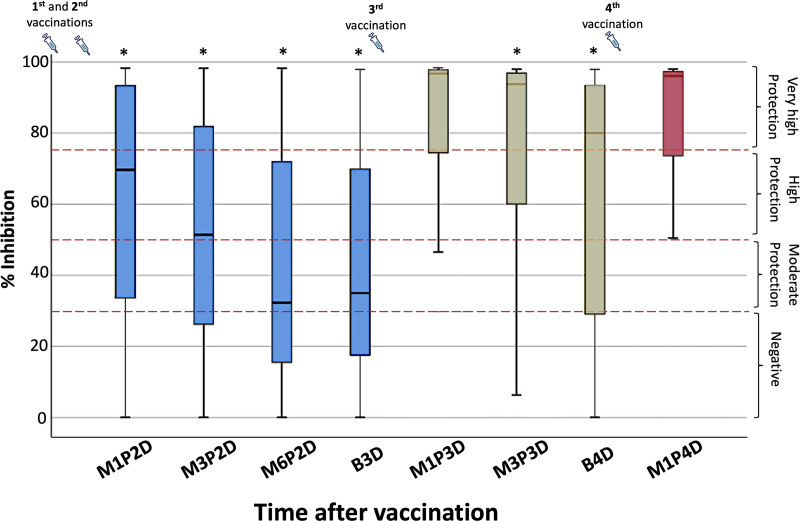
Figure 1 shows the percent inhibition of NAbs starting from 1 month after the second vaccination (M1P2D) and ending up to 1 month after the fourth dose (M1P4D). Between 2 consecutive vaccinations, the anticipated motif of decreasing neutralizing activity is observed. Since the kinetics of NAbs against SARS-CoV-2 following the first booster dose in patients with MM have already been discussed,7 this study focused on the investigation of the immune response after the fourth (second booster) dose. Overall, the median inhibition levels at B4D were 80.0% (±3.5%) and at M1P4D they increased to a median value of 96.1% (±3.7%). Before the fourth dose, the proportions of patients having NAbs titers higher than 30%, 50%, and 75% were 74.2%, 68.0%, and 55.7%, respectively. After the fourth dose, the corresponding proportions increased to 81.2%, 81.2%, and 74.1%, respectively. Indeed, the NAb levels increased in 89.4% of the whole patient cohort after the fourth vaccination. Statistical comparison of the neutralizing inhibition titers at M1P4D against inhibition activities at all previous timepoints revealed statistically significant differences (the Wilcoxon P values were <0.05). The only exception was the case of M1P3D (i.e., 1 month after the third vaccination), where the NAbs were found to be very similar to M1P4D (96.7% versus 96.1%, respectively, P = 0.082).
Figure 1.
Inhibition (%) of SARS-CoV-2 binding to the human host receptor angiotensin converting enzyme-2 after vaccination with the BNT162b2 mRNA vaccine in patients with MM. Antibodies were measured at 1 month (M1P2D), 3 months (M3P2D), and 6 months (M6P2D) after the second vaccination, before the third booster dose (B3D), as well as at 1 (M1P3D) and 3 (M3P3D) months after the third vaccination, and before the fourth dose (B4D). The last measurement point was 1 month after the fourth dose (M1P4D). The asterisks (*) indicate statistically significant differences (P < 0.05) between the compared group and the inhibition levels at M1P4D. The boundaries of the box plot refer to the quartiles of the distribution, while the dashed lines of the graph indicate the limits of inhibition, that is, 30%, 50%, and 75%. The syringe symbol represents vaccination.
Furthermore, before the fourth dose, the median NAbs levels for the COVID-19 positive patients were 83.42% (±7.5%), while for the COVID-19 negative patients were 78.8% (±3.7%). Similarly, 1 month after the fourth dose, the median NAb response increased to 97.0% (±6.6%) and 96.1% (±3.9%) for the COVID-19 positive and negative groups, respectively. The Mann-Whitney test revealed that both differences were not statistically significant, with P values of 0.832 and 0.752 for B4D and M1P4D, respectively. As a result, because both groups (COVID-19 positive and negative) consisted of patients with similar characteristics who also showed similar responses to vaccine before and after the fourth vaccination, these 2 groups were combined in the analyses.
The effect of treatment on NAbs levels
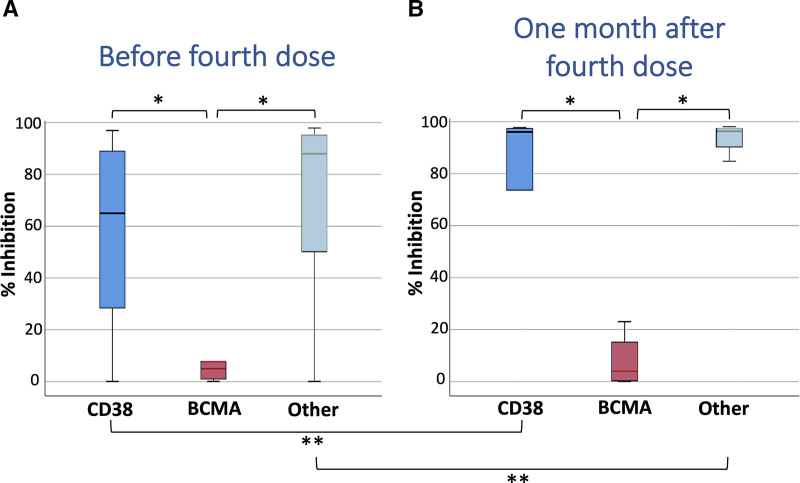
The immune response to the fourth vaccination, for each treatment group, is depicted in Figure 2 where the NAbs levels before and after the fourth vaccination are presented. Before the fourth dose (Figure 2A), the median NAbs for the CD38, BCMA, and the other treatment groups were 71.0%, 3.0%, and 89.6%, respectively. The proportion of patients under CD38-based treatment with NAbs higher than 30%, 50%, and 75% was 72.0%, 64.0%, and 48.0%, respectively. Slightly higher proportions were found for the “other treatment” group, where the corresponding 3 proportions were 80.6%, 74.6%, and 62.7%, respectively. In the case of patients under anti-BCMA treatment, all of them exhibited very low NAbs before the fourth dose, and none of the patients exceeded the 30% inhibition cutoff. Statistical comparison among the 3 treatment groups (CD38-based vs. BCMA-based vs. Other treatment) showed no significant difference (P = 0.263) between the CD38-based and the “other treatment” group, while both of them showed superior inhibition activity than the BCMA group (both P values <0.001).
Figure 2.
Inhibition (%) of SARS-CoV-2 binding after vaccination with the BNT162b2 mRNA vaccine in patients with MM, before and 1 month after the fourth dose. Patients were classified into 3 categories based on their treatment: (A) those anti-CD38 based treatment (N = 43), (B) those receiving anti-BCMA-based treatment (N = 21), and (C) those receiving any other treatment (N = 137). The single asterisk (*) indicates statistically significant differences (P < 0.05) between the compared groups. The double asterisk (**) indicates a significant difference for the same group between the 2 occasions (before and after the fourth vaccination). The boundaries of the box plot refer to the quartiles of the distribution, while the dashed lines of the graph indicate the limits of inhibition, that is, 30%, 50%, and 75%.
One month after the fourth dose (M1P4D) (Figure 2B), the NAbs levels of all the treatment groups increased substantially, apart from the anti-BCMA group. For the CD38-based group, a significant (Wilcoxon P value = 0.001) increase in NAbs was observed from 71.0% to 96.0%. Regarding the “other treatment” group, the increase in NAbs was found to be significant from 89.6% to 96.3% (P< 0.001). For the BCMA-based group, the increase (from 3.0% to 4.0%) was rather small and, as expected, it was not significant (P = 0.263). The percentages of patients receiving CD38-based therapy and exerting neutralizing activity greater than 30%, 50%, and 75% were 77.8%, 77.8%, and 72.2%, respectively. The highest proportions were observed for patients under treatment with medications other than CD38- or BCMA-based; namely, the corresponding percentages were 87.3%, 87.3%, and 79.4%, respectively. Interestingly, none of the patients receiving anti-BCMA-based treatment showed a NAb value higher than 30%. As expected, statistical comparison with the Mann-Whitney test revealed significant differences between the CD38-based and the BCMA-based groups (P = 0.001), as well as between the “other treatment” and the BCMA-based groups (P < 0.001). No statistically significant difference was found between the CD38-based group and the group of patients under treatment with “other” drug combinations (P = 0.758).
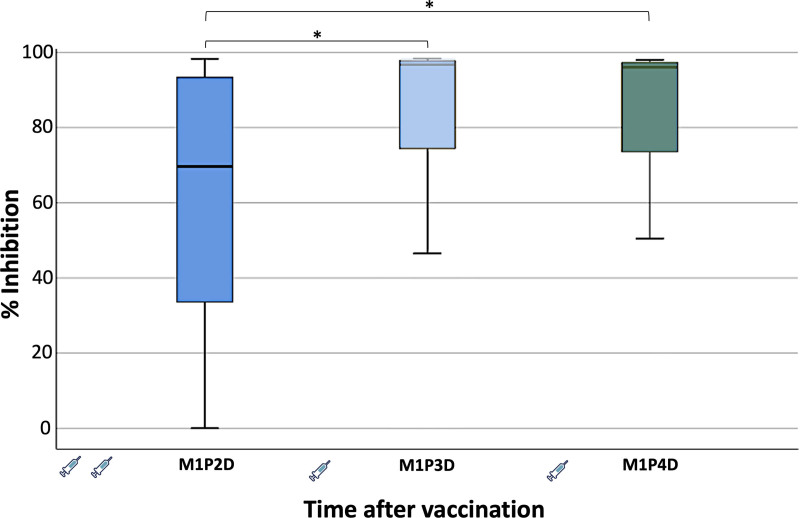
Comparative effect of each vaccine dose on NAb levels
Another interesting issue to investigate, was the comparison of the humoral immune responses at 1 month after each distinct vaccination (second, third, and fourth dose, respectively). Figure 3 shows the neutralizing antibody inhibition activity at M1P2D, M1P3D, and M1P4D. The median inhibition at M1P2D was 69.7% (±2.4%), which was the lowest among the 3 occasions. In particular, 1 month after the third and fourth vaccinations, the median inhibitions were quite similar and more specifically reached 96.7% (±2.6%) and 96.1% (±3.7%), respectively. Statistical comparison using the Friedman’s test revealed that NAbs levels at M1P2D differed significantly in respect to those at M1P3D (P < 0.001) and at M1P4D (P < 0.001).
Figure 3.
Inhibition (%) of SARS-CoV-2 binding after vaccination with the BNT162b2 mRNA vaccine in patients with MM at 1 month after the second (M1P2D), third (M1P3D), and fourth (M1P4D) vaccination, respectively. The asterisk (*) indicates statistically significant differences (P < 0.05) between the different timepoints. The boundaries of the box plot refer to the quartiles of the distribution, while the dashed lines of the graph indicate the limits of inhibition, that is, 30%, 50%, and 75%. The syringe symbol represents vaccination.
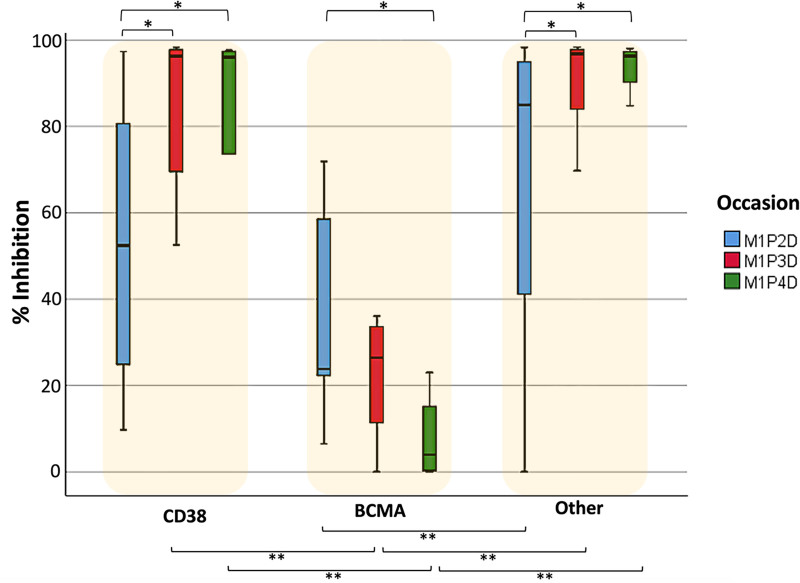
Humoral immune response data were also analyzed according to treatment group to further investigate the response at 1 month after vaccination (Figure 4). In the case of CD38-based and “other” treatment groups, a similar pattern is observed where NAbs levels at M1P3D and M1P4D are significantly higher than those at M1P2D; the P values for the 2 comparisons were <0.001 and equal to 0.006, respectively. For the BCMA-based group, a different pattern is observed, where NAbs have the lowest median value at M1P4D and the highest median value at M1P2D (P = 0.028). At M1P2D, only the BCMA-based group had significantly lower median NAb levels (P = 0.002) compared to the “other treatment” group. At M1P3D, the BCMA-based group had an inferior median NAb value compared with both the CD38-based (P = 0.005) and the “other” (P < 0.001) treatment groups. Similar results emerged at M1P4D; the P values were 0.015 and 0.002 for the comparisons between the BCMA-based with the CD38-based and the “other treatment” groups, respectively.
Figure 4.
Inhibition (%) of SARS-CoV-2 binding after vaccination with the BNT162b2 mRNA vaccine in patients with MM at 1 month after the second (M1P2D), third (M1P3D), and fourth (M1P4D) vaccination, respectively. Patients were classified into 3 categories based on their treatment: (A) those anti-CD38 based treatment (N = 43), (B) those receiving anti-BCMA-based treatment (N = 21), and (C) those receiving any other treatment (N = 137). The single asterisk (*) indicates statistically significant differences (P < 0.05) between the different occasions. The double asterisk (**) indicates a significant difference between 2 different treatment groups on the same occasion. The boundaries of the box plot refer to the quartiles of the distribution, while the dashed lines of the graph indicate the limits of inhibition, that is, 30%, 50% and 75%.
The effect of age, BMI, and disease stage on NAbs levels
A supplemental analysis was also performed with respect to gender to determine possible gender differences in the development of humoral immune responses after the fourth dose. However, in none of the subgroup analyses based on treatment type or timepoint examined gender was found to play a significant role in the development of NAbs levels. In all these occasions, the Mann-Whitney test resulted in significance levels higher than 5%. Furthermore, age and BMI were investigated for their potential effect on NAbs levels before and at 1 month after the fourth vaccine dose. The cutoff values for age and BMI were set at 67 years and 25.8 kg/m2, respectively, which correspond to the median estimate of these 2 variables. However, neither age (P = 0.206), nor BMI (P = 0.318) were found to exert a significant effect on NAbs values. The ISS and RISS stage of the disease at study entry was evaluated, as well. However, no significant effect on NAb levels was found (P = 0.641 and P = 0.882 for ISS and RISS, respectively).
DISCUSSION
Evaluating the kinetics of humoral response over time after each vaccination is important to determine a tailored vaccination and prevention strategy against COVID-19 for patients with MM.14 Our results demonstrated that the second BNT162b2 booster vaccine increased significantly the median NAb value from 80% before to 96% at 1 month post the fourth dose among patients with MM. In line with our findings, Munro et al showed a significant increase in the geometric mean antispike protein IgG concentration following a second booster vaccine against SARS-CoV-2 with either BNT162b2 or mRNA-1273. All 166 individuals who participated in this substudy of the COV-BOOST clinical trial had received BNT162b2 as a first booster shot.15 Both humoral and T-cell immune responses against SARS-CoV-2 are improved following a fourth BNT162b2 vaccine in individuals 60 years of age or older.15,16 This is reflected on the anticipated protection against severe COVID-19 following the second booster shot according to available data stemming from Israel.17 Although the protection against COVID-19 wanes relatively rapidly after the fourth dose, the protection against severe illness remains over time.18–20
Furthermore, we showed that NAbs titers in patients with MM decline even at 3 months after the third vaccine shot. The kinetics of NAb decay after the second and the third BNT162b2 seem to be quite similar, although the humoral response after the first booster dose is superior than after the second vaccine shot.7,21,22 Interestingly, we found that the median NAbs levels at 1 month after the second booster BNT162b2 are similar to those detected at 1 month following the first booster BNT162b2 in patients with MM. Another recent study has also demonstrated that the second booster shot with a mRNA-based vaccine restores the NAb levels to the high values that had been previously achieved after the first booster shot with BNT162b2 in individuals without MM.2
In addition to the above, we confirmed the adverse predictive role of anti-BCMA therapy on NAb response following each vaccine shot. Treatment with anti-BCMA and anti-CD38 agents has been previously associated with inferior humoral responses following both the initial 2-dose vaccination scheme and the third BNT162b2 booster dose.7,13,21–24 Herein, we showed that patients under treatment with anti-BCMA-based regimens did not have any NAb activity before the fourth dose and this did not change after the second booster vaccination. Although patients under treatment with anti-CD38-based combinations had a lower median NAb value before the fourth dose compared with the patients who did not receive anti-BCMA- or anti-CD38-based treatment, the median NAb values after the second booster dose were similar between the 2 patient groups. In contrast to the anti-CD38 and “other treatment” groups, which showed the highest median NAb response at 1 month after each booster dose, the anti-BCMA treatment group showed the highest median NAb response at 1 month after the second vaccine shot. This finding may indicate a cumulative effect of BCMA-targeting therapies in the sustained depletion of B-cells, which in turn results in defective antibody production.25,26
Regarding the role of COVID-19 infection prior to the second booster dose, it should be noted that the proportion of infected patients (16.9%) in the interval between the third and fourth BNT162b2 dose is much less than the uninfected subjects. However, this imbalance did not affect the analysis since patients in both groups had quite similar characteristics in terms of age, BMI, etc. Although prior COVID-19 leads to a high NAb response in patients with MM,27 there was no difference in the humoral responses after the fourth vaccination between those with and without a history of COVID-19 before the second booster shot. It has to be also noted that almost one-fifth of the patients experienced a breakthrough infection after the third BNT162b2 in the era of the Omicron variant of concern. Our finding coincide with the previously reported increased incidence of breakthrough infections following COVID-19 vaccination in patients with MM and other hematologic malignancies.8,28,29 The suboptimal and waning immune response of patients with hematologic cancer to vaccination against SARS-CoV-2 compared with healthy individuals renders them a particularly vulnerable population to breakthrough infections.9,30,31
One of our study’s key features is the examination of NAbs, which have been demonstrated to have a significant predictive value for immunological protection against symptomatic COVID-19.32,33 As a result, NAb levels can be used as surrogate markers for vaccination effectiveness. A limitation of this study is the limited sample size, especially regarding patients receiving treatment based on anti-BCMA regimens. Therefore, the subgroup analyses performed in this study should be considered rather exploratory. Furthermore, we did not assess the kinetics of cellular immune response against SARS-CoV-2 following each dose of the primary and the booster vaccinations. During the short follow-up period after the second booster dose, no new COVID-19 instances were detected. A longer follow-up period will demonstrate any effect of the booster immunization on COVID-19 hospitalization and mortality rate among patients with MM.
In conclusion, fourth vaccination with the BNT162b2 results in substantially improved humoral response against SARS-CoV-2 in patients with MM. Anti-BCMA treatment remains an adverse predictive factor for NAbs response following the second booster vaccine. All patients with MM, but especially those on anti-BCMA therapy, should be prioritized for receiving pre-exposure prophylaxis with monoclonal antibodies and booster shots with variant-specific vaccines. Last but not least, they should be encouraged to wear a mask and avoid crowded places as long as the pandemic is ongoing.
ACKNOWLEDGMENTS
We thank Ioanna Charitaki, RN; Tina Bagratuni, PhD; Christine Ivy Liacos, PhD; Nikoletta-Aikaterini Kokkali, RN; Nefeli Mavrianou-Koutsoukou, PhD; Dimitrios Patseas, PhD; and Mrs Stamatia Skourti for administrative, technical, or material support; Sentiljana Gumeni, PhD and Eleni-Dimitra Papanagnou, PhD for acquisition, analysis, or interpretation of data. We also thank all study participants for donating their time and samples.
AUTHOR CONTRIBUTIONS
ET and INS designed research; INS, MG, PM, ADS, MM, MR, DF, HA, EEP, FT, VAI, EK, and ET performed research; VK, IPT, and MAD contributed vital new reagents or analytical tools; VK analyzed data; INS, VK, and ET wrote the first draft of the paper. All authors have revised the final version of the article and consented to submission.
DISCLOSURES
ET is an editor for HemaSphere. The authors have no other conflicts of interest to disclose.
SOURCES OF FUNDING
We thank SYN-ENOSIS (Greece) and IEMBITHEK (Greece) for partially funding this study.
REFERENCES
- 1.Chemaitelly H, Tang P, Hasan MR, et al. Waning of BNT162b2 vaccine protection against SARS-CoV-2 infection in Qatar. N Engl J Med. 2021;385:e83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hein S, Mhedhbi I, Zahn T, et al. Quantitative and qualitative difference in antibody response against Omicron and ancestral SARS-CoV-2 after third and fourth vaccination. Vaccines (Basel). 2022;10:796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wagner A, Garner-Spitzer E, Schötta AM, et al. SARS-CoV-2-mRNA booster vaccination reverses non-responsiveness and early antibody waning in immunocompromised patients - a phase four study comparing immune responses in patients with solid cancers, multiple myeloma and inflammatory bowel disease. Front Immunol. 2022;13:889138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ito Y, Honda A, Kurokawa M. COVID-19 mRNA vaccine in patients with lymphoid malignancy or anti-CD20 antibody therapy: a systematic review and meta-analysis. Clin Lymphoma Myeloma Leuk. 2022;22:e691-e707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Terpos E, Trougakos IP, Gavriatopoulou M, et al. Low neutralizing antibody responses against SARS-CoV-2 in older patients with myeloma after the first BNT162b2 vaccine dose. Blood. 2021;137:3674–3676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ludwig H, San-Miguel J, Munshi N, et al. Covid-19 vaccination in patients with multiple myeloma: focus on immune response. Am J Hematol. 2021;96:896–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Terpos E, Gavriatopoulou M, Ntanasis-Stathopoulos I, et al. Booster BNT162b2 optimizes SARS-CoV-2 humoral response in patients with myeloma: the negative effect of anti-BCMA therapy. Blood. 2022;139:1409–1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Song Q, Bates B, Shao YR, et al. Risk and outcome of breakthrough COVID-19 infections in vaccinated patients with cancer: real-world evidence from the national COVID cohort collaborative. J Clin Oncol. 2022;40:1414–1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Levine-Tiefenbrun M, Yelin I, Alapi H, et al. Waning of SARS-CoV-2 booster viral-load reduction effectiveness. Nat Commun. 2022;13:1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vijenthira A, Gong IY, Fox TA, et al. Outcomes of patients with hematologic malignancies and COVID-19: a systematic review and meta-analysis of 3377 patients. Blood. 2020;136:2881–2892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Krejci M, Pour L, Adam Z, et al. Outcome of COVID-19 infection in 50 multiple myeloma patients treated with novel drugs: single-center experience. Ann Hematol. 2021;100:2541–2546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ludwig H, Sonneveld P, Facon T, et al. COVID-19 vaccination in patients with multiple myeloma: a consensus of the European Myeloma Network. Lancet Haematol. 2021;8:e934–e946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Terpos E, Gavriatopoulou M, Ntanasis-Stathopoulos I, et al. The neutralizing antibody response post COVID-19 vaccination in patients with myeloma is highly dependent on the type of anti-myeloma treatment. Blood Cancer J. 2021;11:138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Terpos E, Rajkumar SV, Leung N. Neutralizing antibody testing in patients with multiple myeloma following COVID-19 vaccination. JAMA Oncol. 2022;8:201–202. [DOI] [PubMed] [Google Scholar]
- 15.Munro APS, Feng S, Janani L, et al. Safety, immunogenicity, and reactogenicity of BNT162b2 and mRNA-1273 COVID-19 vaccines given as fourth-dose boosters following two doses of ChAdOx1 nCoV-19 or BNT162b2 and a third dose of BNT162b2 (COV-BOOST): a multicentre, blinded, phase 2, randomised trial. Lancet Infect Dis. 2022;22:1131–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bar-Haim E, Eliakim-Raz N, Stemmer A, et al. Humoral and T-cell response before and after a fourth BNT162b2 vaccine dose in adults ≥60 years. J Clin Med. 2022;11:2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arbel R, Sergienko R, Friger M, et al. Effectiveness of a second BNT162b2 booster vaccine against hospitalization and death from COVID-19 in adults aged over 60 years. Nat Med. 2022. Apr 25. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 18.Bar-On YM, Goldberg Y, Mandel M, et al. Protection by a fourth dose of BNT162b2 against Omicron in Israel. N Engl J Med. 2022;386:1712–1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Magen O, Waxman JG, Makov-Assif M, et al. Fourth dose of BNT162b2 mRNA Covid-19 vaccine in a nationwide setting. N Engl J Med. 2022;386:1603–1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gazit S, Saciuk Y, Perez G, et al. Short term, relative effectiveness of four doses versus three doses of BNT162b2 vaccine in people aged 60 years and older in Israel: retrospective, test negative, case-control study. BMJ. 2022;377:e071113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Enssle JC, Campe J, Büchel S, et al. Enhanced but variant-dependent serological and cellular immune responses to third-dose BNT162b2 vaccination in patients with multiple myeloma. Cancer Cell. 2022;40:587–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aleman A, Van Oekelen O, Upadhyaya B, et al. Augmentation of humoral and cellular immune responses after third-dose SARS-CoV-2 vaccination and viral neutralization in myeloma patients. Cancer Cell. 2022;40:441–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aleman A, Upadhyaya B, Tuballes K, et al. Variable cellular responses to SARS-CoV-2 in fully vaccinated patients with multiple myeloma. Cancer Cell. 2021;39:1442–1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Van Oekelen O, Gleason CR, Agte S, et al. Highly variable SARS-CoV-2 spike antibody responses to two doses of COVID-19 RNA vaccination in patients with multiple myeloma. Cancer Cell. 2021;39:1028–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shah N, Chari A, Scott E, et al. B-cell maturation antigen (BCMA) in multiple myeloma: rationale for targeting and current therapeutic approaches. Leukemia. 2020;34:985–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kleber M, Ntanasis-Stathopoulos I, Terpos E. BCMA in multiple myeloma-a promising key to therapy. J Clin Med. 2021;10:4088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gavriatopoulou M, Terpos E, Malandrakis P, et al. Myeloma patients with COVID-19 have superior antibody responses compared to patients fully vaccinated with the BNT162b2 vaccine. Br J Haematol. 2022;196:356–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang L, Berger NA, Xu R. Risks of SARS-CoV-2 breakthrough infection and hospitalization in fully vaccinated patients with multiple myeloma. JAMA Netw Open. 2021;4:e2137575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang L, Kaelber DC, Xu R, et al. COVID-19 breakthrough infections, hospitalizations and mortality in fully vaccinated patients with hematologic malignancies: a clarion call for maintaining mitigation and ramping-up research. Blood Rev. 2022;54:100931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fendler A, Shepherd STC, Au L, et al. Immune responses following third COVID-19 vaccination are reduced in patients with hematological malignancies compared to patients with solid cancer. Cancer Cell. 2022;40:114–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Oster Y, Benenson S, Nir-Paz R, et al. The effect of a third BNT162b2 vaccine on breakthrough infections in health care workers: a cohort analysis. Clin Microbiol Infect. 2022;28:735.e1–735.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cromer D, Steain M, Reynaldi A, et al. Neutralising antibody titres as predictors of protection against SARS-CoV-2 variants and the impact of boosting: a meta-analysis. Lancet Microbe. 2022;3:e52–e61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Khoury DS, Cromer D, Reynaldi A, et al. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat Med. 2021;27:1205–1211. [DOI] [PubMed] [Google Scholar]