Abstract
Objectives
SARS-CoV-2 vaccination has been shown to reduce infection severity; however, the reinfection frequency among unvaccinated, partially vaccinated, and fully vaccinated individuals remains unclear. This study aims to elucidate the rates of and factors associated with such occurrences.
Methods
This retrospective epidemiological report included 1362 COVID-19 reinfection cases in Bahrain between April 2020 and July 2021. We analyzed differences in disease severity and reinfection characteristics among various vaccination statuses: fully vaccinated, interrupted vaccination, one-dose vaccination, postreinfection vaccination, and unvaccinated.
Results
Reinfection cases increased from zero per month in April-June 2020 to a sharp peak of 579 in May 2021. A significantly larger proportion of reinfected individuals were male (60.3%, P <0.0001). Reinfection episodes were highest among those 30-39 years of age (29.7%). The fewest reinfection episodes occurred at 3-6 months after the first infection (20.6%) and most occurred ≥9 months after the initial infection (46.4%). Most individuals were asymptomatic during both episodes (35.7%). Reinfection disease severity was mild, with vaccinated patients less likely to have symptomatic reinfection (odds ratio 0.71, P = 0.004). Only 6.6% of reinfected patients required hospitalization. One death was recorded; the patient belonged to the unvaccinated group.
Conclusion
Vaccine-induced immunity and previous infection with or without vaccination were effective in reducing reinfection disease severity.
Keywords: SARS-CoV-2, Covid-19, Reinfection, Public health, Vaccination
Introduction
COVID-19 began as an outbreak in Wuhan, China, in December 2019 and was declared a pandemic by the World Health Organization (WHO) in March 2020 (WHO, 2020). The disease, caused by SARS-CoV-2, has affected >271,900,000 people worldwide and led to >5,000,000 deaths as of December 19, 2021 (WHO, 2021c). There are currently five SARS-CoV-2 variants that are classified as variants of concern: Alpha (B.1.1.7), Beta (B.1.351), Gamma (P.1), Delta (B.1.617.2), and more recently, Omicron (B.1.1.529) (WHO, 2021b). COVID-19 disease manifests with significant variation; however, the classic symptoms include fever, cough, shortness of breath, fatigue, headache, sore throat, and changes to taste or smell that may appear 2-14 days after viral exposure (CDC, 2021b, CDC, 2021).
Global pandemic control and national policies have been constructed based on herd immunity theory and the assumption that viral exposure would be sufficient to provide long-standing immunity (Aschwanden, 2020). With the availability of vaccines, demonstrating the efficacy of viral exposure against infection became possible. Research showed that vaccine-induced immunity against the Alpha (Chemaitelly et al., 2021; Ikegame et al., 2021), Beta (Lefèvre et al., 2021), Gamma (Vignier et al., 2021), and Delta (Lopez Bernal et al., 2021) variants was achievable. However, there have recently been an increasing number of reports of SARS-CoV-2 reinfections. The first case of reinfection was reported in the United States by Tillett et al., where a 25-year-old man had positive test results on two occasions 48 days apart, separated by two PCR tests with negative results (Tillett et al., 2021). After genetic analysis, the authors concluded that the genetic variation between the two SARS-CoV-2 specimens was too significant to be explained by short-term in vivo evolution. Currently, there are over 900 studies published regarding reinfection in COVID-19 (Dhillon et al., 2021), highlighting the genuine possibility of reinfection that needs to be taken into account by researchers and policymakers.
With the emergence of the Omicron variant, another global infection wave with the risk of national restrictions and lockdowns is likely. If reinfection is indeed a feature of COVID-19, it poses a significant obstacle in tackling the pandemic because it jeopardizes the assumption of herd immunity that many control measures have adopted for containing the spread of SARS-CoV-2. In this study, we investigated the characteristics of patients with recorded reinfection in the Kingdom of Bahrain to understand reinfection and epidemiologically compared vaccine-induced and natural infection–induced immunities.
Methods
Study design
This was a retrospective epidemiological study that analyzed SARS-CoV-2 reinfection cases in Bahrain between April 1, 2020, and July 23, 2021, obtained from the Bahrain National COVID-19 database of individuals who had positive test results for SARS-CoV-2 on two or more episodes at least 3 months apart. Information collected in this study included reinfection status, vaccination status, age, symptoms, time to reinfection, and hospitalization. Symptomatic and asymptomatic patients of any age, identified in several screening and contact-tracing programs as well as in travel testing and random screening were included. A total of 1390 cases of reinfection were identified for the study period, of which 28 were excluded because of incomplete information. A total of 1362 reinfection cases were further analyzed.
We first examined the general characteristics of individuals with reinfection, including gender, age group, symptom, hospitalization status, and SARS-CoV-2 variant of interest available from the national genome database. Disease severity and hospitalization status were characterized based on the Bahrain COVID-19 National Protocol (National Taskforce for Combating the Coronavirus, 2021) as follows: (a) isolation (home isolation without hospitalization), (b) moderate disease status (temperature of ≥38°C with shortness of breath, chest pain, change in mental status, or respiratory rate >30), or (c) severe disease status (requiring ≥15 l oxygen in addition to moderate-status criteria). Symptom status was defined per the US CDC (CDC, 2021a). Symptomatic status describes an infection of SARS-CoV-2 where symptoms have developed, including fever, cough, shortness of breath, fatigue, headache, a new loss of taste or smell, sore throat, and other symptoms. Asymptomatic status describes a SARS-CoV-2 infection where no symptoms have developed throughout the episode for the duration of 14 days of isolation.
Next, we analyzed the differences in these characteristics in reinfection among differing vaccination statuses. We defined vaccinated individuals as those who received two doses of a COVID-19 vaccine ≥14 days before the reinfection episode. We defined reinfected individuals as those with positive RT-PCR test results (cycle threshold [Ct] <35) ≥90 days after the first episode of infection, regardless of symptoms and supported by close-contact exposure or outbreak settings. We categorized reinfection by vaccination status (Table 1 ).
Table 1.
Description of the categories used to classify the data in the study.
| Fully vaccinated | Individuals who had positive test results for reinfection episode ≥14 days after receiving the second dose of vaccine |
| Interrupted vaccination | Individuals who had positive test results for reinfection episode <14 days after receiving the second dose of vaccine |
| One-dose vaccination | Individuals who had positive test results for reinfection episode after receiving only one dose of vaccine |
| Postreinfection vaccination | Individuals who began vaccination after their reinfection episode |
| Unvaccinated | Individuals who did not receive any vaccine dose during the study period |
Individuals were sorted into five different categories according to vaccination status; reinfection was then compared among categories.
In addition to general demographics, we analyzed time to reinfection and Ct values by vaccination status.
Data collection
Beginning in February 2020, the National COVID-19 Task Force of Bahrain began testing all travelers upon arrival into the country, suspected cases, symptomatic individuals, asymptomatic contacts (including family members) of those who had positive test results for SARS-CoV-2, and all hospitalized and critically ill patients suspected of being infected with SARS-CoV-2, and through large-scale random testing of individuals. The following categories of persons underwent PCR testing per the testing strategy: (a) incoming travelers (all travelers, regardless of vaccination status, were required to have a PCR test upon arrival and a second PCR test 10 days later); (b) symptomatic patients (patients exhibiting symptoms suggestive of COVID-19 underwent PCR testing at a medical facility or a designated drive-through testing site after reporting their symptoms through the “BeAware” mobile application or by calling the toll-free COVID-19 hotline); (c) admitted and preoperative patients (all patients admitted to the hospital were required to undergo a test regardless of the medical condition that prompted admission); and (4) close contacts (all close contacts identified in contact tracing were required to quarantine for 10 days from the date of last exposure and to undergo a PCR test at the beginning of and after the quarantine period).
In addition, random tests were conducted daily. Text messages were sent to citizens and residents randomly, inviting them to undergo a free test at their nearest testing site. Mobile units were also dispatched to supermarkets, malls, banks, construction sites, markets, and other areas where people from diverse socio-economic, cultural, and national backgrounds gathered.
We collected the data from the National COVID-19 Contact Tracing Team Database of individuals with positive test results for SARS-CoV-2. The diagnosis followed the national Validation Protocol of Novel Coronavirus Nucleic Acid detection method. The process was based on reverse-transcriptase (RT)-PCR testing of nasopharyngeal samples using Thermo Fisher Scientific (Waltham, MA) TaqPath 1-Step RT-qPCR Master Mix, CG (catalog number A15299) on the Applied Biosystems (Foster City, CA) 7500 Fast Dx Real-time PCR Instrument. The assay followed the WHO protocol and targeted the E gene. If positive, the results were confirmed by testing for the RdRP and N genes. The E gene Ct value was reported and used in this study. Ct values <35 were considered positive.
SARS-CoV-2 variant sequencing was undertaken at the National COVID-19 Molecular Public Health Laboratory. Whole-genome sequencing was used to identify the standard variants of interest and of concern using Congenica Illumina/ARTIC and COVID-Seq protocols. The data were analyzed with the Abiomix platform. Spike gene target status by PCR was used as a second approach for identifying each variant. Data regarding sequencing of the variant were available for only 145 patients, of whom none were excluded from the analysis. This was because of laboratory limitations that led to the prioritization of critical cases and deaths.
Vaccines administered
Four vaccines were being offered to the public during the study period and hence constitute the vaccines included in this study. BNT162b2 vaccines (Pfizer) were being administered through intramuscular injection in two doses, 21 days apart, of 0.3 ml (30 μg) each. BBIBP-CorV vaccines (Sinopharm) were also offered in two doses, 21 days apart, of 4 μg adsorbed to 0.5 mg aluminum. Sputnik V vaccines (Sputnik) were offered as a liquid formulation in two doses, 21 days apart, containing 1011 virus particles per 0.5 ml/dose. COVISHIELD vaccines (Covishield) were offered in two 0.5-ml doses, 8 weeks apart, containing 5 × 1010 adenovirus particles each.
Data handling and statistical analysis
Epidemiological data are presented as (n, %), where n is the sample size corresponding to each category and % is the category sample size as a proportion of the total cohort studied, unless otherwise stated. We report proportions calculated using Agresti-Coull 95% confidence intervals (CIs) and statistical significance calculated using Pearson's chi-square test, unless otherwise indicated. We used z-test of proportion to compare vaccine breakdown in reinfection cases versus the general population, and we used analysis of variance (ANOVA) to statistically analyze the difference in time to reinfection by vaccination status. P-values <0.05 were considered statistically significant. SPSS version 26 was used for statistical analysis (StataCorp. 2017. Stata Statistical Software: Release 15. College Station, Texas: IBM Corp. Released 2019. IBM SPSS Statistics for Windows, Version 26.0. Armonk, New York: IBM Corp). Figures were created using GraphPad Prism version 9.3.1 (350) for macOS (GraphPad Software, La Jolla, California, USA, www.graphpad.com).
Ethical approval
The protocol and manuscript for this study were reviewed and approved by the National COVID-19 Research Committee in Bahrain (CRT-COVID2021-148). All methods and retrospective analyses of data were approved by the National COVID-19 Research and Ethics Committee. Informed consent was obtained from all participants, and the study was carried out in accordance with local guidelines and ethical guidelines of the Declaration of Helsinki 1975.
Results
Epidemiological patterns of reinfection
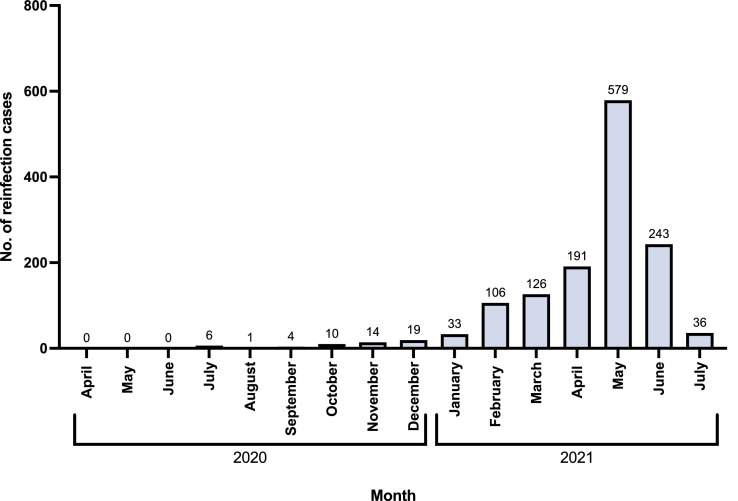
The first SARS-CoV-2 infection case in Bahrain was detected on February 23, 2020. During the study period, the number of reinfection cases steadily increased from zero cases per month in April-June 2020 to a peak of 579 in May 2021. The cases decreased to 36 by July 2021 (Figure 1 ).
Figure 1.
Graphical representation of number of reinfection cases reported per month during the study period. The number of cases reported is represented by the number above the bar corresponding to the month.
Demographics
Within the study cohort of 1362 reinfection cases, male individuals constituted a significantly greater proportion of reinfections (n = 821, 60.3%) than female individuals (39.7%) (P <0.0001). Reinfection episodes were highest among those 30-39 years of age (n = 405, 29.7%) and lowest among those 0-9 years of age (86, 6.3%) (Table 2 ).
Table 2.
Summary of data from 1362 cases of reinfection in Bahrain.
| Total n [%] | Fully vaccinated n [%] | Interrupted vaccination n [%] | One-dose vaccination n [%] | Postreinfection vaccination n [%] | Unvaccinated n [%] | ||
|---|---|---|---|---|---|---|---|
| No. of reinfection cases | 1362 | 387 | 23 | 116 | 171 | 665 | |
| Gender | Male Female |
821 [60.3] 541 [39.7] |
287 [74.2] 100 [25.8] |
17 [73.9] 6 [26.1] |
74 [63.8] 42 [36.2] |
113 [66.1] 58 [33.9] |
330 [49.6] 335 [50.4] |
| Age group, years | 0-9 | 86 [6.3] | 0 [0.0] | 0 [0.0] | 0 [0.0] | 0 [0.0] | 86 [12.9] |
| 10-19 | 128 [9.4] | 3 [0.8] | 0 [0.0] | 1 [0.9] | 3 [1.8] | 121 [18.2] | |
| 20-29 | 363 [26.7] | 106 [27.4] | 7 [30.4] | 35 [30.2] | 52 [30.4] | 163 [24.5] | |
| 30-39 40-49 50+ |
405 [29.7] 226 [16.6] 154 [11.3] |
133 [34.4] 85 [22.0] 60 [15.4] |
10 [43.5] 3 [13.0] 3 [13.0] |
47 [40.5] 21 [18.1] 12 [10.3] |
59 [34.5] 31 [18.1] 26 [15.2] |
156 [23.5] 86 [12.9] 53 [8.0] |
|
| Period between infections, months | 3-6 | 281 [20.6] | 33 [8.5] | 3 [13.0] | 24 [20.7] | 68 [39.8] | 153 [23.0] |
| 6-9 | 449 [33.0] | 98 [25.3] | 9 [39.1] | 31 [26.7] | 63 [36.8] | 248 [37.3] | |
| 9+ | 632 [46.4] | 256 [66.1] | 11 [47.8] | 61 [52.6] | 40 [23.4] | 264 [39.7] | |
| Symptom status | Symptomatic first infection episode only | 224 [16.4] | 70 [18.1] | 4 [17.4] | 17 [14.7] | 30 [17.5] | 103 [15.5] |
| Symptomatic second infection episode only | 387 [28.4] | 90 [23.3] | 7 [30.4] | 39 [33.6] | 53 [31.0] | 198 [29.8] | |
| Symptomatic at both episodes | 265 [19.5] | 64 [16.5] | 2 [8.7] | 25 [21.6] | 28 [16.4] | 146 [22.0] | |
| Asymptomatic at both episodes | 486 [35.7] | 163 [42.1] | 10 [43.5] | 35 [30.2] | 60 [35.1] | 218 [32.8] | |
| Hospitalization status | Isolation | 1273 [93.5] | 378 [97.7] | 23 [100.0] | 110 [94.8] | 156 [91.2] | 606 [91.1] |
| Moderate | 80 [5.9] | 9 [2.3] | 0 [0.0] | 4 [3.4] | 15 [8.8] | 52 [7.8] | |
| Severe | 9 [0.7] | 0 [0.0] | 0 [0.0] | 2 [1.7] | 0 [0.0] | 7 [1.1] |
Data are stratified by vaccination status, which is indicated in column headings.
“Total” column includes all individuals with reinfection, independent of vaccination status.
Values are presented as “n [%]”, where n is the total number of individuals in each category and [%] is n as a proportion of the total shown in the first column.
Time to reinfection
Regarding the time from initial infection to reinfection, the number of cases of reinfection showed a linear decrease with decreasing time between infection and reinfection. Most reinfection episodes occurred at ≥9 months after initial infection (n = 632, 46.4%). The fewest cases of reinfection occurred within a period of 3-6 months after the initial infection (n = 281, 20.6%) (Table 2).
Presentation and outcome
We compared each individual's symptom status between initial infection and reinfection episodes. Most individuals were asymptomatic during both episodes (n = 486, 35.7%), whereas 265 individuals (19.5%) were symptomatic during both episodes. The differences in symptoms between episodes were significant across the cohort (P = 0.003). Only 89 patients with reinfection (6.6%) required hospitalization, of which 80 (5.9%) were of moderate disease status and 9 (0.7%) were of severe disease status (P <0.0001) (Table 2). Only one death was recorded for the cohort; this patient required hospitalization with severe disease.
Variant sequencing
Data regarding sequencing of the SARS-CoV-2 variant were available for only 145 individuals (Table 3 ). The highest number of cases of reinfection involved the Delta variant (B.1.617) (n = 67, 46.2%), followed by the Alpha variant (B.1.17) (n = 60, 41.3%).
Table 3.
Breakdown of data by variant causing reinfection in 145 individuals.
| Variant | n [%] |
|---|---|
| B.1.617 | 67 [46.2] |
| B.1.17 | 60 [41.3] |
| B.1.351 | 4 [2.8] |
| B.1.281 | 1 [0.7] |
| B.1 | 4 [2.8] |
| Other | 9 [6.2] |
Data are presented as n [%], where n is the total number of individuals in each category and [%] is n as a proportion of the total (N = 145).
Analysis by vaccination status
Of the 1362 individuals studied, 388 (28.5%) were vaccinated and 974 (71.5%) were unvaccinated before reinfection. We further stratified these into five levels of vaccination, as described above in the Study Design section and listed in Table 1.
Demographics
In addition to representing the majority of reinfection cases overall, male individuals represented a higher proportion of cases across all vaccination statuses (P <0.0001); this is in accord with Bahrain's demographics. Reinfection was highest in the 30-39 age group across most vaccination statuses; however, the 20-29 age group predominated in the unvaccinated status group. Similarly, reinfection rate was lowest in the 0-9 age group for most vaccination statuses; in the unvaccinated group, the 50+ age group showed the lowest reinfection rate.
Time to reinfection
We observed an increase in the number of reinfection cases with increasing time after the initial infection episode. The lowest number of cases corresponded to a period of 3-6 months between the two infection episodes. The highest number of cases occurred at ≥9 months after initial infection. This was observed across the majority of vaccination statuses. However, in the postreinfection vaccination status, this trend was reversed (P <0.0001); most reinfection cases in this group occurred 3-6 months after initial infection (n = 68, 39.8%) and the fewest occurred at ≥9 months after initial infection (n = 40, 23.4%).
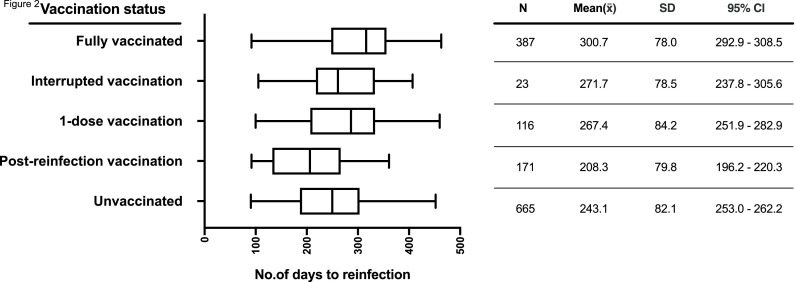
To further dissect time to reinfection, we compared the average number of days to reinfection among the various vaccination statuses. The highest number of days to reinfection was observed in the fully vaccinated group (mean = 300.7, SD = 78.0, 95% Cl 292.9-308.5), and the lowest was observed in the postreinfection vaccination group (mean = 208.3, SD = 79.8, 95% Cl 196.2-221.3). The data are illustrated in Figure 2 . ANOVA analysis showed that the average number of days to reinfection was significantly different among the various vaccination statuses (P <0.0001).
Figure 2.
Summary of number of days to reinfection across different vaccination statuses for 1362 individuals. Boxplot shows minimum, interquartile range, median, and maximum for each status on the y-axis, with corresponding descriptive statistics tabulated. CI, confidence interval.
Presentation and outcome
Most patients with reinfection were asymptomatic during both initial infection and reinfection. This was observed in all vaccination status groups (P =0.001) except individuals who received one-dose vaccination before reinfection. In this group the majority were symptomatic but only during their reinfection episode (n = 39, 33.6%) (Table 2). We used logistic regression analysis to examine the effect of symptom status at first infection, gender, and age on the probability of being symptomatic on reinfection. The risk of symptomatic presentation of reinfection rose with age (odds ratio [OR] for a 1-decade increase in age 1.13, P = 0.002) and was higher in female individuals (OR 1.3, P = 0.022). Patients who were symptomatic with first infection were more likely to be symptomatic with second infection (OR 1.5, P = 0.001). Adjusted for these factors, unvaccinated patients were more likely to be symptomatic on reinfection (OR 1.62, P = 0.001).
Investigation of the effect of vaccination status on hospitalization showed that most patients with reinfection required self-isolation; this was observed in all vaccination status groups (P <0.0001). Patients with moderate and severe disease statuses who required hospitalization were predominantly those with one dose of vaccine postreinfection or those who were unvaccinated. Absence of vaccination was a significant predictor of the need for hospital treatment; rates were 4.3% in the vaccinated group and 8.9% in the unvaccinated group, giving a preventable fraction in the vaccinated group of 52% (95% CI 26-67%, P <0.001). The risk of requiring hospital treatment increased with age (OR for a 1-decade increase in age 1.7, P <0.001) but did not differ significantly between male and female individuals. Although nine vaccinated individuals (2.3%) required hospitalization, no deaths were recorded in the vaccination group (Poisson exact upper 97.5% Cl 2.7 deaths per 1000); 1 death was recorded among the unvaccinated (P = 0.009).
Vaccines in reinfection
Analysis of vaccines used was conducted for all individuals in the fully vaccinated (n = 387), interrupted vaccination (n = 23), and one-dose vaccinated (n = 116) groups. Among the 387 fully vaccinated individuals, 87.3% of reinfection cases (n = 338) were with the Sinopharm vaccine. We adjusted for population proportions of each vaccine by comparing the proportion of each vaccine within the group to the proportion of each vaccine within the general population. The relative proportions of each vaccine in the fully vaccinated group were significantly different from relative proportions in the general population. The Sinopharm vaccine was observed at a higher frequency among fully vaccinated individuals with reinfections compared with the proportion of the population administered Sinopharm vaccine (P <0.0001). All other vaccines (Covishield, Sputnik, Pfizer) occurred at a lower frequency among individuals with reinfection compared with the population proportion for each (Table 4 ).
Table 4.
Breakdown of data by vaccine among cases of reinfection in vaccinated individuals compared with the general population in the kingdom, stratified by vaccination category.
| Vaccine | Sample breakdown n, [%] | Population breakdown n, [%] | Z-statistic | P-value | |
|---|---|---|---|---|---|
| Fully vaccinated | Sinopharm | 338 [87.3] | 575,159 [55.7] | 12.51 | <0.0001 |
| Covishield | 20 [5.2] | 192,357 [18.6] | 6.78 | <0.0001 | |
| Sputnik | 20 [5.2] | 186,275 [18.0] | 6.55 | <0.0001 | |
| Pfizer | 9 [2.3] | 79,287 [7.7] | 3.99 | 0.0001 | |
| Total | 387 | 1,033,078 | |||
| Interrupted vaccination | Sinopharm | 14 [60.9] | 575,159 [55.7] | 0.50 | 0.616 |
| Covishield | 3 [13.0] | 192,357 [18.6] | 0.69 | 0.490 | |
| Sputnik | 6 [26.1] | 186,275 [18.0] | 1.01 | 0.312 | |
| Pfizer | 0 [0.0] | 79,287 [7.7] | 1.39 | 0.166 | |
| Total | 23 | 1,033,078 | |||
| One-dose vaccination | Sinopharm | 68 [58.6] | 575,159 [55.7] | 0.63 | 0.530 |
| Covishield | 6 [5.2] | 192,357 [18.6] | 3.71 | 0.0002 | |
| Sputnik | 25 [21.6] | 186,275 [18.0] | 1.01 | 0.313 | |
| Pfizer | 17 [14.7] | 79,287 [7.7] | 2.83 | 0.0047 | |
| Total | 116 | 1,033,078 |
Data are presented as n [%], where n is the total number of individuals in each category and [%] is n as a proportion of the total shown in the last row for that vaccination category. Z-test of proportions was used to compare vaccine breakdown among cases of reinfection with the proportion of the population receiving each vaccine.
The Sinopharm vaccine was also the most common vaccine among the remaining vaccination categories, although the proportion was not significantly different from the population proportion. Among the 23 individuals with interrupted vaccination, 60.9% of reinfection cases (n = 14) were with Sinopharm (P = 0.616); in the 116 individuals with one-dose vaccination before reinfection, 58.60% of reinfection cases (n = 68) were with Sinopharm (P = 0.530). Among one-dose vaccinations, Covishield was significantly less frequent than in the general population (P = 0.0002), and Pfizer vaccine was more frequent (P = 0.0047). These data are listed in Table 4.
Discussion
The epidemiological data presented in this study indicate that reinfection can occur in both vaccinated and unvaccinated individuals. Through analysis of reinfection by vaccination status, we found that vaccine-induced immunity may be more effective at reducing reinfection episodes, as indicated by the relatively lower proportion of vaccinated individuals with reinfection in the studied population. These results are in contrast with the notion that vaccine-induced and infection-induced immunity are comparable (Cromer et al., 2021). Although it is difficult to compare effectiveness without analyzing incidence, the relative benefit of vaccine-induced immunity has already been observed and reported extensively (CDC, 2021a). Additional studies showed that those who recovered from COVID-19 were twice as likely to become reinfected as those who were vaccinated (Cavanaugh et al., 2021). Another study showed the relative risk to be five times greater in the recovered than in those who were vaccinated (Bozio et al., 2021). It had been suggested that there is a difference in the immune response in these two groups, with lower antibody level but longer memory-cell response in infection-induced immunity and vice versa in vaccination-induced immunity (Cho et al., 2021; Gazit et al., 2021). We observed no deaths among vaccinated individuals with reinfections; however, this observed rate showed an upper 97% Cl of 2.7 deaths per 1000. Nonetheless, the number recorded for unvaccinated reinfections is very small. Hence, caution must be taken in interpreting mortality from the data presented in this study.
The rate of reinfection reported in previous studies has been persistently low. A systematic review on reinfection involving 113,715 patients highlighted that most reports quote a low reinfection rate of 1% among populations (Tang et al., 2021). This finding suggests that the risk of reinfection to the public health is low. However, it also challenges the perception of herd immunity and its application in policies for tackling COVID-19. For instance, Pinto et al. suggested that immunity passports based on the assumption that reinfection is unlikely may need to be re-evaluated. Pinto et al. also argued that the perspective of vaccine failure and booster requirements should include iatrogenic immune response decay similar to that of the natural immune response (Pinto et al., 2021).
Interestingly, we observed a steady increase in reinfection cases with increased time after the first infection. This was true for reinfections in both vaccinated and unvaccinated individuals. It may be due to natural waning of immunity and particularly related to Sinopharm in fully vaccinated individuals, as Sinopharm occurred at a significantly higher frequency in this category even compared with vaccine uptake in the country's population. However, the peak of reinfection in May 2021 may also be influenced by behavioral factors due to concurrence with the Ramadan period with its increased communal gatherings. We also observed reinfections at periods >6 months after primary infection, mostly among unvaccinated individuals, in accord with CDC reports stating that “vaccine-induced immunity was more protective than infection-induced immunity” (Bozio et al., 2021). However, some reports show persistent immunological memory >6 months after primary infection (Dan et al., 2021; Zuo et al., 2021). The dominant theory in current studies is that of immune decay. Protective immunity is assessed as a correlate of neutralizing antibody responses, which recognize the viral spike protein exclusively (Wheatley et al., 2021). A recent study showed that the risk of reinfection among vaccinated and recovered individuals remained low, at least within the first 6 months after infection or vaccination (CDC, 2021a). Several studies show a decrease in these antibodies within 2-3 months after SARS-CoV-2 infection (Beaudoin-Bussières et al., 2020; Crawford et al., 2021; Ibarrondo et al., 2020). A study by Cromer et al. suggested that the rapid initial decay of immunity was due to the short half-life of serum antibodies and of antibody-secreting cells (Cromer et al., 2021). This may explain the increased risk of reinfection with longer period from the primary infection. Nonetheless, immune decay is an expected phenomenon with both vaccine-induced and infection-induced immunity and is characteristic of many viral infections (Cohen and Burbelo, 2021). In the context of SARS-CoV-2, acquired immunity may be protective for only months rather than years, although this is likely to be a reflection of the interplay between immune decay and improved immune evasion with novel SARS-CoV-2 variants.
We report that 46% of reinfection cases were due to the Delta (B.1.617) variant, closely followed by the Alpha (B.1.1.7) variant at 41%. Both variants have evolved immune evasion for both vaccine-induced and natural immunity (Planas et al., 2021). However, because of data limitations, the variant breakdown results reported in our study correspond to a small sample size of 145.
Through analysis of reinfection by age, we found the highest reinfection episode rate among the 20-39 age group. This finding is consistent with reports of increased susceptibility to the infection among individuals aged >20 years (Davies et al., 2020; Zhang et al., 2020). More specifically, the highest proportion corresponded to the 30-39 age group. This result is also directly comparable to results from our previous report on SARS-CoV-2 cases in Bahrain (Almadhi et al., 2021). We argued that this increased susceptibility was attributable to the fact that this is the working-age group in Bahrain and that these individuals therefore have greater exposure to social interactions. Moreover, the spike and subsequent reduction coincided with Ramadan (the month of fasting), when traditional large communal gatherings occur. The enhanced social interaction, coinciding with the lifting of restrictions on gatherings in restaurants, coffee shops, and cinemas, may have influenced the rate of interaction and hence the transmission of COVID-19 among these age groups. Interestingly, this trend was observed across reinfections in both vaccinated and unvaccinated individuals, further suggesting that the reinfection risk was driven by environmental factors rather than vaccination status.
However, these findings contradict those from a large study by Hansen et al. investigating reinfection among 4 million individuals in Denmark (Hansen et al., 2021). The study reports higher protection against reinfection in individuals aged <65 years old and lower protection in those >65 years old. Although our results do not assess protection, we report higher proportions of reinfection among individuals aged 20-39 years than among those aged 50+ years. We believe our findings are influenced by the proactive vaccination policy that prioritized those >65 years old in Bahrain and possibly by increased precautionary measures in this age group. In addition, we believe these differences may be due to factors such as environmental effects, demographics, way of life, and differences in the phase of the pandemic between Denmark and Bahrain. In addition, this research was conducted between September and December 31, 2020; the first cases of Alpha (B.1.1.7) and Delta (B.1.617) variants appeared in Denmark on November 9 and December 5, 2020, respectively (Latif et al., 2021b). Because of the difference in cohort sampling times, it is possible that the results also report characteristics of different variants. In contrast, the first cases of Alpha (B.1.1.7) and Delta (B.1.617) variants in Bahrain were reported on February 14 and April 5, 2021, respectively (Latif et al., 2021a). This may explain the sharp rise in cases of reinfection in both February and May (Figure 1). It should be noted that our results reflect reinfection before emergence of the Omicron (B.1.1.529) variant, which was announced as a variant of concern by the WHO in November 2021 (WHO, 2021a). This is important to consider when interpreting the results from this study, as the Omicron variant has been reported to have a high rate of infection in unvaccinated individuals and of reinfection in previously infected individuals (Altarawneh et al., 2022; Cele et al., 2022; Chaguza et al., 2022; Pulliam et al., 2022). The risk of reinfection is significantly higher with the Omicron variant and is reported to be more than five-fold higher than the risk with the Delta variant (Ferguson et al., 2021).
Analysis of reinfection among vaccinated individuals with regard to the vaccine administered showed that the highest proportion of reinfections (87.4%) occurred with the Sinopharm vaccine, despite Sinopharm accounting for only 55.7% of the vaccinated population. Furthermore, the remaining vaccines (Covishield, Sputnik, and Pfizer) were significantly less frequently represented among fully vaccinated individuals with reinfection compared with the population. However, this may not reflect protection, as the Sinopharm vaccine was the first to be approved for use in Bahrain (Ministry of Health, Kingdom of Bahrain, 2021). Combining this with immune decay, it is possible that the individuals who had taken early vaccination with Sinopharm during the pandemic were the most likely to be reinfected because of the longer period of immune decay. Nevertheless, the number of subjects analyzed with the remaining three vaccines was relatively low, and additional data are needed to confirm these findings. We also found that for reinfections after an interrupted vaccination, all four vaccines reflected their respective population proportions. It is difficult to know whether this is because of a general underperformance or overperformance of the vaccines; however, it is clear that at this stage all vaccines display similar effects on infection. Interestingly, our results show that Covishield vaccine is significantly less frequently represented among individuals reinfected after one-dose vaccination compared with the other vaccinations. On the other hand, the Pfizer vaccine was significantly more frequently represented in this group compared with its proportion of uptake in the general population. These results may portray a protective and adverse effect, respectively, for each vaccine after administration of one dose; however, the sample sizes were small (n = 6 and n = 17, respectively).
It is interesting to note that patients in this cohort had mild symptoms or were asymptomatic (93.5%). This finding is comparable to results from a systematic review of reinfection cases, which stated that 75% of patients were categorized as “mildly symptomatic” (Dhillon et al., 2021). However, asymptomatic infection was 42.1% among fully vaccinated individuals versus 32.8% among those who were not vaccinated. Notably, the interrupted vaccination (reinfected <14 days after the second dose) group did not show the same result. The vaccine had been developed and tested to decrease severe disease and mortality. In a systematic analysis, the vaccine efficacy/effectiveness in fully vaccinated individuals was 80-90% against symptomatic and asymptomatic infections (Harder et al., 2021). In addition, one study among healthcare workers showed a decline in symptomatic and asymptomatic SARS-CoV-2 infections after vaccination despite high rates of COVID-19 disease nationally (Gohil et al., 2021).
It is expected that vaccination protects against moderate and severe disease. In this cohort, moderate and severe disease rates were 2.3% among those fully vaccinated and 8.9% among those not vaccinated, showing that vaccinated individuals had hospitalization rates lower than those of unvaccinated individuals. One study of an mRNA vaccine showed lower hospitalization rates among individuals with vaccine- breakthrough infections than among those without vaccination (Tenforde et al., 2021). In addition, another study of an inactivated SARS-CoV-2 vaccine showed a significant reduction in symptomatic disease after vaccination (Al Kaabi et al., 2021). As noted above, COVID-19 testing in Bahrain has been extensive and includes a large proportion of random testing conducted daily. We believe this ensures that the number and nature of confirmed cases accurately reflect actual incidence rates.
Finally, it is essential to note that reinfection with SARS-CoV-2 is not unexpected, as this is an established characteristic of viruses causing mucosal infections (Cohen and Burbelo, 2021). Hence, it is an important factor moving forward to establish better COVID-19 policies to address the pandemic effectively.
Limitations and further work
The main limitation of this study is that vaccination status was determined by time since the first infection. This introduces the possibility of immortal time bias, wherein the longer the period before reinfection, the higher the chance that the person will have attained a full vaccination status. Hence, time to reinfection may be influencing vaccination status while vaccination status is influencing time to reinfection. Second, our primary cohort analysis included only 1362 reinfection cases; the sample size was further minimized when the cohort was divided into different analysis categories. In addition, the majority of vaccinations in the cohort were with Sinopharm, meaning analysis of vaccination was mainly a reflection of Sinopharm. It would be important to replicate this study with a larger cohort and with different ethnic populations to expand our understanding of SARS-CoV-2 reinfection patterns. Epidemiological confirmation of reinfection and viral genotyping of the first and second specimens are needed. These were not possible in this study, however, and we were therefore unable draw reliable conclusions. Vaccine analysis suggested that the Sinopharm vaccine was associated with more reinfection, but this needs to be confirmed in a larger population or by meta-analysis. Breakdown of data by variant and vaccine simultaneously could improve our understanding of vaccine efficacy with variant evolution. Finally, we believe that replicating this study with added focus on differentiating reinfection and reactivation would be instrumental to understanding and controlling SARS-CoV-2 spread.
In conclusion, vaccine-induced immunity and previous infection with or without vaccination were effective in reducing disease severity of reinfection episodes.
Author contributions
AAA provided the data from the National COVID-19 Database. MAM and MAQ analyzed and interpreted the data and wrote the manuscript. ASA and RC conducted the statistical analysis and contributed to the writing of the manuscript. SA and JAAT contributed to the interpretation and writing of the manuscript. All authors reviewed and approved the final version of the manuscript.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Ethical approval
Approval granted after review from National COVID-19 Research and Ethics Committee (Approval Code: CRT-COVID2021-148).
References
- Al Kaabi N, Zhang Y, Xia S, Yang Y, Al Qahtani MM, Abdulrazzaq N, et al. Effect of 2 inactivated SARS-CoV-2 vaccines on symptomatic COVID-19 infection in adults: a randomized clinical trial. JAMA. 2021;326:35–45. doi: 10.1001/jama.2021.8565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almadhi MA, Abdulrahman A, Sharaf SA, AlSaad D, Stevenson NJ, Atkin SL, et al. The high prevalence of asymptomatic SARS-CoV-2 infection reveals the silent spread of COVID-19. Int J Infect Dis. 2021;105:656–661. doi: 10.1016/j.ijid.2021.02.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altarawneh HN, Chemaitelly H, Hasan MR, Ayoub HH, Qassim S, AlMukdad S, et al. Protection against the Omicron variant from previous SARS-CoV-2 infection. N Engl J Med. 2022;386:1288–1290. doi: 10.1056/NEJMc2200133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aschwanden C. The false promise of herd immunity for COVID-19. Nature. 2020;587:26–28. doi: 10.1038/d41586-020-02948-4. [DOI] [PubMed] [Google Scholar]
- Beaudoin-Bussières G, Laumaea A, Anand SP, Prévost J, Gasser R, Goyette G, et al. Decline of humoral responses against SARS-CoV-2 spike in convalescent individuals. mBio. 2020;11:e02590. doi: 10.1128/mBio.02590-20. -20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozio CH, Grannis SJ, Naleway AL, Ong TC, Butterfield KA, DeSilva MB, et al. Laboratory-confirmed COVID-19 among adults hospitalized with COVID-19-like illness with infection-induced or mRNA vaccine-induced SARS-CoV-2 immunity - nine states, January–September 2021. MMWR Morb Mortal Wkly Rep. 2021;70:1539–1544. doi: 10.15585/mmwr.mm7044e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanaugh AM, Spicer KB, Thoroughman D, Glick C, Winter K. Reduced risk of reinfection with SARS-CoV-2 after COVID-19 vaccination - Kentucky, May–June 2021. MMWR Morb Mortal Wkly Rep. 2021;70:1081–1083. doi: 10.15585/mmwr.mm7032e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC. Science brief: SARS-CoV-2 infection-induced and vaccine-induced immunity. Atlanta: CDC; 2021a.
- CDC Symptoms of COVID-19. Centers for Disease Control and Prevention. 2021 Atlanta. [Google Scholar]
- CDC. Symptoms of COVID-19. 2021. https://www.cdc.gov/coronavirus/2019-ncov/symptoms-testing/symptoms.html. (Accessed 05 June 2022)
- Cele S, Jackson L, Khoury DS, Khan K, Moyo-Gwete T, Tegally H, et al. Omicron extensively but incompletely escapes Pfizer BNT162b2 neutralization. Nature. 2022;602:654–656. doi: 10.1038/s41586-021-04387-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaguza C, Coppi A, Earnest R, Ferguson D, Kerantzas N, Warner F, et al. Rapid emergence of SARS-CoV-2 Omicron variant is associated with an infection advantage over Delta in vaccinated persons. Med (N Y) 2022;3:325–334. doi: 10.1016/j.medj.2022.03.010. e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chemaitelly H, Yassine HM, Benslimane FM, Al Khatib HA, Tang P, Hasan MR, et al. mRNA-1273 COVID-19 vaccine effectiveness against the B.1.1.7 and B.1.351 variants and severe COVID-19 disease in Qatar. Nat Med. 2021;27:1614–1621. doi: 10.1038/s41591-021-01446-y. [DOI] [PubMed] [Google Scholar]
- Cho A, Muecksch F, Schaefer-Babajew D, Wang Z, Finkin S, Gaebler C, et al. Anti-SARS-CoV-2 receptor-binding domain antibody evolution after mRNA vaccination. Nature. 2021;600:517–522. doi: 10.1038/s41586-021-04060-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen JI, Burbelo PD. Reinfection with SARS-CoV-2: implications for vaccines. Clin Infect Dis. 2021;73:e4223–e4228. doi: 10.1093/cid/ciaa1866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford KHD, Dingens AS, Eguia R, Wolf CR, Wilcox N, Logue JK, et al. Dynamics of neutralizing antibody titers in the months after severe acute respiratory syndrome coronavirus 2 infection. J Infect Dis. 2021;223:197–205. doi: 10.1093/infdis/jiaa618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cromer D, Juno JA, Khoury D, Reynaldi A, Wheatley AK, Kent SJ, et al. Prospects for durable immune control of SARS-CoV-2 and prevention of reinfection. Nat Rev Immunol. 2021;21:395–404. doi: 10.1038/s41577-021-00550-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dan JM, Mateus J, Kato Y, Hastie KM, Yu ED, Faliti CE, et al. Immunological memory to SARS-CoV-2 assessed for up to 8 months after infection. Science. 2021;371:eabf4063. doi: 10.1126/science.abf4063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies NG, Klepac P, Liu Y, Prem K, Jit M. CMMID COVID-19 working group, et al. Age-dependent effects in the transmission and control of COVID-19 epidemics. Nat Med. 2020;26:1205–1211. doi: 10.1038/s41591-020-0962-9. [DOI] [PubMed] [Google Scholar]
- Dhillon RA, Qamar MA, Gilani JA, Irfan O, Waqar U, Sajid MI, et al. The mystery of COVID-19 reinfections: a global systematic review and meta-analysis. Ann Med Surg (Lond) 2021;72 doi: 10.1016/j.amsu.2021.103130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson N, Ghani A, Cori A, Hogan A, Hinsley W, Volz E. Imperial College Press; London: 2021. Growth, population distribution and immune escape of Omicron in England. 16 December. [Google Scholar]
- Gazit S, Shlezinger R, Perez G, Lotan R, Peretz A, Ben-Tov A, et al. Comparing SARS-CoV-2 natural immunity to vaccine-induced immunity: reinfections versus breakthrough infections. 2021. https://www.medrxiv.org/content/10.1101/2021.08.24.21262415v1 (accessed 11 December 2021).
- Gohil SK, Olenslager K, Quan KA, Dastur CK, Afsar N, Chang W, et al. Asymptomatic and symptomatic COVID-19 infections among health care personnel before and after vaccination. JAMA Netw Open. 2021;4 doi: 10.1001/jamanetworkopen.2021.15980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen CH, Michlmayr D, Gubbels SM, Mølbak K, Ethelberg S. Assessment of protection against reinfection with SARS-CoV-2 among 4 million PCR-tested individuals in Denmark in 2020: a population-level observational study. Lancet. 2021;397:1204–1212. doi: 10.1016/S0140-6736(21)00575-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harder T, Koch J, Vygen-Bonnet S, Külper-Schiek W, Pilic A, Reda S, et al. Efficacy and effectiveness of COVID-19 vaccines against SARS-CoV-2 infection: interim results of a living systematic review, 1 January to 14 May 2021. Euro Surveill. 2021;26 doi: 10.2807/1560-7917.ES.2021.26.28.2100563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibarrondo FJ, Fulcher JA, Goodman-Meza D, Elliott J, Hofmann C, Hausner MA, et al. Rapid decay of anti-SARS-CoV-2 antibodies in persons with mild Covid-19. N Engl J Med. 2020;383:1085–1087. doi: 10.1056/NEJMc2025179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikegame S, Siddiquey MNA, Hung CT, Haas G, Brambilla L, Oguntuyo KY, et al. Neutralizing activity of Sputnik V vaccine sera against SARS-CoV-2 variants. Nat Commun. 2021;12:4598. doi: 10.1038/s41467-021-24909-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefèvre B, Tondeur L, Madec Y, Grant R, Lina B, van der Werf S, et al. Beta SARS-CoV-2 variant and BNT162b2 vaccine effectiveness in long-term care facilities in France. Lancet Healthy Longev. 2021;2:e685–e687. doi: 10.1016/S2666-7568(21)00230-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez Bernal J, Andrews N, Gower C, Gallagher E, Simmons R, Thelwall S, et al. Effectiveness of Covid-19 vaccines against the B.1.617.2 (Delta) variant. N Engl J Med. 2021;385:585–594. doi: 10.1056/NEJMoa2108891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gangavarapu K, Latif AA, Mullen JL, Alkuzweny M, Hufbauer E, Tsueng G et al. Bahrain variant report. https://outbreak.info/location-reports?loc=BHR, 2021a. (accessed 14 September 2021).
- Ministry of Health, Kingdom of Bahrain . Kingdom of Bahrain; 2021. The official website for the latest health developments. https://healthalert.gov.bh/en/category/latest-decWoSons. (accessed 11 December 2021) [Google Scholar]
- Pinto LM, Nanda V, Sunavala A, Rodriques C. Reinfection in COVID-19: a scoping review. Med J Armed Forces India. 2021;77:S257–S263. doi: 10.1016/j.mjafi.2021.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Planas D, Veyer D, Baidaliuk A, Staropoli I, Guivel-Benhassine F, Rajah MM, Planchais C, Porrot F, Robillard N, Puech J, Prot M, Gallais F, Gantner P, Velay A, Le Guen J, Kassis-Chikhani N, Edriss D, Belec L, Seve A, Courtellemont L, Péré H, Hocqueloux L, Fafi-Kremer S, Prazuck T, Mouquet H, Bruel T, Simon-Lorière E, Rey FA, Schwartz O. Reduced sensitivity of SARS-CoV-2 variant Delta to antibody neutralization. Nature. 2021;596:276–280. doi: 10.1038/s41586-021-03777-9. [DOI] [PubMed] [Google Scholar]
- Pulliam JRC, van Schalkwyk C, Govender N, von Gottberg A, Cohen C, Groome MJ, et al. Increased risk of SARS-CoV-2 reinfection associated with emergence of Omicron in South Africa. Science. 2022;376:eabn4947. doi: 10.1126/science.abn4947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang X, Musa SS, Zhao S, He D. Reinfection or reactivation of severe acute respiratory syndrome coronavirus 2: a systematic review. Front Public Health. 2021;9 doi: 10.3389/fpubh.2021.663045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenforde MW, Self WH, Adams K, Gaglani M, Ginde AA, McNeal T, et al. Association between mRNA vaccination and COVID-19 hospitalization and disease severity. JAMA. 2021;326:2043–2054. doi: 10.1001/jama.2021.19499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tillett RL, Sevinsky JR, Hartley PD, Kerwin H, Crawford N, Gorzalski A, et al. Genomic evidence for reinfection with SARS-CoV-2: a case study. Lancet Infect Dis. 2021;21:52–58. doi: 10.1016/S1473-3099(20)30764-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vignier N, Bérot V, Bonnave N, Peugny S, Ballet M, Jacoud E, et al. Breakthrough infections of SARS-CoV-2 Gamma variant in fully vaccinated gold miners, French Guiana, 2021. Emerg Infect Dis. 2021;27:2673–2676. doi: 10.3201/eid2710.211427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheatley AK, Juno JA, Wang JJ, Selva KJ, Reynaldi A, Tan HX, et al. Evolution of immune responses to SARS-CoV-2 in mild-moderate COVID-19. Nat Commun. 2021;12:1162. doi: 10.1038/s41467-021-21444-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Taskforce for Combating the Coronavirus (COVID-19). Bahrain COVID-19 national protocols. 2021. https://www.nhra.bh/Media/Announcement/MediaHandler/GenericHandler/documents/Announcements/NHRA_News_MOH%20ALERT_Bahrain%20COVID-19%20National%20Protocols_20210505.pdf. (accessed 09 August 2021).
- WHO. WHO Director-General's opening remarks at the media briefing on COVID-19 - 11 March 2020. Geneva: World Health Organization, 2020.
- Gangavarapu K, Latif AA, Mullen JL, Alkuzweny M, Hufbauer E, Tsueng G et al. Denmark variant report. https://outbreak.info/location-reports?loc=DNK, 2021b. (accessed 14 September 2021).
- WHO. Classification of Omicron (B.1.1.529): SARS-CoV-2 variant of concern. https://www.who.int/news/item/26-11-2021-classification-of-omicron-(b.1.1.529)-sars-cov-2-variant-of-concern, 2021a (accessed 19 June 2022).
- WHO. Tracking SARS-CoV-2 variants. https://www.who.int/en/activities/tracking-SARS-CoV-2-variants/, 2021b (accessed 19 December 2021).
- WHO. WHO Coronavirus Disease (COVID-19) Dashboard. https://covid19.who.int, 2021c (accessed 19 December 2021).
- Zhang J, Litvinova M, Liang Y, Wang Y, Wang W, Zhao S, et al. Changes in contact patterns shape the dynamics of the COVID-19 outbreak in China. Science. 2020;368:1481–1486. doi: 10.1126/science.abb8001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo J, Dowell AC, Pearce H, Verma K, Long HM, Begum J, et al. Author correction: robust SARS-CoV-2-specific T cell immunity is maintained at 6 months following primary infection. Nat Immunol. 2021;22:928. doi: 10.1038/s41590-021-00957-7. [DOI] [PMC free article] [PubMed] [Google Scholar]