Abstract
Vibrio harveyi regulates the expression of bioluminescence (lux) in response to cell density, a phenomenon known as quorum sensing. In V. harveyi, two independent quorum-sensing systems exist, and each produces, detects, and responds to a specific cell density-dependent autoinducer signal. The autoinducers are recognized by two-component hybrid sensor kinases called LuxN and LuxQ, and sensory information from both systems is transduced by a phosphorelay mechanism to the response regulator protein LuxO. Genetic evidence suggests that LuxO-phosphate negatively regulates the expression of luminescence at low cell density in the absence of autoinducers. At high cell density, interaction of the sensors with their cognate autoinducers results in dephosphorylation and inactivation of the LuxO repressor. In the present report, we show that LuxN and LuxQ channel sensory information to LuxO via a newly identified phosphorelay protein that we have named LuxU. LuxU shows sequence similarity to other described phosphorelay proteins, including BvgS, ArcB, and Ypd1. A critical His residue (His 58) of LuxU is required for phosphorelay function.
Regulation of the expression of bioluminescence in Vibrio harveyi is a complex process involving multiple signalling circuits (7, 29). Several environmental cues are detected by the bacterium, and partial regulation of lux occurs in response to each input. Cell density-dependent regulation of lux is the best-understood mechanism of control in V. harveyi. Density-dependent regulation of gene expression, or quorum sensing, was first described in Vibrio fischeri and V. harveyi but is now known to exist in many genera of bacteria (5, 14, 32, 33, 34). Usually, quorum sensing is controlled by two regulatory proteins similar to LuxI and LuxR from V. fischeri. The LuxI-like proteins are autoinducer synthases and are responsible for production of acyl homoserine lactone autoinducer signal molecules, and the LuxR-like proteins are transcriptional activators whose activity is regulated by interaction with a cognate acyl homoserine lactone autoinducer (16, 20). Unlike other known density-sensing systems, the regulatory components controlling quorum sensing in V. harveyi are not similar to LuxI and LuxR but are members of the family of bacterial two-component adaptive regulators. And signal transduction occurs via a phosphorylation-dephosphorylation mechanism (7).
In the V. harveyi quorum-sensing circuit, two endogenously produced autoinducer signals are used to control light emission in response to changes in cell density (7). Both autoinducers are released into the surrounding medium as the cells grow. At a critical cell density, the autoinducer concentrations become sufficient to be detected by the cognate sensors. A signal transduction cascade is subsequently initiated, resulting in an exponential increase in the expression of the luciferase structural operon luxCDABEGH. Biochemical and genetic analyses have demonstrated that one V. harveyi autoinducer, designated AI-1, is N-(3-hydroxybutanoyl)-l-homoserine lactone, and its synthesis is dependent on the activities of the products encoded by the luxL and luxM genes (8, 13). AI-1 is detected by a sensor protein called LuxN, which is a two-component hybrid sensor kinase (8). V. harveyi mutants defective in the production of AI-1 remain capable of production of another compound (designated AI-2) that also stimulates the density-dependent expression of luxCDABEGH. Response to AI-2 is dependent on the AI-2 sensor LuxPQ (9). LuxP is similar to the periplasmic ribose binding proteins of Escherichia coli and Salmonella typhimurium. LuxQ is a two-component hybrid sensor kinase protein similar to LuxN. We propose that the primary receptor for AI-2 is LuxP and that the LuxP–AI-2 complex interacts with LuxQ. AI-2 of V. harveyi has not been purified, nor has the gene encoding the AI-2 synthase been identified. Mutant analyses have shown that the two V. harveyi quorum-sensing systems function in parallel, because either system alone is sufficient for the density-dependent expression of luminescence.
In V. harveyi, autoinducer signalling from both sensory systems is channeled to the lux operon through a shared response regulator protein called LuxO (10). LuxO is a repressor of lux expression at low cell density. Our data indicate that at low cell density, LuxO is phosphorylated at Asp 47, and in this form, LuxO has repressor activity (18). Autoinducer-stimulated dephosphorylation of LuxO at high cell density inactivates the LuxO repressor. Both phosphorylation and dephosphorylation of LuxO are dependent on the sensors LuxN and LuxQ. A positive transcription factor called LuxR is also required for expression of luxCDABEGH (28, 38, 40). LuxR is not a two-component protein, nor is it similar to the V. fischeri LuxR transcriptional activator protein.
Sequence analysis of the two sensor proteins LuxN and LuxQ showed that they each possess an N-terminal periplasmic domain, presumably for interaction with an autoinducer, a central cytoplasmic histidine kinase domain, and a C-terminal response regulator domain (7). It was not clear what function the LuxN and LuxQ response regulator domains play in intermolecular signalling to LuxO. However, we now understand that four-step phosphorelay mechanisms exist and are common in two-component signalling systems containing hybrid sensor kinases similar to LuxN and LuxQ (2, 12). These relays involve sequential phosphotransfer from the autophosphorylated His residue on the sensor kinase (His 1) to a conserved Asp residue in a response regulator protein or domain (Asp 1). In the next step, the phosphoryl group is transferred from Asp 1 to a His residue (His 2) on a newly identified two-component enzyme module called the phosphorelay or phosphotransferase protein (12). Finally, transfer from His 2 of the phosphorelay protein to Asp 2 of a second response regulator occurs. The consequence of phosphorylation at Asp 2 of this response regulator is an alteration in activity of the protein which is translated into a change in the gene expression or behavior of the bacterium.
We have identified a new component of the V. harveyi Lux quorum-sensing circuit, and we call it LuxU. The genetic analysis presented in this report suggests that LuxU is a phosphorelay protein and is responsible for coupling signalling events from the hybrid sensor kinases LuxN and LuxQ to the response regulator protein LuxO.
MATERIALS AND METHODS
Bacterial strains and media.
A description of the bacterial strains and plasmids used in this study is presented in Table 1. V. harveyi strains were grown in HI medium (8) at 30°C with shaking prior to chromosomal preparation. The density-dependent bioluminescence assay has been previously described (8). These assays were performed with AB (autoinducer bioassay) medium (21). Cloning, mutagenesis, and sequencing of V. harveyi DNA were performed by using E. coli JM109 [supE Δ(lac-proAB) hsdR17 recA1 F′ traD36 proAB+ lacIq lacZΔM15) as the host. As previously described (8), recombinant cosmids were conjugated into V. harveyi recipient strains by using a triparental mating technique. Allelic replacements in the V. harveyi chromosome were accomplished by using a previously published biparental mating procedure (8). Mobilizable IncP plasmids pRK2013 (15) and pPH1JI (11) were maintained in E. coli CC118 [araD139 Δara leu76a7 ΔlacX74 ΔphoA20 galE galK thi rpsE rpoB argE (Am) recA1]. E. coli strains were grown in LB at 37°C with shaking. LB contains 10 g of NaCl, 10 g of Difco Bacto Tryptone, and 5 g of Difco Bacto Yeast Extract per liter. Antibiotics (obtained from Sigma) were used at the following concentrations: ampicillin, 100 mg/liter; chloramphenicol, 10 mg/liter; gentamicin, 100 mg/liter; kanamycin, 100 mg/liter; tetracycline, 10 mg/liter.
TABLE 1.
V. harveyi strains and plasmids used in this study
| Strain or plasmid | Relevant genotype or feature | Reference or source |
|---|---|---|
| BB120 | Wild type | 6 |
| JAF78 | ΔluxOU-Cmr | 18 |
| JAF483 | luxO D47A linked to Knr | 18 |
| JAF548 | luxO D47E linked to Knr | 18 |
| JAF549 | luxN L166R linked to Knr | 18a |
| JAF536 | luxU::Knr | This study |
| JAF553 | luxU H58A linked to Knr | This study |
| JAF558 | luxN L166R luxU::Cmr | This study |
| JAF552 | luxO D47E luxU::Knr | This study |
| pLAFR2 | Broad host range; mob Tetr | 19 |
| pPH1JI | Broad host range; tra mob | 11 |
| pRK2013 | Broad host range; tra | 15 |
| pALTER | For site-directed mutagenesis | Promega |
| pUC4K | Knr cassette | Pharmacia |
| pPHP45Ω | Cmr cassette | 17 |
| pJAF182 | luxOU in pALTER | 18 |
| pJAF806 | pLAFR2 with luxU::Knr | This study |
| pJAF829 | pLAFR2 with luxO D47E, luxU::Knr | This study |
| pJAF830 | pLAFR2 with luxU H58A linked to Knr | This study |
| pJAF962 | pLAFR2 with luxU H103A linked to Knr | This study |
Recombinant DNA techniques.
The methods used for the isolation and analysis of V. harveyi DNA have been reported previously (18). Standard molecular techniques involving E. coli were performed as described by Sambrook et al. (36). New England Biolabs supplied restriction endonucleases and T4 DNA ligase. Taq DNA polymerase, T4 polynucleotide kinase, calf intestine alkaline phosphatase, and lysozyme were supplied by Boehringer Mannheim Biochemicals. Pfu polymerase and Pfu ligase were purchased from Stratagene. All biochemical reagents were used in accordance with the recommendations of the suppliers. Site-directed mutagenesis (SDM) of luxU to alter His 58 and His 103 to Ala was performed by the PCR method of Michael (30). As noted previously (18), Pfu polymerase and ligase were used in the PCRs instead of Taq polymerase and ligase. Oligonucleotides used in site-directed mutageneses were purchased from Midland Certified Reagent Company. The Amersham Multiprime DNA labeling procedure was used to prepare radioactive probes for Southern blotting. [32P]dCTP (Dupont, NEN) was incorporated into DNA probes.
Construction of V. harveyi luxU null strains.
The luxU gene is located downstream of luxO and was isolated from a cosmid identified in the original analysis of luxO (10). A 4.3-kb EcoRI DNA fragment containing both the luxO and luxU genes was subcloned from this cosmid into the vector pALTER (Promega), making plasmid pJAF182 (18). Next, a 1.2-kb EcoRI DNA fragment encoding a kanamycin resistance (Knr) cassette from plasmid pUC4K (Pharmacia) was inserted into an MfeI site internal to luxU. This site corresponds to codon 85 of the luxU open reading frame (ORF) and resulted in an EcoRI fragment containing luxO+, luxU::Knr, and flanking V. harveyi genomic DNA sequences. This EcoRI fragment was subsequently subcloned into pLAFR2. This construction was called pJAF806 and was used for allelic replacement of luxO+ and luxU::Knr in the V. harveyi chromosome. V. harveyi JAF78 (18) was used as the recipient for the allelic replacement procedure. Strain JAF78 carries a chromosomal deletion encompassing both luxO and luxU and is designated ΔluxOU-Cmr (chloramphenicol resistance). Substitution of luxO+ and luxU::Knr from pJAF806 for ΔluxOU-Cmr in the V. harveyi chromosome was accomplished by using selection for inheritance of Knr, followed by screening for loss of Cmr. The luxO+ luxU::Knr V. harveyi strain constructed by this method was called JAF536. Southern blot analysis was used to verify that the JAF536 strain construction was correct.
Double-mutant strain JAF552, containing luxU::Knr and luxO D47E, was constructed as follows. The luxO D47E mutation was constructed in pJAF182 as previously described (18). A Knr cassette was next inserted into the MfeI site located in luxU (see Fig. 1). The EcoRI fragment containing luxO D47E and luxU::Knr was subsequently subcloned into pLAFR2. This construction, pJAF829, was used for allelic replacement into V. harveyi JAF78. Mutant strain JAF549, containing the dark luxN L166R allele, was constructed in a way similar to that reported for luxO alleles (18). The double luxN L166R luxU null strain was constructed by substitution of a Cmr cassette for the Knr cassette at the MfeI site in pJAF806 (18a). The Cmr cassette was obtained from plasmid pHP45Ω Cm (17). The luxU::Cmr gene was subsequently replaced in V. harveyi JAF549 to create double luxN L166R luxU::Cmr null strain JAF558.
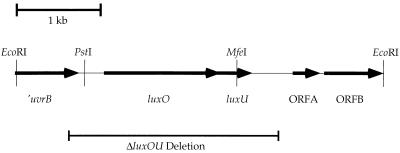
FIG. 1.
Genetic organization of the luxO-luxU region of the V. harveyi chromosome. The region encompassing luxO and luxU in the V. harveyi chromosome is shown. Sequence analysis suggests that a partial ORF encoding a V. harveyi uvrB homologue is located upstream of luxO and extends 692 bp into the sequenced fragment. The luxO gene is encoded by bp 1084 to 2445, and the luxU gene extends from bp 2442 to bp 2787. Two other ORFs, designated ORFA and ORFB, reside downstream of luxU and extend from bp 3302 to 3655 and from bp 3682 to bp 4312, respectively. Arrows designate the direction of transcription of each ORF. The extent of the chromosomal deletion in strain JAF78 is also depicted, showing that it encompasses both luxO and luxU. The PstI and MfeI sites used to insert Knr and Cmr cassettes are also shown (see Materials and Methods).
SDM and analysis of luxU mutants.
His 58 and His 103 of LuxU were mutated to Ala by using an adaptation of the method of Michael (30). For the H58A construction, DNA encoding the 3′ region of luxO and the entire luxU gene was amplified by PCR using primers 5′-CCCAGACGTGCCAATCATC-3′ and 5′-CGCTCGTCTCCATCCCCTGC-3′. In the same PCR, the luxU H58A mutation was constructed by inclusion of mutagenic primer 5′-TTAAAAGAGATCAGCGCTGCACTGAAAAGTAGTGCTGCC-3′. In this reaction, a CAC codon was altered to a GCT codon, which was verified by the introduction of a novel restriction site at the site of the mutation.
The PCR product was subcloned into a pLAFR2 derivative that contained the remainder of the luxO gene and upstream and downstream flanking V. harveyi DNA. This step regenerated the luxO and luxU loci and moved both luxO and luxU H58A back into a larger V. harveyi genomic fragment in pLAFR2. The region of DNA amplified in the PCR was sequenced to ensure that only the desired mutation was incorporated. A 1.2-kb PstI DNA fragment containing the Knr cassette from pUC4K was next cloned into an existing PstI site 307 bp upstream of luxO (Fig. 1). This final construction was called pJAF830. Substitution of this Knr-linked luxO+ luxU H58A locus for ΔluxOU-Cmr in the chromosome of V. harveyi JAF78 was performed exactly as previously described (18). Southern blots were used to show that Knr, wild-type luxO (luxO+), and the luxU H58A missense alleles had all been incorporated at the appropriate location in the V. harveyi chromosome. The resulting strain was called V. harveyi JAF553.
The LuxU His 103-to-Ala mutation was constructed similarly, by alteration of a CAT codon to a GCG codon. In this case, both the amplification and the mutagenesis were accomplished by using two primers. The upstream primer, which incorporated the mutation, was 5′-CCGCAATTGCAAGAGCAGGGGATGGAGACGAGCGAAATGC TCGC T T TACT TGC TATCAC TCGTGACGCC-3′, and the downstream primer was 5′-CGCCAATTGCTATCAAGCTCGAATTCAGTCAGGGTAAACAC-3′. The luxU H103A construct linked to Knr was called pJAF962.
Sequence analysis of luxU.
The luxU gene was sequenced during the analysis of luxO by using the dideoxy-chain termination method of Sanger et al. (37). The 4.3-kb V. harveyi EcoRI fragment containing luxO and luxU was subcloned into M13mp18 and M13mp19. Nested deletions were made by using the Cyclone 1 Biosystems Kit (International Biotechnologies Inc.), and the resulting DNA fragments were sequenced. The sequencing strategy was reported in full by Bassler et al. (10). At that time, luxU was not analyzed. In the present report, BLAST database analysis (1) was performed to search for both DNA and protein similarity to luxU and LuxU.
Nucleotide sequence accession number.
The luxU sequence has been deposited in the EMBL, GenBank, and DDBJ nucleotide sequence data libraries under accession no. L26221, along with information about luxO.
RESULTS
LuxU is required for quorum sensing in V. harveyi.
The sequence of the luxO gene revealed a small ORF located immediately downstream (10). We hypothesized that this putative ORF could encode a protein involved in lux regulation in V. harveyi. We named the gene luxU.
The genetic organization of the V. harveyi chromosome surrounding the luxO-luxU locus is shown in Fig. 1. The V. harveyi EcoRI fragment pictured was subcloned from a larger 25-kb genomic clone because it complemented a constitutively repressing allele of luxO. This fragment was sequenced, and the function of luxO was analyzed by Tn5 mutagenesis and gene replacement as previously described (10). Figure 1 shows the result of our further analysis of this region of the V. harveyi chromosome. A portion of a gene with homology to uvrB (4) is contained at the extreme 5′ end of the EcoRI fragment. The luxO gene is encoded by bp 1084 to 2445, and the putative luxU gene resides at bp 2442 to 2787 (this numbering refers to GenBank entry L26221 and reference 10). Two other putative ORFs are located on the 4.3-kb EcoRI fragment, but they do not show similarity to anything currently in the bacterial database. In the figure, we refer to them as ORFA and ORFB. All of the genes are transcribed from left to right as depicted in Fig. 1.
To test whether luxU encodes a protein involved in quorum sensing, we constructed a luxU null allele on the V. harveyi chromosome and analyzed its effect on Lux expression. To engineer the null mutation, we first constructed a chromosomal deletion encompassing both luxO and luxU (designated ΔluxOU-Cmr). This strain is called JAF78 (18), and the region of the V. harveyi chromosome encompassed by the deletion is shown in Fig. 1. A luxU null mutation was constructed by introduction of a Knr cassette at codon 85 of the cloned luxU gene. We incorporated this luxU::Knr null mutation by allelic replacement onto the V. harveyi chromosome by using V. harveyi JAF78 as the recipient. The gene replacement procedure used to construct the luxU::Knr mutation also restored the wild-type luxO gene in the chromosome.
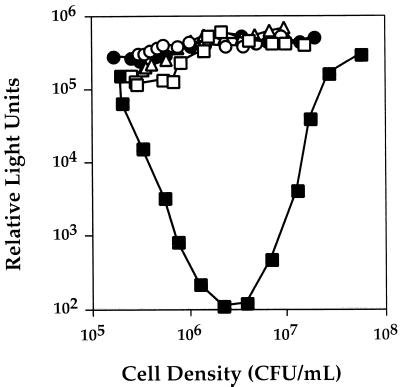
The Lux phenotypes of wild-type V. harveyi BB120, ΔluxOU-Cmr strain JAF78, luxU::Knr null strain JAF536, and luxO D47A missense mutant strain JAF483 are shown in Fig. 2. Overnight cultures of the different V. harveyi strains were diluted 1:5,000 into fresh medium, and light production was subsequently measured as the cells grew. In Fig. 2, the data are reported as relative light units, which is light emission per cell. Immediately after dilution of the wild-type strain (closed squares), light emission per cell decreased dramatically (over 1,000-fold). The decrease in light production occurred because dilution of the wild-type V. harveyi culture caused a reduction in the level of extracellular autoinducers to below the threshold stimulatory concentrations. The absence of the autoinducer signals results in repression of transcription of the luciferase structural operon, so light production drops. However, as the diluted wild-type culture grew, the cells released endogenously synthesized autoinducers. At a critical cell density, which corresponds to the buildup of the minimum stimulatory autoinducer concentrations in the medium, induction of expression of the luxCDABEGH operon occurred. An exponential increase in light emission followed, and light production of the wild-type culture increased to the predilution level.
FIG. 2.
LuxU is involved in quorum sensing in V. harveyi. Cultures of wild-type and mutant V. harveyi strains were grown overnight in AB medium at 30°C. The strains were diluted 1:5,000 into fresh AB medium, and light production was subsequently measured at 30-min intervals during growth of the cultures. Symbols: ■, BB120 (wild type); •, JAF78 (ΔluxOU-Cmr); ▵, JAF483 (luxO D47A); □, JAF536 (luxU::Knr); ○, JAF553 (luxU H58A). Relative light units are defined as 103 counts per minute per milliliter divided by CFU per milliliter.
Figure 2 also shows the phenotype of ΔluxOU-Cmr strain JAF78 (closed circles). The deletion strain produced light constitutively. We have reported previously that in the presence of wild-type luxU, missense mutations that encode nonphosphorylatable LuxO proteins result in a null phenotype identical to that of JAF78 (18). An example of such a strain is also shown in Fig. 2. V. harveyi JAF483 contains a point mutation in luxO (luxO D47A) that renders the LuxO protein incapable of phosphorylation and, therefore, incapable of repression of Lux (open triangles). Strain JAF483 is luxU+. Furthermore, Fig. 2 shows that luxO+ luxU null V. harveyi JAF536 also produced light constitutively (open squares). Therefore, null mutations in either luxO or luxU completely abolished the density-dependent expression of the luxCDABEGH operon and resulted in maximal light production.
LuxU is homologous to other two-component phosphorelay proteins.
The luxU gene was sequenced, and the putative ORF was translated. Both the DNA and protein sequences are shown in Fig. 3A. The predicted LuxU protein is 114 amino acids. The organization of the luxO and luxU genes shows that the luxO termination codon and the initiation codon of luxU overlap; this is a hallmark of translationally coupled proteins.
FIG. 3.
Sequence analysis of luxU and alignment of LuxU with other phosphorelay proteins. The nucleotide and deduced amino acid sequences of LuxU are shown in panel A. The initiation and termination codons of the LuxU ORF are in boldface. The termination codon for the LuxO ORF is underlined. Panel B shows an alignment of a portion of LuxU with other phosphorelay modules over the 20-amino-acid conserved phosphorelay region (3, 23–27, 31, 35, 39, 41, 44). The most highly conserved residues in this region are in boldface. A theoretical consensus sequence is shown in the bottom line of the alignment (24). In the consensus sequence, the conserved histidine is represented by H, the letter h denotes a hydrophobic residue, and a plus sign denotes a positively charged residue.
Figure 3B shows an alignment of the region surrounding each histidine residue of LuxU with other identified phosphorelay domains. A theoretical phosphorelay consensus sequence has been derived (24) and is shown in the figure. Only weak homology over a 20-amino-acid span has been observed between phosphorelay proteins, indicating that the regions encompassing the His 2 residues could correspond to some structural motif that is critical for function, but the remainder of these domains can tolerate variations in amino acid composition. The figure shows that His 103 of LuxU is not located in a region similar to other phosphorelay active sites, while the region around His 58 does resemble other identified phosphorelay active sites. The alignment suggests that His 58 could be the site of phosphorylation, i.e., His 2, in LuxU. Interestingly, except for LuxU, all of the bacterial phosphorelay proteins containing this region of similarity exist as internal domains of hybrid sensor kinase proteins. The only other known detached bacterial phosphorelay protein is Spo0B of Bacillus subtilis (12). Spo0B is not shown in Fig. 3B because it does not contain any sequence similar to the hypothetical phosphorelay consensus sequence. A detached phosphorelay protein, called Ypd1, has been identified in the yeast Saccharomyces cerevisiae (35). Ypd1 is involved in a two-component signal transduction system that regulates the response of S. cerevisiae to osmolarity. As shown in Fig. 3B, Ypd1 possesses a recognizable phosphorelay consensus region.
LuxU couples signalling events from the sensors LuxN and LuxQ to the response regulator LuxO.
The alignment shown in Fig. 3B suggests that LuxU could be a phosphorelay protein. If it is, we hypothesize that it acts downstream of the sensors LuxN and LuxQ and upstream of LuxO. We designed genetic epistasis tests to provide further evidence of the position and function of LuxU in the quorum-sensing hierarchy of V. harveyi.
If LuxU acts downstream of the sensors LuxN and LuxQ, we predict that luxU mutations should be epistatic to LuxN and LuxQ mutations. Strain JAF549 carries a luxN L166R mutation, and this mutation confers a dark, autoinducer-blind phenotype on V. harveyi (18a). LuxN L166R has constitutive kinase activity because it does not respond to AI-1. We have reported that in wild-type cells, the interaction of AI-1 with LuxN stimulates a switch in activity in the protein from kinase to phosphatase. The LuxN L166R protein apparently does not undergo this switch. Therefore, in the luxN L166R mutant, LuxO is continuously phosphorylated and luxCDABEGH transcription is constitutively repressed. The luxN L166R mutation is dominant to wild-type luxQ. If, as we propose, LuxU is situated in the signalling circuit after the sensors and before LuxO, and LuxU acts to relay a signal from LuxN (and LuxQ) to LuxO, phosphorylation of LuxO by LuxN L166R should be dependent on LuxU. Therefore, a luxU null allele should be epistatic to the luxN L166R allele.
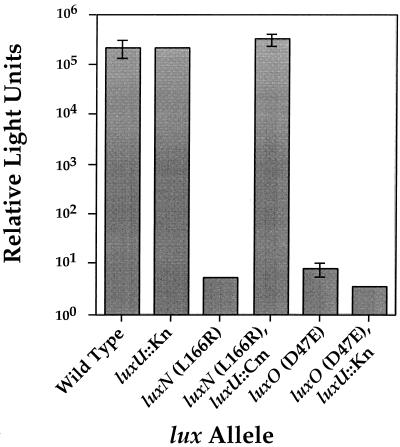
In the experiment presented in Fig. 4, wild-type V. harveyi and different V. harveyi mutant strains containing mutations in luxN, luxO, or luxU or combinations of these mutations were grown to high cell density and the light emission of each strain was subsequently measured and compared. The figure shows that the wild-type V. harveyi strain emitted over 105 relative light units at high cell density. Under these conditions, our results suggest that LuxO exists predominantly in the unphosphorylated form in wild-type cells, and maximal light is produced because unphosphorylated LuxO cannot repress lux expression. The luxU::Knr null strain JAF536, which produces light constitutively, emitted roughly 105 relative light units at high cell density, similar to the wild type. V. harveyi JAF549, which has the luxN L166R allele on the chromosome, emits almost no light (<101 relative light units). However, V. harveyi JAF558, containing both luxN L166R and luxU::Cmr, produced maximal light (>105 relative light units) at high cell density. This result shows that the luxU mutation is epistatic to the luxN mutation and suggests that LuxU functions downstream of LuxN (and, by analogy, LuxQ).
FIG. 4.
Epistasis analysis to determine the Lux signalling pathway. V. harveyi cultures were grown overnight in AB medium and diluted 1:5,000 on the next morning. The diluted cultures were subsequently grown to a high cell density (∼108 CFU/ml). The light production per cell of each strain was quantified as described in the legend to Fig. 2. The strains are BB120 (wild type), JAF536 (luxU::Knr), JAF549 (luxN L166R), JAF558 (luxN L166R, luxU::Cmr), JAF548 (luxO D47E), and JAF552 (luxO D47E luxU::Knr).
In earlier work, we constructed and analyzed LuxO missense mutations that locked LuxO into forms simulating phospho-LuxO (P-LuxO) and unphosphorylated LuxO (18). Mutations rendering LuxO in a form mimicking P-LuxO were constitutive repressors and resulted in a dark phenotype. In contrast, mutations rendering LuxO incapable of phosphorylation inactivated the protein and resulted in a constitutive bright phenotype. We further demonstrated that in the absence of both the LuxN and LuxQ sensors, LuxO could not be phosphorylated, so light was produced constitutively. All of the P-LuxO-like mutations we constructed were phosphorylation independent, because they conferred a dark phenotype on V. harveyi when analyzed in a luxN luxQ double sensor mutant (18).
Here we used one of these P-LuxO-like mutations (luxO D47E) in an epistasis analysis to test if LuxU is positioned upstream of LuxO in the Lux signalling relay. If it is, mutations in LuxO should be epistatic to mutations in LuxU. If this is the case, we predict that a LuxU null (constitutive bright) mutation in combination with the LuxO D47E repressing (dark) mutation would result in a dark phenotype. Alternatively, if LuxU acts after LuxO in the circuit, then we predict that light would be produced constitutively when we combined a LuxU null mutation with the repressing LuxO D47E mutation. Our results are shown in Fig. 4.
V. harveyi JAF548 contains the luxO D47E mutation. Strain JAF548 repressed lux constitutively because this mutation locks LuxO in the P-LuxO-like form (18). Figure 4 shows that JAF548 produced almost no light (∼101 relative light units). In the epistasis test, double mutant V. harveyi JAF552, with both luxO D47E and luxU::Knr on the chromosome, also produced less than 101 relative light units. This result shows that the repressing LuxO D47E mutation is epistatic to a LuxU null mutation, indicating that LuxU can be placed upstream of LuxO in the regulatory hierarchy. Therefore, the path of the Lux phosphorylation cascade is LuxN-LuxQ to LuxU to LuxO.
His 58 is required for LuxU function.
If LuxU is a phosphorelay protein, then we predict that its activity should require a His residue. The translated sequence in Fig. 3A shows that there are only two His residues in LuxU, and Fig. 3B indicates that only His 58 is situated appropriately to act as His 2 in a phosphorelay protein. By using SDM, we changed His 58 and His 103 to alanine residues and analyzed the resulting Lux phenotypes.
Figure 2 shows that similar to the luxU::Knr null mutation (open squares, strain JAF536), introduction of the luxU (H58A) allele onto the V. harveyi chromosome also resulted in maximal constitutive lux expression, suggesting that His 58 is required for LuxU function (open circles, strain JAF553). Presumably, LuxU lacking His 2 is defective in phosphotransfer to LuxO because LuxU cannot undergo phosphorylation. In contrast, alteration of His 103 to Ala had no effect on Lux signalling, suggesting that His 103 is not involved in the phosphorelay (data not shown).
DISCUSSION
Quorum sensing in V. harveyi is regulated by a complex, multicircuit, two-component signalling relay. Two parallel density-sensing systems produce and respond to distinct autoinducer signals and subsequently channel this environmental information to a shared response regulator protein called LuxO. In this report, we have identified and analyzed LuxU, another member of this intercellular signalling circuit. Our data suggest that LuxU is a phosphorelay protein and that LuxU is responsible for collecting sensory information from the sensor kinases LuxN and LuxQ and transducing a signal to the response regulator LuxO.
We constructed a luxU null mutation by insertion of a Knr cassette at codon 85 of the cloned luxU gene, and we introduced this mutation into the V. harveyi chromosome by allelic replacement of a luxU deletion. The luxU::Knr null strain was incapable of density-dependent regulation of lux expression and, instead, produced maximal light at all cell densities, regardless of the autoinducer concentration (Fig. 2). The luxU null phenotype is identical to the luxO null phenotype. Genetic analysis indicates that the response regulator protein LuxO represses lux expression when phosphorylated at Asp 47. Missense mutations in LuxO that lock the protein into an unphosphorylated state are also sufficient to cause the LuxO null phenotype (18). Our interpretation of the LuxU null phenotype is that no repression of lux expression occurs because LuxO cannot be phosphorylated in the absence of LuxU.
Sequence analysis of the luxU gene predicts a small protein of 114 amino acids (Fig. 3A). The majority of the protein does not share significant similarity to any other protein in the GenBank database. However, a 20-amino-acid region of LuxU does have some degree of similarity to other proteins or domains of proteins that have been reported to function as phosphorelay proteins in two-component signalling systems (Fig. 3B). These proteins include BvgS (43), ArcB (24, 42), and yeast protein Ypd1 (35), among others. In most cases, phosphorelays exist as internal modules of two-component hybrid sensor kinases. Besides LuxU, only two other detached two-component phosphorelay proteins have been identified, Spo0B of B. subtilis (12) and Ypd1 of S. cerevisiae (35). We do not know if there is any significance to the molecular organization of these different enzymatic functions. Many other possible domain organizations combining the four critical residues His 1, Asp 1, His 2, and Asp 2 could exist. The genetic organization of luxO and luxU suggests that these proteins are coupled transcriptionally and translationally. Conceivably, at some point in evolution, LuxO and LuxU could have been a single polypeptide. Until additional phosphorelay proteins or modules are identified and their functions are analyzed, the consequence, if any, for signal transduction to a system possessing an intramolecular phosphorelay domain compared to a system containing a detached phosphorelay protein remains unknown.
Our epistasis results suggest that LuxU is positioned in the Lux signalling pathway downstream of the LuxN and LuxQ sensors and upstream of the LuxO response regulator. Figure 4 shows that a luxU null mutation is epistatic to a dark luxN mutation, while a luxO dark mutation is epistatic to a luxU null mutation. And a V. harveyi strain harboring the luxU H58A missense mutation expressed luminescence constitutively, similarly to a LuxO null strain (Fig. 2). This result suggests that the His 58 residue of LuxU is critical for phosphorelay function. Apparently, without His 58 of LuxU, LuxO cannot be phosphorylated in V. harveyi. The LuxU His 58 residue is located appropriately to act as His 2 in the phosphorelay.
Because the phenotype of V. harveyi JAF553 carrying the LuxU H58A mutation is identical to a LuxU null phenotype, this could indicate that the LuxU H58A protein is not expressed or is unstable. We have recently undertaken a biochemical analysis of the Lux two-component system with the aim of demonstrating that LuxU is phosphorylated on His 58. These experiments are in the early stages, so we cannot assert unequivocally that the phenotype shown by strain JAF553 is due to mutation of His 58. However, the genetic evidence outlined in this report has led us to suspect that this is the case.
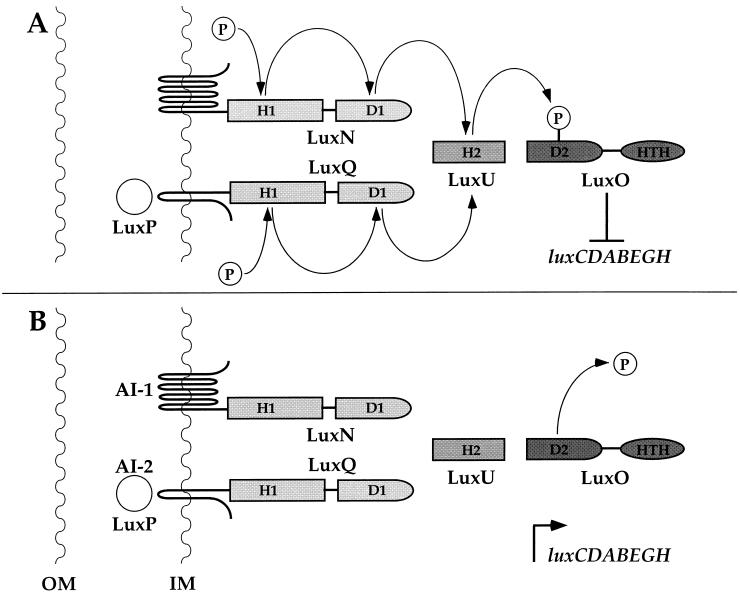
A model for regulation of quorum sensing in V. harveyi by our hypothesized phosphorylation-dephosphorylation cascade is presented in Fig. 5. Panel A shows the low cell density situation. We have already reported that in the absence of autoinducers, our genetic data indicate that the sensors LuxN and LuxQ possess kinase activity (18). Autophosphorylation of the conserved His 1 residues on LuxN and LuxQ occurs, and this phosphoryl group is subsequently transferred intramolecularly to the corresponding Asp 1 residues of the hybrid sensors. The data presented here imply that the next phosphorylation reaction is intermolecular, from the Asp 1 residues of LuxN and LuxQ to His 58 of LuxU. The His 58 residue of LuxU corresponds to His 2 in the signalling nomenclature. Finally, transfer from LuxU to LuxO at Asp 47 (i.e., Asp 2) occurs. Our model predicts that LuxO-phosphate is the active lux repressor. Under these conditions, luxCDABEGH is not transcribed and no light is produced.
FIG. 5.
Model for the regulation of quorum sensing in V. harveyi. (A) Genetic analysis (18) suggests that under conditions of low cell density, in the absence of autoinducers, the two hybrid sensor kinases LuxN and LuxQ autophosphorylate at conserved histidine residues (H1). Intramolecular phosphotransfer from the sensor kinase domains to conserved aspartate residues (D1) in the response regulator domains of the hybrid proteins occurs next. Intermolecular transfer from D1 of both LuxN and LuxQ to His 58 (H2) of the phosphorelay protein LuxU occurs, with subsequent phosphotransfer to the conserved Asp 47 residue (D2) of the response regulator protein LuxO. Our evidence suggests that phosphorylation of LuxO activates its repressor function, luxCDABEGH transcription is repressed, and no light is produced. (B) Under conditions of high cell density, the presence of autoinducers stimulates the LuxN and LuxQ sensors to switch from kinases to phosphatases. This switch ultimately results in dephosphorylation of LuxO and inactivation of its repressor function. Dephosphorylation could occur by several different mechanisms, and these terminal reactions have not been characterized. Inactivation of LuxO allows transcription of the luxCDABEGH operon and light production. Outer membrane and inner membrane are abbreviated OM and IM, respectively. HTH, helix-turn-helix.
As the V. harveyi culture grows, autoinducers AI-1 and AI-2 are produced and released by the bacteria and accumulate in the extracellular environment (panel B). At a critical autoinducer concentration, which reflects the population density of the culture, the sensors LuxN and LuxPQ presumably bind their cognate autoinducers AI-1 and AI-2, respectively. Interaction of the sensors with the autoinducers is proposed to stimulate the sensors to switch activities from kinase to phosphatase (18). The phosphatase activity of the sensors ultimately results in dephosphorylation of LuxO, which inactivates the repressor. Our data suggest that dephosphorylation of the LuxO response regulator domain results in a conformational change in the protein. This change promotes an interaction of the unphosphorylated response regulator domain and the LuxO DNA binding domain (18). This interaction inhibits the DNA binding activity of LuxO, so luxCDABEGH is transcribed and light is produced.
We do not know where the LuxN and LuxQ phosphatases act. Conceivably, the phosphatases could act on His 1, Asp 1, His 2, Asp 2, or all of these residues. Furthermore, there is no a priori reason to suggest that the two systems are completely symmetrical. One or both phosphatases could act at more than one phosphorylated residue. Analogous to what has been suggested in the B. subtilis system (22), if phosphate could be drained from several sites in the Lux system, it could enable V. harveyi to rapidly and efficiently inactivate the LuxO repressor in response to an autoinducer.
The role of the LuxU phosphorelay could be twofold. First, when V. harveyi exists under conditions in which light production is not beneficial, LuxU could act as a reservoir for the collection of sensory information from many sources. In this model, LuxU would be phosphorylated by several sensors and transfer this information to LuxO to form the P-LuxO repressor as needed. Our evidence suggests that at least two kinases, LuxN and LuxQ, relay a signal to LuxO via LuxU. Other kinases could also act on LuxU. Besides cell density, several other environmental cues are known to influence the expression of luminescence in V. harveyi. For example, light production by V. harveyi is sensitive to the concentrations of iron, oxygen, and carbohydrate in the medium. It is not known how these cues are detected. Conceivably, LuxU could be the point at which all of this environmental information converges. A second potential role for LuxU could be in the rapid inactivation of the repressor P-LuxO. Increasing the number of phosphorylated residues in signalling systems from two in standard two-component systems (i.e., EnvZ and OmpR) to four in hybrid two-component systems (i.e., BvgS and BvgA) and to six in the Lux multichannel two-component system may enable bacteria to more efficiently react to fluctuations in environmental conditions. If the LuxN and LuxQ phosphatases act at all possible sites, the Lux system would have six sinks from which to drain phosphate when rapid elimination of the P-LuxO repressor is necessary. In this model, inclusion of LuxU in the circuit could increase the responsiveness of the system to changes in the environment.
ACKNOWLEDGMENTS
This work was supported by National Science Foundation grant MCB-9506033.
We thank T. J. Silhavy and B. Lilley for many informative discussions.
REFERENCES
- 1.Altschul S F, Thomas L M, Alejandro A S, Jinghui Z, Zheng Z, Webb M, David J L. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Appleby J L, Parkinson J S, Bourret R B. Signal transduction via the multi-step phosphorelay: not necessarily a road less traveled. Cell. 1996;86:845–848. doi: 10.1016/s0092-8674(00)80158-0. [DOI] [PubMed] [Google Scholar]
- 3.Arico B, Miller J F, Roy C, Stibitz S, Monack D, Falkow S, Gross R, Rappouli R. Sequences required for expression of Bordetella pertussis virulence factors share homology with prokaryotic signal transduction proteins. Proc Natl Acad Sci USA. 1989;86:6671–6675. doi: 10.1073/pnas.86.17.6671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arikan E, Kulkarni M S, Thomas D C, Sancar A. Sequences of the Escherichia coli uvrB gene and protein. Nucleic Acids Res. 1986;14:2637–2650. doi: 10.1093/nar/14.6.2637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bainton N J, Bycroft B W, Chhabra S, Stead P, Gledhill L, Hill P J, Rees C E D, Winson M K, Salmond G P C, Stewart G S A B, Williams P. A general role for the lux autoinducer in bacterial cell signalling: control of antibiotic biosynthesis in Erwinia. Gene. 1992;116:87–91. doi: 10.1016/0378-1119(92)90633-z. [DOI] [PubMed] [Google Scholar]
- 6.Bassler B L, Greenberg E P, Stevens A M. Cross-species induction of luminescence in the quorum-sensing bacterium Vibrio harveyi. J Bacteriol. 1997;179:4043–4045. doi: 10.1128/jb.179.12.4043-4045.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bassler B L, Silverman M R. Intercellular communication in marine Vibrio species: density-dependent regulation of the expression of bioluminescence. In: Hoch J A, Silhavy T J, editors. Two-component signal transduction. Washington, D.C: American Society for Microbiology; 1995. pp. 431–445. [Google Scholar]
- 8.Bassler B L, Wright M, Showalter R E, Silverman M R. Intercellular signalling in Vibrio harveyi: sequence and function of genes regulating expression of luminescence. Mol Microbiol. 1993;9:773–786. doi: 10.1111/j.1365-2958.1993.tb01737.x. [DOI] [PubMed] [Google Scholar]
- 9.Bassler B L, Wright M, Silverman M R. Multiple signalling systems controlling expression of luminescence in Vibrio harveyi: sequence and function of genes encoding a second sensory pathway. Mol Microbiol. 1994;13:273–286. doi: 10.1111/j.1365-2958.1994.tb00422.x. [DOI] [PubMed] [Google Scholar]
- 10.Bassler B L, Wright M, Silverman M R. Sequence and function of luxO, a negative regulator of luminescence in Vibrio harveyi. Mol Microbiol. 1994;12:403–412. doi: 10.1111/j.1365-2958.1994.tb01029.x. [DOI] [PubMed] [Google Scholar]
- 11.Beringer J E, Beynon J L, Buchanan-Wollaston A V, Johnston A W B. Transfer of the drug resistance transposon Tn5 to Rhizobium. Nature. 1978;276:633–634. [Google Scholar]
- 12.Burbulys D K, Trach A, Hoch J A. The initiation of sporulation in Bacillus subtilis is controlled by a multicomponent phosphorelay. Cell. 1991;64:545–552. doi: 10.1016/0092-8674(91)90238-t. [DOI] [PubMed] [Google Scholar]
- 13.Cao J, Meighen E A. Purification and structural identification of an autoinducer for the luminescence system of Vibrio harveyi. J Biol Chem. 1989;264:21670–21676. [PubMed] [Google Scholar]
- 14.Cubo M T, Economou A, Murphy G, Johnston A W B, Downie J A. Molecular characterization and regulation of the rhizosphere-expressed genes rhiABCR that can influence nodulation of Rhizobium leguminosarum biovar viciae. J Bacteriol. 1992;174:4026–4035. doi: 10.1128/jb.174.12.4026-4035.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ditta G, Stanfield S, Corbin D, Helinski D. Broad host range DNA cloning system for gram-negative bacteria: construction of a gene bank of Rhizobium meliloti. Proc Natl Acad Sci USA. 1980;77:7347–7351. doi: 10.1073/pnas.77.12.7347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Engebrecht J, Silverman M R. Identification of genes and gene products necessary for bacterial bioluminescence. Proc Natl Acad Sci USA. 1984;81:4154–4158. doi: 10.1073/pnas.81.13.4154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fellay R, Frey J, Krisch H. Interposon mutagenesis of soil and water bacteria: a family of DNA fragments designed for in vitro insertional mutagenesis of Gram-negative bacteria. Gene. 1987;52:147–154. doi: 10.1016/0378-1119(87)90041-2. [DOI] [PubMed] [Google Scholar]
- 18.Freeman J A, Bassler B L. A genetic analysis of the function of LuxO, a two-component response regulator involved in quorum sensing in Vibrio harveyi. Mol Microbiol. 1999;31:665–678. doi: 10.1046/j.1365-2958.1999.01208.x. [DOI] [PubMed] [Google Scholar]
- 18a.Freeman, J. A., et al. Unpublished data.
- 19.Friedman A, Long S R, Brown S E, Buikema W J, Ausubel F. Construction of a broad host range cosmid cloning vector and its use in the genetic analysis of Rhizobium mutants. Gene. 1982;18:289–296. doi: 10.1016/0378-1119(82)90167-6. [DOI] [PubMed] [Google Scholar]
- 20.Fuqua W C, Winans S C, Greenberg E P. Quorum sensing in bacteria: the LuxR-LuxI family of cell density-responsive transcriptional regulators. J Bacteriol. 1994;176:269–275. doi: 10.1128/jb.176.2.269-275.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Greenberg E P, Hastings J W, Ulitzur S. Induction of luciferase synthesis in Beneckea harveyi by other marine bacteria. Arch Microbiol. 1979;120:87–91. [Google Scholar]
- 22.Hoch J A. Control of cellular development in sporulating bacteria by the phosphorelay two-component signal transduction system. In: Hoch J A, Silhavy T J, editors. Two-component signal transduction. Washington, D.C: American Society for Microbiology; 1995. pp. 129–144. [Google Scholar]
- 23.Hrabak E M, Willis D K. The lemA gene required for pathogenicity of Pseudomonas syringae pv. syringae on bean is a member of a family of two-component regulators. J Bacteriol. 1992;174:3011–3020. doi: 10.1128/jb.174.9.3011-3020.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ishige K, Nagasawa S, Tokishita S, Mizuno T. A novel device of bacterial signal transducers. EMBO J. 1994;13:5195–5202. doi: 10.1002/j.1460-2075.1994.tb06850.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Iuchi S, Matsuda Z, Fujiwara T, Lin E C C. The arcB gene of Escherichia coli encodes a sensor-regulator protein for anaerobic repression of the arc modulon. Mol Microbiol. 1990;4:715–727. doi: 10.1111/j.1365-2958.1990.tb00642.x. [DOI] [PubMed] [Google Scholar]
- 26.Jourlin C, Bengrine A, Chippaux M, Mejean V. An unorthodox sensor protein (TorS) mediates the induction of the tor structural genes in response to trimethylamine N-oxide in Escherichia coli. Mol Microbiol. 1996;20:1297–1306. doi: 10.1111/j.1365-2958.1996.tb02648.x. [DOI] [PubMed] [Google Scholar]
- 27.Kehoe D M, Grossman A R. New classes of mutants in complementary chromatic adaptation provide evidence for a novel four-step phosphorelay system. J Bacteriol. 1997;179:3914–3921. doi: 10.1128/jb.179.12.3914-3921.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Martin M, Showalter R, Silverman M R. Identification of a locus controlling expression of luminescence genes in Vibrio harveyi. J Bacteriol. 1989;171:2406–2414. doi: 10.1128/jb.171.5.2406-2414.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Meighen E A. Molecular biology of bacterial bioluminescence. Microbiol Rev. 1991;55:123–142. doi: 10.1128/mr.55.1.123-142.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Michael S F. Mutagenesis by incorporation of a phosphorylated oligo during PCR amplification. BioTechniques. 1994;16:410–412. [PubMed] [Google Scholar]
- 31.Nagasawa S, Ishige K, Mizuno T. Novel members of the two-component signal transduction genes in Escherichia coli. J Biochem. 1993;114:350–357. doi: 10.1093/oxfordjournals.jbchem.a124180. [DOI] [PubMed] [Google Scholar]
- 32.Nealson K H, Platt T, Hastings J W. Cellular control of the synthesis and activity of the bacterial luminescent system. J Bacteriol. 1970;104:313–322. doi: 10.1128/jb.104.1.313-322.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Passador L, Cook J M, Gambello M J, Rust L, Iglewski B H. Expression of Pseudomonas aeruginosa virulence genes requires cell to cell communication. Science. 1993;260:1127–1130. doi: 10.1126/science.8493556. [DOI] [PubMed] [Google Scholar]
- 34.Piper K R, Beck von Bodman S, Farrand S K. Conjugation factor of Agrobacterium tumefaciens regulates Ti plasmid transfer by autoinduction. Nature. 1993;362:448–450. doi: 10.1038/362448a0. [DOI] [PubMed] [Google Scholar]
- 35.Posas F, Wurgler-Murphy S M, Maeda T, Witten E A, Thai T C, Saito H. Yeast HOG1 MAP kinase cascade is regulated by a multistep phosphorelay mechanism in the SLN1-YPD1-SSK1 “two-component” osmosensor. Cell. 1996;86:865–875. doi: 10.1016/s0092-8674(00)80162-2. [DOI] [PubMed] [Google Scholar]
- 36.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 37.Sanger F, Nicklen S, Coulson A R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Showalter R E, Martin M O, Silverman M R. Cloning and nucleotide sequence of luxR, a regulatory gene controlling luminescence in Vibrio harveyi. J Bacteriol. 1990;172:2946–2954. doi: 10.1128/jb.172.6.2946-2954.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stevens A M, Sanders J M, Shoemaker N B, Salyers A A. Genes involved in production of plasmidlike forms by a Bacteroides conjugal chromosomal element share amino acid homology with two-component regulatory systems. J Bacteriol. 1992;174:2935–2942. doi: 10.1128/jb.174.9.2935-2942.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Swartzman E, Silverman M R, Meighen E A. The luxR gene product of Vibrio harveyi is a transcriptional activator of the lux promoter. J Bacteriol. 1992;174:7490–7493. doi: 10.1128/jb.174.22.7490-7493.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tang J L, Liu Y N, Barber C E, Dow J M, Wooten J C, Daniels M J. Genetic and molecular analysis of a cluster of rpf genes involved in positive regulation of synthesis of extracellular enzymes and polysaccharide in Xanthomonas campestris pathovar campestris. Mol Gen Genet. 1991;226:409–417. doi: 10.1007/BF00260653. [DOI] [PubMed] [Google Scholar]
- 42.Tsuzuki M, Ishige K, Mizuno T. Phosphotransfer circuity of the putative multi-signal transducer, ArcB, of Escherichia coli: in vitro studies with mutants. Mol Microbiol. 1995;18:953–962. doi: 10.1111/j.1365-2958.1995.18050953.x. [DOI] [PubMed] [Google Scholar]
- 43.Uhl M A, Miller J F. Integration of multiple domains in a two-component sensor protein: the Bordetella pertussis BvgAS phosphorelay. EMBO J. 1996;15:1028–1036. [PMC free article] [PubMed] [Google Scholar]
- 44.Utsumi R, Katayama S, Taniguchi M, Horie T, Ikeda M, Igaki S, Nakagawa H, Miwa A, Tanebe H, Noda M. Newly identified genes involved in the signal transduction of Escherichia coli K-12. Gene. 1994;140:73–77. doi: 10.1016/0378-1119(94)90733-1. [DOI] [PubMed] [Google Scholar]