Abstract
Archaea-specific radA primers were used with PCR to amplify fragments of radA genes from 11 cultivated archaeal species and one marine sponge tissue sample that contained essentially an archaeal monoculture. The amino acid sequences encoded by the PCR fragments, three RadA protein sequences previously published (21), and two new complete RadA sequences were aligned with representative bacterial RecA proteins and eucaryal Rad51 and Dmc1 proteins. The alignment supported the existence of four insertions and one deletion in the archaeal and eucaryal sequences relative to the bacterial sequences. The sizes of three of the insertions were found to have taxonomic and phylogenetic significance. Comparative analysis of the RadA sequences, omitting amino acids in the insertions and deletions, shows a cladal distribution of species which mimics to a large extent that obtained by a similar analysis of archaeal 16S rRNA sequences. The PCR technique also was used to amplify fragments of 15 radA genes from uncultured natural sources. Phylogenetic analysis of the amino acid sequences encoded by these fragments reveals several clades with affinity, sometimes only distant, to the putative RadA proteins of several species of Crenarcheota. The two most deeply branching archaeal radA genes found had some amino acid deletion and insertion patterns characteristic of bacterial recA genes. Possible explanations are discussed. Finally, signature codons are presented to distinguish among RecA protein family members.
DNA repair and recombination are fundamental molecular processes that were most likely present in the earliest life. Supporting this view is the observation that Bacteria, Archaea, and Eucarya all have phylogenetically related DNA repair and recombination genes that encode a crucial protein involved in synapsing two parental DNA molecules. The first and archetypal member of this protein family was identified by mutations in the recA gene of the bacterium Escherichia coli (5); therefore, the protein it encodes is called RecA. Homologues of the E. coli recA gene have now been found in all bacterial divisions in which they have been sought (7, 12, 20). Two budding-yeast genes, RAD51 and DMC1, have been recognized to be homologues of bacterial recA genes (22, 26). Since then, homologues of these two genes have been found in all Eucarya species tested (24). Finally, Sandler et al. (21) identified genes from three archaeal genera whose putative proteins are similar to RecA proteins but are even more similar to the eucaryal Rad51 and Dmc1 proteins. These genes and proteins are called radA and RadA, respectively.
There were two objectives to this research: a protein structure-function objective and a phylogenetic objective. The protein structure-function objective was to determine how consistent the differences are between the RecA group of proteins and the RadA-Rad51-Dmc1 group observed by Sandler et al. (21) and Brendel et al. (3). These authors noted that the archaeal RadA proteins provided additional information that allowed a better alignment of the core regions of eucaryl Rad51 and Dmc1 proteins with the bacterial RecA proteins. Existence of a homologous core region in all of the RecA, Rad51, and Dmc1 proteins was already known (22). Also known was the fact that, relative to the core, the Rad51 and Dmc1 proteins have longer amino-terminal ends and the bacterial RecA proteins have longer carboxy-terminal ends (22). Unclear, however, was the alignment of amino acids within the core. Sandler et al. (21) and Brendel et al. (3) contended that the cores of RadA-Rad51-Dmc1 proteins have four highly conserved insertions and one deletion relative to the cores of the RecA proteins. Furthermore Sandler et al. (21) stated that there might be functional significance in the locations of the insertions. They located these insertions in the context of the X-ray crystal structure of E. coli RecA protein complexed with ADP and found them to be on the outside of the protein, away from the putative DNA binding site. This is consistent with the insertions not compromising the synaptase function of RadA while adding features that potentially might interact with accessory proteins.
Phylogenetic relationships among RecA proteins from greater than 65 different species of bacteria have been the focus of several studies (7, 12, 13, 17). The trees are robust and correlate well with the trees formed by 16S rRNAs from the corresponding bacteria, leading to the conclusion that RecA is a useful genetic marker for reconstructing bacterial phylogeny. Others have found that the eucaryal Dmc1 and Rad51 proteins are not good phylogenetic markers because they appear to have unequal rates of evolution (24). Thus, we wanted to determine if the RadA proteins would be useful in deciphering the phylogeny of the Archaea. In addition, we wanted to test the hypothesis of Sandler et al. (21) that there might be taxonomic significance in the number of amino acids present in one or more of the insertions.
MATERIALS AND METHODS
Archaeal genomic DNA.
DNA from cultured archaea was obtained for the strains listed in Table 1. Environmental DNAs were obtained from three different hot springs in Yellowstone National Park—Obsidian Pool, hot pool N10 in the Norris Geyser Basin, and hot pool O1 in the White Creek area—as previously described (11).
TABLE 1.
Abbreviations and accession numbers of sequences
| Protein | Species or isolate | Abbreviation in Fig. 1–4 | Accession no.
|
|
|---|---|---|---|---|
| RecA-like protein | 16S RNA | |||
| Cultured | ||||
| Euryarchaeota | ||||
| RadA | Methanococcus jannaschii | Mc.jan | U45311 | M59126 |
| Methanococcus voltaeb | Mc.vol | AF090200 | U38461 | |
| Methanococcus maripaludisb,e | Mc.mar | AF090204 | U38486 | |
| Methanococcus vannieliib | Mc.van | AF090203 | M32222 | |
| Methansarcinia mazeib | Ms.maz | AF090201 | U20151 | |
| Methanothermus fervidusb,e | Mt.fer | AF090202 | M32222 | |
| Picrophilius torridusb | Pp.tor | AF090205 | Unknown | |
| Picrophilus oshimaeb,e | Pp.osh | AF090206 | X84901 | |
| Haloferax volcanii | Hf.vol | U45312 | K00421 | |
| Halobacterium halobiumc | Hb.hal | AF090196 | M11583 | |
| Haloarcula hispanicaa | Ha.his | AF090199 | U68541 | |
| Archeoglobus fulgidusb | Ag.ful | AF090198 | Y00275 | |
| Crenarcheota | ||||
| Sulfolobus solfataricus | Sul.sol | U45310 | X90478 | |
| Sulfolobus shibataeb,f | Sul.shi | AF090207 | M32504 | |
| Sulfolobus acidocaldariusb,f | Sul.aci | AF090208 | D14876 | |
| Pyrobacculum aerophiliumb | Pb.aer | Unknown | L07510 | |
| Cenarchaeum symbiosuma | Ca.sym | AF090197 | U51469 | |
| Uncultured | ||||
| Norris Geyser Basin 1b | NGB#1 | AF090209 | nag | |
| Norris Geyser Basin 4b | NGB#4 | AF090210 | na | |
| Norris Geyser Basin 6b | NGB#6 | AF090211 | na | |
| Norris Geyser Basin 8b | NGB#8 | AF090212 | na | |
| Norris Geyser Basin 9b | NGB#9 | AF090213 | na | |
| Norris Geyser Basin 13b | NGB#13 | AF090214 | na | |
| Norris Geyser Basin 14b | NGB#14 | AF090215 | na | |
| Norris Geyser Basin 16b | NGB#16 | AF090216 | na | |
| White Creek 28b | WC#28 | AF090217 | na | |
| Obsidian Pool 1b | OP#1 | AF090218 | na | |
| Obsidian Pool 3b | OP#3 | AF090219 | na | |
| Obsidian Pool 4b | OP#4 | AF090220 | na | |
| Obsidian Pool 6b | OP#6 | AF090221 | na | |
| Obsidian Pool 9b | OP#9 | AF090222 | na | |
| Obsidian Pool 10b | OP#10 | AF090223 | na | |
| Antarctica 17d,f | Ant#17 | AF090224 | na | |
| Outgroups | ||||
| Bacteria | ||||
| RecA | Escherichia coli | E. coli | V00328 | E05005 |
| Deinococcus radiodurans | Dc.rad | U01876 | M21413 | |
| Thermotoga maritima | Tt.mar | L23425 | M21774 | |
| Eucarya | ||||
| RAD51 | Homo sapiens | H.sap | D13804 | U13369 |
| RAD51 | Saccharomyces cerevisiae | S.cer | D10023 | Z75578 |
| DMC1 | Homo sapiens | H.sap | D64108 | U13369 |
| DMC1 | Saccharomyces cerevisiae | S.cer | U18922 | Z75578 |
Isolated in this study by using prSJS252 and prSJS253 as oligonucleotide primers for PCR.
Isolated in this study by using prSJS254 and prSJS255 as oligonucleotide primers for PCR.
prSJS152 and prSJS153 were the oligonucleotides used for PCR. These primers were defined by Sandler et al. (21). The gene was cloned and sequenced from a genomic fragment by a process similar that described by Sandler et al. (21).
Isolated in this study by using prSJS247 and prSJS248a as oligonucleotide primers for PCR.
DNA fragments were amplified by the touchdown PCR method defined in Materials and Methods.
Touchdown PCR, as described in Materials and Methods, was used, except the starting hybridization temperature was 47°C instead of 50°C.
na, not applicable.
Antarctic bacterioplankton samples were collected in nearshore surface waters off of Anvers Island, Antarctica. Samples were filtered and DNA was extracted as previously described (16). A fosmid library was prepared from the archaeal symbiont Cenarchaeum symbiosum. Symbiont cells were initially dissociated from host tissues of the sponge Axinella mexicana and enriched by differential and density gradient centrifugation, as previously described (18). DNA extraction, preparation of fosmid libraries, and PCR-based screening were performed as previously described (18, 25). Individual fosmids which tested positive with RadA-specific primers were purified and used for further sequencing.
PCR, cloning, and sequencing.
The primers used to amplify portions of genomic DNA in this work are listed in Table 2. For most PCR amplifications, we followed the procedure of Sandler et al. (21), which specifies an initial three cycles in which hybridization is carried out at temperatures increasing at 1°C per 10 s from 37 to 72°C. These cycles are followed by 26 cycles in which hybridization is carried out at 43°C. Six DNA preparations, however, did not yield amplification products by this procedure. For them (see Table 1) we used an alternative called “touchdown PCR” (9). In this procedure, hybridization is performed during the first cycle at 50°C for 1.5 min. After that cycle, the hybridization temperature is decreased by 0.5°C to 40°C in each of 19 successive cycles. An additional 20 cycles in which the hybridization temperature was 43°C were performed. In every cycle there was a denaturation step of 1 min at 94°C preceding and an extension step of 1 min at 71°C following hybridization. Lastly, the reactions were incubated at 71°C for 10 min before storage at 4°C. An MJ Research thermocycler, PTC-150, was used for PCR.
TABLE 2.
Oligonucleotide primers for PCR
| Primer | Sequence (5′ to 3′)a | Amino acid sequence | Avg GC content (%) |
|---|---|---|---|
| prSJS247 | ACMGARTTCTWCGGMGARTTCGGMTCKGGMAA | TE F F GEFGSGK | 51.6 |
| prSJS252 | ACSGARKTSTWCGGSGARTTCGGSKCSGGSAA | TE V/F Y/F GEFGSGK | 62.5 |
| prSJS254 | ACWGARTTYKYWGGWGARTTYGGWWSYGGWAA | TE F F/A GEFGSGK | 43.8 |
| prSJS248a | RTCKGGKYTKGCKGAKACYTGRTTKGT | TNQV S A N/R PD | 51.9 |
| prSJS253 | GTCSGGGTTSGMSAMSACCTGGTTSGT | TNQV A/S A/S N PD | 63.0 |
| prSJS255 | RTCWGGYCTWGCWGCWACYTGRTTWGT | TNQV A A R PD | 48.1 |
Conforms to the standard DNA alphabet as follows: W, A or T; Y, T or C; S, G or C; M, A or C; K, T or G; R, A or G; B, T or C or G; H, A or T or C; D, A or T or G; V, A or C or G; N, A or T or C or G.
DNA fragments generated by PCR amplification were cloned by using the Pharmacia SureClone kit and pUC18 as a vector. Individual plasmids with single inserts were identified and subjected to automated DNA sequence analysis with vector-specific primers. The sequences of both strands of each insert were determined.
Sequence alignment and phylogenetic inference.
The RadA alignment used in this study is based on our previous alignment (21). One hundred thirty-two unambiguously alignable amino acid positions, excluding the N and C termini, or 264 first- and second-codon positions of the corresponding nucleotide alignment were used in all RadA analyses. A total of 1,206 unambiguously alignable nucleotide positions were used in 16S rRNA analyses.
Maximum-likelihood (ML) analyses were conducted on the radA nucleotide and 16S rRNA datasets by using fastDNAml (version 1.1.1a [15]) with empirical base frequencies, optimized transition/transversion ratios (T-0.8 and 1/1 for RadA and 16S rRNA datasets, respectively), random sequence input order, and global branch swapping. Rate-corrected ML analyses were performed by using DNA rates (15) to estimate site-to-site rate variations, which were then reincorporated into the ML analysis.
Maximum-parsimony (MP) analyses were conducted on RadA amino acid and nucleotide datasets and the 16S rRNA dataset by using test version 4.0d55 of PAUP*, written by David L. Swofford. Default parameters were used in all analyses with the exception of random sequence addition with 10 repetitions per addition, TBR, and the steepest descent tree-building option with a heuristic search.
Evolutionary-distance (ED) trees were constructed from the radA nucleotide and 16S rRNA datasets by using PAUP* test version 4.0d55 (Kimura two-parameter or log Det distance matrix algorithms/neighbor joining) and from the RadA amino acid dataset by using PHYLIP version 3.57c (8) (Protdist [Dayhoff PAM or Kimura algorithms]/neighbor).
Transversion analysis also was performed on the 16S rRNA dataset for one set of MP and ED trees to compensate for known G+C bias in archaeal 16S rRNA datasets (29).
Bootstrap resampling (100 replicates) of the ML, MP, and ED trees was performed in all analyses to provide confidence estimates for the inferred topologies.
Nucleotide sequence accession numbers.
GenBank accession numbers for the nucleotide sequences determined in this study are listed in Table 1.
RESULTS
Design of primers.
Sandler et al. (21) used one set of primers to isolate a fragment of the radA gene of Sulfolobus solfataricus and another set to isolate fragments of radA genes of two other genera. Only 18 amino acids of the E. coli RecA protein lay between the equivalent positions of the primers in the E. coli recA gene. Our goal in this study required that we maximize the number of amino acids between the conserved regions used to design primers so that meaningful phylogenetic data could be extracted from the fragments isolated. Consequently, we chose two regions separated by 120 E. coli RecA amino acids for our primer design. The upstream region consists of portions of beta strand b1 and the Walker phosphate binding hole motif. The downstream region consists of portions of beta strand b5 and loop L2. The region in between comprises an integral domain of the E. coli RecA protein (27).
Amplification of radA gene fragments and alignment of their putative protein products.
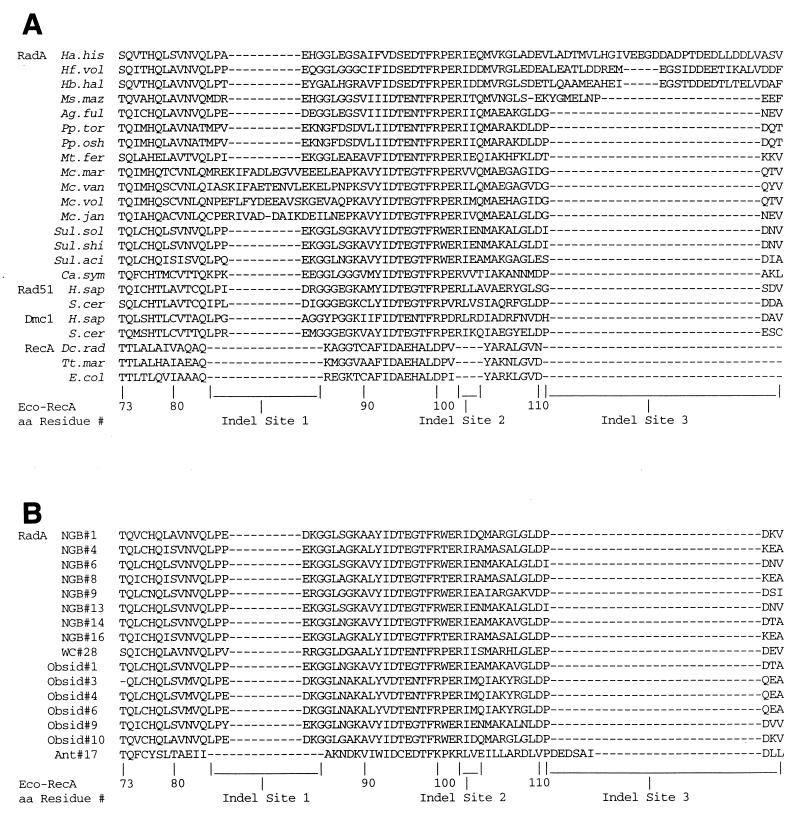
Three sets of primers were designed to be used in conjunction with DNAs containing high, low, and medium levels of G+C, as shown in Table 2. We used these primers to clone fragments of radA genes from 13 cultured species of Archaea. Alignments of the amino acids that are encoded by the fragments are shown in Fig. 1A and C. Evident from these panels is the occurrence of inserted or deleted amino acids in the RadA, Rad51, and Dmc1 sequences in comparison to the RecA sequences. Inserted amino acids occur between residues equivalent to residues 84 and 85, 102 and 103, 110 and 111, and 137 and 138 of E. coli RecA; these are designated “Indel sites” 1 to 4, respectively. Deleted are amino acids equivalent to residues 152 to 155 of E. coli RecA; this is called Indel site 5. The numbers of amino acids inserted at Indel site 2 and deleted at Indel site 5 are the same in all of the RadA, Rad51, and Dmc1 sequences shown in Fig. 1A and C. The numbers of amino acids at the other Indel sites differ, which indicates that multiple events may have occurred. Four sequences show 15- or 16-amino-acid insertions between E. coli RecA residues 84 and 85 rather than the 6 amino acids characteristic of the others (Indel site 1); we call these four sequences EL1 insertions (for extra length at site 1). These four are all from members of the genus Methanococcus. Four RadA sequences have between 11 and 33 amino acids between E. coli RecA residues 110 and 111 (Indel 3) rather than the 4 amino acids characteristic of the others; we call these sequences EL3 insertions. Three of these are from extreme halophiles. The same four sequences also have eight amino acid residues at Indel site 4 (EL4), thus differing from the three to five amino acids present in the others, except the sequence for Archaeoglobus fulgidus. Further information on the way these patterns of insertions and deletions are distributed phylogenetically is presented below.
FIG. 1.
Alignment of amino acids encoded by PCR-amplified fragments of radA genes. (A) Residues 1 to 90 of radA fragments from 15 cultivated and 1 enriched archaeal species. Thirteen fragments were isolated by the PCR technique and cloned. Those from H. volcanii (Hf. vol), M. jannaschii (Mc. jan), and S. solfataricus (Sul. sol) were taken from cognate parts of the sequences published by Sandler et al. (21). Cognate parts of Rad51, Dmc1, and RecA have previously been published (2, 10). (B) Residues 1 to 90 of radA fragments from environmental samples. These residues are shown in their proper alignment to the residues of the RecA, Rad51, and Dmc1 fragments in panel A. (C) Residues 91 to 180 of radA fragments from 15 cultivated and 1 enriched archaeal species. See the legend to panel A for further information. (D) Residues 91 to 180 of radA fragments from environmental samples shown in appropriate alignment to residues of the RecA, Rad51, and Dmc1 fragments in panel C. Abbreviations are defined in Table 1.
Also shown in Fig. 1 (panels B and D) are the sequences of 16 clones isolated from environmental DNA samples. These show much more uniformity with regard to the numbers of amino acids at Indel sites 1 and 3 than the RadA sequences from cultured and enriched species. The only exception is the sequence isolated from the Antarctic Ocean DNA sample. In this case, there is no insertion at Indel site 1, thereby distinguishing this sequence from all other RadA, Rad51, and Dmc1 sequences. On the other hand, the Antarctic Ocean sample has a longer insertion at Indel site 3, like the extreme halophiles. The phylogenetic significance of these characteristics is discussed below.
Sequences of complete RadA genes.
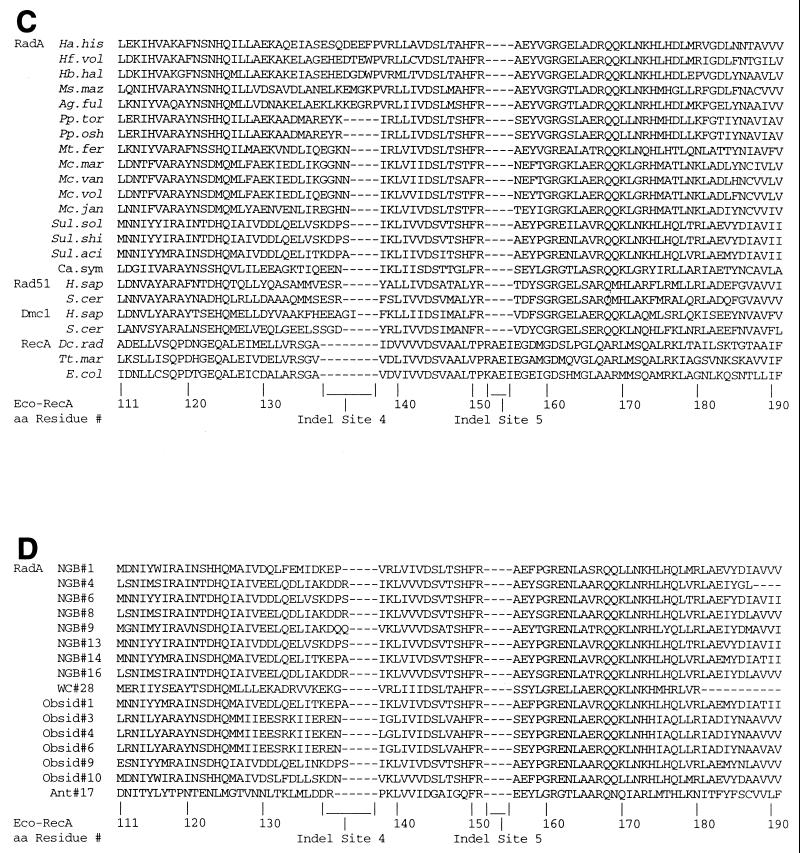
Using cloned radA fragments (21) as hybridization probes, we cloned the entire radA genes of Halobacterium halobium and C. symbiosum. The protein sequences are presented in Fig. 2. Portions of the sequences, called “domains” by Brendel et al. (3), are indicated. A distinctive feature of the C. symbiosum sequence is that it does not have a complete domain A sequence. This distinguishes it from the H. halobium sequence and all other RadA, Rad51, and Dmc1 sequences so far examined (Fig. 2) (3). Another distinctive feature is the existence of a carboxy-terminal sequence similar in length, but not in sequence, to that of E. coli RecA protein and not possessed by any other RadA, Rad51, or Dmc1 sequence so far studied (3, 24).
FIG. 2.
Sequences of two complete putative RadA proteins (from H. halobium [Hb. hal] and C. symbiosum [Ca. sym]) aligned with E. coli RecA, S. cerevisiae Rad51, and S. cerevisiae Dmc1 proteins.
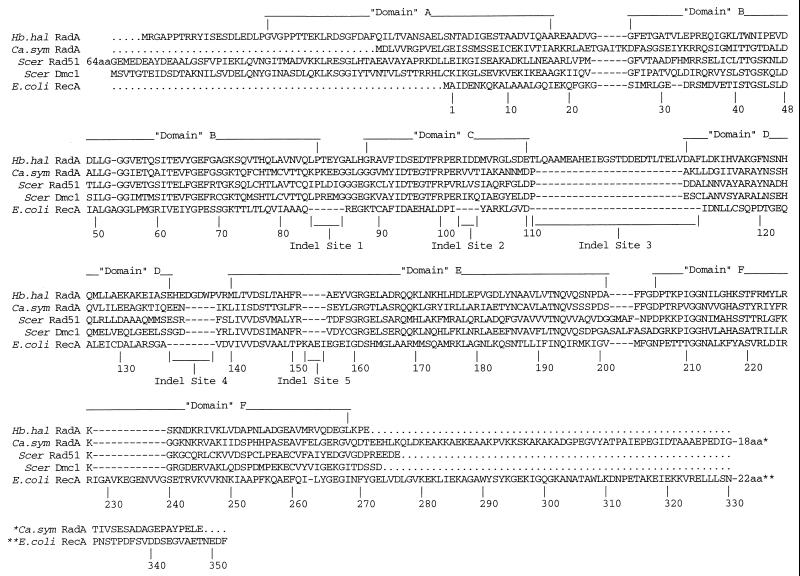
Phylogeny of the Sequences: RadA versus 16S rDNA.
The sequences of the RadA fragments shown in Fig. 1A and C were analyzed phylogenetically, and the phylogeny obtained was compared to that obtained with cognate 16S ribosomal DNA (rDNA) sequences (Fig. 3). In general there is a high degree of consistency between the two phylogenies. The shaded regions in this figure show that the two molecules reveal similar clades of halophiles, Methanococcus spp. and Sulfolobus spp., with minor branching-order discrepancies. In performing this analysis, we excluded the extra amino acids at Indel sites 1 to 5 (Fig. 1). Nonetheless, certain features of the inserts are consistent with the clade structure based on the RadA fragments. In Fig. 3, the numbers in circles refer to corresponding extra-length (EL) numbers and show the common phylogenetic ancestry of these extra-long insertions. The most notable inconsistency between the RadA and 16S rDNA phylogenies is the position of C. symbiosum in the Crenarchaeota by 16S phylogeny but in an independent lineage by RadA phylogeny. The implications of this are discussed below.
FIG. 3.
Comparison of 16S rRNA and radA trees generated by rate-corrected ML analyses of nucleotide sequences (first and second codon positions only) for radA encoding the amino acid sequences shown in Fig. 1A and C. One additional unpublished sequence, that of P. aerophilium, was generously provided by S. Fitz-Gibbon (see reference 28). Branch points supported (bootstrap values, ≥75%) by most or all phylogenetic analyses (see Materials and Methods) are indicated by filled circles. Open circles indicate branch points supported by some analyses but marginally supported (bootstrap, 50 to 74%) or unsupported (bootstrap, <50%) by others (see Results and Discussion). Strongly supported monophyletic groups consistent between the two molecules are indicated by shading. Three bacterial outgroup sequences (from E. coli, Deinococcus radiodurans and Thermotoga maritima) were used for all 16S rRNA analyses, and four eucaryal outgroup sequences (Homo sapiens RAD51 and DMC1 and S. cerevisiae RAD51 and DMC1) were used for all radA analyses. Three secondary structural features of radA (see Fig. 1) are indicated at branch points; that is, all sequences to the right of the indicated branch point share the secondary structural feature. 1, 3, and 4 represent extra-long inserts EL1, EL3, and EL4 at Indel sites 1, 3, and 4 respectively (see Fig. 1 and text). Abbreviations are defined in Table 1.
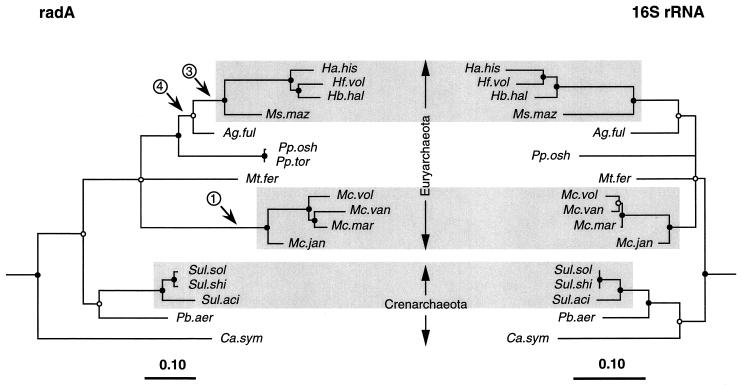
Phylogeny of environmental samples.
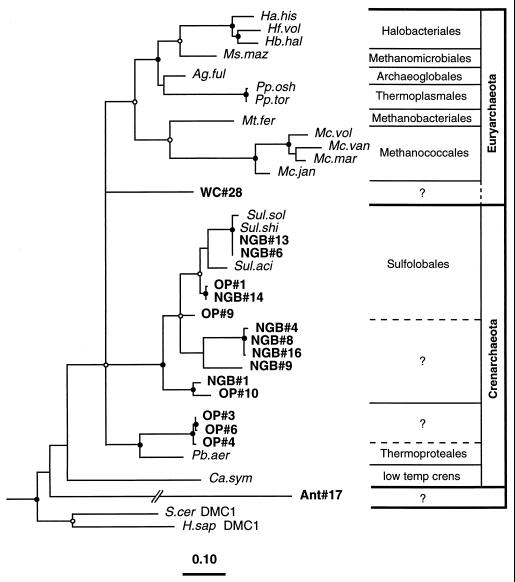
We determined phylogenetic relationships of the radA nucleotide sequences extracted from environmental samples (Fig. 1B and D) with the radA nucleotide sequences from known archaeal species (Fig. 4). The results show that 14 of the 16 sequences cluster unambiguously with Crenarchaeota sequences. Two of the 14 (NGB#6 and NGB#13) are probably from representatives of the genus Sulfolobus because they occur within the radiation of reference Sulfolobus species in Fig. 4. Nine others are monophyletic with the Sulfolobales representatives, but at present there are insufficient reference RadA sequences to identify their generic affiliations. However, based on a proposed generic lower limit of 78% identity (data not shown), clones OP#1, NGB#14, and OP#9 may represent Sulfolobus species, as indicated by the dashed line in Fig. 4. Three others (OP#3, OP#4, and OP#6) are monophyletic with Pyrobacculum aerophilium, but again there are insufficient reference sequences at this time to conclude their generic affiliations.
FIG. 4.
Phylogenetic relationships of the 17 radA sequences from cultivated archaeal species and 16 environmental radA sequences determined by using rate-corrected ML analyses of first- and second-position nucleotide sequences. The radA nucleotide sequences used were those encoding the amino acid sequences in Fig. 1 with the exception of the P. aerophilium sequence (see the legend to Fig. 3). Eucaryal outgroup sequences were as described for Fig. 3. Filled and open circles indicating phylogenetic integrity of branch points are as in Fig. 3. Branch points without circles were not resolved (bootstrap, <50%) as specific groups in different analyses. Abbreviations are defined in Table 1.
Of the two sequences which do not belong to the Crenarchaeota, one, WC#28, is marginally supported (ca., 70% bootstrap values) as a member of the Euryarchaeota by evolutionary distance analysis. The final sequence, Ant#17, is very different, appears to branch independently, and shows only weak similarity to the other RadA sequence from a low-temperature crenarchaeotan, C. symbiosum.
Signature amino acid codons.
Signature amino acids derived from aligned RecA family sequences are useful for deducing the subfamily to which each protein belongs without resort to formal phylogenetic analysis. Comparative analysis of the sequences in Fig. 1 reveals that nine amino acids can be used to distinguish five groups of RecA-like proteins. The first set of three amino acids (Table 3, group A) separates the family into three groups consisting of RecA in one group, RadA, Rad51, and Dmc1 in another, and RadB in a third. The next set of two amino acids (Table 3, group B) separates RadA, Rad51, and Dmc1 from each other and increases the definition of RecA and RadB from each other and the other three family members. Finally, there are four additional amino acids that enhance the definitions still further.
TABLE 3.
Codon signatures distinguishing recognized groups of recA-like sequences
| Group | E. coli codon position | Codon signature
|
||||
|---|---|---|---|---|---|---|
| RecA (bacterial)a | RadA (archaeal)b | Rad51 (eucaryal)c | Dmc1 (eucaryal)c | RadB (archaeal)d | ||
| A | 74 | T | Q | Q | Q | N/T |
| 98 | A(S) | T | T | T | G | |
| 100 | D(E) | R(K) | R | R | S | |
| B | 150 | T(V) | F | Y | F | Y |
| 157 | G | E(S) | D | D | E/K | |
| C | 122 | G | S(T) | T(S/A) | S(Y) | F |
| 160 | G(S/K) | G | G | G | N/D/R | |
| 169 | R | Q(N) | M(N/T) | Q(K) | L/K/R/A | |
| 174 | A | H(Y/L) | F | M(H) | Q | |
We have applied this signature amino acid analysis to the C. symbiosum and Ant#17 sequences because they differ so markedly from the other RadA sequences. For example, the predicted C. symbiosum protein has N- and C-terminal sequences whose lengths differ from the other RadA, Rad51, and Dmc1 proteins and resemble the lengths of the N- and C-terminal sequences of bacterial RecA proteins. The C. symbiosum sequence, however, possesses the signature amino acids at E. coli RecA codon positions 74, 98, 100, 150, and 157 that all other RadA sequences possess (Table 3). The Ant#17 sequence lacks the six-or-more amino acid insertion at Indel site 1, which is characteristic of all other RadA proteins. Nonetheless, it has four of the five signature amino acids that characterize RadA sequences and at the fifth, E. coli RecA codon position 100, it has the basic amino acid lysine in place of the basic amino acid arginine. Thus, we think both of these sequences belong to the RadA subfamily, although we cannot rule out the possibility that they belong to a new subfamily paralogous to RadA.
DISCUSSION
Sandler et al. (21) proposed that three putative archaeal RadA proteins, although about 20% identical to bacterial RecA proteins, possess certain primary structural features that set them apart. We have found evidence to support this proposal by sequencing 2 complete and 11 partial radA genes from different archaeal species. In particular, we found that all archaeal RadA species have four regions of inserted and one region of deleted amino acids relative to bacterial RecA sequences (Fig. 1A and C). In addition, we found that the lengths of three of the inserts have taxonomic significance and distinguish particular phylogenetic clades determined by either RadA or 16S rRNA sequences (Fig. 3). Furthermore, all 13 new sequences share these diagnostic features with Rad51 and Dmc1 sequences from representative eukaryotes. This strengthens the proposal of Sandler et al. (21) that RadA is orthologous to the common ancestor of Rad51 and Dmc1.
Close correspondence of the phylogenies obtained for archaeal species with either the RadA or 16S rRNA sequences (Fig. 3) is also noteworthy because it suggests that RadA sequences can be used as an independent measure of archaeal diversity. Indeed, RadA clearly resolves the phylogenetic position of A. fulgidus as monophyletic with Halobacteriales and Methanomicrobiales. Furthermore, a secondary structural feature (the length of the insertion of Indel site 4) corroborates this association. This analysis independently supports the conclusion of Woese et al. (29), who based their finding on compensation for G+C bias in the 16S rDNA sequences. However, the failure of the C. symbiosum fragment to be monophyletic with the Crenarchaeota may indicate that distant phylogenetic relationships cannot be resolved by using this small RadA fragment. An alternative explanation is that we have sequenced a RadA paralog from C. symbiosum. We can test this alternative by screening more completely the C. symbiosum genome for additional RadA-like sequences.
As another test of the phylogenetic utility of the RadA molecule, we examined DNAs obtained from four different natural sources: three ecologically distinct hot pools in Yellowstone National Park (11) and the Antarctic Ocean. Fourteen of the 15 hot pool sequences cluster with members of the Crenarcheota. Six of these were from Obsidian Pool, whose analysis by 16S rDNA phylogeny had also shown a dominance of crenarchaeotan sequences (1). The other eight were from a pool in the Norris Geyser Basin for which there is no equivalent 16S rDNA analysis. However, we would predict that such an analysis would reveal a majority of crenarchaeotan sequences.
Two of the sequences, for C. symbiosum and Ant#17, stand out by their unique characteristics. Figure 4 demonstrates the novelty of these sequences by their independent branching in the archaeal tree. Although they may represent proteins paralogous to RadA, we are encouraged to anticipate the discovery of more diversity in RecA family sequences, with others possibly showing mixtures of the features of RecA and RadA proteins and perhaps representing evolutionary intermediates.
The RecA family actually contains two different member classes in the Archaea: radA and radB. In this study, we have been concerned mainly with radA. radB, however, was originally found in the genome sequence of Methanococcus janaschii (4), where it was identified as a Rad51 relative. Other members of the radB subfamily have been found in A. fulgidus (14), Pyrococcus sp. KOD1 (19), Pyrococcus furiosus (6), and Methanobacterium thermoautotrophicum (23). Putative RadB proteins differ from RadA in two major features. First, they are only about 70% as big, having neither an N- nor a C-terminal extension. Second, their sequences differ appreciably, and we have shown in Table 3 the signature sequences that distinguish RadA from RadB. The sequences are different enough that special primers would have to be designed to amplify them by the PCR technique. The primers that we used to amplify radA sequences (Table 1) would have had a very low probability of amplifying radB sequences. It seems worthwhile in the future to search for radB sequences, given the similarity of their proteins in sequence and possibly in function to the Rad55 and Rad57 proteins of Saccharomyces cerevisiae (17a).
ACKNOWLEDGMENTS
We thank Mike Dyall-Smith, Ken Jarrell, Shil DasSarma, Everly Conway de Macario, Patricia Hartzell, Wolfram Zillig, and Dennis Grogan for strains or purified genomic DNA used in this study. We also thank David Swofford for permission to publish results from a PAUP test version.
S.J.S. and A.J.C. were supported by grant AI05371 from the National Institutes of Health (NIH). P.H. and N.R.P. were supported by grants from the U.S. Department of Energy and NIH. E.F.D. was supported by NSF grants OCE95-29804 and OPP94-18442. C.S. was supported by a fellowship from the Deutsche Forschungsgemeinschaft.
REFERENCES
- 1.Barns S M, Delwiche C F, Palmer J D, Pace N R. Perspectives on archaeal diversity, thermophily and monophyly from environmental rRNA sequences. Proc Natl Acad Sci USA. 1996;93:9188–9193. doi: 10.1073/pnas.93.17.9188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bishop D K, Park D, Xu L, Kleckner N. DMC1: a meiosis-specific yeast homolog of E. coli recA required for recombination, synaptonemal complex formation, and cell cycle progression. Cell. 1992;69:439–456. doi: 10.1016/0092-8674(92)90446-j. [DOI] [PubMed] [Google Scholar]
- 3.Brendel V, Brocchieri L, Sandler S J, Clark A J, Karlin S. Evolutionary comparisons of RecA-like proteins across all major kingdoms of living organisms. J Mol Evol. 1997;44:528–541. doi: 10.1007/pl00006177. [DOI] [PubMed] [Google Scholar]
- 4.Bult C, White O, Olsen G J, Zhou L, Fleischmann R D, Gocayne J D, Kerlavage A R, Dougherty B A, Tomb J R, Adams M D, et al. Complete genome sequence of the methanogenic archaeon Methanococcus jannaschii. Science. 1996;273:1058–1073. doi: 10.1126/science.273.5278.1058. [DOI] [PubMed] [Google Scholar]
- 5.Clark A J, Margulies A D. Isolation and characterization of recombination-deficient mutants of Escherichia coli K-12. Proc Natl Acad Sci USA. 1965;53:451. doi: 10.1073/pnas.53.2.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.DiRuggiero, J., J. R. Brown, A. P. Bogert, and F. T. Robb. DNA repair systems in archaea: momentos from the last universal common ancestor? J. Mol. Evol., in press. [DOI] [PubMed]
- 7.Eisen J A. The RecA protein as a model molecule for molecular systematic studies of bacteria: comparison of RecAs and 16S rRNAs from the same species. J Mol Evol. 1995;41:1105–1123. doi: 10.1007/BF00173192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Felsenstein J. PHYLIP (Phylogeny Inference Package), version 3.5c. Seattle: Department of Genetics, University of Washington; 1993. [Google Scholar]
- 9.Hecker K H, Roux K H. High and low annealing temperatures increase both specificity and yield in touchdown and stepdown PCR. BioTechniques. 1996;20:478–485. doi: 10.2144/19962003478. [DOI] [PubMed] [Google Scholar]
- 10.Horii T, Ogawa T, Ogawa H. Organization of the recA gene of Escherichia coli. Proc Natl Acad Sci USA. 1980;77:313–317. doi: 10.1073/pnas.77.1.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hugenholtz P, Pitulle C, Hershberger K L, Pace N R. Novel division level bacterial diversity in a Yellowstone hot spring. J Bacteriol. 1998;180:366–376. doi: 10.1128/jb.180.2.366-376.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Karlin S, Brocchieri L. Evolutionary conservation of recA genes in relation to protein structure and function. J Bacteriol. 1996;178:1881–1894. doi: 10.1128/jb.178.7.1881-1894.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Karlin S, Weinstock G M, Brendel V. Bacterial classifications derived from recA protein sequence comparisons. J Bacteriol. 1995;177:6881–6893. doi: 10.1128/jb.177.23.6881-6893.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Klenk H P, Clayton R A, Tomb J F, White O, Nelson K E, Ketchum K A, Dodson R J, Gwinn M, Hickey E K, Peterson J D, et al. The complete genome sequence of the hypertheromophilic sulphate-reducing archaeon Archeoglobus fulgidus. Nature. 1997;390:364–370. doi: 10.1038/37052. [DOI] [PubMed] [Google Scholar]
- 15.Maidak B L, Olsen G J, Larsen N, Overbeck R, McCaughey M J, Woese C R. The RDP (Ribosomal Database Project) Nucleic Acids Res. 1997;25:109–110. doi: 10.1093/nar/25.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Massana R, Murray A E, Preston C M, DeLong E F. Vertical distribution and phylogenetic characterization of marine planktonic archaea in the Santa Barbara Channel. Appl Environ Microbiol. 1997;63:50–56. doi: 10.1128/aem.63.1.50-56.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miller R V, Kokjohn T A. General microbiology of recA: environmental and evolutionary significance. Annu Rev Microbiol. 1990;44:365–394. doi: 10.1146/annurev.mi.44.100190.002053. [DOI] [PubMed] [Google Scholar]
- 17a.Olsen, G. Personal communication.
- 18.Preston C M, Wu K Y, Molinski T F, DeLong E F. A psychrophilic crenarchaeon inhabits a marine sponge: Cenarchaeum symbiosum, gen. nov., sp. nov. Proc Natl Acad Sci USA. 1996;93:6241–6246. doi: 10.1073/pnas.93.13.6241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rashid N, Morikawa M, Imanaka T. A RecA/RAD51 homologue from a hyper-thermophilic archaeon retains the major RecA domain only. Mol Gen Genet. 1996;253:397–400. doi: 10.1007/s004380050337. [DOI] [PubMed] [Google Scholar]
- 20.Roca A I, Cox M M. RecA protein: structure, function, and role in recombinational DNA repair. Prog Nucleic Acid Res Mol Biol. 1997;56:129–223. doi: 10.1016/s0079-6603(08)61005-3. [DOI] [PubMed] [Google Scholar]
- 21.Sandler S J, Satin L H, Samra H S, Clark A J. recA-like genes from three archaean species with putative protein products similar to Rad51 and Dmc1 proteins of the yeast Saccharomyces cerevisiae. Nucleic Acids Res. 1996;24:2125–2132. doi: 10.1093/nar/24.11.2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shinohara A, Ogawa H, Ogawa T. Rad51 protein involved in repair and recombination in S. cerevisiae is a RecA-like protein. Cell. 1992;69:457–470. doi: 10.1016/0092-8674(92)90447-k. [DOI] [PubMed] [Google Scholar]
- 23.Smith D R, Doucette-Stamm L A, Deloughery C, Lee H, Dubois J, Aldredge T, Rashirzadeh R, Blakely D, Cook R, Gilbert K, et al. Complete genome sequence of Methanobacterium thermoautotrophicum Δ H: functional analysis and comparative genomics. J Bacteriol. 1997;179:7135–7155. doi: 10.1128/jb.179.22.7135-7155.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stassen N Y, Logsdon J M, Jr, Vora G J, Offenberg H H, Palmer J D, Zolan M E. Isolation and characterization of rad51 orthologs from Coprinus cinercus and Lycopersicon esculentum and phylogenetic analysis of eukaryotic recA homologs. Curr Genet. 1997;31:144–157. doi: 10.1007/s002940050189. [DOI] [PubMed] [Google Scholar]
- 25.Stein J L T L M, Wu K Y, Shizuya H, DeLong E F. Characterization of uncultivated prokaryotes: isolation and analysis of a 40-kilobase-pair genome fragment from a planktonic marine archaeon. J Bacteriol. 1996;178:591–599. doi: 10.1128/jb.178.3.591-599.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Story R M, Bishop D K, Kleckner N, Steitz T A. Structural relationship of bacterial RecA proteins to recombination proteins from bacteriophage T4 and yeast. Science. 1993;259:1892–1896. doi: 10.1126/science.8456313. [DOI] [PubMed] [Google Scholar]
- 27.Story R M, Steitz T A. Structure of the recA protein-ADP complex. Nature. 1992;355:374–376. doi: 10.1038/355374a0. [DOI] [PubMed] [Google Scholar]
- 28.Volkl P, Markiewicz P, Baikalov C, Fitz-Gibbon S, Stetter K O, Miller J H. Genomic and cDNA sequence tags of the hyperthermophilic archaean Pyrobaculum aierophilum. Nucleic Acids Res. 1996;24:4373–4378. doi: 10.1093/nar/24.22.4373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Woese C R, Achenbach L, Rouviere P, Mandelco L. Archaeal phylogeny: reexamination of the phylogenetic position of Archaeoglobus fulgidus in light of certain composition-induced artifacts. Syst Appl Microbiol. 1991;14:364–371. doi: 10.1016/s0723-2020(11)80311-5. [DOI] [PubMed] [Google Scholar]