Abstract
Dysphagia is a common debilitating symptom in people with Parkinson’s Disease (PD), adequate screening of swallowing disorders is fundamental. The DYMUS questionnaire has shown very good characteristics for the screening of dysphagia in Multiple Sclerosis, and it might also prove useful for screening dysphagia in PD. The primary aim was to test and validate the DYMUS questionnaire in PD patients. This is an observational multicentric study involving 103 patients affected by PD. All subjects filled in the DYMUS and the Eating Assessment Tool (EAT-10) questionnaires. A subgroup of patients (n = 53) underwent a fiber-optic endoscopic evaluation of swallowing (FEES) and their dysphagia was scored by means of the Dysphagia Outcome Severity Scale (DOSS). DYMUS showed a relatively high level of internal consistency (Cronbach’s alpha 0.79). A significant positive correlation was found between the DYMUS and the EAT-10 scores (p < 0.001), while a negative correlation was found between the DYMUS and the DOSS scores (p < 0.001). DYMUS showed a good sensitivity and specificity compared to FEES for detecting dysphagia (area under the curve: 0.82, p < 0.001). The ROC curve analysis showed that a DYMUS score ≥ 6 represents a reliable cut-off for the risk of dysphagia. The DYMUS questionnaire proved to be a reliable screening tool to detect dysphagia in patients suffering from PD. It is easy to understand, it can be self-administered and therefore adequate for adoption in the clinical practice with the more convenient name of DYPARK.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00455-021-10332-1.
Keywords: Dysphagia, Parkinson’s disease, Deglutition disorders, Screening
Introduction
Parkinson’s Disease (PD) is a progressive neurodegenerative disorder primarily characterized by the hallmarks of motor symptoms, such as tremor, bradykinesia, rigidity, and postural instability. Beside these cardinal symptoms, dysphagia is frequent and clinically relevant [1]. The vast majority of PD patients develop swallowing impairment during the course of their disease and more than 50% of those who are subjectively asymptomatic for dysphagia show swallowing dysfunction when assessed with fiber-optic endoscopic evaluation of swallowing (FEES) or videofluoroscopic swallowing study (VFSS) [2, 3]. It is estimated that up to 80% of patients with early-stage PD experience oropharyngeal dysphagia. At advanced stages, the incidence may be as high as 95% [2, 4].
The presence of swallowing dysfunction in PD patients can be very disabling and have a relevant impact on the quality of life [5]. Dysphagia also leads to insufficient medication intake, malnutrition and aspiration with subsequent pneumonia, which is a major cause of death in PD patients [6]. Although a frequent and debilitating symptom, dysphagia is still underestimated and overlooked in clinical practice. This is possibly due to multiple causes. First of all, swallowing disorders are subjectively reported by only one third of parkinsonian patients [7], a mismatch that likely reflects the fact that patients tend to underreport swallowing difficulties unless specifically questioned about it. Secondly, the careful clinical evaluation of dysphagia requires resources (both in terms of time and of trained professionals) and methodologies (FEES, VFSS, etc.) that are not widely available.
In this frame, screening assessment focused on identifying risk factors are highly needed, in order to identify early patients who should be referred to further, more specific, clinical and/or instrumental investigations. In 2009 the task force of the Movement Disorders Society (MDS) assessed clinical rating scales for PD by means of systematic reviews [8]. Two dysphagia scales, the Swallowing Disturbance Questionnaire (SDQ) and the Dysphagia-Specific Quality of Life (SWAL-QOL) scale, met the criteria for being “suggested” but not the “recommended”. The lower strength of endorsement was motivated by methodological limitations: SDQ was tested on a small size of PD patients, while the SWAL-QOL was tested for dysphagia in neurodegenerative diseases in general, but not specifically in PD. Among available screening questionnaires, the DYMUS has already been validated for the early screening of dysphagia in Multiple Sclerosis [9, 10]. DYMUS is composed by 10 items that require a binary response and has proved to be easily self-administered. It is useful to preliminary select patients who should be referred for more specific and comprehensive clinical and instrumental assessments and, if required, to rehabilitation [9].
The primary aim of this study was to validate the DYMUS questionnaire in PD patients. The secondary aim was to compare the performance of DYMUS in screening dysphagia with the Eating Assessment Tool (EAT-10) [11] and with the instrumental assessment by FEES.
Our hypothesis is that DYMUS may be a reliable and easy-to-use tool to detect dysphagia in PD patients.
Materials and Methods
This is a prospective multicentric, cross-sectional, observational study conducted in 5 Italian Neurological/Neurorehabilitation Centers (Pavia, Verona, Catania, Zingonia and Moncrivello) between January 2017 and June 2019. The protocol was approved by the local Ethics Committees and patients signed the informed consent before being enrolled in the study.
Patients with PD were included in the study if they satisfied the MDS Clinical Diagnostic Criteria for PD [12] and they had an age range between 40 and 85 years.
Exclusion criteria were:
Concomitance of other neurological diseases or any clinical conditions potentially causing dysphagia;
Presence of percutaneous endoscopic gastrostomy (PEG);
Psychiatric disorders;
Moderate-severe cognitive impairment (MMSE ≤ 21).
The patients were recruited unselectively and consecutively in each center and underwent examination in a single visit, irrespective of self-reported or doctor-suspected swallowing symptoms.
The patients underwent anamnestic evaluation: diagnosis, disease duration, pharmacological treatment, height and weight were noted.
The clinical stage of disease was defined according to the modified Hoehn and Yahr staging system, global motor disability was evaluated by the MDS-Unified Parkinson’s Disease Rating Scale.
The Questionnaire
All the patients were asked to fill in the DYMUS and the EAT-10 questionnaires. We also asked the caregiver of each patient to fill in the DYMUS separately.
DYMUS is composed by 10 items, all the answers are dichotomous, coded as 1 or 0, depending on the presence or absence of the event (range of score: 0–10; higher = worse) [1] (Supplementary figure).
EAT-10 is a clinical scale used to document dysphagia symptoms severity in patients with swallowing disorders [11]. EAT-10 includes 10 questions, each scored from 0 to 4 (“no problem” to “severe problem”); the total score is calculated by adding the scores of each question, with a higher score indicating higher self-perceived dysphagia severity.
Instrumental Examination
For the comparison of DYMUS with FEES, patients enrolled in the centers where FEES was available underwent the instrumental evaluation after completing DYMUS and EAT-10. Based on FEES findings, dysphagia severity was rated according to the Dysphagia Outcome Severity Scale (DOSS) [13]. FEES was performed by an experienced otolaryngology specialist. The images of the structures assigned to the swallowing function were evaluated directly through the lens of the fiber-optic laryngoscope head. During the execution of the exam, the patient was in the upright seated position. Only in a few cases, was it necessary to perform the examination with the patient in a semi-seated position. The procedure generally did not require any preparation. The instrument was inserted transnasally through the path of least resistance, generally along the nasal floor or between the inferior and middle turbinate. For the study of the swallowing act, the distal end of the fiberscope was positioned above the epiglottis at the level of the uvula. This “high” position allows a good visualization of the base of the tongue, of the lateral and posterior pharyngeal walls, of the epiglottis, of the vallecula, of the larynx and of the piriform sinuses. The observation point is ideal for assessing airway closure during swallowing. The swallowing test was carried out with foods of different consistencies, from liquid to solid. More specifically, we recorded 3 swallows with a 2-ml volume of liquid, 3 swallows with 1-ml volume of semisolid and 3 swallows of solid bolus (one bite of biscuit-type food).
Based on endoscopic results, patients were classified using the DOSS into seven levels, subdivided into three different degrees of swallowing function:
grade 0 = no dysphagia, no diet limitations (levels 6 and 7);
grade 1 = diet with limitations (levels 3, 4 and 5);
grade 2 = nutrition with the enteral route (levels 1 and 2).
A score ≤ 5 at the DOSS was used to separate dysphagic from non-dysphagic patients.
FEES is a minimally invasive test that represents the non-radiologic gold standard to verify the severity of dysphagia and is necessary to determine any limitations in the diet.
An additional group of 20 PD patients who were hospitalized in the Neurorehabilitation Unit of the Pavia Centre were evaluated to assess the test–retest validity of the DYMUS scale 1 week apart. None of these patients underwent speech or swallowing therapy during this period, nor were they allowed to change their pharmacological treatment.
Statistical Analysis
Statistical analysis was performed using “R Statistics Software”. Demographic and clinical characteristics were expressed as mean ± standard deviation. The distribution of DYMUS, EAT-10 and DOSS scores was not normal, and data are therefore presented as median and interquartile range.
For the analysis of the internal and construct validity and reliability of DYMUS we calculated:
the Cronbach’s alpha coefficient (of the entire questionnaire and subsequently by removing one item at a time). The value of 0.7 was considered the cut-off for defining a relatively high internal consistency;
the Cohen Kappa (of the entire questionnaire and of each item) calculated for the test–retest of 20 patients.
Correlation coefficient and logistic regression were used to compare the DYMUS score with EAT-10 or DOSS scores. The sensitivity and specificity for the association between DYMUS and EAT-10 and DYMUS and DOSS scales were calculated.
We also computed the correlation coefficient to analyze the DYMUS scores obtained when the questionnaire was filled in by the patients or by their caregivers.
We considered statistically significant a p value < 0.05.
Finally, we sought the cut-off score for a reliable identification of potentially dysphagic patients who are candidate for instrumental investigations.
Results
We enrolled a total of 103 PD patients in the study: 56 males (54.4%) and 47 females (45.6%). The mean age was 68.0 ± 2.8 years (range 40–83 years), the mean MMSE score was 24.9 ± 8.5 and the mean disease duration was 11.5 ± 4.9 years (range 1–31 years) (Table 1). Fifty-one patients underwent FEES evaluation. The median score at the DYMUS Questionnaire was 2 (IQR 1;4), at EAT-10 2 (IQR 0;5) and at DOSS 6 (IQR 5;7).
Table 1.
Demographic and clinical characteristics of the population enrolled in the study
| Variable | Value |
|---|---|
|
N. Subjects Sex (M/F) |
103 56/47 |
| Age (years), mean ± SD | 68.0 ± 2.8 |
| Disease duration (years), mean ± SD | 11.5 ± 4.9 |
To assess the validity of DYMUS, we evaluated preliminarily the reliability of the Questionnaire using the Cronbach’s alpha coefficient. The 10-item DYMUS questionnaire showed a relatively high internal consistency with a Cronbach’s alpha of 0.79. The coherence analysis was also repeated by calculating Cronbach’s alpha while eliminating an item at a time: the scores obtained confirmed the internal consistency (Table 2).
Table 2.
Cronbach’s alpha for each item of DYMUS: Coherence analysis repeated by calculating Cronbach’s alpha when eliminating one of the 10 items at a time
| Item | Cronbach’s alpha |
|---|---|
| 1 | 0.7560596 |
| 2 | 0.7847757 |
| 3 | 0.7668145 |
| 4 | 0.7899099 |
| 5 | 0.7726620 |
| 6 | 0.7516790 |
| 7 | 0.7608186 |
| 8 | 0.7738234 |
| 9 | 0.7639014 |
| 10 | 0.7961238 |
As regards test–retest reliability, the Cohen Kappa of the entire questionnaire was 0.69, which indicates a substantial agreement between the two administrations. We also calculated the Cohen Kappa for each item and found substantial repeatability in 8 out of the 10 items, moderate for items 6 and 7 (Table 3).
Table 3.
Cohen Kappa for test–retest reliability of the DYMUS items
| Item | Cohen kappa |
|---|---|
| 1 | 0.69 |
| 2 | 0.57 |
| 3 | 0.69 |
| 4 | 0.77 |
| 5 | 1 |
| 6 | 0.48 |
| 7 | 0.47 |
| 8 | 0.68 |
| 9 | 0.69 |
| 10 | 0.78 |
As regards the score obtained when the DYMUS questionnaire was filled in separately by the patients and their caregivers, we did not find statistically significant differences as regards the total score and the individual items. When analyzing the relation between the scores obtained with the two groups (patients and caregivers), we found a significant positive correlation (p value < 0.001 R 0.68).
Correlations Between DYMUS and EAT-10 Scores, and DYMUS and DOSS Scores
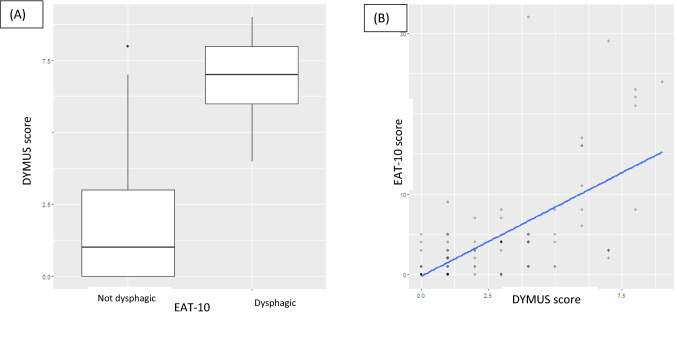
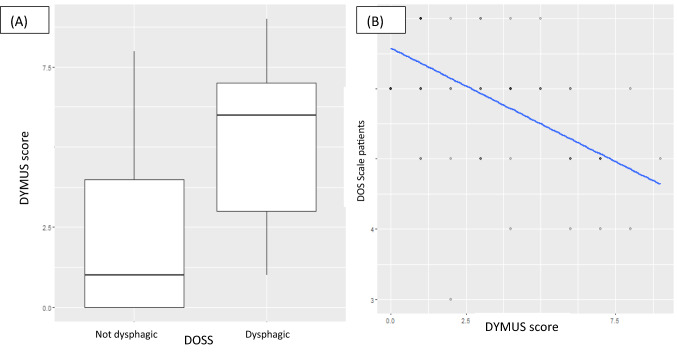
We found a significant positive correlation between the DYMUS and the EAT-10 scores (p < 0.001 R 0.66) and a negative correlation between the DYMUS and DOSS scores (p < 0.001 R − 0.58) (Figs. 1, 2).
Fig. 1.
Correlations between EAT-10 and DYMUS scores: we found a significant positive correlation between the DYMUS and the EAT-10 scores (p < 0.001 R 0.66). A Box-plot; B scatter-plot
Fig. 2.
Correlations between EAT-10 and DOSS scores: we found a significant negative correlation between the DYMUS and the DOSS scores (p < 0.001 R − 0.58). A Box-plot; B scatter-plot
The DYMUS proved capable of detecting dysphagic patients (area under the curve: 0.95, p < 0.001).
When the DYMUS questionnaire was anchored to DOSS score, we found a DYMUS score of 6 as the cut-off for identifying patients at higher risk of dysphagia with a sensibility of 0.7 and a specificity of 0.84 (Fig. 3).
Fig. 3.
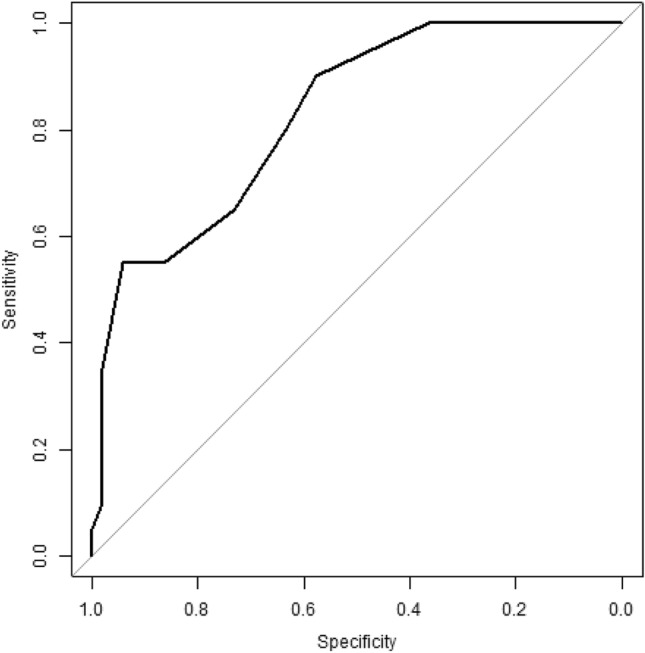
ROC curves for the ability of the DYMUS Scale to identify PD patients with an increased risk of dysphagia. We identified the DYMUS score of 6 as the cut-off for identifying patients at higher risk of dysphagia with a sensibility of 0.7 and a specificity of 0.84
Discussion
The main finding of this study is that the DYMUS questionnaire proved a useful screening tool to detect dysphagia in PD patients. Furthermore, it showed a reliable performance when compared to other clinical (EAT-10) and instrumental (FEES) dysphagia assessments.
The presence of swallowing dysfunction in PD patients can be very disabling, negatively affect their quality of life and, in some cases, lead to a fatal outcome [1, 4–6]. Dysphagia may manifest with several symptoms, e.g., difficulties in bolus formation, residual food in oral cavity, multiple swallow, and/or signs, e.g., increased oral transit, decreased swallowing reflex, reduced pharyngeal and oesophageal motility, oesophageal sphincter dysfunction [4–6, 14], which unfortunately are frequently underreported by patients or may go undetected by physicians.
In this context, the early detection of dysphagia in PD patients is of the utmost importance to reduce the risk of complications such as malnutrition and aspiration pneumonia. The diagnostic process and management of dysphagia in PD may require, especially in advances cases, a complex instrumental approach that encompasses VFSS, FEES, electrophysiological investigations and, possibly, the study of the oesophageal motility [15–17]. These investigations are minimally invasive, but often not readily accessible and require expert operators in order to ensure reliability, which makes them non-suitable for the wide-scale use in the daily clinical practice. Hence the importance of a reliable tool for screening patients at risk of dysphagia. The use of such an instrument would prompt the identification of the subjects who actually deserve being referred for more specific investigations.
In the past years, several tests have been proposed for screening dysphagia in PD patients, but these presented some limits. Some of the screening tests have been validated for neurogenic dysphagia in general, not specifically for PD, such as the Sydney Swallowing Questionnaire [18]. Other proposed tests required clinical or instrumental evaluation, such as respectively the Functional Dysphagia Scale [19] and the Swallowing Clinical Assessment Score in Parkinson’s Disease (SCAS-PD) [20].
Finally, very recently two different tools for dysphagia screening in PD failed to show a reliable performance as compared to a non invasive swallowing-respiration assessment system or to FEES, respectively [21, 22]. Among the screening tests, the DYMUS had been already validated for another chronic and progressive neurological disorder, multiple sclerosis [9, 10].
In the present study, we demonstrated that DYMUS has a relatively high internal consistency when used in PD patients (Cronbach’s alpha: 0.79). It also has a substantial repeatability. Only in the DYMUS items that tests swallowing of solid food, we obtained a moderate level of repeatability. A possible interpretation of this finding is that swallowing of solid boluses may be more affected by motor fluctuations than swallowing of liquids. We took care to administer DYMUS in the ON phase during both test and retest sessions, but it is very difficult to fully control for motor performances in sessions taken 7 days apart. Then we compared DYMUS with EAT-10, a validated test for dysphagia screening in neurological patients. For its dichotomic type of responses, we hypothesized that DYMUS could more easily understood by subjects as compared to EAT-10, which has more degrees of variability and requires a subjective evaluation of severity. We also compared DYMUS with DOSS, whose score is based on FEES evaluation.
In doing so, we were able to identify a cut-off score of DYMUS for discriminating PD subjects who are at higher risk of dysphagia. When this cut-off was adopted, DYMUS Questionnaire, compared with the DOSS, showed a good specificity (84%) and sensitivity (70%) to identify patients at higher risk of dysphagia. Hence, DYMUS allows to screen subjects for whom further evaluations is highly recommended.
Another interesting aspect that emerged from our study is the significant correlation of scores when DYMUS was filled in by patients or by their caregivers: this observation has important implications in the clinical practice because it suggests the possibility to use the caregiver as a proxy rater in those conditions where the patient’s reliability may be compromised, i.e., subjects with cognitive impairment.
In this study we focused on PD and therefore, at present, we do not know whether DYMUS is also suitable for atypical parkinsonisms, where dysphagia may be present at disease onset or in the very early phases [22, 23].
In conclusion, DYMUS proved a reliable tool to detect dysphagic PD subjects. It is also easy to self-administer or can be filled in by the caregiver. These characteristics make DYMUS a good tool for the adoption in the daily clinical practice for dysphagia screening, possibly with the more captivating acronym of DYPARK (DYsphagia in PARKinson’s disease).
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We will like to thank Elena Ballante and the BioData Science Center (IRCCS Mondino Foundation, Pavia) for the statistical analysis. Pavia: M. Berlangieri, S. Cristina, E. Alfonsi, E. Monti, IRCCS Mondino Foundation—G. Bertino, IRCCS Policlinico San Matteo. Verona: M. Tinazzi, G. Busselli, C. De Paoli, N. Smania. Moncrivello: K. Inglese, S. De Santi. Zingonia di Ciserano: N. Taiocchi, B. Zucchelli, G. Ronzoni. Venezia: S. Nordio, P. Tonin.
Biographies
Carlotta Dagna
MD
Micol Avenali
MD
Roberto De Icco
MD, PhD
Marialuisa Gandolfi
MD
Claudio Solaro
MD
Domenico Restivo
MD, PhD
Michelangelo Bartolo
MD
Francesca Meneghello
MD, PhD
Giorgio Sandrini
MD, PhD
Cristina Tassorelli
MD, PhD
M. Berlangieri
BS
S. Cristina
MD
E. Alfonsi
MD
E. Monti
BS
M. Tinazzi
MD, PhD
G. Busselli
MD
C. De Paoli
MD
N. Smania
MD
K. Inglese
MD
S. De Santi
MD
N. Taiocchi
MD
B. Zucchelli
MD
G. Ronzoni
MD
S. Nordio
MD
P. Tonin
MD
Author Contributions
CD: drafting the article, data analysis and critical interpretation of findings. MA: manuscript preparation (data analysis and critical interpretation of findings). RDI: collecting data and interpretation of the findings. MG: collecting data and critical revision of the paper. CS: collecting data and critical revision of the paper. DR: collecting data and critical revision of the paper. MB: collecting data and critical revision of the paper. FM: collecting data and critical revision of the paper. GS: critical revision of the paper. CT: conception and design, coordination of activities, revising the paper and final approval of the manuscript.
Funding
Open access funding provided by Università degli Studi di Pavia within the CRUI-CARE Agreement. This study was supported by a Grant of SIRN Italian Society of NeuroRehabilitation.
Declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
The members of DYPARK SIRN Group are listed in Acknowledgements section.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Carlotta Dagna, Email: dagna.carlotta@gmail.com.
DYPARK SIRN Group:
M. Berlangieri, S. Cristina, E. Alfonsi, E. Monti, M. Tinazzi, G. Busselli, C. De Paoli, N. Smania, K. Inglese, S. De Santi, N. Taiocchi, B. Zucchelli, G. Ronzoni, S. Nordio, and P. Tonin
References
- 1.Suttrup I, Warnecke T. Dysphagia in Parkinson’s disease. Dysphagia. 2016;31(1):24–32. doi: 10.1007/s00455-015-9671-9. [DOI] [PubMed] [Google Scholar]
- 2.Kalf JG, de Swart BJM, Bloem BR, Munneke M. Prevalence of oropharyngeal dysphagia in Parkinson’s disease: a meta-analysis. Parkinsonism Relat Disord. 2012;18(4):311–315. doi: 10.1016/j.parkreldis.2011.11.006. [DOI] [PubMed] [Google Scholar]
- 3.Fuh JL, Lee RC, Wang SJ, et al. Swallowing difficulty in Parkinson’s disease. Clin Neurol Neurosurg. 1997;99(2):106–112. doi: 10.1016/S0303-8467(97)80006-6. [DOI] [PubMed] [Google Scholar]
- 4.Ayres A, Jotz GP, Rieder CRDM, Schuh AFS, Olchik MR. The impact of dysphagia therapy on quality of life in patients with Parkinson’s disease as measured by the Swallowing Quality of Life Questionnaire (SWALQOL) Int Arch Otorhinolaryngol. 2016;20(3):206–216. doi: 10.1055/s-0036-1582450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Leow LP, Huckabee ML, Anderson T, Beckert L. The impact of dysphagia on quality of life in ageing and Parkinson’s disease as measured by the Swallowing Quality of Life (SWAL-QOL) questionnaire. Dysphagia. 2010;25(3):216–220. doi: 10.1007/s00455-009-9245-9. [DOI] [PubMed] [Google Scholar]
- 6.Plowman-Prine EK, Sapienza CM, Okun MS, et al. The relationship between quality of life and swallowing in Parkinson’s disease. Mov Disord. 2009;24(9):1352–1358. doi: 10.1002/mds.22617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pflug C, Bihler M, Emich K, et al. Critical dysphagia is common in Parkinson disease and occurs even in early stages: a prospective cohort study. Dysphagia. 2018;33(1):41–50. doi: 10.1007/s00455-017-9831-1. [DOI] [PubMed] [Google Scholar]
- 8.Evatt ML, Chaudhuri KR, Chou KL, et al. Dysautonomia rating scales in Parkinson’s disease: sialorrhea, dysphagia, and constipation—critique and recommendations by movement disorders task force on rating scales for Parkinson’s disease. Mov Disord. 2009;24:635–646. doi: 10.1002/mds.22260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bergamaschi R, Crivelli P, Rezzani C, et al. The DYMUS questionnaire for the assessment of dysphagia in multiple sclerosis. J Neurol Sci. 2008;269(1–2):49–53. doi: 10.1016/j.jns.2007.12.021. [DOI] [PubMed] [Google Scholar]
- 10.Bergamaschi R, Rezzani C, Minguzzi S, et al. Validation of the DYMUS questionnaire for the assessment of dysphagia in multiple sclerosis. Funct Neurol. 2009;24(3):159–162. [PubMed] [Google Scholar]
- 11.Belafsky PC, Mouadeb DA, Rees CJ, et al. Validity and reliability of the eating assessment tool (EAT-10) Ann Otol Rhinol Laryngol. 2008;117(12):919–924. doi: 10.1177/000348940811701210. [DOI] [PubMed] [Google Scholar]
- 12.Postuma RB, Berg D, Stern M, et al. MDS clinical diagnostic criteria for Parkinson’s disease. Mov Disord. 2015;30(12):1591–1601. doi: 10.1002/mds.26424. [DOI] [PubMed] [Google Scholar]
- 13.Neil KHO, Purdy M, Falk J, Gallo L. The Dysphagia Outcome and Severity Scale. Dysphagia. 1999;14(3):139–145. doi: 10.1007/PL00009595. [DOI] [PubMed] [Google Scholar]
- 14.Umay E, Ozturk E, Gurcay E, Delibas O, Celikel F. Swallowing in Parkinson’s disease: how is it affected? Clin Neurol Neurosurg. 2019;177:37–41. doi: 10.1016/j.clineuro.2018.12.015. [DOI] [PubMed] [Google Scholar]
- 15.Dziewas R, Baijens L, Schindler A, Verin E, Michou E, Clave P. European Society for swallowing disorders FEES Accreditation Program for neurogenic and geriatric oropharyngeal dysphagia. Dysphagia. 2017;32(6):725–733. doi: 10.1007/s00455-017-9828-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alfonsi E, Merlo IM, Ponzio M, et al. An electrophysiological approach to the diagnosis of neurogenic dysphagia: implications for botulinum toxin treatment. J Neurol Neurosurg Psychiatry. 2010;81(1):54–60. doi: 10.1136/jnnp.2009.174698. [DOI] [PubMed] [Google Scholar]
- 17.Jones CA, Ciucci MR. Multimodal swallowing evaluation with high-resolution manometry reveals subtle swallowing changes in early and mid-stage Parkinson disease. J Parkinsons Dis. 2016;6(1):197–208. doi: 10.3233/JPD-150687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Simons JA, Fietzek UM, Waldmann A, Warnecke T, Schuster T, Ceballos-Baumann AO. Development and validation of a new screening questionnaire for dysphagia in early stages of Parkinson’s disease. Parkinsonism Relat Disord. 2014;20:992–998. doi: 10.1016/j.parkreldis.2014.06.008. [DOI] [PubMed] [Google Scholar]
- 19.Lee JH, Lee KW, Kim SB, Lee SJ, Chun SM, Jung SM. The functional dysphagia scale is a useful tool for predicting aspiration pneumonia in patients with Parkinson disease. Ann Rehabil Med. 2016;40(3):440–446. doi: 10.5535/arm.2016.40.3.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Branco LL, Trentin S, Helena C, Schwanke A, Gomes I, Loureiro F. The Swallowing Clinical Assessment Score in Parkinson’s disease (SCAS-PD) is a valid and low-cost tool for evaluation of dysphagia: a gold-standard comparison study. J Aging Res. 2019;2019:7984635. doi: 10.1155/2019/7984635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Buhmann C, Flügel T, Bihler M, et al. Is the Munich dysphagia Test–Parkinson’s disease (MDT-PD) a valid screening tool for patients at risk for aspiration? Parkinsonism Relat Disord. 2019;61:138–143. doi: 10.1016/j.parkreldis.2018.10.031. [DOI] [PubMed] [Google Scholar]
- 22.Finger ME, Madden LL, Haq IU, McLouth CJ, Siddiqui MS. Analysis of the prevalence and onset of dysphonia and dysphagia symptoms in movement disorders at an academic medical center. J Clin Neurosci. 2019;64:111–115. doi: 10.1016/j.jocn.2019.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alfonsi E, Versino M, Merlo IM, et al. Electrophysiologic patterns of oral-pharyngeal swallowing in Parkinsonian syndromes. Neurology. 2007;68(8):583–589. doi: 10.1212/01.wnl.0000254478.46278.67. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.