Abstract
Background
Prostate cancer (PCa) is the most frequently diagnosed cancer in men in Europe. The impact of PCa natural history and therapeutic management on the outcomes of castration-resistant prostate cancer patients with metastasis (mCRPC) remains unclear.
Objective
The objective of this study was to describe retrospectively patterns of clinical progression through diagnosis sequences before the mCRPC stage and to assess how these sequences impacted patients’ disease progression and overall survival at mCRPC stage.
Patients and Methods
Patients with mCRPC were identified from the Prostate Cancer Registry (PCR), an observational study in a real-world setting in 16 countries between 2013 and 2016. Patients were grouped in diagnosis sequences before mCRPC and defined by date of PCa diagnosis, first metastasis, and castration resistance. Distribution of time-to-event variables were estimated using Kaplan-Meier product-limit survival curves for overall survival (OS) and progression-free survival (PFS). Non-adjusted Cox models were conducted for efficacy endpoints (OS, PFS) to estimate hazard ratios between diagnosis sequences.
Results
At the end of study, 2859 mCRPC patients were included in this analysis. Among mCRPC four diagnosis sequences were identified: 35% developed metastases (mHSPC) before becoming castration resistant (sequence 1, metachronous mHSPC), 10% developed castration resistance (nmCRPC) before metastases (sequence 2), 27% developed metastases and castration resistance within 4 months (sequence 3) and 28% of patients were de novo mHSPC (sequence 4). Median OS was 17.7 months (interquartile range (IQR): 8.8–29.9) and PFS was 6.4 months (IQR: 3.2–12.0). The univariate analyses showed no correlation between mCRPC patients’ OS or PFS and the diagnosis sequence.
Conclusion
This large European study describe four different patterns of prostate cancer progression to mCRPC stage. Our results indicate that patient survival becomes comparable after progression to mCRPC, regardless of the diagnosis sequence.
Trial Registration
ClinicalTrials.gov identifier NCT02236637; registered September 2014.
Supplementary Information
The online version contains supplementary material available at 10.1007/s11523-022-00899-6.
Key Points
| Question: How patient disease history in terms of diagnosis sequences before mCRPC impact patients’ disease progression and overall survival? |
| Findings: OS and PFS at mCRPC stage were not significantly influenced by disease history of PCa, identified into four diagnosis sequences as follows: 28% of mCRPC patients were de novo mHSPC, and the other 72% were diagnosed at a local/locally advanced disease stage, with 10% nmCRPC before metastases, 35% with metastases before mCRPC, and 27% with metastases and castration-resistance. |
| Meaning: Patient survival becomes comparable after progression to mCRPC, regardless of the diagnosis sequence. |
Background
Prostate cancer (PCa) is the most frequently diagnosed cancer in men in the EU with an incidence rate of 103.2 cases per 100,000 men in 2018, greater than the incidence rates of both lung (63.5 cases per 100,000 men) and colorectal cancer (57.5 cases per 100,000 men). In 2018, there were 81,540 deaths due to PCa in the EU, making it the third most lethal cancer among men in the EU [1].
Prostate-specific antigen (PSA) screening tests have allowed earlier detection of the disease, leading to stage migration toward low-risk, low-grade disease and allowing most patients to be offered local treatment with curative intent (surgery or radiotherapy) [2–4]. However, around 30% of patients undergoing surgery and 40% of patients undergoing radiation therapy will experience rising PSA levels (biochemical recurrence) within 10 years following local therapy [5]. Patients with biochemical recurrence have a variable prognosis with metastasis-free survival ranging from 1 to more than 15 years [6].
Management of biochemical recurrence usually consists of androgen deprivation therapy (ADT), achieved chemically with gonadotropin-releasing hormone (GnRH) agonists or antagonists [4]. As the cancer progresses the androgen receptors may develop some mechanisms (mutations, splicing, or gene amplification), allowing increasing numbers to function without androgen, leading to rising PSA levels and signaling castration-resistant PCa (CRPC) [7, 8].
Patients who progress to metastatic disease mostly develop bone metastases [6]. Non-metastatic (nm) patients who are not treated with ADT progress to metastatic hormone-sensitive PCa (mHSPC), which will become mCRPC within 1–3 years. [9–13]. Non-metastatic CRPC and nmHSPC patients who are treated with ADT alone often progress to mCRPC, the final stage of PCa that is also associated with an elevated mortality risk [7, 8, 14]. Recommended therapeutic options in mCRPC patients consist of sequences of chemotherapy (docetaxel, cabazitaxel), recent novel hormonal therapies like abiraterone or enzalutamide, and Radium-223, allowing for median overall survivals (OS) of up to 3 years [15, 16], or olaparib, a poly(adenosine diphosphate–ribose) polymerase (PARP) inhibitor. However, the impact that the sequence of diagnosis has on the outcomes for mCRPC patients remains unclear.
We analyzed data from the Prostate Cancer Registry (PCR) from 2013 to 2016 to describe treatment patterns of mCRPC in current practice and to document how patient demographics, clinical characteristics, disease history, and treatment history impacted patients’ disease progression and OS.
Materials and Methods
Materials
The PCR (NCT02236637) is the first international, prospective observational study of patients with mCRPC, and was initiated to examine retrospectively the care of patients with mCRPC in a real-world setting [17, 18]. It is a non-interventional, multicentre, observational registry of patients with a confirmed diagnosis of mCRPC based on the documented presence of metastatic PCa and castration resistance.
Observational methodology was used to capture data from 199 participating centres specializing in the treatment of PCa by both medical oncologists and urologists in 16 countries (Online Supplementary Material (OSM) Table 1). Adults with a confirmed diagnosis of adenocarcinoma of the prostate, documented metastatic PCa, and documented castration resistance were eligible for enrolment in the registry. Treatment decisions were made at the discretion of the treating physician, per routine clinical practice. This includes prescription of treatments according to their labelled indication or local clinical guidelines. The study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement.
Collected Data
Data collected at entry of study (baseline) included patient demographics (country, age, gender) and comorbid medical conditions. Retrospective data related to the patient’s PCa characteristics and treatments included: dates of initial diagnosis, castration resistance and first metastatic diagnosis, Gleason score and TNM stage at diagnosis, and PCa treatments (local and systemic therapy) received since diagnosis. Diagnosis of mCRPC was defined by disease progression despite testosterone < 50 ng/dL and/or ADT and/or a history of orchiectomy. Data related to the patient’s current PCa characteristics at baseline included: symptoms of disease and current treatment(s), ECOG performance status (ECOG PS), biological parameters (PSA, LDH, serum testosterone) and, where permitted, EQ-5D-5L and FACT-P questionnaires.
All subjects enrolled in the registry were included in the analysis. The eligibility period extended from the index date of 1 June 2013 until 11 March 2016. Data cut-off date was 31 December 2018. Patient demographics and disease characteristics were collected at baseline, and patients were followed for up to 3 years. Only data available from routine clinical practice were collected. Quality control measures were followed to ensure the accuracy and reliability of the data recorded on case report forms (CRFs), including regular on-site monitoring and data verification. Routine monitoring of data performed by trained clinical study professionals ensured congruence between data entered in the electronic CRF (eCRF) by site personnel and into patients’ medical records.
However, in line with non-interventional methodology, the protocol did not stipulate any structured treatment, clinic visit or assessment schedules. Thus, the frequency of data collection, the presence and completeness of assessments—and hence data availability—was subject to variability from clinic to clinic, and from patient to patient. Furthermore, at the start of the PCR, abiraterone acetate plus prednisone and enzalutamide were not routinely available in the mCRPC setting in all 16 countries.
Statistical Analyses
For the purposes of the current analysis, patients were grouped retrospectively in different clinical stages according to their diagnosis sequences before the mCRPC stage. Date of cancer diagnosis, first metastasis and castration resistance determined five different clinical stages, defining four different diagnosis sequences: patients diagnosed with localized or locally advanced PCa, whose disease progressed through mHSPC before mCRPC (sequence 1, metachronous mHSPC), nmCRPC before mCRPC (sequence 2), mCRPC without a prior diagnosis of nmCRPC or mHSPC (sequence 3) and newly diagnosed (de novo) mHSPC who were diagnosed with mCRPC (sequence 4, de novo mHSPC). A ≤ 4 months’ delay between metastases and castration-resistance diagnoses were considered to distinguish between sequences 1 or 2 and 3 [19]. Patients with metastases and castration-resistance diagnoses within 4 months after a diagnosis of PrCA constituted sequence 3.
Natural history patterns until mCRPC were then distinguished by studying the diagnosis sequences. Data collected at baseline, patient characteristics listed above, and change in biological parameters were analyzed descriptively (summary statistics for continuous variables, counts and percentages for categorical and binary variables, missing data). Distribution of time-to-event variables since mCRPC diagnosis were estimated using Kaplan-Meier product-limit survival curves for OS and progression-free survival (PFS). The median times to event with two-sided 95% confidence intervals were estimated and compared between groups using log-rank tests. Data were right-censored at the last follow-up visit and no replacement of missing data was performed.
Non-adjusted Cox models were conducted for efficacy endpoints [OS, progression-free survival (PFS)] to estimate hazard ratios between diagnosis-sequence. Multivariate analyses, including clinical meaningful covariates at diagnosis (ECOG performance score, age, Gleason score, number of bone lesions, number of previous mCRPC treatments), period of inclusion (before/after 1 January 2014) were also performed for diagnosis-sequence.
Results
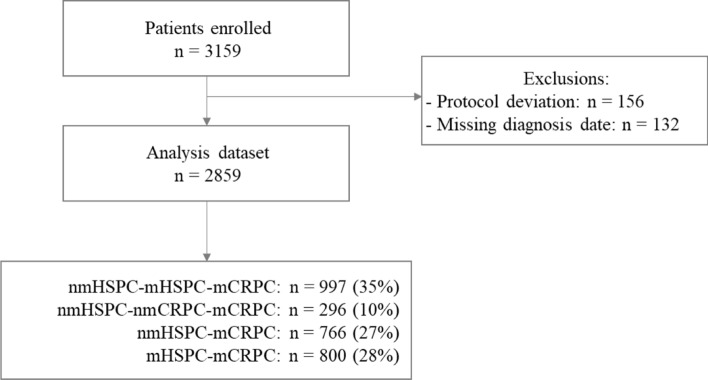
At the end of study, 2859 patients were evaluated; 288 patients included in the registry were excluded from the analysis, either due to protocol deviation or missing diagnosis date (Fig. 1). The study duration was 5.6 years, with a median patient follow-up of 18.5 months (range 8.8–31.5).
Fig. 1.
Flowchart of included patients. mCRPC metastatic castration resistance prostate cancer, mHSPC metastatic hormone-sensitive prostate cancer, nmCRPC non-metastatic castration-resistant prostate cancer
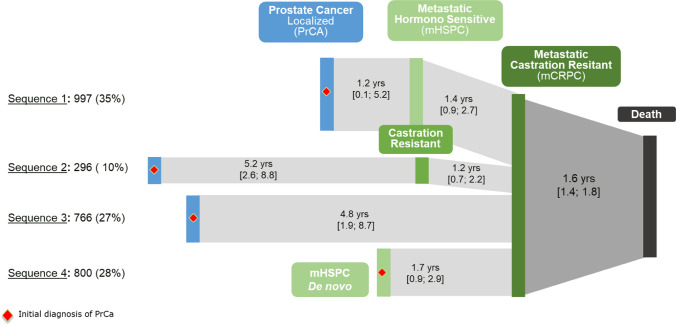
Figure 2 presents the distribution of patients between treatment sequences and the median duration between the disease stages. Four diagnosis sequences were identified: 35% (n = 997) developed metastases (mHSPC) before becoming castration-resistant (sequence 1, metachronous mHSPC), 10% (n = 269) developed castration-resistance (nmCRPC) before metastases (sequence 2), 27% (n = 766) developed metastases and castration-resistance within 4 months (sequence 3), and 28% (n = 800) of patients were de novo mHSPC (sequence 4, de Novo mHSPC).
Fig. 2.
Progression of patients until mCRPC in the European Cancer Prostate Registry–Median [Q1–Q3] in years. mCRPC metastatic castration-resistant prostate cancer, mHSPC metastatic hormone-sensitive prostate cancer, nmCRPC non-metastatic castration-resistant prostate cancer, PrCa prostate cancer
Table 1 presents the baseline characteristics of patients by diagnosis sequence. Mean age was 72.6 years, with patients in sequence 4 (de novo mHSPC) being younger than patients in sequences 1, 2 and 3. At mCRPC stage, 63.4% of patients had a bone metastasis, 34.7% had a lymph node metastasis, and 12.6% had visceral metastasis (liver 5.6% and lung 7.0%). A minority of patients (24.7%) had oligometastatic bone disease (< five lesions). The median PSA level was 175 ng/mL. The average duration between initial PCa diagnosis and the start of the study was shorter in de novo mHSPC patients with a more severe disease: compared to other groups, > 20% of these patients had ≥ 20 bone lesions, their Gleason score were more frequently ≥ 8 at diagnosis with a higher PSA at baseline (mean PSA 231 ng/mL).
Table 1.
Characteristics at the time of entry into the PC Registry by diagnosis sequence
| nmHSPC -mHSPC-mCRPC | nmHSPC-nmCRPC-mCRPC | nmHSPC -mCRPC | mHSPC-mCRPC | Total | |
|---|---|---|---|---|---|
| N = 997 | N = 296 | N = 766 | N = 800 | N = 2859 | |
| Follow-up (mo.) Mean (SD) | 20.4 (11.9) | 19.9 (11.9) | 19.5 (12.1) | 19.0 (11.9) | 19.7 (12.0) |
| Median | 19.4 | 18.4 | 18.1 | 17.2 | 18.5 |
| Age, Mean (y) (SD) | 72.5 (7.9) | 75.8 (7.0) | 74.1 (8.0) | 70.2 (8.9) | 72.6 (8.3) |
| Gleason score at PrCa diagnosis | |||||
| ≤ 6 | 126 (12.6%) | 42 (14.2%) | 122 (15.9%) | 39 (4.9%) | 329 (11.5%) |
| 7 | 310 (33.4%) | 82 (31.9%) | 254 (36.3%) | 200 (27.9%) | 846 (32.5%) |
| 8 | 198 (21.3%) | 57 (22.2%) | 138 (19.7%) | 192 (26.7%) | 585 (22.5%) |
| 9+ | 295 (31.7%) | 76 (29.5%) | 186 (26.6%) | 287 (39.9%) | 844 (32.4%) |
| ECOG Performance Status | |||||
| 0 | 353 (39.3%) | 108 (39.6%) | 288 (40.2%) | 267 (36.2%) | 1016 (38.7%) |
| 1 | 443 (49.3%) | 126 (46.2%) | 340 (47.5%) | 357 (48.4%) | 1266 (48.2%) |
| 2 | 83 (9.2%) | 34 (12.5%) | 65 (9.1%) | 91 (12.3%) | 273 (10.4%) |
| 3 | 20 (2.2%) | 5 (1.8%) | 22 (3.1%) | 20 (2.7%) | 67 (2.6%) |
| 4 | 0 | 0 | 1 (0.1%) | 2 (0.3%) | 3 (0.1%) |
| Site of lesion | |||||
| Bone | 636 (63.8%) | 162 (54.7%) | 453 (59.1%) | 563 (70.4%) | 1814 (63.4%) |
| Node | 325 (32.6%) | 125 (42.2%) | 294 (38.4%) | 249 (31.1%) | 993 (34.7%) |
| Prostate | 123 (12.3%) | 29 (9.8%) | 91 (11.9%) | 129 (16.1%) | 372 (13.0%) |
| Liver | 48 (4.8%) | 15 (5.1%) | 46 (6.0%) | 51 (6.4%) | 160 (5.6%) |
| Lung | 69 (6.9%) | 20 (6.8%) | 58 (7.6%) | 52 (6.5%) | 199 (7.0%) |
| Other | 71 (7.1%) | 40 (13.5%) | 56 (7.3%) | 40 (5.0%) | 207 (7.2%) |
| Number of bone lesion(s) | |||||
| 0 lesions | 56 (7.9%) | 28 (13.1%) | 64 (11.6%) | 18 (3.1%) | 166 (8.1%) |
| 1 lesion | 61 (8.6%) | 31 (14.5%) | 53 (9.6%) | 20 (3.4%) | 165 (8.0%) |
| 2 lesions | 35 (5.0%) | 13 (6.1%) | 48 (8.7%) | 35 (6.0%) | 131 (6.4%) |
| 3–4 lesions | 76 (10.7%) | 27 (12.6%) | 60 (10.8%) | 49 (8.4%) | 212 (10.3%) |
| 5–9 lesions | 111 (15.7%) | 37 (17.3%) | 92 (16.6%) | 93 (16.0%) | 333 (16.2%) |
| 10–20 lesions | 84 (11.9%) | 26 (12.1%) | 67 (12.1%) | 79 (13.6%) | 256 (12.5%) |
| > 20 lesions | 104 (14.7%) | 14 (6.5%) | 56 (10.1%) | 122 (21.0%) | 296 (14.4%) |
| Present, nb unknown | 176 (24.9%) | 37 (17.3%) | 110 (19.9%) | 161 (27.8%) | 484 (23.6%) |
| Not evaluable | 4 (0.6%) | 1 (0.5%) | 3 (0.5%) | 3 (0.5%) | 11 (0.5%) |
| Missing | 290 (29.1%) | 82 (27.7%) | 213 (27.8%) | 220 (27.5%) | 805 (28.2%) |
| Biological parameters at baseline | |||||
| Prostate-specific antigen (PSA) (ng/mL), Mean (sd) | 146 (321) | 155 (297) | 164 (394) | 231 (744) | 175 (491) |
| Haemoglobin (Hb) (g/dL), Mean (SD) | 12.4 (1.7) | 12.4 (1.7) | 12.4 (1.6) | 12.2 (1.8) | 12.3 (1.7) |
| Serum testosterone (ng/dL), Mean (SD) | 2.7 (8.2) | 2.3 (5.8) | 2.2 (6.5) | 1.8 (4.7) | 2.3 (6.7) |
mCRPC metastatic castration-resistant prostate cancer, mHSPC metastatic hormone-sensitive prostate cancer, nmCRPC non-metastatic castration-resistant prostate cancer, PrCa prostate cancer
The different diagnosis-sequence groups were comparable in terms of number of mCRPC treatments received before inclusion in the Prostate Cancer Registry. In the management care before the mCRPC stage, 32.5% of patients received chemotherapy, 35.9% received bone-targeted therapy, and 53.2% received radiation therapy. Patients who transited by nmCRPC before mCRPC had received more surgery, radiotherapy and chemotherapy between PCa and mCRPC diagnoses (56.4%, 65.2% and 40.3%, respectively), while de novo mHSPC had received less (19.8%, 36.6% and 32.9%, respectively). Consistent with the number of bone lesions, de novo mHPSC patients had received more bone-targeted agents (Table 2). In addition, de novo mHPSC had higher Gleason scores and higher PSA at baseline.
Table 2.
Prior anticancer therapy by diagnosis sequence
| nmHSPC -mHSPC-mCRPC | nmHSPC-nmCRPC-mCRPC | nmHSPC -mCRPC | mHSPC-mCRPC | Total | |||||
|---|---|---|---|---|---|---|---|---|---|
| Surgery since diagnosis | 432 (43.3%) | 167 (56.4%) | 374 (48.8%) | 158 (19.8%) | 1131 (39.6%) | ||||
| Type of prior surgery | |||||||||
| Radical prostatectomy | 237 (23.8%) | 108 (36.5%) | 193 (25.2%) | 18 (2.3%) | 556 (19.4%) | ||||
| Orchiectomy | 52 (5.2%) | 14 (4.7%) | 42 (5.5%) | 58 (7.3%) | 166 (5.8%) | ||||
| Other | 147 (14.7%) | 47 (15.9%) | 145 (18.9%) | 85 (10.6%) | 424 (14.8%) | ||||
| Radiotherapy since diagnosis | 588 (59.0%) | 193 (65.2%) | 447 (58.4%) | 293 (36.6%) | 1521 (53.2%) | ||||
| Type of radiotherapy since diagnosis | |||||||||
| Prostate | 382 (38.3%) | 161 (54.4%) | 329 (43.0%) | 74 (9.3%) | 946 (33.1%) | ||||
| Rachis | 140 (14.0%) | 22 (7.4%) | 64 (8.4%) | 120 (15.0%) | 346 (12.1%) | ||||
| Limb | 46 (4.6%) | 6 (2.0%) | 11 (1.4%) | 30 (3.8%) | 93 (3.3%) | ||||
| Costal | 22 (2.2%) | 2 (0.7%) | 11 (1.4%) | 14 (1.8%) | 49 (1.7%) | ||||
| Brain | 3 (0.3%) | 1 (0.3%) | 2 (0.3%) | 0 | 6 (0.2%) | ||||
| Other | 227 (22.8%) | 57 (19.3%) | 148 (19.3%) | 132 (16.5%) | 564 (19.7%) | ||||
| Number of previous mCRPC treatments | |||||||||
| 0 | 616 (61.8%) | 166 (56.1%) | 500 (65.3%) | 498 (62.3%) | 1780 (62.3%) | ||||
| 1 | 237 (23.8%) | 74 (25.0%) | 160 (20.9%) | 198 (24.8%) | 669 (23.4%) | ||||
| 2 | 103 (10.3%) | 34 (11.5%) | 74 (9.7%) | 66 (8.3%) | 277 (9.7%) | ||||
| 3 | 26 (2.6%) | 18 (6.1%) | 23 (3.0%) | 28 (3.5%) | 95 (3.3%) | ||||
| 4 | 14 (1.4%) | 3 (1.0%) | 9 (1.2%) | 8 (1.0%) | 34 (1.2%) | ||||
| 5 | 1 (0.1%) | 1 (0.3%) | 0 | 2 (0.3%) | 4 (0.1%) | ||||
| Docetaxel since diagnosis | 163 (16.3%) | 49 (16.6%) | 97 (12.7%) | 144 (18.0%) | 453 (15.8%) | ||||
| Type anticancer therapy since diagnosis | |||||||||
| Bone-targeted agents | 393 (40.3%) | 75 (26.0%) | 169 (23.1%) | 357 (46.2%) | 994 (35.9%) | ||||
| Chemotherapy | 318 (32.6%) | 116 (40.3%) | 210 (28.7%) | 254 (32.9%) | 898 (32.5%) | ||||
| Hormonal treatment | 968 (99.4%) | 288 (100%) | 730 (99.7%) | 754 (97.5%) | 2740 (99.0%) | ||||
| Novel hormonal therapiesa | 65 (6.5%) | 22 (7.4%) | 57 (7.5%) | 47 (5.9%) | 191 (6.7%) | ||||
mCRPC metastatic castration-resistant prostate cancer, mHSPC metastatic hormone-sensitive prostate cancer, nmCRPC non-metastatic castration-resistant prostate cancer, PrCa prostate cancer
aAt the start of the Prostate Cancer Registry, next-generation hormonal treatment (i.e., abiraterone acetate and enzalutamide) were not routinely available for patients with mCRPC in all 16 countries
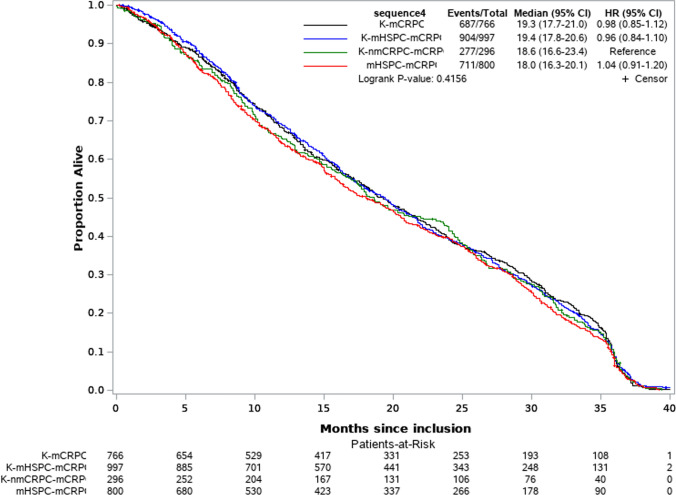
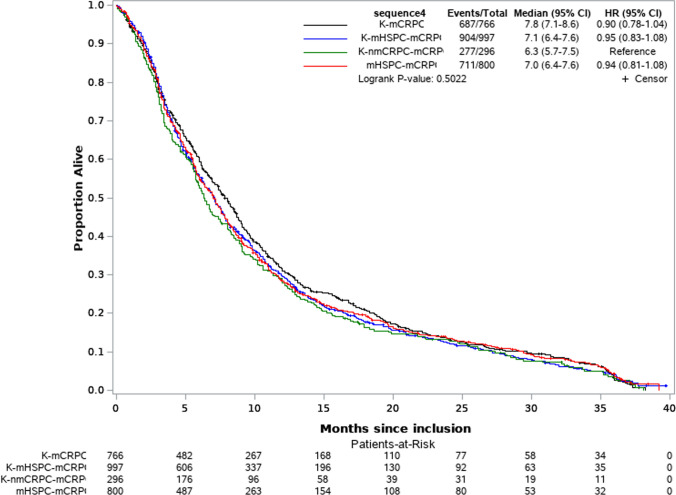
Overall, in patients at mCRPC stage median OS was 17.7 months (interquartile range (IQR): 8.8–29.9) and PFS was 6.4 months (IQR: 3.2–12.0), while univariate analyses did not show a significant impact of treatment sequence on OS (Fig. 3) or PFS (Fig. 4).
Fig. 3.
Overall survival by diagnosis sequence - Kaplan Meier curve and hazard ratio. K prostate cancer; mCRPC metastatic castration resistance prostate cancer, mHSPC metastatic hormone-sensitive prostate cancer, nmCRPC non-metastatic castration-resistant prostate cancer
Fig. 4.
Progression-free survival by diagnosis sequence- Kaplan Meier curve and hazard ratio. K prostate cancer; mCRPC metastatic castration-resistant prostate cancer, mHSPC metastatic hormone-sensitive prostate cancer, nmCRPC non-metastatic castration-resistant prostate cancer
Discussion and Conclusion
Using the European Prostate Cancer Registry—the largest cohort of patients with mCRPC receiving first-line treatment—we analyzed the characteristics, disease and treatment sequence of 2859 European patients with a mCRPC diagnosis between 2013 and 2016, and studied how these characteristics impact patients’ outcomes. The main finding was the repartition of mCRPC pathways into four diagnosis sequences, where 28% of mCRPC patients were de novo mHSPC. The other 72% were diagnosed at a local/locally advanced disease stage. Among mCRPC patients, 10% developed castration-resistance (nmCRPC) before metastases, within a median duration of 6.4 years, 35% developed metastases (mHSPC) before becoming castration-resistant (metachronous mHSPC), and 27% developed metastases and castration-resistance within 4 months or less.
The sequence 1 with a previous local treatment and mHSPC stage is the most common pattern in our study (35%). This pattern corresponds to the evolution of the disease after a local treatment and the occurrence of metastases. Notably those patients who progressed to mCRPC went through a rapid progression from localized disease to mHSPC (1.2 years [IQR: 0.1–5.2]).
The number of patients (27%) who developed metastases and castration resistance within 4 months or less (sequence 3) was surprising, indicating they had late diagnosis of metastases when the disease was already castration resistant, which may not intuitively reflect the clinical setting. These patients were most likely treated by hormone therapy (HT) without prior local treatment, and mostly were patients over 75 years with comorbidities.
The relatively low proportion (10%) of patients with castration resistance (nmCRPC) before metastases (sequence 2) demonstrated that only a small proportion of PCa patients with biochemical progression after HT without visible metastases. Besides, it is very likely that their proportion will decrease over time with the improvement of imaging techniques, especially PET imaging.
Our secondary finding is that OS and PFS of metastatic PCa patients after castration resistance was not significantly influenced by the natural history of PCa and diagnosis sequence prior to mCRPC stage. Multivariate analyses adjusting for patients’ characteristics and proxies for disease severity at diagnosis found that patients who went through mHSPC (newly diagnosed or progressive) had better OS and PFS compared to other diagnosis sequences (OSM Tables S2 and S3). However, these significant differences were likely due to adjustment on parameters determinant of the treatment sequence itself, adding bias to the analysis by undermining the underlying differences between sequences [20]. Consistent with the literature, de novo mHSPC were found to have a higher Gleason score, higher tumor burden (> 20 bone lesions), higher PSA at baseline, and greater use of bone-targeted agents [11].
Overall survival was relatively poor in our real-world study, since all men died within 3 years with a median OS of 17.7 months, in comparison to existing clinical trials such as PREVAIL [21] and COU-AA-302 [22], where median OS was 30 months. If most patients from this study were not treated according to actual best standard of care, results from this largest European cohort (2859 patients) highlight the poor prognosis for patients at the mCRPC stage in a real-world setting, with higher ECOG, higher Gleason, more bone metastasis, and more comorbidities, in comparison to a randomized study with restricted inclusion criteria.
The Prostate Cancer Registry (PCR) allowed the determination of time spent by patients at the different disease stages. These durations were consistent with the PFS and metastasis-free survival reported in placebo arms of published randomized controlled trials in mHSPC and nmCRPC patients [12, 13, 23–25]. Indeed, due to the period of inclusion of the PCR, it can be assumed that very few patients received novel hormonal therapies before mCRPC diagnosis, while only 15.8% of patients received docetaxel prior to castration resistance. Besides, the 2,059 patients diagnosed at a local/locally advanced stage were analyzed based on the type of treatment received before mCRPC diagnosis.
This study benefits from robust monitoring data, the size and scope of the PCR allowing for inclusion of patient subgroups under-represented in clinical trials, such as some cardiovascular disease requiring treatment, or diabetes mellitus, or visceral metastases, and a duration that allows mature data to be collected. This is the largest PCR describing natural diagnosis sequences for mCRPC patients. Collected data were rich enough to reconstitute diagnosis sequences based on date of cancer diagnosis, first metastasis, and castration resistance occurring from 2010 to 2016. However, a limitation may arise from the nature of the collected data. Delay for ascertainment regarding the presence of metastases and castration resistance may be variable depending on the patient’s profile and the period of diagnosis. Hence, a 4-month period between metastases and castration-resistance diagnoses was considered to distinguish between sequences 1 or 2 and 3. However, as highlighted by the proportion of patients in sequence 1, some patients still had a diagnosis of PCa and metastases very close together.
In addition, due to the non-interventional design of the registry, the PSA threshold triggering metastasis detection as well as imaging modalities (PET, bone scan, etc.) were varied between centres and periods. However, these discrepancies may have modestly impacted the reported delay between initial diagnosis and metastasis since, during the study period (2013–2016), PET imaging was not routinely used in PCa and only indicated in a castration-resistant setting.
Overall, our results indicate that patients’ survival becomes comparable after progression to mCRPC, regardless of the diagnosis sequence, although the natural history of the disease is indicative of the overall prognosis, as reflected by the patients’ distribution among the treatment sequences.
Even though the median sequence durations identified in our analysis reflect the more severe nature of de novo mHSPC–mCRPC and progressive mHSPC–mCRPC sequences, our results indicate that this classification of treatment sequences based on clinical trials is not relevant after disease progression to mCRPC, where all patients are associated with similar PFS and OS.
This result is indicative of the biologic evolution of PCa at later stages through which tumoral cells become castration resistant and blur the differences between patients with different diagnosis sequences, leading to similar prognoses. This is consistent with results from Hatakeyama et al. who compared the evolution of low- and high-volume mHSPC patients until and after castration resistance: even though the latter had shorter CRPC-free survival, both groups had similar OS after castration resistance [26]. Similarly, trial results in mHSPC and nmCRPC showed that delayed progression and castration resistance were associated with significant OS improvements [12, 13, 23–25], leading to the current recommended use of chemotherapy and novel hormonal therapies before mCRPC [4].
Over the past few years, the treatment landscape of advanced prostate has rapidly and dramatically evolved with multiple novel therapeutic classes: androgen-axis inhibition, PARP inhibitors, immune checkpoint inhibitors, and molecularly targeted therapies [27]. Emergence of additional new treatments targeting tumor markers in the future may allow for further efficacy improvements. In this fast-evolving environment, additional investigations are required to determine how global OS after progression to mCRPC is positively impacted.
In many countries, routine clinical practice is already influenced by the introduction of some of these new alternatives, but also by novel classifications and diagnostic tools that have been paving the way for the development of precision medicine in prostate cancer. Further research is needed to identify patients who would benefit the most from a broader treatment approach and who need a more targeted therapy.
Supplementary Information
Below is the link to the electronic supplementary material.
Declarations
Funding
The Prostate Cancer Registry (PCR) secondary analyses were funded by Janssen-Cilag France. Janssen Cilag, France contributed to the study design, the collection, analysis, and interpretation of data, the writing of the report, and the decision to submit the paper for publication.
Conflict of interest
Camille Verry, Sébastien Vincendeau, and Marc-Olivier Timsit received fees from Janssen-Cilag to participate in the conception, design, interpretations, and the writing of these secondary analyses presented in this manuscript. Marie-Laure Bazil, Pauline Bernardini, and Ségolène Pettré are employees of Janssen-Cilag, Issy les Moulineaux, France. Marc Massetti, Martin Blachier, and Alexandre Vimont are employees of Public Health Expertise which was contracted by Janssen-Cilag, France to perform the Prostate Cancer Registry (PCR) secondary analyses and participate in the writing of the manuscript.
Ethics approval
The Prostate Cancer Registry (PCR) was approved by the ethics committees of every country that participated. The study was conducted in accordance with the Declaration of Helsinki.
Consent to participate
All patients provided written informed consent.
Availability of data and material
The data-sharing policy of Janssen Pharmaceutical Companies of Johnson & Johnson is available at https://www.janssen.com/clinical-trials/transparency. As noted on this site, requests for access to the study data can be submitted through Yale Open Data Access (YODA) Project site at https ://yoda.yale.edu.
Author contributions
All authors contributed to the Prostate Cancer Registry (PCR) secondary analyses including study conception, design, and writing of the manuscript. All authors read and approved the final manuscript. Public Health Expertise which was contracted by J-C, France to perform the Prostate Cancer Registry (PCR) secondary registry analyses and participate in the writing of the manuscript. The funder of the study (J-C) had a role in study design, data collection, data analysis, data interpretation (in collaboration with all authors), and writing of the manuscript. The corresponding author had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Contributor Information
Camille Verry, Email: cverry@chu-grenoble.fr.
Sébastien Vincendeau, Email: svincendeau@vivalto-sante.com.
Marc Massetti, Email: marc.massetti@ph-expertise.com.
Martin Blachier, Email: martin.blachier@ph-expertise.com.
Alexandre Vimont, Email: alexandre.vimont@ph-expertise.com.
Marie-Laure Bazil, Email: mbazil@its.jnj.com.
Pauline Bernardini, Email: pbernar2@its.jnj.com.
Ségolène Pettré, Email: spettre@its.jnj.com.
Marc-Olivier Timsit, Email: marc-olivier.timsit@aphp.fr.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Cooperberg MR, Lubeck DP, Meng MV, Mehta SS, Carroll PR. The changing face of low-risk prostate cancer: trends in clinical presentation and primary management. J Clin Oncol. 2004;22(11):2141–2149. doi: 10.1200/JCO.2004.10.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cooperberg MR, Broering JM, Kantoff PW, Carroll PR. Contemporary trends in low risk prostate cancer: risk assessment and treatment. J Urol. 2007;178(3 Pt 2):S14–S19. doi: 10.1016/j.juro.2007.03.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Parker C, Castro E, Fizazi K, Heidenreich A, Ost P, Procopio G, et al. Prostate cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2020;31(9):1119–1134. doi: 10.1016/j.annonc.2020.06.011. [DOI] [PubMed] [Google Scholar]
- 5.Paller CJ, Antonarakis ES. Management of biochemically recurrent prostate cancer after local therapy: evolving standards of care and new directions. Clin Adv Hematol Oncol. 2013;11(1):14–23. [PMC free article] [PubMed] [Google Scholar]
- 6.Antonarakis ES, Feng Z, Trock BJ, Humphreys EB, Carducci MA, Partin AW, et al. The natural history of metastatic progression in men with prostate-specific antigen recurrence after radical prostatectomy: long-term follow-up. BJU Int. 2012;109(1):32–39. doi: 10.1111/j.1464-410X.2011.10422.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chang KH, Ercole CE, Sharifi N. Androgen metabolism in prostate cancer: from molecular mechanisms to clinical consequences. Br J Cancer. 2014;111(7):1249–1254. doi: 10.1038/bjc.2014.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Scher HI, Heller G. Clinical states in prostate cancer: toward a dynamic model of disease progression. Urology. 2000;55(3):323–327. doi: 10.1016/S0090-4295(99)00471-9. [DOI] [PubMed] [Google Scholar]
- 9.Sweeney CJ, Chen YH, Carducci M, Liu G, Jarrard DF, Eisenberger M, et al. Chemohormonal therapy in metastatic hormone-sensitive prostate cancer. N Engl J Med. 2015;373(8):737–746. doi: 10.1056/NEJMoa1503747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gravis G, Fizazi K, Joly F, Oudard S, Priou F, Esterni B, et al. Androgen-deprivation therapy alone or with docetaxel in non-castrate metastatic prostate cancer (GETUG-AFU 15): a randomised, open-label, phase 3 trial. Lancet Oncol. 2013;14(2):149–158. doi: 10.1016/S1470-2045(12)70560-0. [DOI] [PubMed] [Google Scholar]
- 11.James ND, Spears MR, Clarke NW, Dearnaley DP, De Bono JS, Gale J, et al. Survival with newly diagnosed metastatic prostate cancer in the "Docetaxel Era": data from 917 Patients in the Control Arm of the STAMPEDE Trial (MRC PR08, CRUK/06/019) Eur Urol. 2015;67(6):1028–1038. doi: 10.1016/j.eururo.2014.09.032. [DOI] [PubMed] [Google Scholar]
- 12.Chi KN, Agarwal N, Bjartell A, Chung BH, Pereira de Santana Gomes AJ, Given R, et al. Apalutamide for metastatic, castration-sensitive prostate cancer. N Engl J Med. 2019;381(1):13–24. doi: 10.1056/NEJMoa1903307. [DOI] [PubMed] [Google Scholar]
- 13.Fizazi K, Tran N, Fein L, Matsubara N, Rodriguez-Antolin A, Alekseev BY, et al. Abiraterone acetate plus prednisone in patients with newly diagnosed high-risk metastatic castration-sensitive prostate cancer (LATITUDE): final overall survival analysis of a randomised, double-blind, phase 3 trial. Lancet Oncol. 2019;20(5):686–700. doi: 10.1016/S1470-2045(19)30082-8. [DOI] [PubMed] [Google Scholar]
- 14.Ingrosso G, Detti B, Scartoni D, Lancia A, Giacomelli I, Baki M, et al. Current therapeutic options in metastatic castration-resistant prostate cancer. Semin Oncol. 2018;45(5–6):303–315. doi: 10.1053/j.seminoncol.2018.10.001. [DOI] [PubMed] [Google Scholar]
- 15.Ryan CJ, Smith MR, Fizazi K, Saad F, Mulders PF, Sternberg CN, et al. Abiraterone acetate plus prednisone versus placebo plus prednisone in chemotherapy-naive men with metastatic castration-resistant prostate cancer (COU-AA-302): final overall survival analysis of a randomised, double-blind, placebo-controlled phase 3 study. Lancet Oncol. 2015;16(2):152–160. doi: 10.1016/S1470-2045(14)71205-7. [DOI] [PubMed] [Google Scholar]
- 16.Armstrong AJ, Lin P, Tombal B, Saad F, Higano CS, Joshua AM, et al. Five-year survival prediction and safety outcomes with enzalutamide in men with chemotherapy-naive metastatic castration-resistant prostate cancer from the PREVAIL Trial. Eur Urol. 2020;78(3):347–357. doi: 10.1016/j.eururo.2020.04.061. [DOI] [PubMed] [Google Scholar]
- 17.Chowdhury S, Bjartell A, Lumen N, Maroto P, Paiss T, Gomez-Veiga F, et al. Real-world outcomes in first-line treatment of metastatic castration-resistant prostate cancer: the prostate cancer registry. Target Oncol. 2020;15(3):301–315. doi: 10.1007/s11523-020-00720-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bjartell A, Lumen N, Maroto P, Paiss T, Gomez-Veiga F, Birtle A, et al. Real-world safety and efficacy outcomes with abiraterone acetate plus prednisone or prednisolone as the first- or second-line treatment for metastatic castration-resistant prostate cancer: data from the Prostate Cancer Registry. Target Oncol. 2021;16(3):357–367. doi: 10.1007/s11523-021-00807-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thurin NH, Rouyer M, Gross-Goupil M, Rebillard X, Soulié M, Haaser T, et al. Epidemiology of metastatic castration-resistant prostate cancer: a first estimate of incidence and prevalence using the French nationwide healthcare database. Cancer Epidemiol. 2020;69:101833. doi: 10.1016/j.canep.2020.101833. [DOI] [PubMed] [Google Scholar]
- 20.Schisterman EF, Cole SR, Platt RW. Overadjustment bias and unnecessary adjustment in epidemiologic studies. Epidemiology. 2009;20(4):488–495. doi: 10.1097/EDE.0b013e3181a819a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Beer TM, Armstrong AJ, Rathkopf DE, Loriot Y, Sternberg CN, Higano CS, et al. Enzalutamide in metastatic prostate cancer before chemotherapy. N Engl J Med. 2014;371(5):424–433. doi: 10.1056/NEJMoa1405095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miller K, Carles J, Gschwend JE, Van Poppel H, Diels J, Brookman-May SD. The phase 3 COU-AA-302 study of abiraterone acetate plus prednisone in men with chemotherapy-naive metastatic castration-resistant prostate cancer: stratified analysis based on pain, prostate-specific antigen, and gleason score. Eur Urol. 2018;74(1):17–23. doi: 10.1016/j.eururo.2017.08.035. [DOI] [PubMed] [Google Scholar]
- 23.Davis ID, Martin AJ, Stockler MR, Begbie S, Chi KN, Chowdhury S, et al. Enzalutamide with standard first-line therapy in metastatic prostate cancer. N Engl J Med. 2019;381(2):121–131. doi: 10.1056/NEJMoa1903835. [DOI] [PubMed] [Google Scholar]
- 24.Kyriakopoulos CE, Chen YH, Carducci MA, Liu G, Jarrard DF, Hahn NM, et al. Chemohormonal therapy in metastatic hormone-sensitive prostate cancer: long-term survival analysis of the randomized phase III E3805 CHAARTED Trial. J Clin Oncol. 2018;36(11):1080–1087. doi: 10.1200/JCO.2017.75.3657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sternberg CN, Fizazi K, Saad F, Shore ND, De Giorgi U, Penson DF, et al. Enzalutamide and survival in nonmetastatic, castration-resistant prostate cancer. N Engl J Med. 2020;382(23):2197–2206. doi: 10.1056/NEJMoa2003892. [DOI] [PubMed] [Google Scholar]
- 26.Hatakeyama S, Narita S, Takahashi M, Sakurai T, Kawamura S, Hoshi S, et al. Association of tumor burden with the eligibility of upfront intensification therapy in metastatic castration-sensitive prostate cancer: A multicenter retrospective study. Int J Urol. 2020;27(7):610–617. doi: 10.1111/iju.14258. [DOI] [PubMed] [Google Scholar]
- 27.Marshall CH, Antonarakis ES. Emerging treatments for metastatic castration-resistant prostate cancer: immunotherapy, PARP inhibitors, and PSMA-targeted approaches. Cancer Treat Res Commun. 2020;7(23):100164. doi: 10.1016/j.ctarc.2020.100164. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.