Abstract
Background
We performed a systematic review of mechanically ventilated patients with COVID-19, which analysed the effect of tracheostomy timing and technique (surgical vs percutaneous) on mortality. Secondary outcomes included intensive care unit (ICU) and hospital length of stay (LOS), decannulation from tracheostomy, duration of mechanical ventilation, and complications.
Methods
Four databases were screened between January 1, 2020 and January 10, 2022 (PubMed, Embase, Scopus, and Cochrane). Papers were selected according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) and the Population or Problem, Intervention or exposure, Comparison, and Outcome (PICO) guidelines. Meta-analysis and meta-regression for main outcomes were performed.
Results
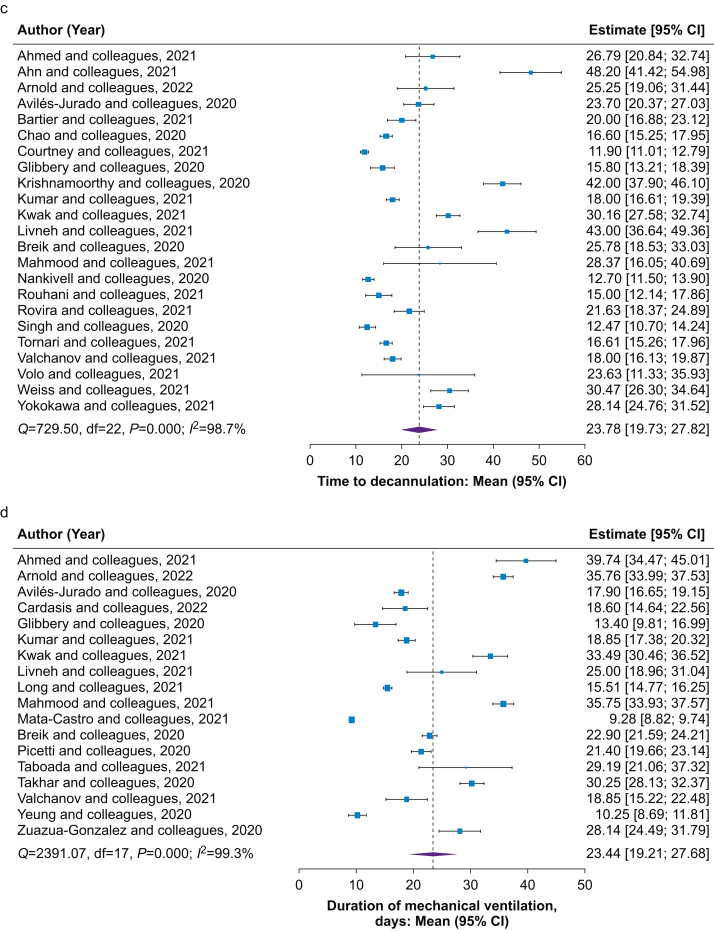
The search yielded 9024 potentially relevant studies, of which 47 (n=5268 patients) were included. High levels of between-study heterogeneity were observed across study outcomes. The pooled mean tracheostomy timing was 16.5 days (95% confidence interval [CI]: 14.7–18.4; I2=99.6%). Pooled mortality was 22.1% (95% CI: 18.7–25.5; I2=89.0%). Meta-regression did not show significant associations between mortality and tracheostomy timing, mechanical ventilation duration, time to decannulation, and tracheostomy technique. Pooled mean estimates for ICU and hospital LOS were 29.6 (95% CI: 24.0–35.2; I2=98.6%) and 38.8 (95% CI: 32.1–45.6; I2=95.7%) days, both associated with mechanical ventilation duration (coefficient 0.8 [95% CI: 0.2–1.4], P=0.02 and 0.9 [95% CI: 0.4–1.4], P=0.01, respectively) but not tracheostomy timing. Data were insufficient to assess tracheostomy technique on LOS. Duration of mechanical ventilation was 23.4 days (95% CI: 19.2–27.7; I2=99.3%), not associated with tracheostomy timing. Data were insufficient to assess the effect of tracheostomy technique on mechanical ventilation duration. Time to decannulation was 23.8 days (95% CI: 19.7–27.8; I2=98.7%), not influenced by tracheostomy timing or technique. The most common complications were stoma infection, ulcers or necrosis, and bleeding.
Conclusions
In patients with COVID-19 requiring tracheostomy, the timing and technique of tracheostomy did not clearly impact on patient outcomes.
Systematic Review Protocol
PROSPERO CRD42021272220.
Keywords: ARDS, COVID-19, mortality, outcomes, SARS-CoV-2, tracheostomy
Editor's key points.
-
•
Tracheostomy practice has changed during the COVID-19 pandemic. The benefits of a tracheostomy, its optimal timing, and technique are yet to be thoroughly investigated.
-
•
In this systematic review and meta-analysis, the authors explore important outcomes in critically ill patients with COVID-19 and a tracheostomy, examining associations with the timing of tracheostomy and the technique (percutaneous or surgical) used.
-
•
The timing and type of tracheostomy appeared to have no impact on outcome. The authors conclude that decisions around timing and technique should include the multidisciplinary team, considering patient and their family's wishes.
During exponential increases of novel coronavirus (COVID-19) spread, extraordinary pressure on hospitals has been exerted worldwide.1 Patients with severe disease, requiring intensive care unit (ICU) admission and mechanical ventilation (MV) presented similar mortality rates in different pandemic periods.2 Prolonged MV has been frequently observed in COVID-19,3,4 with tracheostomy being commonly used to facilitate weaning from respiratory support and accelerate discharge from ICU. Tracheostomy practice has changed during the pandemic with a higher rate of performance compared with patients without COVID-19.5, 6, 7, 8, 9 Considering that patients with COVID-19 typically experience longer periods of MV than those with other pneumonias, it is possible that tracheostomy yields a potential survival benefit,7 perhaps by facilitating weaning from ventilatory support8 and by streamlining the critical care management of airways.10 Nevertheless, the potential benefits of a tracheostomy; its optimal timing and technique choice; and burden for patients, staff, and resources in COVID-19 have yet to be defined.11
Few meta-analyses of tracheostomy practice in COVID-19 have been published at the beginning of the pandemic,12, 13, 14 but none of them was able to compare outcomes beyond mortality facing the issues around timing and technique of tracheostomy. We performed an updated systematic review and meta-analysis at the tail end of the COVID-19 pandemic to summarise and assess all published evidence regarding overall mortality in patients with COVID-19 and a tracheostomy, also investigating tracheostomy timing and technique (surgical [open] vs percutaneous). Secondary outcomes included ICU and hospital length of stay (LOS); timing of tracheotomy from intubation, proportion, and timing of decannulation after tracheostomy; MV duration; and tracheostomy complications.
Methods
This systematic review was reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA)15,16 and the Joanna Briggs Institute (JBI Reviewers' Manual for Systematic Reviews of Literature17 (Supplementary material item S1). The protocol was registered in the International Prospective Register of Systematic Reviews (PROSPERO) on August 20, 2021 (Registration number: CRD42021272220).
Search strategy and study selection
Two reviewers (DB and LP) systematically searched PubMed, Embase, Scopus, and the Cochrane trial registry for all published observational studies as of January 10, 2022, aiming to investigate the timing of tracheotomy from intubation, mortality, length of hospital and ICU stay, prevalence and timing of decannulation, duration of MV, and complications in a critically ill population with COVID-19. We used a combination of headings and keywords specific for each database based on the following Medical Subject Headings (MeSH) ‘(tracheostomy OR tracheotomy OR trachea∗) AND (COVID-19 OR ncov OR coronavirus OR COVID OR coronavirus disease OR SARS-CoV-2 OR acute severe respiratory syndrome coronavirus)’. The extended list of MeSH terms is reported in the Supplementary material item S2. Titles and abstracts of all identified studies were independently screened by two authors (DB and LP) and retrieved for duplication checking. The references of all these papers were also reviewed to identify other studies of interest potentially missed during the primary search. In addition, peer-reviewed publications, preprints, and press releases were eligible for inclusion. There were no restrictions placed on language or geographic region. After screening the titles and abstracts, the same two authors independently screened the full text of all selected articles for possible inclusion. In the case of uncertain selection, discrepancies were resolved by a consensus. If a consensus was not reached, a third reviewer was involved in the process (IDG, FB, or MS).
The selected studies included (i) observational studies and randomised trials if present; (ii) adult patients with suspected or confirmed SARS-CoV-2 infection; (iii) patients with COVID-19, who received a tracheostomy during their ICU course; and (iv) studies including 20 or more patients. Exclusion criteria were the paediatric population and non-compliance with the aforementioned inclusion criteria.
Definitions
Time to tracheostomy was defined as the mean time between initiation of MV and tracheostomy performance (days). Time to decannulation was defined as the mean timing between tracheostomy performance and tracheostomy removal (days). Prevalence of decannulation was defined as the number of alive patients who underwent decannulation from tracheostomy during their hospital stay. MV duration was defined as the timing between initiation of MV and its discontinuation. Primary analysis treated time to tracheostomy as a continuous variable to maximise information reported across studies. As a sensitivity analysis, we considered meta-analysis of outcomes based on published definitions of early vs late tracheostomy. Time to tracheostomy was defined as early when <14 or <16.5 days and late when ≥14 or ≥16.5 days. These cut-offs were based on the COVID-19 literature to date (14 days)9,18, 19, 20 and the mean tracheostomy time in our cohort of patients (16.5 days).
Outcomes
Primary outcome was all-cause mortality in patients with COVID-19 and tracheostomy. For this outcome, we applied meta-regression to mortality as a function of mean time to tracheostomy, study follow-up time, study start date, and study time frame to examine associations related to pandemic phase and hospital stress. Additionally, outcomes for surgical vs percutaneous tracheostomy groups were also compared. Secondary outcomes included ICU and hospital LOS in patients with COVID-19 and a tracheostomy, timing of insertion of tracheostomy, timing of decannulation of tracheostomy, duration of MV, and complications after tracheostomy.
Data extraction and risk-of-bias assessment
According to the Population or Problem, Intervention or exposure, Comparison, and Outcome (PICO) approach, two reviewers independently extracted data (DB and LP) on tracheostomy specifics and outcomes. The following data were extracted for each study: study design characteristics (case–control, cohort studies, or case series), study information (first author, date of publication, publication type, study site, and first/second wave), COVID-19 population characteristics (number of patients with or without tracheostomy, with surgical/percutaneous tracheostomy, with early/late tracheostomy, decannulated, with complications, and who died), patient characteristics (age, country, sex, total sample size, missing patients, severity of COVID-19, death, ICU LOS, and hospital LOS), and tracheostomy characteristics (type of tracheostomy, definition of timing, setting, duration of tracheostomy, duration of MV, time to decannulation, and type of tracheostomy complications [bleeding, infection of stoma, air leak, lower respiratory tract infections, hypoxia, closure, and others]). When necessary, the corresponding authors of the included studies were contacted to obtain missing data related to trial demographics, methods, and outcomes.
For each study, two reviewers (DB and LP) independently assessed the risk of bias for type and timing of tracheostomy, and outcome features, such as decannulation, ventilator weaning, complications, and mortality using the modified 8-item Newcastle–Ottawa scale (NOS) and the COVID-19 adapted NOS21 (see Supplementary material item S3). Disagreements amongst reviewers were discussed with a third author until a consensus was reached (IDG, FB, or MS).
Strategy for data synthesis
We provided a narrative and tabular synthesis of the findings from the included studies, structured with the aim to assess the characteristics and outcomes of critically ill patients with COVID-19 and a tracheostomy. Numerical data on the prevalence of time and type of tracheostomy and outcome features, such as mortality, LOS, decannulation, MV ventilation duration, and complications, were collected for pooled prevalence analysis.
Statistical analysis
Data were expressed as mean (standard deviation [sd]) for continuous variables and number (percentages, %) for categorical variables. Transformation from medians (inter-quartile range) to estimated means (sd) was performed using the following formula (l, lower; m, median; ss, sample size; u, upper)22:
| Estimate mean=(l+2m+u)/4+(l–2m+u)/4ss |
| Estimate sd=1/12 {[(l–2m+u)2/4]+(u–l)2} |
A meta-analysis was conducted to obtain pooled estimates for timing of tracheostomy, type of tracheostomy, mortality, ICU and hospital LOS, decannulation, MV, and complications in critically ill patients with COVID-19. Pooled estimates were obtained using a random effects model to account for expected study heterogeneity using the inverse variance method. Heterogeneity was assessed using both Cochrane Q test, τ2, and Higgins I2 statistic. Confidence intervals (CIs) for binary outcomes were calculated using Wilson scores with between-study variation estimated using the DerSimonian–Laird estimator.23
We utilised meta-regression to assess evidence of associations between outcomes and moderators as sources of between-study heterogeneity, including the possible effects of study duration and study start date. Random effects meta-regression with residual maximum likelihood (REML) model was used; residual Q statistic and Wald's χ2 test results were also displayed. Binary outcomes were analysed on the logit scale. Ninety-five percent CIs were calculated for individual studies, and pooled estimates with 95% CI were displayed using Forest plots. Statistical significance was set at P<0.05. All statistical analyses were computed with STATA® (StataCorp LLC, College Station, TX, USA) and R® software (R Foundation for Statistical Computing, Vienna, Austria).
Results
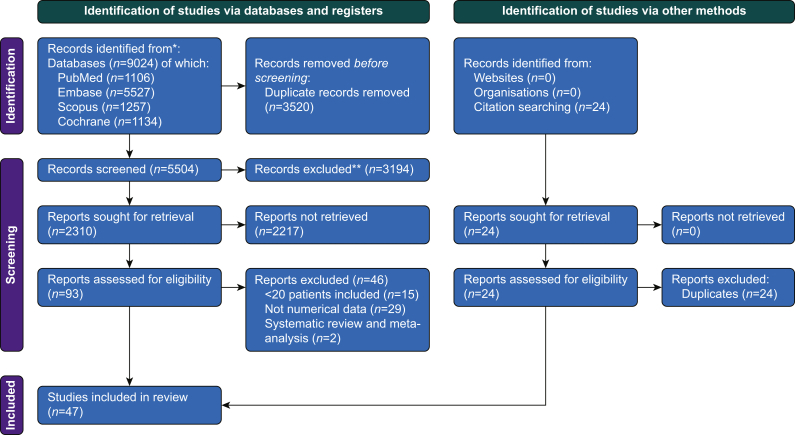
The initial search yielded 9024 potentially relevant studies, of which 3520 were excluded as duplicate studies and 3194 were excluded after revision of titles and abstracts. After full-text review, 47 studies were included in this systematic review and meta-analysis.6, 7, 8, 9,18,19,24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64 The search and selection strategies are shown in Fig 1.
Fig 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flowchart. PRISMA 2020 flow diagram for systematic reviews, which include searches of databases, registers, and other sources. This figure depicts the number of records identified from each database or register searched (rather than the total number across all databases/registers); from Page and colleagues.16
Characteristics of included studies and patients
All included studies were published in English between January 1, 2020 and January 10, 2022. Table 1 depicts the main characteristics of the 47 included studies, comprising 5268 patients (2252 males, 891 females, and 2125 unknowns, with a male/female ratio of 2.53). The mean age (sd) of patients was 60.1 (25.3) yr. In the overall study population, the mean pooled estimate timing of tracheostomy was 16.5 (95% CI: 14.7–18.4; I2=99.6%) days. There were 2202 patients who underwent percutaneous tracheostomy and 2798 surgical tracheostomies, and for 268 patients the method of insertion was not clearly defined (surgical technique adopting percutaneous dilators or percutaneous technique completed by surgical approach). Forty-five studies reported mortality data, including 5218 patients of whom 1067 died (12 studies comprised the surgical group and 19 the percutaneous group). ICU and hospital LOS were reported in 10 and 15 studies, respectively. Thirty-three studies provided information on decannulation outcome; of 3093 patients, 1785 were decannulated (six studies in the surgical and 10 in the percutaneous groups). Duration of MV was reported in 18 studies.
Table 1.
Characteristics of included studies. NC, not clear; NR, not reported; P, percutaneous; P/S, percutaneous and surgical; S, surgical. Data are expressed as mean (standard deviation [sd]).
| Authors | Year | Country | Age, mean (sd) (yr) | Overall population, n patients | Tracheostomy timing, mean, (sd) (days) | S, P, or p/s | Mortality, n patients |
|---|---|---|---|---|---|---|---|
| Ahmed and colleagues63 | 2021 | USA | 63.00 (11.85) | 64.00 | 20.00 (7.04) | P/S | 21.00 |
| Ahn and colleagues64 | 2021 | Korea | 68.80 (41.72) | 27.00 | 15.80 (9.00) | P/S | 11.00 |
| Angel and colleagues18 | 2021 | USA | NR | 178.00 | NR | P | 44.00 |
| Arnold and colleagues24 | 2022 | USA | 66.00 (7.41) | 59.00 | 19.00 (5.19) | P | 23.00 |
| Avilés-Jurado and colleagues60 | 2020 | Spain | 63.80 (9.70) | 50.00 | 9.00 (16.30) | P | 8.00 |
| Bartier and colleagues61 | 2021 | France | 56.00 (12.00) | 59.00 | NR | P/S | 6.00 |
| Battaglini and colleagues9 | 2021 | Italy | 63.40 (9.34) | 153.00 | 15.00 (15.75) | P/S | 65.00 |
| Botti and colleagues25 | 2021 | Italy | 64.00 (11.25) | 47.00 | NR | P/S | 14.00 |
| Boujaoude and colleagues26 | 2021 | USA | 54.00 (12.00) | 32.00 | 22.00 (8.00) | P | 9.00 |
| Breik and colleagues7 | 2020 | UK | 55.00 (12.00) | 100.00 | 13.90 (4.50) | P/S | 15.00 |
| Cagino and colleagues27 | 2021 | USA | 56.00 (15.75) | 25.00 | 22.00 (17.03) | P/S | NR |
| Cardasis and colleagues28 | 2022 | USA | 61.10 (10.00) | 24.00 | 18.60 (10.37) | S | 3.00 |
| Chao and colleagues29 | 2020 | USA | 62.00 (14.30) | 53.00 | 19.70 (6.90) | P/S | 6.00 |
| Cohen and colleagues30 | 2022 | USA | 59.90 (15.10) | 24.00 | 31.90 (12.30) | P | 9.00 |
| Courtney and colleagues32 | 2021 | UK | 54.00 (8.60) | 20.00 | 16.50 (3.70) | S | 0.00 |
| COVIDTrach Collaborative33 | 2020 | UK | NR | 563.00 | 16.75 (6.94) | P/S | 62.00 |
| Floyd and colleagues34 | 2020 | USA | NR | 38.00 | 24.00 (5.33) | P/S | 2.00 |
| Forni and colleagues62 | 2020 | Switzerland | NR | 53.00 | NR | P | 8.00 |
| Glibbery and colleagues19 | 2020 | UK | 60.50 (12.40) | 28.00 | 17.00 (4.40) | P/S | 2.00 |
| Illuzzi and colleagues35 | 2020 | Not clear | NR | 111.00 | NR | P | 33.00 |
| Krishnamoorthy and colleagues36 | 2020 | USA | 62.54 (13.55) | 143.00 | 25.00 (6.60) | P/S | 13.00 |
| Kumar and colleagues37 | 2021 | India | 45.50 (9.59) | 38.00 | 11.60 (4.63) | P | 6.00 |
| Kwak and colleagues38 | 2021 | USA | 58.10 (15.80) | 148.00 | 12.23 (6.82) | P/S | 30.00 |
| Livneh and colleagues39 | 2021 | USA | 64.00 (21.33) | 38.00 | 7.50 (4.08) | NC | 22.00 |
| Long and colleagues59 | 2021 | USA | 62.00 (0.00) | 67.00 | 20.00 (22.96) | P/S | 5.00 |
| Mahmood and colleagues40 | 2021 | USA | 53.87 (42.19) | 118.00 | 21.75 (4.10) | P/S | 18 |
| Martin-Villares and colleagues8 | 2021 | Spain | NR | 1890.00 | 17.50 (130.42) | P/S | 383.00 |
| Mata-Castro and colleagues41 | 2021 | Spain | 66.40 (6.20) | 29.00 | 15.20 (9.50) | P/S | 5.00 |
| Picetti and colleagues42 | 2020 | Italy | 58.70 (8.70) | 66.00 | 6.10 (2.10) | S | 9.00 |
| Riestra-Ayora and colleagues43 | 2020 | Spain | 67.55 (10.60) | 27.00 | NR | P/S | 11.00 |
| Rosano and colleagues44 | 2022 | Italy | 64.00 (9.00) | 121.00 | 6.00 (1.48) | P | 54.00 |
| Rouhani and colleagues45 | 2021 | UK | 57.00 (11.25) | 41.00 | 24.00 (29.63) | P/S | 4.00 |
| Rovira and colleagues46 | 2021 | UK | 55.60 (11.20) | 201.00 | 17.00 (5.93) | P/S | 29.00 |
| Šifrer and colleagues47 | 2022 | Slovenia | 65.50 (26.67) | 25.00 | NR | S | NR |
| Singh and colleagues31 | 2020 | UK | 55.70 (9.50) | 47.00 | 18.60 (6.70) | S | 1.00 |
| Taboada and colleagues48 | 2021 | Spain | 69.59 (8.16) | 29.00 | 15.00 (4.07) | NC | 12.00 |
| Takhar and colleagues49 | 2020 | UK | 52.94 (172.10) | 87.00 | 16.00 (5.19) | NC | 7.00 |
| Tang and colleagues50 | 2020 | China | 63.90 (14.00) | 80.00 | 17.50 (11.63) | P/S | 43.00 |
| Tornari and colleagues60 | 2021 | UK | 57.28 (45.77) | 78.00 | 16.25 (4.10) | P/S | 0.00 |
| Turri-Zanoni and colleagues51 | 2020 | Italy | 62.00 (53.00) | 32.00 | 15.00 (15.00) | P/S | 5.00 |
| Valchanov and colleagues52 | 2021 | India | 45.50 (9.59) | 38.00 | 11.66 (4.63) | P | 9.00 |
| Volo and colleagues53 | 2021 | Italy | 69.00 (31.11) | 23.00 | 13.00 (0.00) | P/S | 9.00 |
| Weiss and colleagues54 | 2021 | USA | 55.19 (253.10) | 28.00 | 26.00 (9.00) | P/S | 3.00 |
| Williamson and colleagues55 | 2021 | UK | 66.00 (8.15) | 29.00 | 4.00 (8.88) | P | 7.00 |
| Yeung and colleagues56 | 2020 | UK | 57.70 (10.48) | 72.00 | 17.00 (5.19) | P/S | 7.00 |
| Yokokawa and colleagues57 | 2021 | Japan | NR | 35.00 | NR | S | 17.00 |
| Zuazua-Gonzalez and colleagues58 | 2020 | Spain | 60.80 (8.43) | 30.00 | NR | S | 17.00 |
Quality and bias assessment
Supplementary material item S4 presents the methods used for the assessment of methodological quality of the included studies using the NOS. Twenty-six studies were rated as high quality, 21 as moderate, and no studies were indicated as low quality. Supplementary material item S5 provides funnel plots for each outcome of interest.
Mortality
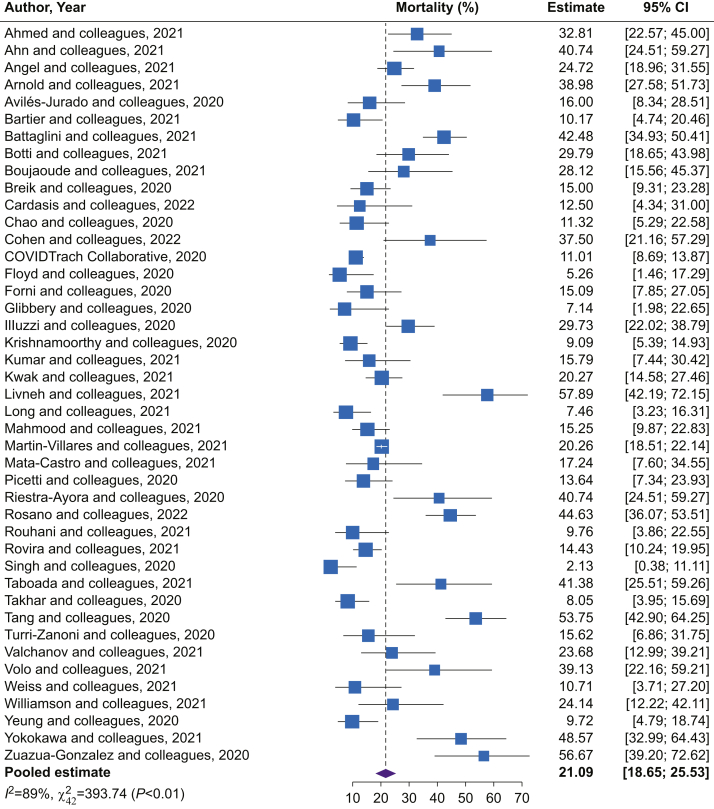
Mortality data were reported in 45 of 47 included studies. The pooled mortality in the overall population of patients who received tracheostomy was 22.1% (95% CI: 18.7–25.5; I2=89.0%; Fig 2). Where study characteristics (start date and duration) were included as moderators, meta-regression failed to indicate any association between tracheostomy timing and mortality (coefficient –0.6 [95% CI: –1.5 to 0.2]; P=0.13) (Fig 3; Supplementary material item S6). Similarly, time to decannulation (coefficient 0.4 [95% CI: –0.2 to 1.0]; P=0.15) and MV duration (coefficient 0.6 [95% CI: –0.3 to 1.4]; P=0.20) were not associated with mortality (after accounting for study characteristics) (Supplementary material item S6). When applying definitions of early and late tracheostomy timing, the late group showed higher mortality than the early group (cut-off 16.5 days; P=0.02; Supplementary material item S7).
Fig 2.
Forest plot of mortality in the overall population. This figure depicts the forest plots of prevalence of mortality in the overall population of patients with COVID-19 and a tracheostomy. CI, confidence interval.
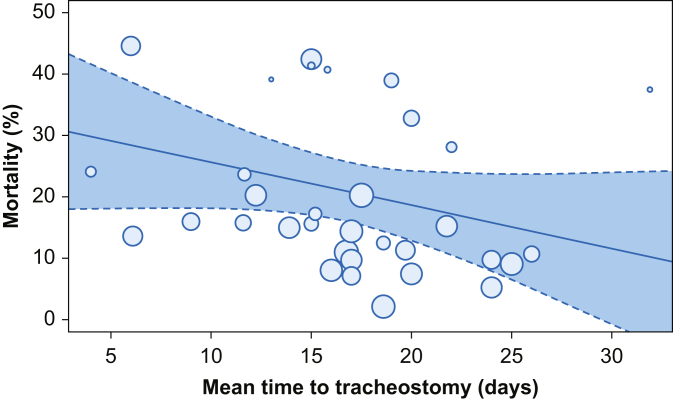
Fig 3.
Meta-regression of mortality with mean time to tracheostomy as moderator. Red line represents proposed linear trend, where coefficient 0.70 (–1.55 to 0.15); P=0.10; and constant 32.64 (17.67–47.60); P=0.00 (meta-regression outputs with moderators are reported in Supplementary material item S3).
Grouping by follow-up time revealed a small mortality increase in studies with follow-up time >30 days compared with those with ≤30 days (Supplementary material items S8 and S9). In Supplementary material item S10, mortality is represented by study. Pooled mortality estimates were similar between percutaneous and surgical tracheostomy groups (22.8 [95% CI: 17.2–28.3; I2=80.9%] vs 16.5 [95% CI: 9.4–23.52; I2=87.7%]; P=0.17).
Hospital and ICU lengths of stay
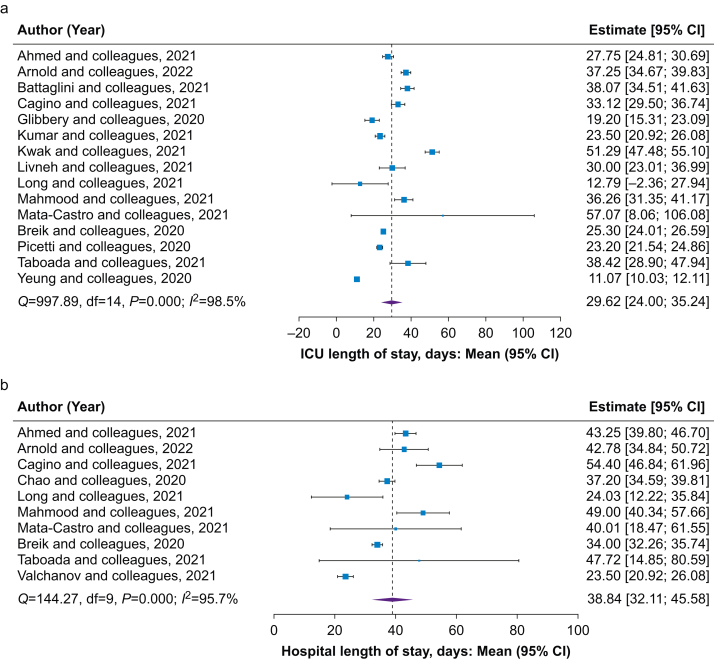
The pooled estimated mean ICU and hospital LOS of all patients with a tracheostomy were 29.6 (95% CI: 24.0–35.2; I2=98.5%) and 38.8 (95% CI: 32.1–45.6; I2=95.7%) days, respectively (Fig 4). Neither hospital LOS nor ICU LOS was associated with mean time to tracheostomy or time to decannulation (Supplementary material item S6). Notably, ICU and hospital LOS were significantly associated with duration of MV, although the strength of correlation was weak (coefficient 0.8 [95% CI: 0.2–1.4], P=0.02 and 0.9 [95% CI: 0.4–1.4], P=0.01, respectively). Applying definitions of early and late tracheostomy timing indicated differences in hospital LOS favouring the early tracheostomy group. Between-group comparisons for ICU LOS indicate similar outcomes between the early and late groups (Supplementary material item S7). Hospital and ICU LOS were not computable between surgical and percutaneous groups because of insufficient data.
Fig 4.
Forest plots of secondary outcomes in the overall population. Forest plots of mean estimate of (a) ICU and (b) hospital length of stay; (c) decannulation after tracheostomy and (d) mean duration of mechanical ventilation in overall patients with COVID-19 and a tracheostomy. CI, confidence interval.
Decannulation
The pooled prevalence of decannulation in the overall population was 47.5% (95% CI: 35.4–59.6; I2=98.6%). Average times to tracheostomy did not appear to impact the prevalence of decannulation (Supplementary material item S6), with no differences between early and late tracheostomy (Supplementary material item S7). Prevalence of decannulation did not significantly differ between percutaneous and surgical groups (47.5 [95% CI: 26.6–68.4; I2=98.0%] vs 46.6 [95% CI: 20.2–72.9; I2=96.2%]; P=0.96). In the overall study population, the time to decannulation was 23.8 (95% CI: 19.7–27.8; I2=98.7%) days (Fig 4). Time to decannulation was not associated with timing of tracheostomy (Supplementary material item S6). No differences in decannulation time were observed based on definitions of early and late tracheostomy timing (Supplementary material item S7). Time to decannulation was similar for percutaneous and surgical groups (21.0 [95% CI: 15.7–26.3] vs 18.4 [95% CI: 12.4–24.3] days; difference: 2.6 [95% CI: –5.4 to 10.6]; P=0.52).
Duration of mechanical ventilation
In the overall population of patients with COVID-19 and a tracheostomy, the mean MV duration was 23.4 days (95% CI: 19.2–27.7; I2=99.3%; Fig 4). MV duration was not associated with tracheostomy timing (Supplementary material item S6), and no differences in MV duration were found between early and late tracheostomy timing (Supplementary material item S7). MV duration was not computable between surgical and percutaneous groups because of insufficient data. MV duration did not correlate with decannulation timing, ICU LOS, or hospital LOS (Supplementary material item S11).
Tracheostomy complications
Data regarding complications were not always available for comparison between the groups. Table 2 presents pooled prevalence of tracheostomy complications. Where available for comparison, tracheostomy complications for timing and surgical vs percutaneous tracheostomy groups are reported in the table legend.
Table 2.
Tracheostomy complications. ∗False passage includes cases of dislodgement and replacement. No differences in bleeding were found between the early and late groups (6.4 [95% CI: 3.8–8.9; I2=11.0%) vs 5.0 [95% CI: 2.8–7.3; I2=100.0%]; P=0.44), but early tracheostomy (14 days cut-off) was more likely associated with bleeding (OR 1.6 [95% CI: 1.0–2.4]) than late. No differences in bleeding were found between percutaneous vs surgical groups (5.6 [95% CI: 2.0–9.1; I2=0.0%] vs 6.9 [95% CI: –1.6 to 15.4; I2=100.0%]; P=0.83; OR 0.9 [95% CI: 0.6–1.4]). Early tracheostomy (14 days cut-off) was less likely associated with false passage/dislodgement (OR 0.5 [95% CI: 0.2–1.5]) than late. CI, confidence interval; OR, odds ratio.
| Complication | Overall prevalence (95% CI) (%) | Heterogeneity I2 (%) |
|---|---|---|
| Bleeding | 7.0 (7.4–8.7) | 52.6 |
| Cuff or air leak | 2.4 (1.1–3.7) | 31.7 |
| False passage or dislodgement∗ | 2.3 (1.0–3.6) | 100.0 |
| Peri-procedural hypoxaemia or desaturation | 3.1 (0.8–5.4) | 74.0 |
| Pneumothorax or pneumomediastinum | 0.0 (0.0–0.0) | 0.03 |
| Stenosis or obstruction | 2.0 (0.5–3.6) | 14.7 |
| Stoma infection, breakdown/ulcers, or necrosis | 7.6 (3.5–11.8) | 90.0 |
Discussion
The main findings of our study were (i) the pooled prevalence of mortality in patients with COVID-19 and a tracheostomy was 22.1% without influence of timing to tracheostomy, tracheostomy technique, time to decannulation, and duration of MV. When applying definitions for early and late tracheostomy timing, the late tracheostomy group showed greater mortality than the early group (cut-off 16.5 days). (ii) The pooled estimated mean ICU and hospital LOS of all patients with a tracheostomy were 29.6 and 38.8 days, respectively, being both influenced by MV duration but not tracheostomy timing. No data for surgical vs percutaneous comparison were available. (iii) The mean time to decannulation was 23.8 days, and the pooled prevalence of decannulation was 47.5% of patients, both without influence by tracheostomy timing and technique. (iv) The mean duration of MV was 23.4 days, and it was not influenced by tracheostomy time. No data for surgical vs percutaneous comparison were available. (v) The most prevalent complications of tracheostomy were stoma infection/breakdown/ulcers or necrosis, followed by bleeding.
To the best of our knowledge, this is the first systematic review, meta-analysis, and meta-regression in critically ill patients with COVID-19 and a tracheostomy, which reports the associations between outcomes and moderators as sources of between-study heterogeneity accounting for the effects of study duration (partial accounting for time-varying associations) and study start date (hospital strain), and the comparison between early vs late at different cut-offs and technique (surgical vs percutaneous tracheostomy) for several outcomes, including mortality, hospital and ICU LOS, decannulation, duration of MV, and complications. Previous meta-analyses reported the comparison between such subgroups of patients only for a few outcomes.12, 13, 14,65
Mortality
Considering 45 studies for the outcome mortality, our results showed a pooled tracheostomy mortality in COVID-19 of 22.1% (95% CI: 18.7–25.5; I2=89.0%), similarly to that previously reported by Ferro and colleagues13 of 19.2% (95% CI: 15.2–23.6), including 37 studies, and Ji and colleagues,14 including 14 studies but higher than that reported by Benito and colleagues12 of 13.1% (95% CI: 8.5–18.4), including 14 studies. Ferro and colleagues13 reported no differences in cumulative mortality between early and late tracheostomy (relative risk [RR] 1.6; 95% CI: 0.2–11.8) and surgical vs percutaneous tracheostomy (RR 2.0; 95% CI: 0.2–20.4). Other meta-analyses by Benito and colleagues12 and Chong and Tan66 used a cut-off at 7 and 14 and 10 and 14 days, finding no differences in mortality between the early and late groups. In our study, the mean timing of tracheostomy in patients with COVID-19 was 16.5 days. To reduce heterogeneity and the bias related to pandemic phase and hospital stress, and to face the unclear benefit of using the tracheostomy timing as dichotomic variable, we performed a meta-regression for mortality using the mean time to tracheostomy as continuous variable, and accounting for the effects of study duration (partial accounting for time-varying associations) and study start date (hospital strain). We found that neither time to tracheostomy nor tracheostomy technique (percutaneous vs surgical) explained the heterogeneity in mortality results. Adopting the statistical inverse variance REML method and the generalised linear mixed model method, a small difference in mortality was observed between groups followed-up for >30 and ≤30 days, whereas an overlap in CIs suggests no notable effect. Further significant heterogeneity was observed in both groups. The existing literature in COVID-19 reported a mean tracheostomy timing to be closer to 14 rather than 10 or 7 days described in the non-COVID-19 literature.67, 68, 69 When applying definitions for early and late tracheostomy timing (14 or 16.5 days as cut-offs), only 16.5 days cut-off reported differences in mortality being higher in the late group than in the early tracheostomy group, not confirming the results of other meta-analyses.12, 13, 14,66 An early tracheostomy performance demonstrated possible beneficial effects on outcome in patients without COVID-19.70,71 However, this does not really account for the real impact in terms of benefits or harm on patients' outcomes, because critically ill patients present a high likelihood of evolving to multiple organ dysfunction that cannot be optimally predicted during the first days of ICU admission.11 Early tracheostomy is often thought to be accompanied by reduced laryngeal injury and laryngeal dysfunction associated with prolonged tracheal intubation; reduced cumulative burden of sedative agents; better pulmonary hygiene through secretion clearance; earlier return to eating, drinking, and talking; and earlier rehabilitation.72 However, a late tracheostomy could be considered in some patients who are clinically unstable and may require prone positioning that might be at risk of tracheostomy dislodgement or who present with multi-organ failure.73,74 During the pandemic, some factors that may have influenced the choice of performing a tracheostomy have changed. Hence, a tracheostomy procedure in patients with COVID-19 could have been associated with staff procedural risks as a result of aerosol generation, thus delaying the procedure earlier in the pandemic.75,76 Therefore, we can assume that a late tracheostomy timing could have been selected in some patients with COVID-19 who were believed to be survivors or to facilitate a difficult weaning from MV, whilst early on in the COVID-19 pandemic many authorities were not recommending early tracheostomy because of infection control issues.73 The selection of patients and their severity of illness may have contributed to a possible selection bias when analysing mortality in the early vs late groups and the surgical vs percutaneous, thus explaining these contrasting results on outcomes. Therefore, the optimal timing for tracheostomy insertion remains controversial.
ICU and hospital lengths of stay
In the present study, the mean ICU and hospital LOS for patients with a tracheostomy were significantly influenced by the duration of MV. A recent meta-analysis by Deng and colleagues77 in patients without COVID-19 in the ICU revealed that early tracheostomy was associated with MV duration and a shorter ICU stay.77 Other studies in patients without COVID-19 reported that early tracheostomy was associated with shorter overall ICU stay when compared with late tracheostomy,78,79 confirming an association between tracheostomy timing and hospital and ICU LOS,80 but this was not clearly confirmed in our study. A late group might be expected to survive and be less sick at the time of tracheostomy, whereas an early group of patients might be expected to be sicker, being less clear whether they will survive, thus with possible longer hospital LOS.11 However, our results did not confirm the influence of tracheostomy timing on ICU and hospital LOS. In COVID-19, two previous meta-analyses reported reduced ICU stay with early tracheostomy (less than 14 days)14,65; this contrasting result can be explained by the limited number of studies included and the different definition of early and late tracheostomy timing. However, despite its other potential advantages, early tracheostomy in patients in general ICU and in COVID-19 to shorten the LOS is not clearly supported by the literature.77,79,81, 82, 83 However, based on our new findings, we can suppose that the duration of MV and its consequences might play a pivotal role on the outcomes of patients with COVID-19.
Tracheostomy decannulation
This study found that time to decannulation was 23.8 days with a prevalence of 47.5%, with no significant impact of timing and technique of tracheostomy, not different from Ferro and colleagues.13 Benito and colleagues12 reported an incidence of decannulation of 34.9 (25.4–44.9) by including 15 studies with a mean duration of 18.6 days (sd 5.7). Staibano and colleagues65 reported no association between early tracheostomy (<14 days) and decannulation. A pre-pandemic survey revealed that patient level of consciousness, cough effectiveness, secretions, and oxygenation are important determinants of clinicians' decision to decannulate84; however, the association between tracheostomy timing, decannulation, and outcome remains unclear.
Tracheostomy and duration of mechanical ventilation
In the current study, the mean duration of MV was 23.4 days, and the duration of MV significantly influenced the ICU and hospital LOS. Additionally, the duration of MV was not influenced by the tracheostomy timing. Previous study in patients without COVID-19 found a correlation between tracheostomy timing and duration of MV,80 a result not duplicated in this study. A systematic review in non-COVID-19 concluded a shorter duration of MV for early tracheostomy,85 again not confirmed by other studies.82 In COVID-19, Staibano and colleagues66 found no association between early tracheostomy (<14 days) and duration of MV, whereas in our study we found an interesting effect of duration of MV on LOS. The supposed benefit of earlier tracheostomy is that it allows for decreased sedation and earlier mobilisation. However, this analysis suggests that even with early tracheostomy, patients are subjected to prolonged periods of ventilatory support, likely because of protracted severe respiratory failure that might affect the patients' outcomes.
Tracheostomy complications
The most prevalent complication of tracheostomy was stoma infection/breakdown/ulcers or necrosis, followed by bleeding. These findings are not clearly supported by the non-COVID-19 and previous COVID-19 literature.13,86 Our results showed that early tracheostomy was more likely associated with bleeding and less likely associated with stoma infection/breakdown or ulcers than late. The reason for such results could be explained by the increased risk of bleeding, anticoagulant therapies, or anti-platelet medications that often characterise the initial phase of disease treatment.87, 88, 89 No clear advantages for percutaneous or surgical technique were found with regard to bleeding. A percutaneous approach involves less dissection and a smaller stoma but with limited direct visualisation that may have an impact on peri-procedural complications.73
Limitations
This study has several limitations to address. First, possibly related to the nature of the COVID-19 pandemic, the qualities of some included studies were low with absence of long-term follow-up, short periods of available research, and incomplete data. Second, the weighted means are estimated with assumption of normal distribution, probably causing a selection bias. Moreover, it was not possible to report the values as medians because of insufficient data from the selected studies. The selection of patients (not evaluated for severity of illness) included in the mortality analysis may lead to a significant bias, impossible to control for by meta-regression analysis. We were not able to look at trends in tracheostomy performance, but it may change with time. Important, we found a huge heterogeneity across studies, which was impossible to explain even accounting immortality time bias in meta-regression. Inferring causal relationships from observational studies is difficult to ascertain, particularly in the context of meta-analyses, which are constrained by the availability of published data on both potential moderators and outcomes. In our analysis, we have examined evidence of potential associations between the mean time to tracheostomy (as a continuous variable) and expected outcomes, without implying causality attributable to limited data. Finally, by pooling studies from across the world across the period examined, it was impossible to control for the effect of the evolving treatment strategies and infection control measures (impacting tracheostomy practice) used by the various studies (i.e. the introduction of steroids, etc.), even when assessing a meta-regression analysis. An analysis able to adjust for such secular trends would be of interest.
Conclusions
Our findings suggest that in mechanically ventilated patients with COVID-19, the timing (early vs late) and type (surgical vs percutaneous) of tracheostomy have no clear impact on outcome. Decisions surrounding optimal timing and technique should include a multidisciplinary team, and patients' and families' wishes, and be informed by further evidence generation.
Authors' contributions
Study conception: DB
Study design: DB, PP
Database screening: DB, LP, AS, CR, S-MC, IDG, FB, BHC, MS
Literature search: DB, LP
Statistical analysis: DB, LP, NW
Writing of article: DB
Editing of article: DB, AS, CR, S-MC, IDG, FB, BHC, MS, GLB, JS, JFF, PP
Approval of article: AS, CR, S-MC, IDG, FB, BHC, MS, NW, GLB, JS, JFF, PP
Acknowledgements
GLB acknowledges receipt of a Biomedicine International Training Research programme for Excellent Clinician-Scientists (BITRECS) fellowship. The BITRECS project has received funding from the European Union's Horizon 2020 research and innovation program under the Marie Skłodowska-Curie grant agreement no. 754550 and from the “La Caixa” Foundation (ID 100010434) under the agreement LCF/PR/GN18/50310006.
Handling editor: Jonathan Hardman
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bja.2022.07.032.
Declarations of interest
MS received consultancy fees from Teleflex Medical (Athlone, Ireland) and from Verathon Medical (USA), Boston Scientific (France), and MSD (Italy). The other authors declare that they have no conflicts of interest.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Iacobucci G. Covid-19: how is vaccination affecting hospital admissions and deaths? BMJ. 2021;374:n2306. doi: 10.1136/bmj.n2306. [DOI] [PubMed] [Google Scholar]
- 2.Karagiannidis C., Windisch W., McAuley D.F., Welte T., Busse R. Major differences in ICU admissions during the first and second COVID-19 wave in Germany. Lancet Respir Med. 2021;9:e47–e48. doi: 10.1016/S2213-2600(21)00101-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gamberini L., Tonetti T., Spadaro S., et al. Factors influencing liberation from mechanical ventilation in coronavirus disease 2019: multicenter observational study in fifteen Italian ICUs. J Intensive Care. 2020;8:80. doi: 10.1186/s40560-020-00499-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Richards-Belle A., Orzechowska I., Gould D.W., et al. COVID-19 in critical care: epidemiology of the first epidemic wave across England, Wales and Northern Ireland. Intensive Care Med. 2020;46:2035–2047. doi: 10.1007/s00134-020-06267-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mehta A.B., Syeda S.N., Bajpayee L., Cooke C.R., Walkey A.J., Wiener R.S. Trends in tracheostomy for mechanically ventilated patients in the United States, 1993–2012. Am J Respir Crit Care Med. 2015;192:446–454. doi: 10.1164/rccm.201502-0239OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McGrath B.A., Wallace S., Lynch J., et al. Improving tracheostomy care in the United Kingdom: results of a guided quality improvement programme in 20 diverse hospitals. Br J Anaesth. 2020;125:e119–e129. doi: 10.1016/j.bja.2020.04.064. [DOI] [PubMed] [Google Scholar]
- 7.Breik O., Nankivell P., Sharma N., et al. Safety and 30-day outcomes of tracheostomy for COVID-19: a prospective observational cohort study. Br J Anaesth. 2020;125:872–879. doi: 10.1016/j.bja.2020.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Martin-Villares C., Perez Molina-Ramirez C., Bartolome-Benito M., et al. Outcome of 1890 tracheostomies for critical COVID-19 patients: a national cohort study in Spain. Eur Arch Otorhinolaryngol. 2021;278:1605–1612. doi: 10.1007/s00405-020-06220-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Battaglini D., Missale F., Schiavetti I., et al. Tracheostomy timing and outcome in severe COVID-19: the WeanTrach multicenter study. J Clin Med. 2021;10:2651. doi: 10.3390/jcm10122651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Avilés-Jurado F.X., Prieto-Alhambra D., González-Sánchez N., et al. Timing, complications, and safety of tracheotomy in critically ill patients with COVID-19. JAMA Otolaryngol Head Neck Surg. 2020;147:1–8. doi: 10.1001/jamaoto.2020.3641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Williams T., McGrath B.A. Tracheostomy for COVID-19: evolving best practice. Crit Care. 2021;25:316. doi: 10.1186/s13054-021-03674-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Benito D.A., Bestourous D.E., Tong J.Y., Pasick L.J., Sataloff R.T. Tracheotomy in COVID-19 patients: a systematic review and meta-analysis of weaning, decannulation, and survival. Otolaryngol Head Neck Surg. 2021;165:398–405. doi: 10.1177/0194599820984780. [DOI] [PubMed] [Google Scholar]
- 13.Ferro A., Kotecha S., Auzinger G., Yeung E., Fan K. Systematic review and meta-analysis of tracheostomy outcomes in COVID-19 patients. Br J Oral Maxillofac Surg. 2021;59:1013–1023. doi: 10.1016/j.bjoms.2021.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ji Y., Fang Y., Cheng B., Li L., Fang X. Tracheostomy timing and clinical outcomes in ventilated COVID-19 patients: a systematic review and meta-analysis. Crit Care. 2022;26:40. doi: 10.1186/s13054-022-03904-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liberati A., Altman D.G., Tetzlaff J., et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700. doi: 10.1136/bmj.b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Page M.J., McKenzie J.E., Bossuyt P.M., et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aromataris E., Munn Z. In: JBI manual for evidence synthesis. Aromataris E., Munn Z., editors. 2020. [Google Scholar]
- 18.Angel L.F., Amoroso N.E., Rafeq S., et al. Percutaneous dilational tracheostomy for coronavirus disease 2019 patients requiring mechanical ventilation. Crit Care Med. 2021;49:1058–1067. doi: 10.1097/CCM.0000000000004969. [DOI] [PubMed] [Google Scholar]
- 19.Glibbery N., Karamali K., Walker C., Fitzgerald O’Connor I., Fish B., Irune E. Tracheostomy in the coronavirus disease 2019 patient: evaluating feasibility, challenges and early outcomes of the 14-day guidance. J Laryngol Otol. 2020;134:688–695. doi: 10.1017/S0022215120001759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McGrath B.A., Brenner M.J., Warrillow S.J. Tracheostomy for COVID-19: business as usual? Br J Anaesth. 2020;125:867–871. doi: 10.1016/j.bja.2020.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lo C.K.-L., Mertz D., Loeb M. Newcastle-Ottawa scale: comparing reviewers’ to authors’ assessments. BMC Med Res Methodol. 2014;14:45. doi: 10.1186/1471-2288-14-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hozo S.P., Djulbegovic B., Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. 2005;5:13. doi: 10.1186/1471-2288-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nyaga V.N., Arbyn M., Aerts M. Metaprop: a Stata command to perform meta-analysis of binomial data. Arch Public Health. 2014;72:39. doi: 10.1186/2049-3258-72-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Arnold J., Gao C.A., Malsin E., et al. Outcomes of percutaneous tracheostomy for patients with SARS-CoV-2 respiratory failure. J Bronchology Interv Pulmonol. 2022 doi: 10.1101/2021.02.23.21252231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Botti C., Lusetti F., Neri T., et al. Comparison of percutaneous dilatational tracheotomy versus open surgical technique in severe COVID-19: complication rates, relative risks and benefits. Auris Nasus Larynx. 2021;48:511–517. doi: 10.1016/j.anl.2020.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boujaoude Z., Madisi N., Patel B., Rachoin J.-S., Dellinger R.P., Abouzgheib W. Safety and feasibility of a novel protocol for percutaneous dilatational tracheostomy in patients with respiratory failure due to COVID-19 infection: a single center experience. Pulm Med. 2021;2021:1–5. doi: 10.1155/2021/8815925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cagino L.M., Kercheval J.B., Kenes M.T., et al. Association of tracheostomy with changes in sedation during COVID-19: a quality improvement evaluation at the University of Michigan. Ann Am Thorac Soc. 2021;18:907–909. doi: 10.1513/AnnalsATS.202009-1096RL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cardasis J.J., Rasamny J.K., Berzofsky C.E., Bello J.A., Multz A.S. Outcomes after tracheostomy for patients with respiratory failure due to COVID-19. Ear Nose Throat J. 2022;101:354–358. doi: 10.1177/0145561321993567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chao T.N., Harbison S.P., Braslow B.M., et al. Outcomes after tracheostomy in COVID-19 patients. Ann Surg. 2020;272:e181–e186. doi: 10.1097/SLA.0000000000004166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cohen S.E., Lopez A.R., Ng P.K., Friedman O.A., Chaux G.E. Percutaneous tracheostomy in respiratory failure due to COVID-19. J Bronchology Interv Pulmonol. 2022;29:125–130. doi: 10.1097/LBR.0000000000000800. [DOI] [PubMed] [Google Scholar]
- 31.Singh S., Hind M., Jordan S., et al. Weaning by surgical tracheostomy and portable ventilators released ICU ventilators during coronavirus disease 2019 surge in London. Crit Care Explor. 2020;2:e0193. doi: 10.1097/CCE.0000000000000193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Courtney A., Lignos L., Ward P.A., Vizcaychipi M.P. Surgical tracheostomy outcomes in COVID-19-positive patients. OTO Open. 2021;5 doi: 10.1177/2473974X20984998. 2473974X20984998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.COVIDTrach Collaborative COVIDTrach; the outcomes of mechanically ventilated COVID-19 patients undergoing tracheostomy in the UK: interim report. Br J Surg. 2020;107:e583–e584. doi: 10.1002/bjs.12020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Floyd E., Harris S.S., Lim J.W., Edelstein D.R., Filangeri B., Bruni M. Early data from case series of tracheostomy in patients with SARS-CoV-2. Otolaryngol Head Neck Surg. 2020;163:1150–1152. doi: 10.1177/0194599820940655. [DOI] [PubMed] [Google Scholar]
- 35.Illuzzi E., Bassily-Marcus A., Kohli-Seth R., Leibner E., Mohammed A. Tracheostomy timing and outcomes in patients with COVID-19 pneumonia. Chest. 2020;158:A598. [Google Scholar]
- 36.Krishnamoorthy S., Polanco A., Coleman N., et al. The safety and efficacy of tracheostomy in patients diagnosed with COVID-19: an analysis of 143 patients at a major NYC medical center. Ann Surg Online Ahead of Print Published on November. 2020;17 doi: 10.1097/SLA.0000000000004612. [DOI] [PubMed] [Google Scholar]
- 37.Kumar N., Kumar A., Kumar A., Kumar S. Coronavirus disease-2019: modified underwater seal chest drain system. J Cardiothorac Vasc Anesth. 2021;35:347–348. doi: 10.1053/j.jvca.2020.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kwak P.E., Connors J.R., Benedict P.A., et al. Early outcomes from early tracheostomy for patients with COVID-19. JAMA Otolaryngol Head Neck Surg. 2021;147:239–244. doi: 10.1001/jamaoto.2020.4837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Livneh N., Mansour J., Kassif Lerner R., Feinmesser G., Alon E. Early vs. late tracheostomy in ventilated COVID-19 patients—a retrospective study. Am J Otolaryngol. 2021;42 doi: 10.1016/j.amjoto.2021.103102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mahmood K., Cheng G.Z., Van Nostrand K., et al. Tracheostomy for COVID-19 respiratory failure: multidisciplinary, multicenter data on timing, technique, and outcomes. Ann Surg. 2021;274:234–239. doi: 10.1097/SLA.0000000000004955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mata-Castro N., Sanz-López L., Pinacho-Martínez P., Varillas-Delgado D., Miró-Murillo M., Martín-Delgado M.C. Tracheostomy in patients with SARS-CoV-2 reduces time on mechanical ventilation but not intensive care unit stay. Am J Otolaryngol. 2021;42 doi: 10.1016/j.amjoto.2020.102867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Picetti E., Fornaciari A., Taccone F.S., et al. Safety of bedside surgical tracheostomy during COVID-19 pandemic: a retrospective observational study. PLoS One. 2020;15 doi: 10.1371/journal.pone.0240014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Riestra-Ayora J., Yanes-Diaz J., Penuelas O., Molina-Quiros C., Sanz-Fernández R., Martin-Sanz E. Safety and prognosis in percutaneous vs surgical tracheostomy in 27 patients with COVID-19. Otolaryngol Head Neck Surg. 2020;163:462–464. doi: 10.1177/0194599820931801. [DOI] [PubMed] [Google Scholar]
- 44.Rosano A., Martinelli E., Fusina F., et al. Early percutaneous tracheotomy in coronavirus disease 2019 (COVID-19) and infection in healthcare personnel: a cohort study. Infect Control Hosp Epidemiol. 2022;43:271–272. doi: 10.1017/ice.2020.1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rouhani M.J., Clunie G., Thong G., et al. A prospective study of voice, swallow, and airway outcomes following tracheostomy for COVID-19. Laryngoscope. 2021;131:E1918–E1925. doi: 10.1002/lary.29346. [DOI] [PubMed] [Google Scholar]
- 46.Rovira A., Tricklebank S., Surda P., et al. Open versus percutaneous tracheostomy in COVID-19: a multicentre comparison and recommendation for future resource utilisation. Eur Arch Otorhinolaryngol. 2021;278:2107–2114. doi: 10.1007/s00405-020-06597-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Šifrer R., Benedik J., Aničin A. Elective open “shield tracheostomy” in patients with COVID-19. Eur Arch Otorhinolaryngol. 2022;279:891–897. doi: 10.1007/s00405-021-06820-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Taboada M., Moreno E., Leal S., et al. Long-term outcomes after tracheostomy for COVID-19. Arch Bronconeumol. 2021;57:54–56. doi: 10.1016/j.arbres.2021.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Takhar A., Surda P., Ahmad I., et al. Timing of tracheostomy for prolonged respiratory wean in critically ill coronavirus disease 2019 patients: a machine learning approach. Crit Care Explor. 2020;2:e0279. doi: 10.1097/CCE.0000000000000279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tang Y., Wu Y., Zhu F., et al. Tracheostomy in 80 COVID-19 patients: a multicenter, retrospective, observational study. Front Med (Lausanne) 2020;7 doi: 10.3389/fmed.2020.615845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Turri-Zanoni M., Battaglia P., Czaczkes C., Pelosi P., Castelnuovo P., Cabrini L. Elective tracheostomy during mechanical ventilation in patients affected by COVID-19: preliminary case series from Lombardy, Italy. Otolaryngol Head Neck Surg. 2020;163:135–137. doi: 10.1177/0194599820928963. [DOI] [PubMed] [Google Scholar]
- 52.Valchanov K., Salaunkey K., Parmar J. Percutaneous dilatational tracheostomy in coronavirus disease 2019 extracorporeal membrane oxygenation patients: a case series. J Cardiothorac Vasc Anesth. 2021;35:348–350. doi: 10.1053/j.jvca.2020.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Volo T., Stritoni P., Battel I., et al. Elective tracheostomy during COVID-19 outbreak: to whom, when, how? Early experience from Venice, Italy. Eur Arch Otorhinolaryngol. 2021;278:781–789. doi: 10.1007/s00405-020-06190-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Weiss K.D., Coppolino A., Wiener D.C., et al. Controlled apneic tracheostomy in patients with coronavirus disease 2019 (COVID-19) JTCVS Tech. 2021;6:172–177. doi: 10.1016/j.xjtc.2020.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Williamson A., Roberts M.T., Phillips J., Saha R. Early percutaneous tracheostomy for patients with COVID-19. Anaesthesia. 2021;76:138–139. doi: 10.1111/anae.15197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yeung E., Hopkins P., Auzinger G., Fan K. Challenges of tracheostomy in COVID-19 patients in a tertiary centre in inner city London. Int J Oral Maxillofac Surg. 2020;49:1385–1391. doi: 10.1016/j.ijom.2020.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yokokawa T., Ariizumi Y., Hiramatsu M., et al. Management of tracheostomy in COVID-19 patients: the Japanese experience. Auris Nasus Larynx. 2021;48:525–529. doi: 10.1016/j.anl.2021.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zuazua-Gonzalez A., Collazo-Lorduy T., Coello-Casariego G., et al. Surgical tracheostomies in COVID-19 patients: indications, technique, and results in a second-level Spanish hospital. OTO Open. 2020;4 doi: 10.1177/2473974X20957636. 2473974X2095763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Long S.M., Chern A., Feit N.Z., et al. Percutaneous and open tracheostomy in patients with COVID-19. Ann Surg. 2021;273:403–409. doi: 10.1097/SLA.0000000000004428. [DOI] [PubMed] [Google Scholar]
- 60.Tornari C., Surda P., Takhar A., et al. Tracheostomy, ventilatory wean, and decannulation in COVID-19 patients. Eur Arch Otorhinolaryngol. 2021;278:1595–1604. doi: 10.1007/s00405-020-06187-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bartier S., La Croix C., Evrard D., et al. Tracheostomies after SARS-CoV-2 intubation, performed by academic otorhinolaryngologists in the Paris area of France: preliminary results. Eur Ann Otorhinolaryngol Head Neck Dis. 2021;138:443–449. doi: 10.1016/j.anorl.2021.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Forni R., Besana T., Amitrano A., Voinea C., Ogna A. Ventilatory weaning and early rehabilitation in COVID-19-related acute respiratory distress syndrome: the experience at Locarno hospital, canton of Ticino, Switzerland. Swiss Med Wkly. 2020;150 doi: 10.4414/smw.2020.20397. [DOI] [PubMed] [Google Scholar]
- 63.Ahmed Y., Cao A., Thal A., et al. Tracheotomy outcomes in 64 ventilated COVID-19 patients at a high-volume center in Bronx, NY. Laryngoscope. 2021;131 doi: 10.1002/lary.29391. E1797–804. [DOI] [PubMed] [Google Scholar]
- 64.Ahn D., Lee G.J., Choi Y.S., et al. Timing and clinical outcomes of tracheostomy in patients with COVID-19. Br J Surg. 2021;108:e27–e28. doi: 10.1093/bjs/znaa064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Staibano P., Levin M., McHugh T., Gupta M., Sommer D.D. Association of tracheostomy with outcomes in patients with COVID-19 and SARS-CoV-2 transmission among health care professionals. JAMA Otolaryngol Head Neck Surg. 2021;147:646–655. doi: 10.1001/jamaoto.2021.0930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chong W.H., Tan C.K. Clinical outcomes of early versus late tracheostomy in coronavirus disease 2019 patients: a systematic review and meta-analysis. J Intensive Care Med. 2022;37:1121–1132. doi: 10.1177/08850666221098930. [DOI] [PubMed] [Google Scholar]
- 67.Bier-Laning C., Cramer J.D., Roy S., et al. Tracheostomy during the COVID-19 pandemic: comparison of international perioperative care protocols and practices in 26 countries. Otolaryngol Head Neck Surg. 2021;164:1136–1147. doi: 10.1177/0194599820961985. [DOI] [PubMed] [Google Scholar]
- 68.Trouillet J.L., Collange O., Belafia F., et al. Tracheotomy in the intensive care unit: guidelines from a French expert panel. Ann Intensive Care. 2018;8:37. doi: 10.1186/s13613-018-0381-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.McGrath B.A., Ashby N., Birchall M., et al. Multidisciplinary guidance for safe tracheostomy care during the COVID-19 pandemic: the NHS national patient safety improvement programme (NatPatSIP) Anaesthesia. 2020;75:1659–1670. doi: 10.1111/anae.15120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nahar T., Roberts A., Brigode W., et al. HYPER-EARLY” tracheostomy within 48 hours has less complications and better prognosis compared to traditional tracheostomy. Am Surg. 2022;88:1517–1521. doi: 10.1177/00031348221082288. [DOI] [PubMed] [Google Scholar]
- 71.Chorath K., Hoang A., Rajasekaran K., Moreira A. Association of early vs late tracheostomy placement with pneumonia and ventilator days in critically ill patients. JAMA Otolaryngol Head Neck Surg. 2021;147:450–459. doi: 10.1001/jamaoto.2021.0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sutt A.L., Tronstad O., Barnett A.G., Kitchenman S., Fraser J.F. Earlier tracheostomy is associated with an earlier return to walking, talking, and eating. Aust Crit Care. 2020;33:213–218. doi: 10.1016/j.aucc.2020.02.006. [DOI] [PubMed] [Google Scholar]
- 73.McGrath B.A., Brenner M.J., Warrillow S.J., et al. Tracheostomy in the COVID-19 era: global and multidisciplinary guidance. Lancet Respir Med. 2020;8:717–725. doi: 10.1016/S2213-2600(20)30230-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Brodsky M.B., Levy M.J., Jedlanek E., et al. Laryngeal injury and upper airway symptoms after oral endotracheal intubation with mechanical ventilation during critical care. Crit Care Med. 2018;46:2010. doi: 10.1097/CCM.0000000000003368. –7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zou L., Ruan F., Huang M., et al. SARS-CoV-2 viral load in upper respiratory specimens of infected patients. N Engl J Med. 2020;382:1177–1179. doi: 10.1056/NEJMc2001737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sorbello M., Rosenblatt W., Hofmeyr R., Greif R., Urdaneta F. Aerosol boxes and barrier enclosures for airway management in COVID-19 patients: a scoping review and narrative synthesis. Br J Anaesth. 2020;125:880–894. doi: 10.1016/j.bja.2020.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Deng H., Fang Q., Chen K., Zhang X. Early versus late tracheotomy in ICU patients. Medicine (Baltimore) 2021;100 doi: 10.1097/MD.0000000000024329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hosokawa K., Nishimura M., Egi M., Vincent J.-L. Timing of tracheotomy in ICU patients: a systematic review of randomized controlled trials. Crit Care. 2015;19:424. doi: 10.1186/s13054-015-1138-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Meng L., Wang C., Li J., Zhang J. Early vs late tracheostomy in critically ill patients: a systematic review and meta-analysis. Clin Respir J. 2016;10:684–692. doi: 10.1111/crj.12286. [DOI] [PubMed] [Google Scholar]
- 80.Freeman B.D., Borecki I.B., Coopersmith C.M., Buchman T.G. Relationship between tracheostomy timing and duration of mechanical ventilation in critically ill patients. Crit Care Med. 2005;33:2513–2520. doi: 10.1097/01.ccm.0000186369.91799.44. [DOI] [PubMed] [Google Scholar]
- 81.Griffiths J., Barber V.S., Morgan L., Young J.D. Systematic review and meta-analysis of studies of the timing of tracheostomy in adult patients undergoing artificial ventilation. BMJ. 2005;330:1243. doi: 10.1136/bmj.38467.485671.E0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Huang H., Li Y., Ariani F., Chen X., Lin J. Timing of tracheostomy in critically ill patients: a meta-analysis. PLoS One. 2014;9 doi: 10.1371/journal.pone.0092981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Siempos, Ntaidou T.K., Filippidis F.T., Choi A.M.K. Effect of early versus late or no tracheostomy on mortality and pneumonia of critically ill patients receiving mechanical ventilation: a systematic review and meta-analysis. Lancet Respir Med. 2015;3:150–158. doi: 10.1016/S2213-2600(15)00007-7. [DOI] [PubMed] [Google Scholar]
- 84.Stelfox H., Crimi C., Berra L., et al. Determinants of tracheostomy decannulation: an international survey. Crit Care. 2008;12:R26. doi: 10.1186/cc6802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Adly A., Youssef T.A., El-Begermy M.M., Younis H.M. Timing of tracheostomy in patients with prolonged endotracheal intubation: a systematic review. Eur Arch Otorhinolaryngol. 2018;275:679–690. doi: 10.1007/s00405-017-4838-7. [DOI] [PubMed] [Google Scholar]
- 86.Putensen C., Theuerkauf N., Guenther U., Vargas M., Pelosi P. Percutaneous and surgical tracheostomy in critically ill adult patients: a meta-analysis. Crit Care. 2014;18:544. doi: 10.1186/s13054-014-0544-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lavinio A., Ercole A., Battaglini D., et al. Safety profile of enhanced thromboprophylaxis strategies for critically ill COVID-19 patients during the first wave of the pandemic: observational report from 28 European intensive care units. Crit Care. 2021;25:155. doi: 10.1186/s13054-021-03543-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lazzaroni M.G., Piantoni S., Masneri S., et al. Coagulation dysfunction in COVID-19: the interplay between inflammation, viral infection and the coagulation system. Blood Rev. 2021;46 doi: 10.1016/j.blre.2020.100745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Robba C., Battaglini D., Ball L., et al. Coagulative disorders in critically ill COVID-19 patients with acute distress respiratory syndrome: a critical review. J Clin Med. 2021;10:140. doi: 10.3390/jcm10010140. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.