Abstract
Acute kidney injury (AKI) induced by cisplatin (cis-AKI) involves indicators such as inflammation and oxidative stress (OS) in proximal tubules, although its underlying mechanisms remain largely unknown so far. Exploration of the molecular mechanisms underlying cisplatin-induced AKI is of great significance for AKI prevention and also for preventing its progression into chronic kidney disease (CKD) or end-stage renal disease (ESRD). OS and ferroptosis are mutually causal; they finally lead to the regulatory cell injury and death induced by the accumulation of reactive oxygen species (ROS). GPX4 is critical not only in OS, but studies established as the key regulator of ferroptosis. In this context, the present study focused on determining the biological function of miR-214-3p in the cisplatin-induced ferroptosis of tubular epithelial cell (TEC) and the underlying molecular mechanism. The relationship between TEC ferroptosis and cisplatin-induced AKI was investigated in vitro and in vivo. Ferrostatin-1(Fer-1), an inhibitor of ferroptosis, was observed to confer a protective effect against the renal tubular injury and renal failure induced by cisplatin. MicroRNAs (miRNAs) regulate the genes that have important functions in the development of cis-AKI. In the present study, GPX4 was predicted as a target of miR-214–3p. Moreover, inhibiting miR-214-3p enhanced the expressions of GPX4 and SLC7A11 while decreasing the ACSL4 expression. Furthermore, miR-214-3p down-regulation protected against TEC death and renal tubule damage both in vitro and in vivo. According to these findings, inhibiting miR-214-3p would alleviate TEC ferroptosis in cis-AKI via GPX4.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12192-022-01271-3.
Keywords: Acute kidney injury, Ferroptosis, Cisplatin, miR-214-3p, GPX4, Oxidative stress
Introduction
Cisplatin has been applied extensively as a postoperative chemotherapeutic in cancer. However, there are certain instances of undesirable after-effects of this drug. For instance, adjuvant chemotherapy containing cisplatin has been reported to enhance the prognosis of non-small cell lung cancer (NSCLC) in patients undergoing radical resection (Arriagada et al. 2004). Cisplatin-induced nephrotoxicity, which is characterized by significant cellular damage in the kidney tubules due to oxidative stress, is emerging as the main limitation in the application of cisplatin, affecting 30% of the cisplatin recipient patients (Pabla and Dong 2008; Holditch et al. 2019). Acute kidney injury (AKI) is a kidney disease characterized by rapidly declining renal function, which promotes toxin and metabolic waste deposition and may lead to further complications or even organ failure (Lameire et al. 2013). Even the patients who survive AKI have a double risk of chronic kidney disease (CKD) and end-stage renal disease (ESRD) (Holditch et al. 2019), thereby having to deal with heavy economic burdens. AKI induced by cisplatin involves multiple factors and several phases. Therefore, it is vital to explore the molecular mechanisms underlying the AKI caused by cisplatin (cis-AKI).
Ferroptosis is a type of programmed cell death that is dependent on iron. Ferroptosis is caused by the accumulation of lipid-based reactive oxygen species (ROS) at a lethal dose due to impairment of the lipid peroxide repair systems that are dependent on glutathione (GSH) (Dixon et al. 2012). Meanwhile, as an important ferroptosis suppressor and non-enzymatic antioxidant, GSH provides a vital defense system to protect cells from different types of oxidative stress. In pathological events, the activity of glutathione peroxidase is inhibited directly or indirectly via the upstream pathway, which results in a loss of the antioxidant activity and an increase in the lipid peroxidation reaction and ROS levels, leading to ferroptosis. Studies report TEC ferroptosis as an important pathological process, and the inhibition of which has been demonstrated to protect against AKI (Belavgeni et al. 2020; Hu et al. 2019). Glutathione peroxidase 4 (GPX4) is speculated to be the main glutathione peroxidase molecule that protects the biofilm from hydrogen peroxidation. Genetic studies performed in cells and mice established the selenoenzyme GPX4 as the key regulator of this form of cell death and plays a further important role in ferroptosis (Dixon et al. 2012; Linkermann et al. 2014). Research shows that GSH/Gpx4 axis plays an essential role in preventing lipid-oxidation-induced acute renal failure (Friedmann Angeli et al. 2014). Therefore, ferroptosis connecting oxidative stress associated with iron and lipid metabolism plays a pathological role in cis-AKI, and developing novel therapeutics that regulate GPX4 and thereby reduce ferroptosis in the renal tubular epithelial cells might be a promising approach for treating cis-AKI.
MicroRNAs (miRNAs) are non-coding small RNAs containing only 19–25 nucleotides. The literature documents several miRNAs involved in the regulation of various biological processes occurring in the renal tubular epithelial cells, including ferroptosis, pyroptosis, and endoplasmic reticulum stress (ERS) (Ding et al. 2020; Wang et al. 2020, 2019). However, few studies have reported the mechanisms underlying the regulation of ferroptosis by miRNAs in cis-AKI.
The present study focused on exploring the function of miR-214-3p in mice kidney and TCMK-1 cells treated with cisplatin. In addition, the role of miR-214–3p in preventing TEC ferroptosis via GPX4 regulation was investigated. Collectively, the results of the present study unraveled the precise mechanism underlying the regulation of TEC ferroptosis in cis-AKI by miR-214–3p.
Materials and methods
Animals and experimental protocols
The study protocols used in the present work were approved by the Institutional Animal Care and Use Committee of Qingdao Municipal Hospital. Cisplatin was provided by Qingdao Municipal Hospital. The C57BL/6 male mice (8-week-old, weighing approximately 20–25 g) were procured from Beijing Huafukang Bioscience Co. Inc. The mice were raised under the SPF condition. Cisplatin was injected into the mice intraperitoneally and only once at a dose of 30 mg/kg to induce AKI, while the control mice were injected with PBS. Intravenous administration of 10 mg/kg of agomir negative control (agomir NC) or agomir miR-214-3p (GenePharma Co. Ltd, Shanghai, China) was performed for the control group mice and model group mice, respectively. The ferroptosis inhibitor named Fer-1 (#S7243, Selleck Chemicals, Houston, TX, USA) was dissolved in DMSO and then diluted in 0.9% NaCl to prepare separate Fer-1 solutions each with a concentration of 0.2 mg/mL. Fer-1 was injected into the mice intraperitoneally, 1 h prior to injecting cisplatin, while the control mice received the injection of only 0.9% NaCl in 0.1% DMSO. Both experimental and control group mice were sacrificed at Day 1, 2, and 3, separately, post-cisplatin injection. The mice were sacrificed using pentobarbital sodium (200 mg/kg) after the completion of the experiment. The resected tissues from the left kidney of each mouse were immediately frozen in liquid nitrogen and then preserved in 4% paraformaldehyde or stored at − 80 °C until used for the subsequent biochemical and pathological analyses.
Cell culture and transfection
The TCMK-1 cell line (the immortalized mice tubule epithelial cell line) obtained from the American Type Culture Collection (no. CCL-139) was cultured in the complete medium under 5% CO2 and 95% air atmosphere. After 24 h of culture, the cells were collected for subsequent experiments. The cells assigned to the cisplatin group were stimulated with cisplatin (20 µmol/L) for different durations (6, 12, and 24 h), while the cells assigned to the control group were treated with PBS under the same conditions. These treated TCMK-1 cells were then transfected with agomir NC or agomir miR-214–3p using Lipofectamine 2000 (Invitrogen, Shanghai, China).
TUNEL assay
The TUNEL kit (cat no. 11684817910, Roche) was utilized to stain the 4-µm sections using specific protocols. The nuclei of the TUNEL-positive cells are stained brown. The stained sections were observed under a microscope at 400 × magnification in five randomly selected fields of vision.
GSH detection in cell
The GSH activity assay kits were utilized to measure the GSH contents within the cells by following specific instructions, using a microplate fluorometer at 405 nm and 450 nm. Bradford’s method (catalog no. S0053, Beyotime Institute of Biotechnology, Haimen, China) was employed to determine the total protein content.
Cell Counting Kit-8 (CCK-8) assay
The CCK-8 kit (catalog no. C0038, Beyotime) was employed to detect cell viability by following specific protocols. A microplate reader (Molecular Devices) was used for measuring the absorbance at 450 nm.
qRT-PCR
Total cellular (TCMK-1) and renal tissue RNAs were isolated using TRIzol reagent (Invitrogen). Later, Revert Aid First-Strand cDNA Synthesis kit (catalog no. K1622, Invitrogen) was used for quantifying and reverse transcribing the isolated RNAs by following specific protocols. The sequences of the primers used in the experiments are listed below:
GPX4, 5′-ATACGCTGAGTGTGGTTTGC-3′ (forward),
5′-CTTCATCCACTTCCACAGCG-3′ (reverse);
ACSL4, 5′-GAGGCUUCCUAUCUGAUUATT-3′ (forward),
5′-UAAUCAGAUAGGAAGCCUCTT-3′ (reverse);
SLC7A11, 5′-GCTGACACTCGTGCTATT-3′ (forward),
5′-ATTCTGGAGGTCTTTGGT-3′ (reverse);
β-actin, 5′-TACCTGAAGCCCCAACTACAAA-3′ (forward),
5′-GTGCCCTGCCACATGATAAA-3′ (reverse);
miR-214–3p, 5′-GAGTGTTGGCCTGTCCTCAA-3′ (forward),
5′-TTGTGCCCAGTTGCCTGTAT-3′ (reverse);
U6, 5′-GCTTCGGCAGCACATATACTAAAAT-3′ (forward),
5′-CGCTTCACGAATTTGCGTGTCAT-3′ (reverse).
U6 was used as a reference for normalizing the miR-214-3p level, while β-actin was used for normalizing the GPX4, SLC7A11, and ACSL4 levels. The gene levels were calculated using the 2–(Cq) method (Livak and Schmittgen 2001).
Hematoxylin and Eosin (H&E) staining
After fixing the tissue specimens in 4% paraformaldehyde, the paraffin-embedded renal tissue specimens were excised into 4-µm sections. The excised sections were stained using the H&E kit (catalog no. C0105M, Beyotime) by following specific protocols. The pathological changes were analyzed under a microscope at 200 × or 400 × magnification in five randomly selected fields of vision. The proportion of the tubular cell affected was calculated in terms of the tubular injury score — 0 = ≤ 10%, 1 = 10–25%, 2 = 26–50%, 3 = 51–75%, and 4 = ≥ 75%.
Iron assay
The Fe2+ content in the cells was measured using the iron assay kit (catalog no. ab83366, Abcam) by following specific protocols. Briefly, the kidney samples were dissected and rinsed with pre-chilled PBS. Subsequently, the samples were homogenized in five volumes of the iron assay buffer on ice. The supernatants were collected, mixed with the iron reducer, and incubated for 30 min. After the addition of the iron probe into each sample, all the samples were mixed and incubated for 60 min. Finally, the output absorbance was measured at 593 nm using a microplate reader.
ROS measurement
The cells were incubated with the DCFH-DA probe (10 µM; Beyotime, Shanghai, China) for 25 min and then washed twice with PBS. The fluorescence was measured at the emission and excitation wavelengths of 525 nm and 488 nm, respectively, using a fluorescence microplate reader.
Serum creatinine and BUN content measurements
Commercial assay kits were employed to measure the creatinine and BUN contents (LabAssay™ Creatinine for creatinine, Wako, Osaka; Urea Nitrogen B Test for BUN) in the cells.
Flow cytometry analysis
The stimulated cells were stained sequentially with Annexin V and propidium iodide (PI) using the Annexin V-FITC Apoptosis Detection kit (catalog no. 558064, BD Biosciences). The stained apoptotic cells were visualized using flow cytometry.
Immunohistochemistry (IHC) analysis
After blocking the paraffinized sections using 3% hydrogen peroxide, followed by deparaffinizing and hydrating the sections (thickness 4 µm) using 10% normal goat serum (catalog no. 16210072, Gibco, Thermo Fisher Scientific, Inc.), the sections were probed with anti-GPX4 (1:200; catalog no. ab125066) and anti-ACSL4 (1:200; catalog no. ab155282, Abcam). Subsequently, each section was incubated with the HRP-labeled goat anti-rabbit IgG (Thermo Fisher Scientific Inc.) utilized as the secondary antibody. Finally, the sections were stained with 3,3′-Diaminobenzidine (DAB), and after color development, were observed and photographed under a microscope at 400 × magnification.
Western Blotting (WB) analysis
The extracted protein was quantified for GPX4, ACSL4, and SLC7A11 using the BCA assay kit (Invitrogen). The membranes were incubated overnight with primary antibodies, including anti-ACSL4 (1:200; catalog no. ab155282), anti-GPX4 (1:200; catalog no. ab125066), β-actin (1:500; catalog no. ab8224), and anti-SLC7A11 (1:500; catalog no. ab37185; all from Abcam). Subsequently, the membranes were incubated with the HRP-labeled secondary antibody (1:500; catalog no. ab6789, Abcam). Finally, the protein bands were analyzed using the Odyssey infrared imaging system (LI-COR Biotechnology).
Mitochondrial morphology observation using the electron microscope
Briefly, 1 mm3 of the freshly prepared kidney tissue samples were dissected and immediately fixed with the electron microscopy fixative at 4 °C. Subsequently, the paraffin-embedded ultrathin sections of size 60–80 nm were prepared and double-stained with uranium lead. The renal tubular epithelial cells were observed and photographed under a transmission electron microscope (TEM).
Statistical analysis
The obtained result data were expressed as mean ± SD. The SPSS 22.0 was employed to conduct the statistical analysis of the obtained data. The linearity of the variables was evaluated using Pearson’s correlation test. Each experiment was conducted in triplicate. The significance of the difference was evaluated through variance analysis. P < 0.05 was utilized as the statistical significance threshold.
Results
Ferroptosis participates in the cisplatin-induced AKI
In order to establish the AKI model, cisplatin (30 mg/kg) was injected into 8-week-old wild-type C57BL/6 male mice, and cellular changes were observed at Day 1, 2, and 3 after the injection. The cisplatin-induced tubular injuries (excessive death of tubular cells, interstitial edema, serious cell shedding, and disruption of the brush border in the renal cortex) were aggravated upon the H&E staining of the kidney tissues; H&E injury scores were calculated (Fig. 1A and B). As depicted in Fig. 1A and C, the number of TUNEL-positive TECs demonstrated a gradual increase with time, reaching its maximum value on Day 3. In addition, consistent with the tubular injury, the SCr and BUN contents decreased steadily with prolonged time intervals compared to the control group (Fig. 1D and E).
Fig. 1.
Ferroptosis is involved in cisplatin induced AKI. A Representative photomicrographs of tubular cell injury in mouse kidney tissue sections with HE and TUNEL staining, 400 × , scale bar = 20 µm. B Statistical quantification analysis showed the injury score of HE staining in the kidney tissues exposed to cisplatin at 1, 2, or 3, day respectively. C Statistical analysis showed the percentage of TUNEL positive TECs in the kidney tissues exposed to cisplatin at 1, 2, or 3 day, respectively. The levels of SCr (D) and BUN (E) were detected in mice. F The level of iron was detected in the kidney tissues exposed to cisplatin at 1, 2, or 3 day, respectively. G qRT-PCR analysis of GPX4 expression at different times exposed to cisplatin in kidney samples. H, I Western blot analysis of GPX4 protein expression at different times exposed to cisplatin in kidney samples. Data are expressed as the mean ± SD. n = 6 per group. *P < 0.05 vs. Con group, #P < 0.05 vs. cisplatin treatment group at 2 day, one-way ANOVA
In order to demonstrate the role of ferroptosis in cis-AKI, the changes in the Fe concentration were evaluated. It was revealed that Fe concentration increased dramatically in renal tubular cells (Fig. 1F). In addition, the mRNA and protein expressions of glutathione peroxidase 4 (GPX4, the key factor regulating ferroptosis) were evaluated (Fig. 1G–I). It was revealed that the GPX4 mRNA and protein levels decreased in a dose dependent manner after the cisplatin treatment, which suggested a connection between the cisplatin-mediated tubular cell injury and GPX4.
Ferroptosis plays a key role in the cisplatin-induced AKI and the role of GPX4 in the ferroptosis in cisplatin-stimulated TCMK-1 cells
The biological role of ferroptosis in cis-AKI was investigated. The H&E staining and TUNEL assay were conducted to assess the pathology of the cisplatin-induced mice, and the results of which suggested the role of Fer-1 in alleviating the kidney cell death and tissue injury in cis-treated kidneys by Day 3 (Fig. 2A–C). Moreover, pretreatment with Fer-1 remarkably declined the BUN and SCr levels in the cells (Fig. 2D and E). Furthermore, Fer-1 reduced the Fe2+ expression (Fig. 2F). In addition, the qRT-PCR and western blot analysis revealed that Fer-1 dramatically decreased the expression of ACSL4 while increasing the expressions of GPX4 and SLC7A11 in the kidneys induced by cisplatin (Fig. 2G–I). These findings indicated that ferroptosis played a key role in cis-AKI and that the inhibition of ferroptosis could effectively alleviate cis-AKI. In vitro experiments were conducted using the TCMK-1 cell line (a kind of immortalized murine tubular epithelial cell line). The TCMK-1 cells were treated with cisplatin for 6, 12, and 24 h, followed by performing the CCK-8 assay to assess TEC death and viability. The results presented a significant decrease in the viability of TCMK-1 cells with the increased duration of cisplatin intervention in comparison to control (Fig. 2J). Again, it was revealed that the ROS levels increased gradually with a prolonged duration of time (Fig. 2K). Similarly, the data from the iron level analysis revealed a gradual increase in the Fe2+ levels in the cisplatin-exposed TECs (Fig. 2L). Furthermore, the expressions of both mRNA and protein of GPX4 in the TCMK-1 cells decreased steadily with the prolonged intervals of cisplatin intervention (Fig. 2M–O). These results further indicated that GPX4 was involved as an important regulatory factor in cis-AKI.
Fig. 2.
Ferroptosis is involved in cisplatin induced AKI and the role of GPX4 in ferroptosis in cisplatin-stimulated TCMK-1 Cells. A Representative photomicrographs of tubular cell injury in mouse kidney tissue sections with HE and TUNEL staining, 400 × , scale bar = 20 µm. B Statistical quantification analysis showed the injury score of HE staining in the kidney tissues. C Statistical analysis showed the percentage of TUNEL positive TECs in the kidney tissues. The levels of SCr (D) and BUN (E) were detected in each group. F The levels of iron was detected in each group. G qRT-PCR analysis of GPX4, ACSL4, and SLC7A11 mRNA expression in each group. (H&I) Western blot analysis of GPX4, ACSL4, and SLC7A11 protein expression in each group. J Cell viability was detected by CCK-8 assay. The levels of ROS (K) and iron (L) were detected at different times exposed to cisplatin in TCMK-1 cells. M qRT-PCR analysis of GPX4 expression at different times exposed to cisplatin in TCMK-1 cells. N, O Western blot analysis of GPX4 protein expression at different times exposed to cisplatin in TCMK-1 cells. Data are expressed as the mean ± SD. n = 6 per group in vivo and n = 5 per group in vitro. *P < 0.05 vs. Con group, #P < 0.05 vs. cisplatin treatment group, ^P > 0.05 vs. cisplatin treatment group. #P < 0.05 vs. cisplatin treatment group at 12 h, one-way ANOVA
GPX4 is a potential target of miR-214–3p
As revealed by the electron microscopy analysis, the renal tissues exposed to cisplatin exhibited excess mitochondrial swelling, decreased number of cristae, and a lamellar phenotype relative to the healthy controls (Fig. 3A). The flow cytometric analysis revealed that cisplatin enhanced cell death in vitro (Fig. 3B and C).
Fig. 3.
GPX4 is a potential target of miR-214-3p. A Representative photomicrographs of tubular cell injury in mouse kidney tissue preparation for visualization in transmission electron microscopy (× 12.0 k). B Flow cytometry assays were performed to show the cell death. C Statistical analysis was used to show the levels of cell death. D qRT-PCR analysis of miR-214-3p expression in vivo and in vitro. E Pearson’s correlation analysis revealed that the expression of miR-214-3p was inversely correlated with the expression of GPX4 mRNA. F Dual-reporter luciferase assay of miR-214-3p expression in wt-pGL3-GPX4-3′UTR or mut-pGL3-GPX4-3′UTR constructs. G Database predicted the existence of binding sites between GPX4 3′UTR and miR-214-3p. Data are expressed as the mean ± SD. n = 6 per group in vivo and n = 5 per group in vitro. *P < 0.05 vs. Con group in vivo or in vitro or scramble wt-pGL3-GPX4-3′UTR group. ∧P > 0.05 vs. scramble mut-pGL3-GPX4-3′UTR group, one-way ANOVA
The bioinformatics analyses (TargetScan, Diana, and miRDB) predicted a conserved binding site for miR-214-3p in the 3′UTR of GPX4. In order to confirm this prediction, qRT-PCR was performed to determine the miR-92a-3p levels. It was revealed that miR-214-3p was significantly overexpressed in the cisplatin group compared to the controls both in vitro and in vivo (Fig. 3D). Moreover, the mRNA level of GPX4 was negatively correlated to the miR-214-3p level in the TCMK-1 cells (Fig. 3E). When the mutant (mut-pGL3-GPX4–3′UTR) or wild-type (wt-pGL3-GPX4–3′UTR) plasmid was co-transfected with miR-214-3p mimic or the corresponding NC into the TCMK-1 cells, the miR-214-3p overexpression markedly decreased the GPX4 level in the wild type compared to the mutant type plasmid (Fig. 3F and G). Therefore, it was inferred that miR-214-3p possibly modulated the GPX4 level in cis-AKI.
Suppression of miR-214-3p alleviates ferroptosis in the cisplatin-induced TCMK-1 cells
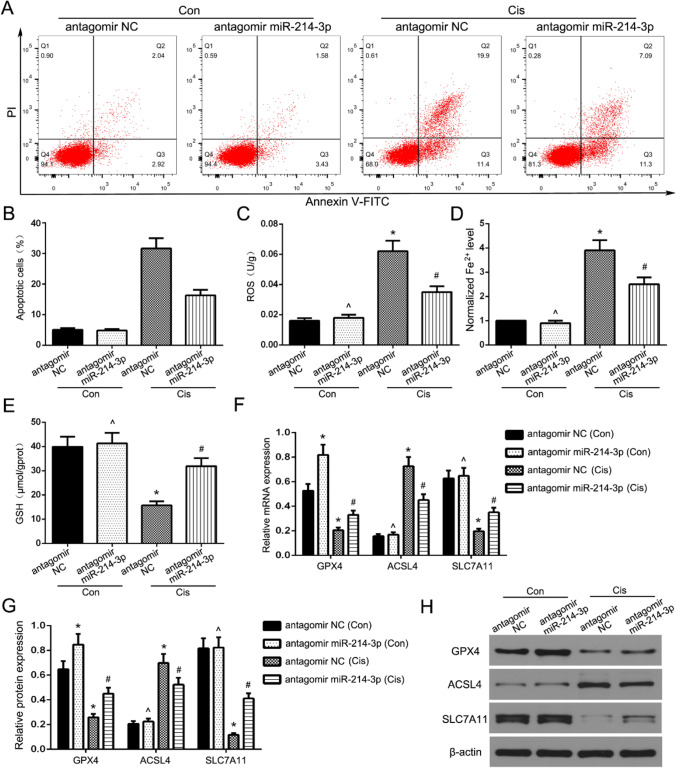
Since GPX4 plays an important role in the regulation of ferroptosis, the regulatory role of miR-214-3p in cis-AKI was also investigated. It was revealed that cisplatin promoted ferroptosis in the TCMK-1 cells transfected with antagomir NC or antagomir miR-214–3p. Furthermore, downregulation of the miR-214-3p expression significantly reduced ferroptosis in the TCMK-1 cells induced by cisplatin (Fig. 4A–D). The inhibition of miR-214-3p significantly elevated the GSH expression in the cisplatin-induced TCMK-1 cells compared to the normal cells (Fig. 4E). Moreover, decreased miR-214-3p expression upregulated the SLC7A11 and GPX4 levels while downregulating the ACSL4 level in comparison to the cisplatin-induced antagomir NC group (Fig. 4F–H).
Fig. 4.
Inhibition of miR-214-3p alleviates ferroptosis in cisplatin -induced TCMK-1 Cells. A Flow cytometry assays were performed to show the cell death. B Statistical analysis was used to show the levels of cell death. The levels of ROS (C), iron (D), and GSH (E) in TCMK-1 cells. F qRT-PCR analysis of GPX4, ACSL4, and SLC7A11 expression in TCMK-1 cells. G, H Western blot analysis of GPX4, ACSL4, and SLC7A11 expression in TCMK-1 cells. Data are expressed as the mean ± SD. n = 5 per group. *P < 0.05 and ∧P > 0.05 vs. con antagomir NC group, #P < 0.05 vs. antagomir NC group treated by cisplatin, one-way ANOVA
Suppression of miR-214-3p reduces TEC ferroptosis in the cisplatin-induced mice kidney
In order to better assess the biological significance of miR-214–3p, mice kidney samples were collected for analysis 3 days after the cisplatin stimulation. Interestingly, the antagomir miR-214-3p caused no alteration in the kidney structure upon exposure to cisplatin, as revealed by the H&E staining. Nonetheless, the cisplatin-induced kidney samples exhibited significant renal tubular damage, although this effect was significantly alleviated in the antagomir miR-214-3p group (Fig. 5A and B). As a consequence, the number of TUNEL-positive TECs declined by the greatest extent in the cisplatin-exposed kidneys, followed by the miR-214-3p inhibition group (Fig. 5A and C). The IHC analysis revealed that the downregulation of miR-214-3p decreased the ACSL4 level and increased the GPX4 level in the cisplatin-treated group compared to the antagomir NC group (Fig. 5A, D–E). Meanwhile, the Fe2+ content declined significantly in the renal cisplatin group, although this effect was mitigated with the inhibition of miR-214-3p (Fig. 5F). The inhibition of miR-214–3p, on the other hand, elevated the SLC7A11 and GPX4 expressions and declined the ACSL4 level in the cisplatin-exposed kidneys (Fig. 5G–I). Collectively, these results suggested that miR-214-3p suppression mitigates tubular ferroptosis in AKI via GPX4.
Fig. 5.
Inhibition of miR-214-3p alleviates TEC ferroptosis in the cisplatin-induced kidney of mice. A Representative photomicrographs of tubular cell injury in mouse kidney tissue sections with HE staining, TUNEL staining, GPX4, and ACSL4 expression in mouse kidney tissue sections by immunohistochemistry( 400 × , scale bar = 20 µm). B Statistical quantification analysis showed the injury score of HE staining in the kidney tissues. C Statistical analysis showed the percentage of TUNEL-positive TECs in the kidney tissues. D, E Statistical analysis showed the positive area of GPX4 and ACSL4 in the kidney tissues. The level of iron (F) was detected in kidney samples. G qRT-PCR analysis of GPX4 and ACSL4 expression in kidney samples. H, I Western blot analysis of GPX4, ACSL4, and SLC7A11 protein expression in kidney samples. Data are expressed as the mean ± SD. n = 6 per group. *P < 0.05 and ^P > 0.05 vs. con antagomir NC groups. #P < 0.05 vs. antagomir NC group treated by cisplatin, one-way ANOVA
Discussion
Cisplatin is a chemotherapeutic that has been used extensively in the treatment of several cancers. It is reported that the amount of the constituent metabolites of cisplatin in the kidney is five times higher compared to that in the blood due to the differences in the pharmacokinetic characteristics of these tissues (Xiang et al. 2019). This suggests that the innate renal cells, particularly the renal tubular epithelial cells, are capable ofactively adsorbing drugs. The elevated intracellular content of cisplatin impairs the mitochondrial and nuclear DNA and generates ROS, which may activate the apoptotic and necrotic pathways, thereby causing nephrotoxicity (Pabla and Dong 2008). Several studies have proposed the possible mechanism for cis-AKI, primarily including oxidative stress (Chirino and Pedraza-Chaverri 2009), lipid peroxidation (Mata-Miranda et al. 2019), and disturbance of calcium homeostasis (Goren 2003), although the precise molecular mechanism remains largely unknown so far. In the present study, our findings provide a molecular mechanism by which the suppression of miR-214-3p protected against TEC ferroptosis by oxidative stress-induced lipid peroxidation in cis-AKI via GPX4.
Ferroptosis is induced by lipid peroxidation that is dependent on Fe2+ and ROS. Under the catalysis of Fe2+, the metabolism is abnormal, and lipids are produced in huge amounts, which damages the intracellular redox balance, leading to attacks on the biological macromolecules and finally inducing cell death (Dixon et al. 2012; Yang et al. 2014). Recent studies have revealed that inhibiting TEC ferroptosis could alleviate renal injury (Hu et al. 2020). The present study discovered a significant level of ferroptosis in the kidneys of the AKI mouse model induced with 30 mg/kg of cisplatin which was characterized by a remarkable increase in the Fe2+ content and ROS production by the end of 24 h in vitro and by the end of the 3rd day in vivo. The results of the present study confirmed that TEC ferroptosis might be an important pathological process in cis-AKI, in addition to providing a model choice for subsequent research on the ferroptosis induced by cisplatin.
The literature suggests that the peroxidation of the phospholipids (PLs) containing polyunsaturated fatty acids (PUFAs) is the main cause of ferroptosis. ACSL4, a long-chain fatty-acid-coenzyme A ligase, plays a vital role in the biosynthesis of PUFA-PL (Doll et al. 2017). GPX4 is a glutathione peroxidase capable of utilizing reduced glutathione containing the rate-limiting precursor cysteine to convert lipid hydroperoxides into lipid alcohols, thereby alleviating lipid peroxidation while suppressing ferroptosis (Friedmann Angeli et al. 2014; Seibt et al. 2019). Considering the crucial role of GPX4 in regulating lipid peroxidation, there is no doubt that the enzyme has emerged as a pivotal regulator of ferroptosis. Indeed, many ferroptosis modulators are directly or indirectly related to GPX4 function. Most of the cells acquire cysteine through the extracellular cystine import via SLC7A11 (an amino acid transporter also referred to as xCT) (Koppula et al. 2018). Accordingly, the inactivation of Glutathione (GSH)-dependent detoxication enzyme system via various pharmacological or genetic approaches would induce TEC ferroptosis (Friedmann Angeli et al. 2014). The present study revealed that GPX4 expression was negatively correlated to renal tubular injury and positively correlated to renal function in cisplatin-stimulated mice. These results indicated that GPX4 could serve as an important indicator of the degree of renal injury and the clinical prognosis of cis-AKI patients. Furthermore, cisplatin injection could remarkably increase the Fe2 + content and ROS production in the kidneys of cis-AKI mice in the present study. In addition, ACSL4 expression was upregulated while the expressions of GPX4 and SLC7A11 were inhibited. However, these effects were reversed by Fer-1 along with alleviation in tissue damage and improvement in renal function. Therefore, it was inferred that ferroptosis could serve as an effective therapeutic target in cis-AKI.
In the context of cis-AKI, microRNAs are reported to exhibit differential expression associated with the molecular mechanisms of programmed cell death. For instance, after cisplatin exposure, the miR-375 expression remarkably increased with p53 and NF-κB, thereby inducing the death of renal tubular cells (Guo et al. 2019). The inhibition of miR-449 restrained the cisplatin-induced cell apoptosis in vitro by preserving the SIRT1–p53–BAX pathway (Qin et al. 2016). The inhibition of miR-146b was reported to mitigate the nephrotoxicity in the cultivated tubular epithelial cells induced by cisplatin by promoting the ErbB4 expression while accelerating cell proliferation (Zhu et al. 2016). In the present work, target genes were predicted using TargetScan, Diana, and miRDB, which revealed GPX4 as the target of miR-214–3p. As one of the conserved mammalian miRNAs, miR-214-3p is localized in the intron 14 of the Dynamin-3 (DNM3) gene located on chromosome 1q24.3 and is encoded via the miR-214 gene. This miR has been investigated extensively in diverse renal disorders or injuries. It is reported that miR-214-3p enhances kidney fibrosis, injuries, and inflammation in hyperlipidemic pancreatitis secondary to AKI (Yan et al. 2020). However, the role of miR-214-3p in cis-AKI has not been investigated in any previous study. The results of the present study revealed an up-regulation of miR-214-3p in cisplatin-treated TECs, which was also observed to be negatively correlated with the GPX4 mRNA level. Therefore, it was inferred that miR-214-3p could serve as a novel therapeutic target in the treatment of cis-AKI. The present study also analyzed the effect of miR-214–3p on the TEC ferroptosis in cis-AKI. It was revealed that miR-214-3p inhibition increased the SLC7A11 and GPX4 expressions and decreased the ACSL4 expression both in vitro and in vivo. The inhibition of miR-214-3p also mitigated the TEC death and renal tubular injury in the cisplatin-exposed kidneys, which suggested that miR-214-3p could be utilized as a marker for diagnosing and treating cis-AKI.
Interestingly, Xie M has quite recently reported that miR-214-3p downregulation protects the vascular endothelial cells (VECs) against ROS-mediated endothelial impairment by inhibiting the GPX4 suppression induced by miRNA (Xie et al. 2021), although the role of miR-214-3p in ferroptosis was not studied specifically by this author. The experimental results of the present study confirmed GPX4 as a target of miR-214-3p in TEC. Furthermore, the present study revealed that miR-214-3p inhibition significantly improved ferroptosis in TECs, protected renal function, and reduced renal injury in cis-AKI. Nonetheless, to establish the potential of miR-214-3p as a diagnostic marker for cis-AKI, it is necessary to conduct further investigation involving the extraction of total miR-214-3p from urine and serum samples and the statistical analysis of its relationship with the decline in renal function and the degree of kidney damage.
In conclusion, suppression of miR-214-3p reduces tubular ferroptosis in cis-AKI by inhibiting the genetic suppression of GPX4.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We acknowledge the assistance from Qingdao Municipal Hospital, the Affiliated Hospital of Qingdao University and Qingdao Chengyang People’s Hospital.
Author contribution
Junran Zhou and Chengcheng Xiao contribute equally to this paper and should be considered as co-first author. Renhe Wang, Junran Zhou, and Chengcheng Xiao designed the research, analyzed the data, and drafted the manuscript. Shuaishuai Zheng and Qian Wang performed the experiments. Chengcheng Xiao, Hai Zhu, and Yingyu Zhang helped with data acquisition and discussion. Junran Zhou analyzed the data and prepared the figures. All authors contributed to manuscript writing and editing.
Funding
This work was financially supported by the National Natural Science Foundation of China (82104789) and Health and Family Planning Commission of Shandong Province Fund (202004050826).
Data availability
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Declarations
Ethics approval and consent to participate
This study was conducted according to the guidelines of the Declaration of Helsinki. All animal experiments were performed according to protocols approved by the Institutional Animal Care and Use Committee of Qingdao Municipal Hospital and were performed with strict adherence to the American Association for the Accreditation of Laboratory Animal Care International and National Institutes of Health Guidelines embodied in the Guide for the Care and Use of Laboratory Animals. The above experiments have been put on record in Qingdao Municipal Hospital Ethics Committee.
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Junran Zhou and Chengcheng Xiao contributed equally to this work.
References
- Arriagada R, Bergman B, Dunant A, Le Chevalier T, Pignon JP, Vansteenkiste J. Cisplatin-based adjuvant chemotherapy in patients with completely resected non-small-cell lung cancer. N Engl J Med. 2004;350(4):351–360. doi: 10.1056/NEJMoa031644. [DOI] [PubMed] [Google Scholar]
- Belavgeni A, Meyer C, Stumpf J, Hugo C, Linkermann A. Ferroptosis and necroptosis in the kidney. Cell Chem Biol. 2020;27(4):448–462. doi: 10.1016/j.chembiol.2020.03.016. [DOI] [PubMed] [Google Scholar]
- Chirino YI, Pedraza-Chaverri J. Role of oxidative and nitrosative stress in cisplatin-induced nephrotoxicity. Exp Toxicol Pathol. 2009;61(3):223–242. doi: 10.1016/j.etp.2008.09.003. [DOI] [PubMed] [Google Scholar]
- Ding C, Ding X, Zheng J, Wang B, Li Y, Xiang H, Dou M, Qiao Y, Tian P, Xue W. miR-182-5p and miR-378a-3p regulate ferroptosis in I/R-induced renal injury. Cell Death Dis. 2020;11(10):929. doi: 10.1038/s41419-020-03135-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon SJ, Lemberg KM, Lamprecht MR, Skouta R, Zaitsev EM, Gleason CE, Patel DN, Bauer AJ, Cantley AM, Yang WS, Morrison B, 3rd, Stockwell BR. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell. 2012;149(5):1060–1072. doi: 10.1016/j.cell.2012.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doll S, Proneth B, Tyurina YY, Panzilius E, Kobayashi S, Ingold I, Irmler M, Beckers J, Aichler M, Walch A, Prokisch H, Trümbach D, Mao G, Qu F, Bayir H, Füllekrug J, Scheel CH, Wurst W, Schick JA, Kagan VE, Angeli JP, Conrad M. ACSL4 dictates ferroptosis sensitivity by shaping cellular lipid composition. Nat Chem Biol. 2017;13(1):91–98. doi: 10.1038/nchembio.2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FriedmannAngeli JP, Schneider M, Proneth B, Tyurina YY, Tyurin VA, Hammond VJ, Herbach N, Aichler M, Walch A, Eggenhofer E, Basavarajappa D, Rådmark O, Kobayashi S, Seibt T, Beck H, Neff F, Esposito I, Wanke R, Förster H, Yefremova O, Heinrichmeyer M, Bornkamm GW, Geissler EK, Thomas SB, Stockwell BR, O'Donnell VB, Kagan VE, Schick JA, Conrad M. Inactivation of the ferroptosis regulator Gpx4 triggers acute renal failure in mice. Nat Cell Biol. 2014;16(12):1180–91. doi: 10.1038/ncb3064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goren MP. Cisplatin nephrotoxicity affects magnesium and calcium metabolism. Med Pediatr Oncol. 2003;41(3):186–189. doi: 10.1002/mpo.10335. [DOI] [PubMed] [Google Scholar]
- Guo C, Dong G, Liang X, Dong Z. Epigenetic regulation in AKI and kidney repair: mechanisms and therapeutic implications. Nat Rev Nephrol. 2019;15(4):220–239. doi: 10.1038/s41581-018-0103-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holditch SJ, Brown CN, Lombardi AM, Nguyen KN, Edelstein CL. Recent advances in models, mechanisms, biomarkers, and interventions in cisplatin-induced acute kidney injury. Int J Mol Sci. 2019;20(12):3011. doi: 10.3390/ijms20123011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Z, Zhang H, Yang SK, Wu X, He D, Cao K, Zhang W. Emerging role of ferroptosis in acute kidney injury. Oxid Med Cell Longev. 2019;2019:8010614. doi: 10.1155/2019/8010614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Z, Zhang H, Yi B, Yang S, Liu J, Hu J, Wang J, Cao K, Zhang W. VDR activation attenuate cisplatin induced AKI by inhibiting ferroptosis. Cell Death Dis. 2020;11(1):73. doi: 10.1038/s41419-020-2256-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koppula P, Zhang Y, Zhuang L, Gan B. Amino acid transporter SLC7A11/xCT at the crossroads of regulating redox homeostasis and nutrient dependency of cancer. Cancer Commun (London, England) 2018;38(1):12. doi: 10.1186/s40880-018-0288-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lameire NH, Bagga A, Cruz D, De Maeseneer J, Endre Z, Kellum JA, Liu KD, Mehta RL, Pannu N, Van Biesen W, Vanholder R. Acute kidney injury: an increasing global concern. Lancet (london, England) 2013;382(9887):170–179. doi: 10.1016/S0140-6736(13)60647-9. [DOI] [PubMed] [Google Scholar]
- Linkermann A, Skouta R, Himmerkus N, Mulay SR, Dewitz C, De Zen F, Prokai A, Zuchtriegel G, Krombach F, Welz PS, Weinlich R, VandenBerghe T, Vandenabeele P, Pasparakis M, Bleich M, Weinberg JM, Reichel CA, Bräsen JH, Kunzendorf U, Anders HJ, Stockwell BR, Green DR, Krautwald S. Synchronized renal tubular cell death involves ferroptosis. Proc Natl Acad Sci U S A. 2014;111(47):16836–41. doi: 10.1073/pnas.1415518111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-delta delta C(T)) method. Methods (San Diego, CA) 2001;25(4):402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Mata-Miranda MM, Bernal-Barquero CE, Martinez-Cuazitl A, Guerrero-Robles CI, Sanchez-Monroy V, Rojas-Lopez M, Vazquez-Zapien GJ. Nephroprotective effect of embryonic stem cells reducing lipid peroxidation in kidney injury induced by cisplatin. Oxid Med Cell Longev. 2019;2019:5420624. doi: 10.1155/2019/5420624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pabla N, Dong Z. Cisplatin nephrotoxicity: mechanisms and renoprotective strategies. Kidney Int. 2008;73(9):994–1007. doi: 10.1038/sj.ki.5002786. [DOI] [PubMed] [Google Scholar]
- Qin W, Xie W, Yang X, Xia N, Yang K. Inhibiting microRNA-449 attenuates cisplatin-induced injury in NRK-52E cells possibly via regulating the SIRT1/P53/BAX pathway. Med Sci Monit. 2016;22:818–823. doi: 10.12659/MSM.897187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seibt TM, Proneth B, Conrad M. Role of GPX4 in ferroptosis and its pharmacological implication. Free Radical Biol Med. 2019;133:144–152. doi: 10.1016/j.freeradbiomed.2018.09.014. [DOI] [PubMed] [Google Scholar]
- Wang C, Zhu G, He W, Yin H, Lin F, Gou X, Li X. BMSCs protect against renal ischemia-reperfusion injury by secreting exosomes loaded with miR-199a-5p that target BIP to inhibit endoplasmic reticulum stress at the very early reperfusion stages. FASEB J. 2019;33(4):5440–5456. doi: 10.1096/fj.201801821R. [DOI] [PubMed] [Google Scholar]
- Wang R, Zhao H, Zhang Y, Zhu H, Su Q, Qi H, Deng J, Xiao C. Identification of MicroRNA-92a-3p as an essential regulator of tubular epithelial cell pyroptosis by targeting Nrf1 via HO-1. Front Genet. 2020;11:616947. doi: 10.3389/fgene.2020.616947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang X, Guo C, Tang C, Cai J, Dong Z. Epigenetic regulation in kidney toxicity: insights from cisplatin nephrotoxicity. Semin Nephrol. 2019;39(2):152–158. doi: 10.1016/j.semnephrol.2018.12.005. [DOI] [PubMed] [Google Scholar]
- Xie M, Huang P, Wu T, Chen L, Guo R. Inhibition of miR-214-3p protects endothelial cells from ox-LDL-induced damage by targeting GPX4. Biomed Res Int. 2021;2021:9919729. doi: 10.1155/2021/9919729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Z, Zang B, Gong X, Ren J, Wang R. MiR-214–3p exacerbates kidney damages and inflammation induced by hyperlipidemic pancreatitis complicated with acute renal injury. Life Sci. 2020;241:117118. doi: 10.1016/j.lfs.2019.117118. [DOI] [PubMed] [Google Scholar]
- Yang WS, SriRamaratnam R, Welsch ME, Shimada K, Skouta R, Viswanathan VS, Cheah JH, Clemons PA, Shamji AF, Clish CB, Brown LM, Girotti AW, Cornish VW, Schreiber SL, Stockwell BR. Regulation of ferroptotic cancer cell death by GPX4. Cell. 2014;156(1–2):317–331. doi: 10.1016/j.cell.2013.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Yu J, Yin L, Zhou Y, Sun Z, Jia H, Tao Y, Liu W, Zhang B, Zhang J, Wang M, Zhang X, Yan Y, Xue J, Gu H, Mao F, Xu W, Qian H. MicroRNA-146b, a sensitive indicator of mesenchymal stem cell repair of acute renal injury. Stem Cells Transl Med. 2016;5(10):1406–1415. doi: 10.5966/sctm.2015-0355. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.