Abstract
Chronic myeloid leukemia (CML) is a hematological tumor marked by the bcr-abl fusion gene formed by t (9;22) (q34; q11), which translated into the BCR-ABL protein. Tyrosine kinase inhibitors (TKIs) have been widely used to cure CML patients. Nevertheless, the emergence of TKI resistance has become the problem to the outcome of CML patients. Histone deacetylase 6 (HDAC6), a kind of Hsp90α deacetylase, was detected to be overexpressed in chronic myeloid leukemia stem cells. Besides, the loss of HDAC6 enzymatic activity can result in the degradation of Hsp90α’s client proteins, such as BCR-ABL, the oncoprotein of CML. Here, we explored the expression of HDAC6 and discovered that it was upregulated compared with control in CML. Then we explored the effect of Rocilinostat (ACY-1215), a specific HDAC6 inhibitor, on CML cells. Our results proved that ACY-1215 could induce apoptosis and cell cycle arrest in a ROS-dependent manner. Moreover, we detected a downregulation of the BCR-ABL signaling pathway in the ACY-1215 treatment group. Mechanistically, we noted that the upregulation of PTEN was induced after being treated by ACY-1215 and its downstream protein p-Akt was decreased. The Akt activator SC79 can partially reverse the influence of ACY-1215 on CML cells. Besides, our results also proved that ACY-1215 can synergize with imatinib to suppress chronic myeloid leukemia in vitro and in vivo. On the whole, our study revealed that HDAC6 is a possible therapeutic target in CML, and the combination therapy of TKI and HDAC6 inhibitor may improve the outcome of CML patients.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12192-022-01280-2.
Keywords: Chronic myeloid leukemia, HDAC6, BCR-ABL, Reactive oxygen species, PTEN
Introduction
Chronic myeloid leukemia is a myeloproliferative neoplasm characterized by the translocation t (9;22) (q34; q11), which leads to the formation of the bcr-abl gene and translated into the oncogenic BCR-ABL fusion protein (Apperley 2015). Multiple pro-survival signaling pathways could be activated by BCR-ABL, such as Stat5, Crkl and PI3K/Akt, which causes the abnormal proliferative and survival of leukemia cells (Kang et al. 2016). A variety of tyrosine kinase inhibitors (TKIs) have been developed for the treatment of CML, such as imatinib (IM), can effectively inhibit the tyrosine kinase activity of BCR-ABL and its downstream signaling pathways (Cuellar et al. 2018). However, there are still some patients who do not respond to TKI treatment, and some of them can be explained by the amplification and mutation of bcr-abl gene and these patients then become resistant to TKI treatment, while the reason for TKI failure of the remaining patients is unknown. In subsequent studies, it was found that due to the presence of leukemia stem cells (LSCs) CML patients could not be completely cured and patients of deep molecular responses may relapse after TKI discontinuation. What is more, LSCs are insensitive to TKI. The features of LSCs make eradicating LSC a challenge (Holyoake and Vetrie 2017). For those patients with poor response to TKI therapy, hematopoietic stem cell transplant (HSCT) provides another treatment option (Innes et al. 2016). However, patients receiving HSCT are at a risk of developing graft-versus-host disease (GVHD) (Malik et al. 2001). Therefore, a new treatment strategy or target should be found.
More and more studies have used non-BCR-ABL inhibitors to overcome TKI resistance of chronic myeloid leukemia, such as the inhibitor of mTOR, JAK2, Hsp90, HDAC and sirtuin (Massimino et al. 2018). Among them, Hsp90 is an important chaperone protein of many oncoproteins, include BCR-ABL. Hsp90 inhibitor showed excellent anticancer effects on many cancers, include AML (Katayama et al. 2018), ALL (Mshaik et al. 2021), CML (Bhatia et al. 2018) and myeloproliferative neoplasms (Hobbs et al. 2018). And studies have shown that high expression of Hsp90 suggests a strong aggressiveness of the disease and Hsp90 level could serve as a risk factor in CML (Zackova et al. 2013). Hsp90 inhibitor have been used for the treatment of chronic myeloid leukemia and the inhibition of Hsp90 could kill TKI-resistant CML cells (He et al. 2016; Bhatia et al. 2018). Therefore, targeting the cheperone activity of Hsp90 may be a strategy to overcome TKI resistance of CML patients.
The acetylated state of Hsp90 is regulated by HDAC, and deacetylated Hsp90 is able to exert its chaperone activity. Histone deacetylases (HDACs) can catalyze the deacetylation of proteins. Eighteen HDACs had been identified in mammals and can be classified into four categories. There are four members of class I, including HDAC1, HDAC2, HDAC3 and HDAC8. And HDAC4, HDAC5, HDAC6, HDAC7, HDAC9 and HDAC10 belong to class II, while sirtuins (SIRT1-7) belong to class III, and HDAC 11 is the only member of class IV.(Zhao et al. 2020). HDAC inhibitor was found to have anticancer effect against CML, including these cells resistant to TKI treatment (Fiskus et al. 2006; Lernoux et al. 2020; Nguyen et al. 2011). An early study revealed that vorinostat (SAHA), a pan-HDAC inhibitor, could synergize with IM and significantly suppress the viability of drug-resistant CD34+/CD38− CML stem cells. This study demonstrated that HDACs might be a new target to treat TKI-resistant CML patients. What is more, LAQ824, another inhibitor of HDAC, induced cell cycle arrest and apoptosis of CML blast crisis cells and inhibited BCR-ABL expression, including BCR-ABL with T315I mutation. Besides, LAQ824 also resulted in the acetylation of Hsp90 (Nimmanapalli et al. 2003). HDAC6 was discovered to be a Hsp90α deacetylase in 2005, and hyperacetylation of Hsp90α influences its chaperone activity (Aoyagi and Archer 2005). And a recent investigation found that the inhibition of HDAC6 can trigger ubiquitination of BCR-ABL and its degradation (Losson et al. 2020).
PTEN is a potent tumor suppressor, and the loss of function is frequently observed in cancers (Eritja et al. 2021; Xia et al. 2021; Du et al. 2021). Even a subtle decrease in PTEN level and activity result in increased cancer susceptibility and favors tumor progression. Previous studies had demonstrated that PTEN expression was inhibited by BCR-ABL and PTEN deletion caused acceleration of CML development (Peng et al. 2010). Besides, PTEN can inhibit the PI3K/Akt pathway, which is activated in CML. Therefore, activation of PTEN could be a strategy to eliminate CML cells.
ACY-1215 is a specific HDAC6 inhibitor that has been proved exert anticancer effects on a lot of cancers, including multiple myeloma (Santo et al. 2012), gallbladder cancer (Ruan et al. 2021), non-small cell lung cancer (Deskin et al. 2020) and so on (Lee et al. 2018; Tan et al. 2019). Besides, it has been used in clinical trials (Santo et al. 2012). However, there is no study to research the effects and the corresponding mechanism of ACY-1215 on CML. Here, we explored the antitumor effects of ACY-1215 on CML cells, especially TKI-resistant CML cells, alone or combined with IM and its underlying mechanism, which might provide new ideas for the therapy of CML patients.
Materials and methods
Reagents and antibodies
Ricolinostat (ACY-1215), imatinib (IM) and SC79 were bought from Topscience (China). DMSO was used to dissolve the drugs used in this study, and the final concentration was 50 mM. RPMI 1640 medium was used to dilute the drugs to the working concentration. Double-distilled water was used to dissolve the N-Acetyl-L-cysteine (NAC) (Beyotime, China) at a final concentration of 500 mM.
The related antibodies were described below: anti-HDAC6 (Bimake, China); anti-P21, anti-c-Myc, anti-Caspase3, anti-PARP, anti-c-abl, anti-phospho-c-abl, anti-phospho-Stat5 and anti-β-Actin (CST, USA); anti-phospho-Crkl and anti-phospho-Akt1 (Abcam, UK); anti-CCND1 and anti-PTEN (HuaBio, China); anti-Hsp90α (GENETEX, USA); and anti-Ac-lysine (Santa Cruz, USA).
Cell lines and clinical samples
The CML cell lines used in this study were K562, K562/G01 and KCL22. And other cell lines used in this study were TK6 (non-leukemic lymphoblasts cell line), Sup-B15 (acute lymphocytic leukemia cell line) and THP-1 (acute monocytic leukemia cell line). K562, K562/G01, KCL22, TK6 and THP1 were cultured in RPMI 1640 medium (Gibco, USA) supplemented with 10% FBS (Gibco, USA). Sup-B15 was grown in IMDM medium (Gibco, USA), which containing 20% FBS. Both of them were cultured under a condition of 5% CO2 at 37 °C.
The clinical samples of healthy donors and CML patients were collected from the Second Affiliated Hospital of Chongqing Medical University. Bone marrow mononuclear cells (BMMNCs) of clinical samples were separated according to the instructions of human BMMNCs isolation kit (Tbdscience, China).
Real-Time polymerase chain reaction
TRIzol reagent was used to extract total RNA following the protocol. cDNA was synthesized using the EvoM-MLV RT kit (Accurate Biology, China). Q-PCR was carried out using SYBR® Green Premix (Accurate Biology, China). Table 1 displays the primer sequences.
Table 1.
Primers used for Q-PCR
| Gene | Forward/Reverse | Primer Sequence (5'-3') |
|---|---|---|
| HDAC6 | F | AAGACCTAATCGTGGGACTGC |
| R | ACGTGGTTGAACATGCAATAG | |
| PTEN | F | AGAGCGAGGGGCATCAG |
| R | GCAGGAAATCCCATAGCAATAA | |
| β-actin | F | ACTTAGTTGCGTTACACCCTT |
| R | TGTCACCTTCACCGTTCC |
Western blotting
After being treated by ACY-1215 for 48 h, the cells were collected and lysed by using RIPA lysis buffer supplemented with PMSF (1%), NaF (1%) and Na3VO4 (1%). BCA (Bytotime, China) assay was performed to determine the protein concentration. 10% SDS-PAGE was used to separate the proteins. After that, the wet transfer method was used to transfer the proteins onto the methanol-activated PVDF membranes. Then 5% skimmed milk was used to block the membranes at RT for 2 h. Briefly rinse the membranes in water and incubated overnight with the primary antibody against the target protein at 4 °C. The membranes were then rinsed by TBST for twice and then incubated at RT for 1.5 h with the corresponding secondary antibodies. The enhanced chemiluminescence substrate was used for detection.
Cell viability assay
The CCK-8 test was performed to determine the cell number of each group. CCK-8 was purchased from Topscience (China). K562 and K562/G01 cells were treated with ACY-1215 for a specific time. To detect the combination effect of ACY-1215 and IM, CML cells were treated by both of them. Add 10 μl CCK-8 reagent to each well after a certain period of ACY-1215 treatment. The optical density (OD) value of each well was recorded at 450 nm after incubation at 37 °C for 2 h.
Colony formation assays
In a 96-well plate, K562 and K562/G01 cells were planted. After being treated by ACY-1215 for 7 days, the cell colony number was counted and photographed.
Cell cycle analysis
The cell cycle distribution was assessed by the cell cycle detection kit (SIGMA, USA) and analyzed by flow cytometry after being treated by ACY-1215.
Cellular apoptosis detection
To detect the apoptosis of CML cells, apoptosis was assessed by the Annexin V-PE/7-AAD apoptosis detection kit (Yeasen, China) or the Annexin V-PE/Rednucleus II apoptosis detection kit (Bioscience, China).
Immunofluorescence assay
Cells were spread on slides, and then, there was a 15 min of paraformaldehyde fixation at room temperature. After that, the cells were permeabilized for 15 min at 37 °C with Triton X-100 and blocked for 1 h at 4 °C using goat serum. The cells were then treated overnight with the primary antibody at 4 °C, followed by 1 h at 37 °C with the Cy3-labeled secondary antibody (Introvigen, USA). Then 4',6-diamidino-2-phenylindole (DAPI) was used to stain the nucleus at 37 °C for 15 min in the dark. In the end, the slides were sealed using glycerin and photographed under a confocal microscope.
Co-Immunoprecipitation
Cells were collected after being treated by 5 μM ACY-1215 for 48 h while the untreated cells were collected as control. Cells were harvested and lysed using IP lysis buffer (Beyotime, China) on ice for 10 min. Add the corresponding antibodies to protein A/G magnetic beads (MCE, USA) and rotated tube at room temperature for 0.5 h. Then, the magnetic beads were rinsed with PBST for six times. After that, the proteins were mixed with the magnetic beads and rotated at 4 °C overnight. Then rinsing the complex of bead–antibody–protein for six times. Finally, 2 × loading buffer was added to the magnetic beads, and the proteins were denatured at 100 °C for 5 min. The antibodies used in this experiment were as follows: anti-c-abl (1:100, CST, USA), anti-Hsp90α (1:100, GENETEX, USA), anti-Ac-lysine (1:20, Santa Cruz, USA), rabbit IgG (1:100, Beyotime, China) and mouse IgG (1:100, Beyotime, China).
Reactive oxygen species detection
ROS generation was analyzed using the Reactive Oxygen Species Assay Kit (Beyotime, China) after being treated with ACY-1215 according to the instructions.
Murine tumor models
5-week-old female NOD/SCID mice were irradiated by 250 cGy X-ray before intravenous injection. Twenty mice were distributed into 4 groups randomly and injected with 5 × 106 K562/G01 cells through the tail vein. After 1 week of xenograft leukemogenesis, ACY-1215 (50 mg/kg) or imatinib (50 mg/kg) was injected intraperitoneally (i.p.) every two days and for eight times in total. All procedures in the experiment were approved by the Ethics Committee of Chongqing Medical University.
Statistical analysis
The statistical difference between the two groups was compared using an unpaired Student’s t-test using GraphPad Prism 8.0. A p-value < 0.05 was considered statistically significant.
Results
HDAC6 is upregulated in CML
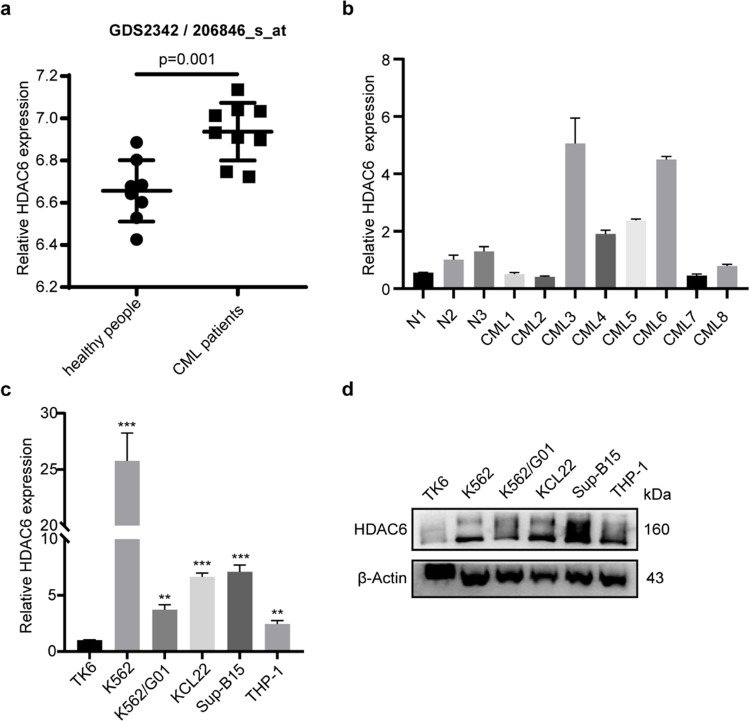
To understand the difference of HDAC6 expression between normal and CML patients, we analyzed the mRNA expression level of HDAC6 in CML patients by searching the GEO database (GDS2342/206846_s_at). The result showed that HDAC6 was overexpressed in CD34+ hematopoietic stem and progenitor cells of CML patients compared to normal people (Fig. 1a). After that, we collected bone marrow cell samples from three healthy individuals and eight CML patients and then detected HDAC6 expression. Higher mRNA level of HDAC6 in some CML patients was confirmed by Q-PCR (Fig. 1b). To further analyze the HDAC6 expression level, several leukemia cell lines were used. As shown in Fig. 1c-d, HDAC6 expression levels were much higher in leukemia cell lines compared with TK6, including CML cell lines. In summary, these results showed that HDAC6 was upregulated in CML, suggesting HDAC6 might play an important role in CML.
Fig. 1.
HDAC6 is overexpressed in CML. (a) The HDAC6 mRNA expression level was analyzed in the GEO database using GDS2342/206846_s_at. There were eight samples of healthy people and nine samples of CML patients. (b) Bone marrow mononuclear cells of CML patients (8) and healthy donors (3) were separated to analyze the HDAC6 mRNA expression level. (c) The HDAC6 mRNA expression levels were detected in leukemia cells THP-1, Sup-B15, K562, K562/G01, KCL22 and TK6 control. (d) The protein levels of HDAC6 were examined in leukemia cells THP-1, Sup-B15, K562, K562/G01, KCL22 and TK6. **p < 0.01, ***p < 0.001
ACY-1215 inhibits the proliferation of CML cells
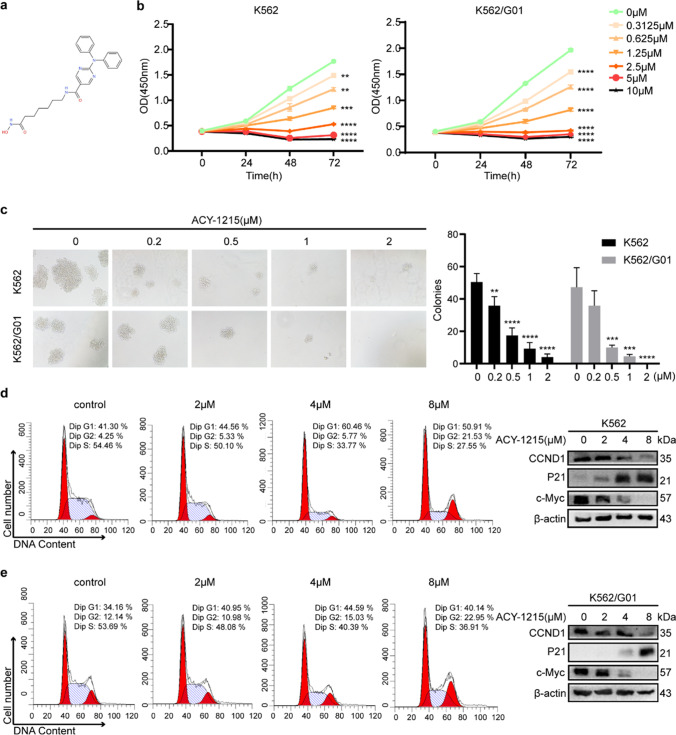
ACY-1215 (Fig. 2a) is a specific HDAC6 inhibitor. To learn more about HDAC6's function in CML, K562 (imatinib-sensitive) and K562/G01 (imatinib-resistant) cells were treated with ACY-1215. As shown in Fig. 2b, ACY-1215 suppressed the cell viability of CML cells significantly, while it had little effect on the cell activity of TK6 cells with low expression of HDAC6 (sFigure 1a). As the concentration of ACY-1215 increased, the colony formation assay also showed fewer and smaller colonies after being treated by ACY-1215 (Fig. 2c). We next analyzed the cell cycle distributions of each group and found that there are fewer cells in S-phase in the ACY-1215 treated group, which indicated that the ability of cells to proliferate is weakened. A western blot also showed an increased level of P21 and a decreased level of CCND1 and c-Myc (Fig. 2d, e). These findings suggested that ACY-1215 could suppress the proliferation of CML cells.
Fig. 2.
ACY-1215 inhibits the growth of CML cells. (a) ACY-1215’s chemical structure. (b) CCK-8 experiment was performed to determine the cell viability of CML cells that had been exposed to a range of ACY-1215 concentrations. (c) The proliferation potential of CML cells treated with ACY-1215 was determined using a colony formation assay. Colony numbers and sizes were counted and photographed 7 days later. (d, e) Cell cycle distributions of CML cells were examined using flow cytometry after being treated with ACY-1215 for 48 h. Cell cycle-related proteins were investigated using a western blot assay. **p < 0.01, ***p < 0.001, ****p < 0.0001
ACY-1215 induces apoptosis of CML cells
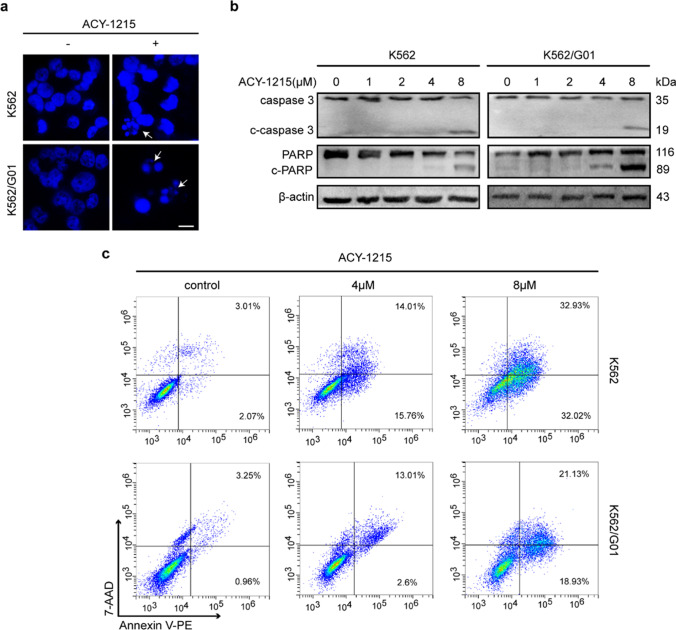
To further test whether ACY-1215 can induce apoptosis of CML cells. CML cells received a 48-h treatment of ACY-1215. DAPI staining was performed, and the morphological changes indicated apoptosis in the ACY-1215 treated group (Fig. 3a). A western blot showed the activation of caspase-3 and PARP in K562 and K562/G01 cells after treatment of ACY-1215 (Fig. 3b), suggesting the apoptosis was caspase-3-related. Besides, we also performed flow cytometry to detect cell apoptosis. Our results showed an increased apoptosis rate after treated by ACY-1215 (Fig. 3c). Meanwhile, 8 μM ACY-1215 treatment for 48 h could not induce apoptosis of TK6 cells (sFigure 1b). Taken together, these results showed that ACY-1215 can induce apoptosis of CML cells.
Fig. 3.
ACY-1215 promotes the apoptosis of CML cells. (a) The nuclear morphology was examined using DAPI labeling. The white arrow indicates cell apoptosis. Scale bar, 10 μm. (b) The activation of caspase-3 and PARP were examined using western blot. (c) CML cells were treated with ACY-1215 for 48 h, and then, FCM was used to detect the cell apoptosis
ACY-1215 inhibits BCR-ABL signaling pathway
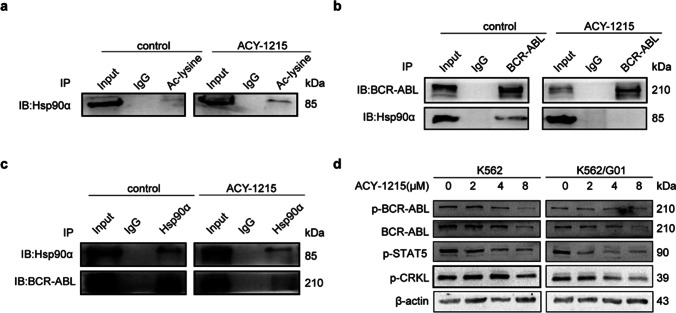
Hsp90α is a substrate of HDAC6 and also an important chaperone protein of BCR-ABL. We found that ACY-1215 can result in the hyperacetylation of Hsp90α (Fig. 4a), thus losing its chaperone activity and reducing the combination with BCR-ABL (Fig. 4b, c). Next, we detected the BCR-ABL signaling pathway. Our findings indicated that the expression of BCR-ABL and its key downstream proteins, such as p-Stat5 and p-Crkl, was decreased (Fig. 4d). In summary, our results showed that ACY-1215 can inhibit the BCR-ABL signaling pathway.
Fig. 4.
ACY-1215 inhibits the BCR-ABL signaling pathway. (a) Co-IP was performed to detect the acetylation of Hsp90α. (b, c) Co-IP was used to detect the binding of BCR-ABL and Hsp90α with or without ACY-1215 treatment. (d) Western blot was used to detect the expression of p-BCR-ABL, p-STAT5, p-CRKL in CML cells
ACY-1215's anticancer effects are associated with ROS production
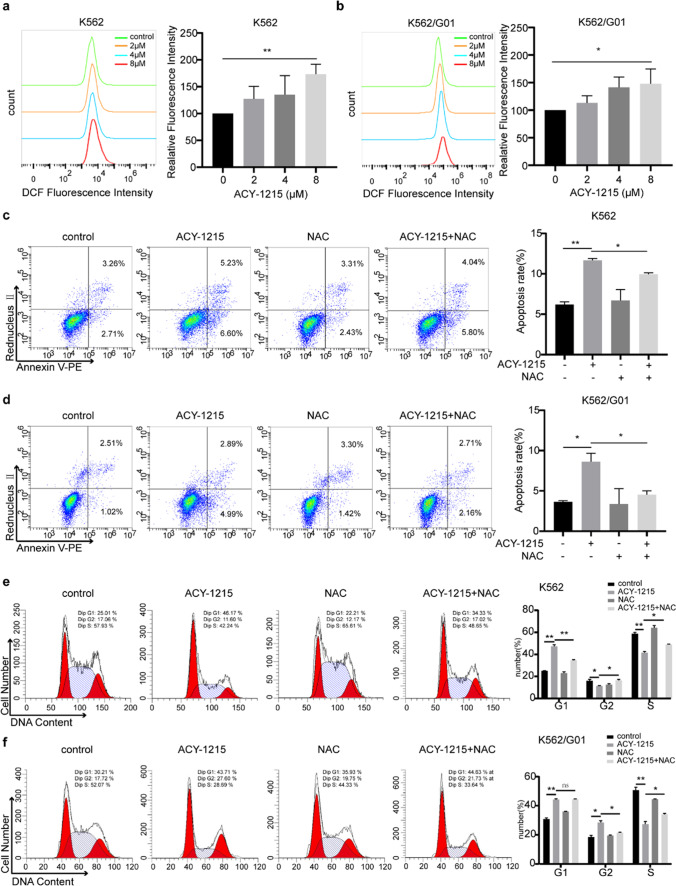
Chemotherapy drugs usually exert antitumor activities by stimulating the generation of ROS. Our results found that ACY-1215 treatment can significantly result in the ROS generation of CML cells in a dose dependent manner (Fig. 5a, b). Next ROS was scavenged using NAC, an antioxidant, to investigate the function of ROS in the anticancer effects of ACY-1215 on CML cells. NAC was given to CML cells for a one-hour pretreatment, and then, ACY-1215 was added for another 24 h. Results of flow cytometry showed that ROS inhibition can partially rescue CML cells from ACY-1215-induced apoptosis (Fig. 5c, d). Besides, NAC treatment also partially prevented CML cells from cell cycle arrest induced by ACY-1215 (Fig. 5e, f). In conclusion, our results indicated that the anticancer activities of ACY-1215 on CML cells are associated with ROS generation.
Fig. 5.
ACY-1215 induces apoptosis and cell cycle arrest by ROS generation. (a, b) CML cells were treated with ACY-1215 for 48 h. Then ROS production of each group was detected by the DCFH-DA fluorescent probe. Fluorescence intensity was measured by flow cytometry. ROS was scavenged by NAC, a ROS inhibitor. NAC was applied to CML cells for 1 h before cells were treated by ACY-1215 for another 24 h. Apoptosis rates (c, d) and cell cycle distributions (e, f) were detected by FCM. *p < 0.05, **p < 0.01
ACY-1215 exerts anticancer effects through the ROS/PTEN/Akt pathway
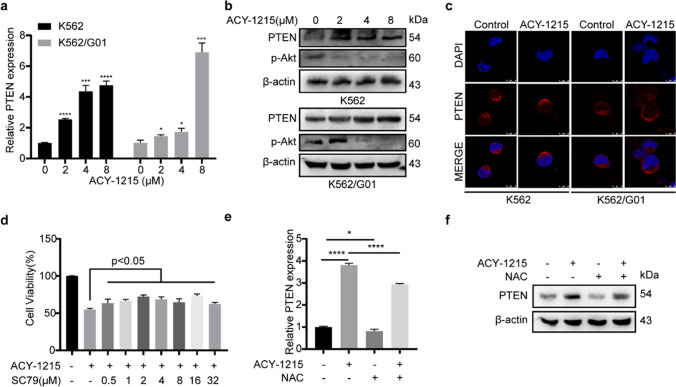
PTEN is a crucial tumor suppressor protein of CML. Here, we found that ACY-1215 induced PTEN expression in not only mRNA but also protein level (Fig. 6a, b). Besides, PTEN membrane translocation occurred in CML cells after ACY-1215 treatment (Fig. 6c), which contributed to its cancer suppressor function. PTEN is a suppressor of the PI3K/Akt pathway, which is usually activated in CML. The result of the western blot revealed that the activity of p-Akt was inhibited in the ACY-1215 treatment group (Fig. 6b). And SC79, an Akt activator, can partially prevent CML cells from ACY-1215-induced cell viability inhibition (Fig. 6d). And western blot showed that SC79 could effectively activate Akt (sFigure 2). Then we explore the relationship between ROS and PTEN, the results showed that ROS inhibition decreased the expression level of PTEN, including mRNA and protein levels, and the increased PTEN expression induced by ACY-1215 treatment could partially be rescued by NAC treatment (Fig. 6e, f). To sum up, our results indicated that ACY-1215 kills CML cells by inhibiting the PI3K/Akt pathway and ROS acts as a positive regulator of PTEN expression.
Fig. 6.
ACY-1215 inhibits CML cells by ROS/PTEN/Akt pathway. (a, b) The expression of PTEN in mRNA and protein level were confirmed using Q-PCR and western blot, respectively. p-Akt expression of each group was examined by western blot. (c) PTEN location was detected by immunofluorescent and photographed by confocal microscope. (d) SC79, an Akt activator, was used. CCK-8 was used to determine the cell viability. (e) PTEN expression of mRNA was determined by Q-PCR. (f) The protein level of PTEN was examined using western blot
ACY-1215 synergizes with imatinib to show antileukemic activity in vitro and in vivo
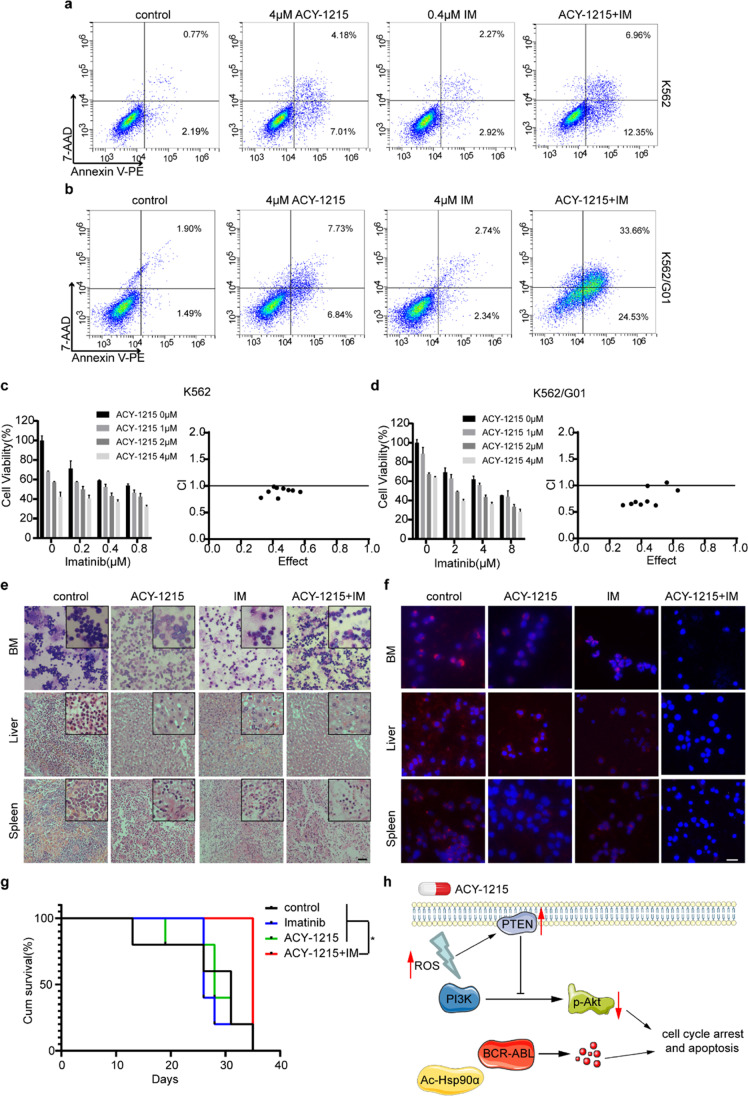
Drug combination therapy is often used to overcome single-drug resistance. Hence, we combined ACY-1215 with IM to explore the combined effect. Flow cytometry results showed that the co-treatment can result in a higher apoptosis rate of CML cells, including K562/G01, an IM-resistant CML cell line (Fig. 7a, b). In order to analyze whether ACY-1215 and IM have a synergistic effect, we used CompuSyn to calculate the combination index (CI) of ACY-1215 and IM. If CI < 1, it means that the two drugs have a synergistic effect. Our results demonstrated that ACY-1215 and IM can synergistically inhibit CML cell viability (Fig. 7c, d).
Fig. 7.
ACY-1215 and IM synergistically inhibit CML cells in vitro and in vivo. (a, b) CML cells were exposed to ACY-1215 and IM for 48 h, apoptosis rate was detected by FCM. (c, d) In order to determine the cell viability, CCK-8 experiment was carried out. CompuSyn software was used to calculate the combination index (CI) values. CI < 1 means that the two drugs have a synergistic effect. (e) H&E was used to examine murine liver and spleen infiltration in four groups, and Wright's stain was used to examine bone marrow cells. Scale bar, 100 μm. (f) An immunofluorescence test was used to detect BCR/ABL expression. Scale bar, 50 μm. (g) Survival curves were analyzed by Kaplan–Meier methods using GraphPad Prism 8.0. *p < 0.05. (h) The working model of ACY-1215-induced cell apoptosis and cycle arrest in CML. ACY-1215 induced acetylation of Hsp90α, thus resulting in degradation of BCR-ABL. Besides, ACY-1215 increased the production of ROS and PTEN expression, which inhibited the PI3K/Akt pathway.
To further observe the effect of combination treatment, we constructed a xenograft model of chronic myeloid leukemia. NOD/SCID mice were injected intravenously with K562/G01 cells and randomly divided into four groups. The mice received ACY-1215 and IM treatment at a dosage of 50 mg/kg each time for three weeks (8 times in total, i.p.) at 7 days post-construction of tumor model, while the mice of control group received the same volume of cosolvent. Mice were killed 5 weeks after the injection of cells. As shown in Fig. 7e, the infiltration of leukemia cells in mice of the combined treatment group was much less. Furthermore, the immunofluorescent assay findings demonstrated that the combination therapy group had lower BCR/ABL protein expression in tissues and BM than the other groups (Fig. 7f). Correspondingly the mice in the combined treatment group have a longer survival time (Fig. 7g). In general, our results demonstrated that the combination therapy of ACY-1215 and IM has an excellent therapeutic effect on IM-resistant mouse models.
Discussion
It is now considered that cancer is regulated by epigenetics, and epigenetic disorders provide preconditions for the occurrence and development of tumors (Feinberg, Ohlsson, and Henikoff 2006). In CML, as the disease progresses, a variety of epigenetic mechanisms have changed, including DNA methylation, histone modification and non-coding RNAs (Koschmieder and Vetrie 2018). TKI resistance has been attributed mostly to the mutations of BCR-ABL and the existence of leukemia stem cells (LSCs) (Linev et al. 2018; Zhou and Xu 2015). However, it has been found that epigenetic disorders also play a role in TKI resistance. Drugs targeting epigenetics-related proteins combined with TKI can overcome the resistance of CML to TKI, indicating the important role of those proteins in CML (Bugler et al. 2019; Florean et al. 2011).
Many proteins undergo acetylation modification on their lysine residues. Histone acetyltransferases (HATs) add acetyl groups, whereas HDACs remove them. Acetylation modifications of histones can regulate gene expression. Acetylation is involved in the formation of open chromatin and gene expression, while hypoacetylation is related to condensed chromatin and transcriptional inhibition. Besides, HATs and HDACs can regulate the acetylation of many non-histone proteins, such as Hsp90, ERK1 and p53, and are involved in a variety of cellular processes (Florean et al. 2011; Di Gennaro et al. 2004). Up to now, there have been many studies on the role of HDACs inhibitors and TKI in CML, such as SAHA (Dai et al. 2008; Bu et al. 2014), LAQ824 (Nimmanapalli et al. 2003) and MAKV-8 (Lernoux et al. 2020), and the results are encouraging. However, the pan-HDACi has many side effects, so it is necessary to target a specific HDAC.
HDAC6 belongs to Class IIB HDAC. It can catalyze the deacetylation of a variety of proteins, such as ERK1, Ku70, Hsp90α, LC3II and Prx I/II, and is involved in apoptosis, autophagy, oxidative stress and so on (Losson, Schnekenburger, et al. 2020). Among them, Hsp90α is an important chaperone protein of BCR-ABL. Acetylated Hsp90α will not be able to exert its chaperone activity, leading to the degradation of its client protein (Aoyagi and Archer 2005; Losson, Gajulapalli, et al. 2020). These results suggest that HDAC6 might be another therapeutic target of CML.
ACY-1215 is a hydroxamic acid derivative that has a particular inhibitory effect on HDAC6. It has been used in clinical trials of several cancers, such as relapsed chronic lymphocytic leukemia and relapsed or refractory lymphoid malignancies (Santo et al. 2012; Losson et al. 2020). Based on the above research, we reasonably speculated that ACY-1215 possesses the potential to treat CML. Our study demonstrated that ACY-1215 can induce apoptosis and cell cycle arrest of CML cells (Fig. 2, 3). To further elucidate the mechanism of the anticancer effect of ACY-1215 on CML, we first detected ROS production in vitro. ACY-1215 caused a considerable increase in ROS generation, according to our findings. Then, in order to highlight ROS’s importance in ACY-1215-induced apoptosis and cell cycle arrest, we used NAC to inhibit ROS. We found that NAC can partially prevent CML cells from ACY-1215-induced apoptosis and cell cycle arrest, which indicates the important role of ROS in the anticancer effect of ACY-1215 (Fig. 5). Interestingly, we found that PTEN, a crucial tumor suppressor, was upregulated in the ACY-1215 treated group. Furthermore, ROS regulated PTEN at the transcription and translation levels. Corresponding to the upregulation of PTEN, the PI3K/Akt axis was inhibited and the Akt activator SC79 could partially rescue the antitumor effect (Fig. 6).
BCR-ABL is a client protein of Hsp90α, the degradation of BCR-ABL cells was detected after treating with ACY-1215. And the downstream proteins that participated in CML’s development were also decreased. Hsp90α was in a hyperacetylated state and the combination of Hsp90α and BCR-ABL was also reduced (Fig. 4), which explains why BCR-ABL has been decreased.
More and more researches have illustrated that combined treatment exert excellent antitumor activity on drug-resistant tumor models (Xiao et al. 2020; Li et al. 2021). We found that the combination of ACY-1215 and IM had a stronger antitumor effect toward CML, which led to a higher apoptosis rate than a single drug. The combination index further clarified that these two drugs had a synergy effect. We demonstrated the synergy effect not only in vitro but also in vivo. Consistent with in vitro experimental results, though a single drug had little effect on CML mice, the combined treatment showed an excellent antitumor effect against IM-resistant CML mice (Fig. 7).
Our research also has some limitations. First, the underlying mechanism was not complete. The inhibition of ROS and activation of Akt only partially rescued the effect of ACY-1215, indicating that there are other mechanisms involved in the antitumor effect of ACY-1215. Besides we have carried out certain explorations at the cellular and animal level, but we lack the support of clinical trials. There are more need to be explored.
Nonetheless, our research still has a certain meaning. Our results demonstrated that the HDAC6 inhibitor ACY-1215 could induce BCR-ABL degradation and kill CML cells through the ROS/PTEN/Akt pathway in vitro. Moreover, the combination of ACY-1215 and IM could synergistically show an antitumor effect in IM-resistant CML mouse model, providing a theoretical basis for targeting HDAC6 to treat TKI-resistant CML patients. Finally, HDAC6 inhibitor ACY-1215 has been used in clinical trials of a variety of cancers, it is hopeful that HDAC6 inhibitors would be applied to treat CML in the future.
Supplementary Information
Below is the link to the electronic supplementary material.
Author contributions
The idea for this study was proposed by YQ. YQ and YL designed the experiments. YQ, YL, GJ and YP conducted the experiments. YQ drew the all figures and wrote the manuscript. WF revised this manuscript. All authors contributed to the article and approved the submitted version.
Declarations
Competing Interests
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Yuefeng Qin and Yang Liang contributed equally to this work.
References
- Aoyagi S, Archer TK (2005). "Modulating molecular chaperone Hsp90 functions through reversible acetylation." Trends Cell Biol 15 (11): 565–710.1016/j.tcb.2005.09.003. https://www.ncbi.nlm.nih.gov/pubmed/16199163. [DOI] [PubMed]
- Apperley JF. Chronic myeloid leukaemia. The Lancet. 2015;385(9976):1447–1459. doi: 10.1016/s0140-6736(13)62120-0. [DOI] [PubMed] [Google Scholar]
- Bhatia S Diedrich D Frieg B, Ahlert H Stein S Bopp B Lang F, Zang T, Kroger T, Ernst T, Kogler G, Krieg A. Ludeke S, Kunkel H, Rodrigues Moita AJ, Kassack MU, Marquardt V, Opitz FV, Oldenburg M, Remke M, Babor F, Grez M, Hochhaus A, Borkhardt A, Groth G, Nagel-Steger L, Jose J, Kurz T, Gohlke H, Hansen FK, Hauer J 2018. "Targeting HSP90 dimerization via the C terminus is effective in imatinib-resistant CML and lacks the heat shock response." Blood 132 (3): 307-32010.1182/blood-2017-10-810986. https://www.ncbi.nlm.nih.gov/pubmed/29724897 [DOI] [PMC free article] [PubMed]
- Bu Q, Cui L, Li J, Du X, Zou W, Ding K, Pan J (2014). "SAHA and S116836, a novel tyrosine kinase inhibitor, synergistically induce apoptosis in imatinib-resistant chronic myelogenous leukemia cells." Cancer Biol Ther 15 (7): 951–6210.4161/cbt.28931. https://www.ncbi.nlm.nih.gov/pubmed/24759597. [DOI] [PMC free article] [PubMed]
- Bugler J, Kinstrie R, Scott MT, Vetrie D. 2019. "Epigenetic Reprogramming and Emerging Epigenetic Therapies in CML." Front Cell Dev Biol 7: 13610.3389/fcell.2019.00136. https://www.ncbi.nlm.nih.gov/pubmed/31380371. [DOI] [PMC free article] [PubMed]
- Cuellar S, Vozniak M, Rhodes J, Forcello N, Olszta D (2018). "BCR-ABL1 tyrosine kinase inhibitors for the treatment of chronic myeloid leukemia." J Oncol Pharm Pract 24 (6): 433–45210.1177/1078155217710553. https://www.ncbi.nlm.nih.gov/pubmed/28580869. [DOI] [PMC free article] [PubMed]
- Dai Y, Chen S, Venditti CA, Pei XY, Nguyen TK Dent P, Grant S (2008). "Vorinostat synergistically potentiates MK-0457 lethality in chronic myelogenous leukemia cells sensitive and resistant to imatinib mesylate." Blood 112 (3): 793–80410.1182/blood-2007–10–116376. https://www.ncbi.nlm.nih.gov/pubmed/18505786. [DOI] [PMC free article] [PubMed]
- Deskin B, Yin Q, Zhuang Y, Saito S, Shan B, Lasky JA (2020). "Inhibition of HDAC6 Attenuates Tumor Growth of Non-Small Cell Lung Cancer." Transl Oncol 13 (2): 135–14510.1016/j.tranon.2019.11.001. https://www.ncbi.nlm.nih.gov/pubmed/31865176. [DOI] [PMC free article] [PubMed]
- Di Gennaro E, Bruzzese F, Caraglia M, Abruzzese A, Budillon A (2004) "Acetylation of proteins as novel target for antitumor therapy: review article." Amino Acids 26 (4): 435–4110.1007/s00726–004–0087–3. https://www.ncbi.nlm.nih.gov/pubmed/15290351. [DOI] [PubMed]
- Du MG, Peng ZQ, Gai WB, Liu F, Liu W, Chen YJ, Li, HC, Zhang X, Liu CH, Zhang LQ, Jiang H, Xie P (2021). "The Absence of PTEN in Breast Cancer Is a Driver of MLN4924 Resistance." Front Cell Dev Biol 9: 66743510.3389/fcell.2021.667435. https://www.ncbi.nlm.nih.gov/pubmed/33996822. [DOI] [PMC free article] [PubMed]
- Eritja N, Navaridas R, Ruiz-Mitjana A, Vidal-Sabanes M, Egea J, Encinas M, Matias-Guiu X, Dolcet X. (2021). "Endometrial PTEN Deficiency Leads to SMAD2/3 Nuclear Translocation." Cancers (Basel) 13 (19)10.3390/cancers13194990. https://www.ncbi.nlm.nih.gov/pubmed/34638474. [DOI] [PMC free article] [PubMed]
- Feinberg AP, Ohlsson R, Henikoff S (2006) "The epigenetic progenitor origin of human cancer." Nat Rev Genet 7 (1): 21–3310.1038/nrg1748. https://www.ncbi.nlm.nih.gov/pubmed/16369569. [DOI] [PubMed]
- Fiskus W, Pranpat M, Balasis M, Bali P, Estrella V, Kumaraswamy S, Rao R, Rocha K, Herger B, Lee F, Richon V, Bhalla K. (2006). "Cotreatment with vorinostat (suberoylanilide hydroxamic acid) enhances activity of dasatinib (BMS-354825) against imatinib mesylate-sensitive or imatinib mesylate-resistant chronic myelogenous leukemia cells." Clin Cancer Res 12 (19): 5869–7810.1158/1078–0432.CCR-06–0980. https://www.ncbi.nlm.nih.gov/pubmed/17020995. [DOI] [PubMed]
- Florean C, Schnekenburger M, Grandjenette C, Dicato K, Diederich M (2011). "Epigenomics of leukemia: from mechanisms to therapeutic applications." Epigenomics 3 (5): 581–60910.2217/epi.11.73. https://www.ncbi.nlm.nih.gov/pubmed/22126248. [DOI] [PubMed]
- He W, Ye X, Huang X, Lel W, You L, Wang L, Chen X, Qian W (2016). "Hsp90 inhibitor, BIIB021, induces apoptosis and autophagy by regulating mTOR-Ulk1 pathway in imatinib-sensitive and -resistant chronic myeloid leukemia cells." Int J Oncol 48 (4): 1710–2010.3892/ijo.2016.3382. https://www.ncbi.nlm.nih.gov/pubmed/26892093. [DOI] [PubMed]
- Hobbs GS, Hanasoge Somasundara AV, Kleppe M, Litvin R, Arcila M, Ahn J, McKenney AS, Knapp K, Ptashkin R, Weinstein H, Heinemann MH, Francis J, Chanel S, Berman E, Mauro M Tallman MS, Heaney ML, Levine RL, Rampal RK (2018). "Hsp90 inhibition disrupts JAK-STAT signaling and leads to reductions in splenomegaly in patients with myeloproliferative neoplasms." Haematologica 103 (1): e5-e910.3324/haematol.2017.177600. https://www.ncbi.nlm.nih.gov/pubmed/29051283. [DOI] [PMC free article] [PubMed]
- Holyoake TL, Vetrie D. (2017). "The chronic myeloid leukemia stem cell: stemming the tide of persistence." Blood 129 (12): 1595–160610.1182/blood-2016–09–696013. https://www.ncbi.nlm.nih.gov/pubmed/28159740. [DOI] [PubMed]
- Innes AJ, Milojkovic D, Apperley JF (2016). "Allogeneic transplantation for CML in the TKI era: striking the right balance." Nat Rev Clin Oncol 13 (2): 79–9110.1038/nrclinonc.2015.193. https://www.ncbi.nlm.nih.gov/pubmed/26573423. [DOI] [PubMed]
- Kang ZJ, Liu YF, Xu LZ, Long ZJ, Huang D, Yang Y, Liu B, Feng JX, Pan YJ, Yan JS, Liu Q (2016). "The Philadelphia chromosome in leukemogenesis." Chin J Cancer 35: 4810.1186/s40880–016–0108–0. https://www.ncbi.nlm.nih.gov/pubmed/27233483. [DOI] [PMC free article] [PubMed]
- Katayama K, Noguchi K, SugimotoY (2018). "Heat shock protein 90 inhibitors overcome the resistance to Fms-like tyrosine kinase 3 inhibitors in acute myeloid leukemia." Oncotarget 9 (76): 34240–3425810.18632/oncotarget.26045. https://www.ncbi.nlm.nih.gov/pubmed/30344940. [DOI] [PMC free article] [PubMed]
- Koschmieder S, Vetrie D (2018). "Epigenetic dysregulation in chronic myeloid leukaemia: A myriad of mechanisms and therapeutic options." Semin Cancer Biol 51: 180–19710.1016/j.semcancer.2017.07.006. https://www.ncbi.nlm.nih.gov/pubmed/28778403. [DOI] [PubMed]
- Lee DH, Won HR, Ryu HW, Han JM, Kwon SH. (2018). "The HDAC6 inhibitor ACY1215 enhances the anticancer activity of oxaliplatin in colorectal cancer cells." Int J Oncol 53 (2): 844–85410.3892/ijo.2018.4405. https://www.ncbi.nlm.nih.gov/pubmed/29749542. [DOI] [PubMed]
- Lernoux M, Schnekenburger M, Losson H, Vermeulen K, Hahn H, Gerard D, Lee JY, MazumderA, Ahamed M, Christov C, Kim DW, Dicato M, Bormans G, Han BW, Diederich M. (2020). "Novel HDAC inhibitor MAKV-8 and imatinib synergistically kill chronic myeloid leukemia cells via inhibition of BCR-ABL/MYC-signaling: effect on imatinib resistance and stem cells." Clin Epigenetics 12 (1): 6910.1186/s13148–020–00839-z. https://www.ncbi.nlm.nih.gov/pubmed/32430012. [DOI] [PMC free article] [PubMed]
- Li X, Su Y, Hege K, Madlambayan G, Edwards H, Knight T, Polin L, Kushner J, Dzinic SH, White K, Yang J, Miller R, Wang G, Zhao L, Wang Y, Lin H, Taub JW, Ge T (2021). "The HDAC and PI3K dual inhibitor CUDC-907 synergistically enhances the antileukemic activity of venetoclax in preclinical models of acute myeloid leukemia." Haematologica 106 (5): 1262–127710.3324/haematol.2019.233445. https://www.ncbi.nlm.nih.gov/pubmed/32165486. [DOI] [PMC free article] [PubMed]
- Linev AJ, Ivanov HJ, Zhelyazkov IG, Ivanova H, Goranova-Marinova VS, Stoyanova VK (2018). "Mutations Associated with Imatinib Mesylate Resistance - Review." Folia Med (Plovdiv) 60 (4): 617–62310.2478/folmed-2018–0030. https://www.ncbi.nlm.nih.gov/pubmed/31188765. [DOI] [PubMed]
- Losson H, Gajulapalli SR, Lernoux M, Lee JY, Mazumder A, Gerard D, Seidel C, Hahn H, Christov C, Dicato M, Kirsch G, Han BW, Schnekenburger M, Diederich M (2020a). "The HDAC6 inhibitor 7b induces BCR-ABL ubiquitination and downregulation and synergizes with imatinib to trigger apoptosis in chronic myeloid leukemia." Pharmacol Res 160: 10505810.1016/j.phrs.2020a.105058. https://www.ncbi.nlm.nih.gov/pubmed/32619722. [DOI] [PubMed]
- Losson H, Schnekenburger M, Dicato M, Diederich M (2020b) "HDAC6-an Emerging Target Against Chronic Myeloid Leukemia?" Cancers (Basel) 12 (2)10.3390/cancers12020b318. https://www.ncbi.nlm.nih.gov/pubmed/32013157. [DOI] [PMC free article] [PubMed]
- Malik AH, Collins RH Jr., Saboorian MH, Lee WM (2001) "Chronic graft-versus-host disease after hematopoietic cell transplantation presenting as an acute hepatitis." Am J Gastroenterol 96 (2): 588–9010.1111/j.1572–0241.2001.03563.x. https://www.ncbi.nlm.nih.gov/pubmed/11232714. [DOI] [PubMed]
- Massimino M, Stella S, Tirro E, Romano C, Pennisi MS, Puma A, Manzella L, Zanghi S, Stagno F, Di Raimondo F, Vigneri P (2018). "Non ABL-directed inhibitors as alternative treatment strategies for chronic myeloid leukemia." Mol Cancer 17 (1): 5610.1186/s12943–018–0805–1. https://www.ncbi.nlm.nih.gov/pubmed/29455672. [DOI] [PMC free article] [PubMed]
- Mshaik R, Simonet J, Georgievski A, Jamal L, Bechoua S, Ballerini P, Bellaye PS, Mlamla Z, Pais de Barros JP, Geissler S, Francin PJ, Girodon F, Garrido C, Quere R (2021). "HSP90 inhibitor NVP-BEP800 affects stability of SRC kinases and growth of T-cell and B-cell acute lymphoblastic leukemias." Blood Cancer J 11 (3): 6110.1038/s41408–021–00450–2. https://www.ncbi.nlm.nih.gov/pubmed/33737511. [DOI] [PMC free article] [PubMed]
- Nguyen T, Dai Y, Attkisson E, Kramer L, Jordan N, Nguyen N, Kolluri N, Muschen M, Grant S (2011). "HDAC inhibitors potentiate the activity of the BCR/ABL kinase inhibitor KW-2449 in imatinib-sensitive or -resistant BCR/ABL+ leukemia cells in vitro and in vivo." Clin Cancer Res 17 (10): 3219–3210.1158/1078–0432.CCR-11–0234. https://www.ncbi.nlm.nih.gov/pubmed/21474579. [DOI] [PMC free article] [PubMed]
- Nimmanapalli R, Fuino L, Bali P, Gasparetto M, Glozak M, Tao J, Moscinski L, Smith C, Wu J, Jove R, Atadja P, Bhalla K (2003) "Histone Deacetylase Inhibitor LAQ824 Both Lowers Expression and Promotes Proteasomal Degradation of Bcr-Abl and Induces Apoptosis of Imatinib Mesylate-sensitive or -refractory Chronic Myelogenous Leukemia-Blast Crisis Cells." Cancer Research 63 (16): 5126–5135. https://cancerres.aacrjournals.org/content/canres/63/16/5126.full.pdf. [PubMed]
- Peng C, Chen Y, Yang Z, Zhang H, Osterby L, Rosmarin AG, Li S (2010) "PTEN is a tumor suppressor in CML stem cells and BCR-ABL-induced leukemias in mice." Blood 115 (3): 626–3510.1182/blood-2009–06–228130. https://www.ncbi.nlm.nih.gov/pubmed/19965668. [DOI] [PMC free article] [PubMed]
- Ruan Y, Wang L, Lu Y (2021) "HDAC6 inhibitor, ACY1215 suppress the proliferation and induce apoptosis of gallbladder cancer cells and increased the chemotherapy effect of gemcitabine and oxaliplatin." Drug Dev Res 82 (4): 598–60410.1002/ddr.21780. https://www.ncbi.nlm.nih.gov/pubmed/33428788. [DOI] [PubMed]
- Santo L, Hideshima T, Kung LA, Tseng JC, Tamang D, Yang M, Jarpe M, van Duzer JH, Mazitschek R, Ogier WC, Cirstea D, Rodig S, Eda H, Scullen T, Canavese M, Bradner J, Anderson KC, Jones SS, Raje N (2012) "Preclinical activity, pharmacodynamic, and pharmacokinetic properties of a selective HDAC6 inhibitor, ACY-1215, in combination with bortezomib in multiple myeloma." Blood 119 (11): 2579–8910.1182/blood-2011–10–387365. https://www.ncbi.nlm.nih.gov/pubmed/22262760. [DOI] [PMC free article] [PubMed]
- Tan Y, Zhang S, Zhu H, Chu Y, Zhou H, Liu D, Huo J (2019) "Histone deacetylase 6 selective inhibitor ACY1215 inhibits cell proliferation and enhances the chemotherapeutic effect of 5-fluorouracil in HCT116 cells." Ann Transl Med 7 (1): 210.21037/atm.2018.11.48. https://www.ncbi.nlm.nih.gov/pubmed/30788349. [DOI] [PMC free article] [PubMed]
- Xia RM, Liu T, Li WG, Xu XQ (2021). "RNA-binding protein RBM24 represses colorectal tumourigenesis by stabilising PTEN mRNA." Clin Transl Med 11 (10): e38310.1002/ctm2.383. https://www.ncbi.nlm.nih.gov/pubmed/34709758. [DOI] [PMC free article] [PubMed]
- Xiao X, Liu P, Li D, Xia Z, Wang P, Zhang X, Liu M, Liao L, Jiao B, Ren R (2020). "Combination therapy of BCR-ABL-positive B cell acute lymphoblastic leukemia by tyrosine kinase inhibitor dasatinib and c-JUN N-terminal kinase inhibition." J Hematol Oncol 13 (1): 8010.1186/s13045–020–00912–3. https://www.ncbi.nlm.nih.gov/pubmed/32552902. [DOI] [PMC free article] [PubMed]
- Zackova M, Mouckova D, Lopotova T, Ondrackova Z, Klamova H, Moravcova J (2013). "Hsp90 - a potential prognostic marker in CML." Blood Cells Mol Dis 50 (3): 184–910.1016/j.bcmd.2012.11.002. https://www.ncbi.nlm.nih.gov/pubmed/23190580. [DOI] [PubMed]
- Zhao C, Dong H, Xu Q, Zhang Y (2020). "Histone deacetylase (HDAC) inhibitors in cancer: a patent review (2017-present)." Expert Opin Ther Pat 30 (4): 263–27410.1080/13543776.2020.1725470. https://www.ncbi.nlm.nih.gov/pubmed/32008402. [DOI] [PubMed]
- Zhou H, Xu R (2015). "Leukemia stem cells: the root of chronic myeloid leukemia." Protein Cell 6 (6): 403–1210.1007/s13238–015–0143–7. https://www.ncbi.nlm.nih.gov/pubmed/25749979. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.