Abstract
The prevalence of both obesity and hypogonadism in the United States has increased over the past two decades. While prior studies have shown an association between obesity and secondary hypogonadism—low testosterone and luteinizing hormone—few have used a large enough sample size to determine prevalence at each body mass index class. We aimed to compare rates of secondary hypogonadism among body mass index classes by constructing a retrospective database with men who had their body mass index, morning testosterone and luteinizing hormone levels measured during a visit to a urology clinic at a tertiary academic medical center between 2011–2020. Men previously on testosterone replacement therapy, Clomiphene, or Anastrozole were excluded. Chi-squared analysis was conducted in “R”.
We found that among the 7 211 men studied, 45.7%, 22.6%, and 4.4% were classified as having diagnosis of secondary, primary, and compensated hypogonadism, respectively. We found that obese men and underweight men had increased prevalence of secondary hypogonadism as compared to men with normal BMI. These findings support the need for routine screening criteria and personalized advice to patients dealing with secondary hypogonadism.
Introduction
The prevalence of obesity in the United States (U.S.) has been increasing at an alarming rate over the past two decades, coinciding with the global obesity epidemic. Between 1999 and 2015 alone, U.S. females and males saw an 8.2% and 11.4% increase in obesity prevalence, respectively (1). More recent studies project that nearly half of U.S. adults will be obese by the year 2030 (2). It is well understood that obese individuals have a higher risk of suffering from type 2 diabetes mellitus, dyslipidemia, hypertension, and cardiovascular disease (3). Obesity has also been associated with male hypogonadism, a clinical syndrome resulting from failure of the testis to produce physiological concentrations of testosterone (T), sperm, or both (4).
Hypogonadism can be classified as primary, secondary, or compensated, depending on the affected organ and corresponding hormone levels. Primary hypogonadism is defined by a deficiency at the level of the testes whereas secondary hypogonadism corresponds to abnormalities of the hypothalamus or the pituitary, and thereby low production of luteinizing hormone (LH). Compensated hypogonadism has been recognized more recently as a term that represents a subclinical state that may eventually develop into overt primary hypogonadism (5). In compensated hypogonadism, T levels are within the normal range but LH levels are increased. Hypogonadism attributed to obesity is commonly classified as secondary hypogonadism and thus termed as male obesity-associated secondary hypogonadism (MOSH) (6).
Several mechanisms have been proposed as the pathogenesis of MOSH, including increased aromatase activity that facilitates peripheral aromatization of T to estradiol in adipose tissue, the production of adipokines, and leptin resistance in the hypothalamus (7, 8). Decreased levels of sex hormone binding globulin (SHBG) previously shown in obese men is also thought to contribute to MOSH by reducing levels of total T (9). Clinical features of hypogonadism commonly present when T levels fall below 300 ng/dL and include excessive fatigue, depressed mood, impaired concentration, and sexual dysfunction manifested as erectile dysfunction and/or poor libido (10). Symptomatic men who are found to have hypogonadism are typically treated with testosterone replacement therapy (TRT). Off-label clomiphene citrate (CC), a selective estrogen receptor modulator, is a commonly used alternative therapy that indirectly increases serum T in men with functioning Leydig cells by inhibiting the negative feedback of estradiol onto the hypothalamus (11). The variability in treatment approach highlights the importance of correctly identifying the underlying mechanism of hypogonadism in men presenting with the indistinguishable symptoms of hypogonadism and low T.
In 2006, the Hypogonadism in Males study estimated the prevalence of hypogonadism to be 38.7% among men over the age of 45 years presenting to primary care clinics (12). A more recent study of 100 men with moderate to severe obesity found that approximately 45% of them suffered from MOSH (13). Although the association between elevated body mass index (BMI) and secondary hypogonadism is well-documented, few studies have sampled large enough populations to determine the prevalence and classification of hypogonadism at the various BMI classes (5). We hypothesized that as BMI increases, the prevalence of secondary hypogonadism also increases. Therefore, the goal of our study was to compare the prevalence of secondary hypogonadism among men in each BMI class who presented to a urology clinic at a tertiary academic center between 2011–2020.
Methods
Subjects
We constructed an IRB-approved retrospective database with men who had their BMI, morning T and LH levels measured during a visit to the urology clinic at a tertiary academic medical center between 2011–2020. Men previously on testosterone replacement therapy (TRT), Clomiphene, or Anastrozole for any period were excluded. We utilized data from the earliest chronologic encounter for men who had multiple visits recorded.
Hormone Measurements
All T and LH levels for patients included in our study were drawn on the same day prior to 10AM. T levels were measured via one of two methods: (1) liquid chromatography/tandem mass spectrometry (LC/MS/MS) or (2) electrochemiluminescence immunoassay (ECLIA) per Quest Diagnostics and LabCorp protocols, respectively. LH was measured by ECLIA per Quest Diagnostics and LabCorp protocols.
Experimental Design
We stratified men by BMI based on CDC BMI classification (Table 1) (14). Gonadal state was determined using Total T or Free T and LH levels (Table 2). The cutoff level of Total T of 300 ng/dL is consistent with the American Urological Association to support the diagnosis of low T and the cut-off established by the laboratory which measured our population’s T level (15). For Free T, an established cutoff level < 64 was used to identify patients (16). The LH level of 8 mIU/mL is the cut-off established by the laboratory which measured our population’s LH level.
Table 1:
BMI classification
| Classification | BMI |
|---|---|
| Underweight | <18.5 |
| Normal Weight | 18.5–24.9 |
| Overweight | 25–29.9 |
| Obesity Class I | 30.0–34.9 |
| Obesity Class II | 35–39.9 |
| Obesity Class III | >40 |
Table 2:
Gonadal State Classification
| Gonadal State | Total T (ng/dL) | Free T (pg/mL) | LH (mIU/mL) |
|---|---|---|---|
| Eugonadal | ≥ 300 | ≥64 | < 8 |
| Primary Hypogonadism | < 300 | <64 | ≥ 8 |
| Secondary Hypogonadism | < 300 | <64 | < 8 |
| Compensated Hypogonadism | ≥ 300 | ≥64 | ≥ 8 |
Statistical Methods
A Chi-squared analysis was performed to examine the relationship between BMI classification and gonadal state. All statistical analyses were conducted using R version 4.1.1 (17).
Results
This study analyzed data from 7 211 men (Table 3). Based on Total T and LH levels, 3 293 (45.7%), 1 627 (22.6%), and 314 (4.4%) patients were classified as secondary, primary, and compensated hypogonadism, respectively.
Table 3:
Study Demographics (N = 7211)
| Category | Total |
|---|---|
| Underweight | 374 |
| Normal Weight | 487 |
| Overweight | 2221 |
| Obesity Class I | 2204 |
| Obesity Class II | 1275 |
| Obesity Class III | 650 |
| Total T mIU/mL (μ) | 203.1 |
| Free T pg/mL (μ) | 21.53 |
| LH ng/dL (μ) | 7.785 |
5 252 patients had both Total T and Free T levels measured. Of 2 421 men with secondary hypogonadism based on Total T levels, 2 396 (98.9%) were also classified as having secondary hypogonadism when using Free T. 1.1% of patients previously identified as secondary hypogonadism based on Total T, were re-classified as eugonadal.
We found that 56% and 61% of men in obesity class II and III, respectively, and 63% underweight men had secondary hypogonadism (Figure 1). The Pearson’s Chi-Squared test had a X2 = 374.1 (p < .001, df = 15).
Figure 1:
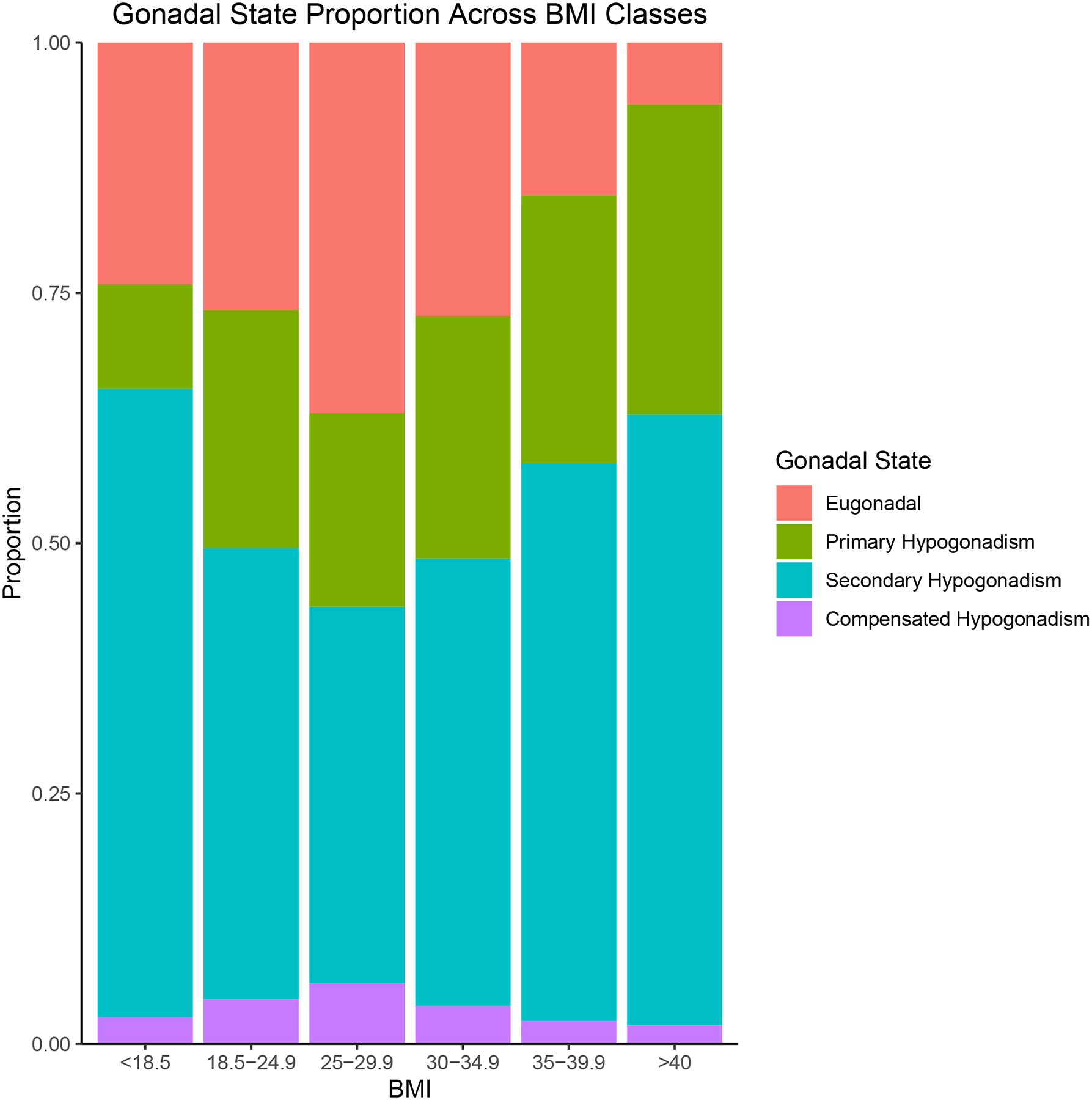
Prevalence of secondary hypogonadism based on BMI class
Discussion
Previous studies have identified that secondary hypogonadism is associated with elevated BMI in the general population. The purpose of our study was to compare the prevalence of secondary hypogonadism among men in each BMI class who presented to a urology clinic at a tertiary academic center. Among our study participants, we found that obesity class II and III as well as underweight men had a higher prevalence of secondary hypogonadism compared to normal BMI controls. To our knowledge, this is the first study to identify this U-shaped distribution of secondary hypogonadism in men presenting to a tertiary care center.
Our study findings are aligned with those presented in the European Male Ageing Study (EMAS), which demonstrated that men with a BMI > 30kg/m2 had an 8-times higher rate of secondary hypogonadism (5). While their study showed that 11.8%, 2.0%, and 9.5% of men were classified into the secondary, primary, and compensated hypogonadism categories, respectively, we found significantly higher rates. This discrepancy may be explained by the fact that our patients uniformly presented to a tertiary care center with hypogonadal symptoms. The association between BMI and secondary hypogonadism has been well-documented, and several mechanisms exist to explain this relationship.
In population-based studies, obesity is the most important contributor to testosterone deficiency (5). According to the hypogonadal–obesity–adipocytokine hypothesis, obese individuals have lower testosterone and higher estrogen hormones secondary to adipose tissues producing enhanced aromatase enzymes. Testosterone deficiency further facilitates adipocyte differentiation and inflammation, which results in hypothalamo–pituitary–testicular (HPT) axis suppression (18). Additionally, insulin resistance is commonly seen in testosterone-deficient patients. Increased adipocyte differentiation, increased pro-inflammatory cytokines (TNF-α, IL-1 and IL-6) and decreased adiponectin all contribute to this phenomenon (19). The hyperinsulinemia decreases kisspeptin signaling, which results in decreased GnRH release and low LH and T levels (20).
Underweight men may experience secondary hypogonadism due to an adaptive response to an energy deficit, involving suppression of the hypothalamus due to dysregulation of ghrelin, leptin, and pro-inflammatory cytokines. This process akin to starvation-associated amenorrhea often reported in adolescent females, who are unable to mobilize energy from limited body fat to maintain ovulatory function (16). Previous studies have shown that obesity is associated with leptin resistance and suppressed hypothalamic kisspeptin gene expression. In fact, obese men experience an interaction between circulating leptin and testosterone that promotes a cycle of adiposity and reproductive dysfunction (16, 19).
The study findings are well supported by our large sample size and availability of both Total T and Free T levels for men. Additionally, the strict inclusion criteria of including only men with labs drawn on the same morning and excluding those who were previously on testosterone replacement therapy (TRT), Clomiphene, or Anastrozole strengthen our findings. We observed a high consistency rate in secondary hypogonadism diagnosis when using both Total T and Free T. Limitations of this study include our inability to document the hypogonadal symptoms experienced by patients using a validated questionnaire. Additionally, we do not have measurements of sex-hormone binding globulin—an important transporter of sex hormones—which are known to be decreased in obese patients because of the high lipid content of the liver (21). Future analyses looking at the changes in BMI classifications over time, especially in the underweight population, and variations in gonadal state would be of interest.
Conclusions
Males at BMI extremes (i.e., Obesity Class II-III and underweight) have a higher prevalence of secondary hypogonadism. Obese men may have secondary hypogonadism due to decreased levels of circulating estradiol and sex hormone binding globulin, and leptin resistance in the hypothalamus. For underweight men, this process may represent an adaptive response to an energy deficit, involving suppression of the hypothalamus due to dysregulation of ghrelin, leptin, and pro-inflammatory cytokines. These findings support screening for secondary hypogonadism in obese men to recommend weight loss and TRT to reduce cardiovascular related mortality, while increasing caloric intake in underweight men to improve libido and sexual function.
Acknowledgements
We would like to thank members from the University of Miami Miller School of Medicine Urology and Endocrinology departments for their contribution to this work.
Funding
This work was supported by National Institutes of Health Grant R01 DK130991 and Clinician Scientist Development Grant from the American Cancer Society to Ranjith Ramasamy.
Footnotes
Ethical Approval
The study protocol was approved by the IRB of The University of Miami (IRB No. 20170849). Informed consent was confirmed by the IRB.
Competing Interests
The authors have no competing interests to disclose.
References
- 1.Wang Y, Beydoun MA, Min J, Xue H, Kaminsky LA, Cheskin LJ. Has the prevalence of overweight, obesity and central obesity levelled off in the United States? Trends, patterns, disparities, and future projections for the obesity epidemic. Int J Epidemiol. 2020;49(3):810–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ward ZJ, Bleich SN, Cradock AL, Barrett JL, Giles CM, Flax C, et al. Projected U.S. State-Level Prevalence of Adult Obesity and Severe Obesity. N Engl J Med. 2019;381(25):2440–50. [DOI] [PubMed] [Google Scholar]
- 3.Nguyen NT, Magno CP, Lane KT, Hinojosa MW, Lane JS. Association of hypertension, diabetes, dyslipidemia, and metabolic syndrome with obesity: findings from the National Health and Nutrition Examination Survey, 1999 to 2004. J Am Coll Surg. 2008;207(6):928–34. [DOI] [PubMed] [Google Scholar]
- 4.Bhasin S, Brito JP, Cunningham GR, Hayes FJ, Hodis HN, Matsumoto AM, et al. Testosterone Therapy in Men With Hypogonadism: An Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2018;103(5):1715–44. [DOI] [PubMed] [Google Scholar]
- 5.Tajar A, Forti G, O’Neill TW, Lee DM, Silman AJ, Finn JD, et al. Characteristics of secondary, primary, and compensated hypogonadism in aging men: evidence from the European Male Ageing Study. J Clin Endocrinol Metab. 2010;95(4):1810–8. [DOI] [PubMed] [Google Scholar]
- 6.Masterson JM, Soodana-Prakash N, Patel AS, Kargi AY, Ramasamy R. Elevated Body Mass Index Is Associated with Secondary Hypogonadism Among Men Presenting to a Tertiary Academic Medical Center. World J Mens Health. 2019;37(1):93–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Porte D Jr., Baskin DG, Schwartz MW. Insulin signaling in the central nervous system: a critical role in metabolic homeostasis and disease from C. elegans to humans. Diabetes. 2005;54(5):1264–76. [DOI] [PubMed] [Google Scholar]
- 8.Mammi C, Calanchini M, Antelmi A, Cinti F, Rosano GM, Lenzi A, et al. Androgens and adipose tissue in males: a complex and reciprocal interplay. Int J Endocrinol. 2012;2012:789653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gautier A, Bonnet F, Dubois S, Massart C, Grosheny C, Bachelot A, et al. Associations between visceral adipose tissue, inflammation and sex steroid concentrations in men. Clin Endocrinol (Oxf). 2013;78(3):373–8. [DOI] [PubMed] [Google Scholar]
- 10.Isidori AM, Giannetta E, Greco EA, Gianfrilli D, Bonifacio V, Isidori A, et al. Effects of testosterone on body composition, bone metabolism and serum lipid profile in middle-aged men: a meta-analysis. Clin Endocrinol (Oxf). 2005;63(3):280–93. [DOI] [PubMed] [Google Scholar]
- 11.Whitten SJ, Nangia AK, Kolettis PN. Select patients with hypogonadotropic hypogonadism may respond to treatment with clomiphene citrate. Fertil Steril. 2006;86(6):1664–8. [DOI] [PubMed] [Google Scholar]
- 12.Mulligan T, Frick MF, Zuraw QC, Stemhagen A, McWhirter C. Prevalence of hypogonadism in males aged at least 45 years: the HIM study. Int J Clin Pract. 2006;60(7):762–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Calderon B, Gomez-Martin JM, Vega-Pinero B, Martin-Hidalgo A, Galindo J, Luque-Ramirez M, et al. Prevalence of male secondary hypogonadism in moderate to severe obesity and its relationship with insulin resistance and excess body weight. Andrology. 2016;4(1):62–7. [DOI] [PubMed] [Google Scholar]
- 14.Defining Adult Overweight & Obesity: Centers for Disease Control and Prevention; 2021. [Available from: https://www.cdc.gov/obesity/adult/defining.html.
- 15.Mulhall JP, Trost LW, Brannigan RE, Kurtz EG, Redmon JB, Chiles KA, et al. Evaluation and Management of Testosterone Deficiency: AUA Guideline. J Urol. 2018;200(2):423–32. [DOI] [PubMed] [Google Scholar]
- 16.Wu FC, Tajar A, Beynon JM, Pye SR, Silman AJ, Finn JD, et al. Identification of late-onset hypogonadism in middle-aged and elderly men. N Engl J Med. 2010;363(2):123–35. [DOI] [PubMed] [Google Scholar]
- 17.R: A language and environment for statistical computing Vienna, Austria: R Foundation for Statistical Computing; 2021. [Available from: https://www.R-project.org/. [Google Scholar]
- 18.Kelly DM, Jones TH. Testosterone: a metabolic hormone in health and disease. J Endocrinol. 2013;217(3):R25–45. [DOI] [PubMed] [Google Scholar]
- 19.Grossmann M. Testosterone and glucose metabolism in men: current concepts and controversies. J Endocrinol. 2014;220(3):R37–55. [DOI] [PubMed] [Google Scholar]
- 20.Fernandez CJ, Chacko EC, Pappachan JM. Male Obesity-related Secondary Hypogonadism - Pathophysiology, Clinical Implications and Management. Eur Endocrinol. 2019;15(2):83–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Glass AR, Swerdloff RS, Bray GA, Dahms WT, Atkinson RL. Low serum testosterone and sex-hormone-binding-globulin in massively obese men. J Clin Endocrinol Metab. 1977;45(6):1211–9. [DOI] [PubMed] [Google Scholar]


