Abstract
Background
Bloodstream infections (BSIs) among people with human immunodeficiency virus (PWH) remain a poorly studied source of morbidity and mortality. We characterize the epidemiology, microbiology, and clinical outcomes including reinfection, hospitalization, and mortality rates of both community-acquired and hospital-acquired BSI in PWH.
Methods
We identified all BSI, between January 1, 2000 and December 31, 2017 in PWH in care at Southern Alberta Clinic, by linking data from laboratory and clinical databases. Crude incidence rates per 1000 person-years for BSI and death were calculated. Cox proportional hazards models estimated crude and adjusted hazard ratios (HRs) and 95% confidence intervals (CIs) to conduct a risk factor analysis of BSI in PWH. Logistic regression models with generalized estimating equations estimated crude and adjusted odds ratios (aORs) to identify characteristics associated with 1-year mortality after BSI.
Results
Among 2895 PWH, 396 BSI episodes occurred in 228 (8%) PWH. There were 278 (72%) Gram-positive and 109 (28%) Gram-negative BSI. People with human immunodeficiency virus with lower CD4 nadirs, higher Charlson comorbidity indices, and hepatitis C virus were at highest risk for BSI. Long-term all-cause mortality was greater in those experiencing BSI (HR, 5.25; 95% CI, 4.21–6.55). CD4 count <200 cells/mm3 measured closest to the time of BSI was associated with 1-year mortality after BSI (aOR, 3.88; 95% CI, 1.78–8.46). Repeat episodes (42%) and polymicrobial BSI (19%) were common.
Conclusions
Bloodstream infections continue to occur at an elevated rate among PWH with high reoccurrence rates and associated morbidity and mortality. To risk stratify and develop targeted interventions, we identified PWH at greatest risk for BSI. People with human immunodeficiency virus with low immunity at the time of BSI are at highest risk of poor outcomes.
Keywords: bacteremia, bloodstream infection, Canada, HIV/AIDS, longitudinal
Despite a reduction with time, bloodstream infections continue to occur at an elevated rate among PWH with high reoccurrence, morbidity, and mortality. PWH with low immunity at the time of BSI are at highest risk of poor outcomes.
After the introduction of modern antiretroviral therapy (ART) and continued improvements in human immunodeficiency virus (HIV) care, people with HIV (PWH) have achieved a life expectancy approaching that of the general population in North America [1]. In PWH attending the Southern Alberta HIV Clinic (SAC), the acquired immune deficiency syndrome (AIDS)-related annual mortality rate declined between 1994 and 2017 from 11.0% to 0.1%, respectively [2]. However, invasive bacterial infections remain a significant cause of morbidity among PWH leading to increased length of hospital stay, intensive care unit admissions, and mortality [3]. A systematic review published by Huson et al [4] in 2014 demonstrated that bacterial infections accounted for 15% of mortality among PWH in the USA, 16%–18% in Europe, and 25%–43% in Asia. Since the widespread introduction and use of ART, however, the incidence of bloodstream infections (BSIs) has decreased [5, 6].
Bloodstream infection is associated with HIV acquisition risk factors (ie, injection drug use) as well as the severity of a patient’s immunosuppression [4, 7]. Altered cell-mediated immunity predisposes PWH to invasive bacterial and fungal infections for up to 6–10 years after ART initiation, with a higher-risk of recurrent infection and death [8–10]. Limited longitudinal studies of incident BSI in PWH have been reported, with no known population-based studies having assessed both community- and hospital-acquired cases. With unique databases compiled over the past 2 decades, we performed a population-based retrospective cohort study of BSI in all PWH in our region by linking 2 comprehensive databases of PWH and all microbiology.
Our objective was to characterize the epidemiology, microbiology, and clinical outcomes including, reinfection, hospitalization, and mortality of both community-acquired BSI (CA-BSI) and hospital-acquired BSI (HA-BSI) among PWH. Understanding of the epidemiology, microbiology, and outcomes of BSI in PWH will identify those at greatest risk to develop targeted intervention strategies.
METHODS
Ethics Statement
The use of nonnominal SAC cohort data for research has been approved by the University of Calgary Conjoint Health Research Ethics Board along with the microbiology research database (REB15-0629) and the current research protocol (REB17-2283).
Patient Consent Statement
All participants of the SAC cohort signed a general consent for research, and the research ethics board deemed specific consent to conduct this retrospective analysis unnecessary.
Participants and Setting
All PWH in southern Alberta, Canada receive exclusive HIV care under universal healthcare through a centralized program at SAC. All PWH ≥18 years old engaged in HIV care at SAC between January 1, 2000 and December 31, 2017 were included. People with HIV were considered to be engaged in care if they had attended at least 1 clinic appointment within the year included. Patients were followed until they moved out of the region, were lost to follow-up, died, or until December 31, 2017. Lost to follow-up was defined as not having attended at least 1 SAC clinic appointment for 1 year. Mortality among participants was evaluated until December 31, 2018 to identify full 1-year mortality rates post-BSI among those diagnosed with BSI in 2017.
Outcome: Blood Stream Infection Ascertainment
All PWH who experienced a BSI during the study period were retrospectively identified through the research database at Calgary Laboratory Services (CLS) and matched to the SAC database. All diagnostic microbiology services were provided for Calgary and surrounding rural areas (currently ∼1.5 M people) by CLS during the study period. Blood cultures were collected using a standard regional adult protocol of a 2-set, 4-bottle draw for each order [11]. The BacTAlert (bioMérieux, Laval, Quebec) automated system was used throughout the study, and each bottle consisted of FAN Plus Media for optimal aerobic and anaerobic organism recovery. Blood cultures were evaluated by a medical doctor (R.L. and D.C.), identifying those with 1 of 4 bottles positive for organisms that may be consistent with contamination. A total of 128 (32%) isolates were deemed contaminants and were excluded.
Covariates
Self-reported HIV acquisition risks were categorized into people who inject drugs (PWID), gay/bisexual men who have sex with men (gbMSM), heterosexual contact, or other (blood transfusion or perinatal transmission). Self-reported race/ethnicity was analyzed by the most common groupings of White, Indigenous/Métis, and Black. All other ethnicities including East Asian, West Asian, Indo Asian, and Hispanic were grouped as “other”. Hepatitis C virus (HCV) coinfection was defined by presence of a positive HCV antibody test. The AIDS-defining conditions were obtained through diagnosis codes collected by the SAC database and based on the Centers for Disease Control and Prevention list [12]. Participants diagnostic codes from clinical care were used to calculate a Charlson comorbidity index (CCI) [13, 14]. However, data on 2 of the CCI comorbidities were not available (hemiplegia and connective tissue disease) because they were not recorded in the SAC database.
Blood stream infection occurring in ambulatory patients or within 72 hours of their hospital admission were categorized as CA-BSI. If BSI occurred >72 hours after hospitalization, it was considered HA-BSI [15–17]. Hospitalization status was defined as being admitted at the time of the initial blood culture or within 7 days after the initial blood culture draw. Polymicrobial BSI occurred when blood cultures yielded ≥2 different pathogenic organisms within 48 hours of each other within the 5-day incubation period. CD4 counts and HIV ribonucleic acid (RNA) measurements closest to a diagnosis of BSI episodes were categorized.
Outcomes After Blood Stream Infection
Clinical outcomes data included hospitalization, BSI recurrence, endocarditis, and all-cause mortality. Blood stream infection recurrence was defined as another BSI >14 days after the first. Endocarditis diagnosis was established based on hospital discharge diagnosis codes in the SAC database. Death and cause of death among all patients at SAC are ascertained from Alberta Health and Office of the Medical Examiner. Specific mortality causes were classified according to the Cause of Death (CoDe) protocol [18].
Statistical Analysis
Descriptive analysis was performed on demographic data and risk factors for those with BSI compared with those without BSI. Univariable analyses were conducted using all data, but observations with missing data were excluded for multivariate analysis. Incidence rates (IRs) for BSI were calculated each year. Blood stream infection was categorized by Gram-negative and Gram-positive organisms for further comparison. Due to its rarity, fungemia was assessed through descriptive statistics. Categorical variables were compared using the χ2 test and continuous variables were compared using t tests.
Crude hazard ratios (HRs) and adjusted HRs were calculated using a Cox proportional hazards model to compare characteristics of participants with at least 1 BSI episode versus those who did not have BSI. A priori covariates were incorporated including age at HIV diagnosis, sex, HIV risk factor, and CD4 nadir. Logistic regression models with generalized estimating equations (allowing for multiple BSI episodes within a single person) were used to assess characteristics of participants with Gram-positive compared with Gram-negative BSI and those with BSI who died within 1-year compared with those who survived. A priori confounders included in this latter model included age at BSI diagnosis, BSI source, CD4 closest to BSI, CCI index, and whether the culture was polymicrobial. The remainder of both models included covariates with significant univariate associations with the outcome. All P values are 2-tailed tests with the statistical significance level set at P = .05 including 95% confidence intervals (CIs). All analyses were performed using STATA version 16.0 (StataCorp, College Station, TX).
RESULTS
Characteristics of People With Human Immunodeficiency Virus and Risk Factors for Blood Stream Infection
Among 2895 PWH meeting the above inclusion criteria, 228 (8%) experienced at least 1 episode of BSI between 2007 and 2017 with 396 total episodes of BSI (Table 1). A total of 22 008 person-years (PY) were observed with slightly longer mean follow-up in those without BSI compared with those with BSI (7.9 years vs 7.2 years, respectively; P = .547). The study population was predominately male (77%) and of White race (57%). The number of participants with any missing values was 233 (7%), with 107 missing values for ethnicity, 155 for HCV status, 28 for CD4 nadir, 11 for HIV risk factor, 7 for HIV date of diagnosis, 1 for sex, and 1 for date of birth.
Table 1.
Characteristics of PWH Who Experience At Least 1 Episode of BSI Compared With Those Who Do Not Between 2000 and 2017 in Persons Actively Followed at SAC
| Characteristics | Total N = 2895 (%) |
Total With No BSI n = 2667 (92%) |
Total With BSI n = 228 (8%) |
HR (95% CI) | aHR (95% CI) | |
|---|---|---|---|---|---|---|
| Age at HIV diagnosis, years | *Median (IQR) | 33 (27–41) | 33 (27–41) | 34 (27–44) | ||
| ≤30 | 1037 (36) | 963 (36) | 74 (34) | 1.00 | 1.00 | |
| 31–40 | 1156 (37) | 986 (37) | 70 (32) | .94 (.68–1.30) | .87 (.61–1.22) | |
| >40 | 795 (28) | 718 (27) | 77 (35) | 1.40 (1.02–1.92) | 1.17 (.81–1.69) | |
| Year of HIV Diagnosis | <2000 | 1023 (35) | 898 (34) | 125 (57) | 1.00 | 1.00 |
| ≥2000 | 1865 (65) | 1769 (66) | 96 (43) | .64 (.48–.85) | .86 (.62–1.19) | |
| Sex | *Male | 2220 (77) | 2057 (77) | 163 (72) | 1.00 | 1.00 |
| Female | 674 (23) | 610 (23) | 64 (28) | 1.43 (1.07–1.91) | 1.36 (.97–1.93) | |
| Race/Ethnicity | White | 1622 (57) | 1498 (57) | 124 (55) | 1.00 | 1.00 |
| Indigenous/Métis | 293 (10) | 232 (9) | 61 (27) | 3.58 (2.63–4.87) | 1.90 (1.32–2.73) | |
| Black | 632 (22) | 608 (23) | 24 (11) | .64 (.41–.99) | .90 (.53–1.53) | |
| Other | 285 (10) | 270 (10) | 15 (7) | .91 (.53–1.56) | .95 (.51–1.78) | |
| Median (IQR) | 195 (72–316) | 205 (83–324) | 64 (15–174) | |||
| CD4 Nadir | > 200 cells/mm3 | 1407 (49) | 1359 (51) | 48 (22) | 1.00 | 1.00 |
| ≤ 200 cells/mm3 | 1483 (51) | 1307 (49) | 175 (79) | 3.13 (2.27–4.30) | 1.76 (1.22–2.54) | |
| HIV Acquisition Risk Factor | gbMSM | 1379 (48) | 1303 (49) | 76 (34) | 1.00 | 1.00 |
| Heterosexual | 1463 (51) | 1326 (50) | 137 (61) | 2.07 (1.56–2.75) | 1.23 (.85–1.76) | |
| Other risk | 47 (2) | 37 (1) | 10 (5) | 4.31 (2.23–8.33) | 1.88 (.82–4.29) | |
| AIDS-Defining Diagnosis | No | 2139 (74) | 2031 (76) | 108 (47) | 1.00 | |
| Yes | 756 (26) | 636 (24) | 120 (53) | 3.09 (2.38–4.01) | - | |
| Charlson Comorbidity Index | Median (IQR) | 0 (0–6) | 0 (0–3) | 6 (1–8) | ||
| 0 | 1616 (56) | 1562 (59) | 54 (24) | 1.00 | 1.00 | |
| 1–4 | 504 (17) | 456 (17) | 48 (21) | 2.53 (1.71–3.73) | 2.21 (1.41–3.46) | |
| >4 | 775 (27) | 649 (24) | 126 (55) | 4.60 (3.35–6.34) | 4.16 (2.82–6.13) | |
| PWID | No | 2431 (84) | 2299 (86) | 132 (59) | 1.00 | 1.00 |
| Yes | 458 (16) | 367 (14) | 91 (41) | 3.84 (2.94–5.02) | 1.32 (.86–2.02) | |
| Hepatitis C Coinfection | No | 2396 (85) | 2286 (88) | 110 (52) | 1.00 | 1.00 |
| Yes | 424 (15) | 323 (12) | 101 (48) | 5.79 (4.43–7.59) | 2.99 (1.96–4.56) | |
| Hepatitis B Coinfection | No | 2847 (99) | 2647 (99) | 227 (100) | 1.00 | |
| Yes | 21 (1) | 20 (1) | 1 (0) | .92 (.12–6.57) | - | |
Abbreviations: aHR, adjusted hazard ratio; AIDS, acquired immune deficiency syndrome; BSI, bloodstream infection; CCI, Charlson comorbidity index; CI, confidence interval; gbMSM, gay/bisexual, men who have sex with men; HIV, human immunodeficiency virus; HR, crude hazard ratio; IQR, interquartile range; PWH, people with HIV; PWID, people who inject drugs; SAC, Southern Alberta Clinic.
*, P values calculated from χ2 test for categorical and binary variables and t tests for continuous variables, all <.05 unless. Hazard ratios calculated from Cox regression based on follow-up years to first episode BSI, with a total of 21 207.8 person years of follow-up. Values with P < .05 are bolded.
NOTES: AIDS-defining diagnoses were not included in the adjusted model to limit collinearity because all persons with CCI > 4 will have an AIDS-defining diagnosis. For Race/Ethnicity, category “Other” includes East Asian, West Asian, Indo Asian, and Hispanic. For HIV risk factor, category Other includes blood transfusion, perinatal transmission, or unknown.
In adjusted analyses, PWH with CCI scores of >4 had a 4-fold increased risk of BSI compared with those with CCI scores of zero after adjustment for age, sex, race/ethnicity, CD4 nadir, HIV risk factors, HCV, and PWID. When compared with White PWH, Indigenous/Métis PWH had an 90% increased risk of BSI, after adjusting for the factors listed above. People with HIV who were over 40 years old at the time of HIV diagnosis had an increased risk of BSI compared with PWH diagnosed before age 30 years, as did females; however, these associations were statistically nonsignificant. People diagnosed with HIV after the year 2000 compared with before 2000 had a statistically nonsignificant reduced risk of BSI in adjusted analysis. Individuals with a CD4 nadir of ≤200 cells/mm3 had a 76% increased risk for BSI, and those with HCV coinfection had over 3-fold increased risk for BSI in adjusted analyses (Table 1).
Incidence Rates of Blood Stream Infection and Death in People With Human Immunodeficiency Virus
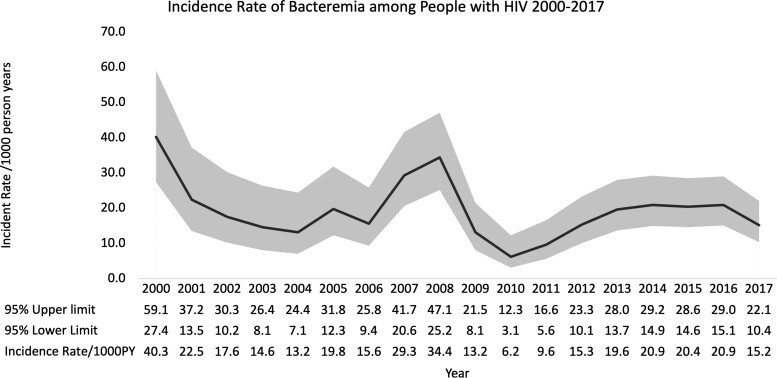
The overall average IR for BSI among PWH was 18.7/1000 PY (95% CI, 17.0–20.7). Over time, the IR of BSI decreased, and in 2000 it was 40.3/1000 PY compared with 15.2/1000 PY in 2017. In 2007 and 2008 there were large spikes in BSI rates (Figure 1), driven by Staphylococcus aureus and Streptococcus pneumoniae BSI episodes.
Figure 1.
Incidence rate per 1000 person-years (PY) of bloodstream infections among people with human immunodeficiency virus active in care at Southern Alberta Clinic between 2000 and 2017. The overall incidence is 18.7/1000 PY (95% confidence interval, 17.0–20.7), from 21 203 PY and 396 episodes occurring.
The IR for all-cause mortality for all PWH between 2000 and 2017 was 15.0/1000 PY (95% CI, 13.5–16.6). This was greater among those with BSI (IR = 58.5/1000 PY; 95% CI, 48.7–17.2) compared with those without BSI (IR = 11.2/1000; 95% CI, 9.9–12.7). The IR for AIDS-related death was 13.2/1000 PY for PWH who had a BSI compared with 2.7/1000 PY for PWH with no BSI. Those with BSI had a 5.3-fold (95% CI, 4.2–6.5) greater likelihood of dying from all causes over the 19-year follow-up than those without BSI (Figure 2).
Figure 2.
Cumulative all-cause mortality over 19 years of follow-up comparing people with human immunodeficiency virus who experience bloodstream infection (BSI) with those who do not. The risk for death occurring over the entire follow-up period is greater among those with BSI (hazard ratio, 5.25; 95% confidence interval [CI], 4.21–6.55).
Microbiology and Epidemiology of Blood Stream Infection
Most Common Organisms
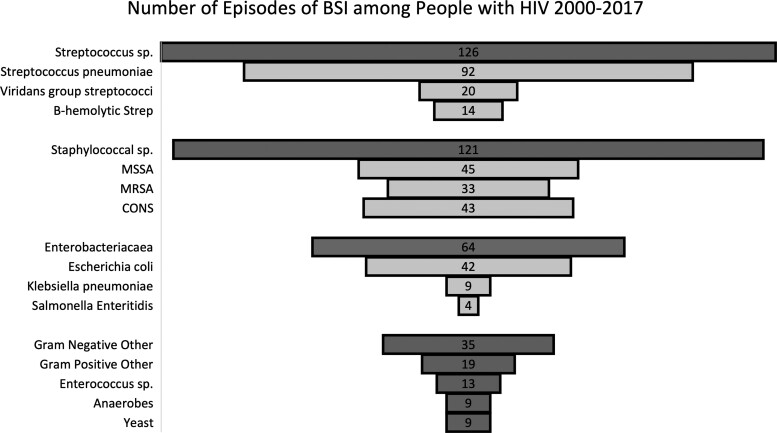
Streptococcus spp caused one third (32%) of BSI episodes over the 18-year study period with S pneumoniae (23%) being the most common organism (Figure 3). Staphylococcus spp caused another one third (31%) of BSI episodes, and the most common organism was methicillin-sensitive S aureus (MSSA) (11%). Enterobacterales caused 16% of BSI episodes, and the most common organism was Escherichia coli (11%). There were only 9 episodes of fungal BSI. Supplementary Table 1 provides data for the microbial etiology of all 396 BSI episodes.
Figure 3.
Different categories of bloodstream infections (BSI) among people with human immunodeficiency virus (HIV) between 2000 and 2017 receiving care at Southern Alberta Clinic (N = 396). CONS, coagulase-negative staphylococci; MRSA, methicillin-resistant Staphylococcus aureus; MSSA, methicillin-sensitive S aureus.
Bacterial Blood Stream Infection (BSI): Gram-Positive Versus Gram-Negative BSI
Bacteremia was categorized by being caused by either a Gram-positive (278, 72%) or Gram-negative (109, 28%) organism. There was minimal difference between Gram-negative and Gram-positive BSI according to year of BSI, age at BSI, sex, race/ethnicity, and HIV risk factors including PWID status (see Supplementary Table 2). There was also no significant difference between Gram-positive and Gram-negative BSI for being hospitalized or acquiring nosocomial infection. Over 50% of those with BSI were prescribed antimicrobial prophylaxis, but this did not impact acquiring Gram-positive compared with Gram-negative BSI. Those with HCV coinfection had 2.5-fold greater odds of having Gram-positive BSI compared with Gram-negative infection.
Fungal Bacterial Blood Stream Infection
Nine episodes of fungemia occurred among 7 participants; 4 were due to Candida spp and 5 were due to Cryptococcus neoformans variety neoformans. Of the 2 participants with repeat episodes of fungemia, both were due to Cryptococcus and separated by more than 1 month. There were 3 episodes that met the definition for being hospital-acquired. Most patients (75%) were not on ART at the time of their fungal infection, and most (67%) also had detectable HIV viremia (HIV RNA >200 copies/mL). Four episodes of fungemia required hospitalization (median duration of 36 days; interquartile range [IQR], 27–124.5). One PWH died of a stroke within 30 days of having Candidemia.
Outcomes After Bacterial Blood Stream Infection
Of the 230 episodes of BSI that resulted in hospitalization, the median length of stay was 11 (IQR, 7–30) days for Gram-positive BSI and 10 (IQR, 7–28) days for Gram-negative BSI. There were 24 episodes of infective endocarditis (IE) diagnosed during the follow-up period, occurring in 17 (8%) persons with documented BSI and 7 (0.3%) with no documented BSI. Among those with IE and BSI, 16 (94%) occurred with Gram-positive BSI and 1 (6%) occurred with Gram-negative BSI.
Risk of Recurrent Bacterial Blood Stream Infection
There were 229 (58%) initial cases of BSI and 167 (42%) recurrent cases. Of recurrent episodes, 80 (48%) were 1st repeats, 36 (22%) were 2nd repeats, 25 (15%) were 3rd repeats, and 27 (16%) had 4 or more BSI episodes. The most recurrent BSI episodes in one individual were 10 that occurred between 2010 and 2017, due to a variety of organisms including S pneumoniae (n = 4), methicillin-resistant S aureus (MRSA) (n = 2), MSSA (n = 1), Group A Streptococcus (n = 2), and Campylobacter jejuni (n = 1), all separated by at least 2 weeks. This individual had a CD4 nadir of 59 cells/mm3 and was not on ART after their HIV diagnosis. If repeated organisms were the same as previously isolated, they were 5-fold more likely to be Gram-positive organisms. People with HIV who had recurrent episodes of BSI were more likely to have HCV coinfection (40 of 61, 66%), have either injection drug use (43 of 78, 55%), or heterosexual sexual activity (59 of 78, 76%) as their HIV acquisition risk compared with gbMSM and have CCI scores of ≥4 (51 of 80, 64%) compared with those with scores of zero.
All-Cause Mortality After Bacterial Blood Stream Infection
There were 365 of 2895 (13%) deaths observed in this population during the study period; 115 of 228 (50%) occurred among individuals with BSI and 250 of 2667 (9%) occurred among those without BSI. Among those with BSI, 33 (15%) died within 1 month and 65 (29%) died within 1 year after BSI.
In unadjusted analyses, those with Gram-negative BSI had 72% higher odds of 1-year mortality compared with Gram-positive BSI (Table 2). People who inject drugs and those identifying as heterosexual had lower odds of 1-year mortality after BSI compared with non-PWID and gbMSM persons. Those with CD4 counts of ≤200 cells/mm3 closest to their BSI diagnosis had 3-fold higher odds of 1-year mortality. However, there was no association with virologic suppression on the 1-year mortality risk after BSI. Per each 1-point increase in CCI score, there was 8% increased odds in 1-year mortality. There was an over 2-fold increased odds of 1-year mortality among those with polymicrobial BSI episodes compared with monomicrobial.
Table 2.
Logistic Regression Models Using Generalized Estimating Equations to Estimate Unadjusted and Adjusted Odds of All-Cause 1-Year Mortality Among PWH After BSI (N = 387)
| Characteristic | OR | 95% CI | aOR | 95% CI | |
|---|---|---|---|---|---|
| Bacteremia Category | Gram Positive | 1.00 | 1.00 | ||
| Gram Negative | 1.72 | 1.00–2.95 | 1.52 | .90–2.57 | |
| Age at BSI | Per each 10-year increase | 1.13 | .88–1.45 | 1.00 | .74–1.33 |
| Sex | Female | 0.55 | .29–1.02 | .81 | .36–1.80 |
| Race/Ethnicity | White | 1.00 | 1.00 | ||
| Indigenous/Métis | .68 | .36–1.29 | .84 | .39–1.79 | |
| Black | .55 | .19–1.58 | .34 | .08–1.45 | |
| Other | 1.28 | .47–3.50 | 1.15 | .23–5.67 | |
| CD4 Nadir | ≤ 200 cells/mm3 | 1.65 | .80–3.42 | .77 | .27–2.16 |
| CD4 at Time of BSI | ≤200 cells/mm3 | 2.99 | 1.73–5.15 | 3.88 | 1.78–8.46 |
| HIV RNA at Time of BSI | >200 copies/mL | 1.03 | .59–1.81 | - | - |
| On ART | Yes | 1.92 | 1.10–3.34 | 1.13 | .55–2.28 |
| PWID | Yes | 0.46 | .24–.82 | .50 | .21–1.18 |
| HIV Acquisition Risk Factor | gbMSM | 1.00 | 1.00 | ||
| Heterosexual | 0.42 | .24–.75 | .64 | .25–1.59 | |
| Other/Unknown | 0.77 | .24–2.47 | 2.06 | .45–9.76 | |
| Hospitalized | Admitted to hospital | 1.42 | .93–2.17 | - | - |
| BSI Source | Hospital-Acquired | 1.65 | .92–2.96 | 1.43 | .60–3.42 |
| HCV Status | HCV Coinfected | 0.65 | .36–1.17 | - | - |
| Year of BSI | 2000–2005 | 1.00 | - | ||
| 2006–2011 | 1.84 | .82–4.11 | - | - | |
| 2012–2017 | 1.51 | .69–3.32 | - | - | |
| CCI | Per 1 point increase | 1.08 | 1.01–1.17 | 1.03 | .93–1.13 |
| Polymicrobial | Yes | 2.17 | 1.04–4.52 | 2.01 | 0.85–4.79 |
Abbreviations: aOR, adjusted odds ratio; ART, antiretroviral therapy; BSI, bloodstream infection; CCI, Charlson comorbidity index; CI, confidence interval; gbMSM, gay/bisexual, men who have sex with men; HCV, hepatitis C virus; HIV, human immunodeficiency virus; OR, odds ratio; PWH, people with HIV; PWID, people who inject drugs; RNA, ribonucleic acid. Values with P < .05 are bolded.
NOTES: Odds ratios calculated from logistic regression using generalized estimating equations to account for multiple episodes within an individual. For Race/Ethnicity, category “Other” includes East Asian, West Asian, Indo Asian, and Hispanic. For HIV risk factor, category Other includes blood transfusion, perinatal transmission, or unknown.
Following adjustement for BSI category, age at BSI, ART use, HIV risk category, BSI source, CCI, and polymicrobial BSIs, having a CD4 count of ≤200 cells/mm3 closest to their BSI diagnosis was the only characteristic that remained to have a statistically significant association with an approximately 4-fold increased odds of 1-year mortality after BSI (Table 2).
Specific-Cause Mortality After Bacterial Blood Stream Infection
Individuals who experienced BSI had a higher likelihood of dying from a bacterial infection (23% vs 4%) or other infectious cause (13% vs 8%) compared with those with no bacteremia. Of the persons who died within 1 month after BSI, the predominant cause of death was bacterial infection (39%), followed by AIDS-related causes (15%) and malignancy (12%). Of those who died within 1 year, the most likely cause of death remained bacterial infection (28%); however, AIDS-related causes accounted for 26% of deaths. After evaluating specific causes of death at 1 year after BSI, we found that 83% of deaths due to bacterial infections occurred after Gram-positive BSI. Although approximately 70% of deaths due to malignancy, other infectious causes and AIDS-related causes occurred after Gram-negative BSI (Supplementary Figure 1).
DISCUSSION
We used longitudinal, comprehensive databases to evaluate clinical, demographic, and laboratory characteristics of PWH who experience BSI as well as their outcomes. The incidence rates of BSI among PWH, although fluctuating annually, has declined between 2000 and 2017. In 2007, there was a large increase in BSI among PWH, predominantly due to either Staphylococcus spp or Streptococcus spp infections. In addition, there was a documented large population-based outbreak in Alberta, Canada of community-associated (CA)-MRSA and S pneumoniae that both peaked in 2007 [19–21]. In Calgary, between 2000 and 2004, the annual incidence of CA-BSI was 0.82/1000 PY, which was substantially less than the average seen in our PWH population of 18.72/1000 PY [22].
Prior evidence suggests that altered cell-mediated immunity, B-cell dysfunction, impaired neutrophil function, and skin and mucous membrane breakdown among PWH result in increased risk of BSI [10]. An increased risk of BSI was found among those with CD4 nadirs <200 cells/mm3, with prior AIDS-defining diagnoses, HCV coinfection, and more comorbidities. Despite immune recovery with ART, HIV causes persistent inflammation and immune activation that are associated with the development of non-AIDS comorbidities [23]. As in this study and several others, the comorbid status and severity of immunocompromise of PWH, even if it was in the past, has an impact on BSI risk [10, 24, 25]; therefore, long-lasting immune effects may remain despite treatment with ART.
In our population, Black individuals had a reduced risk of BSI compared with White individuals. Studies of the general population and in PWH in the United States have shown Black individuals have higher incidence of BSI and worse outcomes compared with White individuals [26, 27]. Ramraj et al [28] have suggested that “the relationship between race and health varies as a function of the societal context in which it operates” and found that Black-White and Hispanic-White inequities were greater in the United States, whereas Indigenous-White inequities were larger in Canada. We found that individuals of Indigenous/Métis ethnicity were at a higher risk of BSI, possibly associated with experiencing health and social inequities [29, 30]; however, further studies are needed to ensure reproducibility and identify explanations for this association.
Within the general population of Calgary, the most common causes of CA-BSI are E coli followed by S aureus and S pneumoniae, and these 3 pathogens account for more than half of all BSI cases [15]. The most common causes of BSI reported in PWH in the United States and Europe are S pneumoniae (23%), S aureus (20%), and E coli (11%), which is consistent with our study [4, 10]. In Asia, the most common BSI pathogen was nontyphoidal Salmonella (47%), whereas the prevalence of S pneumoniae was low (1%), illustrating (1) the epidemiological differences in geographic distributions of pathogens by region and (2) variations in antibiotic usage [4]. In our study, we only identified 4 (1%) cases of BSI due to nontyphoidal Salmonella, which is in keeping with the low prevalence among our general population (∼1/100 000 year) [31].
The elevated long-term mortality of those with BSI likely reflects the complexity of social factors, underlying comorbidities, and inflammation leading to an immune compromised state [24]. The 1-year mortality after BSI was 29% with over half of those dying within the first month, which is in keeping with other studies [5]. People who inject drugs had a reduced risk of 1-year mortality after BSI compared with non-PWID. This association attenuated with multivariable analysis and is likely explained by BSI in PWID typically occurring in younger age groups with fewer comorbidities, because age and comorbid status are predictors of mortality associated with BSI [32]. The only factor that was significantly associated with an increased 1-year mortality was having a CD4 count <200 cells/mm3 measured closest to the time of BSI. Therefore, PWH with low immunity at the time of BSI should be followed closely to identify and manage complications after infection to try to improve outcomes.
Bacterial infection was the most common cause of death among those with BSI, whereas among those without BSI the most common cause of death was due to AIDS-related factors. Of those who died of bacterial infection within 1 year of their BSI, deaths occurred more often after Gram-positive BSI. However, the all-cause 1-year mortality rate was greater after Gram-negative BSI, which is consistent with other studies [25]. The increased mortality among those with Gram-negative BSI was likely related to their older age, increased CCI, and greater frequency of polymicrobial episodes [32, 33].
There are several limitations to this work. First, this was a single-center study with a geographically defined cohort; our findings may not be generalizable to other areas. Second, there may be reporting bias among covariates that were self-reported. Third, in calculation of the CCI, hemiplegia and connective tissue diagnoses were not available, therefore likely leading to lower CCI scores. Fourth, blood stream infection is a rare event; therefore, despite 18 years of follow-up and rates being higher in PWH than reported in the general population, we had relatively few BSI in our population, which may have resulted in nonsignificant analysis when trends were seen. Fifth, blood stream infection episodes would not have been captured if these events occurred outside of the Calgary region or surrounding area. Each isolate was evaluated and excluded if it met the predetermined criteria for contamination; therefore, there is low risk of contaminants being included. Finally, if the participant died while outside of the province, mortality may not have been captured.
CONCLUSIONS
Blood stream infections continue to occur at a greater rate among PWH than the general population with high reoccurrence despite incidence decreasing over time. To risk stratify and develop targeted interventions, we have identified PWH at greatest risk for BSI. Both the subsequent short-term and long-term mortality is high among PWH who experience BSI. People with HIV with low immunity at the time of BSI are at highest risk of poor outcomes, and efforts should be made to engage them in HIV care and treatment with regular follow-up to identify and manage complications after infection.
Supplementary Material
Acknowledgments
We thank Quang Vu for help with this project. We also thank all the staff and patients at Southern Alberta Clinic for making this work possible.
Contributor Information
Raynell Lang, Departments of Medicine and Community Health Sciences, Cumming School of Medicine, University of Calgary, Calgary, Alberta, Canada; Southern Alberta HIV Clinic, Alberta Health Services, Calgary, Alberta, Canada.
M John Gill, Southern Alberta HIV Clinic, Alberta Health Services, Calgary, Alberta, Canada; Department of Medicine, Cumming School of Medicine, University of Calgary, Calgary, Alberta, Canada.
Jeannine Viczko, Department of Pathology & Laboratory Medicine, Cumming School of Medicine, University of Calgary, Calgary, Alberta, Canada.
Christopher Naugler, Departments of Pathology & Laboratory Medicine and Community Health Sciences, Cumming School of Medicine, University of Calgary, Calgary, Alberta, Canada.
Deirdre Church, Departments of Medicine and Pathology & Laboratory Medicine, Cumming School of Medicine, University of Calgary, Calgary, Alberta, Canada; Calgary Laboratory Services, Alberta Health Services, Calgary, Alberta, Canada.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Author contributions. All authors have read and approved the final manuscript.
Potential conflicts of interest. M. J. G. has received honoraria as an ad hoc member of national HIV advisory boards to Merck, ViiV, and Gilead. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Samji H, Cescon A, Hogg RS, et al. Closing the gap: increases in life expectancy among treated HIV-positive individuals in the United States and Canada. PLoS One 2013; 8:e81355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hanhoff N, Vu Q, Lang R, et al. Impact of three decades of antiretroviral therapy in a longitudinal population cohort study. Antivir Ther 2019; 24:153–65. [DOI] [PubMed] [Google Scholar]
- 3. Afessa B, Green B. Bacterial pneumonia in hospitalized patients with HIV infection: the Pulmonary Complications, ICU Support, and Prognostic Factors of Hospitalized Patients with HIV (PIP) Study. Chest 2000; 117:1017–22. [DOI] [PubMed] [Google Scholar]
- 4. Huson MA, Stolp SM, van der Poll T, Grobusch MP. Community-acquired bacterial bloodstream infections in HIV-infected patients: a systematic review. Clin Infect Dis 2014; 58:79–92. [DOI] [PubMed] [Google Scholar]
- 5. Declercq S, De Munter P, Derdelinckx I, et al. Characteristics, causes, and outcome of 54 episodes of bloodstream infections in a cohort of HIV patients. Infect Dis (Lond) 2015; 47:611–7. [DOI] [PubMed] [Google Scholar]
- 6. Ortega M, Almela M, Soriano A, et al. Bloodstream infections among human immunodeficiency virus-infected adult patients: epidemiology and risk factors for mortality. Eur J Clin Microbiol Infect Dis 2008; 27:969–76. [DOI] [PubMed] [Google Scholar]
- 7. Lang R, Gill MJ, Vu Q, Viczko J, Naugler C, Church D. Longitudinal evaluation of risk factors and outcomes of blood stream infections due to Staphylococcus species in persons with HIV: an observational cohort study. EClinicalMedicine 2021; 31:100675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Opportunistic Infections Project Team of the Collaboration of Observational HIV, Epidemiological, Research, in, Europe, COHERE, in, EuroCoord, Young J, Psichogiou M, et al. CD4 cell count and the risk of AIDS or death in HIV-Infected adults on combination antiretroviral therapy with a suppressed viral load: a longitudinal cohort study from COHERE. PLoS Med 2012; 9:e1001194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mocroft A, Furrer HJ, Miro JM, et al. The incidence of AIDS-defining illnesses at a current CD4 count ≥200 cells/muL in the post-combination antiretroviral therapy era. Clin Infect Dis 2013; 57:1038–47. [DOI] [PubMed] [Google Scholar]
- 10. Taramasso L, Tatarelli P, Di Biagio A. Bloodstream infections in HIV-infected patients. Virulence 2016; 7:320–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kirn TJ, Weinstein MP. Update on blood cultures: how to obtain, process, report, and interpret. Clin Microbiol Infect 2013; 19:513–20. [DOI] [PubMed] [Google Scholar]
- 12. Schneider E, Whitmore S, Glynn KM, et al. Revised surveillance case definitions for HIV infection among adults, adolescents, and children aged <18 months and for HIV infection and AIDS among children aged 18 months to <13 years–United States, 2008. MMWR Recomm Rep 2008; 57(RR-10):1–12. [PubMed] [Google Scholar]
- 13. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987; 40:373–83. [DOI] [PubMed] [Google Scholar]
- 14. Quan H, Li B, Couris CM, et al. Updating and validating the Charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am J Epidemiol 2011; 173:676–82. [DOI] [PubMed] [Google Scholar]
- 15. Laupland KB, Church DL. Population-based epidemiology and microbiology of community-onset bloodstream infections. Clin Microbiol Rev 2014; 27:647–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lenz R, Leal JR, Church DL, Gregson DB, Ross T, Laupland KB. The distinct category of healthcare associated bloodstream infections. BMC Infect Dis 2012; 12:85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lam JC, Gregson DB, Robinson S, Somayaji R, Conly JM, Parkins MD. Epidemiology and outcome determinants of Staphylococcus aureus bacteremia revisited: a population-based study. Infection 2019; 47:961–71. [DOI] [PubMed] [Google Scholar]
- 18. Ingle SM, May MT, Gill MJ, et al. Impact of risk factors for specific causes of death in the first and subsequent years of antiretroviral therapy among HIV-infected patients. Clin Infect Dis 2014; 59:287–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gill VC, Ma I, Guo M, Gregson DB, Naugler C, Church DL. Sociodemographic and geospatial associations with community-associated methicillin-resistant Staphylococcus aureus (CA-MRSA) infections in a large Canadian city: an 11 year retrospective study. BMC Public Health 2019; 19:914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kellner JD, Vanderkooi OG, MacDonald J, Church DL, Tyrrell GJ, Scheifele DW. Changing epidemiology of invasive pneumococcal disease in Canada, 1998–2007: update from the Calgary-area Streptococcus pneumoniae research (CASPER) study. Clin Infect Dis 2009; 49:205–12. [DOI] [PubMed] [Google Scholar]
- 21. Maheden R, Simmonds K, Lohman T, et al. Alberta notifiable disease incidence- a historical record (1919–2014). Alberta Government, 2015. Available at: https://open.alberta.ca/dataset/09ff0f40-1cfc-48fd-b888-4357104c3c32/resource/c5ceca04-ccda-4811-9ed0-03a3cbe8c0fb/download/7019844-notifiable-disease-incidence-1919-2014.pdf. Accessed 25 October 2021. [Google Scholar]
- 22. Laupland KB, Gregson DB, Flemons WW, Hawkins D, Ross T, Church DL. Burden of community-onset bloodstream infection: a population-based assessment. Epidemiol Infect 2007; 135:1037–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zicari S, Sessa L, Cotugno N, et al. Immune activation, inflammation, and non-AIDS co-morbidities in HIV-infected patients under long-term ART. Viruses 2019; 11:200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Taramasso L, Liggieri F, Cenderello G, et al. Bloodstream infections in patients living with HIV in the modern cART era. Sci Rep 2019; 9:5418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Franceschini E, Santoro A, Menozzi M, et al. Epidemiology and outcomes of bloodstream infections in HIV-patients during a 13-year period. Microorganisms 2020; 8:1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Barnato AE, Alexander SL, Linde-Zwirble WT, Angus DC. Racial variation in the incidence, care, and outcomes of severe sepsis: analysis of population, patient, and hospital characteristics. Am J Respir Crit Care Med 2008; 177:279–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Afessa B, Morales I, Weaver B. Bacteremia in hospitalized patients with human immunodeficiency virus: a prospective, cohort study. BMC Infect Dis 2001; 1:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ramraj C, Shahidi FV, Darity W Jr, Kawachi I, Zuberi D, Siddiqi A. Equally inequitable? A cross-national comparative study of racial health inequalities in the United States and Canada. Soc Sci Med 2016; 161:19–26. [DOI] [PubMed] [Google Scholar]
- 29. Kim PJ. Social determinants of health inequities in Indigenous Canadians through a life course approach to colonialism and the residential school system. Health Equity 2019; 3:378–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Browne AJ, Varcoe C, Lavoie J, et al. Enhancing health care equity with Indigenous populations: evidence-based strategies from an ethnographic study. BMC Health Serv Res 2016; 16:544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Laupland KB, Schonheyder HC, Kennedy KJ, et al. Salmonella enterica bacteraemia: a multi-national population-based cohort study. BMC Infect Dis 2010; 10:95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Jensen US, Knudsen JD, Wehberg S, Gregson DB, Laupland KB. Risk factors for recurrence and death after bacteraemia: a population-based study. Clin Microbiol Infect 2011; 17:1148–54. [DOI] [PubMed] [Google Scholar]
- 33. Lin JN, Tsai YS, Lai CH, et al. Risk factors for mortality of bacteremic patients in the emergency department. Acad Emerg Med 2009; 16:749–55. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.