Abstract
Background
To investigate the impact of station 3A lymph node dissection (LND) on overall survival (OS) and disease‐free survival (DFS) in completely resected right‐side non‐small‐cell lung cancer (NSCLC) patients.
Methods
A total of 1661 cases with completely resected right‐side NSCLC were included. Propensity score matching (PSM) was performed to minimize selection bias, and a logistic regression model was conducted to investigate the risk factors associated with station 3A lymph node metastasis (LNM). The Kaplan–Meier method and Cox proportional hazards model were used to evaluate the impact of station 3A LND on survival.
Results
For the entire cohort, 503 patients (30.3%) underwent station 3A LND. Of those, 11.3% (57/503) presented station 3A LNM. Univariate and multivariate logistic analyses showed that station 10 LNM, tumor location, and the number of resected lymph nodes were independent risk factors associated with station 3A LNM. Before PSM, patients with station 3A LND had worse 5‐year OS (p = 0.002) and DFS (p = 0.011), and more drainage on postoperative day 1 (p = 0.041) than those without. After PSM, however, station 3A LND was not associated with the 5‐year OS (65.7% vs. 63.6%, p = 0.432) or DFS (57.4% vs. 56.0%, p = 0.437). The multivariate analysis further confirmed that station 3A LND was not a prognostic factor (OS, p = 0.361; DFS, p = 0.447).
Conclusions
Station 3A LND could not improve long‐term outcomes and it was unnecessary to dissect station 3A lymph nodes during surgery of right‐side NSCLC.
Keywords: mediastinal lymph node dissection, non‐small‐cell lung cancer, prognosis
Patients with 3A lymph node dissection (LND) had a worse survival before propensity score matching (PSM). Station 3A LND had no impact on survival after PSM. Therefore, station 3A LND was unnecessary during surgery for right‐side non‐small‐cell lung cancer.

INTRODUCTION
Anatomical lobectomy combined with mediastinal lymph node dissection (MLND) has been confirmed as the standard surgical treatment for early‐stage non‐small‐cell lung cancer (NSCLC). 1 , 2 , 3 , 4 , 5 It has been widely accepted that MLND could improve staging accuracy and long‐term survival. 6 , 7 , 8 However, the optimal extent of MLND has not yet been well determined. 9 , 10 , 11 , 12 , 13 According to clinical guidelines, at least three mediastinal lymph node stations (including station 7) should be examined, but which station except for station 7 should be routinely resected has not been well elucidated. 3 , 4 , 5
In our previous studies, we demonstrated that the metastasis of station 3A in operatable right‐side NSCLC was not rare (11.7%), 14 even in tumors ≤3 cm (9.6%), 15 which indicated that station 3A LND might be helpful to improve long‐term outcomes in these patients. Unfortunately, strong evidence on this issue is lacking, and station 3A LND is often neglected by thoracic surgeons in real‐world practice. 14 , 15 , 16 It is therefore very important to investigate if station 3A LND is essential for surgical resection of right‐side NSCLCs.
In this study, we used a large cohort of patients with right‐side NSCLC to investigate the impact of station 3A LND on accurate staging and long‐term survival. The large number, long‐term follow‐up, and propensity score matching (PSM) between groups improve the reliability of this study.
METHODS
Patient selection
We used a database that included 5346 patients with operable NSCLC who underwent consecutive pulmonary resections at Sun Yat‐sen University Cancer Center from January 2001 to December 2014, as previously described. 14 , 15 , 17 The authenticity of this article has been validated by uploading the key raw data on the Research Data Deposit (RDD) public platform (www.researchdata.org.cn), with approval of RDD number RDDA2021002024 authorized by the Institutional Review Board of Sun Yat‐sen University Cancer Center. The inclusion criteria were as follows: (i) patients presented with primary right‐side NSCLC; (ii) complete resection was performed based on the International Association for the Study of Lung Cancer (IASLC) criteria 18 ; and (iii) the tumor pathological stage was T1‐4N0‐2M0. Patients with the following characteristics were excluded: (i) other concurrent or previous primary cancers; (ii) preoperative neoadjuvant therapy; (iii) sublobar resection (including segmentectomy and wedge resection); and (iv) operative mortality. Operative mortality was defined as death within 30 days of operation or at any time after the operation if the patient did not leave the hospital alive. 19 Finally, 1661 patients were included in this study. These patients were further divided into two groups: patients with station 3A LND (station 3A LND+ group) and patients without station 3A LND (station 3A LND− group). The 8th edition of the lung cancer stage classification system of the American Joint Committee on Cancer was used to restage all of these patients. 20
Preoperative evaluations for staging have been described elsewhere. 15 , 17 All patients underwent preoperative evaluation that included a chest computed tomography (CT), brain CT, or magnetic resonance imaging, abdominal ultrasonography or CT scan, and bone scan. Positron emission tomography (PET) was not routinely performed since it is not covered by medical insurance in mainland China. For those patients who underwent CT scans or PET scans of mediastinal lymph nodes that yielded a positive result, an endobronchial ultrasound biopsy or mediastinoscopic examination was recommended. 15 , 17
Assessment of MLNM
Mediastinal lymph nodes were grouped into different “stations” and “zones” (upper zone [stations 2R, 3A, and 4R], subcarinal [SC] zone [station 7], and lower zone [stations 8 and 9]) based on the IASLC lymph node map. 21 The MLNM rate of a certain station/zone was defined as the number of patients whose lymph nodes in this station/zone were involved divided by the number of patients whose lymph nodes in this station/zone were resected for examination, as we described previously. 14 , 15 , 17 The MLNM rates of each station/zone were calculated and compared between different lobes. Furthermore, the clinical characteristics associated with MLNM were identified to determine which type of patients were more likely to have N2 disease.
Follow‐up
In general, follow‐up examinations were recommended every 3 months for the first 2 years, every 6 months for the next 3–5 years, and once a year thereafter. At each follow‐up visit, a physical examination, serum tumor marker test, spiral contrast‐enhanced chest CT scan, and abdominal sonography were carried out. If the patient had specific symptoms, the examination was performed as soon as possible for a more careful assessment. 15 , 17 Abdomen CT scans, bone scans, and brain magnetic resonance imaging scans were carried out when clinically indicated. Follow‐up information was last updated in April 2019 or on the date of death. Patients without an event were censored at the time last known to be alive. The median time from the date of surgery to the last contact with the patients was 55 months (range 1–210 months). During the follow‐up period, 42.6% (708/1661) of included patients occurred death/recurrence and 5.3% (88/1661) of included patients were lost to follow‐up.
PSM analysis
PSM analysis was used to minimize selection bias. 22 A logistic regression model that included sex, age, tumor location, anatomical type, smoking history, comorbidity, surgical resection, surgical approach, histology, cell differentiation, adjuvant therapy, complications, pT category, pN category, and resected lymph node number was used to calculate the propensity score of each case. Patients from the two groups (station 3A LND+ group vs. station 3A LND− group) were matched in a 1:1 ratio according to propensity scores using a nearest‐neighbor approach with caliper restrictions. R version 4.0.2 software (Bell Laboratories, https://cran.r-project.org/bin/windows/base/R-4.0.2-win.exe) was used to perform the PSM analysis.
Statistical analysis
Continuous variables were compared between groups by the Mann–Whitney U‐test. The Pearson test was used to determine significant differences between groups for categorical variables. Univariate and multivariate logistic regression models were used to reveal the clinical factors associated with station 3A LNM. The variates whose p < 0.15 in the univariate logistic regression model were further included in the multivariate logistic regression model. Overall survival (OS) is the time between the date of surgery and the date of death. Disease‐free survival (DFS) is the time from surgery until recurrence or death from any cause. The Kaplan–Meier method was used to assess OS and DFS. The log‐rank test was used to compare the differences in OS and DFS between groups. The Cox proportional hazards regression model was used to determine independent prognostic factors impacting OS and DFS. IBM SPSS Statistics (version 25.0, IBM Corp.) was used to conduct all statistical analyses. Two‐sided p < 0.05 was considered statistically significant.
RESULTS
Patient characteristics
A total of 1661 patients were included in this study, and 494 pairs were successfully matched. The clinicopathological characteristics for both the entire cohort and matched cohorts are summarized in Table 1. For the entire cohort, 30.3% (503/1661) of patients underwent 3A LND. Patients with 3A LND were more likely to be older (p = 0.003), have pathological N2 disease (p = 0.004), have tumors in the middle lobes (p = 0.021), present other pathological types than adenocarcinoma or squamous cell carcinoma (p = 0.023), present central‐type NSCLC (p = 0.024), have more drainage on postoperative day 1 (p = 0.041), and have more lymph nodes harvested (p < 0.001) (Table 1). Other clinicopathological characteristics were well balanced between groups. After PSM, all of the clinicopathological characteristics were comparable between the 3A LND+ group and the 3A LND− group (Table 1).
TABLE 1.
General clinicopathological characteristics of patients with and without station 3A LND before and after propensity score matching
| Characteristics | Entire cohort (N = 1661) | p | Propensity score matching (N = 988) | p | ||
|---|---|---|---|---|---|---|
| Without 3A LND (N = 1158) | With 3A LND (N = 503) | Without 3A LND (N = 494) | With 3A LND (N = 494) | |||
| Sex | 0.254 | 0.790 | ||||
| Male | 786 (67.9) | 327 (65.0) | 318 (64.4) | 322 (65.2) | ||
| Female | 372 (32.1) | 176 (35.0) | 176 (35.6) | 172 (34.8) | ||
| Age (years) | 0.003 | 0.564 | ||||
| ≤60 | 588 (50.8) | 216 (42.9) | 233 (45.1) | 214 (43.3) | ||
| >60 | 570 (49.2) | 287 (57.1) | 271 (54.9) | 280 (56.7) | ||
| Mean ± SD | 58.9 ± 9.8 | 60.1 ± 8.9 | 0.025 a | 59.5 ± 9.8 | 60.0 ± 8.9 | 0.844 a |
| Median (min., max.) | 59.0 (25, 81) | 61.0 (24, 81) | 61.0 (25, 79) | 60.5 (24, 81) | ||
| Pathological stage | 0.003 | 0.719 | ||||
| I | 542 (46.8) | 208 (41.4) | 198 (40.1) | 206 (41.7) | ||
| II | 259 (22.4) | 97 (19.3) | 107 (21.7) | 97 (19.6) | ||
| III | 357 (30.8) | 198 (39.4) | 189 (38.3) | 191 (38.7) | ||
| Pathological T category | 0.238 | 0.726 | ||||
| T1 | 370 (32.0) | 152 (30.2) | 155 (31.4) | 151 (30.6) | ||
| T2 | 541 (46.7) | 228 (45.3) | 224 (45.3) | 225 (45.5) | ||
| T3 | 175 (15.1) | 78 (15.5) | 80 (16.2) | 74 (15.0) | ||
| T4 | 72 (6.2) | 45 (8.9) | 35 (7.1) | 44 (8.9) | ||
| Pathological N category | 0.004 | 0.671 | ||||
| N0 | 743 (64.2) | 287 (57.1) | 277 (56.1) | 285 (57.7) | ||
| N1 | 139 (12.0) | 57 (11.3) | 64 (13.0) | 55 (11.1) | ||
| N2 | 276 (23.8) | 159 (31.6) | 153 (31.0) | 154 (31.2) | ||
| Tumor location | 0.021 | 0.455 | ||||
| RUL | 619 (53.5) | 259 (51.5) | 270 (54.7) | 256 (51.8) | ||
| RML | 126 (10.9) | 79 (15.7) | 64 (13.0) | 77 (15.6) | ||
| RLL | 413 (35.7) | 165 (32.8) | 160 (32.4) | 161 (32.6) | ||
| Anatomical type | 0.024 | 0.927 | ||||
| Central | 127 (11.0) | 75 (14.9) | 69 (14.0) | 70 (14.2) | ||
| Peripheral | 1031 (89.0) | 428 (85.1) | 425 (86.0) | 424 (85.8) | ||
| Smoking history | 0.528 | 0.848 | ||||
| Never | 549 (47.4) | 230 (45.7) | 223 (45.1) | 226 (45.7) | ||
| Current/former | 609 (52.6) | 273 (54.3) | 271 (54.9) | 268 (54.3) | ||
| Comorbidity | 0.981 | 0.949 | ||||
| No | 574 (49.6) | 249 (49.5) | 243 (49.2) | 244 (49.4) | ||
| Yes | 584 (50.4) | 254 (50.5) | 251 (50.8) | 250 (50.6) | ||
| Surgical approach | 0.590 | 0.458 | ||||
| Thoracotomy | 851 (73.5) | 376 (74.8) | 379 (76.7) | 369 (74.7) | ||
| Thoracoscopic | 307 (26.5) | 127 (25.2) | 115 (23.3) | 125 (25.3) | ||
| Surgical resection | 0.055 | 0.921 | ||||
| Lobectomy | 991 (85.6) | 408 (81.1) | 398 (80.6) | 403 (81.6) | ||
| Bilobectomy | 136 (11.7) | 74 (14.7) | 77 (15.6) | 73 (14.8) | ||
| Pneumonectomy | 31 (2.7) | 21 (4.2) | 19 (3.8) | 18 (3.6) | ||
| Histology | 0.023 | 0.561 | ||||
| Adenocarcinoma | 770 (66.5) | 321 (63.8) | 303 (61.3) | 317 (64.2) | ||
| Squamous cell carcinoma | 317 (27.4) | 132 (26.2) | 143 (28.9) | 128 (25.9) | ||
| Others | 71 (6.1) | 50 (9.9) | 48 (9.7) | 49 (9.9) | ||
| Cell differentiation | 0.495 | 0.949 | ||||
| Well | 76 (6.6) | 34 (6.8) | 32 (6.5) | 34 (6.9) | ||
| Moderate | 477 (41.2) | 187 (37.2) | 191 (38.7) | 184 (37.2) | ||
| Poor | 539 (46.5) | 251 (49.9) | 238 (48.2) | 245 (49.6) | ||
| NA | 66 (5.7) | 31 (6.2) | 33 (6.7) | 31 (6.3) | ||
| Tumor size (cm) | 0.164 a | 0.869 a | ||||
| Mean ± SD | 3.6 ± 2.0 | 3.8 ± 2.1 | 3.7 ± 2.0 | 3.8 ± 2.1 | ||
| Median (min., max.) | 3.0 (0.5, 14.0) | 3.0 (0.3, 13.0) | 3.0 (0.5, 14.0) | 3.0 (0.3, 13.0) | ||
| Adjuvant therapy | 0.220 | 0.949 | ||||
| No | 657 (56.7) | 269 (53.5) | 263 (53.2) | 262 (53.0) | ||
| Yes | 501 (43.3) | 234 (46.5) | 231 (46.8) | 232 (47.0) | ||
| Complication | 0.920 | 0.533 | ||||
| No | 1079 (93.2) | 468 (93.0) | 457 (92.5) | 462 (93.5) | ||
| Yes | 79 (6.8) | 35 (7.0) | 37 (7.5) | 32 (6.5) | ||
| No. of resected TLNs | <0.001 a | 0.688 a | ||||
| Mean ± SD | 22.3 ± 10.0 | 25.0 ± 11.2 | 24.8 ± 10.8 | 24.7 ± 10.9 | ||
| Median (min, max) | 21.0 (4, 84) | 23.0 (5, 78) | 23.0 (4, 77) | 23.0 (5, 78) | ||
| No. of resected MLNs | <0.001 a | 0.478 a | ||||
| Mean ± SD | 15.0 ± 7.7 | 17.7 ± 8.5 | 17.3 ± 8.7 | 17.4 ± 8.0 | ||
| Median (min, max) | 14.0 (3, 60) | 17.0 (3, 67) | 16.0 (3, 55) | 16.0 (3, 52) | ||
| Drainage of POD1 (ml) | 0.041 | 0.145 | ||||
| Mean ± SD | 481.3 ± 238.4 | 516.1 ± 281.5 | 484.3 ± 231.9 | 515.9 ± 282.7 | ||
| Median (min, max) | 460.0 (0, 2410) | 500.0 (0, 2710) | 460.0 (0, 1450) | 500.0 (0, 2710) | ||
| Hospital stays (days) | 0.851 | 0.239 | ||||
| Mean ± SD | 10.2 ± 6.2 | 12.5 ± 29.3 | 10.7 ± 6.8 | 10.0 ± 5.3 | ||
| Median (min, max) | 9.0 (2, 89) | 9.0 (1, 379) | 9.0 (2, 79) | 9.0 (3, 65) | ||
Abbreviations: LND, lymph node dissection; SD, standard deviations; RUL, right upper lobe; RML, right middle lobe; RLL, right lower lobe; NA, not available, TLNs, total lymph nodes; MLNs, mediastinal lymph nodes; POD1, postoperative day 1.
Mann–Whitney U test.
Assessment of mediastinal lymph node metastases
As predicted, station 4R (17.1%, 233/1365; Table 2) lymph nodes were most likely to metastasize throughout the entire cohort, but the metastasis rate of station 3A lymph nodes was also as high as 11.3% (57/503) for the entire cohort. The most common sites of MLNM for tumors in the right upper lobe (RUL), right middle lobe (RML), and right lower lobe (RLL) were station 4R (21.6%, 160/742), station 7 (22.0%, 45/205), and station 7 (23.2%, 134/578) lymph nodes, respectively (Table 2). RUL and RML tumors were more likely to have upper zone (station 2R/3A/4R) MLNM than RLL tumors (p < 0.001; Table 2). RLL tumors were more likely to have lower zone (station 8/9) MLNM than tumors in other lobes (p < 0.001; Table 2). It is not surprising that RML and RLL tumors had a significantly higher rate of station 7 MlNM than RUL tumors (p < 0.001; Table 2).
TABLE 2.
Occurrence and distribution of MLNM by lymph node stations and zones for the entire cohort stratified by tumor location (N = 1661)
| Stations/zones | Lymph node metastatic rate % (involved/resected) | p | |||
|---|---|---|---|---|---|
| Total | RUL | RML | RLL | ||
| Station 2R | 11.6 (176/1516) | 13.2 (108/817) | 13.0 (24/184) | 8.5 (44/515) | 0.028 |
| Station 3A | 11.3 (57/503) | 12.7 (33/259) | 17.7 (14/79) | 6.1 (10/165) | 0.016 |
| Station 4R | 17.1 (233/1365) | 21.6 (160/742) | 14.6 (25/171) | 10.6 (48/452) | <0.001 |
| Station 7 (SC zone) | 12.5 (208/1661) | 3.3 (29/878) | 22.0 (45/205) | 23.2 (134/578) | <0.001 |
| Station 8 | 3.7 (7/189) | 1.2 (1/86) | 0 (0/20) | 7.2 (6/83) | 0.045 a |
| Station 9 | 4.5 (35/780) | 0.8 (3/377) | 2.8 (2/72) | 9.1 (30/331) | <0.001 |
| Upper zone | 19.1 (316/1651) | 23.8 (208/873) | 20.5 (42/205) | 11.5 (66/573) | <0.001 |
| Lower zone | 4.6 (40/877) | 1.0 (4/420) | 2.3 (2/88) | 9.2 (34/369) | <0.001 |
Abbreviations: MLNM, mediastinal lymph node metastasis; RUL, right upper lobe; RML, right middle lobe; RLL, right lower lobe.
Fisher's exact test.
Furthermore, we calculated the single‐station MLNM rate for each station in all patients with N2 disease. As Supporting Information Table S1, online only) shows, the incidence of single station 3A LNM was only 3.0% (13/436), which was much lower than that of station 2R (9.9%, 43/436), station 4R (20.2%, 88/436), and station 7 (22.2%, 97/436) LNM. Moreover, in 57 patients with station 3A LNM, 44 patients (77.2%) showed multistation MLNM and only 13 patients (22.8%) did not show MLNM in other stations.
Risk factors for station 3A LNM
To identify the risk factors influencing station 3A LNM, we performed univariate and multivariate logistic regression models. As showed in Supporting Information Table S2 (online only), a univariate logistic model found that tumor location, surgical approach, station 2R/4R/7/8/9/10 LNM, the number of examined N2 stations, and the number of examined N2 lymph nodes were associated with station 3A LNM. The multivariate logistic model further confirmed that tumor location (RML: odds ratio [OR] = 6.442, 95% confidence interval [CI], 1.315–31.569, p = 0.022), station 4R LNM (OR = 33.544, 95% CI, 6.728–167.233, p < 0.001), station 7 LNM (OR = 8.783, 95% CI, 1.933–39.907, p = 0.005), and the number of examined N2 lymph nodes (OR = 0.895, 95% CI, 0.817–0.982, p = 0.019) were all independent risk factors associated with station 3A LNM (Supporting Information Table S2, online only).
Survival comparison
Before PSM, it was surprising that patients with 3A LND had a significantly worse OS (5‐year OS 70.1% vs. 63.1%, p = 0.002; Figure 1a) and DFS (5‐year DFS 61.7% vs. 55.7%, p = 0.011; Figure 1b) than those without 3A LND. Multivariate analysis suggested that 3A LND was an independent factor associated with worse OS (hazard ratio [HR] = 1.214, 95% CI, 1.016–1.452, p = 0.033; Table 3) but not DFS (HR = 1.162, 95% CI, 0.988–1.367, p = 0.070; Table 3).
FIGURE 1.
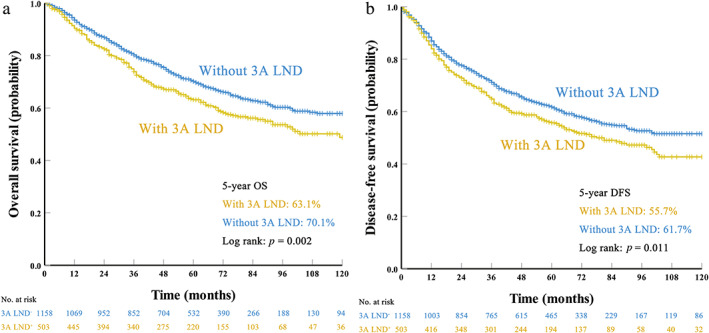
Survival curves for patients with and without station 3A lymph node dissection (LND) before propensity score matching: (a) overall survival (OS) and (b) disease‐free survival (DFS)
TABLE 3.
Multivariate Cox regression analyses for prognostic factors before propensity score matching (N = 1661)
| Characteristics | OS | DFS | ||
|---|---|---|---|---|
| HR (95% CI) | p | HR (95% CI) | p | |
| Sex | ||||
| Male | Ref | Ref | ||
| Female | 0.859 (0.664–1.111) | 0.247 | 0.924 (0.737–1.160) | 0.497 |
| Age (years) | ||||
| ≤60 | Ref | Ref | ||
| >60 | 1.369 (1.153–1.625) | <0.001 | 1.190 (1.019–1.389) | 0.028 |
| Tumor location | ||||
| RUL | Ref | Ref | ||
| RML | 1.231 (0.939–1.613) | 0.133 | 1.106 (0.865–1.415) | 0.422 |
| RLL | 1.222 (1.011–1.477) | 0.038 | 1.187 (1.002–1.405) | 0.048 |
| Anatomical type | ||||
| Central | Ref | Ref | ||
| Peripheral | 0.785 (0.611–1.010) | 0.059 | 0.852 (0.673–1.079) | 0.184 |
| Smoking history | ||||
| Never | Ref | Ref | ||
| Current/Former | 1.314 (1.040–1.659) | 0.022 | 1.336 (1.083–1.648) | 0.007 |
| Comorbidity | ||||
| No | Ref | Ref | ||
| Yes | 0.892 (0.754–1.054) | 0.180 | 1.012 (0.870–1.178) | 0.877 |
| Surgical resection | ||||
| Lobectomy | Ref | Ref | ||
| Bilobectomy | 0.983 (0.760–1.272) | 0.898 | 1.056 (0.836–1.335) | 0.646 |
| Pneumonectomy | 0.869 (0.559–1.351) | 0.532 | 0.888 (0.582–1.355) | 0.581 |
| Histology | ||||
| Adenocarcinoma | Ref | Ref | ||
| Squamous cell carcinoma | 0.792 (0.643–0.976) | 0.028 | 0.678 (0.558–0.824) | <0.001 |
| Others | 0.840 (0.601–1.174) | 0.308 | 0.843 (0.622–1.142) | 0.270 |
| Cell differentiation | ||||
| Well | Ref | Ref | ||
| Moderate | 1.328 (0.851–2.072) | 0.212 | 1.415 (0.954–2.097) | 0.084 |
| Poor | 1.817 (1.170–2.820) | 0.008 | 1.608 (1.086–2.381) | 0.018 |
| NA | 1.384 (0.780–2.457) | 0.267 | 1.349 (0.815–2.231) | 0.244 |
| Adjuvant therapy | ||||
| No | Ref | Ref | ||
| Yes | 0.814 (0.677–0.978) | 0.028 | 0.978 (0.830–1.153) | 0.793 |
| Pathological T category | ||||
| T1 | Ref | Ref | ||
| T2 | 1.419 (1.143–1.761) | 0.001 | 1.351 (1.117–1.634) | 0.002 |
| T3 | 2.072 (1.593–2.694) | <0.001 | 2.012 (1.586–2.552) | <0.001 |
| T4 | 2.078 (1.489–2.899) | <0.001 | 1.904 (1.404–2.583) | <0.001 |
| Pathological N category | ||||
| N0 | Ref | Ref | ||
| N1 | 1.477 (1.120–1.947) | 0.006 | 1.559 (1.224–1.987) | <0.001 |
| N2 | 2.755 (2.259–3.360) | <0.001 | 2.400 (2.005–2.872) | <0.001 |
| Postoperative complications | ||||
| No | Ref | Ref | ||
| Yes | 1.221 (0.908–1.643) | 0.187 | 1.208 (0.918–1.590) | 0.177 |
| No. of resected N1 LNs | 1.003 (0.985–1.022) | 0.732 | 1.005 (0.988–1.022) | 0.555 |
| No. of resected N2 LNs | 0.995 (0.985–1.006) | 0.361 | 0.992 (0.982–1.001) | 0.093 |
| 3A dissection | ||||
| 3A LND− | Ref | Ref | ||
| 3A LND+ | 1.214 (1.016–1.452) | 0.033 | 1.162 (0.988–1.367) | 0.070 |
Abbreviations: OS, overall survival; DFS, disease‐free survival; HR, hazard ratio; CI, confidence interval; Ref, reference; RUL, right upper lobe; RML, right middle lobe; RLL, right lower lobe; NA, not available; LND, lymph node dissection.
After PSM, however, the 5‐year OS rates (3A LND− group vs. 3A LND+ group 65.7% vs. 63.6%, p = 0.432; Figure 2a) and DFS rates (3A LND− group vs. 3A LND+ group 57.4% vs. 56.0% p = 0.437; Figure 2b) were comparable between the 3A LND+ group and the 3A LND− group. Multivariate analyses also confirmed that 3A LND had no impact on OS (HR = 1.093, 95% CI, 0.890–1.341, p = 0.397; Table 4) or DFS (HR = 1.071, 95% CI, 0.888–1.291, p = 0.473; Table 4).
FIGURE 2.
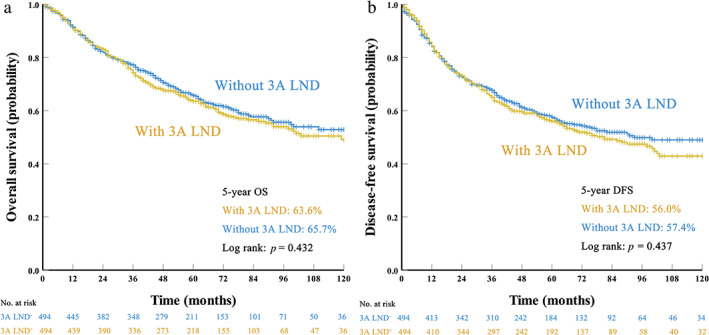
Survival curves for patients with and without station 3A lymph node dissection (LND) after propensity score matching: (a) overall survival (OS) and (b) disease‐free survival (DFS)
TABLE 4.
Multivariate Cox regression analyses for prognostic factors after propensity score matching (N = 988)
| Characteristics | OS | DFS | ||
|---|---|---|---|---|
| HR (95% CI) | p | HR (95% CI) | p | |
| Sex | ||||
| Male | Ref | Ref | ||
| Female | 0.979 (0.700–1.368) | 0.899 | 0.976 (0.723–1.316) | 0.871 |
| Age (years) | ||||
| ≤60 | Ref | Ref | ||
| >60 | 1.354 (1.090–1.681) | 0.006 | 1.160 (0.952–1.413) | 0.141 |
| Tumor location | ||||
| RUL | Ref | Ref | ||
| RML | 1.277 (0.930–1.754) | 0.131 | 1.090 (0.812–1.463) | 0.565 |
| RLL | 1.310 (1.027–1.671) | 0.030 | 1.225 (0.982–1.527) | 0.072 |
| Anatomical type | ||||
| Central | Ref | Ref | ||
| Peripheral | 0.732 (0.541–0.991) | 0.043 | 0.831 (0.624–1.106) | 0.204 |
| Smoking history | ||||
| Never | Ref | Ref | ||
| Current/Former | 1.657 (1.211–2.269) | 0.002 | 1.548 (1.166–2.053) | 0.002 |
| Comorbidity | ||||
| No | Ref | Ref | ||
| Yes | 0.860 (0.698–1.061) | 0.159 | 1.024 (0.845–1.242) | 0.806 |
| Surgical resection | ||||
| Lobectomy | Ref | Ref | ||
| Bilobectomy | 1.013 (0.745–1.376) | 0.935 | 1.089 (0.820–1.447) | 0.557 |
| Pneumonectomy | 0.840 (0.501–1.408) | 0.508 | 0.871 (0.532–1.426) | 0.583 |
| Histology | ||||
| Adenocarcinoma | Ref | Ref | ||
| Squamous cell carcinoma | 0.697 (0.537–0.904) | 0.007 | 0.625 (0.489–0.799) | <0.001 |
| Others | 0.893 (0.621–1.285) | 0.542 | 0.921 (0.661–1.283) | 0.626 |
| Cell differentiation | ||||
| Well | Ref | Ref | ||
| Moderate | 1.329 (0.761–2.323) | 0.318 | 1.517 (0.903–2.549) | 0.115 |
| Poor | 1.957 (1.128–3.393) | 0.017 | 1.831 (1.094–3.066) | 0.021 |
| NA | 1.495 (0.741–3.016) | 0.261 | 1.473 (0.774–2.802) | 0.238 |
| Adjuvant therapy | ||||
| No | Ref | Ref | ||
| Yes | 0.851 (0.677–1.071) | 0.170 | 0.961 (0.779–1.186) | 0.712 |
| Pathological T category | ||||
| T1 | Ref | Ref | ||
| T2 | 1.397 (1.070–1.825) | 0.014 | 1.352 (1.064–1.717) | 0.014 |
| T3 | 1.997 (1.441–2.767) | <0.001 | 1.835 (1.358–2.480) | <0.001 |
| T4 | 1.927 (1.292–2.873) | 0.001 | 1.806 (1.249–2.611) | 0.002 |
| Pathological N category | ||||
| N0 | Ref | Ref | ||
| N1 | 1.268 (0.883–1.820) | 0.198 | 1.551 (1.133–2.124) | 0.006 |
| N2 | 2.474 (1.932–3.168) | <0.001 | 2.430 (1.933–3.054) | <0.001 |
| Postoperative complications | ||||
| No | Ref | Ref | ||
| Yes | 1.202 (0.827–1.747) | 0.335 | 1.112 (0.779–1.585) | 0.559 |
| No. of resected N1 LNs | 1.005 (0.982–1.028) | 0.670 | 1.007 (0.986–1.028) | 0.541 |
| No. of resected N2 LNs | 0.989 (0.976–1.002) | 0.099 | 0.988 (0.976–1.000) | 0.052 |
| 3A dissection | ||||
| 3A LND− | Ref | Ref | ||
| 3A LND+ | 1.100 (0.896–1.352) | 0.361 | 1.076 (0.892–1.297) | 0.447 |
Abbreviations: OS, overall survival; DFS, disease‐free survival; HR, hazard ratio; CI, confidence interval; Ref, reference; RUL, right upper lobe; RML, right middle lobe; RLL, right lower lobe; NA, not available; LND, lymph node dissection.
To identify which group of patients might benefit from station 3A LND after PSM, subgroup analyses based on age, tumor location, anatomical type, smoking history, histology, cell differentiation, pathological T category, and pathological N category were further performed. Unfortunately, the subgroup analyses suggested that station 3A LND had no impact on either OS or DFS in any subset of the matched cohort (all p > 0.05; Supporting Information Table S3, online only).
DISCUSSION
Although anatomical lobectomy combined with MLND has been widely accepted as the standard surgical treatment for early‐stage NSCLC, there is no uniform standard of the extent of MLND. 3 , 4 , 5 Therefore, the decision to dissect specific mediastinal lymph nodes in clinical practice is mainly dependent on individual surgeons' experience and preference. 18 In our previous studies, we demonstrated that station 3A LNM occurred in 11.7% (76/648) of right‐side NSCLC patients who underwent complete resection, 14 and even in patients whose tumors were no more than 3 cm, 9.6% of patients still had station 3A LNM. 15 Nevertheless, previous studies, including ours, suggested that in clinical practice, only approximately 30% of patients underwent station 3A LND during surgery. 14 , 16 , 23 To the best of our knowledge, only one study retrospectively investigated the prognostic significance of station 3A LND using data from a single institution. 23 It is therefore important to clarify the clinical significance of station 3A LND in right‐side NSCLC patients who underwent surgical resection.
In this study, our data suggested that station 3A LNM occurred in 11.3% of right‐side NSCLC patients who underwent complete resection, which is similar to the findings of previous studies. 14 , 15 , 23 The data also showed that in patients with station 3A LNM, 77.2% had multistation MLNM while only 22.8% did not have MLNM of other stations. These findings suggested that the occurrence of station 3A LNM corresponded to widespread disease. Interestingly, the data suggested that station 3A LND had no impact on either OS or DFS in the matched cohorts, and this result was also confirmed by the multivariate analyses and subgroup analyses. One possible explanation for this result is that 3A LNM suggests a widespread disease that requires multimodality therapy, and the role of surgical resection as a local treatment in these patients was compromised. Moreover, our data showed that patients with 3A LND had significantly more drainage on postoperative day 1 than those without 3A LND and after PSM patients with 3A LND still had an average of 31.6 ml more drainage than those without. Based on these findings, we suggest that the dissection of station 3A lymph nodes itself is not associated with long‐term outcomes and it is unnecessary to dissect station 3A lymph nodes during the surgery for right‐side NSCLC.
The MLNM pattern of right‐side NSCLC has also been assessed by previous studies. 16 , 23 , 24 Riquet et al. performed a retrospective study that included 1779 NSCLC patients who underwent lobectomy combined with MLND. 16 Their results showed that 15.4% (159/1035) of patients with right‐side NSCLC had N2 diseases and 120 patients (11.6%, 120/1035) had single‐station LNM. Among the cases of single‐station involvement, only two patients (1.7%) were diagnosed with station 3A LNM. In contrast, 80 (66.7%) patients with single‐station involvement had station 2R/4R LNM and 36 (30.0%) patients had single‐station 7 LNM, which was similar to our results. Liu et al. performed retrospective research using PSM analysis to investigate the impact of station 3A LND on long‐term survival in patients with right‐side NSCLC. 23 They found that the metastasis rate of station 3A lymph nodes was 15.3% (87/570), and this value was second only to that of station 4 LNM (17.3%, 287/1660). Our data in this study also demonstrated that station 3A LNM was not rare (11.3%) but it was more likely to detect multistation MLNM when station 3A LNM occurred, indicating the limited value of station 3A LND for improving long‐term survival.
To the best of our knowledge, only one previous study has focused on the impact of station 3A LND on long‐term outcomes. 23 Liu and his colleagues demonstrated that patients with station 3A LND showed higher DFS (5‐year DFS, 52.4% vs. 37.1%, p = 0.001) and OS (5‐year OS, 58.8% vs. 48.7%, p = 0.007) than those without station 3A LND. 23 The results of their study were not consistent with ours. It is worth noting that the PSM analysis in Liu et al.’s study did not include the number of resected lymph nodes in the propensity score model. While it had already been confirmed that the number of resected lymph nodes was intensely associated with long‐term outcomes, 25 , 26 excluding the number of resected lymph nodes as a variable in PSM may cause bias when comparing the survival prognosis in their study. In our series, the data showed that the number of resected lymph nodes in the station 3A LND+ group was significantly larger than that in the station 3A LND− group (p < 0.001). We therefore believe that including the number of resected lymph nodes in the propensity score model to balance this variable between groups is very important. In addition, as Liu et al. admitted in their article, the follow‐up period of their research was relatively short (median follow‐up of 33 months), and further follow‐up was essential to draw a reliable conclusion. In this study, the median follow‐up period was as long as 55 months, which was much longer than that in their study. We believe that the long follow‐up period and inclusion of the number of resected nodes as a variable in the PSM model improved the reliability of the results of this study, and it may also explain the discrepancies between Liu et al.’s study and ours.
Our study also has its limitations. First, this is a single‐center retrospective study and bias may also exist due to the retrospective nature. For example, patients with station 3A LND were more likely to have more lymph nodes resected, but we could not know the exact reason for this. One possible explanation might be that the surgeons who preferred to dissect station 3A lymph nodes during surgery were more likely to perform extensive systematic MLND. Second, although the PSM method was used to minimize selection bias, this method could only control confounding factors that were already identified, but other unknown factors may impact survival. In short, well‐designed prospective clinical trials are warranted to testify our results.
In conclusion, our results indicated that patients with station 3A LNM were more likely to have multistation MLNM and station 3A LND could not improve long‐term outcomes. We therefore propose that station 3A LND was unnecessary in the surgical treatment of right‐side NSCLC.
DISCLOSURE
The author has no conflicts of interest to declare.
Supporting information
Supporting Information Table S1: Occurrence of Single Station MLNM for Patients with N2 Disease Stratified by Tumor Locations (N = 436).
Supporting Information Table S2: Univariate and Multivariate Analyses of the Risk Factors for Station 3A LNM (N = 503).
Supporting Information Table S3: Subgroup Survival Analyses between Patients with and without Station 3A LND after Propensity Score Matching (N = 988).
ACKNOWLEDGMENTS
This work was supported by the Sun Yat‐sen University Clinical Research 5010 Program (2019012, ChiCTR2000034737), the Natural Science Foundation of Guangdong Province (2018A030313410, 2020A151501311), the National Natural Science Foundation of China (82072572), the Sun Yat‐sen University Young Teacher Plan (19ykpy179), and the Guangzhou Science and Technology Program (202002020074, 202103000023).
Yang M‐Z, Tan Z‐H, Li J‐B, Long H, Fu J‐H, Zhang L‐J, et al. Station 3A lymph node dissection does not improve long‐term survival in right‐side operable non‐small‐cell lung cancer patients: A propensity score matching study. Thorac Cancer. 2022;13(15):2106–2116. 10.1111/1759-7714.14456
†These authors contributed equally to this paper and share the first authorship.
The main content of this paper has been accepted for e‐poster presentation by the 29th European Conference on General Thoracic Surgery on 20–22 June 2021 in virtual format.
Funding information Guangzhou Science and Technology Program, Grant/Award Numbers: 202002020074, 202103000023; Sun Yat‐sen University Clinical Research 5010 Program, Grant/Award Number: 2019012; Sun Yat‐sen University Young Teacher Plan, Grant/Award Number: 19ykpy179; National Natural Science Foundation of China, Grant/Award Number: 82072572; Natural Science Foundation of Guangdong Province, Grant/Award Numbers: 2018A030313410, 2020A151501311
Contributor Information
Xue Hou, Email: houxue@sysucc.org.cn.
Hao‐Xian Yang, Email: yanghx@sysucc.org.cn.
REFERENCES
- 1. Ginsberg RJ, Rubinstein LV. Randomized trial of lobectomy versus limited resection for T1 N0 non‐small cell lung cancer. Lung Cancer Study Group. Ann Thorac Surg. 1995;60(3):615–22. [DOI] [PubMed] [Google Scholar]
- 2. Lardinois D, De Leyn P, Van Schil P, et al. ESTS guidelines for intraoperative lymph node staging in non‐small cell lung cancer. Eur J Cardiothorac Surg. 2006;30(5):787–92. [DOI] [PubMed] [Google Scholar]
- 3. Howington JA, Blum MG, Chang AC, Balekian AA, Murthy SC. Treatment of stage I and II non‐small cell lung cancer: diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence‐based clinical practice guidelines. Chest. 2013;143(5 Suppl):e278S–313S. [DOI] [PubMed] [Google Scholar]
- 4. National Comprehensive Cancer Network . NCCN guideline for non‐small cell lung cancer. Version 2 NCCN; 2021. [DOI] [PubMed]
- 5. Postmus PE, Kerr KM, Oudkerk M, Senan S, Waller DA, Vansteenkiste J, et al. Early and locally advanced non‐small‐cell lung cancer (NSCLC): ESMO clinical practice guidelines for diagnosis, treatment and follow‐up. Ann Oncol. 2017;28(suppl_4):iv1–iv21. [DOI] [PubMed] [Google Scholar]
- 6. Cerfolio RJ, Bryant AS, Minnich DJ. Complete thoracic mediastinal lymphadenectomy leads to a higher rate of pathologically proven N2 disease in patients with non‐small cell lung cancer. Ann Thorac Surg. 2012;94(3):902–6. [DOI] [PubMed] [Google Scholar]
- 7. Gajra A, Newman N, Gamble GP, Kohman LJ, Graziano SL. Effect of number of lymph nodes sampled on outcome in patients with stage I non‐small‐cell lung cancer. J Clin Oncol. 2003;21(6):1029–34. [DOI] [PubMed] [Google Scholar]
- 8. Doddoli C, Aragon A, Barlesi F, et al. Does the extent of lymph node dissection influence outcome in patients with stage I non‐small‐cell lung cancer? Eur J Cardiothorac Surg. 2005;27(4):680–5. [DOI] [PubMed] [Google Scholar]
- 9. Wu Y, Huang ZF, Wang SY, Yang XN, Ou W. A randomized trial of systematic nodal dissection in resectable non‐small cell lung cancer. Lung Cancer. 2002;36(1):1–6. [DOI] [PubMed] [Google Scholar]
- 10. Darling GE, Allen MS, Decker PA, Ballman K, Malthaner RA, Inculet RI, et al. Number of lymph nodes harvested from a mediastinal lymphadenectomy: results of the randomized, prospective American College of Surgeons oncology group Z0030 trial. Chest. 2011;139(5):1124–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Izbicki JR, Passlick B, Pantel K, Pichlmeier U, Hosch SB, Karg O, et al. Effectiveness of radical systematic mediastinal lymphadenectomy in patients with resectable non‐small cell lung cancer: results of a prospective randomized trial. Ann Surg. 1998;227(1):138–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Murthy SC, Reznik SI, Ogwudu UC, Farver CF, Arrossi A, Batizy LH, et al. Winning the battle, losing the war: the noncurative "curative" resection for stage I adenocarcinoma of the lung. Ann Thorac Surg. 2010;90(4):1067–74. [DOI] [PubMed] [Google Scholar]
- 13. Shapiro M, Kadakia S, Lim J, Breglio A, Wisnivesky JP, Kaufman A, et al. Lobe‐specific mediastinal nodal dissection is sufficient during lobectomy by video‐assisted thoracic surgery or thoracotomy for early‐stage lung cancer. Chest. 2013;144(5):1615–21. [DOI] [PubMed] [Google Scholar]
- 14. Liang RB, Yang J, Zeng TS, Long H, Fu JH, Zhang LJ, et al. Incidence and distribution of lobe‐specific mediastinal lymph node metastasis in non‐small cell lung cancer: data from 4511 resected cases. Ann Surg Oncol. 2018;25(11):3300–7. [DOI] [PubMed] [Google Scholar]
- 15. Yang MZ, Hou X, Liang RB, Lai RC, Yang J, Li S, et al. The incidence and distribution of mediastinal lymph node metastasis and its impact on survival in patients with non‐small‐cell lung cancers 3 cm or less: data from 2292 cases. Eur J Cardiothorac Surg. 2019;56(1):159–66. [DOI] [PubMed] [Google Scholar]
- 16. Riquet M, Rivera C, Pricopi C, Arame A, Mordant P, Foucault C, et al. Is the lymphatic drainage of lung cancer lobe‐specific? A surgical appraisal. Eur J Cardiothorac Surg. 2015;47(3):543–9. [DOI] [PubMed] [Google Scholar]
- 17. Yang MZ, Hou X, Li JB, Cai JS, Yang J, Li S, et al. Impact of L4 lymph node dissection on long‐term survival in left‐side operable non‐small‐cell lung cancer: a propensity score matching study. Eur J Cardiothorac Surg. 2020;57(6):1181–8. [DOI] [PubMed] [Google Scholar]
- 18. Rami‐Porta R, Wittekind C, Goldstraw P. Complete resection in lung cancer surgery: proposed definition. Lung Cancer. 2005;49(1):25–33. [DOI] [PubMed] [Google Scholar]
- 19. Yang HX, Woo KM, Sima CS, Bains MS, Adusumilli PS, Huang J, et al. Long‐term survival based on the surgical approach to lobectomy for clinical stage I non‐small cell lung cancer: comparison of robotic, video‐assisted thoracic surgery, and thoracotomy lobectomy. Ann Surg. 2017;265(2):431–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Goldstraw P, Chansky K, Crowley J, Rami‐Porta R, Asamura H, Eberhardt WEE, et al. The IASLC lung cancer staging project: proposals for revision of the TNM stage groupings in the forthcoming (eighth) edition of the TNM classification for lung cancer. J Thorac Oncol. 2016;11(1):39–51. [DOI] [PubMed] [Google Scholar]
- 21. Rusch VW, Asamura H, Watanabe H, Giroux DJ, Rami‐Porta R, Goldstraw P. The IASLC lung cancer staging project: a proposal for a new international lymph node map in the forthcoming seventh edition of the TNM classification for lung cancer. J Thorac Oncol. 2009;4(5):568–77. [DOI] [PubMed] [Google Scholar]
- 22. D'Agostino RB Jr. Propensity score methods for bias reduction in the comparison of a treatment to a non‐randomized control group. Stat Med. 1998;17(19):2265–81. [DOI] [PubMed] [Google Scholar]
- 23. Liu C, Wei S, Guo C, Mei J, Pu Q, Liu L. Clinical significance of station 3A lymph node dissection in patients with right‐side non‐small‐cell lung cancer: a retrospective propensity‐matched analysis. Ann Surg Oncol. 2021;28(1):194–202. [DOI] [PubMed] [Google Scholar]
- 24. Kotoulas CS, Foroulis CN, Kostikas K, Konstantinou M, Kalkandi P, Dimadi M, et al. Involvement of lymphatic metastatic spread in non‐small cell lung cancer accordingly to the primary cancer location. Lung Cancer. 2004;44(2):183–91. [DOI] [PubMed] [Google Scholar]
- 25. Osarogiagbon RU, Ogbata O, Yu X. Number of lymph nodes associated with maximal reduction of long‐term mortality risk in pathologic node‐negative non‐small cell lung cancer. Ann Thorac Surg. 2014;97(2):385–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Liang W, He J, Shen Y, Shen J, He Q, Zhang J, et al. Impact of examined lymph node count on precise staging and long‐term survival of examined non‐small‐cell lung cancer: a population study of the US SEER database and a Chinese multi‐institutional registry. J Clin Oncol. 2017;35(11):1162–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information Table S1: Occurrence of Single Station MLNM for Patients with N2 Disease Stratified by Tumor Locations (N = 436).
Supporting Information Table S2: Univariate and Multivariate Analyses of the Risk Factors for Station 3A LNM (N = 503).
Supporting Information Table S3: Subgroup Survival Analyses between Patients with and without Station 3A LND after Propensity Score Matching (N = 988).


