Abstract
Here, we first report a case of neoadjuvant ceritinib for locally advanced lung adenosquamous carcinoma. In this study, a locally advanced adenosquamous carcinoma (ASC) patient with EML4‐ALK fusion who achieved a partial response with neoadjuvant ceritinib treatment after a cycle of neoadjuvant chemotherapy did not show significant efficacy. A complete surgical resection was performed with mild adhesions and a small amount of bleeding intraoperatively. The EML4‐ALK fusion was detected by targeted next‐generation sequencing (NGS) in both pretreatment biopsy and the postoperative tissue specimens with a dramatic decrease in the allele frequency (26.2% [pre]–2.3% [post]). Pathological examination of the postoperative specimens indicated a diagnosis of ASC but the proportions of adenocarcinoma and squamous cell carcinoma cells in the primary lung tumor and metastatic lymph node site were different, suggesting the various responses to ceritinib. Thus, with the case presented here, we provide the clinical evidence for ALK‐positive locally advanced ASC patients benefiting from neoadjuvant ceritinib treatment with a tolerable safety profile, whereas further cohort studies of the efficacy and safety of neoadjuvant ceritinib in such patients are needed.
Keywords: adenosquamous carcinoma, ALK‐rearranged, ceritinib, neoadjuvant therapy, non‐small cell lung cancer
Here, we report a case of neoadjuvant ceritinib for locally advanced lung adenosquamous carcinoma. The treatment for this patient was replaced with neoadjuvant ceritinib after the failure of neoadjuvant chemotherapy and surgical R0 resection was achieved. We present the argument for neoadjuvant therapeutic strategies as a reasonable option for such patients.
INTRODUCTION
Finding the best neoadjuvant treatment strategy for patients with locally advanced non‐small cell lung cancer (LANSCLC) has been a hot topic of discussion in the medical community. Currently, the treatment modality is mainly based on concurrent chemoradiotherapy. Patients with ALK rearrangements have previously been reported to have a poor prognosis and are insensitive to chemotherapy in LANSCLC. 1 In recent years, the application of ALK TKIs as neoadjuvant treatment in ALK‐positive NSCLC patients has been studied, 2 , 3 , 4 but the timing and the feasibility are still unclear.
Adenosquamous carcinoma (ASC) is a rare type of NSCLC, the origin and the treatment of which is debatable. 5 , 6 Here, we report a case of ALK‐positive locally advanced ASC that failed neoadjuvant chemotherapy but benefited from neoadjuvant ceritinib treatment leading to a complete surgical resection.
CASE REPORT
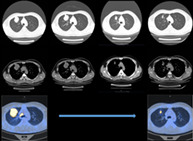
A male 34‐year‐old nonsmoker had no significant symptoms during his illness. Computed tomography (CT) and positron emission tomography (PET) (Figure 1a) revealed a tumor in the upper lobe right lung and an enlarged right upper paratracheal lymph node. After the pathological assessment of CT‐guided biopsy (Figure 2a), the patient was diagnosed with stage IIIB adenocarcinoma (ADC) of the lung (cT3N2M0). The biopsy was also subjected to targeted next‐generation sequencing (NGS) of 14 genes and an EML4‐ALK rearrangement was detected (EML4:exon6‐ALK:exon20) with an allele frequency of 26.2%. Neoadjuvant chemotherapy (pemetrexed 800 mg, carboplatin AUC = 5) was first administered for one cycle but the tumor did not shrink as shown by the follow‐up CT scan (Figure 1a). Therefore, the ALK TKI, ceritinib (450 mg/day) was chosen as the neoadjuvant targeted therapy. The chest CT after 4 weeks (one cycle) of ceritinib treatment revealed a partial response (PR) of disease with a tumor shrinkage of 38% in size (Figure 1a). Another 4 weeks of neoadjuvant ceritinib treatment led to further shrinkage of the lung tumor by 55%, as well as the decrease in SUVmax (28.04 vs. 9.51). The enlarged lymph nodes also subsided. Mild diarrhea was reported as an adverse effect of ceritinib treatment.
FIGURE 1.
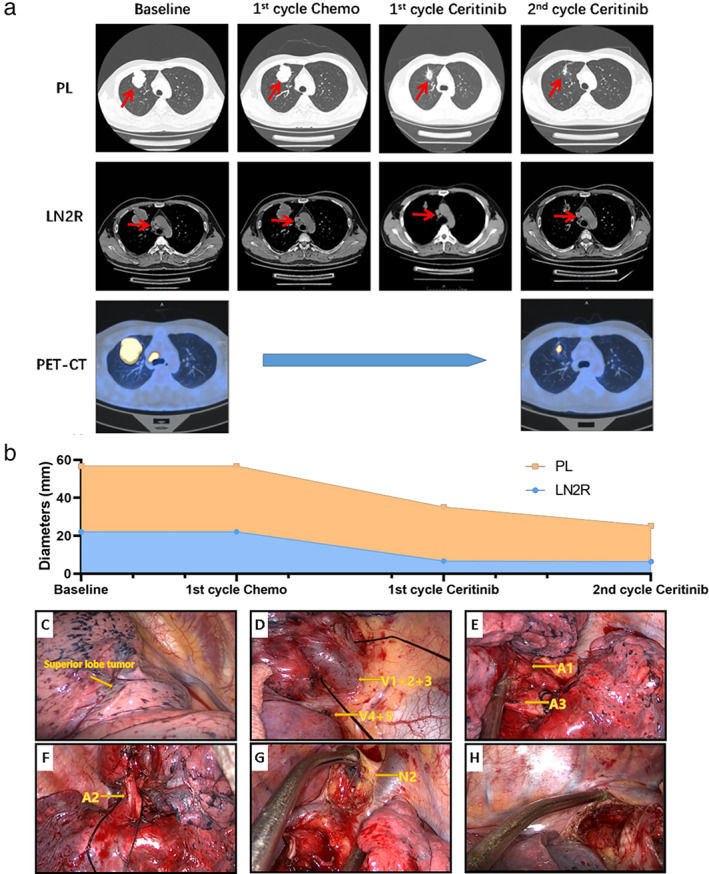
Imageological examinations during neoadjuvant treatments and intraoperative conditions (a) computed tomography (CT) and positron emission tomography (PET) at baseline showed a mass located in upper lobe right lung (max diameter: 56.7 mm; SUVmax: 28.04), and the enlarged LN2R lymph node (max diameter: 22.1 mm; SUVmax: 22.01). After one cycle of chemotherapy, the tumor and the lymph node remained stable. The PL tumor and LN2R lymph node continuously shrunk during ceritinib treatment and after eight‐week ceritinib, the maximum diameters of PL and LN2R are 25.2 and 6.4 mm, respectively, with an SUVmax of 9.51 and 2.88. (b) The changes of maximum diameters of PL and LN2R tumors during neoadjuvant treatment are shown. Images of intraoperative condition (right lung) are shown with only mild tissue adhesions and a small amount of blood leakage. (c) Tumor in the upper lobe right lung. (d) Separation of the upper lobe veins. (e) Exposure of the A1 and A3 arteries. (f) The A2 artery is revealed. (g) Removal of the upper paratracheal lymph node. (h) Subcarinal lymph node trauma. PL, primary lung cancer; LN2R, right upper paratracheal lymph node
FIGURE 2.
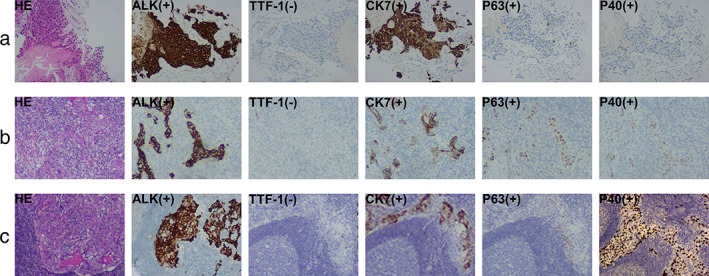
Hematoxylin–eosin (HE) staining and immunohistochemistry (IHC) images (100× magnification). (a) The HE staining of samples punctured before neoadjuvant ceritinib shows nests of tumor cells with necrosis. IHC examinations reveal positive ALK (D5F3), CK7, P63, P40, and negative TTF‐1, supporting a diagnosis of ADC. (b) In the surgically‐resected specimen after neoadjuvant ceritinib, there is a significant decreased amount of tumor cells in the tumor bed, most of the interstitial fibrosis, and more lymphocytes are scattered, where round tumor cell nests with eosinophilic cytoplasm and vesicular nuclei are seen. The IHC of ALK (D5F3), CK7, TTF‐1, P63, and P40 indicated the histology of ASC. (c) The right upper paratracheal lymph node, surgically‐resected after neoadjuvant ceritinib, shows scattered nests of tumor cells with eosinophilic cytoplasm and vesicular nuclei. The IHC of ALK (D5F3), CK7, TTF‐1, P63, and P40 indicated the histology of ASC
Video‐assisted thoracoscopic surgery (VATS) right upper lobectomy was performed seven days after the last dose of ceritinib. Complete surgical resection (R0) was achieved with mild adhesions and a small amount of bleeding intraoperatively (Figure 1c–h). Postoperative pathological examination revealed extensive tumor necrosis with less than 10% residual tumor cells, suggesting a major pathological remission (MPR). However, the postoperative immunohistochemical (IHC) examinations revealed the coexistence of adenocarcinoma cells (ACC) and squamous cell carcinoma cells (SCCC) in the primary lung tumor (Figure 2b; 30% ACC, CK7+; 70% SCCC, P40+, P63+) and the metastatic lymph node site (Figure 2c; 60% ACC; 40% SCCC), which were both positive for ALK protein expression. Therefore, the final diagnosis was stage IIIA ASC (ypT1cN2M0). The EML4‐ALK fusion was detected in the postoperative specimens by targeted NGS of 425 cancer‐related genes with a significant decrease in allele frequency compared with the pretreatment baseline biopsy (2.3% vs. 26.2%). Other concurrent mutations are shown in Table 1. This patient continued to take oral ceritinib (450 mg/day) according to the original regimen seven days after surgery. He underwent CT examinations every three months and showed no signs of tumor recurrence, and had his external plasma examined for cDNA three times within one year after surgery, and no tumor‐specific mutations were found.
TABLE 1.
Genomic alterations detected in the surgically resected tumor by targeted NGS polymorphisms of drug metabolism‐related enzymes. Mutations of XRCC1, TYMS 6 bp, DPYD, NQO1, GSTM1, MTHFR, GSTT1, UGT1A1, and TYMS3R were identified
| Polymorphisms of drug metabolism‐related enzymes | ||
|---|---|---|
| Gene | Mutation | Mutant type |
| DPYD | Homozygous p.I543V polymorphism | c.l627A > G(p.I543V) |
| GSTM1 | Homozygous deletion polymorphism | ‐ |
| GSTT1 | Homozygous deletion polymorphism | ‐ |
| MTHFR | Heterozygous p.A222V polymorphism | c665C > T(p.A222V) |
| NQO1 | Heterozygous p.P187S polymorphism | c.559C > T(p.P187S) |
| TYMS | Homozygous 3R/3R polymorphism | c.‐97_‐70CCGCGCCACTTGGCCTGCCTCCGTCCCG |
| TYMS | Homozygous deletion ‐6 bp/−6 bp polymorphism | c.*450_*455delAAGTTA |
| UGT1A1 | Heterozygous p.G71R polymorphism | c.211G > A(p.G71R) |
| XRCC1 | Homozygous p.Q399R polymorphism | c.1196A > G(p.Q399R) |
DISCUSSION
ASC is an uncommon malignancy, accounting for only 0.4%–4% of lung cancers. 5 There is still controversy regarding the origin of ASC. Hammond et al. 6 pointed out that the ACC and the SCCC are geographically separated at an early stage, while during tumor growth, these two components coinfiltrate together. Thus, ASC is likely to be polyclonal in origin. However, Lin et al. 5 found that almost all ASC patients (27/28) had common variants in ACC and SCCC, which indicated the monoclonal origin of ACC and SCCC. In this study, both ACC and SCCC were positive for ALK protein expression, which supported the theory of monoclonal origin. Furthermore, we observed that the primary lung tumor shrunk more than the metastatic lymph node site (Figure 1a) and the post‐ceritinib lung tumor contained a higher proportion of SCC than the lymph node metastasis (70% vs. 40%). Thus, we speculate that ACC is more sensitive to ceritinib than SCCC, resulting in a larger proportion of SCCC in the postoperative specimens. In contrast, the pathological examinations of the baseline biopsy revealed ACC alone, which might be attributed to the limited sampling. Comparison of the tissue NGS sequencing results before and after drug administration revealed a decrease in the allele frequency of EML4‐ALK, from 26.2 to 2.3%. It also demonstrates the effectiveness of ceritinib.
Response to standard chemotherapy occurs after 12 weeks, whereas response times to ALK inhibitors ranges from 3 to 8 weeks. 7 There is a contradiction between longer treatment cycles and increased risk of intraoperative adhesions and bleeding for neoadjuvant targeted therapy. We decided on 8 weeks of ceritinib tablet therapy based on genetic testing results, taking into account its median response time of 8 weeks in advanced disease. 7 , 8 As the first‐line therapy, ceritinib showed a better efficacy than standard chemotherapy in ALK‐rearranged advanced NSCLC patients. 9 In addition, previous cases have reported the feasibility and good tolerability of crizotinib 3 , 10 and alectinib 2 as neoadjuvant targeted therapies in ALK‐positive locally advanced NSCLC patients. However, there have been no reports on the neoadjuvant TKI treatments for ALK‐positive locally advanced ASC patients. As neoadjuvant treatment is essential for patients with locally advanced diseases to gain an opportunity for surgery, which could also reduce the risk of tissue fibrosis and intraoperative bleeding, studies on the application of neoadjuvant TKI treatments in ASC patients are urgently required.
In conclusion, we first report an ALK‐rearranged locally advanced ASC patient who benefited from neoadjuvant ceritinib treatment. This case indicated the feasibility of ceritinib as neoadjuvant therapy in ASC patients with ALK gene fusions but the efficacy and preoperative dosing schedule need to be studied further in the future.
CONFLICT OF INTEREST
Qiuxiang Ou and Hanlin Chen are full‐time employee of Nanjing Geneseeq Technology Inc. Nanjing, Jiangsu, China.
ACKNOWLEDGMENT
The patient involved in this case report gave his informed consent authorizing use and disclosure of his health information.
Mai S, Wang Y, Wang X, Yang W, Gao H, Xu Z, et al. Neoadjuvant ceritinib treatment in ALK ‐rearranged locally advanced adenosquamous carcinoma: A case report. Thorac Cancer. 2022;13(15):2275–2278. 10.1111/1759-7714.14558
Shixiong Mai and Yue Wang contributed equally to this work.
Funding information Changchun Science and Technology Bureau, Grant/Award Number: 19SS015; Jilin Province Science and Technology Bureau, Grant/Award Number: 20200603006SF
REFERENCES
- 1. Morodomi Y, Takenoyama M, Inamasu E, Toyozawa R, Kojo M, Toyokawa G, et al. Non‐small cell lung cancer patients with EML4‐ALK fusion gene are insensitive to cytotoxic chemotherapy. Anticancer Res. 2014;34(7):3825–30. [PubMed] [Google Scholar]
- 2. Zhang C, Yan LX, Jiang BY, Wu YL, Zhong WZ. Feasibility and safety of Neoadjuvant Alectinib in a patient with ALK‐positive locally advanced NSCLC. J Thorac Oncol. 2020;15(6):e95–9. 10.1016/j.jtho.2019.12.133 [DOI] [PubMed] [Google Scholar]
- 3. Kilickap S, Onder S, Dizdar O, Erman M, Uner A. Short‐time use of crizotinib as neoadjuvant in ALK‐positive non‐small cell lung carcinoma can be a chance for resectability. Cancer Chemother Pharmacol. 2019;83(6):1195–6. 10.1007/s00280-019-03810-9 [DOI] [PubMed] [Google Scholar]
- 4. Dumont D, Do P, Lerouge D, Planchard G, Riffet M, Dubos‐Arvis C, et al. Off‐label use of Crizotinib as a Neoadjuvant treatment for a young patient when conventional chemotherapy gave no benefits in stage IIIA non‐small cell lung cancer. Am J Med Case Rep. 2017;18:890–3. 10.12659/ajcr.903528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lin G, Li C, Li PS, Fang WZ, Xu HP, Gong YH, et al. Genomic origin and EGFR‐TKI treatments of pulmonary adenosquamous carcinoma. Ann Oncol. 2020;31(4):517–24. 10.1016/j.annonc.2020.01.014 [DOI] [PubMed] [Google Scholar]
- 6. Hammond WG, Tesluk H, Benfield JR. Histogenesis of adenosquamous bronchogenic carcinoma. Cancer Lett. 1995;96(2):163–8. 10.1016/0304-3835(95)03931-l [DOI] [PubMed] [Google Scholar]
- 7. Soria JC, Tan DSW, Chiari R, Wu YL, Paz‐Ares L, Wolf J, et al. First‐line ceritinib versus platinum‐based chemotherapy in advanced ALK‐rearranged non‐small‐cell lung cancer (ASCEND‐4): a randomised, open‐label, phase 3 study. Lancet. 2017;389(10072):917–29. 10.1016/S0140-6736(17)30123-X [DOI] [PubMed] [Google Scholar]
- 8. Claxton L, O'Connor J, Woolacott N, Wright K, Hodgson R. Ceritinib for untreated anaplastic lymphoma kinase‐positive advanced non‐small‐cell lung cancer: an evidence review group evaluation of a NICE single technology appraisal. Pharmacoeconomics. 2019;37(5):645–54. 10.1007/s40273-018-0720-8 [DOI] [PubMed] [Google Scholar]
- 9. Shaw AT, Kim TM, Crinò L, Gridelli C, Kiura K, Liu G, et al. Ceritinib versus chemotherapy in patients with ALK‐rearranged non‐small‐cell lung cancer previously given chemotherapy and crizotinib (ASCEND‐5): a randomised, controlled, open‐label, phase 3 trial. Lancet Oncol. 2017;18(7):874–86. 10.1016/s1470-2045(17)30339-x [DOI] [PubMed] [Google Scholar]
- 10. Zhang C, Li SL, Nie Q, Dong S, Shao Y, Yang XN, et al. Neoadjuvant crizotinib in resectable locally advanced non‐small cell lung cancer with ALK rearrangement. J Thorac Oncol. 2019;14(4):726–31. 10.1016/j.jtho.2018.10.161 [DOI] [PubMed] [Google Scholar]


