Abstract
Background
With the development of imaging, the etiology of ocular lesions caused by lung cancer was not found only intraocular. Neuro‐ophthalmological imaging has been found to be useful for the diagnosis of meningeal carcinomatosis, although few studies have previously been published. Our study used magnetic resonance imaging (MRI) to determine if there was a the relationship between ocular symptoms and lung cancer metastasis.
Methods
We carried out a retrospective analysis which included patients with ocular lesions and lung cancer on which orbit MRI was performed together with ocular examination during January 2014 to January 2022. Here, we describe the characteristics of intraocular metastasis and optic nerve sheath lesions.
Results
A total of 21 lung cancer patients had ocular lesions, of which eight cases had choroidal metastasis; a further eight patients had optic nerve sheath lesions. There was one case (12.5%) of choroidal metastasis with brain or meningeal metastasis, and eight cases (100%) with optic nerve sheath lesions with brain or meningeal metastasis. A total of 75.0% patients with choroidal metastasis did not have any history of known lung cancer, and 25.0% of optic nerve sheath lesions in patients were found before a meningeal metastasis diagnosis. The features of optic nerve sheath lesions included thickening and strengthening of the long segment of the orbital optic nerve sheath with a clear boundary.
Conclusions
The pathway of choroidal and optic nerve sheath lesions was different. Optic nerve sheath lesions were associated with brain and meningeal metastasis. When lung cancer‐induced meningeal metastasis is suspected, orbital MRI is recommended to assist in the diagnosis.
Keywords: brain or meningeal metastasis, choroidal metastasis, lung cancer, MRI, optic nerve sheath metastasis
Diagnosis of the latent brain or meningeal metastasis of lung cancer patients is problematic. We found that optic nerve sheath lesions were associated with brain or meningeal metastasis of lung cancer. The imaging of optic nerve sheath lesions by orbit MRI may be beneficial for diagnosing latent brain or meningeal metastasis of lung cancer patients at an early stage.

INTRODUCTION
The combined presentation of lung cancer and the ocular lesion is rare; however, such presentation generally predicts a poor prognosis. 1 Appropriate treatment based on an early diagnosis significantly improves or delays the deterioration of vision leading to a better prognosis. 2 Choroidal metastasis is the most common etiology in lung cancer, 3 and the prognosis is dismal with an annual cancer‐related death rate of 54% in patients.
However, lung ocular lesions were not only found during intraocular metastasis but also other pathogenic ocular lesions have been associated with lung cancer. 4 Carcinoma metastasis rarely leads to intracranial meningioma; the optic nerve sheath (ONS) lesion may be caused by the metastasis to the intracranial meningioma. 5 We have previously found that neuro‐ophthalmological imaging can be useful in the early diagnoses of lung cancer patients with meningeal metastases (MM). 6 MRI results have demonstrated that abnormal imaging findings in the sheath of the optic nerve are one of the characteristics of MM. However, this was a rare case, and a greater sample size is needed to confirm this observation. We hypothesized that either choroidal metastasis is associated with blood metastasis, or that an optic nerve sheath lesion is mediated by the entry of the tumor cells into the subarachnoid space. Therefore, optic nerve sheath lesions should be associated with brain or MM metastasis.
In the current study, we reviewed the orbit MRI of lung cancer patients in order to indentify if optic nerve sheath lesions could be found in patients with brain and meningeal metastases. We also compared the clinical differences between intraocular metastasis and optic nerve sheath lesions. Our observations show clinicians that optic imaging is necessary for lung cancer diagnosis and clinical treatment.
METHODS
This was a retrospective study. A total of 38 cancer patients who underwent orbit MRI in Beijing Friendship Hospital (BFH) were recruited between January 2014 and January 2022. The patients who fulfilled the inclusion parameters were included in the study. All the cases were from patients with eye complications who had been diagnosed by significantly experienced ophthalmologists.
Orbit MR imaging
MRI‐3.0T (TW1WSPEED HDXT, GE) was employed to measure the orbit with the following parameters and scanning sequences: coronal T1‐weighted fast spin‐echo (TR = 660 ms; TE =11.1 ms, matrix size = 256 × 256 mm, FOV = 18 × 18 cm, slice thickness = 2.5–3.0 mm); Gd‐DTPA and fat suppression method increased the contrast of the MRI. Two neuroradiologists with significant experience interpreted the MRI data.
Review of ophthalmic imaging
Slit‐lamp examinations and uni‐ or bilateral pupillary reactions were included. The vision logarithms (standardized, 5 m) were used to measure vision. The patients who failed to decipher letters (1 m) underwent further evaluations. A noncontact tonometer was used to test intraocular pressure. Nonmydriatic fundus photography was used to obtain digital fundus photographs. Fundus fluorescein angiography (FFA) and optical coherence tomography (OCT) were used as needed.
Statistical analysis
Mann–Whitney tests were used to evaluate significant differences between groups. Pearson χ2 or Fisher's exact tests were employed to adjust for variables. Social Sciences software version 19.0 was used to perform all statistics. Results were considered statistically significant if p < 0.05.
RESULTS
Patient characteristics
A total of 38 cancer patients had ocular lesions. There were 21 patients with lung cancer; n = 7 patients with breast cancer, n = 5 patients with lymphoma, n = 3 patients with colon cancer, one with renal carcinoma, and one with thyroid carcinoma (Figure 1a). Among the 21 lung cancer patients, the ratio of males and females was 11:10, and the age range was 30–83 years.
FIGURE 1.

Characteristics of the patient cohort. (a) Types of primary tumors in patients with ocular lesions. (b) The etiologies of ocular lesions in lung cancer
Ocular lesions etiologies
The etiologies of ocular lesions in lung cancer were analyzed (Figure 1b). A total of eight patients had choroidal metastasis (Figure 2), eight had optic nerve sheath lesions (Figures 3, 4), two had papilledema induced by intracranial hypertension, one hadoptic nerve compression by orbital mass, one had immunoglobulin G4‐related disease, one had optic neuritis caused by paraneoplastic syndrome, and one had abducent neuritis. There was one patient who had both intracranial hypertension and optic nerve sheath enhancement. A total of 75.0% of patients with choroidal metastasis had no history of known lung cancer. Furthermore, MRI results showed two optic nerve sheath metastasis cases before a definitive diagnosis of brain or meningeal metastases.
FIGURE 2.

Ocular multimodal imaging features of choroidal metastatic carcinoma. (a) FFA: The boundary of the tumor is clear, dilated retinal capillaries at the margin of the arteriovenous stage, needle‐like hyperfluorescent leakage at the edge of the lesion from early to late angiography, with visible tumor blood vessels. ICG: The images of ICGA revealed fluorescein block in the early phase, and hypofluorescence in the late phase (white arrow). (b) Color doppler ultrasound: Choroidal thickening, medium echo, and detachment of the retina and showed high blood perfusion (white arrow). (c) OCT revealed that the choroid thickened and bulged, protruded into the vitreous cavity, retinal pigment epithelial layer and retinal neuroepithelial layer detachment (white arrow) MRI: (d) Axial T2 weighted image: The intrabulbar mass was equal T2 signal (yellow arrow). (e) Axial T1 weighted image: The intrabulbar mass was equal T1 signal (yellow arrow). (f) Axial gadolinium‐enhanced TI MRI showed that the intrabulbar mass increased with moderate intensity (yellow arrow)
FIGURE 3.
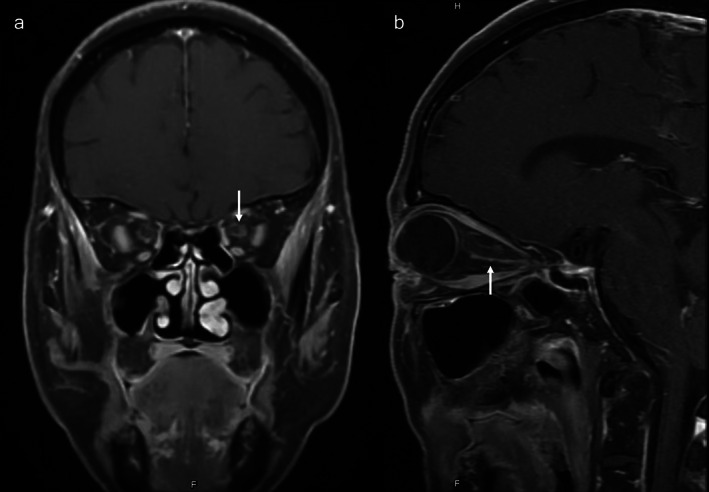
Orbit MRI results in the OSM case (a) coronal gadolinium‐enhanced TI orbit MRI demonstrated that the optic nerve sheath is increased (b) and also in the sagittal gadolinium‐enhanced TI image (white arrow)
FIGURE 4.

Ultra‐wide angle fundus imaging and orbit MRI results in the OSM case (a) The retina arterial vessels are slender and white sheath can be seen, hemorrhage and batt‐like exudation from fundus imaging. (b) Axial gadolinium‐enhanced TI orbit MRI showed abnormal dilation of the superior ophthalmic vein (white arrow), also multiple nodular enhanced metastatic lesions could be found in the brain (yellow arrow). (c) Coronal gadolinium‐enhanced TI orbit MRI showed that the optic nerve sheath was increased (yellow arrow)
Differences between CM and ONS lesions
We found CM and ONS lesions to be the most common lesions in lung cancer, and the differences between them are listed in Table 1. There were six males in the CM group and two males in the ONS group (p = 0.13). There was one patient with brain or meningeal metastasis and eight patients in the ONS group (p = 0.001). Seven patients were diagnosed with adenocarcinoma in CM, and eight were diagnosed in the ONS group (p = 1.0). Four patients with EGFR exon 19 del were found in the CM group and six patients in the ONS group (p = 0.61). One patient had ALK mutation and one patient with RET in the CM group. Seven patients in the CM group and three patients in the ONS group showed severe visual impairment (BCVA < 0.5) (p = 0.12). Seven patients had retinal detachment and vitreous hemorrhage (p < 0.001) and two patients had neovascular glaucoma (p = 0.47). Eight patients had a retinal and choroidal mass in the CM group (p < 0.001), and three patients showed bilateral papilledema in the ONS group (p = 0.2).
TABLE 1.
The differences between choroidal metastasis and optic nerve sheath lesions
| Choroidal metastasis | Optic nerve sheath lesions | p‐value | |
|---|---|---|---|
| Number of patients (n) | 8 | 8 | |
| Male/Female | 6/2 | 2/6 | 0.13 |
| Age (Y, Mean ± SD) | 55.03 ± 16.09 | 64.4 ± 4.98 | 0.03* |
| Brain or meningeal metastasis (n, %) | 1 (12.5%) | 8 (100%) | 0.001** |
| EGFR exon 19 del (n, %) | 4 (50.0%) | 6 (75.0%) | 0.61 |
| ALK mutation (n, %) | 1 (12.5%) | 0 (0%) | 1.00 |
| RET (n, %) | 1 (12.5%) | 0 (0%) | 1.00 |
| Adenocarcinoma (n, %) | 7 (87.5%) | 8 (100%) | 1.00 |
| Ocular as the first symptom (n, %) | 6 (75%) | 1 (12.5%) | 0.04* |
| Ocular symptoms | |||
| Unilateral affection (n, %) | 8 (100%) | 0 (0%) | <0.001** |
| BCVA < 0.5 (n, %) | 7 (87.5%) | 3 (37.5%) | 0.12 |
| BCVA ≥ 0.5 (n, %) | 1 (12.5%) | 5 (62.5%) | 0.12 |
| Retinal detachment (n, %) | 7 (87.5%) | 0 (0%) | 0.001** |
| Vitreous hemorrhage (n, %) | 7 (87.5%) | 0 (0%) | 0.001** |
| Increase in intraocular pressure (n, %) | 2 (25%) | 0 (0%) | 0.47 |
| Retinal and choroidal mass (n, %) | 8 (100%) | 0 (0%) | <0.001** |
| Bilateral papilledema (n, %) | 0 (0%) | 3 (37.5%) | 0.2 |
Abbreviation: BCVA, best corrected visual accuracy.
= p < 0.05,
= p < 0.01.
DISCUSSION
ONS lesions are usually combined with metastasis of the brain and meninges in lung cancer patients. We found that ONS enhancement in MRI was adequate for the prediction of meningeal metastasis. 6 Furthermore, in the current study, the MRI features of ONS metastasis were identified—a long segment of optic nerve sheath in the orbit was thickened and strengthened, with a clear boundary. This was usually combined with brain and meningeal metastasis.
Meningeal metastasis is generally established by the growth of tumor cells along a vessel or nerve linings or the entry of the cancer cells into the leptomeningeal space via blood or the cerebrospinal fluid (CSF). Therefore, patients experience a bevy of neural symptoms, including different regions of the brain and different nerves. 7 Previous studies have shown that the ONS and optic nerve are linked to cancer via paraneoplastic syndrome 8 and metastasis. 9 The nerve sheath and three layers of meninges protect the optic nerve. Metastasis also plagues the ONS and optic nerve. 10 , 11 Lung cancer often results in blood metastasis, which is facilitated by damage to the blood–brain barrier. The anatomical structures of the optic nerve sheath and meninges are the same with the same metastasis pathogenesis. Breast cancer (47%) and lung cancer (21%) are mainly responsible for the majority of eye metastases (88%). 12 The supply to the choroid is via a posterior ciliary artery which has a complex and branched vascular structure and allows increased cancer emboli to the posterior uvea. 13 Further, the loose stack of endothelial cells in the choroidal capillary is filled with fenestrations which creates a fertile environment for metastasis of cancer cells. 14 , 15 Therefore, the metastatic pathologies of the optic nerve sheath and choroid are different.
Metastasis of lung cancer to the orbit may occur during the initial phase of the disease, and the optic nerve sheath may be implicit 16 , 17 in this process. Early diagnosis of meningeal carcinomatosis is usually very difficult. Advanced high contrast MRI scans of the brain and CSF tests are needed for diagnosis. However, delay in the diagnosis often occurs because of the asymptomatic presentation of the disease. 17 CSF tests initially reveal cancer cells in only 50% of patients with MC. 18 In our study, there were two MM patients with optic nerve sheath enhancement, which were detected before brain MRI and CSF test. Scanning sequences and parameters of orbit MRI were more sensitive than brain MRI scans in detecting meningeal dissemination. The majority of ocular symptoms caused by meningeal carcinomatosis were diplopia and vision loss. 19 In the current study, we found that the vision loss was mild in the early phase, and a small amount of retina bleeding, exudation, and papilledema was seen in the fundus. In the late stages, there was irreversible and severe vision loss and optic atrophy, but no neovascular glaucoma. Therefore, early diagnosis is critical in order to improve the prognosis, and an orbit MRI is useful for early diagnosis.
In contrast to optic nerve sheath metastasis, 75% of patients with CM experience the first symptoms before lung cancer diagnosis. A multicenter study found that 44% of CM did not have a history of known lung cancer. 20 The incidence rate in this study was higher than that of the previous study, which may be due to the different races and sample size. This study was also conducted with a small number of patients who underwent ocular MRI test for clinical reasons. Therefore, there is a selection bias. Metamorphopsia and vision loss are commonly experienced by most patients. 21 Severe subretinal fluid, retinal detachment, and papilledema has also been observed in some patients. 21 , 22 Similar to our study, MRI results previously revealed a choroidal mass. 23 Therefore, the fundus imaging, OCT, FFA + ICG, and doppler were useful in CM diagnosis, and the MRI was more useful in identifying optic nerve sheath lesions. Orbit MRI scaning sequences are recommended in asymptomatic patients with suspected MM.
Our study suffered from certain limitations. This was a retrospective single‐center study, with possible selection bias and there was also no control group. The study only analyzed 21 patients and demonstrated a lack of long‐term survival, suggesting that future studies with a larger patient cohort are needed.
In conclusion, optic nerve sheath lesions are associated with the brain or meningeal metastases. Ocular imaging may be beneficial for diagnosing latent brain or meningeal metastasis of lung cancer patients at an early stage. Performing ultra‐wide‐angle fundus imaging, OCT, and orbital enhanced MRI is therefore recommended if a lung cancer patient has ocular symptoms.
AUTHOR CONTRIBUTIONS
Hongyang Li was involved in the conception and design of the study. Shuai Song and Dong Chang contributed to the acquisition, analysis and interpretation of data as well as drafting the manuscript and revising it critically. Hao Li and Yong cui were the duty of diagnosis the disease, Shuai Song and Chunquan Liu have also provided final approval of the version to be published. All authors have given final approval of the version to be published. All authors have agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
ACKNOWLEDGMENTS
We acknowledge MedEditing (www.medediting.com) for editorial services. Capital's Funds for Health Improvement and Research. (2022‐2‐20211).
Song S, Chang D, Li H, Liu C, Cui Y, Li H. Association of optic nerve sheath lesion and brain or meningeal metastasis caused by lung cancer. Thorac Cancer. 2022;13(15):2164–2169. 10.1111/1759-7714.14538
Funding information Capital's Funds for Health Improvement and Research, Grant/Award Number: 2022‐2‐20211
REFERENCES
- 1. Torre LA, Siegel RL, Jemal A. Lung cancer statistics. Adv Exp Med Biol. 2016;893:1–19. 10.1007/978-3-319-24223-1_1 [DOI] [PubMed] [Google Scholar]
- 2. Mathis T, Jardel P, Loria O, Delaunay B, Nguyen AM, Lanza F, et al. New concepts in the diagnosis and management of choroidal metastases. Prog Retin Eye Res. 2019;68:144–76. 10.1016/j.preteyeres.2018.09.003 [DOI] [PubMed] [Google Scholar]
- 3. Lin L, Sun J, Wang J. Lung cancer and intraocular metastasis in gestation: clinical experiences of a rare case. Thorac Cancer. 2020;11(9):2723–6. 10.1111/1759-7714.13572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ookuma T, Kikuchi R, Takoi H, Toriyama K, Abe S. Orbital apex syndrome associated with intraorbital metastasis of lung cancer. Respirol Case Rep. 2022;10(4):e0922. 10.1002/rcr2.922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Arnold AC, Hepler RS, Badr MA, Lufkin RB, Anzai Y, Konrad PN, et al. Metastasis of adenocarcinoma of the lung to optic nerve sheath meningioma. Arch Ophthalmol. 1995;113(3):346–51. 10.1001/archopht.1995.01100030102029 [DOI] [PubMed] [Google Scholar]
- 6. Song S, Chang D, Li H, Liu C, Li H, Cui Y. Application of optic neuro‐ophthalmology imaging in latent meningeal metastases of lung cancer. Thorac Cancer. 2021;12(19):2614–7. 10.1111/1759-7714.14128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hoffman PC, Mauer AM, Vokes EE. Lung cancer. Lancet. 2000;355(9202):479–85. 10.1016/S0140-6736(00)82038-3 [DOI] [PubMed] [Google Scholar]
- 8. Xu Q, Du W, Zhou H, Zhang X, Liu H, Song H, et al. Distinct clinical characteristics of paraneoplastic optic neuropathy. Br J Ophthalmol. 2019;103(6):797–801. 10.1136/bjophthalmol-2018-312046 [DOI] [PubMed] [Google Scholar]
- 9. Fox B, Pacheco P, DeMonte F. Carcinoma of the breast metastatic to the optic nerve mimicking an optic nerve sheath meningioma: case report and review of the literature. Skull Base. 2005;15(4):281–7; discussion 7–9. 10.1055/s-2005-921935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sabater AL, Sadaba LM, de Nova E. Ocular symptoms secondary to meningeal carcinomatosis in a patient with lung adenocarcinoma: a case report. BMC Ophthalmol. 2012;12:65. 10.1186/1471-2415-12-65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hernandez Pardines F, Molina Martin JC, Fernandez Montalvo L, Juarez Marroqui A. Optic nerve metastasis caused by lung adenocarcinoma. Arch Soc Esp Oftalmol. 2017;92(11):552–4. 10.1016/j.oftal.2017.01.007 [DOI] [PubMed] [Google Scholar]
- 12. Shields CL, Shields JA, Gross NE, Schwartz GP, Lally SE. Survey of 520 eyes with uveal metastases. Ophthalmology. 1997;104(8):1265–76. 10.1016/s0161-6420(97)30148-1 [DOI] [PubMed] [Google Scholar]
- 13. Hogan MJ. Diseases of the uveal tract. AMA Arch Ophthalmol. 1951;45(3):334–56. 10.1001/archopht.1951.01700010340008 [DOI] [PubMed] [Google Scholar]
- 14. Ferry AP. The biological behavior and pathological features of carcinoma metastatic to the eye and orbit. Trans Am Ophthalmol Soc. 1973;71:373–425. [PMC free article] [PubMed] [Google Scholar]
- 15. Qu Z, Liu J, Zhu L, Zhou Q. A comprehensive understanding of choroidal metastasis from lung cancer. Onco Targets Ther. 2021;14:4451–65. 10.2147/OTT.S315532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Shankar VA, Bialick SR, Shafer BM. Metastatic squamous cell lung carcinoma to the optic nerve sheath mimicking retrobulbar optic neuritis. Ophth Plast Reconstr Surg. 2020;36(4):e87–90. 10.1097/IOP.0000000000001600 [DOI] [PubMed] [Google Scholar]
- 17. Takahashi H, Isogawa M. Management of breast cancer brain metastases. Chin Clin Oncol. 2018;7(3):30. 10.21037/cco.2018.05.06 [DOI] [PubMed] [Google Scholar]
- 18. Wasserstrom WR, Glass JP, Posner JB. Diagnosis and treatment of leptomeningeal metastases from solid tumors: experience with 90 patients. Cancer. 1982;49(4):759–72. [DOI] [PubMed] [Google Scholar]
- 19. Gleissner B, Chamberlain MC. Neoplastic meningitis. Lancet Neurol. 2006;5(5):443–52. 10.1016/S1474-4422(06)70443-4 [DOI] [PubMed] [Google Scholar]
- 20. Shah SU, Mashayekhi A, Shields CL, Walia HS, Hubbard GB 3rd, Zhang J, et al. Uveal metastasis from lung cancer: clinical features, treatment, and outcome in 194 patients. Ophthalmology. 2014;121(1):352–7. 10.1016/j.ophtha.2013.07.014 [DOI] [PubMed] [Google Scholar]
- 21. Yang CJ, Tsai YM, Tsai MJ, Chang HL, Huang MS. The effect of chemotherapy with cisplatin and pemetrexed for choroidal metastasis of non‐squamous cell carcinoma. Cancer Chemother Pharmacol. 2014;73(1):199–205. 10.1007/s00280-013-2341-4 [DOI] [PubMed] [Google Scholar]
- 22. Lu S, Azada MC, Ou SH. Choroidal metastasis response to crizotinib in a ROS1‐rearranged NSCLC patient. Lung Cancer. 2015;87(2):207–9. 10.1016/j.lungcan.2014.12.011 [DOI] [PubMed] [Google Scholar]
- 23. Peyster RG, Augsburger JJ, Shields JA, Hershey BL, Eagle R Jr, Haskin ME. Intraocular tumors: evaluation with MR imaging. Radiology. 1988;168(3):773–9. 10.1148/radiology.168.3.3406407 [DOI] [PubMed] [Google Scholar]


