Abstract
A 69‐year‐old Japanese man visited our hospital because of worsening shortness of breath. His chest computed tomography (CT) showed a giant left lung mass with a massive left pleural effusion. He could not be treated with chemotherapy and eventually died from a rapidly progressive tumor. He was diagnosed with combined small cell lung carcinoma (C‐SCLC) with spindle‐shaped cell tumor at autopsy. C‐SCLC is characterized by pathologically concurrent SCLC and adenocarcinoma or squamous cell carcinoma, or rarely, spindle‐shaped cell tumor. The clinical course of C‐SCLC with spindle‐shaped cell tumor has not previously been determined. Our patient's tumor increased by 2.59‐fold in 20 days. The combination of C‐SCLC with spindle‐shaped cell tumor suggested rapid progression and a poor prognosis.
Keywords: adenocarcinoma, combined small‐cell lung carcinoma, spindle‐shaped cell tumor
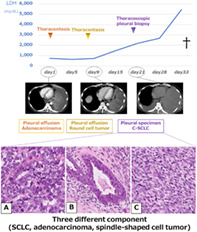
We present a rare case of C‐SCLC with three different components (SCLC, adenocarcinoma, and a spindle‐shaped cell tumor). Two thoracenteses did not allow for a diagnosis, but thoracoscopic pleural biopsy showed features of SCLC (a), adenocarcinoma (b), and a spindle‐shaped cell tumor (c). Autopsy led to a diagnosis of C‐SCLC including those three tumor components. Our patient's tumor increased by 2.59‐fold in size in 20 days, suggesting that the combination of C‐SCLC with a spindle‐shaped cell tumor caused rapid progression and a poor prognosis. Physicians should be aware of the proper diagnosis of C‐SCLC including spindle‐shaped cell tumors in patients with rapidly progressive lung cancer in addition to SCLC.
INTRODUCTION
Combined small cell lung carcinoma (C‐SCLC) is defined as the concurrence of SCLC and any of the histological non‐small cell lung cancers (NSCLCs). 1 , 2 Accounting for only 1%–5% of all SCLCs, 2 , 3 , 4 C‐SCLC is relatively uncommon, although its incidence has recently increased. 3 The most frequent components of C‐SCLC (outside of SCLC) are squamous cell carcinoma, adenocarcinoma, and large cell carcinoma. Among patients with C‐SCLC, spindle‐shaped cell tumor is rarely observed. The clinical impact of C‐SCLC with spindle cell tumor has not been determined. Here, we present a review of the clinical, imaging, and histological features of a rare case of C‐SCLC with three different components (SCLC, adenocarcinoma, and spindle‐shaped cell tumor).
CASE REPORT
A 69‐year‐old Japanese man visited our hospital because of worsening shortness of breath. He was a current smoker (40 pack‐years) with a family history of lung cancer. He had experienced shortness of breath a month prior, but did not visit a doctor due to the coronavirus disease pandemic. Upon admission, his physical examination revealed height, 166.5 cm; bodyweight, 62.0 kg; body temperature, 37.5°C; heart rate, 110 bpm; blood pressure, 146/106 mmHg; and oxygen saturation, 91% (oxygen 4 l/min, at rest). His European Cooperative Oncology Group (ECOG) performance status (PS) was 1. Laboratory results (Table 1) showed high levels of serum carcinoembryonic antigen (7.3 ng/ml), progastrin‐releasing peptide (140 pg/ml), and neuron‐specific enolase (132.0 ng/ml). High‐resolution chest computed tomography (CT) showed a giant left lung mass with a massive left pleural effusion causing mediastinal shift to the right (Figure 1). Left‐sided thoracentesis revealed that the pleural effusion was exudative with lymphocyte predominance. Adenocarcinoma cells negative for thyroid transcription factor‐1 and napsin A were cytologically detected. There were too few tumor cells to make a diagnosis. Dyspnea improved after left‐sided chest tube drainage on the fifth day. Further, cytological examination of the left pleural effusion revealed round tumor cells negative for adenocarcinoma. Subsequent left pleural effusion cytology was nondiagnostic. A transbronchial lung biopsy from his left S4 on the 12th day was also nondiagnostic. A thoracoscopic pleural biopsy on the 21st day showed several features of SCLC, adenocarcinoma, and spindle‐shaped cell tumor, with C‐SCLC (Figure 2). The programmed death‐ligand 1 tumor proportion score was less than 1%. Post‐thoracoscopy, respiratory and general conditions rapidly worsened. The patient died on the 34th day (Figure 3). On autopsy, macroscopic examination revealed a large, erupted tumor occupying almost the entire lower lobe of the left lung with circumferential adhesion and infiltration of the left chest wall and diaphragm. Microscopic findings revealed that the mass was composed of SCLC (60%), spindle‐shaped cell tumor (30%), and adenocarcinoma (10%). Our patient was diagnosed with C‐SCLC including three tumor components.
TABLE 1.
Laboratory data on admission
| <Blood cell counts> | <Blood chemistry> | |||||||
|---|---|---|---|---|---|---|---|---|
| WBC | 11 700 | /μl | TP | 6.2 | g/dl | Glucose | 258 | mg/dl |
| Neutrophils | 86.5 | % | Alb | 2.8 | g/dl | HbA1c | 7.8 | % |
| Lymphocytes | 7.7 | % | T‐bil | 1.4 | mg/dl | CRP | 6.46 | mg/dl |
| Eosinophils | 0.3 | % | AST | 51 | IU/l | |||
| Monocytes | 5.2 | % | ALT | 38 | IU/l | <Tumor marker> | ||
| RBC | 4.65 × 106 | /μl | LDH | 737 | IU/l | CEA | 7.3 | ng/ml |
| Hb | 14.4 | g/dl | BUN | 4.2 | mg/dl | CYFRA21‐1 | 1.7 | ng/ml |
| Ht | 41.9 | % | Cre | 0.72 | mg/dl | Pro‐GRP | 140.0 | pg/ml |
| Platelets | 30.9 × 104 | /μl | Na | 137 | mEq/l | SLX | 25.1 | U/ml |
| K | 4.3 | mEq/l | NSE | 132 | ng/ml | |||
| Cl | 103 | mEq/l | ||||||
Abbreviations: Alb, albumin; ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; BUN, blood urea nitrogen; CEA, carcinoembryonic antigen; CRP, C‐reactive protein; CYFRA, cytokeratin fragment; Hb, hemoglobin; Ht, hematocrit; LDH, lactate dehydrogenase; NSE, neuron‐specific enolase; Pro‐GRP, Pro‐gastrin‐releasing peptide; RBC, red blood cell; SLX, sialic Lewis X‐i antigen; T‐bil, total bilirubin; TP, total protein; WBC, white blood cell; γ‐GTP, gamma‐glutamyl transferase.
FIGURE 1.
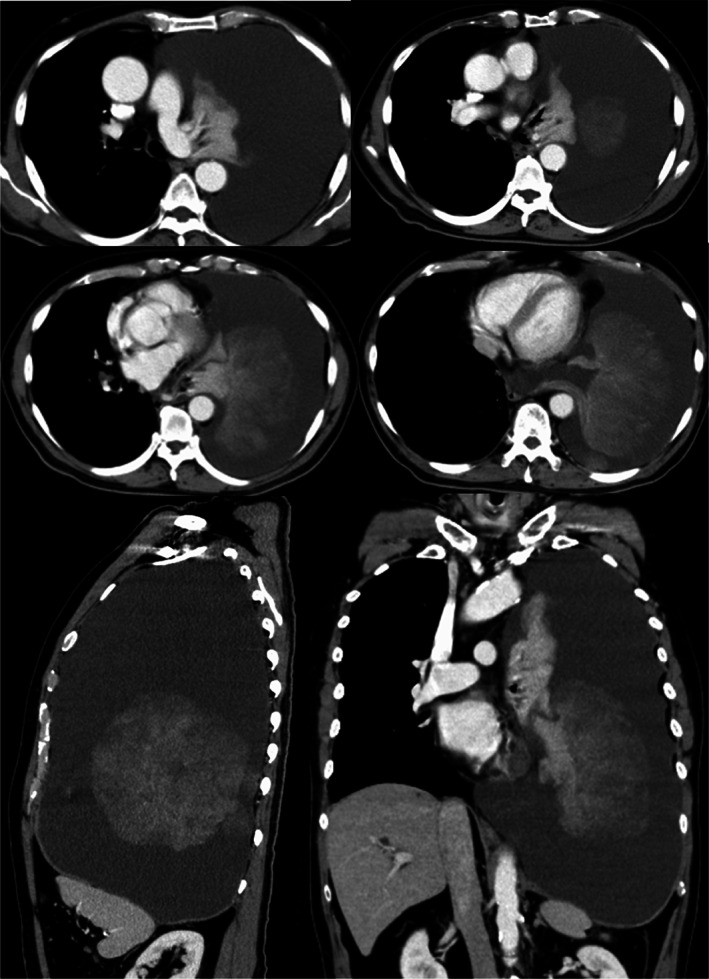
Chest computed tomography revealed a giant mass in the left lung and left pleural effusion with a prominent mediastinal shift
FIGURE 2.
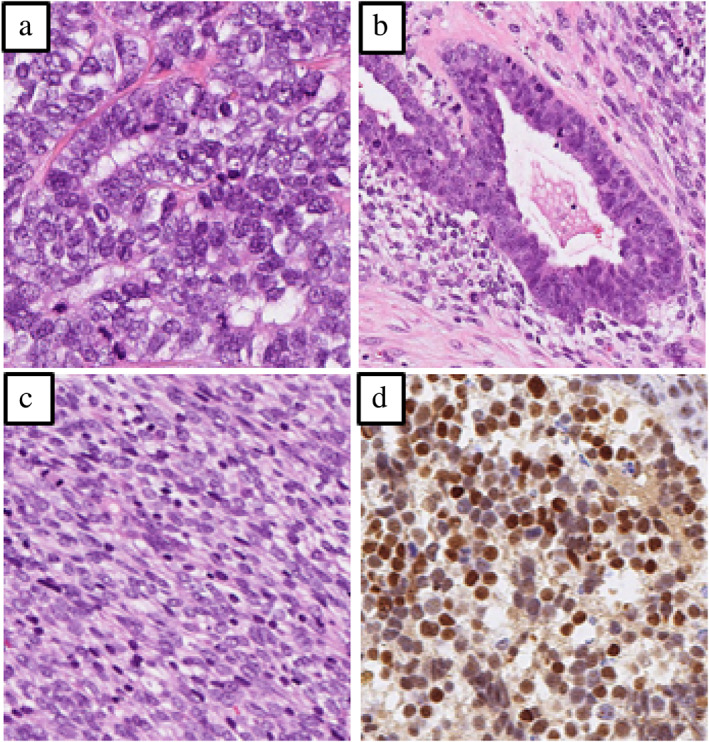
Histopathological findings of the pleural biopsy specimen based on hematoxylin–eosin staining showed (a) small‐cell lung carcinoma, (b) adenocarcinoma, and (c) spindle‐shaped cell tumors. (d) The small‐cell lung carcinoma lesion was positive for insulinoma‐associated protein 1
FIGURE 3.
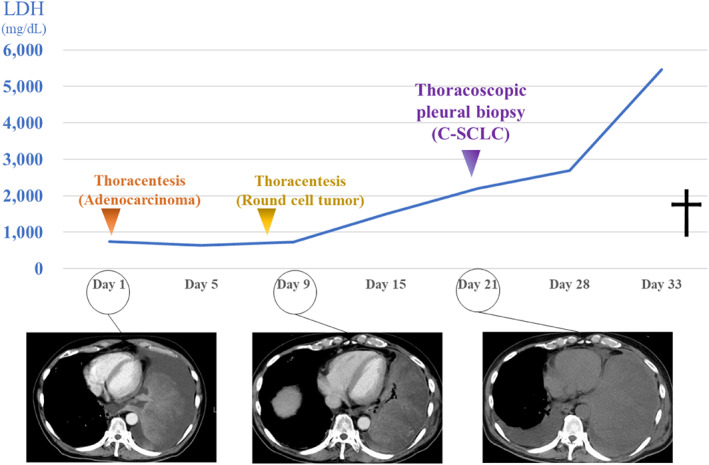
The clinical course of the patient. The tumor increased in size rapidly with worsening of his respiratory condition and performance status. C‐SCLC, combined small cell lung carcinoma
DISCUSSION
C‐SCLC is a rare type of cancer with an incidence of approximately 1%–5% of SCLC patients. Low incidence and insufficient biopsy specimens make it difficult to obtain an accurate diagnosis. 5 The rate of misdiagnosis of C‐SCLC through biopsy specimens is high. An adequate sample and a thorough examination are mandatory for a proper diagnosis. 4 Making a final diagnosis of C‐SCLC while our patient was alive would have been difficult because of the differing cytology and thoracoscopic pleural biopsy results. Autopsy findings revealed the diagnosis of C‐SCLC.
C‐SCLC is defined as the concurrence of SCLC and any histological type of NSCLC, which is usually adenocarcinoma and squamous cell carcinoma, 6 or less commonly, spindle‐shaped cell tumor. 2 Including our patient, there have been seven case reports of C‐SCLC with spindle‐shaped cell tumor (Table 2), 7 , 8 , 9 , 10 , 11 , 12 and four cases of C‐SCLC with three different components (Table 2).
TABLE 2.
Reported cases of combined small cell lung carcinoma with spindle‐shaped cell tumor/spindle cell carcinoma
| Case | Age/sex | Components of combined small cell lung carcinoma | Treatment | Survival | Authors | Reported year | |||
|---|---|---|---|---|---|---|---|---|---|
| Small cell lung carcinoma | Adenocarcinoma | Squamous cell carcinoma | Spindle‐shaped cell tumor/spindle cell carcinoma | ||||||
| 1 | 62/M | ○ | ○ | Sleeve resection and RT | Unknown | Tsubota et al. 7 | 1992 | ||
| 2 | 63/M | ○ | ○ | Lobectomy | 2 years and 4 months | Niho et al. 8 | 1999 | ||
| 3 | 61/M | ○ | ○ | ○ | Lobectomy | Unknown | Gotoh et al. 9 | 2004 | |
| 4 | 54/M | ○ | ○ | ○ | Lobectomy | Unknown | Hsiao et al. 10 | 2006 | |
| 5 | 76/M | ○ | ○ | Lobectomy | Unknown | Pelosi et al. 11 | 2011 | ||
| 6 | 76/M | ○ | ○ | ○ | Pneumonectomy | Unknown | Asahina et al. 12 | 2018 | |
| Present case | 69/M | ○ | ○ | ○ | BSC | 34 days | Morimoto et al. | ||
Abbreviations: BSC: best supportive care; F: female; M: male; RT: radiation therapy.
Men et al. 13 reported that, among 114 patients with C‐SCLC, 66 underwent surgery (57.9%), 48 received radiation therapy (42.1%), and 96 received systemic chemotherapy (84.2%) that included cisplatin/carboplatin combined with etoposide (57.3%) and platinum combined with paclitaxel (12.5%), irinotecan (7.3%), pemetrexed (5.2%), and other regimens (17.7%), with relatively low responses. The reported six patients with C‐SCLC with spindle‐shaped cell tumors were treated with radiation therapy and surgery, and none with chemotherapy (Table 2). Both C‐SCLC and pleomorphic carcinoma of the lung, which includes spindle‐shaped/giant cell tumors, are reportedly resistant to chemotherapy. 1 , 14 Based on this, C‐SCLC including spindle‐shaped cell tumor possibly resistant to systemic chemotherapy. Further clinical investigations are needed to determine the most appropriate chemotherapeutic regimens for treating patients with C‐SCLC including a spindle‐shaped cell tumor.
Regarding the prognosis of C‐SCLC, few factors are available for predicting the prognosis of patients with C‐SCLC. The survival rate is 31% for stage 1 with no 5‐year survivors observed in stages 2 and 3, making the prognosis poor for advanced cancer. 4 Pathological heterogeneity significantly affects prognosis, especially in tumors containing sarcomatous/spindle cell components. 15 , 16 , 17 As there have been no reports on the prognosis of patients with C‐SCLC including spindle‐shaped cell components, we only discuss the prognosis of pleomorphic carcinoma, which is pathologically similar to C‐SCLC in that it has spindle cells. Pleomorphic carcinoma is a poorly differentiated NSCLC containing at least 10% spindle and/or giant cells, or a carcinoma consisting only of spindle and giant cells in combination with SCC, adenocarcinoma, or undifferentiated NSCLC. When SCLC is combined with these components, it is called C‐SCLC instead of pleomorphic carcinoma. Hence, our patient was diagnosed with C‐SCLC. The overall survival rate of patients with pleomorphic (spindle/giant cell) carcinoma of the lung is poor. The median survival time is 10 months with a five‐year survival rate of 10%. 15 However, the clinical course of C‐SCLC (including spindle‐shaped cell tumors) remains unclear (Table 2). Our patient's tumor rapidly increased in size by 1.46‐fold in nine days and 2.59‐fold in 20 days. The serum lactate dehydrogenase (LDH) concentration is reportedly associated with tumor invasion and metastasis. 18 Increasing serum LDH levels were accompanied by disease progression in the patient reported here (Figure 3). This finding suggests that LDH is a viable biomarker for disease progression in patients with C‐SCLC patients with spindle cell tumor. This might have been because of the rapid progression and poor prognosis of C‐SCLC including a spindle‐shaped cell tumor compared with that of SCLC. Physicians should be aware of the proper diagnosis of C‐SCLC including spindle‐shaped cell tumors in patients with rapidly progressive lung cancer in addition to SCLC. Performing the relevant examinations for early and sufficient diagnosis to provide appropriate treatment are recommended.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
AUTHOR CONTRIBUTION
Toshiki Morimoto, Kei Yamasaki and Kazuhiro Yatera drafted the manuscript. Toshiki Morimoto, Tatsuya Shingu, Tomoki Sato, Takumu Uryu, Kaori Kato were in charge of this patient. Takanobu Jotatsu, Hiroki Kawabata and Chinatsu Nishida helped to draft the manuscript. All authors read and approved the final manuscript.
Morimoto T, Yamasaki K, Shingu T, Sato T, Uryu T, Jotatsu T, et al. Autopsy case of a patient with rapidly progressive combined small‐cell lung carcinoma with spindle‐shaped cell tumor. Thorac Cancer. 2022;13(15):2279–2282. 10.1111/1759-7714.14559
REFERENCES
- 1. Qin J, Lu H. Combined small‐cell lung carcinoma. Onco Targets Ther. 2018;11:3505–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Nicholson SA, Beasley MB, Brambilla E, Hasleton PS, Colby TV, Sheppard MN, et al. Small cell lung carcinoma (SCLC): a clinicopathologic study of 100 cases with surgical specimens. Am J Surg Pathol. 2002;26:1184–97. [DOI] [PubMed] [Google Scholar]
- 3. Babakoohi S, Fu P, Yang M, Linden PA, Dowlati A. Combined SCLC clinical and pathologic characteristics. Clin Lung Cancer. 2013;14:113–9. [DOI] [PubMed] [Google Scholar]
- 4. Hage R, Elbers JRJ, Brutel de la Riviere A, et al. Surgery for combined type small cell lung carcinoma. Thorax. 1998;53:450–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Roggli VL, Vollmer RT, Greenberg SD, McGavran MH, Spjut HJ, Yesner R. Lung cancer heterogeneity: a blinded and randomized study of 100 consecutive cases. Hum Pathol. 1985;16:569–79. [DOI] [PubMed] [Google Scholar]
- 6. Hirsch FR, Matthews MJ, Aisner S, Campobasso O, Elema JD, Gazdar AF, et al. Histopathologic classification of small cell lung cancer. Changing concepts and terminology. Cancer. 1988;62:973–7. [DOI] [PubMed] [Google Scholar]
- 7. Tsubota YT, Kawaguchi T, Hoso T, Nishino E, Travis WD. A combined small cell and spindle cell carcinoma of the lung. Report of a unique case with immunohistochemical and ultrastructural studies. Am J Surg Pathol. 1992;16:1108–15. [DOI] [PubMed] [Google Scholar]
- 8. Niho S, Yokose T, Nagai K, Nishiwaki Y, Kodama T, Mukai K. A case of synchronous double primary lung cancer with neuroendocrine features. Jpn J Clin Oncol. 1999;29:219–25. [DOI] [PubMed] [Google Scholar]
- 9. Gotoh M, Yamamoto Y, Huang CL, Yokomine H. A combined small cell carcinoma of the lung containing three components: small cell, spindle cell and squamous cell carcinoma. Eur J Cardiothorac Surg. 2004;26:1047–9. [DOI] [PubMed] [Google Scholar]
- 10. Hsiao HH, Tsai HJ, Liu YC, Tseng SB, Chai CY, Lin SF. A rare case of combined small‐cell lung cancer with unusual soft tissue metastasis. Kaohsiung J Med Sci. 2006;22:352–6. [DOI] [PubMed] [Google Scholar]
- 11. Pelosi G, Sonzogni A, Galetta D, Perrone F, Braidotti P, Manzotti M, et al. Combined small‐cell carcinoma of the lung with quadripartite differentiation of epithelial, neuroendocrine, skeletal muscle, and myofibroblastic type. Virchows Arch. 2011;458:497–503. [DOI] [PubMed] [Google Scholar]
- 12. Asahina M, Fukumura Y, Mamat O, Saito T, Hayashi T, Uekusa T, et al. A case of combined small cell lung carcinoma with unique morphology: investigation of tumorigenesis. Pathol Int. 2018;68:618–23. [DOI] [PubMed] [Google Scholar]
- 13. Men Y, Hui Z, Liang J, Feng Q, Chen D, Zhang H, et al. Further understanding of an uncommon disease of combined small cell lung cancer: clinical features and prognostic factors of 114 cases. Chin J Cancer Res. 2016;28:486–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bae HM, Min HS, Lee SH, Kim DW, Chung DH, Lee JS, et al. Palliative chemotherapy for pulmonary pleomorphic carcinoma. Lung Cancer. 2007;57:112–5. [DOI] [PubMed] [Google Scholar]
- 15. Fishback NF, Travis WD, Moran CA, Guinee DG Jr, McCarthy WF, Koss MN. Pleomorphic (spindle/giant cell) carcinoma of the lung. A clinicopathologic correlation of 78 cases. Cancer. 1994;73:2936–45. [DOI] [PubMed] [Google Scholar]
- 16. Ruffini E, Rena O, Oliaro A, et al. Lung tumors with mixed histologic patterns. Clinico‐pathologic characteristics and prognostic significance. Eur J Cardiothorac Surg. 2002;22:701–7. [DOI] [PubMed] [Google Scholar]
- 17. Nappi O, Glasner SD, Swanson PE, Wick MR. Biphasic and monophasic sarcomatoid carcinomas of the lung: a reappraisal of “carcinosarcomas” and “spindle‐cell carcinomas”. Am J Clin Pathol. 1994;102:331–40. [DOI] [PubMed] [Google Scholar]
- 18. de Jong C, Deneer VHM, Kelder JC, Ruven H, Egberts TCG, Herder GJM. Association between serum biomarkers CEA and LDH and response in advanced non‐small cell lung cancer patients treated with platinum‐based chemotherapy. Thorac Cancer. 2020;11:1790–800. [DOI] [PMC free article] [PubMed] [Google Scholar]


