Abstract
Background
Falls are inherent to Parkinson's disease (PD) progression, and risk assessment is mandatory for optimal long term management.
Objective
To determine if the telehealth application of two observer‐based, objective measures of fall‐risk in PD—Five‐Times‐Sit‐To‐Stand (FTSTS) and 360° Rapid‐Turns‐Test (RTT)—is feasible and safe.
Methods
Following in‐clinic training, 15 people with Hoehn and Yahr Stage 2 (n = 8) and 3 (n = 7) PD, median MoCA score 25 (range 14–29), and subjective freezing‐of‐gait (n = 13), participated in four televisits with care partners biweekly for 10 weeks where virtual FTSTS/RTT assessments were performed.
Results
Participants completed all protocol‐driven 120 virtual FTSTS and 60 RTT assessments with effective ratability (feasibility) and zero adverse events (safety). 22% virtual FTSTS and 55% RTT met criteria for high fall‐risk designation.
Conclusions
Objective fall‐risk assessment with virtual FTSTS and RTT through telehealth among HY2‐3 PD patients, with varying motor and cognitive function, is feasible and safe following introductory in‐clinic training.
Keywords: Parkinson's disease, telemedicine
In response to the SARS‐CoV‐2 pandemic, telehealth utilization increased globally within Movement Disorders Neurology, even in countries without prior experience. 1 In addition to shifting towards more remote‐based clinical solutions, telemedicine is now being applied to clinical research and rehabilitative services to ensure follow‐up and improve access. 2 , 3 In a sub‐study of the phase 3 STEADY‐PD trial of isradipine in Parkinson's Disease (PD) patients, 95% of 40 participants completed research televisits, and 75% expressed higher likelihood of future research enrollment if some visits were conducted remotely. 4
In PD, safe and validated remote, or “virtual,” assessment of fall risk through the detection of postural instability and PD‐specific gait dysfunction, including freezing‐of‐gait (FOG), would be particularly useful and relevant to clinical care and research. It is well‐known that there is a strong correlation between the onset of postural instability (ie Hoehn & Yahr stage 3 [HY3]) and falls. 5 , 6 FOG is another common reason for falls, occurring episodically both in ON‐ and OFF‐dopaminergic states and triggered by specific challenging environments. 7 , 8 , 9 , 10 For clinical care, the consequences of falls include accelerated immobility due to fractures and other injuries, reduced cardiovascular health, quality‐of‐life, and independence, and increased likelihood of institutionalization. 11 , 12 , 13 For research, progression to HY3 is a relevant motor endpoint for observational and interventional trials. 14 , 15 To enhance both clinical care and research, we report efforts to establish a safe, validated at‐home fall‐risk assessment protocol that can be rated virtually and executed by PD patients and their care partners without on‐site professional oversight.
Methods
Virtual Assessments
We conducted a systematic review of objective validated measures of gait and balance function in PD. 16 , 17 We considered objectivity, simplicity, training and equipment needs, ease‐of‐execution, and ratability by observation only. We prioritized measures with published evidence of high test–retest and interrater reliability and validity in PD, and those that could evaluate the most influential physical factors in PD related to fall‐risk. 18 , 19 , 20 , 21 , 22 Of 10 measures considered (Table S1), we selected the Five‐times‐sit‐to‐stand (FTSTS) that assesses fall‐risk by challenging postural instability and lower extremity strength, and the 360° rapid‐turns‐test (RTT) that assesses fall‐risk by identifying underlying FOG. Both measures have cut‐off designations for high fall‐risk and are ratable through observation. 18 , 19 , 20
Participants
This feasibility study was one component of a pilot 10‐week virtual fall‐prevention program for PD patients involving tele‐physical therapy (PT) and tele‐occupational therapy (OT), developed by the first author. The overarching study was approved by the Rush University Institutional Review Board and the final results will be reported separately. The feasibility study enrolled sequential English‐speaking PD patients over the age of 18 with HY2‐3 PD (as rated during the OFF‐dopaminergic medication state) who had been identified by their Movement Disorder neurologist as likely to benefit from PT and OT to reduce fall‐risk, between January 2020–March 2021. Participants were required to have a care partner to provide stand‐by assistance at all televisits, home WiFi connectivity, and videoconferencing technology for televisits, as well as a stable PD medication regimen for 1 month preceding and during study participation. Exclusion criteria included unstable medical or other neurological conditions affecting mobility or committed participation, including a clinical diagnosis of dementia.
Study Protocol
All participants had an initial in‐person clinic evaluation, followed by four televisits every 2 weeks and then a final in‐person visit (Fig. 1). Televisits were performed on the HIPAA‐compliant secure VidyoConnect Epic platform. Participants used their own or a loaned Apple iPad and connected to their home WiFi to participate in televisits. At the baseline in‐person visit, the study physical therapist demonstrated the FTSTS and RTT measures and obtained baseline scores. Participants learned how to execute measures at home, and care partners were instructed on stand‐by assistance measures. At all televisits, participants were in an ON‐dopaminergic medication state and wore a gait belt. The same physical therapist provided the training and virtual FTSTS and RTT assessments.
FIG. 1.
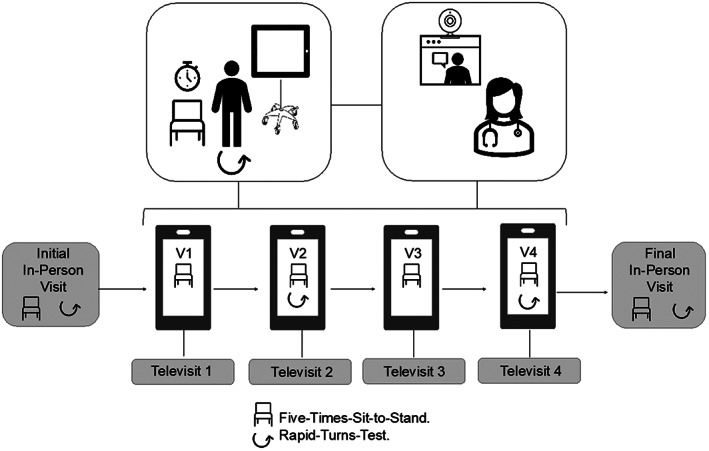
Schematic of virtual assessments. Participants were instructed on the how to perform the FTSTS and RTT maneuvers at their initial in‐person evaluation and then asked to perform them independently over video with care partner supervision over four biweekly televisits across 10 weeks (FTSTS: Televisits 1–4, RTT: Televisits 2 and 4).
For the FTSTS, participants were instructed to use the same free‐standing dining chair with or without armrests of reasonable height (43–45 cm) from their home in an open space, sit with arms folded across the chest and back against the chair, and stand up and sit down five times as quickly as possible. Participants were instructed to straighten legs completely upon standing and to sit fully, but they did not need to touch the back of the chair each time. Two timed trials were rated for the FTSTS, with high fall‐risk designation defined by an inability to complete five repetitions without upper extremity support or assistance within 16 seconds. 18 For the RTT, participants performed 360° narrow turns with small rapid steps starting from standstill, twice rightward and twice leftward. If freezing occurred at any point, the patient was identified as a “definite freezer,” automatically designating them as high fall‐risk. 20 , 21 , 22 , 23
Data Analysis
Descriptive statistics examined demographic data (Table 1). Feasibility was determined by the proportion of effectively completed tests without limitations (adherence and ratability), and safety was determined by the number and nature of adverse events related to the virtual measures. In the case of failures or limitations, the cause was categorized as patient‐based, environmental, or technical. Ratability was assessed as YES/NO by the physical therapist immediately after each measure.
TABLE 1.
Baseline participant demographics and clinical characteristics
| Characteristic | n (%) |
|---|---|
| Age | |
| 60–64 years | 4 (26.7%) |
| ≥65 | 11 (73.3%) |
| Median [IQR]: 67 [64–73] | |
| Gender | |
| Male | 10 (66.7%) |
| Race | |
| White | 11 (73.3%) |
| Black | 1 (6.7%) |
| Asian | 3 (20.0%) |
| MoCA score | |
| 0–25 | 8 (53.3%) |
| ≥26 | 7 (46.7%) |
| Median [IQR]: 25 [21–27] | |
| HY stage 1 | |
| Stage 2 | 8 (53.3%) |
| Stage 3 | 7 (46.7%) |
| MDS‐UPDRS part III score 1 | |
| 1–19 | 0 (0%) |
| 20–38 | 6 (40%) |
| ≥39 | 9 (60%) |
| Median [IQR]: 41 [31–50] | |
| Disease duration 2 | |
| 0–4 years | 2 (13.3%) |
| ≥5 | 13 (86.7%) |
| Median [IQR]: 8 [5–8] | |
| LEDD | |
| 0–100 mg/day | 1 (6.7%) |
| 101–399 | 2 (13.3%) |
| 400–999 | 10 (66.7%) |
| ≥1000 | 2 (13.3%) |
| Median [IQR]: 550 [400–850] | |
| History of a fall within the last year | |
| Yes | 12 (80%) |
| No | 3 (20%) |
| History of recurrent falls within the last year | |
| Yes | 11(73.3%) |
| No | 4 (26.7%) |
| Subjective history of freezing‐of‐gait 3 | |
| Yes | 13 (86.7%) |
| No | 2 (13.3%) |
| History of falls during 10‐week study period | |
| Yes | 10 (66.7%) |
| No | 5 (33.3%) |
1Assessed in the OFF‐dopaminergic medication state.
2From initial motor symptoms.
Based on interview and response to the New Freezing of Gait Questionnaire.
Abbreviations: IQR, Interquartile range; MoCA, Montreal Cognitive Assessment; HY, Hoehn and Yahr Scale; MDS‐UPDRS, Movement Disorders Society Unified Parkinson's Disease Rating Scale; LEDD, total levodopa‐equivalent daily dose.
Results
Of the 15 participants, eight had HY2 PD, seven had HY3 PD (Table 1), and the majority had a PD duration from initial symptom‐onset of greater than 5 years (n = 13, 86.7%). Most had a history of more than one fall within the last year (n = 11, 73%) and had fallen within the 10‐week study period (n = 10, 66.7%). All but two subjects endorsed FOG by self‐report (upon interview and the New Freezing of Gait‐Questionnaire) at baseline (n = 13, 86.7%). On cognitive testing, the median MoCA score was 25 [IQR 21–27, RNG 14–29] with a near even split between normal and impaired (n = 7 with ≥26, n = 8 with <26). No participants carried dementia diagnoses.
Feasibility. All participants completed baseline in‐person training and four televisits, providing data from 60 televisits where 120 virtual FTSTS and 60 virtual RTT were performed with 100% participant compliance and 100% ratability over video. No limitations were encountered related to patient and care partner participation, home environment, equipment, and web‐based videoconferencing. Of note, of 63 PD patients screened for eligibility based on disease severity alone (HY 2–3), 98% had access to home WiFi, 94% had a smartphone, and 68% even owned an iPad. Only five of the 15 participating pairs required a loaned iPad.
Safety. No falls, near‐falls, or any other adverse event occurred. Care partners, though present, did not need to intervene in any instance for safety concerns.
Ratings. 26/120 virtual FTSTS (22%) assessments met the criteria of high fall‐risk (>16 seconds or requiring upper extremity support), resulting from 9/15 participants (60%). Of the 60 virtual RTT performed, more than half of the assessments demonstrated FOG (n = 33, 55%), resulting from 9/15 participants (60%). An exploratory analysis of the association between FTSTS and RTT results revealed that high fall‐risk based on virtual FTSTS time did not make participants more likely to exhibit FOG detected by virtual RTT at the same televisit [2nd televisit: RR = 0.38, 95% CI = 0.05–2.59]; 4th televisit: RR = 0.44, 95% CI = 0.05–3.85]. For instance, the patient who was deemed to be high fall‐risk during all of his televisits based on high FTSTS times did not exhibit FOG at any of his televisits, nor his in‐person visits in the OFF‐medication state.
Discussion
Individuals with chronic neurological diseases like PD that impact gait and balance require frequent fall‐risk assessment, especially as disability advances. 6 , 22 , 23 Travel time and safety concerns limit in‐person clinic visits, a pattern accentuated by public health emergencies like the SARS‐CoV‐2 pandemic. Clinic visits alone can give a false impression of PD patients' actual day‐to‐day performance. For example, FOG, an environmentally‐sensitive and intermittent phenomenon can be much less pronounced during in‐clinic evaluations, even among individuals severely affected by FOG at home. 24 , 25 , 26 If feasible and safe, at‐home objective assessments using web‐based videoconferencing would reduce patient burden, provide diagnostic value and ecologically‐valid assessment, and offer continuity‐of‐care while retaining face‐to‐face interaction and personal connection. 27 , 28 Similarly, for research, valid tools that can be applied through telehealth could improve recruitment and retention, reduce costs, and ensure single rater consistency for clinical endpoints. 3 As our screening data suggests, there is also increasing evidence for the ubiquity of home internet and smartphones among the aging population. 29 , 30 , 31
There is a growing body of literature supporting the feasibility, validity, and reliability of conducting motor assessments remotely in PD, but the objective assessment of rigidity, postural stability, and overall fall‐risk have been considered problematic on standard rating scales without in‐person assessment. 27 , 32 , 33 , 34 In this study, we tested the feasibility (adherence and ratability) and safety (adverse events) of two standard observer‐based fall‐risk measures applied virtually following a single in‐person training session in ambulatory PD patients deemed at risk for falls by their neurologist and with a history of falls and/or subjective FOG. Our first finding showed that patient and care partner pairs understood and completed with full safety the two measures in their own homes, providing easily ratable remote data for the presence or absence of professionally‐designated fall‐risk. This was the case despite being high fall‐risk on testing and cognitively‐impaired based on MoCA scores. The incongruence between the results of the two virtual measures appreciated in some patients demonstrated the complimentary nature of these tools (assessing for postural stability and lower extremity strength versus FOG), consistent with what is known about the distinct mechanisms underlying imbalance in PD. 35 , 36 , 37 This highlights the value of both virtual measures in guiding clinical decision‐making. Given that patient self‐reports of perceived FOG are not always reliable, we could consider the use of telehealth‐based objective testing like the virtual RTT as especially important in identifying freezers. 24 , 25 , 26 , 38 , 39 , 40 , 41
We recognize the limitations of this study are the small sample size and protocol tailored to the technological capabilities of a single medical center and its patients. Future investigations could include a larger, multicenter, and cross‐cultural study, testing the use of lower‐resolution smartphones in lieu of tablets, and potentially real‐time ratings with wearable sensors to provide validation. In this study, we focused only on the feasibility and safety of the virtual measures and not specifically on other important clinimetric elements like discriminatory capacity or responsivity to interventional change to address longitudinal value.
Author Roles
(1) Research Project: A. Conception, B. Organization, C. Execution; (2) Statistical Analysis: A. Design, B. Execution, C. Review and Critique; (3) Manuscript Preparation: A. Writing the First Draft, B. Review and Critique.
MA: 1A, 1B, 1C, 2A, 2B, 2C, 3A, 3B.
AVH: 1B, 1C, 2A, 2B, 2C, 3B.
JN: 1A, 3B.
BRB: 1A, 3B.
CGG: 1A, 1B, 1C, 2A, 2B, 2C, 3B.
Disclosures
Ethical Compliance Statement
This study was approved by the Rush University Medical Center Institutional Review Board. Written informed consent was obtained from all participants. We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this work is consistent with those guidelines.
Funding Sources and Conflicts of Interest
This work was funded by grants from the Rush University Department of Neurological Sciences and the Consolidated Anti‐Aging Foundation awarded to MA. The authors declare that there are no conflicts of interest relevant to this work.
Financial Disclosures for the Previous 12 Months
MA: MA has received research grant support from the Rush University Department of Neurological Sciences and the Illinois Consolidated Anti‐Aging Foundation to support this work. AVH: None. JN: JN reports grants from ZonMW (OffRoad grant, Veni grant), Michael J. Fox Foundation, Ipsen Pharmaceuticals, and Gossweiler Foundation outside the submitted work. He serves at the medical advisory board of Cue2Walk and Ceriter. BRB: BRB has received fees from serving on the scientific advisory board for UCB, Kyowa Kirin, Zambon and the Critical Path Institute (paid to the Institute), has received fees for speaking at conferences from AbbVie, Biogen, UCB, Zambon, Roche, GE Healthcare, Oruen and Bial (paid to the Institute), and has received research support from the Netherlands Organization for Health Research and Development, the Michael J Fox Foundation, UCB, the Stichting Parkinson Fonds, Hersenstichting Nederland, de Stichting Woelse Waard, Stichting Alkemade‐Keuls, de Maag Lever Darm Stichting, Parkinson NL, Davis Phinney Foundation, the Parkinson's Foundation, Verily Life Sciences, Horizon 2020, the Topsector Life Sciences and Health, Nothing Impossible and the Parkinson Vereniging, outside the submitted work. CGG: CGG has received grant support from the National Institutes of Health, US Department of Defense, and Michael J. Fox Foundation outside the submitted work, faculty and speaker honoraria from the International Parkinson and Movement Disorder Society, Charlotte County Medical Society, and Oruen Ltdk London UK, and publishing royalties from Elsevier Publishers, Wolters Kluwer Publishers, and Oxford University Press.
Supporting information
Table S1. Observer‐Based Gait and Balance Measures Validated in PD. 16 , 17 The authors systematically reviewed 10 objective validated measures of gait and balance function in PD when considering which measures could best be performed virtually by participant and care partner pairs in this study. 16 , 17 This table presents the authors’ ratings on each measure's objectivity, simplicity, training and equipment needs, ease‐of‐execution, ratability by observation, equipment needs, and independence from on‐site professional involvement.
References
- 1. Hassan A, Mari Z, Gatto EM, et al. Global survey on telemedicine utilization for movement disorders during the COVID‐19 pandemic. Mov Disord 2020;35:1701–1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Dorsey ER, Okun MS, Bloem BR. Care, convenience, comfort, confidentiality, and contagion: The 5 C's that will shape the future of telemedicine. J Parkinsons Dis 2020;10(3):893–897. [DOI] [PubMed] [Google Scholar]
- 3. Naito A, Wills A, Tropea T, et al. Expediting telehealth use in clinical research studies: Recommendations for overcoming barriers. NPJ Parkinsons Dis 2021;7(1):34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tarolli CG, Andrzejewski K, Zimmerman GA. Feasibility, reliability, and value of remote video‐based trial visits in Parkinson's disease. J Parkinsons Dis 2020;10:1779–1786. [DOI] [PubMed] [Google Scholar]
- 5. Pickering RM, Grimbergen YA, Rigney U, et al. A meta‐analysis of six prospective studies of falling in Parkinson's disease. Mov Disord 2007;22:1892–1900. [DOI] [PubMed] [Google Scholar]
- 6. Hely MA, Morris JG, Reid WG, et al. Sydney multicenter study of Parkinson's disease: non‐L‐dopa‐responsive problems dominate at 15 years. Mov Disord 2005;20:190–199. [DOI] [PubMed] [Google Scholar]
- 7. Okuma Y. Freezing of gait and falls in Parkinson's disease. J Parkinsons Dis 2014;4(2):255–260. [DOI] [PubMed] [Google Scholar]
- 8. Latt MD, Lord SR, Morris JG, Fung VS. Clinical and physiological assessments for elucidating falls risk in Parkinson's disease. Mov Disord 2009;24(9):1280e9–1280e1289. [DOI] [PubMed] [Google Scholar]
- 9. Kerr GK, Worringham CJ, Cole MH, Lacherez PF, Wood JM, Silburn PA. Predictors of future falls in Parkinson disease. Neurology 2010;75(2):116e24–116e124. [DOI] [PubMed] [Google Scholar]
- 10. Okuma Y, Silva de Lima AL, Fukae J, Bloem BR, Snijders AH. A prospective study of falls in relation to freezing of gait and response fluctuations in Parkinson's disease. Parkinsonism Relat Disord 2018;46:30–35. [DOI] [PubMed] [Google Scholar]
- 11. Muslimovic D, Post B, Speelman JD, Schmand B, De Haan RJ, (CARPA study group) . Determinants of disability and quality of life in mild to moderate Parkinson disease. Neurology 2008;70(23):2241–2247. [DOI] [PubMed] [Google Scholar]
- 12. Pressley JC, Louis ED, Tang MX, Cote L, Cohen PD, Glied S, Mayeux R. The impact of comorbid disease and injuries on resource use and expenditures in parkinsonism. Neurology 2003;60(1):87–93. [DOI] [PubMed] [Google Scholar]
- 13. Paul SS, Sherrington C, Canning CG, Fung VSC, Close JC, Lord SR. The relative contribution of physical and cognitive fall‐risk factors in people with Parkinson's disease: a large prospective cohort study. Neurorehabilit Neural Repair 2014;28:282–290. [DOI] [PubMed] [Google Scholar]
- 14. Marsili L, Mahajan A. Clinical milestones in Parkinson's disease: past, present, and future. J Neurol Sci 2021;432:120082. [DOI] [PubMed] [Google Scholar]
- 15. Goetz CG, Stebbins GT, Blasucci LM. Differential progression of motor impairment in levodopa‐treated Parkinson's disease. Mov Disord 2000;15:479–484. [DOI] [PubMed] [Google Scholar]
- 16. Keus SHJ, Munneke M, Graziano M, et al. European Physiotherapy Guideline for Parkinson's Disease. Royal Dutch Society of Physiotherapy (KNGF)/ParkinsonNet; the Netherlands: 2014. [Google Scholar]
- 17. The Parkinson Evidence Database to Guide Effectiveness (PD‐EDGE) Task Force 2014; Recommendations for patients with Parkinson disease. Accessed December 5, 2018. http://www.neuropt.org/docs/default-source/parkinson-edge/pdedge-all-documents-combined.pdf?sfvrsn=2
- 18. Duncan RP, Leddy AL, Earhart GM. Five times sit‐to‐stand test performance in Parkinson's disease. Arch Phys Med Rehabil 2011;92(9):1431–1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Buatois S, Miljkovic D, Manckoundia P, et al. Five times sit to stand test is a predictor of recurrent falls in healthy community‐living subjects aged 65 and older. J Am Geriatr Soc 2008;56(8):1575–1577. [DOI] [PubMed] [Google Scholar]
- 20. Snijders AH, Haaxma CA, Hagen YJ, Munneke M, Bloem BR. Freezer or non‐freezer: clinical assessment of freezing of gait. Parkinsonism Relat Disord 2012;18(2):149–154. [DOI] [PubMed] [Google Scholar]
- 21. Nonnekes J, Janssen AM, Mensink SH, Oude Nijhuis LB, Bloem BR, Snijders AH. Short rapid steps to provoke freezing of gait in Parkinson's disease. J Neurol 2014;261(9):1763–1767. [DOI] [PubMed] [Google Scholar]
- 22. Macht M, Kaussner Y, Möller JC, Stiasny‐Kolster K, Eggert KM, Krüger HP, Ellgring H. Predictors of freezing in Parkinson's disease: a survey of 6,620 patients. Mov Disord 2007;22(7):953–956. [DOI] [PubMed] [Google Scholar]
- 23. Allen NE, Schwarzel AK, Canning CG. Recurrent falls in Parkinson's disease: a systematic review. Parkinsons Dis 2013;2013:906274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Snijders AH, Nijkrake MJ, Bakker M, Munneke M, Wind C, Bloem BR. Clinimetrics of freezing of gait. Mov Disord 2008;23(Suppl 2):S468–S474. [DOI] [PubMed] [Google Scholar]
- 25. Nutt JG, Bloem BR, Giladi N, Hallett M, Horak FB, Nieuwboer A. Freezing of gait: moving forward on a mysterious clinical phenomenon. Lancet Neurol 2011;10:734–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Morris TR, Cho C, Dilda V, Shine JM, Naismith SL, Lewis SJ, Moore ST. A comparison of clinical and objective measures of freezing of gait in Parkinson's disease. Parkinsonism Relat Disord 2012;18:572–577. [DOI] [PubMed] [Google Scholar]
- 27. Dorsey ER, Deuel LM, Voss TS. Increasing access to specialty care: a pilot, randomized controlled trial of telemedicine for Parkinson's disease. Mov Disord 2010;25(11):1652–1659. [DOI] [PubMed] [Google Scholar]
- 28. Picillo KN, Barone P, Fasano A. Recruitment strategies and patient selection in clinical trials for Parkinson's disease: going viral and keeping science and ethics at the highest standards. Parkinsonism Relat Disord 2015;21(9):1041–1048. [DOI] [PubMed] [Google Scholar]
- 29. Berenguer A, Goncalves J, Hosio S, Ferreira D, Anagnostopoulos T, Kostakos V. Are smartphones ubiquitous? An in‐depth survey of smartphone adoption by seniors. IEEE Consum Electron Mag 2017;6:104–110. 10.1109/MCE.2016.2614524. [DOI] [Google Scholar]
- 30. Pheeraphuttharangkoon S, Choudrie J, Zamani ED, & Giaglis GM. Investigating the adoption and use of smartphones in the UK: a silver‐surfers perspective. 22nd European Conference on Information Systems (ECIS 2014).
- 31. Mohadisdudis HM, Mohamad Ali N. A study of smartphone usage and barriers among the elderly. The institute of electrical and electronics engineers, Inc (IEEE) Conference Proceedings 2014; 109. [Google Scholar]
- 32. Biglan KM, Voss TS, Deuel LM. Telemedicine for the care of nursing home residents with Parkinson's disease. Mov Disord 2009;24(7):1073–1076. [DOI] [PubMed] [Google Scholar]
- 33. Abdolahi A, Scoglio N, Killoran A. Potential reliability and validity of a modified version of the unified Parkinson's disease rating scale that could be administered remotely. Parkinsonism Relat Disord 2013;19:218–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Goetz CG, Stebbins GT, Luo S. Movement Disorder Society–unified Parkinson's disease rating scale use in the Covid‐19 era. Mov Disord 2020;35(6):911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Goetz CG, Luo S, Wang L, Tilley BC, LaPelle NR, Stebbins GT. Handling missing values in the MDS‐UPDRS. Mov Disord 2015;30(12):1632–1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bloem BR, Marinus J, Almeida Q, et al. Movement disorders society rating scales committee. Measurement instruments to assess posture, gait, and balance in Parkinson's disease: critique and recommendations. Mov Disord 2016;31(9):1342–1355. [DOI] [PubMed] [Google Scholar]
- 37. Coomans D, Spildooren J, Vercruysse S, Nieuwboer A, Kerckhofs E. Freezing of gait in Parkinson's disease: disturbances in automaticity and control. Front Hum Neurosci 2013;6:356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Vervoort G, Nackaerts E, Mohammadi F, Heremans E, Verschueren S, Nieuwboer A, Vercruysse S. Which aspects of postural control differentiate between patients with Parkinson's disease with and without freezing of gait? Parkinsons Dis 2013;2013:971480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Nieuwboer A, Giladi N. Characterizing freezing of gait in Parkinson's disease: models of an episodic phenomenon. Mov Disord 2013;28(11):1509–1519. [DOI] [PubMed] [Google Scholar]
- 40. Nieuwboer A, Giladi N. The challenge of evaluating freezing of gait in patients with Parkinson's disease. Br J Neurosurg 2008;22(Suppl 1):S16–S18. [DOI] [PubMed] [Google Scholar]
- 41. Hulzinga F, Nieuwboer A, Dijkstra BW, Mancini M, Strouwen C, Bloem BR, Ginis P. The new freezing of gait questionnaire: unsuitable as an outcome in clinical trials? Mov Disord Clin Pract 2020;7(2):199–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Observer‐Based Gait and Balance Measures Validated in PD. 16 , 17 The authors systematically reviewed 10 objective validated measures of gait and balance function in PD when considering which measures could best be performed virtually by participant and care partner pairs in this study. 16 , 17 This table presents the authors’ ratings on each measure's objectivity, simplicity, training and equipment needs, ease‐of‐execution, ratability by observation, equipment needs, and independence from on‐site professional involvement.


