Abstract
Various techniques for regional anesthesia and analgesia of the thorax are currently being used in clinical practice. A recent international consensus has anatomically classified paraspinal blocks in the thoracic spinal region into the following four types: paravertebral, retrolaminar, erector spinae plane, and intertransverse process blocks. These blocks have different anatomical targets; thus, the spreading patterns of the injectates differ and can consequently exhibit different neural blockade characteristics. The paravertebral block directly targets the paravertebral space just outside the neuraxial region and has an analgesic efficacy comparable to that of the epidural block; however, there are multiple potential risks associated with this technique. Retrolaminar and erector spinae plane blocks target the erector spinae plane on the vertebral lamina and transverse process, respectively. In anatomical studies, these two blocks showed different injectate spreading patterns to the back muscles and the fascial plane. In cadaveric studies, paravertebral spread was identified, but variable. However, numerous clinical reports have shown paravertebral spread with erector spinae plane blocks. Both techniques have been found to reduce postoperative pain compared to controls; however, the results have been more inconsistent than with the paravertebral block. Finally, the intertransverse process block targets the tissue complex posterior to the superior costotransverse ligament. Anatomical studies have revealed that this block has pathways that are more direct and closer to the paravertebral space than the retrolaminar and erector spinae plane blocks. Cadaveric evaluations have consistently shown promising results; however, further clinical studies using this technique are needed to confirm these anatomical findings.
Keywords: Analgesia, Anatomy, Anesthesia, Nerve block, Postoperative pain, Ultrasonography
Introduction
Performing a paravertebral block (PVB) in the thoracic region is a well-established technique for perioperative analgesia and chronic pain management of the thorax [1–3]. PVBs directly target the thoracic paravertebral space (TPVS), which contains the roots of the spinal nerves, making PVBs distinct from peripheral nerve blocks [4]. Clinically, successful PVBs result in a blockade of the ipsilateral, segmental, somatic, and sympathetic nerves in the dermatomes adjacent to the hemithorax [3]. This technique was first described by Hugo Sellheim in 1905 [1] and has since been modified and improved. However, even with ultrasound guidance, the potential risk of a pneumothorax, neurovascular damage, or unintentional neuraxial injection remain a concern [5–7].
Ultrasound-guided regional anesthesia techniques are fundamental components of multimodal perioperative care [8]. The utility of ultrasound has led to the development of many novel approaches of anesthesia and analgesia delivery, including the interfascial plane block, in which local anesthesia is injected into a fascial plane to indirectly access target nerves [9]. Since the erector spinae plane (ESP) block was first described by Forero et al. [10] in 2016, it has attracted considerable attention and has stimulated an explosion of interest in interfascial plane blocks. As a result, many block techniques have since been introduced; however, similar techniques carry different names, and techniques with the same name can have different technical approaches and targets. To standardize this, a recent international consensus has anatomically classified paraspinal blocks in the thoracic region into four types: PVBs, ESP blocks, retrolaminar blocks (RLBs), and intertransverse process (ITP) blocks [11]. Each paraspinal block is associated with different spreading patterns of injectate following different anatomical target points and consequently have different neural blockade characteristics when used clinically.
The purpose of this review is to outline the proposed mechanisms of action of each thoracic paraspinal block based on the available anatomical evidence and discuss clinical findings that have been reported to date.
Paravertebral block
Anatomical description
A PVB is anatomically described as a block injected into the paravertebral space between the superior costotransverse ligament (SCTL) and the parietal pleura in the thoracic region (Fig. 1) [11].
Fig. 1.
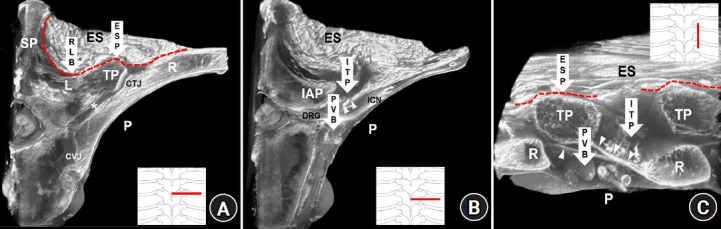
Anatomical targets of paraspinal blocks at the mid-thoracic region on sectional images of micro-computed tomography. (A) A cross-sectional image at the level of the transverse process. (B) A cross-sectional image of the intertransverse process region. (C) A sagittal-sectional image of the intertransverse process region. Arrows indicate the paraspinal block techniques. The arrowheads indicate the superior costotransverse ligament (SCTL). The asterisk indicates the costotransverse space between the rib (R) and transverse process (TP). The red dotted lines indicate the erector spinae fascial plane. PVB: paravertebral block, RLB: retrolaminar block, ESP: erector spinae plane block, ITP: intertransverse process block, SP: spinous process, L: vertebral lamina, P: pleura, CTJ: costotransverse joint, CVJ: costovertebral joint, ES: erector spinae muscles, IAP: inferior articular process, DRG: dorsal root ganglion, ICN: intercostal nerve.
Anatomical considerations
The TPVS contains the roots of the spinal nerve along with its dorsal and ventral branches and their plexuses, white and gray rami communicantes, the sympathetic ganglion, and sympathetic chain (Fig. 2) [2,4]. Thus, PVBs may be the closest anatomical approach to the concept of ‘paraneuraxial’ nerve blocks [12]. Even established anatomy textbooks, such as Gray’s Anatomy, do not use the term ‘paravertebral space’ [13]. The concept of the TPVS appears to have been shaped by clinical needs, as it has not been fully elucidated as a clearly delineated space from an anatomical perspective. Posteriorly, the TPVS is bounded by the transverse process, a rib, and the SCTL, and the needle should pass the SCTL to reach the TPVS for the conventional PVB [1,2]. In the classic literature, a subtle ‘pop’ or ‘click’ is felt upon the needle piercing the SCTL, and a loss of resistance is described [1,2]. In the T4–T5 region, for example, the SCTL originates from the superior surface of the fifth rib (Fig. 2) [14]. The anterior and posterior layers diverge from the common origin of the SCTL on the rib [14]. The anterior and posterior layers of the SCTL are attached to the inferior surfaces of the fourth rib and transverse process of T4, respectively [14]. In addition, the morphology of the SCTL with some anatomical variations seems to differ depending on the location of the upper or lower thoracic spine [15]. Therefore, although the SCTL is the most important landmark for the PVB, it is occasionally difficult to identify using ultrasonography. The SCTL is located very close to the pleura; in one anatomical report, the mean distance between the pleura and the attachment of the SCTL was only 7.8 mm [14]. Furthermore, the posterior intercostal artery and vein within the TPVS are located close to the pleura. The unique anatomy of the TPVS could explain the risks associated with PVBs.
Fig. 2.
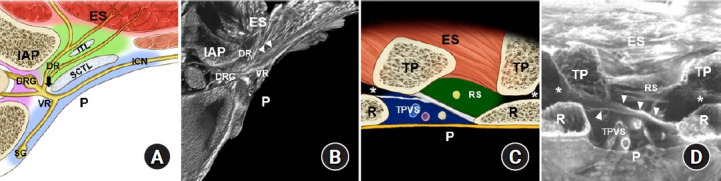
Paravertebral space and adjacent anatomical structures at the mid-thoracic region. (A) Illustrated diagram of a transverse sectional view of the intertransverse/intercostal region. (B) Micro-computed tomography image corresponding to illustration A [14]. (C) Illustrated diagram of a sagittal sectional view of the intertransverse process region. (D) Micro-computed tomography image corresponding to illustration C [14]. The arrow indicates the costotransverse foramen, and arrowheads indicate the anterior and posterior layer of the superior costotransverse ligament (SCTL). The asterisks indicate the costotransverse space between the rib (R) and transverse process (TP). ES: erector spinae muscle, IAP: inferior articular process, ITL: intertransverse ligament, DRG: dorsal root ganglion, DR: dorsal rami, VR: ventral rami, ICN: intercostal nerve, SG: sympathetic ganglion, P: pleura, TPVS: thoracic paravertebral space (bluish area), RS: retro-SCTL space (greenish area).
Potential risks of block-related complications
Although the reported incidence of procedure-related complications following PVBs varies among studies, it appears to be low when ultrasonography is used [5]. However, a recent meta-analysis reported a similar incidence of pneumothorax, pleural puncture, and vascular puncture at 0.3% with ultrasound-guided PVBs [7], although this is likely an underestimation due to under-reporting. Indeed, the incidence of procedure-related complications was higher with PVB than with other regional techniques for breast surgery [7]. Previously reported incidences of pneumothorax, pleural puncture, and vascular puncture after a PVB using the classic landmark technique were 0.5%, 1.1%, and 3.8%, respectively [16]. Therefore, while ultrasonography might reduce the failure rate of the PVB, clinicians should be aware of these potential procedural risks.
Spreading pattern of the injectate
Typically for PVBs, there is both an anteromedial spread of injectate within the TPVS and a lateral intercostal spread. A PVB with 20 ml of injectate has been shown to spread paravertebrally and intercostally over approximately 3-4 segments in cadavers [17]. A single injection of a PVB with 25 ml of local anesthetic and multiple injections of PVB with 5 ml at each of the five levels provided a similar sensory block over 4–6 dermatomes in patients undergoing a unilateral mastectomy [18]. The ventral rami of the spinal nerve and sympathetic ganglion were shown to be involved in successful thoracic PVBs, and epidural spread via the intervertebral foramen is often observed [17].
The endothoracic fascia seems to be significantly involved in the variability of injectate spreading patterns following a thoracic PVB. The endothoracic fascia, which is the deep fascia of the thorax, is a fibroelastic structure lining the thoracic cage. It is interposed between the parietal pleura and the innermost intercostal muscle in the chest wall, and between the parietal pleura and the SCTL or transverse process in the TPVS [19]. The endothoracic fascia divides the TPVS into two potential fascial compartments: the anterior (extrapleural) and the posterior (subendothoracic) compartment [2,19]. The sympathetic ganglion is contained in the extraplueral compartment, whereas the spinal nerve is located in the subendothoracic compartment [2,19]. This anatomy was confirmed by electron microscopy in rats [20]. However, the endothoracic fascia appears to be difficult to observe using a dissection technique in human tissues because it is a very thin fascia that is indistinguishable from the parietal pleura [21]. An injection in the extrapleural compartment may produce extensive longitudinal, prevertebral, and contralateral diffusion of the injectate with a sympathetic blockade [19,20]. In contrast, injections in the subendothoracic compartment of the TPVS may result in a cloud-like spreading pattern, with only limited distribution over adjacent segments, but with a greater chance of epidural spread [19,20]. The endothoracic fascia is superiorly continuous with the suprapleural membrane (Sibson’s fascia) that is attached to the inner border of the first rib and costal cartilage anteriorly, the C7 transverse process posteriorly, and the mediastinal pleura medially [19]. Inferiorly, the endothoracic fascia is continuous with the abdominal transversalis fascia [21,22]. This continuity occurs dorsal to the diaphragm through the lumbocostal arches and aortic hiatus [19]. The transversalis fascia blends medially with the fascia of the anterior layer of the quadratus lumborum and psoas fascia [22]. Anatomically, an injection into the subendothoracic compartment of the TPVS at the lower thoracic levels can spread caudally via the medial and lateral arcuate ligaments to the retroperitoneal space in the abdomen [22]. This spread can affect peripheral nerves originating from the lumbar plexus [19–21].
Clinical evidence
The PVB is the oldest paraspinal block technique, and thus there are numerous clinical reports of PVBs in patients with surgical and chronic pain. The PVB can also be used for surgical anesthesia. Historically, PVB was designed to replace spinal anesthesia and was frequently performed to control pain during abdominal surgery [1]. According to data taken from six randomized controlled trials, thoracic PVBs for surgical anesthesia are associated with lower pain intensity during the immediate postoperative period, less postoperative nausea and vomiting, a shorter length of hospital stay, and greater patient satisfaction than general anesthesia in patients undergoing breast surgery [23]. PVBs have shown similar postoperative pain control efficacy after thoracic surgery to thoracic epidural analgesia [24,25]. Moreover, contraindications to thoracic epidural analgesia do not preclude PVBs in most cases. For thoracic surgeries, such as thoracotomies, PVBs provide better postoperative analgesia and lower opioid consumption than ESP blocks and RLBs [26–28]. Although conflicting results have been reported for breast cancer surgery [29], the analgesic efficacy of PVBs was found to be superior to that of ESP and other truncal blocks [7]. The PVB was thus recommended as the first-choice regional analgesic technique for major breast surgery in recently published guidelines [30].
Retrolaminar block
Anatomical description
An RLB is anatomically described as an injection in the plane between the erector spinae muscles and the lamina (Fig. 1) [11].
Anatomical considerations
The RLB, also known as the ‘lamina technique,’ was introduced much earlier than the ESP block. This technique was first described by Pfeiffer in 2006 as a landmark (blind) technique [31]. The main advantage of this technique is that it is a very simple procedure to perform, requiring either a single injection or the use of a catheter. Furthermore, it can be performed in the cervical and lumbar regions, and thus has a wide range of clinical indications. The target injection point for an RLB is the posterior surface of the vertebral lamina [32], while the ESP block targets the fascial plane deep to the erector spinae muscle at the tip of the transverse process (Fig. 3) [10], which is located more laterally than the target point of an RLB. Although the injection locations for these two techniques differ, there are anatomical similarities, because the anterior fascia of the erector spinae muscle group adheres to both the lamina and the transverse process.
Fig. 3.
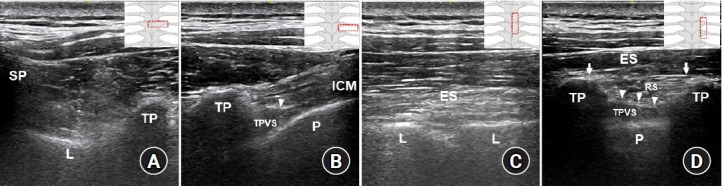
Ultrasound images of the paravertebral space and anatomical structures relevant to a paraspinal block at the mid-thoracic region. (A) A transverse scan to identify bony landmarks. (B) A transverse scan to perform a paravertebral block (PVB). (C) A parasagittal scan to perform a retrolaminar block (RLB). (D) A parasagittal scan of the intertransverse process (ITP) region. Illustrations in the right corner show the probe locations in each ultrasound image. Arrows indicate the erector spinae fascial plane (ESP), and arrowheads indicate the superior costotransverse ligament (SCTL). As shown in Fig. 3D, the erector spinae fascial plane is the target for an ESP block, the tissue complex posterior to the SCTL (retro-SCTL space) is the target for an ITP block, and the TPVS is directly targeted for the PVB. ES: erector spinae muscles, L: vertebral lamina, ICM: intercostal muscles, TP: transverse process, SP: spinous process, P: pleura, TPVS: thoracic paravertebral space, RS: retro-SCTL space.
Spreading pattern of the injectate
Some anatomical studies have demonstrated that with an RLB, the injectate spreads to the TPVS, epidural space, intercostal space, and intervertebral foramina; however, the spreading pattern appears to be quite variable [33,34]. Compared with the conventional PVB, the spread of injectate to the TPVS is much more limited with an RLB [33]. The spinous process, lamina, and facet joint capsule can act as anatomical barriers between the retrolaminar space and the TPVS [34]. The lateral tip of the transverse process is directly and indirectly connected to the two layers of the thoracolumbar fascia [35]. The ESP block injectate is placed directly into the fascial space of the thoracolumbar fascia. For an RLB, however, the injectate is inserted into a plane continuous with the fascial space of the thoracolumbar fascia. Thus, in both techniques, the injectate can spread directly and indirectly into the posterior layer of the thoracolumbar fascia or the posterior fascia of the erector spinae [34]. However, cadaveric evaluation found the injectate of RLBs and ESP blocks to have distinct spreading patterns [34]. With the ESP block, which involves a direct injection into the fascial plane, the dye spreads more laterally, while with the RLB, it spreads vertically along the posterior surface of the lamina [34]. RLBs result in an intensely stained retrolaminar plane beneath the transversospinalis and erector spinae muscles, with wide vertical spread, which may indicate an adequate blockade of the dorsal rami at the affected spinal level [34].
Clinical evidence
The RLB has been reported to be clinically effective in patients with rib fractures and those undergoing breast surgery [32,36,37]; however, clinical data regarding RLBs are more limited than the data available for the ESP block. In a previous report, the analgesic efficacy of the continuous RLB was found to be inferior to that of the continuous PVB in the first 24 h period after a mastectomy [38]. Furthermore, a single injection of an RLB was found to provide analgesia lasting only 2–3 h after breast surgery, which is a much shorter period than that reported for the PVB [39]. Additionally, in thoracoscopic surgery, PVBs provide better analgesia and result in less nausea than RLBs [28].
Erector spinae plane block
Anatomical description
The ESP block is anatomically described as an injection in the plane between the erector spinae muscles and the transverse process (Fig. 1) [11].
Proposed mechanisms of action
The ESP block has gained popularity as a simpler and safer technique than the traditional PVB, given the option of injection through a catheter. However, the exact mechanism of action is still unclear. Some proposed mechanisms of action include analgesic effects mediated by elevated local anesthetic plasma concentrations due to systemic absorption, immunomodulatory analgesic effects via the lymphatic system, or analgesic effects mediated through nerve innervation of the thoracolumbar fascia [40]. However, these findings seem to be inconclusive and require further investigation [40]. The most controversial topic is whether the ESP block produces a neural blockade from the direct spread of the local anesthetic to the TPVS. Paravertebral spread was originally proposed as the primary mechanism of action of the ESP block [10]. However, anatomical study results are inconsistent. Some previous cadaveric studies have revealed limited or even no paravertebral spread with the ESP block, but more predominant posterior back muscle or fascia spread [34,41]. Nevertheless, the available evidence shows that paravertebral spread can and does occur. Magnetic resonance imaging of living subjects have demonstrated contrast medium spread into the paravertebral and even epidural spaces across multi-segmental levels with the ESP block [42,43].
Limitations of cadaveric studies
Some limitations of cadaveric studies should be considered when interpreting these findings. The condition of the cadavers, such as their freeze-thaw status or whether they have been embalmed, can affect the results. Furthermore, owing to the nature of cadaveric studies, the sample size is limited and usually only descriptive results are reported. Additionally, postmortem changes in the muscle, fascia, and ligamentous tissue can significantly affect the diffusion of the injectate. More importantly, cadaveric studies cannot reflect delayed diffusion after an ESP block, as would occur with respiratory movement in living subjects.
Clinical evidence
When the ESP block was first introduced, there was some controversy regarding its anatomical similarity to the RLB [44,45]. However, RLBs and ESP blocks show different spreading patterns in cadavers and different block efficacy in patients [34,46]. Although there are no clear clinical comparisons and more clinical data regarding RLBs are needed, the clinical efficacy of single-shot RLBs is controversial, while ESP blocks have been found to result in a significant reduction in postoperative pain [47-49]. Currently (5–6 years after the ESP block was first introduced), a large number of randomized controlled trials investigating the analgesic efficacy of ESP blocks in almost all types of surgeries performed on the human torso have been published [50]. Overall, the ESP block has provided better pain relief and lower opioid consumption after a variety of surgeries, such as breast surgery, laparoscopic cholecystectomy, and thoracotomy, than sham or no-block groups [47-54]. However, it is uncertain whether the ESP block can replace the conventional PVB, which has been found to afford better pain relief after thoracotomy and breast surgeries in several studies [7,27,53–55], although there were some conflicting results [29,54]. Furthermore, block reproducibility with consistent results is a major concern in clinical practice. Therefore, more controlled studies are needed to compare ESP blocks with robust multimodal analgesic regimens. Dermatomal sensory blockade data on the ESP block should be evaluated according to the injected volume and spinal level. Considering the anatomical differences between the ESP block and PVB, it is reasonable to assume that these approaches differ in terms of their specific clinical indications and risk-benefit ratio. Thus, future clinical studies comparing ESP blocks with other truncal nerve blocks are warranted.
Spreading pattern of the injectate
A significant amount of injectate spread following an ESP block injection has been observed in the back muscles and fascial layer in previous anatomical evaluations [34,41]. This spreading pattern clearly supports the multisegmental involvement of the dorsal rami of the spinal nerves with the ESP block. Certainly, this characteristic of the ESP block, which is similar to that of the RLB, can result in good analgesia during surgical procedures involving the spine or back [52,56]. However, to block the ventral rami of the spinal nerve or sympathetic ganglion, local anesthetics should be spread into the TPVS. The fascia structure is highly permeable to macromolecules, including local anesthetic agents [40]. At the microscopic level, gaps in its largely acellular architecture of interlinked collagen fibers readily permit rapid diffusion [40,57]. The SCTL has a slit structure on both the medial and lateral ends, and its medial slit corresponds to the well-known anatomical ‘costotransverse foramen’ (Fig. 4). Thus, the TPVS is not an anatomically isolated compartment or closed space, as it communicates with the outside posteriorly via the slits of the SCTL [14]. Additionally, some microroutes involving muscular branches of the dorsal rami and intercostal nerves and vessels or through the intercostal membrane could contribute to the spread of the injectate to the TPVS associated with the ESP block [40]. Indeed, there is considerable clinical evidence that although it has been reported sporadically or described as a side effect, that ESP blocks can involve the ventral rami and sympathetic nerves, yielding analgesia for visceral pain and complex regional pain syndrome, some sympathetically mediated symptoms (Harlequin syndrome, priapism, hypotension), and even motor blockade [58–65].
Fig. 4.
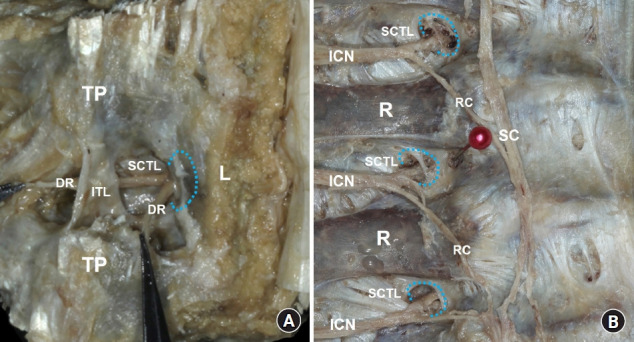
Costotransverse foramen (CTF) at the mid-thoracic region. (A) The erector spinae muscles have been removed, and the dorsal rami of the spinal nerve (DR) emerge from the medial slit of the superior costotransverse ligament (SCTL), corresponding to the CTF (blue-dotted line) beneath the vertebral lamina (L). (B) In the intrathoracic view, the CTFs can be clearly visualized after the pleura and vessels have been removed. The ventral rami of the spinal nerve (intercostal nerve [ICN]) pass through the CTFs (blue-dotted line). TP: transverse process, ITL: intertransverse ligament, R: rib, RC: rami communicants, SC: sympathetic chain.
Clinical considerations
Interfascial plane blocks are generally regarded as volume-dependent blocks [66]. The underlying principle is that a volume of local anesthetic is injected into a fascial plane remote from the intended site of action [9]. There are a few reports of local anesthetic systemic toxicity following ESP blocks with a large volume of local anesthetic [67,68]. However, the safe dose ranges of local anesthetics for ESP blocks have not been specifically evaluated based on serum concentrations. It is difficult to predict the volume of the injectate that will spread to the TPVS anteriorly or the back muscles and the fascial layer posteriorly with the ESP block. A previous cadaveric study showed that continually increasing the volume of injectate for ESP blocks does not guarantee an increase in the extent of paravertebral spread [69]. Therefore, for a safe and effective ESP block, clinical data regarding optimal dose-volume regimens that consider the patient condition, injection site, and types of local anesthetic should be gathered [70].
Intertransverse process block
Anatomical description
The ITP block is anatomically described as a block injected into the tissue between the two transverse processes, posterior to the SCTL, or halfway between the posterior aspect of the transverse process and the pleura (Fig. 1) [11].
Anatomical considerations
The ITP block, recently named by international consensus [11], is a collective name for several reported block techniques, including the mid-point transverse process to pleura block [71], multiple injection costotransverse block [72], subtransverse process interligamentary block [73], and costotransverse foramen block [74]. The concept of the ITP block was first introduced by Costache et al. [71] in 2017 as a midpoint transverse process to the pleura block. The target site for the ITP block is tissue, which is clearly different from that of the fascial plane target for an ESP block or RLB. Thus, an ITP block is not an interfascial plane block. The complex area posterior to the SCTL was first described as the ‘intertransverse tissue complex’ (ITTC) and comprises the intertransverse ligament, fatty tissue, the intertransverse and levatores costarum muscles, and the SCTL, which borders the TPVS [72]. While the ITTC has been designated from an anatomical perspective, ultrasound images have not been able to show its intricacies in detail. The proposed term for the space posterior to the SCTL, the ‘retro-SCTL space’ [14], might clinically represent the appropriate target area of an anatomically intricate tissue complex for the ITP block. The erector spinae fascia, SCTL, the pleura that demarcates the erector spinae muscle compartment, the retro-SCTL space, and the TPVS are important landmarks used during an ultrasound-guided ITP block (Fig. 3) [75]. Recent micro-computed tomography images have provided a detailed three-dimensional anatomical depiction of the TPVS and retro-SCTL space using cadavers (Supplemental Video 1) [14,75].
Spreading pattern of the injectate
In contrast to the ESP block or RLB, anatomical studies of ITP blocks have consistently demonstrated paravertebral spread with sympathetic involvement, which is a very similar pattern to that with the PVB (Fig. 5) [71,72,74,75]. The retro-SCTL space appears to directly communicate with the TPVS via the slit structure of the SCTL (the costotransverse foramen) and via the costotransverse space, which is the space between the transverse process and rib (Fig. 2) [14,75]. The costotransverse space is connected to the roof and base of the retro-SCTL space, and its base is incompletely covered by a part of the SCTL and radiate ligament [75]. Histological examination has revealed that the costotransverse space is mostly occupied by adipose and loose connective tissue [75]. In a cadaveric evaluation, the costotransverse foramen and costotransverse space were found to serve as anatomical conduits for anterior and intersegmental paravertebral spread of the ITP block, respectively [75]. Indeed, on real-time ultrasound images, anterior pleural displacement was observed with most ITP block injections [71,74,75]. However, some spreading of the injectate to the erector spinae fascia was also observed on real-time ultrasound images, indicating posterior spread [71,74,75]. Dye infiltration to the back muscles and fascia following the ITP block, which is similar to the spreading pattern of the ESP block, has been observed in most cadaveric studies [71,72,74,75]. Although this spread can contribute to the blockade of the dorsal rami of the spinal nerve, it can also result in the loss of injectate volume that can spread paravertebrally. A needle replacement technique has previously been proposed to minimize this posterior spread until adequate anterior spread is visualized by ultrasonography [74]. In terms of paravertebral spread, recent reports have suggested that as the ITP block involves injection into the retro-SCTL space, which has direct and close pathways to the TPVS, this could be anatomically more advantageous than the ESP block or RLB [71,72,74,75]. Local anesthetics follow a longer pathway to reach the TPVS, through both the erector spinae fascia and retro-SCTL space, with the RLB and ESP block. The bottom line shows that the ITP block has characteristics that fall somewhere between those of the PVB and ESP block.
Fig. 5.
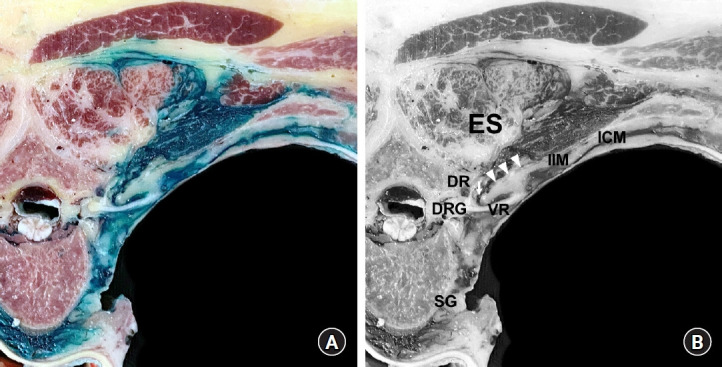
Dye spreading pattern following an intertransverse process (ITP) block. (A) A cross-sectional cut of a non-embalmed cadaver after a dye solution has been injected into the tissue complex posterior to the superior costotransverse ligament (SCTL), the retro-SCTL space, for an ITP block at the T4–T5 level [75]. (B) Anatomical structure annotations corresponding to Fig. 5A. The arrow indicates the costotransverse foramen (CTF; medial slit of the SCTL), and arrowheads indicate the SCTL. The dye was found to spread multidirectionally from the injection site (retro-SCTL space), into the paravertebral space through the CTF, intercostal space, and erector spinae (ES) compartment. The dye fully surrounded the dorsal rami (DR) and ventral rami (VR) of the spinal nerve and sympathetic ganglion (SG). DRG: dorsal root ganglion, IIM: intercostal membrane, ICM: intercostal muscles.
Clinical evidence
Indeed, despite the limited sample sizes of the studies, ITP blocks have been shown to have excellent analgesic efficacy and provide a strong sensory block in patients undergoing breast surgery or thoracotomy [71–74]. However, more data are required concerning the effectiveness of the ITP block compared to the ESP block or RLB. The clinical potential and efficacy of ITP blocks should be evaluated in further clinical studies and compared to those of thoracic paraspinal or truncal nerve blocks.
Conclusions
A precise anatomical understanding of the TPVS is essential for a successful and safe performance of thoracic paraspinal blocks. Despite some potential risks, the PVB is a well-established technique with a wealth of available clinical data and comparable analgesic efficacy to epidural blocks. Thus, education and training regarding thoracic PVB performance in clinical practice should continue. ESP blocks and RLBs, which are more superficial blocks, do not approach the pleura and neurovascular structures, and their procedure-related risks are lower compared to the PVB. Furthermore, the ESP block and RLB can be performed in difficult situations or in the cervical/lumbar regions for a wide range of clinical indications. However, it is uncertain whether the ESP block has the same analgesic efficacy as the conventional PVB. The ITP block targets the retro-SCTL space, which has more direct and closer pathways to the TPVS and appears to be anatomically more advantageous than the ESP block or RLB. Further clinical studies are needed to confirm this anatomical finding for ITP blocks.
Each type of thoracic paraspinal block has a different anatomical basis, resulting in different spreading patterns of the injectate. Consequently, the intrinsic characteristics of the neural blockade differ according to the technique. Understanding the proposed mechanisms of action of each paraspinal block could assist clinicians in further investigating and refining block performance, with the ultimate goal of optimizing analgesic efficacy and improving patient outcomes.
Footnotes
Funding
This work was supported by a grant from the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (No. 2021R1F1A1045873).
Conflicts of Interest
No potential conflict of interest relevant to this article was reported.
Supplementary Material
Serial three-dimensional images at the T4–T5 level are shown in the cross and sagittal sections.
References
- 1.Richardson J, Lönnqvist PA. Thoracic paravertebral block. Br J Anaesth. 1998;81:230–8. doi: 10.1093/bja/81.2.230. [DOI] [PubMed] [Google Scholar]
- 2.Karmakar MK. Thoracic paravertebral block. Anesthesiology. 2001;95:771–80. doi: 10.1097/00000542-200109000-00033. [DOI] [PubMed] [Google Scholar]
- 3.Krediet AC, Moayeri N, van Geffen GJ, Bruhn J, Renes S, Bigeleisen PE, et al. Different approaches to ultrasound-guided thoracic paravertebral block: an illustrated review. Anesthesiology. 2015;123:459–74. doi: 10.1097/ALN.0000000000000747. [DOI] [PubMed] [Google Scholar]
- 4.Boezaart AP, Lucas SD, Elliott CE. Paravertebral block: cervical, thoracic, lumbar, and sacral. Curr Opin Anaesthesiol. 2009;22:637–43. doi: 10.1097/ACO.0b013e32832f3277. [DOI] [PubMed] [Google Scholar]
- 5.Pace MM, Sharma B, Anderson-Dam J, Fleischmann K, Warren L, Stefanovich P. Ultrasound-guided thoracic paravertebral blockade: a retrospective study of the incidence of complications. Anesth Analg. 2016;122:1186–91. doi: 10.1213/ANE.0000000000001117. [DOI] [PubMed] [Google Scholar]
- 6.Kus A, Gurkan Y, Gul Akgul A, Solak M, Toker K. Pleural puncture and intrathoracic catheter placement during ultrasound guided paravertebral block. J Cardiothorac Vasc Anesth. 2013;27:e11–2. doi: 10.1053/j.jvca.2012.10.018. [DOI] [PubMed] [Google Scholar]
- 7.Singh NP, Makkar JK, Kuberan A, Guffey R, Uppal V. Efficacy of regional anesthesia techniques for postoperative analgesia in patients undergoing major oncologic breast surgeries: a systematic review and network meta-analysis of randomized controlled trials. Can J Anaesth. 2022;69:527–49. doi: 10.1007/s12630-021-02183-z. [DOI] [PubMed] [Google Scholar]
- 8.Chitnis SS, Tang R, Mariano ER. The role of regional analgesia in personalized postoperative pain management. Korean J Anesthesiol. 2020;73:363–71. doi: 10.4097/kja.20323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Elsharkawy H, Pawa A, Mariano ER. Interfascial plane blocks: back to basics. Reg Anesth Pain Med. 2018;43:341–6. doi: 10.1097/AAP.0000000000000750. [DOI] [PubMed] [Google Scholar]
- 10.Forero M, Adhikary SD, Lopez H, Tsui C, Chin KJ. The erector spinae plane block: a novel analgesic technique in thoracic neuropathic pain. Reg Anesth Pain Med. 2016;41:621–7. doi: 10.1097/AAP.0000000000000451. [DOI] [PubMed] [Google Scholar]
- 11.El-Boghdadly K, Wolmarans M, Stengel AD, Albrecht E, Chin KJ, Elsharkawy H, et al. Standardizing nomenclature in regional anesthesia: an ASRA-ESRA Delphi consensus study of abdominal wall, paraspinal, and chest wall blocks. Reg Anesth Pain Med. 2021;46:571–80. doi: 10.1136/rapm-2020-102451. [DOI] [PubMed] [Google Scholar]
- 12.Xu JL. Paraneuraxial nerve blocks: a well-defined novel terminology that is clinically essential for regional anesthesia. J Clin Anesth. 2017;43:14. doi: 10.1016/j.jclinane.2017.09.002. [DOI] [PubMed] [Google Scholar]
- 13.Standring S. Gray’s anatomy: the anatomical basis of clinical practice. 42nd ed. London: Elsevier Health Sciences; 2020. pp. 732–939. [Google Scholar]
- 14.Cho TH, Kim SH, O J, Kwon HJ, Kim KW, Yang HM. Anatomy of the thoracic paravertebral space: 3D micro-CT findings and their clinical implications for nerve blockade. Reg Anesth Pain Med. 2021;46:699–703. doi: 10.1136/rapm-2021-102588. [DOI] [PubMed] [Google Scholar]
- 15.Ibrahim AF, Darwish HH. The costotransverse ligaments in human: a detailed anatomical study. Clin Anat. 2005;18:340–5. doi: 10.1002/ca.20102. [DOI] [PubMed] [Google Scholar]
- 16.Lönnqvist PA, MacKenzie J, Soni AK, Conacher ID. Paravertebral blockade. Failure rate and complications. Anaesthesia. 1995;50:813–5. doi: 10.1111/j.1365-2044.1995.tb06148.x. [DOI] [PubMed] [Google Scholar]
- 17.Cowie B, McGlade D, Ivanusic J, Barrington MJ. Ultrasound-guided thoracic paravertebral blockade: a cadaveric study. Anesth Analg. 2010;110:1735–9. doi: 10.1213/ANE.0b013e3181dd58b0. [DOI] [PubMed] [Google Scholar]
- 18.Uppal V, Sondekoppam RV, Sodhi P, Johnston D, Ganapathy S. Single-injection versus multiple-injection technique of ultrasound-guided paravertebral blocks: a randomized controlled study comparing dermatomal spread. Reg Anesth Pain Med. 2017;42:575–81. doi: 10.1097/AAP.0000000000000631. [DOI] [PubMed] [Google Scholar]
- 19.Karmakar MK, Chung DC. Variability of a thoracic paravertebral block. Are we ignoring the endothoracic fascia? Reg Anesth Pain Med. 2000;25:325–7. doi: 10.1016/s1098-7339(00)90028-2. [DOI] [PubMed] [Google Scholar]
- 20.Stopar Pintaric T, Veranic P, Hadzic A, Karmakar M, Cvetko E. Electron-microscopic imaging of endothoracic fascia in the thoracic paravertebral space in rats. Reg Anesth Pain Med. 2012;37:215–8. doi: 10.1097/AAP.0b013e31824451cb. [DOI] [PubMed] [Google Scholar]
- 21.Bouman EA, Sieben JM, Balthasar AJR, Joosten EA, Gramke HF, van Kleef M, et al. Boundaries of the thoracic paravertebral space: potential risks and benefits of the thoracic paravertebral block from an anatomical perspective. Surg Radiol Anat. 2017;39:1117–25. doi: 10.1007/s00276-017-1857-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Saito T, Den S, Tanuma K, Tanuma Y, Carney E, Carlsson C. Anatomical bases for paravertebral anesthetic block: fluid communication between the thoracic and lumbar paravertebral regions. Surg Radiol Anat. 1999;21:359–63. doi: 10.1007/BF01631341. [DOI] [PubMed] [Google Scholar]
- 23.Thavaneswaran P, Rudkin GE, Cooter RD, Moyes DG, Perera CL, Maddern GJ. Brief reports: paravertebral block for anesthesia: a systematic review. Anesth Analg. 2010;110:1740–4. doi: 10.1213/ANE.0b013e3181da82c8. [DOI] [PubMed] [Google Scholar]
- 24.Yeung JH, Gates S, Naidu BV, Wilson MJ, Gao Smith F. Paravertebral block versus thoracic epidural for patients undergoing thoracotomy. Cochrane Database Syst Rev. 2016;2:CD009121. doi: 10.1002/14651858.CD009121.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ding X, Jin S, Niu X, Ren H, Fu S, Li Q. A comparison of the analgesia efficacy and side effects of paravertebral compared with epidural blockade for thoracotomy: an updated meta-analysis. PLoS One. 2014;9:e96233. doi: 10.1371/journal.pone.0096233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xiong C, Han C, Zhao D, Peng W, Xu D, Lan Z. Postoperative analgesic effects of paravertebral block versus erector spinae plane block for thoracic and breast surgery: a meta-analysis. PLoS One. 2021;16:e0256611. doi: 10.1371/journal.pone.0256611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Turhan Ö, Sivrikoz N, Sungur Z, Duman S, Özkan B, Şentürk M. Thoracic paravertebral block achieves better pain control than erector spinae plane block and intercostal nerve block in thoracoscopic surgery: a randomized study. J Cardiothorac Vasc Anesth. 2021;35:2920–7. doi: 10.1053/j.jvca.2020.11.034. [DOI] [PubMed] [Google Scholar]
- 28.Wang Q, Wei S, Li S, Yu J, Zhang G, Ni C, et al. Comparison of the analgesic effect of ultrasound-guided paravertebral block and ultrasound-guided retrolaminar block in Uniportal video-assisted thoracoscopic surgery: a prospective, randomized study. BMC Cancer. 2021;21:1229. doi: 10.1186/s12885-021-08938-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weng WT, Wang CJ, Li CY, Wen HW, Liu YC. Erector spinae plane block similar to paravertebral block for perioperative pain control in breast surgery: a meta-analysis study. Pain Physician. 2021;24:203–13. [PubMed] [Google Scholar]
- 30.Jacobs A, Lemoine A, Joshi GP, Van de Velde M, Bonnet F, PROSPECT Working Group collaborators PROSPECT guideline for oncological breast surgery: a systematic review and procedure-specific postoperative pain management recommendations. Anaesthesia. 2020;75:664–73. doi: 10.1111/anae.14964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pfeiffer G, Oppitz N, Schöne S, Richter-Heine I, Höhne M, Koltermann C. Analgesia of the axilla using a paravertebral catheter in the lamina technique. Anaesthesist. 2006;55:423–7. doi: 10.1007/s00101-005-0969-0. [DOI] [PubMed] [Google Scholar]
- 32.Voscopoulos C, Palaniappan D, Zeballos J, Ko H, Janfaza D, Vlassakov K. The ultrasound-guided retrolaminar block. Can J Anaesth. 2013;60:888–95. doi: 10.1007/s12630-013-9983-x. [DOI] [PubMed] [Google Scholar]
- 33.Sabouri AS, Crawford L, Bick SK, Nozari A, Anderson TA. Is a retrolaminar approach to the thoracic paravertebral space possible?: a human cadaveric study. Reg Anesth Pain Med. 2018;43:864–8. doi: 10.1097/AAP.0000000000000828. [DOI] [PubMed] [Google Scholar]
- 34.Yang HM, Choi YJ, Kwon HJ, O J, Cho TH, Kim SH. Comparison of injectate spread and nerve involvement between retrolaminar and erector spinae plane blocks in the thoracic region: a cadaveric study. Anaesthesia. 2018;73:1244–50. doi: 10.1111/anae.14408. [DOI] [PubMed] [Google Scholar]
- 35.Willard FH, Vleeming A, Schuenke MD, Danneels L, Schleip R. The thoracolumbar fascia: anatomy, function and clinical considerations. J Anat. 2012;221:507–36. doi: 10.1111/j.1469-7580.2012.01511.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jüttner T, Werdehausen R, Hermanns H, Monaca E, Danzeisen O, Pannen BH, et al. The paravertebral lamina technique: a new regional anesthesia approach for breast surgery. J Clin Anesth. 2011;23:443–50. doi: 10.1016/j.jclinane.2010.12.015. [DOI] [PubMed] [Google Scholar]
- 37.Zeballos JL, Voscopoulos C, Kapottos M, Janfaza D, Vlassakov K. Ultrasound-guided retrolaminar paravertebral block. Anaesthesia. 2013;68:649–51. doi: 10.1111/anae.12296. [DOI] [PubMed] [Google Scholar]
- 38.Murouchi T, Yamakage M. Retrolaminar block: analgesic efficacy and safety evaluation. J Anesth. 2016;30:1003–7. doi: 10.1007/s00540-016-2230-1. [DOI] [PubMed] [Google Scholar]
- 39.Onishi E, Murakami M, Nishino R, Ohba R, Yamauchi M. Analgesic effect of double-level retrolaminar paravertebral block for breast cancer surgery in the early postoperative period: a placebo-controlled, randomized clinical trial. Tohoku J Exp Med. 2018;245:179–85. doi: 10.1620/tjem.245.179. [DOI] [PubMed] [Google Scholar]
- 40.Chin KJ, El-Boghdadly K. Mechanisms of action of the erector spinae plane (ESP) block: a narrative review. Can J Anaesth. 2021;68:387–408. doi: 10.1007/s12630-020-01875-2. [DOI] [PubMed] [Google Scholar]
- 41.Ivanusic J, Konishi Y, Barrington MJ. A cadaveric study investigating the mechanism of action of erector spinae blockade. Reg Anesth Pain Med. 2018;43:567–71. doi: 10.1097/AAP.0000000000000789. [DOI] [PubMed] [Google Scholar]
- 42.Schwartzmann A, Peng P, Maciel MA, Forero M. Mechanism of the erector spinae plane block: insights from a magnetic resonance imaging study. Can J Anaesth. 2018;65:1165–6. doi: 10.1007/s12630-018-1187-y. [DOI] [PubMed] [Google Scholar]
- 43.Schwartzmann A, Peng P, Maciel MA, Alcarraz P, Gonzalez X, Forero M. A magnetic resonance imaging study of local anesthetic spread in patients receiving an erector spinae plane block. Can J Anaesth. 2020;67:942–8. doi: 10.1007/s12630-020-01613-8. [DOI] [PubMed] [Google Scholar]
- 44.Ueshima H, Otake H. Similarities between the retrolaminar and erector spinae plane blocks. Reg Anesth Pain Med. 2017;42:123–4. doi: 10.1097/AAP.0000000000000526. [DOI] [PubMed] [Google Scholar]
- 45.Murouchi T. Consideration of block nomenclature: erector spinae plane block or retrolaminar block? Reg Anesth Pain Med. 2017;42:124. doi: 10.1097/AAP.0000000000000525. [DOI] [PubMed] [Google Scholar]
- 46.Onishi E, Toda N, Kameyama Y, Yamauchi M. Comparison of clinical efficacy and anatomical investigation between retrolaminar block and erector spinae plane block. Biomed Res Int. 2019;2019:2578396. doi: 10.1155/2019/2578396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tulgar S, Kapakli MS, Senturk O, Selvi O, Serifsoy TE, Ozer Z. Evaluation of ultrasound-guided erector spinae plane block for postoperative analgesia in laparoscopic cholecystectomy: a prospective, randomized, controlled clinical trial. J Clin Anesth. 2018;49:101–6. doi: 10.1016/j.jclinane.2018.06.019. [DOI] [PubMed] [Google Scholar]
- 48.Gürkan Y, Aksu C, Kuş A, Yörükoğlu UH, Kılıç CT. Ultrasound guided erector spinae plane block reduces postoperative opioid consumption following breast surgery: a randomized controlled study. J Clin Anesth. 2018;50:65–8. doi: 10.1016/j.jclinane.2018.06.033. [DOI] [PubMed] [Google Scholar]
- 49.Krishna SN, Chauhan S, Bhoi D, Kaushal B, Hasija S, Sangdup T, et al. Bilateral erector spinae plane block for acute post-surgical pain in adult cardiac surgical patients: a randomized controlled trial. J Cardiothorac Vasc Anesth. 2019;33:368–75. doi: 10.1053/j.jvca.2018.05.050. [DOI] [PubMed] [Google Scholar]
- 50.Jiao B, Chen H, Chen M, Lu P, Liu J, Chen C. Opioid-sparing effects of ultrasound-guided erector spinae plane block for adult patients undergoing surgery: a systematic review and meta-analysis. Pain Pract. 2022;22:391–404. doi: 10.1111/papr.13091. [DOI] [PubMed] [Google Scholar]
- 51.Zhang Y, Liu T, Zhou Y, Yu Y, Chen G. Analgesic efficacy and safety of erector spinae plane block in breast cancer surgery: a systematic review and meta-analysis. BMC Anesthesiol. 2021;21:59. doi: 10.1186/s12871-021-01277-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liang X, Zhou W, Fan Y. Erector spinae plane block for spinal surgery: a systematic review and meta-analysis. Korean J Pain. 2021;34:487–500. doi: 10.3344/kjp.2021.34.4.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kot P, Rodriguez P, Granell M, Cano B, Rovira L, Morales J, et al. The erector spinae plane block: a narrative review. Korean J Anesthesiol. 2019;72:209–20. doi: 10.4097/kja.d.19.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Saadawi M, Layera S, Aliste J, Bravo D, Leurcharusmee P, Tran Q. Erector spinae plane block: a narrative review with systematic analysis of the evidence pertaining to clinical indications and alternative truncal blocks. J Clin Anesth. 2021;68:110063. doi: 10.1016/j.jclinane.2020.110063. [DOI] [PubMed] [Google Scholar]
- 55.Swisher MW, Wallace AM, Sztain JF, Said ET, Khatibi B, Abanobi M, et al. Erector spinae plane versus paravertebral nerve blocks for postoperative analgesia after breast surgery: a randomized clinical trial. Reg Anesth Pain Med. 2020;45:260–6. doi: 10.1136/rapm-2019-101013. [DOI] [PubMed] [Google Scholar]
- 56.Oh SK, Lim BG, Won YJ, Lee DK, Kim SS. Analgesic efficacy of erector spinae plane block in lumbar spine surgery: a systematic review and meta-analysis. J Clin Anesth. 2022;78:110647. doi: 10.1016/j.jclinane.2022.110647. [DOI] [PubMed] [Google Scholar]
- 57.Yang HM, Kim SH. Injectate spread in interfascial plane block: a microscopic finding. doi: 10.1136/rapm-2019-100693. Reg Anesth Pain Med 2019. Advance Access published on Jul 5, 2019. doi: 10.1136/rapm-2019-100693. [DOI] [PubMed] [Google Scholar]
- 58.Bang S, Choi J, Kim ED. A high thoracic erector spinae plane block used for sympathetic block in patients with upper extremity complex regional pain syndrome. J Clin Anesth. 2020;60:99–100. doi: 10.1016/j.jclinane.2019.09.011. [DOI] [PubMed] [Google Scholar]
- 59.Elkoundi A, Eloukkal Z, Bensghir M, Belyamani L, Lalaoui SJ. Erector spinae plane block for hyperalgesic acute pancreatitis. Pain Med. 2019;20:1055–6. doi: 10.1093/pm/pny232. [DOI] [PubMed] [Google Scholar]
- 60.Aydin ME, Ahiskalioglu A, Tekin E, Ozkaya F, Ahiskalioglu EO, Bayramoglu A. Relief of refractory renal colic in emergency department: a novel indication for ultrasound guided erector spinae plane block. Am J Emerg Med. 2019;37:794. doi: 10.1016/j.ajem.2018.12.042. e1-3. [DOI] [PubMed] [Google Scholar]
- 61.Sullivan TR, Kanda P, Gagne S, Costache I. Harlequin syndrome associated with erector spinae plane block. Anesthesiology. 2019;131:665. doi: 10.1097/ALN.0000000000002733. [DOI] [PubMed] [Google Scholar]
- 62.Elkoundi A, Eloukkal Z, Bensghir M, Belyamani L. Priapism following erector spinae plane block for the treatment of a complex regional pain syndrome. Am J Emerg Med. 2019;37:796. doi: 10.1016/j.ajem.2019.01.012. e3-4. [DOI] [PubMed] [Google Scholar]
- 63.Pak A, Singh P. Epidural-like effects with bilateral erector spinae plane catheters after abdominal surgery: a case report. A A Pract. 2020;14:137–9. doi: 10.1213/XAA.0000000000001164. [DOI] [PubMed] [Google Scholar]
- 64.Selvi O, Tulgar S. Ultrasound guided erector spinae plane block as a cause of unintended motor block. Rev Esp Anestesiol Reanim (Engl Ed) 2018;65:589–92. doi: 10.1016/j.redar.2018.05.009. [DOI] [PubMed] [Google Scholar]
- 65.De Cassai A, Fasolo A, Geraldini F, Munari M. Motor block following bilateral ESP block. J Clin Anesth. 2020;60:23. doi: 10.1016/j.jclinane.2019.08.029. [DOI] [PubMed] [Google Scholar]
- 66.Hamilton DL. Local anesthetic systemic toxicity following erector spinae plane block: sometimes less is more. Korean J Anesthesiol. 2021;74:361–2. doi: 10.4097/kja.20596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yawata S, Imamachi N, Sakura S, Yamamoto H, Saito Y. Local anesthetic systemic toxicity of levobupivacaine in erector spinae plane block. Korean J Anesthesiol. 2021;74:271–2. doi: 10.4097/kja.20560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Karaca O, Pinar HU. Is high dose lumbar erector spinae plane block safe? J Clin Anesth. 2020;62:109721. doi: 10.1016/j.jclinane.2020.109721. [DOI] [PubMed] [Google Scholar]
- 69.Choi YJ, Kwon HJ, O J, Cho TH, Won JY, Yang HM, et al. Influence of injectate volume on paravertebral spread in erector spinae plane block: an endoscopic and anatomical evaluation. PLoS One. 2019;14:e0224487. doi: 10.1371/journal.pone.0224487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tulgar S, Ahiskalioglu A, Balaban O. Reply to Dr. Ueshima: the relationship of local anesthetic volume and dermatomal spread of sensorial block in erector spinae plane blocks: a new dilemma. J Clin Anesth. 2019;52:57. doi: 10.1016/j.jclinane.2018.09.001. [DOI] [PubMed] [Google Scholar]
- 71.Costache I, de Neumann L, Ramnanan CJ, Goodwin SL, Pawa A, Abdallah FW, et al. The mid-point transverse process to pleura (MTP) block: a new end-point for thoracic paravertebral block. Anaesthesia. 2017;72:1230–6. doi: 10.1111/anae.14004. [DOI] [PubMed] [Google Scholar]
- 72.Nielsen MV, Moriggl B, Hoermann R, Nielsen TD, Bendtsen TF, Børglum J. Are single-injection erector spinae plane block and multiple-injection costotransverse block equivalent to thoracic paravertebral block? Acta Anaesthesiol Scand. 2019;63:1231–8. doi: 10.1111/aas.13424. [DOI] [PubMed] [Google Scholar]
- 73.Kilicaslan A, Sarkilar G, Altınok T, Tulgar S. A novel ultrasound-guided technique in peri-paravertebral area: subtransverse process interligamentary (STIL) plane block: the game has not ended yet. J Clin Anesth. 2020;60:76–7. doi: 10.1016/j.jclinane.2019.08.047. [DOI] [PubMed] [Google Scholar]
- 74.Shibata Y, Kampitak W, Tansatit T. The novel costotransverse foramen block technique: distribution characteristics of injectate compared with erector spinae plane block. Pain Physician. 2020;23:E305–14. [PubMed] [Google Scholar]
- 75.Cho TH, Kwon HJ, O J, Cho J, Kim SH, Yang HM. The pathway of injectate spread during thoracic intertransverse process (ITP) block: micro-computed tomography findings and anatomical evaluations. J Clin Anesth. 2022;77:110646. doi: 10.1016/j.jclinane.2022.110646. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Serial three-dimensional images at the T4–T5 level are shown in the cross and sagittal sections.


