ABSTRACT
Rapid technology development, exposure to gadgets, and artificial lights (with different monochromes) have disturbed our lifestyle and the circadian clock, which otherwise confers better regulation of behavioral patterns and sleep:wake cycles in most organisms including Drosophila melanogaster. We assay the effect of LD12:12 h (light:dark) monochromatic lights (violet, blue, green, yellow, orange, and red) on the lifespan, activity, and sleep of the D. melanogaster. We observe a shortened lifespan under 12 h of violet, blue, green, and yellow lights, while significantly reduced activity levels under the light phase of blue and green light as compared to their dark phase is observed. Significant increase in the evening anticipation index of flies under blue and green light alongside increased and decreased sleep depth during the day and night respectively suggests the light avoidance, while there is no effect of colored light on the waking time, daily active time, and sleep time. Thus, our study shows short and long-term exposure to certain colored lights in terms of reduced lifespan and locomotor activity, which cause qualitative as well as quantitative changes in the sleep of flies; probably as a sign of aversion towards a specific light.
This article has an associated First Person interview with the first author of the paper.
KEY WORDS: Drosophila melanogaster, Lifespan, Activity, Monochromatic light, Circadian clocks
Summary: Monochromes influence light:dark phase-dependent changes in active-sleep time, alongside lowering lifespan in fruit flies and dark phase anticipation under blue and green lights, highlighting the detrimental effects of artificial lights.
INTRODUCTION
The circadian clock benefits most of the organisms living under 12:12 h of light: dark (LD) cycles and its precision is seen to evolve across generations upon selection pressures (Kannan et al., 2012). Circadian clock is synchronized when the day is ∼24 h, while it also favors the habitation under constant darkness (DD) or constant light (LL) particularly in the organisms living in the polar region and deep sea oceans (Sharma, 2003; Krittika and Yadav, 2020; references therein). Presence of light:dark cue and light discrimination can confer various advantages for survival, predator avoidance, and benefit the organisms with a sense of food and shelter search (Dominy and Lucas, 2001; Osorio and Vorobyev, 2008). Studying associated responses such as phototaxis and color preference help us understand the pest control, fruit-piercing behavior and mating in fruit flies Drosophila melanogaster (Harada, 1991; Yang et al., 2003; Cowan and Gries, 2009; Díaz-Fleischer and Arredondo, 2011; Hori et al., 2015; Shibuya et al., 2018; references therein). But interestingly, increased exposure to artificial light can interfere with sleep quality and circadian function in humans (Chang et al., 2015; Green et al., 2017); and moreover, D. melanogaster showed color preference (Lazopulo et al., 2019) and the toxicity of blue light (Hori et al., 2015; Nash et al., 2019; Shen et al., 2019). Hence, understanding the toxicity of colored lights on the fitness traits and how the flies perceive colors across time of the day will enable us to understand the clock performance and physiological adaptations and also benefit the agricultural and its related storage sector for effective pest control.
The color identification in flies is ensured by photoreceptors called rhodopsin, wherein certain rhodopsin classes are color-specific (Schnaitmann et al., 2013). Since fruit flies show a color preference across different times of the day (Lazopulo et al., 2019), it is important to test whether this color preference can benefit their lifespan or fitness. Fitness-related studies can be categorized based on the exposure of the experimental conditions (colored lights) during pre-adult (Xiang et al., 2010; Hori et al., 2015; Shibuya et al., 2018) or the adult stages of the flies (Lazopulo et al., 2019; Shen et al., 2019). Due to the tight association between light and the circadian clock, bright light therapy is used to treat sleep:wake cycle disturbances in old people and for mood disorders (Blume et al., 2019; references therein). As colored-light-emitting gadgets and artificial lights have become an integral part of our daily life, especially blue light (high exposure possibility) can aggravate migraine symptoms and also cause sleep disorders (Noseda et al., 2010; Falchi et al., 2011; Green et al., 2017). So, it is important to study their negative effects on the sleep patterns or life of the organism, especially during the evening or night hours (Holzman, 2010).
In the case of D. melanogaster, it is seen that daily blue light exposure alone shortens the lifespan, impairs locomotion, and accelerates aging (Nash et al., 2019). However, there are limited reports on the effect of UV light (100-400 nm) and colors of visible light (400-780 nm) on fruit fly D. melanogaster activity, lifespan, lethality and related traits (Hori et al., 2015; Guntur et al., 2017; Lazopulo et al., 2019; Nash et al., 2019; Shen et al., 2019). Thus, it becomes requisite to understand the effect of other monochromes of shorter (violet) and longer wavelengths (green to red) on the lifespan and locomotor activity of the flies.
In this study, we intend to assess the effect of different colors of visible light range [violet (400-440 nm), blue (460-500 nm), green (500-570 nm), yellow (570-590 nm), orange (590-620 nm), red (620-700 nm)] on the lifespan, activity, and sleep of adult fruit flies D. melanogaster. We observed that the lifespan under shorter wavelengths is shortened when compared with that under LD (12 h:12 h light:dark cycle of white light), DD (24 h constant darkness; control), and red light. As expected, the total activity under DD was higher as compared to all the tested light regimes and controls (LL and LD white light) except under the red LD. Interestingly, activity under blue and yellow LD is higher than that recorded under LL white light control. We also observed that the flies under blue and green LD anticipate dark phase alongside showing higher sleep depth during the light phase and could be assumed to be an avoidance strategy of the flies. They also exhibit shorter active time and extended sleep time during the day, possibly due to the avoidance of light as mentioned earlier. Thus, our study confirms the toxic effect of short-wavelength lights, especially blue and green light on the lifespan of the flies and reveals the activity suppression and sleep enhancement during the blue and green light phase as compared to their dark phase.
RESULTS
Lifespan
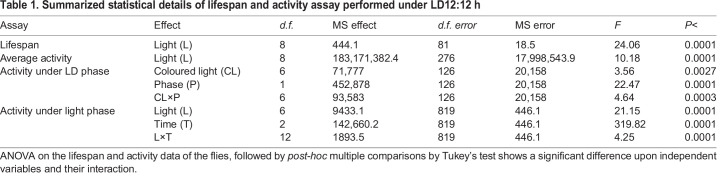
The lifespan of male fruit flies D. melanogaster was assayed under three different control light regimes: LL (constant light), LD (12 h light:12 h dark), DD (constant darkness), and the monochromes of visible light (experimental; violet, blue, green, yellow, orange, red). The survivorship curves and one-way ANOVA on the lifespan data showed a significant effect of light (L; F8,81=24.064, P<0.0001; Table 1, Fig. 1A,B). Multiple comparisons using Tukey's HSD test revealed that lifespan under DD was higher than the other light regimes. Male flies under white LD exhibit a higher lifespan than colored LD lights of violet, blue, green, and yellow. Interestingly, flies exposed to blue LD recorded the lowest lifespan as compared to that under control white light regimes (LL, LD) along with orange and red, but were not different from the lifespan recorded under violet and green light regimes. The lifespan of flies exposed with red light was higher than that exposed with violet, blue, green, and yellow light while being similar to that under orange light and lower than that under DD control. Thus, the results suggest that lifespan is undoubtedly higher under DD conditions followed by red light.
Table 1.
Summarized statistical details of lifespan and activity assay performed under LD12:12 h
Fig. 1.
Lifespan under lights of short wavelength is lower. The survivorship curves (A) and average lifespan (B) under controls of LL, LD and DD and other colored lights (V, violet; B, blue; G, green; Y, yellow; O, orange; R, red) show possible toxic effect of short-wavelength lights on lifespan. The x-axis denotes age in days (A) and light (B), while the y-axis represents percentage (%) survival (A) and average lifespan in days (B). The error bars are standard deviation (SD) and the asterisks indicate statistical significance (P<0.05), n.s. denotes non-significance between DD and red-light lifespan alone.
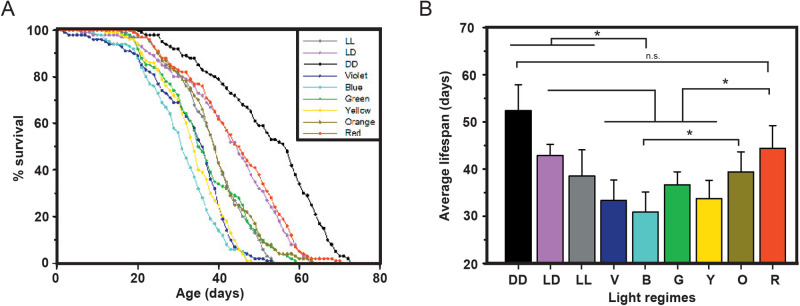
Locomotor activity
Upon differences in lifespan, we assayed the effect of light on the activity levels of flies. One-way ANOVA on the average locomotor activity data with light as an independent factor showed a significant effect of light (L; F8,276=10.177, P<0.0001; Table 1, Fig. 2A). The multiple comparisons by Tukey's test revealed that activity under DD control was higher as compared to all tested lights except red light (wherein it was found to be similar), while white LL control exhibited lower activity as compared to blue, yellow and red. Interestingly, the activity under red light was higher than that observed under white LD and LL activity. Moreover, two-way ANOVA followed by Tukey's HSD test on activity during light:dark phase showed a significant effect of colored light (CL; F6,126=3.56, P<0.0027), phase (P; F1,126=22.47, P<0.0001), and light-phase interaction (L×P; F6,126=4.64, P<0.0003; Table 1, Fig. 2B). The activity levels in red LD during the light phase were found to be higher than those under white LD control, blue, green and orange LD. Under blue and green light, despite the expected difference in the light and dark phase activity, interestingly, we observe a significant increase in the activity at night. Thus, taken together, this study suggests that flies do not prefer being active under the light phases of blue and green light, and thereby compensate for their activity in their dark phase (Fig. 2B; actograms in Fig. S1A-D).
Fig. 2.
The activity of flies under blue and green light phase is lower than their dark phase activity. The total activity over 10 days (A) and average activity per day (B) show lower activity recorded under blue and green light. The x-axis denotes the light regimes imposed, and the y-axis the activity bouts in arbitrary units (a.u.). Other details are the same to Fig. 1.
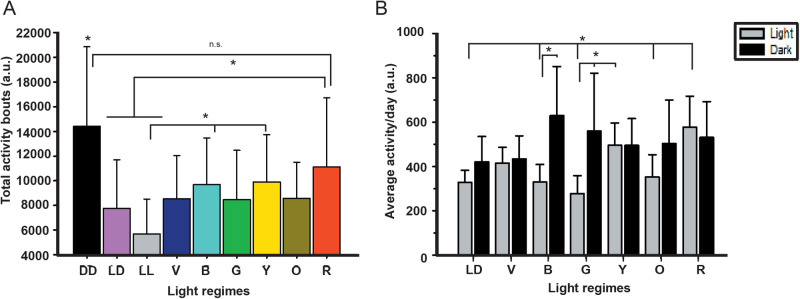
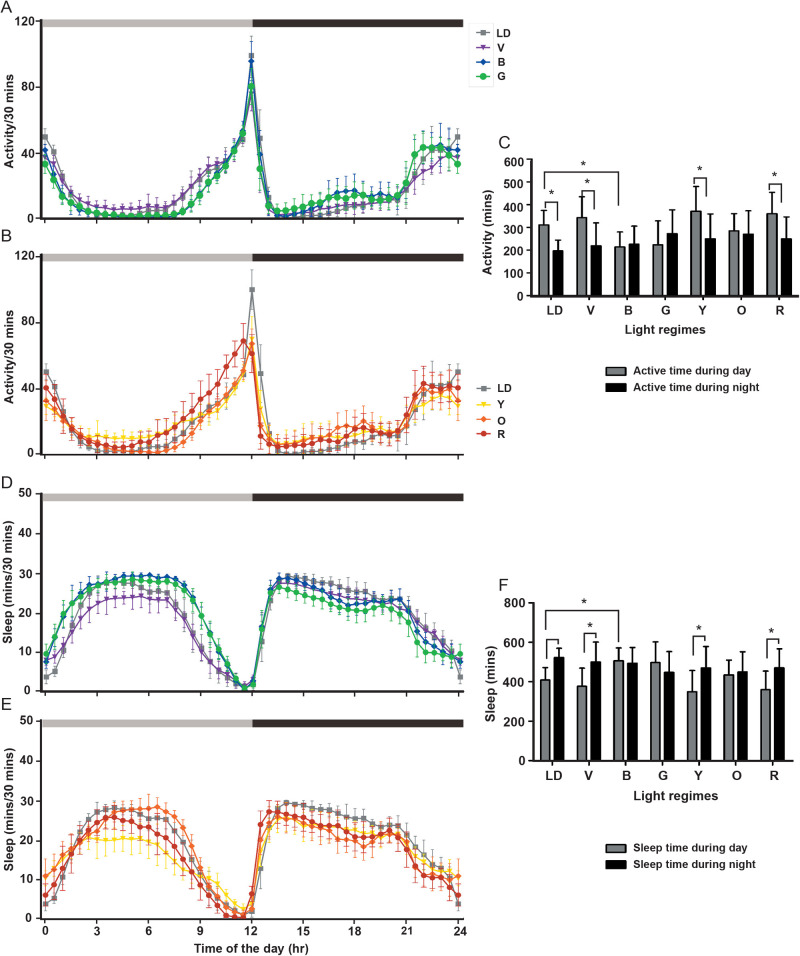
Active and sleep time
Apart from activity, we intended to see whether there were differences in the duration of activity and sleep as light is a major determinant of sleep:wake cycle as discussed earlier. ANOVA on the active time data showed the significant effect of light (L; F6,268=5.85, P<0.0001), time (T; F1,268=30.00, P<0.0001) and their interaction (L×T; F6,268=6.67, P<0.0001; Table 2, Fig. 3A-C). Multiple comparisons using Bonferroni's test revealed that the active time of flies under blue light is shorter than white LD control during the day. Interestingly, the difference in the active time of flies during the day and night is witnessed only under the white LD control, violet, yellow, and red LD lights, while there is no quantitative difference in terms of activity bouts in the light phase versus dark phase under blue, green and orange LD lights (Fig. 3C). ANOVA on the sleep time data showed significant effect of light (L; F6,268=5.85, P<0.0001), time (T; F1,268=30.00, P<0.0001) and their interaction (L×T; F6,268=6.67, P<0.0001; Table 2, Fig. 3D-F). Multiple comparisons by Bonferroni's test revealed that the sleep time of flies under blue light is higher than that under white LD control during the day and the sleep time also shows no phase separation under blue, green, and orange (Fig. 3F, similar to active time). Thus, blue, green, and orange lights are nullifying the effect of LD on the active and sleep time of the flies.
Table 2.
Summarized statistical details of active time, sleep time, and related parameters performed under LD12:12 h
Fig. 3.
No difference in the active and sleep time between day and night under blue and green light. The active time profile during day and night under VBG (A) and YOR (B) and their quantification (C) shows reduced active time during the day under blue light. The sleep time profile during day and night under VBG (D) and YOR (E) and their quantification (F) shows higher sleep time during day under blue light and no difference in the sleep time during day and night under blue, green and orange light. The x-axis denotes the time of the day (A,B,D,E) and the y-axis denotes the activity/30 min (A,B) and sleep/30 min (D,E). The x-axis denotes the light regimes imposed (C,F), and the y-axis the activity (C) and sleep (F) in minutes. The horizontal grey and black bars indicate the light and dark phase respectively for LD 12:12 h. Other details are the same as in Fig. 1.
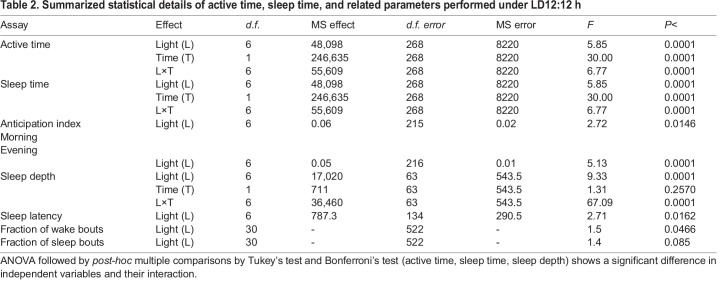
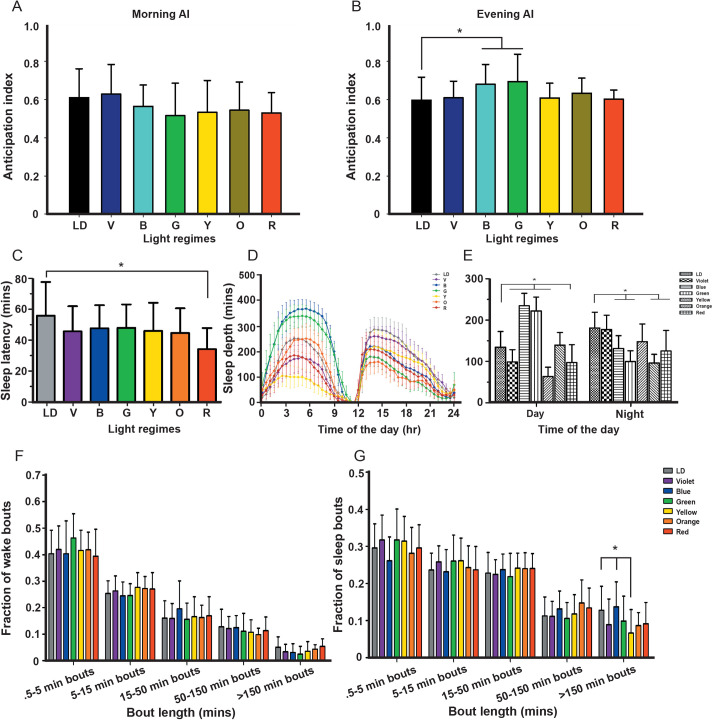
Since, we observed no difference in total active time of the flies under the colored lights or the LD control, we narrowed the assay to assess the fly's preference towards the phase of the day (light or dark phase). We assayed the anticipation index of flies for the evening (dark phase) considering that the flies are less active during the day (light phase). One-way ANOVA followed by multiple comparisons using Tukey's test on anticipation index showed a significant effect of L (morning; F6,215=2.72, P<0.0146; evening; F6,216=5.13, P<0.0001). The morning anticipation was not observed under all colored LD light as compared to the white LD control (Fig. 4A). But, interestingly, flies under blue and green LD light alone showed higher anticipation for the evening (dark phase) as compared to the white LD control and red LD light (Fig. 4B). Further, one-way ANOVA on the sleep latency with light as an independent factor showed a significant effect of light (L; F6,134=2.71, P<0.0162; Table 2, Fig. 4C). The multiple comparisons by Tukey's test revealed that the sleep latency of flies under red light is lower than that of the white LD control, while no other lights showed any effect on sleep latency.
Fig. 4.
Higher sleep depth during the day and anticipation for dark phase under blue and green light. The anticipation index for the morning (A) and evening (B) shows blue and green flies anticipating for dark phase. The sleep latency (C) in red light flies exhibit less time to fall asleep. Flies show increased sleep depth (D,E) during the day under blue and green light, while fraction of wake bouts (F) and sleep bouts (G) show not much effect of color light. The x-axis denotes light (A-C), time of the day (D,E), and the y-axis sleep latency (C) and sleep depth (D,E). The x-axis denotes the minute bouts (F,G) and the y-axis represents the fraction of wake bouts (F) and sleep bouts (G). Other details are the same as in Fig. 1.
In addition to various sleep parameters, the depth of sleep may also be affected by the duration of unfavourable light exposure. One-way ANOVA on the sleep depth showed significant effect of light (L; F6,63=9.33, P<0.0001), and its interaction with time (L×T; F6,63=67.09, P<0.0001; Table 2, Fig. 4D,E), but not of time (T; F1,63=1.31, P=0.2570). The multiple comparisons by Bonferroni's test revealed that the sleep depth of flies under blue and green light is higher during the day and lower at night, while flies under red light show lower sleep depth during day and night (Fig. 4E). This shows that the flies do not prefer blue and green light for activity and thereby prefer being active in the dark as discussed earlier. Moreover, the fraction of wake bouts showed no difference under the tested colors as compared to control (Wilks’ lambda=0.719, F30,522=1.4930, P=0.0467; Fig. 4F), while the fraction of sleep bouts under yellow light (>150 min bouts) is significantly lower than white LD control and blue light (Wilks’ lambda=0.736, F30,522=1.3877, P=0.085; Fig. 4G). Hence, these data suggest that blue and green light are aversive towards the light phase and thereby tend to have higher sleep depth. Interestingly, active time and sleep throughout the day, their average daily activity, and waking time did not show any effect of light (Fig. S3A-D).
DISCUSSION
Given that the presence of light enables the organism to time their daily activity, sleep and feeding pattern (Dominy and Lucas, 2001; Osorio and Vorobyev, 2008; Lazopulo et al., 2019); would the flies modulate their behavior to suit the colored light or dark phase is unexplored. This question made us carry out the current study, and understand the regulatory changes in terms of lifespan, activity, and sleep-related traits under the different colored lights in D. melanogaster. Results of the current study such as shorter lifespan and reduced activity under blue light are similar to the findings of certain studies indicating the toxic effect of blue light (Hori et al., 2015; Nash et al., 2019; Shen et al., 2019). The blue light probably induces the accumulated damage which in turn accelerates the aging rate and thus shorten the lifespan (Nash et al., 2019; Shen et al., 2019), which is still shorter than that observed under LL (white light) control, indicating that the rhythmic blue light is more detrimental for fly's lifespan than the arrhythmic LL (Fig. 1B). In fruit flies, the intensity of visible light exerts sex and diet-specific effects on lifespan and mortality rates (Shen et al., 2019). Interestingly, our study also shows that the lifespan under green light is also shortened in males which is against the report of a recent study that shows that 100 lux of green light do not affect the male lifespan (Shen et al., 2021). This difference in green light effect might be due to the use of DD control; while it is also reported that lifespan under DD is higher than that of white light LD (Allemand et al., 1973), but their fitness is higher upon long-term maintenance in white LD than DD (Kouser et al., 2014). This shows the clear differences in the effect of LD and DD light regimes and their effect on various traits in flies, thereby suggesting us to choose a valid control while comparing monochromes. Moreover, some studies which are focussed entirely on the effect of UV, visible light, and blue light on the pre-adult stages (Hori et al., 2015; Shibuya et al., 2018) have also raised the question of whether the dipterans accept green or its background as a neutral color, because of its relevance in the environment as trees and greenery (Storz and Paul, 1998; Little et al., 2018, 2019). Thus, taken together, lifespan and activity respond to the effect of different monochromes of visible light differently and therefore visible light cannot be considered just the mere average of seven colors.
Our results show that there is no difference in total activity between blue light and that in white LD control (Fig. 2A), which is contrary to the study by Nash et al., (2019), which reported the higher activity under blue light. Additionally, our results also show that the blue and green lights suppress the flies’ activity under the light phase and thereby the flies exhibit higher activity in the dark phase (Fig. 2B). Moreover, the effect of light is well associated with the circadian clock regulations, as the clock gene mutants showed similar blue light avoidance, thereby indicating that the avoidance of the colored light is not essentially associated with the functional clock (Helfrich-Förster, 2019; Lazopulo et al., 2019). However, there exists a significant decrease and increase of active time and sleep time respectively during the day under blue light. There is also no difference in the active and sleep time between the light and dark phases under blue LD (Fig. 3C,F). It is thereby intriguing that the activity under the blue light phase is indifferent as compared with that under white LD control, but their active time during the blue light phase is lower, indicating that the flies might be hyperactive during the lowered active time of their light phase.
Interestingly, the flies under blue and green light show no anticipation for the light phase but shows higher anticipation for the dark phase (Fig. 4A,B), which can be correlated with higher activity in the dark phase, and this can probably be because the flies are aversive to colored light and therefore show a preference towards the absence of light. While, green light exposed flies show significantly higher activity in their dark phase as compared to their light phase, and is not in line with the findings of studies reporting attraction and preference to green (Storz and Paul, 1998; Lazopulo et al., 2019). The blue and green light-based activity reduction can be justified by the aversion of flies towards the blue light and their limited movement probably contributes to the access food throughout the day as similar to Lazopulo et al. (2019), and the same can be the reason for higher activity in the dark phase. Additionally, witnessing the increased and deceased sleep depth in the light and dark phase respectively of the flies under blue and green light (Fig. 4E) can be a point of concern as enhanced night time activity can disrupt the ecological balance by increasing the chances of encountered predation and lack of food availability (Sharma, 2003; Krittika and Yadav, 2020; references therein). The flies under red light show lower sleep latency (Fig. 4C) and is probably consistent with the capacity of red light as a part of light therapy (Videnovic et al., 2017). Moreover, a study on fruit fly larva showed higher avoidance upon green light with a higher intensity as compared to that under the lower intensity of blue and violet lights (Xiang et al., 2010), because rhodopsin 5 (specific for blue light) is indispensable for light avoidance rather than rhodopsin 6 (specific for a green light; Keene et al., 2011). The current study also suggests that under unfavorable lights (like blue light), there might be the possibility of the flies shifting their activity toward the dark phase. Therefore, long-term exposure to the colored light can provoke the flies to shift their active phase, thereby disturbing the crepuscular nature of the flies. Overall, the extent of the colored light effect can be better understood upon 24 h color light exposure (LL of color light) and also various dietary interventions.
MATERIALS AND METHODS
Lifespan assay and experimental design
Egg collection from the running culture of fruit fly D. melanogaster stock (Canton S; CS flies) was done in 10 vials. Freshly emerged flies were collected and only male flies were separated by anesthetizing with mild CO2. The vials were maintained at 25°C temperature and ∼70% relative humidity under the respective light (violet, blue, green, yellow, orange, red) at LD12:12 h throughout the experiment. The control flies for the experiment were kept under white light (LL and LD regimes) alongside DD control. Each setup had ten unmated males per vial with 6 ml of corn food (containing agar from HIMEDIA and dry yeast from Gloripan were used; 100 flies/light) and 10 such vials were used. All the vials were checked for the death of flies every day; wherein the fresh food vials were changed for the surviving flies every 4th day till the death of the last fly in each vial. The lifespan of a fly was calculated as the number of days it survived post-emergence.
Activity and sleep assays and experimental design
The locomotor activity assay of the flies was performed using Drosophila Activity Monitors (DAM; Trikinetics, MA, USA). Freshly eclosed flies were collected and their activity was monitored for 10 days under LD12:12 h with each of the monochromatic light phases and controls of DD and white light regimes (LD and LL). Total 32 virgin males were individually and randomly loaded into the locomotor activity tubes and temperature (∼25°C), relative humidity (∼70%), and intensity of light inside the recording box (15×10×8 cm3; provided with LED lights and corresponding filters if applicable) were found to be stable. The activity data has been plotted with the time of the day on the x-axis and the activity bouts on the y-axis. The actograms of the flies under white light regimes LL, LD (control), blue and green light were analyzed and plotted using CLOCKLAB (Actimetrics, USA). The measurements of activity levels, sleep and related traits were analyzed in a MS-excel sheet using the data extracted from the DAM files. An inactivity period of flies for ≥5 min is defined as sleep, as reported previously (Hendricks et al., 2000; Shaw et al., 2000). Active time and sleep time are taken as the number of minutes the fly exhibited activity or inactivity respectively based on the recording by DAM. The anticipation index (AI) of the flies was calculated by dividing the 3 activity counts of 30 min each by six activity counts of 30 min before lights on (morning AI) and before lights off (evening AI), similar to the method described elsewhere (De et al., 2013). Sleep depth is a deep stage of consolidated sleep, which may be found to be around ∼12 to 15 min of continuous inactivity in flies (van Alphen et al., 2013), which increases upon the conditions like starvation (Brown et al., 2020).
Monochromatic light source
Colored filter transparent sheets (Lee filters) and LED lights were used to reproduce monochromatic colors for the experiments and intensity at 275 to 285 lux. The wavelength of the colors (Lee filters catalog) and the colored sheets used for these experiments were supplied with specific numbers (by Lee filters): violet- 181; blue- 201; green- 738; yellow- 101; orange- 105; red- 106.
Statistical analysis
The data were subjected to ANOVA and post hoc multiple comparisons by Tukey's and Bonferroni's tests. The statistical analyses were performed using STATISTICA for Windows Release 7 (StatSoft Inc. 1995, 2004) and GraphPad Prism version 5.00 for Windows. The statistical significance is considered if P<0.05 and error bars are plotted with mean±s.d.
Supplementary Material
Acknowledgements
P.Y. acknowledges the SASTRA Deemed to be University for the infrastructural facilities. We thank Dr Sheeba Vasu, JNCASR for CLOCKLAB analyses.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Author contributions
Conceptualization: S.K., P.Y. Methodology: S.K., P.Y. Investigation: S.K. Visualization: S.K. Supervision: P.Y. Writing- original draft: S.K., P.Y. Writing- review & editing: S.K., P.Y.
Funding
Department of Science and Technology (DST), India for the INSPIRE fellowship IF170750 (S.K.) Science and Engineering Research Board, DST, Government of India CRG/2019/003184 (P.Y.).
Data availability
All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials.
References
- Allemand, R., Cohet, Y. and David, J. (1973). Increase in the longevity of adult Drosophila melanogaster kept in permanent darkness. Exp. Gerontol. 8, 279-283. 10.1016/0531-5565(73)90040-5 [DOI] [PubMed] [Google Scholar]
- Blume, C., Garbazza, C. and Spitschan, M. (2019). Effects of light on human circadian rhythms, sleep and mood. Somnologie 23, 147-156. 10.1007/s11818-019-00215-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown, E. B., Shah, K. D., Faville, R., Kottler, B. and Keene, A. C. (2020). Drosophila insulin-like peptide 2 mediates dietary regulation of sleep intensity. PLoS Genet. 16, e1008270. 10.1371/journal.pgen.1008270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang, A. M., Aeschbach, D., Duffy, J. F. and Czeisler, C. A. (2015). Evening use of light-emitting eReaders negatively affects sleep, circadian timing, and next-morning alertness. Proc. Natl. Acad. Sci. USA 112, 1232-1237. 10.1073/pnas.1418490112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowan, T. and Gries, G. (2009). Ultraviolet and violet light: attractive orientation cues for the Indian meal moth, Plodia interpunctella. Entomol. Exp. Appl. 131, 148-158. 10.1111/j.1570-7458.2009.00838.x [DOI] [Google Scholar]
- De, J., Varma, V., Saha, S., Sheeba, V. and Sharma, V. K. (2013). Significance of activity peaks in fruit flies, Drosophila melanogaster, under seminatural conditions. Proc. Natl. Acad. Sci. USA 110, 8984-8989. 10.1073/pnas.1220960110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Díaz-Fleischer, F. and Arredondo, J. (2011). Light conditions affect sexual performance in a lekking tephritid fruit fly. J. Exp. Biol. 214, 2595-2602. 10.1242/jeb.055004 [DOI] [PubMed] [Google Scholar]
- Dominy, N. J. and Lucas, P. W. (2001). Ecological importance of trichromatic vision to primates. Nature 410, 363-366. 10.1038/35066567 [DOI] [PubMed] [Google Scholar]
- Falchi, F., Cinzano, P., Elvidge, C. D., Keith, D. M. and Haim, A. (2011). Limiting the impact of light pollution on human health, environment and stellar visibility. J. Environ. Manage 92, 2714-2722. 10.1016/j.jenvman.2011.06.029 [DOI] [PubMed] [Google Scholar]
- Green, A., Cohen-Zion, M., Haim, A. and Dagan, Y. (2017). Evening light exposure to computer screens disrupts human sleep, biological rhythms, and attention abilities. Chronobiol. Int. 34, 855-865. 10.1080/07420528.2017.1324878 [DOI] [PubMed] [Google Scholar]
- Guntur, A. R., Gou, B., Gu, P., He, R., Stern, U., Xiang, Y. and Yang, C. H. (2017). H2O2-sensitive isoforms of Drosophila melanogaster TRPA1 act in bitter-sensing gustatory neurons to promote avoidance of UV during egg-laying. Genetics 205, 749-759. 10.1534/genetics.116.195172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada, T. (1991). Effects of photoperiod and temperature on phototaxis in a water strider, Gerris paludum insularis (Motschulsky). J. Insect Physiol. 37, 27-34. 10.1016/0022-1910(91)90015-R [DOI] [Google Scholar]
- Helfrich-Förster, C. (2019). Flies’ colour preferences depend on the time of day. Nature 574, 43-44. 10.1038/d41586-019-02745-8 [DOI] [PubMed] [Google Scholar]
- Hendricks, J. C., Finn, S. M., Panckeri, K. A., Chavkin, J., Williams, J. A., Sehgal, A. and Pack, A. I. (2000). Rest in Drosophila is a sleep-like state. Neuron 25, 129-138. 10.1016/S0896-6273(00)80877-6 [DOI] [PubMed] [Google Scholar]
- Holzman, D. C. (2010). What's in a color? The unique human health effect of blue light. Environ. Health Perspect. 118, A22-A27. 10.1289/ehp.1184c22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hori, M., Shibuya, K., Sato, M. and Saito, Y. (2015). Lethal effects of short-wavelength visible light on insects. Sci. Rep. 4, 7383. 10.1038/srep07383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kannan, N. N., Vaze, K. M. and Sharma, V. K. (2012). Clock accuracy and precision evolve as a consequence of selection for adult emergence in a narrow window of time in fruit flies Drosophila melanogaster. J. Exp. Biol. 215, 3527-3534. 10.1242/jeb.074534 [DOI] [PubMed] [Google Scholar]
- Keene, A. C., Mazzoni, E. O., Zhen, J., Younger, M. A., Yamaguchi, S., Blau, J., Desplan, C. and Sprecher, S. G. (2011). Distinct visual pathways mediate Drosophila larval light avoidance and circadian clock entrainment. J. Neurosci. 31, 6527-6534. 10.1523/JNEUROSCI.6165-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouser, S., Palaksha, P. and Shakunthala, V. (2014). Study on fitness of Drosophila melanogaster in different light regimes. Biol. Rhythm Res. 45, 293-300. 10.1080/09291016.2013.817138 [DOI] [Google Scholar]
- Krittika, S. and Yadav, P. (2020). Circadian clocks: an overview on its adaptive significance. Biol. Rhythm Res. 51, 1109-1132. 10.1080/09291016.2019.1581480 [DOI] [Google Scholar]
- Lazopulo, S., Lazopulo, A., Baker, J. D. and Syed, S. (2019). Daytime colour preference in Drosophila depends on the circadian clock and TRP channels. Nature 574, 108-111. 10.1038/s41586-019-1571-y [DOI] [PubMed] [Google Scholar]
- Little, C. M., Chapman, T. W. and Hillier, N. K. (2018). Effect of color and contrast of highbush blueberries to host-finding behavior by Drosophila suzukii (Diptera: Drosophilidae). Environ. Entomol. 47, 1242-1251. 10.1093/ee/nvy102 [DOI] [PubMed] [Google Scholar]
- Little, C. M., Rizzato, A. R., Charbonneau, L., Chapman, T. and Hillier, N. K. (2019). Color preference of the spotted wing Drosophila, Drosophila suzukii. Sci. Rep. 9, 16051. 10.1038/s41598-019-52425-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nash, T. R., Chow, E. S., Law, A. D., Fu, S. D., Fuszara, E., Bilska, A., Bebas, P., Kretzschmar, D. and Giebultowicz, J. M. (2019). Daily blue-light exposure shortens lifespan and causes brain neurodegeneration in Drosophila. NPJ Aging Mech. Dis. 5, 8. 10.1038/s41514-019-0038-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noseda, R., Kainz, V., Jakubowski, M., Gooley, J. J., Saper, C. B., Digre, K. and Burstein, R. (2010). A neural mechanism for exacerbation of headache by light. Nat. Neurosci. 13, 239-245. 10.1038/nn.2475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osorio, D. and Vorobyev, M. (2008). A review of the evolution of animal colour vision and visual communication signals. Vision Res. 48, 2042-2051. 10.1016/j.visres.2008.06.018 [DOI] [PubMed] [Google Scholar]
- Schnaitmann, C., Garbers, C., Wachtler, T. and Tanimoto, H. (2013). Color discrimination with broadband photoreceptors. Curr. Biol. 23, 2375-2382. 10.1016/j.cub.2013.10.037 [DOI] [PubMed] [Google Scholar]
- Sharma, V. K. (2003). Adaptive significance of circadian clocks. Chronobiol. Int. 20, 901-919. 10.1081/CBI-120026099 [DOI] [PubMed] [Google Scholar]
- Shaw, P. J., Cirelli, C., Greenspan, R. J. and Tononi, G. (2000). Correlates of sleep and waking in Drosophila melanogaster. Science 287, 1834-1837. 10.1126/science.287.5459.1834 [DOI] [PubMed] [Google Scholar]
- Shen, J., Zhu, X., Gu, Y., Zhang, C., Huang, J. and Xiao, Q. (2019). Toxic effect of visible light on Drosophila life span depending on diet protein content. J. Gerontol. A Biol. Sci. Med. Sci. 74, 163-167. 10.1093/gerona/gly042 [DOI] [PubMed] [Google Scholar]
- Shen, J., Yang, P., Luo, X., Li, H., Xu, Y., Shan, J., Yang, Z. and Liang, B. (2021). Green light extends Drosophila longevity. Exp. Gerontol. 147, 111268. 10.1016/j.exger.2021.111268 [DOI] [PubMed] [Google Scholar]
- Shibuya, K., Onodera, S. and Hori, M. (2018). Toxic wavelength of blue light changes as insects grow. PLoS One 13, e0199266. 10.1371/journal.pone.0199266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storz, U. C. and Paul, R. J. (1998). Phototaxis in water fleas (Daphnia magna) is differently influenced by visible and UV light. J. Comp. Physiol. A 183, 709-717. 10.1007/s003590050293 [DOI] [Google Scholar]
- van Alphen, B., Yap, M. H., Kirszenblat, L., Kottler, B. and van Swinderen, B. (2013). A dynamic deep sleep stage in Drosophila. J. Neurosci. 33, 6917-6927. 10.1523/JNEUROSCI.0061-13.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Videnovic, A., Klerman, E. B., Wang, W., Marconi, A., Kuhta, T. and Zee, P. C. (2017). Timed light therapy for sleep and daytime sleepiness associated with parkinson disease: a randomized clinical trial. JAMA Neurol. 74, 411-418. 10.1001/jamaneurol.2016.5192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang, Y., Yuan, Q., Vogt, N., Looger, L. L., Jan, L. Y. and Jan, Y. N. (2010). Light-avoidance-mediating photoreceptors tile the Drosophila larval body wall. Nature 468, 921-926. 10.1038/nature09576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, E. C., Lee, D. W. and Wu, W. Y. (2003). Action spectra of phototactic responses of flea beetle, Phyllotreta striolata. Physiol. Entomol. 28, 362-368. 10.1111/j.1365-3032.2003.00351.x [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.