Abstract
The hepatitis B virus (HBV) infection remains a global public health problem. This review presents updated recommendations for the optimal current treatment of choice with nucleos(t)ide analogues (NA). Current clinical practice guidelines on the management of chronic hepatitis B (CHB) by the Asian Pacific Association for the Study of the Liver, the European Association for the Study of the Liver and the American Association for the Study of Liver Diseases have been considered. Patients with chronic HBV infection are at increased risk of liver disease progression to cirrhosis and hepatocellular carcinoma (HCC) development. The main goal of therapy is to improve survival preventing disease progression and HCC. The induction of long-term suppression of HBV replication represents the main endpoint of current treatment strategies, while hepatitis B surface antigen (HBsAg) loss is the optimal endpoint. The typical indication for treatment requires elevated HBV desoxyribonucleic acid (DNA), elevated alanine aminotransferase and/or at least moderate histological lesions, while all cirrhotic patients with detectable HBV DNA should be treated. The long-term administration of a potent NA with high barrier to resistance, ie, entecavir, tenofovir disoproxil fumarate or tenofovir alafenamide, represents the treatment of choice. However, HBsAg seroclearance is anecdotal with NA. Treated patients should be monitored for therapy response, adherence, risk of disease progression, and risk of HCC development. This review aims to assess the evolving trends on the potent NA and the new perspectives on finite therapy.
Keywords: antiviral therapy, efficacy, HBsAg loss, kinetics, treatment cessation
Introduction
The hepatitis B virus (HBV) infection remains a global public health problem. Chronic HBV infection is defined as serum detection of hepatitis B surface antigen (HBsAg) for at least 6 months after infection. The World Health Organization (WHO) estimates that there are 257 million people infected with HBV in the world (around 3.5% of the world’s population) causing in 2015 more than 887,000 deaths by cirrhosis and hepatocellular carcinoma (HCC).1
The prevalence of HBV infection varies in different geographical areas. It is higher in the Western Pacific region and in Africa (around 6%) and lower in the Eastern Mediterranean (3.3%), Southeast Asia (2.0%) and Europe (1.6%).1 The prevalence is decreasing in several countries due to improvements in the socioeconomic status, universal vaccination programs, and effective antiviral treatments.2 However, population movements and migration are changing the prevalence and incidence in other countries.
Currently, the long-term administration of a nucleos(t)ide analogue (NA) with high barrier to resistance, ie, entecavir (ETV), tenofovir disoproxil fumarate (TDF) or tenofovir alafenamide (TAF) represents the treatment of choice for chronic hepatitis B (CHB) because these drugs can suppress HBV desoxyribonucleic acid (DNA). However, long-term therapy is needed to maintain the HBV suppression and several issues such as increased cost, reduced adherence, and loss to follow-up should be taken into account. New strategies for limiting the treatment duration should be evaluated.
This review aims to assess the evolving trends in first-line NA and the new perspectives on finite therapy.
HBV Chronic Infection and New Biomarkers
The HBV is a DNA virus that belongs to the Hepadnaviridae family.3 The virus replicates and assembles in the host’s hepatocytes, and the virions are released through the cell secretory pathways. After the virus enters the hepatocytes, the HBV is transported to the nucleus to release the relaxed circular DNA (rcDNA) genome. In the nucleoplasm, the rcDNA becomes a covalently closed circular DNA (cccDNA) that can use the host-cell DNA repair mechanism and can serve as a transcription template for all viral transcripts that are translated into the different viral proteins.4 In addition to complete infectious virions, infected cells produce free, non-infectious sub-viral spherical or filamentous particles.5 Integration of the viral genome into the host genome may occur randomly; it is not necessary for viral replication, but it is one of the mechanisms involved in the hepatocyte transformation and carcinogenesis.6 Phylogenetic analyses of isolated HBV strains have identified 10 major genotypes (A–J) that have a different distribution worldwide.7
Chronic HBV infection is a dynamic process that reflects the interaction between HBV and the host’s immune system. The natural history of chronic infection has been divided into different phases with different prognosis and risk of developing complications, and consequently different need for therapy. The hepatitis B e Antigen (HBeAg)-positive infection usually occurs in younger patients that have a high viral load with normal liver function tests. HBeAg-negative chronic infection phase is characterized by low DNA levels with normal liver function and without significant fibrosis. These are the two phenotypes with the lower risk of developing liver disease or death, and therapy is not generally recommended.8 However, in CHB patients without antiviral treatment, cirrhosis appears to be about twofold higher in HBeAg-negative compared to HBeAg-positive. In untreated cirrhotic patients, the 5-year cumulative risk of developing HCC is 17% in Eastern Asia and 10% in the Western Europe and the United States, and the 5-year liver-related death rate is 15% in Europe and 14% in East Asia.9
An important aspect to consider is the correlation between ethnicity and HBV genotype. The HBV genotypes B and C are endemic in South East Asia, and genotype D is most prevalent in countries bordering the Mediterranean basin.10 This genotype distribution is important for the interpretation of studies on the influence of HBV genotype and other factors in determining the probability of response to treatment strategies.
New biomarkers have been identified during HBV infection. HBV encodes three HBsAg proteins: large, middle, and small. These proteins form the envelope of the virus. The correlation between serum HBsAg and intrahepatic cccDNA is controversial as these subviral particles are derived from both cccDNA and integrated DNA, especially in HBeAg-negative patients.11 However, some studies have demonstrated a parallel decrease in serum HBsAg and intrahepatic cccDNA.12,13 The HBsAg levels vary in the different phases of HBV infection, being higher in chronic HBeAg-positive patients, decreasing in the phase of hepatitis, and being lower in those with chronic HBeAg-negative infection.14,15 The regulation of HBsAg expression is complex and includes more parameters than the amount of cccDNA as its transcriptional activity, the expression of envelope proteins from integrated viral sequences, and the number of infected cells.16 The HBsAg levels have also been described as an indirect marker of the control of the infection by the host’s immune system.15,17 Another interesting marker is the hepatitis B core-related antigen (HBcrAg). It is composed of hepatitis B core antigen (HBcAg), HBeAg, and 22-kDa precore protein (p22cr). HBcrAg has been described as a surrogate marker of intrahepatic cccDNA and its transcriptional activity.18
Goals of Antiviral Therapy
The international guidelines on the management of CHB by the Asian Pacific Association for the Study of the Liver (APASL),19 the European Association for the Study of the Liver (EASL),20 and the American Association for the Study of Liver Diseases (AASLD)21 identify the main goal of therapy as improved survival and quality of life, preventing the progression of the disease, decompensation of cirrhosis, the need for liver transplantation and the development of HCC.
Additionally, other treatment goals are to treat extrahepatic manifestations, prevent mother-to-child transmission, avoid the HBV reactivation in patients on immunosuppressive therapy including transplanted patients, reduce the risk of HCC in those with a family history of HBV-related tumors, and diminish tumor recurrence in patients with HCC.19–21
Treatment Indications
Indications for treatment are generally the same for both HBeAg-positive and HBeAg-negative CHB patients and it is based on the combination of three criteria (HBV DNA levels, alanine aminotransferase (ALT) levels, and liver disease severity). The typical indication requires elevated HBV DNA, elevated ALT and/or at least moderate histological lesions, while all cirrhotic patients with detectable HBV DNA should be treated (Table 1).19–21
Table 1.
Current Recommendations from International Guidelines on Antiviral Treatment in Chronic Hepatitis B Patients
| APASL 201619 | EASL 201720 | AASLD 201821 | |
|---|---|---|---|
| HBeAg-positive | 1) DNA >20,000 IU/mL and ALT >ULN 2) DNA >20,000 IU/mL and normal ALT (treat if moderate or severe inflammation, significant fibrosis or age >30) 3) DNA 2,000–20,000 IU/mL and any ALT: treat only if moderate or severe inflammation or significant fibrosis) 4) DNA <2,000 IU/mL: rule out other causes of liver disease If elevated ALT. Treat only if moderate or severe inflammation or significant fibrosis |
1) ALT >ULN, DNA >2,000 IU/mL and/or at least moderate liver necroinflammation or fibrosis. 2) ALT >ULN and DNA >20,000 IU/mL treat if >30 years 3) ALT >2xULN and DNA >20,000 IU/mL treat regardless degree of fibrosis |
1) ALT 1–2×ULN and DNA >20,000 IU/mL treat if moderate or severe inflammation, significant fibrosis or ALT persistently elevated 2) ALT >2xULN and DNA >20,000 IU/mL 3) ALT >2xULN and DNA 2,000–20,000 IU/mL monitor and treat if persist >6months. |
| HBeAg-negative | 1) DNA >2,000 IU/mL and ALT >2×ULN 2) DNA >2,000 IU/mL and ALT <2×ULN treat if moderate or severe inflammation or significant fibrosis 3) DNA <2,000 IU/mL: rule out other causes of liver disease if elevated ALT. Treat only if moderate or severe inflammation or significant fibrosis |
1) ALT >ULN, DNA >2,000 IU/mL and/or at least moderate liver necroinflammation or fibrosis. 2) ALT >2xULN and DNA >20,000 IU/mL treat regardless degree of fibrosis |
1) ALT >2xULN and DNA >2,000 IU/mL 2) ALT <2xULN and DNA >2,000 IU/mL treat if moderate or severe inflammation or significant fibrosis or if persisting ALT >ULN |
| Compensated cirrhosis | DNA >2,000 IU/mL Any ALT |
DNA detectable Any ALT |
DNA detectable Any ALT |
| Decompensated cirrhosis | DNA detectable Any ALT |
DNA detectable Any ALT |
Treat regardless of DNA or ALT |
Abbreviations: APASL, Asian Pacific Association for the Study of the Liver; EASL, European Association for the Study of the Liver; AASLD, American Association for the Study of Liver Diseases; HBeAg, hepatitis B e antigen; DNA, deoxyribonucleic acid; IU, international units; mL, milliliter; ULN, upper limit normal; ALT, alanine aminotransferase.
Patients who are not candidates for antiviral therapy should be monitored with periodical assessments including serum ALT and HBV DNA levels as well as liver fibrosis severity with non- invasive markers.19–21
There are two currently available treatments for CHB: treatment with oral NA or subcutaneous interferon. The approved interferon formulation for CHB is pegylated interferon alfa-2a (peg-IFN α-2a) that has a modest antiviral activity but can induce a persistent immune control of the infection with limited treatment duration. Twelve months of therapy can induce a sustained response (HBeAg loss with HBV DNA <2,000 IU/mL, 6 months after therapy) in 20–30% of HBeAg-positive patients and the HBsAg loss in 3–7%.22 In HBeAg-negative patients, peg-IFN α-2a can induce the HBsAg loss in 4% of the cases.23 However, peg-IFN α-2a administration is subcutaneous, causes frequent adverse effects, and has a high number of contraindications.
Nucleos(t)ide Analogues and Definitions of Response
The NA approved in Europe for HBV treatment include lamivudine (LAM), adefovir (ADV) telbivudine (LdT), ETV, TDF and TAF, which is an oral prodrug of TDF with lower systemic concentration and higher intracellular concentration.24 The NA can be classified according to their barrier to HBV resistance as “NA with low barrier” (LAM, ADV, LdT) or “NA with high barrier” (ETV, TDF, TAF). International guidelines recommend only the use of NA with high barrier to HBV resistance as the first-line drugs. These drugs are the only treatment option for patients with decompensated liver disease, liver transplants, extrahepatic manifestations, acute hepatitis B or severe chronic HBV exacerbation, and for prevention of HBV reactivation in patients under immunosuppression.19–21
The available NA inhibit the reverse transcription but do not act on cccDNA, so changes in the HBsAg secretory pathway are not expected25 and the sterilizing cure (eradication of cccDNA) is rarely achieved. The functional cure has been defined as the loss of HBsAg (with or without seroconversion of the antibody against HBsAg; anti-HBs) that it is characterized by the presence of normal ALT values and undetectable HBV DNA. The HBsAg loss is considered the optimal goal of the available therapy.20,26 Natural history studies have revealed higher rates of spontaneous HBsAg loss in patients with genotype C than in those with genotype B.27 The HBsAg loss in HBeAg-negative patients treated with long-term TDF was mostly observed in patients with HBV genotypes A or D.28 Similarly, a recent multicenter study, evaluating 1,216 patients after NA withdrawal, demonstrated that patients with genotypes A and D had the highest rates of HBsAg loss and were lower in patients with genotype C, but higher than those with HBV genotype B.29 The loss of HBsAg in patients without advanced fibrosis is associated with minimal risk of cirrhosis,30 decompensation or HCC development, and related to an improvement in survival.31 However, this endpoint is rarely achieved with NA therapy. In a large multicenter cohort receiving ETV or TDF (n=4,769), the 10-year HBsAg loss rate was only 2.1% and the annual incidence was 0.22%.32 Therefore, the virological response, defined as the achievement of undetectable HBV DNA, has been considered as a valid endpoint because it is related to an improvement in clinical outcomes and survival.20 The suppression of HBV DNA to undetectable levels is normally associated with a biochemical response that is defined as the normalization of ALT levels and should be considered as an additional endpoint of the therapy.20,26 Moreover, in HBeAg-positive patients, the NA therapy can induce an HBeAg loss and seroconversion with the antibody against HBeAg (anti-HBe) development, leading to a low replicative phase with a partial immune control that is also considered an advisable endpoint.19–21
In patients with primary non-response to any NA, it is important to check for compliance. If patients receive NA with low barrier to resistance (LAM, LdT or ADV), it is recommended to change to a more potent drug without cross-resistance. In such patients with resistance to LAM, LdT or ETV is recommended switch to TDF or TAF. In patients with ADV resistance, if LAM-naïve, switch to ETV, TDF or TAF and if LAM-resistance switch to TDF or TAF. In patients with TDF or TAF resistance, if LAM-naïve, switch to ETV and if LAM-resistance add ETV. In patients with multidrug resistance, the combination of TDF with ETV appears to be a safe option, but genotypic resistance testing is recommended.19–21
Nucleos(t)ide Analogues Efficacy
Third-generation NA (ETV, TDF and TAF) are drugs with a high barrier to HBV resistance and high efficacy in controlling viral replication (HBV DNA) and inflammatory activity (ALT level). Therefore, first-line NA have demonstrated a high efficacy achieving virological and biochemical response in adherent patients, but HBsAg seroclearance is anecdotic (Table 2).32,33
Table 2.
Efficacy of Approved First-Line Antiviral Therapies in Chronic Hepatitis B Adults. Not Head-to Head Comparisons, and Different Follow-Up
| ETV34,36 | TDF35 | TAF24,37 | |
|---|---|---|---|
| HBeAg-positive | |||
| Follow-up (months) | 60 | 120 | 12 |
| Virological response (%) | 94 | 98 | 73 |
| ALT normalization (%) | 80 | 78 | 72 |
| HBeAg loss (%) | 23 | 52 | 22 |
| HBsAg loss (%) | 1.4 | 4.9 | 1 |
| HBeAg-negative | |||
| Follow-up (months) | 60 | 120 | 12 |
| Virological response (%) | 96 | 100 | 90 |
| ALT normalization (%) | 80 | 83 | 81 |
| HBsAg loss (%) | 4.6 | 3.4 | <1 |
Abbreviations: ETV, entecavir; TDF, tenofovir disoproxil fumarate; TAF, tenofovir alafenamide; HBeAg, hepatitis B e antigen; ALT, alanine aminotransferase; HBsAg, hepatitis B surface antigen.
Virological, Biochemical and Serological Response
In patients with HBeAg-positive CHB, 5 years of antiviral treatment with ETV demonstrated an accumulative probability to develop the virological response of 99%, and HBeAg loss of 53%, but only 1.4% of the included patients achieved the HBsAg loss.34 Similarly, HBeAg-positive patients treated 10 years with TDF lost the HBeAg in 52% but the HBsAg only in 4.9%.35
In patients with HBeAg-negative CHB receiving ETV, the 5-year cumulative probability of virological response was 96% and of HBsAg loss only 4.6%, respectively.36 In the TDF registry study, in HBeAg-negative patients treated for 10 years, 100% of the patients achieved virological response (HBV DNA < 69 IU/mL) and 83% normalized ALT, but only 3.4% achieved HBsAg loss.35
Two non-inferiority randomized controlled trials in HBeAg-positive24 and HBeAg-negative patients37 have demonstrated similar rates of virological response between TDF and TAF.
The combination of NA and peg-IFN α-2a could have synergistic effects. An open-label, active-controlled study, including 740 CHB patients (58% with HBeAg-positive), has shown that NA plus peg-IFN α-2a can achieve a higher proportion of HBsAg loss (9.1%) compared to monotherapy with TDF (0%) or peg-IFN α-2a (2.8%).38 In the PEGAN study,39 185 HBeAg-negative CHB patients were randomly allocated (92 to peg-IFN α-2a plus NA and 93 to NA alone). The study showed that addition of peg-IFN α-2a, 48 weeks, to NA was poorly tolerated and did not result in a significant increase of HBsAg clearance (7.8% vs 3.2%, p=0.15). However, our group showed that the addition of peg-IFN α-2a to NA made a larger and faster decrease of HBsAg levels compared to NA treatment alone, but peg-IFN α-2a side effects limited its use in clinical practice.40 Subsequently, the HERMES study showed similar results in HBeAg-negative CHB patients infected by genotype D.41 In Asian population, the SWAP study42 randomized to switch or add-on peg-IFN α-2b (1.5 mug/kg/weekly) for 48 weeks vs continuing NA. This study showed in Asian HBeAg-negative CHB patients that adding or switching peg-IFN α-2b increased the rate of HBsAg loss compared to NA monotherapy (10.1% or 7.8% vs 0%).
HBV Biomarker Kinetics During NA Therapy
Some studies have evaluated the HBsAg kinetics during long-term NA treatment, and it has been shown that the decline in HBsAg is very slow, with an annual decline around 0.1 log IU/mL.43–45 Therefore, it has been suggested that it would take decades to achieve the functional cure.46,47 However, a larger HBsAg decline during the first years of NA therapy could be an independent predictive factor for achieving low HBsAg levels and HBsAg loss.45,47
Other studies have analyzed the HBcrAg kinetics during NA therapy. In a cohort of 222 patients with CHB (90 HBeAg-positive and 132 HBeAg-negative) receiving long-term ETV, the yearly decline in HBcrAg levels was 0.244 log U/mL.48 However, the HBcrAg levels are significantly lower in HBeAg-negative patients compared to HBeAg-positive patients.48,49 Treatment with TDF or ETV has not shown differences on the HBcrAg decline.49 But the persistence of detectable HBcrAg at the time of treatment withdrawal has been related to severe aminotransferase flares.50
Histological Outcomes
The long-term treatment with NA has demonstrated a significant regression of liver fibrosis and cirrhosis. A study with 57 patients (10 with advanced fibrosis/cirrhosis) receiving ETV and paired liver biopsies51 demonstrated after a median time of 6 years (between biopsies) a histological improvement (decrease ≥2 points in the Knodell necroinflammatory score without increasing the Knodell fibrosis score) in 96% of patients, and fibrosis improvement (decrease ≥1 point in the Ishak fibrosis score) in 88% of them. In an open-label trial with TDF,30 348 patients had paired biopsies (at baseline and week 240) and showed histological improvement in 87% and fibrosis regression (decrease ≥1 point in the Ishak fibrosis score) in 51%. Importantly, among the 96 (28%) patients with cirrhosis (Ishak score 5 or 6) at baseline, 71 (74%) achieved a fibrosis regression and only 3 out of 252 (1.2%) without cirrhosis progressed to Ishak 5 or 6 (p<0.01).
Decrease in liver stiffness measurements (LSM) after long-term treatment with NA has been described. In a systematic review and meta-analyses,52 the decrease in LSM was higher as longer was the treatment duration: from −2.21 kPa at 6 months to −5.19 kPa at 5 years of antiviral therapy (p < 0.001). Some studies have suggested that most of the LSM decline reflect a decrease in necroinflammatory activity and others concluded that LSM decline could be associated with fibrosis improvement.53,54 After treatment initiation, the first LSM decline is faster, mainly related to the necroinflammatory attenuation achieved by the intensive HBV DNA suppression. After 6 months of therapy, the following LSM decrease is slower, which could be related to a true improvement of fibrosis. Therefore, the correlation between changes in LSM and fibrosis stage in CHB-treated patients is controversial probably by this biphasic decline of LSM. Thus, the role of LSM is not well defined in the follow-up of NA-treated patients. Despite this, the international guidelines recommend liver fibrosis assessment with non-invasive markers.19–21
Clinical Outcomes
The main goal of antiviral therapy is to improve survival preventing disease progression, and HCC development. Long-term studies have demonstrated that NA treatment improves clinical outcomes, especially in patients with cirrhosis. Studies comparing untreated patients and those treated with ETV demonstrated that antiviral therapy reduced the incidence of HCC, liver-related complications, and improved survival.55,56 A multicenter study in cirrhotic patients receiving TDF showed a reduced risk of developing HCC, clinical decompensation, or liver transplantation/death compared to an untreated cohort.57 The PAGE-B cohort showed in Caucasian patients treated with ETV or TDF for 5 years an HCC incidence of 5.7–8.4%.58 Therefore, treated patients at high risk of developing HCC (males older than 50 or cirrhotic patients) should continue surveillance.
In recent years, some studies have suggested that patients treated with TDF, compared to ETV, have a lower risk of developing HCC, especially in the Asian population, which has generated an intense debate with conflicting publications.59–64 A large study including 29,350 patients from China showed a reduced HCC incidence in patients receiving TDF but only 1% of those with cirrhosis had received TDF. Moreover, important differences between groups regarding HCC risk factors were found; patients treated with TDF were younger, and more frequently HBeAg-positive, females, without cirrhosis, and without diabetes.60 A recent meta-analysis including 24 studies (37,771 CHB patients treated with TDF and 72,094 treated with ETV) has shown that TDF was associated with a lower HCC risk than ETV in Asian CHB patients (adjusted HR: 0.76, 95% CI: 0.66–0.87; 15 studies) or in NA-naïve patients (adjusted HR: 0.74, 95% CI: 0.65–0.84; 18 studies).61 However, other studies have failed to demonstrate this association.62,63 In Caucasian patients, the largest study including 1,935 patients from the PAGE-B cohort with a median follow-up of 7.5 years did not find differences on 5-year cumulative HCC incidence between ETV (5.4%) or TDF (6.0%).64
A less controversial point is the benefit of achieving HBsAg seroclearance on HCC risk. It has been described that achieving HBsAg seroclearance is associated with improvement in all patients’ outcomes.26 A systematic review including more than 105,000 CHB patients and more than 8,000 with HBsAg clearance showed a significant lower HCC incidence in patients with HBsAg loss (1.86%) compared to those who remained HBsAg-positive (6.56%)(p<0.001). Cirrhosis, male gender, and age ≥50 were the major risks for developing HCC after HBsAg seroclearance.65 It is important to consider that the way in which the HBsAg loss is achieved does not seem to impact on outcomes. A recent study including 1,972 patients with HBsAg loss showed an HCC annual incidence of 0.38 per 100 person-years (during a median follow-up of 5.6 years), without differences between patients who achieved it spontaneously and those with NA.66
Nucleos(t)ide Analogues Safety
The main side effects of NA are the renal tubular dysfunction and the decline of bone mineral density (BMD).67 TDF and ETV are metabolized by the kidneys and should be adjusted in patients with an estimated glomerular filtration rate (eGFR) of less than 50 mL/min per 1.73 m2. TAF is not approved in eGFR below 15 mL/min per 1.73 m2. Despite, a first meta-analysis of studies comparing ETV and TDF did not show differences in serum creatinine level, eGFR, or serum phosphate level.31 More recent studies have demonstrated that TDF is associated with a lower mean eGFR.68,69 A recent multicenter retrospective study including 6,189 treatment-naïve CHB patients with TDF (n= 2,482) or ETV (n = 3,707) showed that TDF vs ETV was associated with higher risk of worsening renal function (adjusted HR 1.26, 95% CI 1.11–1.43).69 In patients with cirrhosis, a systematic review and meta-analysis has shown that both TDF and ETV can influence renal function, but patients under TDF therapy may have more risk to suffer from renal damage and hypophosphatemia.68 The most severe manifestation of tubular TDF toxicity is Fanconi syndrome, which has been reported in sporadic cases of HBV-monoinfected patients, with resolution after withdrawal.70 The increase in serum creatinine levels has been reported in 5% and hypophosphatemia in 1.7% of the patients with TDF for 10 years.35
The effects of TDF in BMD are probably related to the increased tubular phosphate metabolism, but with few clinical implications as they appear to be reversible after withdrawal.67 The TAF has shown lower eGFR reduction and lower decrease in BMD compared to TDF.24,37 Therefore, TAF is especially useful in older patients with chronic kidney disease or low BMD.71
In terms of safety, NA are well tolerated and have a good safety profile.35,36 However, CHB patients usually start treatment at young ages and may be treated for decades. Therefore, it is important to note that treatment adherence can decrease over time. In a systematic review and meta-analyses72 of 30 studies, the adherence with NA was 74.6%. A study including 894 patients showed that the adherence with ETV was <90% in more than 30% of the patients at 5 years of treatment.73 On the other hand, long-term therapy causes financial burden on healthcare systems. Therefore, questions on treatment duration and withdrawal have been gaining interest in recent years.
New Perspectives on Nucleos(t)ide Analogues Cessation
Current international guidelines from the main Scientific Societies differ in the recommendations on the duration of antiviral treatment with NA.19–21
In non-cirrhotic HBeAg-positive CHB patients, the three guidelines (APASL, EASL, AASLD) suggest that NA treatment can be discontinued when HBeAg seroconversion is achieved and HBV is undetectable during at least 1 year of consolidation. In the APASL guideline19 it is suggested that prolong 3 years of consolidation therapy may be beneficial, based on studies that have shown a reduced risk of virological relapse compared to 1 year of consolidation.74,75 In contrast, the AASLD guideline21 suggests that NA therapy could be maintained until achieving the HBsAg loss. The major discrepancy among international guidelines lies in the timing of NA cessation in HBeAg-negative CHB patients.19–21 In these patients, treatment with NA has been reported to induce a slow HBsAg decline43–45,47,76 requiring a long treatment duration, even lifelong, to achieve the HBsAg seroclearance.46
The APASL guideline introduced in 200877 the possibility of stopping NA therapy in HBeAg-negative patients after at least 2 years with undetectable HBV DNA documented on three separate occasions 6 months apart. This discontinuation approach was mostly driven by local reimbursement policies and the first published studies were retrospective. In 2012, Hadzydiannis et al78 reported, in a pilot study with 33 patients, a high rate of HBsAg seroclearance (39% at 5.5 years of follow-up) after stopping ADV. In 2017, the first randomized controlled trial, the FINITE-study79 confirmed that the strategy of stopping therapy increased the HBsAg loss rate 3 years after NA cessation compared to maintain NA (19% vs 0%). Based on these studies, EASL guideline suggested in 2017 that NA could be withdrawn in non-cirrhotic HBeAg-negative CHB patients after 3 years of viral suppression.20 Since then, several studies have evaluated this strategy.80 Studies in Asian patients have shown lower HBsAg loss rate compared to European studies, but there are important differences that need to be taken into account. First, there is a different distribution of HBV genotypes (especially genotype D) in these geographical areas that can explain, in part, the different HBsAg loss rates.81 Second, Asian studies include patients with advanced liver disease and cirrhosis82 that are not recommended by EASL guideline. And third, neither Asian nor European studies have predefined retreatment rules; thus, comparisons between studies are difficult because the timing of retreatment has important implications on HBsAg loss probabilities.83
The evidence of increased HBsAg loss rates after NA cessation in HBeAg-negative CHB patients has caused a paradigm shift in recent years, from indefinite long-term treatment to finite therapy and some experts consider that finite therapy is not only an option but it would be a recommendation.84–86 Hence, new issues rise with this new strategy such as the selection of patients who could benefit the most from stopping therapy, the safety of withdrawal a well-tolerated treatment, and the optimal time to restart the NA.
When to Stop NA Treatment
In clinical guidelines, the stopping rules for NA are mainly based on treatment duration and virological suppression.19,20 It has been suggested that stopping NA treatment can only be successful if cccDNA transcriptional activity has been silenced during treatment depending on the baseline HBV transcriptional activity and the treatment duration.85
After NA discontinuation, it has been postulated that long-term suppression of HBV DNA can revitalize depleted CD8 + T cells and restore immune control against infected hepatocytes by decreasing NK cell killing of HBV-specific T cells and increasing serum cytokines.87–90 Therefore, an immune-mediated long-term control of viral replication can be achieved after treatment discontinuation, with most of the patients remaining in a situation of “inactive carriers”. Moreover, the recurrence of HBV replication after NA discontinuation represents a trigger leading to an activation of immune responses91 that can induce an accelerated HBsAg decline. Recently, our group has observed, in patients treated longer than 6 years, that HBsAg decline accelerated after NA cessation compared to on-treatment decline, even if HBsAg loss was not achieved.92
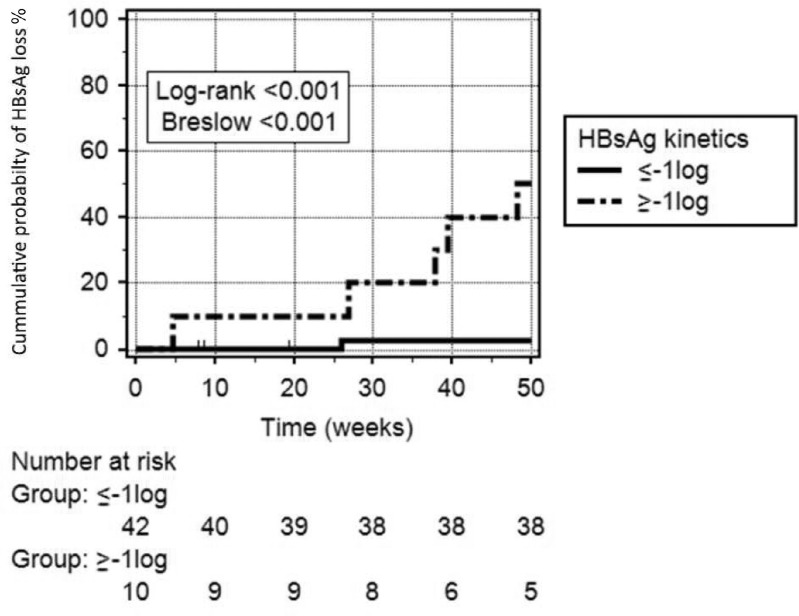
HBV biomarkers as HBsAg and HBcrAg levels have been evaluated to identify better those patients with the highest probability to achieve HBsAg loss. Currently, the HBsAg level is the most reliable predictive marker. It has been postulated that on-treatment HBsAg kinetics and HBsAg levels at the end of therapy (EoT) are good predictors of HBsAg loss after discontinuation.93,94 Our group has recently demonstrated that on-treatment HBsAg kinetics showed a high accuracy to predict the HBsAg loss probability, 1 year after withdrawal.92 Patients with an HBsAg decline ≥1 log IU/mL during NA therapy showed a probability of 50% to achieve HBsAg loss 1 year after discontinuation (Figure 1). The RETRACT-B study,95 a recent international multicenter multiethnic study including 1,552 patients who stopped NA therapy, has demonstrated different accuracy of HBsAg levels at EoT between Asian and Caucasian patients. The study showed that HBsAg <100 IU/mL in Asian patients and <1,000 IU/mL in Caucasian were associated with a 4-year HBsAg loss probability of 33% and 41%, respectively. On the other hand, those patients without these HBsAg levels showed an HBsAg loss probability <10%. The CREATE study,29 a recent multicenter study including 572 patients who stopped NA treatment, has shown lower HBcrAg levels at EoT in those with virological response and HBsAg loss after withdrawal. Patients with undetectable HBcrAg (<2 log U/mL) at EoT showed a virological response rate of 65% and HBsAg loss rate of 12% 1 year after cessation. The same group confirmed in 1,216 patients that non-Asian ethnicity was associated with the highest chance of HBsAg loss and that HBsAg levels (<100 IU/mL) and undetectable HBcrAg were associated with a higher chance of HBsAg loss. Moreover, the study demonstrated that patients with genotypes A or D had the highest rates of HBsAg loss and were lower in patients with genotype C, but higher than those with HBV genotype B.29
Figure 1.
Cumulative probability of HBsAg loss after NA interruption according to HBsAg kinetics during NA therapy.
Notes: Patients with an HBsAg kinetics ≥ −1log10 IU/mL showed an HBsAg loss cumulative probability of 50% 1 year after NA interruption compared to 2.5% in those with an HBsAg kinetics < −1 log10 IU/mL (Log rank= p<0.001; Breslow p=<0.001). Reproduced from Broquetas T, Hernandez JJ, Garcia-Retortillo M et al. On-therapy HBsAg kinetics can predict HBsAg loss after nucleos(t)ide analogues interruption in HBeAg-negative patients. The cup is half full and half empty. Dig Liver Dis. 2022; S1590-8658(21)00926–9. doi:10.1016/j.dld.2021.12.017.92 © 2022 The Authors. Published by Elsevier Ltd on behalf of Editrice Gastroenterologica Italiana S.r.l. CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/).
Safety of NA Withdrawal
Safety of this strategy is a major concern as NA is a well-tolerated treatment with few side effects. An important issue to evaluate after NA interruption is the risk of developing HCC. In the study of Jeng82 that included patients without cirrhosis (n=383) and with cirrhosis (n=308), the HCC incidence at 1-, and 3-years after withdrawal was 0.15% and 1% for non-cirrhotic patients; and 1.3% and 4% for those with cirrhosis. However, the incidence during therapy was similar (0.08% and 0.3% for patients without cirrhosis; and 1.5% and 3.4% for those with cirrhosis). Therefore, treatment cessation did not increase the HCC development. In the RETRACT-B study,95 the HCC incidence at 48 months after NA withdrawal was 2.2% in patients with cirrhosis and only 0.7% in those without cirrhosis.
Apart from the risk of HCC, there is a concern that ALT flares after treatment cessation could induce hepatic decompensation or acute on chronic liver failure. In a study from Taiwan including 85 patients who stopped TDF according to the APASL stopping rules, the clinical relapse (ALT>2xULN and DNA >2,000 IU/mL) occurred in 52% of the patients at 1 year of follow-up.96 The risk factors associated with relapse were higher levels of HBsAg at EoT and shorter treatment duration. In contrast, the CREATE study29 including 572 patients from different ethnicities showed that ALT flare (ALT>3xULN) appeared in only 92 (16%) patients after withdrawal (2 patients developed jaundice without encephalopathy or coagulopathy). The occurrence of hepatic decompensation even death after NA cessation has been described. In the study of Jeng et al82 including 691 patients (44.6% with cirrhosis), a clinical relapse occurred in 60.6% of the patients (7 developed a hepatic decompensation and 3 died). In contrast, in the largest study (RETRACT-B)95 with 1,552 patients, only 19 (1.22%) presented hepatic decompensation during the first 48 months of follow-up (4.3% in patients with cirrhosis vs 0.8% in those without cirrhosis; p<0.01). Death occurred in 7 (0.45%) patients, all with hepatic decompensation, and 4 related to a hepatitis B-associated flare. In view of these results, the treatment cessation is safe, particularly in non-cirrhotic patients, despite a close follow-up is mandatory to avoid severe flares. Therefore, EASL guideline20 does not recommend the withdrawal in cirrhotic patients, whereas the APASL guideline19 suggests that it may be considered if close follow-up is performed.
Retreatment Decision
Probably the most important issue after NA discontinuation is the optimal time to restart treatment. As mentioned above, it has been described that clinical relapse may occur in around 60% of the patients after NA withdrawal.82,95 On the one hand, it has been demonstrated that early initiation of NA retreatment could potentially inhibit the beneficial effect of flare-associated immune activation and no retreatment has been related to HBsAg loss.82,97,98 On the other hand, hepatitis flare after NA cessation may induce hepatic decompensation and even death.82,95 Therefore, the decision of retreatment should not be too late to prevent hepatic decompensation and not too early to allow HBsAg loss.86
A combination of HBsAg and ALT kinetics has been described to be considered to define the different pattern during spontaneous hepatitis flare.99 An HBsAg decline >10% than the preceding level started prior to or around the peak of ALT may reflect that the host is dominating over the virus and the effective immune clearance of HBV is ongoing (“host-dominating flare”). In contrast, HBsAg increasing along with ascending ALT or remaining high after the peak of ALT may reflect that the virus is dominating over the host and the immune response is failing or being ineffective (“virus-dominating flare”). It has been suggested that patients with off-treatment virus dominating flare should be retreated to prevent severe flare and help their ineffective immune response to fight the virus. In contrast, in patients with off-treatment host dominating flare, NA retreatment should be delayed or avoided as it could interrupt the strong endogenous immune clearance response of the host.86
Therefore, immune control against infected hepatocytes after NA cessation is not universal and different flare patterns should be crucial to be recognized in order to identify those patients at higher risk of developing clinical decompensation. On the other hand, the tolerable level of circulating HBV DNA during a post-NA treatment phase and long-term risk of HCC have not been defined. Moreover, different biochemical criteria of retreatment have been applied in the previously published studies making difficult to compare and obtain solid conclusions.79,90,92,100 However, our recommendation is in consonance with Liaw and Chien advice86,101 that retreatment criteria should not be determined at one single time point and careful and close assessment is required to better define the type of hepatitis flare for optimal retreatment decision.
Conclusions
In conclusion, long-term treatment using NA with a high HBV-resistance barrier has demonstrated with low rate of side effects a high efficacy in controlling viral replication and improvement in histology, HCC occurrence, risk of clinical decompensation, and mortality. However, HBsAg seroclearance that is the most desirable endpoint is rarely achieved with NA. Until new therapies will be available, strategies to improve functional cure are under investigation. The NA cessation, after some years of effective treatment, has shown encouraging results. Therefore, a paradigm shift from indefinite NA treatment to a finite NA therapy is emerging. However, accurate predictors to select patients who will benefit the most from this strategy are still under evaluation and retreatment decision is a cornerstone for the NA finite therapy that should be not too late to avoid risk of hepatic decompensation and not too early to improve outcomes. More studies are needed to better answer the questions that this new strategy raises.
Abbreviations
HBV, hepatitis B virus; HBsAg, hepatitis B surface antigen; WHO, World Health Organization; HCC, hepatocellular carcinoma; NA, nucleos(t)ides analogues; ETV, entecavir; TDF, tenofovir disoproxil fumarate; TAF, tenofovir alafenamide; CHB, chronic hepatitis B; DNA, desoxyribonucleic acid; rcDNA, relaxed circular DNA; cccDNA, covalently closed circular DNA; HBeAg, hepatitis B e antigen; HBcrAg, hepatitis B core-related antigen; HBcAg, hepatitis B core antigen; p22cr, 22-kDa precore protein; APASL, Asian Pacific Association for the Study of the Liver; EASL, European Association for the Study of the Liver; AASLD, American Association for the Study of Liver Diseases; ALT, alanine aminotransferase; peg-IFN α-2a, pegylated interferon alfa-2a; LAM, lamivudine; ADV, adefovir; LdT, telbivudine; Anti-HBs, antibody against hepatitis B surface antigen; Anti-HBe, antibody against hepatitis B e antigen; IU, international units; mL, milliliter; ULN, upper limit normal; LSM, liver stiffness measurements; BMD, bone mineral density; eGFR, estimated glomerular filtration rate; EoT, end of therapy.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.World Health Organization (WHO). Global hepatitis report, 2017; 2017: 83.
- 2.Chen CL, Yang JY, Lin SF, et al. Slow decline of hepatitis B burden in general population: results from a population-based survey and longitudinal follow-up study in Taiwan. J Hepatol. 2015;63(2):354–363. doi: 10.1016/j.jhep.2015.03.013 [DOI] [PubMed] [Google Scholar]
- 3.Yuen MF, Chen DS, Dusheiko GM, et al. Hepatitis B virus infection. Nat Rev Dis Primers. 2018;4(1):18035. doi: 10.1038/nrdp.2018.35 [DOI] [PubMed] [Google Scholar]
- 4.Lucifora J, Protzer U. Attacking hepatitis B virus cccDNA–The holy grail to hepatitis B cure. J Hepatol. 2016;64(1 Suppl):S41–S48. doi: 10.1016/j.jhep.2016.02.009 [DOI] [PubMed] [Google Scholar]
- 5.Seeger C, Mason WS. Molecular biology of hepatitis B virus infection. Virology. 2015;479–480:672–686. doi: 10.1016/j.virol.2015.02.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Levrero M, Zucman-Rossi J. Mechanisms of HBV-induced hepatocellular carcinoma. J Hepatol. 2016;64(1 Suppl):S84–S101. doi: 10.1016/j.jhep.2016.02.021 [DOI] [PubMed] [Google Scholar]
- 7.Liu Z, Zhang Y, Xu M, Li X, Zhang Z. Distribution of hepatitis B virus genotypes and subgenotypes: a meta-analysis. Medicine. 2021;100(50):e27941. doi: 10.1097/MD.0000000000027941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.World Health Organization (WHO). Guidelines for the prevention, care and treatment of persons with chronic hepatitis B infection. Geneva: WHO Press through the WHO website; 2015. Available from: www.who.int/about/licensing/copyright_form/en/index.html. Accessed July 13, 2022. [PubMed] [Google Scholar]
- 9.Fattovich G, Bortolotti F, Donato F. Natural history of chronic hepatitis B: special emphasis on disease progression and prognostic factors. J Hepatol. 2008;48(2):335–352. doi: 10.1016/j.jhep.2007.11.011 [DOI] [PubMed] [Google Scholar]
- 10.Rajoriya N, Combet C, Zoulim F, Janssen HLA. How viral genetic variants and genotypes influence disease and treatment outcome of chronic hepatitis B. Time for an individualised approach? J Hepatol. 2017;67(6):1281–1297. doi: 10.1016/j.jhep.2017.07.011 [DOI] [PubMed] [Google Scholar]
- 11.Tong S, Revill P. Overview of hepatitis B viral replication and genetic variability. J Hepatol. 2016;64(1 Suppl):S4–S16. doi: 10.1016/j.jhep.2016.01.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wursthorn K, Lutgehetmann M, Dandri M, et al. Peginterferon alpha-2b plus Adefovir induce strong cccDNA decline and HBsAg reduction in patients with chronic hepatitis B. Hepatology. 2006;44(3):675–684. doi: 10.1002/hep.21282 [DOI] [PubMed] [Google Scholar]
- 13.Janssen HL, Sonneveld MJ, Brunetto MR. Quantification of serum hepatitis B surface antigen: is it useful for the management of chronic hepatitis B? Gut. 2012;61(5):641–645. doi: 10.1136/gutjnl-2011-301096 [DOI] [PubMed] [Google Scholar]
- 14.Jaroszewicz J, Calle Serrano B, Wursthorn K, et al. Hepatitis B surface antigen (HBsAg) levels in the natural history of hepatitis B virus (HBV)-infection: a European perspective. J Hepatol. 2010;52(4):514–522. doi: 10.1016/j.jhep.2010.01.014 [DOI] [PubMed] [Google Scholar]
- 15.Nguyen T, Thompson AJ, Bowden S, et al. Hepatitis B surface antigen levels during the natural history of chronic hepatitis B: a perspective on Asia. J Hepatol. 2010;52(4):508–513. doi: 10.1016/j.jhep.2010.01.007 [DOI] [PubMed] [Google Scholar]
- 16.Zoulim F, Testoni B, Lebosse F. Kinetics of intrahepatic covalently closed circular DNA and serum hepatitis B surface antigen during antiviral therapy for chronic hepatitis B: lessons from experimental and clinical studies. Clin Gastroenterol Hepatol. 2013;11(8):1011–1013. doi: 10.1016/j.cgh.2013.04.010 [DOI] [PubMed] [Google Scholar]
- 17.Chan HL, Wong VW, Tse AM, et al. Serum hepatitis B surface antigen quantitation can reflect hepatitis B virus in the liver and predict treatment response. Clin Gastroenterol Hepatol. 2007;5(12):1462–1468. doi: 10.1016/j.cgh.2007.09.005 [DOI] [PubMed] [Google Scholar]
- 18.Testoni B, Lebosse F, Scholtes C, et al. Serum hepatitis B core-related antigen (HBcrAg) correlates with covalently closed circular DNA transcriptional activity in chronic hepatitis B patients. J Hepatol. 2019;70(4):615–625. doi: 10.1016/j.jhep.2018.11.030 [DOI] [PubMed] [Google Scholar]
- 19.Sarin SK, Kumar M, Lau GK, et al. Asian-Pacific clinical practice guidelines on the management of hepatitis B: a 2015 update. Hepatol Int. 2016;10(1):1–98. doi: 10.1007/s12072-015-9675-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lampertico P, Agarwal K, Berg T; European Association for the Study of the Liver. EASL 2017 clinical practice guidelines on the management of hepatitis B virus infection. J Hepatol. 2017;67(2):370–398. doi: 10.1016/j.jhep.2017.03.021 [DOI] [PubMed] [Google Scholar]
- 21.Terrault NA, Lok ASF, McMahon BJ, et al. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Hepatology. 2018;67(4):1560–1599. doi: 10.1002/hep.29800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sonneveld MJ, Hansen BE, Piratvisuth T, et al. Response-guided peginterferon therapy in hepatitis B e antigen-positive chronic hepatitis B using serum hepatitis B surface antigen levels. Hepatology. 2013;58(3):872–880. doi: 10.1002/hep.26436 [DOI] [PubMed] [Google Scholar]
- 23.Marcellin P, Lau GK, Bonino F, et al. Peginterferon alfa-2a alone, lamivudine alone, and the two in combination in patients with HBeAg-negative chronic hepatitis B. N Engl J Med. 2004;351(12):1206–1217. doi: 10.1056/NEJMoa040431 [DOI] [PubMed] [Google Scholar]
- 24.Chan HL, Fung S, Seto WK, et al. Tenofovir alafenamide versus tenofovir disoproxil fumarate for the treatment of HBeAg-positive chronic hepatitis B virus infection: a randomised, double-blind, Phase 3, non-inferiority trial. Lancet Gastroenterol Hepatol. 2016;1(3):185–195. doi: 10.1016/S2468-1253(16)30024-3 [DOI] [PubMed] [Google Scholar]
- 25.Cornberg M, Wong VW, Locarnini S, Brunetto M, Janssen HLA, Chan HL. The role of quantitative hepatitis B surface antigen revisited. J Hepatol. 2017;66(2):398–411. doi: 10.1016/j.jhep.2016.08.009 [DOI] [PubMed] [Google Scholar]
- 26.Anderson RT, Choi HSJ, Lenz O, et al. Association between seroclearance of hepatitis B surface antigen and long-term clinical outcomes of patients with chronic hepatitis B virus infection: systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2021;19(3):463–472. doi: 10.1016/j.cgh.2020.05.041 [DOI] [PubMed] [Google Scholar]
- 27.Tseng TC, Liu CJ, Chen CL, et al. Higher lifetime chance of spontaneous surface antigen loss in hepatitis B carriers with genotype C infection. Aliment Pharmacol Ther. 2015;41(10):949–960. doi: 10.1111/apt.13170 [DOI] [PubMed] [Google Scholar]
- 28.Heathcote EJ, Marcellin P, Buti M, et al. Three-year efficacy and safety of tenofovir disoproxil fumarate treatment for chronic hepatitis B. Gastroenterology. 2011;140(1):132–143. doi: 10.1053/j.gastro.2010.10.011 [DOI] [PubMed] [Google Scholar]
- 29.Sonneveld MJ, Park JY, Kaewdech A, et al. Prediction of sustained response after nucleo(s)tide analogue cessation using HBsAg and HBcrAg levels: a multicenter study (CREATE). Clin Gastroenterol Hepatol. 2022;20(4):e784–e93. doi: 10.1016/j.cgh.2020.12.005 [DOI] [PubMed] [Google Scholar]
- 30.Marcellin P, Gane E, Buti M, et al. Regression of cirrhosis during treatment with tenofovir disoproxil fumarate for chronic hepatitis B: a 5-year open-label follow-up study. Lancet. 2013;381(9865):468–475. doi: 10.1016/S0140-6736(12)61425-1 [DOI] [PubMed] [Google Scholar]
- 31.Lok AS, McMahon BJ, Brown RS Jr., et al. Antiviral therapy for chronic hepatitis B viral infection in adults: a systematic review and meta-analysis. Hepatology. 2016;63(1):284–306. doi: 10.1002/hep.28280 [DOI] [PubMed] [Google Scholar]
- 32.Hsu YC, Yeh ML, Wong GL, et al. Incidences and determinants of functional cure during entecavir or tenofovir disoproxil fumarate for chronic hepatitis B. J Infect Dis. 2021;224(11):1890–1899. doi: 10.1093/infdis/jiab241 [DOI] [PubMed] [Google Scholar]
- 33.Grossi G, Vigano M, Loglio A, Lampertico P. Hepatitis B virus long-term impact of antiviral therapy nucleot(s)ide analogues (NUCs). Liver Int. 2017;37(Suppl 1):45–51. doi: 10.1111/liv.13291 [DOI] [PubMed] [Google Scholar]
- 34.Chang TT, Lai CL, Kew Yoon S, et al. Entecavir treatment for up to 5 years in patients with hepatitis B e antigen-positive chronic hepatitis B. Hepatology. 2010;51(2):422–430. doi: 10.1002/hep.23327 [DOI] [PubMed] [Google Scholar]
- 35.Marcellin P, Wong DK, Sievert W, et al. Ten-year efficacy and safety of tenofovir disoproxil fumarate treatment for chronic hepatitis B virus infection. Liver Int. 2019;39(10):1868–1875. doi: 10.1111/liv.14155 [DOI] [PubMed] [Google Scholar]
- 36.Ahn J, Lee HM, Lim JK, et al. Entecavir safety and effectiveness in a national cohort of treatment-naive chronic hepatitis B patients in the US - The ENUMERATE study. Aliment Pharmacol Ther. 2016;43(1):134–144. doi: 10.1111/apt.13440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Buti M, Gane E, Seto WK, et al. Tenofovir alafenamide versus tenofovir disoproxil fumarate for the treatment of patients with HBeAg-negative chronic hepatitis B virus infection: a randomised, double-blind, phase 3, non-inferiority trial. Lancet Gastroenterol Hepatol. 2016;1(3):196–206. doi: 10.1016/S2468-1253(16)30107-8 [DOI] [PubMed] [Google Scholar]
- 38.Marcellin P, Ahn SH, Ma X, et al. Combination of tenofovir disoproxil fumarate and peginterferon alpha-2a increases loss of hepatitis B surface antigen in patients with chronic hepatitis B. Gastroenterology. 2016;150(1):134–44 e10. doi: 10.1053/j.gastro.2015.09.043 [DOI] [PubMed] [Google Scholar]
- 39.Bourliere M, Rabiega P, Ganne-Carrie N, et al. Effect on HBs antigen clearance of addition of pegylated interferon alfa-2a to nucleos(t)ide analogue therapy versus nucleos(t)ide analogue therapy alone in patients with HBe antigen-negative chronic hepatitis B and sustained undetectable plasma hepatitis B virus DNA: a randomised, controlled, open-label trial. Lancet Gastroenterol Hepatol. 2017;2(3):177–188. doi: 10.1016/S2468-1253(16)30189-3 [DOI] [PubMed] [Google Scholar]
- 40.Broquetas T, Garcia-Retortillo M, Mico M, et al. Hepatitis B surface antigen and hepatitis B core-related antigen kinetics after adding pegylated-interferon to nucleos(t)ids analogues in hepatitis B e antigen-negative patients. World J Hepatol. 2020;12(11):1076–1088. doi: 10.4254/wjh.v12.i11.1076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lampertico P, Brunetto MR, Craxi A, et al. Add-on peginterferon alfa-2a to nucleos(t)ide analogue therapy for Caucasian patients with hepatitis B ‘e’ antigen-negative chronic hepatitis B genotype D. J Viral Hepat. 2019;26(1):118–125. doi: 10.1111/jvh.12999 [DOI] [PubMed] [Google Scholar]
- 42.Lim SG, Yang WL, Ngu JH, et al. Switching to or add-on peginterferon in patients on nucleos(t)ide analogues for chronic hepatitis B: the SWAP RCT. Clin Gastroenterol Hepatol. 2022;20(2):e228–e50. doi: 10.1016/j.cgh.2021.04.031 [DOI] [PubMed] [Google Scholar]
- 43.Boglione L, D’Avolio A, Cariti G, et al. Kinetics and prediction of HBsAg loss during therapy with analogues in patients affected by chronic hepatitis B HBeAg negative and genotype D. Liver Int. 2013;33(4):580–585. doi: 10.1111/liv.12091 [DOI] [PubMed] [Google Scholar]
- 44.Zoulim F, Carosi G, Greenbloom S, et al. Quantification of HBsAg in nucleos(t)ide-naive patients treated for chronic hepatitis B with entecavir with or without tenofovir in the BE-LOW study. J Hepatol. 2015;62(1):56–63. doi: 10.1016/j.jhep.2014.08.031 [DOI] [PubMed] [Google Scholar]
- 45.Broquetas T, Garcia-Retortillo M, Hernandez JJ, et al. Quantification of HBsAg to predict low levels and seroclearance in HBeAg-negative patients receiving nucleos(t)ide analogues. PLoS One. 2017;12(11):e0188303. doi: 10.1371/journal.pone.0188303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chevaliez S, Hezode C, Bahrami S, Grare M, Pawlotsky JM. Long-term hepatitis B surface antigen (HBsAg) kinetics during nucleoside/nucleotide analogue therapy: finite treatment duration unlikely. J Hepatol. 2013;58(4):676–683. doi: 10.1016/j.jhep.2012.11.039 [DOI] [PubMed] [Google Scholar]
- 47.Seto WK, Wong DK, Fung J, Huang FY, Lai CL, Yuen MF. Reduction of hepatitis B surface antigen levels and hepatitis B surface antigen seroclearance in chronic hepatitis B patients receiving 10 years of nucleoside analogue therapy. Hepatology. 2013;58(3):923–931. doi: 10.1002/hep.26376 [DOI] [PubMed] [Google Scholar]
- 48.Lam YF, Seto WK, Wong D, et al. Seven-year treatment outcome of entecavir in a real-world cohort: effects on clinical parameters, HBsAg and HBcrAg levels. Clin Transl Gastroenterol. 2017;8(10):e125. doi: 10.1038/ctg.2017.51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mak LY, Wong DK, Cheung KS, Seto WK, Fung J, Yuen MF. First-line oral antiviral therapies showed similar efficacies in suppression of serum HBcrAg in chronic hepatitis B patients. BMC Gastroenterol. 2021;21(1):123. doi: 10.1186/s12876-021-01711-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Carey I, Gersch J, Wang B, et al. Pregenomic HBV RNA and hepatitis B core-related antigen predict outcomes in hepatitis B e antigen-negative chronic hepatitis B patients suppressed on nucleos(t)ide analogue therapy. Hepatology. 2020;72(1):42–57. doi: 10.1002/hep.31026 [DOI] [PubMed] [Google Scholar]
- 51.Chang TT, Liaw YF, Wu SS, et al. Long-term entecavir therapy results in the reversal of fibrosis/cirrhosis and continued histological improvement in patients with chronic hepatitis B. Hepatology. 2010;52(3):886–893. doi: 10.1002/hep.23785 [DOI] [PubMed] [Google Scholar]
- 52.Facciorusso A, Garcia Perdomo HA, Muscatiello N, Buccino RV, Wong VW, Singh S. Systematic review with meta-analysis: change in liver stiffness during anti-viral therapy in patients with hepatitis B. Dig Liver Dis. 2018;50(8):787–794. doi: 10.1016/j.dld.2018.05.005 [DOI] [PubMed] [Google Scholar]
- 53.Dong XQ, Wu Z, Li J, Wang GQ, Zhao H. Declining in liver stiffness cannot indicate fibrosis regression in patients with chronic hepatitis B: a 78-week prospective study. J Gastroenterol Hepatol. 2019;34(4):755–763. doi: 10.1111/jgh.14498 [DOI] [PubMed] [Google Scholar]
- 54.Kong Y, Sun Y, Zhou J, et al. Early steep decline of liver stiffness predicts histological reversal of fibrosis in chronic hepatitis B patients treated with entecavir. J Viral Hepat. 2019;26(5):576–585. doi: 10.1111/jvh.13058 [DOI] [PubMed] [Google Scholar]
- 55.Wong GL, Chan HL, Mak CW, et al. Entecavir treatment reduces hepatic events and deaths in chronic hepatitis B patients with liver cirrhosis. Hepatology. 2013;58(5):1537–1547. doi: 10.1002/hep.26301 [DOI] [PubMed] [Google Scholar]
- 56.Su TH, Hu TH, Chen CY, et al. Four-year entecavir therapy reduces hepatocellular carcinoma, cirrhotic events and mortality in chronic hepatitis B patients. Liver Int. 2016;36(12):1755–1764. doi: 10.1111/liv.13253 [DOI] [PubMed] [Google Scholar]
- 57.Liu K, Choi J, Le A, et al. Tenofovir disoproxil fumarate reduces hepatocellular carcinoma, decompensation and death in chronic hepatitis B patients with cirrhosis. Aliment Pharmacol Ther. 2019;50(9):1037–1048. doi: 10.1111/apt.15499 [DOI] [PubMed] [Google Scholar]
- 58.Papatheodoridis G, Dalekos G, Sypsa V, et al. PAGE-B predicts the risk of developing hepatocellular carcinoma in Caucasians with chronic hepatitis B on 5-year antiviral therapy. J Hepatol. 2016;64(4):800–806. doi: 10.1016/j.jhep.2015.11.035 [DOI] [PubMed] [Google Scholar]
- 59.Choi J, Kim HJ, Lee J, Cho S, Ko MJ, Lim YS. Risk of hepatocellular carcinoma in patients treated with entecavir vs tenofovir for chronic hepatitis B: a Korean nationwide cohort study. JAMA Oncol. 2019;5(1):30–36. doi: 10.1001/jamaoncol.2018.4070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yip TC, Wong VW, Chan HL, Tse YK, Lui GC, Wong GL. Tenofovir is associated with lower risk of hepatocellular carcinoma than entecavir in patients with chronic HBV infection in China. Gastroenterology. 2020;158(1):215–25 e6. doi: 10.1053/j.gastro.2019.09.025 [DOI] [PubMed] [Google Scholar]
- 61.Yuan BH, Li RH, Huo RR, Li MJ, Papatheodoridis G, Zhong JH. Lower risk of hepatocellular carcinoma with tenofovir than entecavir treatment in subsets of chronic hepatitis B patients: an updated meta-analysis. J Gastroenterol Hepatol. 2022;37(5):782–794. doi: 10.1111/jgh.15783 [DOI] [PubMed] [Google Scholar]
- 62.Kim SU, Seo YS, Lee HA, et al. A multicenter study of entecavir vs. tenofovir on prognosis of treatment-naive chronic hepatitis B in South Korea. J Hepatol. 2019;71(3):456–464. doi: 10.1016/j.jhep.2019.03.028 [DOI] [PubMed] [Google Scholar]
- 63.Lee SW, Kwon JH, Lee HL, et al. Comparison of tenofovir and entecavir on the risk of hepatocellular carcinoma and mortality in treatment-naive patients with chronic hepatitis B in Korea: a large-scale, propensity score analysis. Gut. 2020;69(7):1301–1308. doi: 10.1136/gutjnl-2019-318947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Papatheodoridis GV, Dalekos GN, Idilman R, et al. Similar risk of hepatocellular carcinoma during long-term entecavir or tenofovir therapy in Caucasian patients with chronic hepatitis B. J Hepatol. 2020;73(5):1037–1045. doi: 10.1016/j.jhep.2020.06.011 [DOI] [PubMed] [Google Scholar]
- 65.Kuang XJ, Jia RR, Huo RR, et al. Systematic review of risk factors of hepatocellular carcinoma after hepatitis B surface antigen seroclearance. J Viral Hepat. 2018;25(9):1026–1037. doi: 10.1111/jvh.12905 [DOI] [PubMed] [Google Scholar]
- 66.Choi J, Yoo S, Lim YS. Comparison of long-term clinical outcomes between spontaneous and therapy-induced HBsAg seroclearance. Hepatology. 2021;73(6):2155–2166. doi: 10.1002/hep.31610 [DOI] [PubMed] [Google Scholar]
- 67.Casado JL, Santiuste C, Vazquez M, et al. Bone mineral density decline according to renal tubular dysfunction and phosphaturia in tenofovir-exposed HIV-infected patients. AIDS. 2016;30(9):1423–1431. doi: 10.1097/QAD.0000000000001067 [DOI] [PubMed] [Google Scholar]
- 68.Han Y, Zeng A, Liao H, Liu Y, Chen Y, Ding H. The efficacy and safety comparison between tenofovir and entecavir in treatment of chronic hepatitis B and HBV related cirrhosis: a systematic review and meta-analysis. Int Immunopharmacol. 2017;42:168–175. doi: 10.1016/j.intimp.2016.11.022 [DOI] [PubMed] [Google Scholar]
- 69.Mak LY, Hoang J, Jun DW, et al. Longitudinal renal changes in chronic hepatitis B patients treated with entecavir versus TDF: a REAL-B study. Hepatol Int. 2022;16(1):48–58. doi: 10.1007/s12072-021-10271-x [DOI] [PubMed] [Google Scholar]
- 70.Vigano M, Brocchieri A, Spinetti A, et al. Tenofovir-induced Fanconi syndrome in chronic hepatitis B monoinfected patients that reverted after tenofovir withdrawal. J Clin Virol. 2014;61(4):600–603. doi: 10.1016/j.jcv.2014.09.016 [DOI] [PubMed] [Google Scholar]
- 71.Fong TL, Lee BT, Tien A, et al. Improvement of bone mineral density and markers of proximal renal tubular function in chronic hepatitis B patients switched from tenofovir disoproxil fumarate to tenofovir alafenamide. J Viral Hepat. 2019;26(5):561–567. doi: 10.1111/jvh.13053 [DOI] [PubMed] [Google Scholar]
- 72.Ford N, Scourse R, Lemoine M, et al. Adherence to nucleos(t)ide analogue therapies for chronic hepatitis B infection: a systematic review and meta-analysis. Hepatol Commun. 2018;2(10):1160–1167. doi: 10.1002/hep4.1247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Shin JW, Jung SW, Lee SB, et al. Medication nonadherence increases hepatocellular carcinoma, cirrhotic complications, and mortality in chronic hepatitis B patients treated with entecavir. Am J Gastroenterol. 2018;113(7):998–1008. doi: 10.1038/s41395-018-0093-9 [DOI] [PubMed] [Google Scholar]
- 74.Pan X, Zhang K, Yang X, et al. Relapse rate and associated-factor of recurrence after stopping NUCs therapy with different prolonged consolidation therapy in HBeAg positive CHB patients. PLoS One. 2013;8(7):e68568. doi: 10.1371/journal.pone.0068568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chi H, Hansen BE, Yim C, et al. Reduced risk of relapse after long-term nucleos(t)ide analogue consolidation therapy for chronic hepatitis B. Aliment Pharmacol Ther. 2015;41(9):867–876. doi: 10.1111/apt.13150 [DOI] [PubMed] [Google Scholar]
- 76.Wong DK, Seto WK, Fung J, et al. Reduction of hepatitis B surface antigen and covalently closed circular DNA by nucleos(t)ide analogues of different potency. Clin Gastroenterol Hepatol. 2013;11(8):1004–10 e1. doi: 10.1016/j.cgh.2013.01.026 [DOI] [PubMed] [Google Scholar]
- 77.Liaw YF, Leung N, Kao JH, et al. Asian-Pacific consensus statement on the management of chronic hepatitis B: a 2008 update. Hepatol Int. 2008;2(3):263–283. doi: 10.1007/s12072-008-9080-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hadziyannis SJ, Sevastianos V, Rapti I, Vassilopoulos D, Hadziyannis E. Sustained responses and loss of HBsAg in HBeAg-negative patients with chronic hepatitis B who stop long-term treatment with Adefovir. Gastroenterology. 2012;143(3):629–36 e1. doi: 10.1053/j.gastro.2012.05.039 [DOI] [PubMed] [Google Scholar]
- 79.Berg T, Simon KG, Mauss S, et al. Long-term response after stopping tenofovir disoproxil fumarate in non-cirrhotic HBeAg-negative patients - FINITE study. J Hepatol. 2017;67(5):918–924. doi: 10.1016/j.jhep.2017.07.012 [DOI] [PubMed] [Google Scholar]
- 80.Papatheodoridis G, Vlachogiannakos I, Cholongitas E, et al. Discontinuation of oral antivirals in chronic hepatitis B: a systematic review. Hepatology. 2016;63(5):1481–1492. doi: 10.1002/hep.28438 [DOI] [PubMed] [Google Scholar]
- 81.Sonneveld MJ, Chiu SM, Park JY, et al. Probability of HBsAg loss after nucleo(s)tide analogue withdrawal depends on HBV genotype and viral antigen levels. J Hepatol. 2022. doi: 10.1016/j.jhep.2022.01.007 [DOI] [PubMed] [Google Scholar]
- 82.Jeng WJ, Chen YC, Chien RN, Sheen IS, Liaw YF. Incidence and predictors of hepatitis B surface antigen seroclearance after cessation of nucleos(t)ide analogue therapy in hepatitis B e antigen-negative chronic hepatitis B. Hepatology. 2018;68(2):425–434. doi: 10.1002/hep.29640 [DOI] [PubMed] [Google Scholar]
- 83.Chien RN, Liaw YF. Re-treatment for severe hepatitis flare in HBeAg-negative chronic hepatitis B: an appraisal with combined HBsAg/ALT kinetics. J Viral Hepat. 2020;27(5):544–547. doi: 10.1111/jvh.13253 [DOI] [PubMed] [Google Scholar]
- 84.Liaw YF. Finite nucleos(t)ide analog therapy in HBeAg-negative chronic hepatitis B: an emerging paradigm shift. Hepatol Int. 2019;13(6):665–673. doi: 10.1007/s12072-019-09989-6 [DOI] [PubMed] [Google Scholar]
- 85.Berg T, Lampertico P. The times they are a-changing - A refined proposal for finite HBV nucleos(t)ide analogue therapy. J Hepatol. 2021;75(2):474–480. doi: 10.1016/j.jhep.2021.04.040 [DOI] [PubMed] [Google Scholar]
- 86.Chien RN, Liaw YF. Current trend in antiviral therapy for chronic hepatitis B. Viruses. 2022;14(2):434. doi: 10.3390/v14020434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Boni C, Laccabue D, Lampertico P, et al. Restored function of HBV-specific T cells after long-term effective therapy with nucleos(t)ide analogues. Gastroenterology. 2012;143(4):963–73 e9. doi: 10.1053/j.gastro.2012.07.014 [DOI] [PubMed] [Google Scholar]
- 88.Honer Zu Siederdissen C, Rinker F, Maasoumy B, et al. Viral and host responses after stopping long-term nucleos(t)ide analogue therapy in HBeAg-negative chronic hepatitis B. J Infect Dis. 2016;214(10):1492–1497. doi: 10.1093/infdis/jiw412 [DOI] [PubMed] [Google Scholar]
- 89.Rivino L, Le Bert N, Gill US, et al. Hepatitis B virus-specific T cells associate with viral control upon nucleos(t)ide-analogue therapy discontinuation. J Clin Invest. 2018;128(2):668–681. doi: 10.1172/JCI92812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Garcia-Lopez M, Lens S, Pallett LJ, et al. Viral and immune factors associated with successful treatment withdrawal in HBeAg-negative chronic hepatitis B patients. J Hepatol. 2020;74(5):1064–1074. doi: 10.1016/j.jhep.2020.11.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Rinker F, Zimmer CL, Honer Zu Siederdissen C, et al. Hepatitis B virus-specific T cell responses after stopping nucleos(t)ide analogue therapy in HBeAg-negative chronic hepatitis B. J Hepatol. 2018;69(3):584–593. doi: 10.1016/j.jhep.2018.05.004 [DOI] [PubMed] [Google Scholar]
- 92.Broquetas T, Hernandez JJ, Garcia-Retortillo M, et al. On-therapy HBsAg kinetics can predict HBsAg loss after nucleos(t)ide analogues interruption in HBeAg-negative patients. The cup is half full and half empty. Dig Liver Dis. 2022. doi: 10.1016/j.dld.2021.12.017 [DOI] [PubMed] [Google Scholar]
- 93.Chen CH, Lu SN, Hung CH, et al. The role of hepatitis B surface antigen quantification in predicting HBsAg loss and HBV relapse after discontinuation of lamivudine treatment. J Hepatol. 2014;61(3):515–522. doi: 10.1016/j.jhep.2014.04.029 [DOI] [PubMed] [Google Scholar]
- 94.Liu J, Li T, Zhang L, Xu A. The role of hepatitis B surface antigen in Nucleos(t)ide analogues cessation among asian patients with chronic hepatitis B: a systematic review. Hepatology. 2019;70(3):1045–1055. doi: 10.1002/hep.30474 [DOI] [PubMed] [Google Scholar]
- 95.Hirode G, Choi HSJ, Chen CH, et al. Off-therapy response after nucleos(t)ide analogue withdrawal in patients with chronic hepatitis B: an international, multicenter, multiethnic cohort (RETRACT-B study). Gastroenterology. 2022;162(3):757–71 e4. doi: 10.1053/j.gastro.2021.11.002 [DOI] [PubMed] [Google Scholar]
- 96.Jeng WJ, Chen YC, Sheen IS, et al. Clinical relapse after cessation of tenofovir therapy in hepatitis B e antigen-negative patients. Clin Gastroenterol Hepatol. 2016;14(12):1813–20 e1. doi: 10.1016/j.cgh.2016.07.002 [DOI] [PubMed] [Google Scholar]
- 97.Seto WK, Hui AJ, Wong VW, et al. Treatment cessation of entecavir in Asian patients with hepatitis B e antigen negative chronic hepatitis B: a multicentre prospective study. Gut. 2015;64(4):667–672. doi: 10.1136/gutjnl-2014-307237 [DOI] [PubMed] [Google Scholar]
- 98.Liaw YF, Jeng WJ, Chang ML. HBsAg kinetics in retreatment decision for off-therapy hepatitis B flare in HBeAg-negative patients. Gastroenterology. 2018;154(8):2280–2281. doi: 10.1053/j.gastro.2018.03.066 [DOI] [PubMed] [Google Scholar]
- 99.Liaw YF. Hepatitis B flare after cessation of nucleos(t)ide analogue therapy in HBeAg-negative chronic hepatitis B: to retreat or not to retreat. Hepatology. 2021;73(2):843–852. doi: 10.1002/hep.31525 [DOI] [PubMed] [Google Scholar]
- 100.Papatheodoridis GV, Rigopoulou EI, Papatheodoridi M, et al. DARING-B: discontinuation of effective entecavir or tenofovir disoproxil fumarate long-term therapy before HBsAg loss in non-cirrhotic HBeAg-negative chronic hepatitis B. Antivir Ther. 2018;23(8):677–685. doi: 10.3851/IMP3256 [DOI] [PubMed] [Google Scholar]
- 101.Liaw YF, Chien RN. Finite nucleos(t)ide analogue therapy in hepatitis B e antigen-negative chronic hepatitis B: from an ”option” to an ”active recommendation”. Kaohsiung J Med Sci. 2022;38(4):295–301. doi: 10.1002/kjm2.12518 [DOI] [PubMed] [Google Scholar]