Abstract
Objective
Parathyroid carcinoma is a rare tumor among parathyroid tumors. Aspiration cytology and needle biopsy are generally not recommended for diagnostic purposes because they cause dissemination. Therefore, it is commonly diagnosed by postoperative histopathological examination. In this study, we investigated whether preoperative inflammatory markers can be used as predictors of cancer in patients with primary hyperparathyroidism.
Design
This was a retrospective study.
Methods
Thirty-six cases of parathyroid carcinoma and 50 cases of parathyroid adenoma (PA) operated with the diagnosis of primary hyperparathyroidism and confirmed histopathologically at Ito Hospital were included in this study. Preoperative clinical characteristics and inflammatory markers (neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio, and lymphocyte-to-monocyte ratio (LMR)) were compared and their values in preoperative prediction were evaluated and analyzed.
Results
Preoperative intact-parathyroid hormone (P = 0.0003), serum calcium (P = 0.0048), and tumor diameter (P = 0.0002) were significantly higher in parathyroid carcinoma than in PA. LMR showed a significant decrease in parathyroid carcinoma (P = 0.0062). In multivariate analysis, LMR and tumor length diameter were independent predictors. In the receiver operating characteristics analysis, the cut-off values for LMR and tumor length diameter were 4.85 and 28.0 mm, respectively, for parathyroid cancer prediction. When the two extracted factors were stratified by the number of factors held, the predictive ability improved as the number of factors increased.
Conclusion
In the preoperative evaluation, a combination of tumor length diameter of more than 28 mm and LMR of less than 4.85 was considered to have a high probability of cancer.
Keywords: lymphocyte-to-monocyte ratio, tumor size, parathyroid carcinoma, preoperative prediction
Introduction
Parathyroid carcinoma (PC) is a rare endocrine tumor that accounts for <0.5–5% of patients with primary hyperparathyroidism (1, 2, 3, 4). Complete surgical resection is the only curative treatment for PC. To prevent local recurrence, the lesions should be removed en bloc to a certain extent (2, 5, 6, 7). Patients with PC often have local recurrence or distant metastases, and most die of uncontrolled severe hypercalcemia (2, 6, 7, 8). Accurate diagnosis before surgery is crucial to reduce the risk of recurrence of PC and allow the required appropriate surgery (9, 10, 11).
Clinical features of PC may include severe hypercalcemia, a palpable neck mass, evidence of local invasion during surgery, or suspicion of distant metastases (2, 10, 12). In contrast, on diagnostic imaging, findings of tumors on cervical ultrasonography (13), diagnoses of PC using tumor depth–width ratio (14), and expected results of PC using short-to-long axis ratio or tumor shape on CT (15) have been found; however, these tests are not specific to PC unless there are clear findings of invasion or distant metastases. In addition, diagnostic fine-needle aspiration cytology or needle biopsy is generally not recommended for parathyroid glands because of the risk of cell seeding due to damage to the parathyroid capsule. Thus, a differential diagnosis between benign and malignant tumors prior to the treatment of primary hyperparathyroidism is difficult. A problem for clinicians is the lack of specific clinical, biochemical, and imaging features that distinguish PC from parathyroid adenoma (PA). Thus, in clinical practice, PC is often diagnosed through histopathological examinations after surgery and diagnosis after recurrence is also not uncommon (3, 16).
Inflammatory activity has long been implicated as having extensive involvement in the progression of cancer (17), and inflammatory biomarkers reflecting the inflammatory status of patients with cancer have been reported to be prognostic predictive factors in various types of cancer. In particular, among these, the neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR), and lymphocyte-to-monocyte ratio (LMR) have been reported to be prognostic predictive factors and markers for differentiating benign tumors from malignant tumors in several types of carcinomas (18, 19, 20, 21, 22, 23, 24). Few articles have described the relationship between parathyroid disease and inflammatory markers, and their value as preoperative screening tools remains unknown (25, 26).
Therefore, this study aimed to investigate whether the screening test could help to distinguish between PC and PA in preoperative diagnosis in surgical cases of primary hyperparathyroidism.
Materials and methods
We retrospectively reviewed the medical data of patients who underwent surgery for primary hyperparathyroidism at Ito Hospital from 1979 to 2019. A total of 1415 patients underwent surgery, and data from 36 cases histopathologically confirmed as PC were retrospectively reviewed. In addition, 50 age- and gender-matched patients with histopathologically confirmed PA were included in the study. Postoperative histopathological analysis was conducted by a pathologist using World Health Organization diagnostic criteria (27). PC was defined as a patient with any one of the following microscopic features necessary for the definitive diagnosis of parathyroid lesion malignancy: (i) vascular invasion, (ii) invasion into adjacent structures or organs, and (iii) metastasis. Patients with secondary or tertiary hyperparathyroidism and those with other causes of vitamin D intoxication, hyperthyroidism, hypercalcemia, and other carcinomas were excluded. In addition, patients with incomplete data and those with active infections, prolonged steroid therapy, chronic inflammatory or autoimmune disease, or hematologic disease were excluded because these may alter neutrophil or lymphocyte counts. For all patients, data on age, sex, blood collection test data (serum calcium, phosphorus, parathyroid hormone (intact PTH), creatinine, alkaline phosphatase (ALP), white blood cell count, and platelet count), and the size of the parathyroid mass were collected from medical charts. The size of the parathyroid mass was determined from the preoperative cervical ultrasonography using the results of the largest diameter of the mass. Inflammation markers were calculated from blood samples taken approximately 2 weeks to the day before the surgery. If multiple measurements were taken, the results from the measurement closer to the date of the surgery were used. LMR was defined as the lymphocyte count divided by the monocyte count, NLR as the neutrophil count divided by the lymphocyte count, and PLR as the platelet count divided by the lymphocyte count.
A receiver operating characteristic (ROC) curve was constructed to estimate the optimal cut-off value for the pre-treatment inflammatory markers. The optimal cut-off value for the ROC curve was determined based on the Youden Index. In addition, analysis of the ability to predict cancer was performed with stratification for the presence or absence of independent predictive factors extracted from the investigation.
Statistical analysis
Continuous variables were expressed as median and interquartile range, and categorical variables were expressed as numbers and percentages. Continuous variables were compared using the Mann–Whitney U-test or Kruskal–Wallis test, and categorical variables were compared using Fisher’s exact test or chi-squared test. Univariate and multivariate logistic regression analyses were used to compare the two groups, and odds ratios and 95% CIs were calculated. Statistical significance was indicated by a value of P < 0.05. Statistical analyses were performed using JMP software (Version 12; SAS Institute Inc., Cary, NC, USA).
This study was approved by the Ethics Committee of Ito Hospital. The study was conducted in accordance with the guidelines described in the Declaration of Helsinki, and consent was obtained after providing verbal explanation of the study to the patients.
Results
Clinical characteristics of parathyroid carcinoma and parathyroid adenoma
The background characteristics of patients in this study are shown in Table 1. In total, 36 patients with a histopathological diagnosis of PC and 50 patients with a diagnosis of PA were included in the analysis. The median age at surgery for patients with carcinoma and adenoma was 55 years and 59.5 years, respectively. The male-to-female ratio was 1:2.6 for patients with carcinoma and 1:2.3 for patients with adenoma, with no difference in the distribution of sex. The median long diameter of tumors on preoperative cervical ultrasonography was 29 mm for PC and 17 mm for PA, which showed that the tumor diameter was considerably larger in patients with carcinoma. Regarding surgery, all patients with PA underwent parathyroidectomy, 16 patients (44.5%) with PC underwent parathyroidectomy, 13 patients (36.1%) underwent parathyroidectomy and hemithyroidectomy, and 7 patients (19.4%) underwent parathyroidectomy, hemithyroidectomy, and lymph node dissection. In about half of the patients with PC, the surrounding organs (thyroid gland and lymph nodes) other than the parathyroid gland were resected. With regard to lymph node dissection, there was only one case with preoperative imaging findings of suspected metastasis. Of 36 patients, 13 underwent dissection, and only one patient had histopathological metastasis, whereas the other patients did not have metastasis. Intraoperative findings of suspected infiltration were mainly in the thyroid gland, and only one case of suspected infiltration into the internal jugular vein was treated with a combined resection. The histological characteristics of PC showed the presence of chief cell-based masses in 30 (83.3%) cases and eosinophilic cell-based masses in 6 (16.7%) cases. In PA, chief cell-based masses were found in 28 (56.0%) cases and eosinophilic cell-based masses were found in 13 (26.0%) cases, with a significant difference in the number of chief cell-based masses in PC (P = 0.0081) (data not shown). Of the patients diagnosed with PC, two (5.6%) underwent external radiation to the neck as an additional postoperative treatment. Postoperative recurrence was observed in four patients (11%). The recurrence site of one patient was local recurrence and distant metastasis, and three patients had distant metastasis. The median follow-up period for PC was 56.7 months.
Table 1.
Comparison of patient and tumor characteristics, including preoperative biochemical laboratory values, in patients with parathyroid carcinoma and parathyroid adenoma.
| Variables | Carcinoma (n = 36) | Adenoma (n = 50) | P - value |
|---|---|---|---|
| Age (years) | 55.0 (46–68.5) | 59.5 (46–68) | 0.8679 |
| Gender (male/female) | 10/26 (27.8/72.2) | 15/35 (30.0/70.0) | 0.8228 |
| Tumor size (mm) | 29 (18–36) | 17 (14–24) | 0.0002 |
| Intact PTH level (pg/mL) | 246.5 (160.0–444.5) | 161.0 (118.0–205.5) | 0.0003 |
| Calcium level (mg/dL) | 11.5 (10.9–12.8) | 10.9 (10.6–11.3) | 0.0048 |
| Creatinine (mg/dL) | 0.74 (0.60–0.90) | 0.76 (0.57–0.86) | 0.6455 |
| ALP (U/L) | 350 (218–512) | 294 (234–386) | 0.3416 |
| WBC (/μL) | 5960 (4880–6900) | 5490 (4743–6605) | 0.3416 |
| Neutrophils (/μL) | 3648 (2936–4384) | 3231 (2698–4207) | 0.1415 |
| Lymphocytes (/μL) | 1784 (1430–2346) | 1789 (1475–2076) | 0.8233 |
| Monocytes (/μL) | 372 (325–479) | 292 (259–352) | 0.0003 |
| Plt (×104/μL) | 23.1 (20.1–28.5) | 24.8 (20.6–27.9) | 0.7109 |
| NLR | 1.90 (1.64–2.46) | 1.89 (1.48–2.22) | 0.4023 |
| PLR | 141.1 (103.6–160.4) | 143.4 (110.4–164.8) | 0.6418 |
| LMR | 4.54 (3.38–6.28) | 6.00 (4.40–7.50) | 0.0062 |
Data were expressed as number (%) or median (interquartile range/IQR). Bold indicates statistical significance, P < 0.05. Normal range; intact PTH: 15–65 pg/mL, calcium: 8.5–10.0 mg/dL, creatinine: 0.46–1.09 mg/dL, ALP: 38–113 U/L.
ALP, alkaline phosphatase; cLND, central lymph node dissection; hTx, hemithyroidectomy; NLR, neutrophil-to-lymphocyte ratio; PTH, parathyroid hormone; Plt, platelet count; PLR, platelet-to-lymphocyte ratio; PTx, parathyroidectomy; LMR, lymphocyte-to-monocyte ratio; WBC, white blood cell.
Analyses of cancer predictive factors
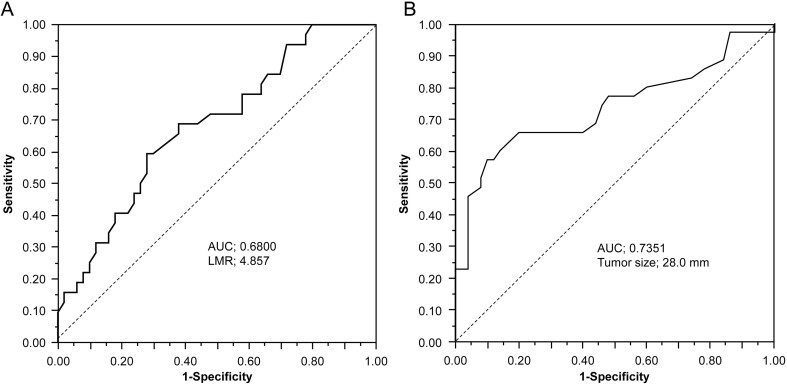
In patients with PC, tumor size, intact PTH, and calcium level were significantly higher than those in patients with PA (P = 0.0002, P = 0.0003, P = 0.0048, respectively). Additionally, LMR was significantly lower in patients with PC than in patients with PA (P = 0.0062). There were no significant differences in NLR or PLR (Table 1). In multivariate analyses, tumor size and LMR were significant independent predictive factors of PC (Table 2). The ROC curve was used to determine the optimal cut-off value for predicting parathyroid cancer in LMR and tumor size (Fig. 1). Results show that the cut-off value for LMR was 4.857, and the area under the curve (AUC) was 0.680 (P = 0.0048). The cut-off value for tumor size was 28.0 mm and that for AUC was 0.7351 (P = 0.0003). The sensitivity and specificity at the optimal LMR cut-off value of 4.85 for the prediction of PC were 59.9 and 72.0%, respectively. At a cut-off value of 28 mm for tumor size, the sensitivity and specificity for predicting carcinoma were 57.1 and 90.0%, respectively.
Table 2.
Multivariate analyses of risk factors for parathyroid carcinoma.
| Variables | Multivariate analysis | |
|---|---|---|
| OR (95% CI) | P - value | |
| Intact PTH | 1.001 (0.998–1.006) | 0.2336 |
| Serum calcium | 1.239 (0.609–2.562) | 0.5512 |
| Tumor size | 1.090 (1.026–1.173) | 0.0035 |
| LMR | 0.763 (0.556–0.994) | 0.0445 |
LMR, lymphocyte/monocyte ratio; OR, odds ratio.
Bold indicates statistical significance, P < 0.05.
Figure 1.
The receiver operating characteristic (ROC) curve to determine cut-off values for lymphocyte/monocyte ratio (LMR) and tumor size. (A) The optimum cut-off point for LMR was 4.857, area under the curve (AUC) was 0.6800, with a sensitivity of 59.4% and a specificity of 72.0%. (B) The optimum cut-off point for tumor size was 28.0 and AUC was 0.7351, with a sensitivity of 57.1% and a specificity of 90.0%.
In addition, the validity of prediction was assessed using different criteria for LMR values (Table 3). The criteria were the first quartile, the high quartile, the median, the criterion obtained by ROC, the low quartile, and the third quartile, and the sensitivity, specificity, positive predictive value, negative predictive value, and accuracy rate were analyzed. An LMR value of 4.85 or less, which was the criterion obtained by ROC, was noted to be highly predictive of PC.
Table 3.
Diagnostic validity of LMR at different cut-off values for discrimination of benign and malignant parathyroid glands.
| Cut-off values for LMR | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | Accuracy rate (%) |
|---|---|---|---|---|---|
| 6.88a | 84.4 | 30 | 43.5 | 75 | 51.2 |
| 6.32b | 78.1 | 42 | 46.3 | 75 | 56.1 |
| 5.26c | 68.8 | 62 | 53.7 | 75.6 | 64.6 |
| 4.85d | 59.9 | 72 | 57.6 | 73.5 | 67.1 |
| 4.39e | 46.9 | 76 | 55.6 | 69.1 | 64.6 |
| 4.17f | 37.5 | 82 | 57.1 | 67.2 | 64.6 |
aThreshold at the point with top 25th percentile (first quartile); bThreshold at the point with the top of three-quarter position; cThreshold at the point with median; dThreshold at a point derived from the receiver operating curve; eThreshold at the point with the bottom of three-quarter position (third quartile); fThreshold at the point with bottom 25th percentile.
LMR, lymphocyte-to-monocyte ratio; NPV, negative predictive value; PPV, positive predictive value.
Stratified analyses of parathyroid carcinoma predictive factors
Two extracted predictive factors (long diameter of the tumor > 28 mm, LMR <4.85) were stratified by the number of factors. Predictions of cancer were analyzed by stratifying patients into a low-risk group (0 factors), an intermediate-risk group (1 factor), and a high-risk group (2 factors) (Table 4). The combination of long diameter of tumors and LMR showed that the predictive power for cancer was higher in the high-risk group with two factors than in the low-risk group. (P < 0.001).
Table 4.
Risk factor stratification in cancer prediction.
| n | OR (95% CI) | P - value | |
|---|---|---|---|
| Low risk | 41 | ref | |
| Intermediate risk | 30 | 4.09 (1.39–12.92) | 0.0101 |
| High risk | 15 | 28.29 (6.10–211.08) | <0.0001 |
OR, odds ratio.
Discussion
Inflammation is strongly associated with cancer, and the levels of inflammatory cells in the peripheral blood, including neutrophils, lymphocytes, and monocytes, have been reported as prognostic factors in various cancers (28, 29). Lymphocytes play an important role in host tumor immunity, for example, by inhibiting cytotoxic cell death and tumor cell proliferation and migration (30, 31, 32). A decline in the level of lymphocytes is believed to undermine the immune response to tumors and promote tumor progression and metastasis (33, 34). Monocytes are known to infiltrate tumors and differentiate into tumor-associated macrophages. Macrophages are involved in tumor growth, invasion, metastasis, neovascularization, and recurrence (35, 36, 37). LMR is an inflammatory marker defined by the ratio of lymphocytes to monocytes; furthermore, low LMR, which reflects the host immune status and tumor progression, may be associated with poor prognosis. In this study, elevated monocyte counts were observed in PC. Higher monocyte counts may indicate more tumor-associated macrophages in the tumor tissue, leading to immunosuppression and tumor growth (38, 39). Many studies till date have suggested that LMR is an independent prognostic factor in other carcinomas (21, 24, 40, 41, 42, 43, 44). LMR has been reported to be useful as a predictive factor for malignancy in other types of carcinomas (45, 46). Till date, however, there have been few reports on parathyroid disease. Lam et al. reported that in patients undergoing surgery for primary hyperparathyroidism, the NLR before parathyroidectomy was associated with serum Ca and PTH levels, and that the NLR levels rapidly decrease after parathyroidectomy; however, this was only observed in patients with PA (26). Although Zeren et al. reported that preoperative NLR was significantly correlated with Ca level, PTH level, size of adenoma, and presence or absence of cancer, only three patients with cancer were studied (25).
To our knowledge, this is the first study to assess the association with LMR as a predictive factor for malignancy in parathyroid disease. In this study, lower the LMR, higher the probability of PC, which indicates that this factor was a predictive marker for cancer preoperatively. In this study, the optimal cut-off value for LMR was calculated as 4.85 from the ROC curve, which was used as a reference value and evaluated as a predictive factor for cancer. Setting a cut-off value based on the ROC curve can help determine the degree to which sensitivity should be sought, but it depends on the disease and medical condition. In this study, sensitivity and specificity were evaluated using different cut-off values as shown in Table 4, and the cut-off value of 4.85 for LMR was found to be optimal. In utilizing these indices, it is important to reevaluate the interpretation of the values at all times while fully considering the purpose of use, characteristics of the target population, and disease stage.
In this study, parathyroid tumor size was also shown to be an important predictive factor for PC in patients with primary hyperparathyroidism. Bae et al. reported that a large parathyroid tumor is a risk factor for malignancy (47). Quin et al. also showed that patients with PC had considerably larger tumors (48). In addition, Hsu et al. demonstrated that tumors that were at least 3 cm in size were associated with an increased risk of lymph node metastases at disease onset (49). In the present study, 17 (47.2%) of 36 patients with PC had a parathyroid mass that was 30 mm or larger. In the ROC analysis, based on the tumor diameter cut-off value of 28 mm as a predictive factor for PC, the sensitivity and specificity were noted to be 57.1 and 90.0%, respectively. This study supports the findings of a previous report (50) that states that particular attention should be paid to patients with hyperparathyroidism who have parathyroid masses that are 3.0 cm or larger.
The clinician should be aware of hyperparathyroidism-jaw cancer (HPT-JT) syndrome as an inherited disorder with an increased risk of PC (51). HPT-JT is an autosomal dominant disorder characterized by hyperparathyroidism caused by adenoma or carcinoma and tumors of the jaw and kidney. The causative gene is the HRPT2/CDC73 gene located on chromosome 1q31.2. Approximately 80–90% of patients with HPT-JT develop hyperparathyroidism and approximately 20% develop PC (52, 53). Most of the patients with HPT-JT develop hyperparathyroidism at a young age. In the present study, we did not include the results of genetic testing because of the older age of onset as well as the older age of the patients. When PC is suspected in a young patient, hereditary disease should be considered. We believe that the results of the present study reveal suitable risk factors for relatively elderly patients.
The purpose of our study was to investigate the possibility of a predictive indicator of cancer diagnosis preoperatively based on preoperative clinical information including inflammatory markers in patients with primary hyperparathyroidism. The LMR can be obtained from peripheral blood and can be easily evaluated. Thus, if cancer can be predicted during the treatment of primary hyperparathyroidism, the LMR can help guide treatment. The addition of tumor size to LMR was shown to increase the ability to predict cancer.
This study has several limitations. First, this is a retrospective single-center study. Secondly, the relatively small sample size inevitably may result in uncontrolled or unrecognized bias. Thirdly, the study was not intended to elucidate the mechanism of decreased LMR in patients with PC. Fourthly, as for the results of hematological parameters, it cannot be denied that there may be some differences in the accuracy of the results due to differences in the equipment used over the years. Thus, a prospective multicenter study with a large sample size is needed to further evaluate the clinical applicability of the present results. However, the inability to prospectively study very rare tumors may also be a limitation of this research.
Conclusion
In patients with primary hyperparathyroidism, a preoperative LMR lower than 4.85 and a tumor larger than 28 mm in diameter may indicate PC. This is the first study to investigate the accuracy of inflammatory markers in the preoperative prediction of PC. It is a simple and optimal combination of markers for predicting the possibility of PC and may help in the selection of appropriate treatment.
Declaration of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Funding
This work did not receive any specific grant from any funding agency in the public, commercial, or not-for-profit sector.
Acknowledgements
The authors would like to express our gratitude to all the staff members of Ito Hospital who cooperated with this research.
References
- 1.Ozolins A, Narbuts Z, Vanags A, Simtniece Z, Visnevska Z, Akca A, Wirowski D, Gardovskis J, Strumfa I, Goretzki PE. Evaluation of malignant parathyroid tumours in two European cohorts of patients with sporadic primary hyperparathyroidism. Langenbeck’s Archives of Surgery 2016401943–951. ( 10.1007/s00423-015-1361-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Talat N, Schulte KM. Clinical presentation, staging and long-term evolution of parathyroid cancer. Annals of Surgical Oncology 2010172156–2174. ( 10.1245/s10434-010-1003-6) [DOI] [PubMed] [Google Scholar]
- 3.Cetani F, Pardi E, Marcocci C. Parathyroid carcinoma. Frontiers of Hormone Research 20195163–76. ( 10.1159/000491039) [DOI] [PubMed] [Google Scholar]
- 4.Obara T, Fujimoto Y. Diagnosis and treatment of patients with parathyroid carcinoma: an update and review. World Journal of Surgery 199115738–744. ( 10.1007/BF01665308) [DOI] [PubMed] [Google Scholar]
- 5.Villar-del-Moral J, Jiménez-García A, Salvador-Egea P, Martos-Martínez JM, Nuño-Vázquez-Garza JM, Serradilla-Martín M, Gómez-Palacios A, Moreno-Llorente P, Ortega-Serrano J, de la Quintana-Basarrate A. Prognostic factors and staging systems in parathyroid cancer: a multicenter cohort study. Surgery 20141561132–1144. ( 10.1016/j.surg.2014.05.014) [DOI] [PubMed] [Google Scholar]
- 6.Sadler C, Gow KW, Beierle EA, Doski JJ, Langer M, Nuchtern JG, Vasudevan SA, Goldfarb M. Parathyroid carcinoma in more than 1000 patients: a population-level analysis. Surgery 20141561622–1629; discussion 1629–1630. ( 10.1016/j.surg.2014.08.069) [DOI] [PubMed] [Google Scholar]
- 7.Xue S, Chen H, Lv C, Shen X, Ding J, Liu J, Chen X. Preoperative diagnosis and prognosis in 40 parathyroid carcinoma patients. Clinical Endocrinology 20168529–36. ( 10.1111/cen.13055) [DOI] [PubMed] [Google Scholar]
- 8.Harari A, Waring A, Fernandez-Ranvier G, Hwang J, Suh I, Mitmaker E, Shen W, Gosnell J, Duh QY, Clark O. Parathyroid carcinoma: a 43-year outcome and survival analysis. Journal of Clinical Endocrinology and Metabolism 2011963679–3686. ( 10.1210/jc.2011-1571) [DOI] [PubMed] [Google Scholar]
- 9.Schulte KM, Gill AJ, Barczynski M, Karakas E, Miyauchi A, Knoefel WT, Lombardi CP, Talat N, Diaz-Cano S, Grant CS. Classification of parathyroid cancer. Annals of Surgical Oncology 2012192620–2628. ( 10.1245/s10434-012-2306-6) [DOI] [PubMed] [Google Scholar]
- 10.Schulte KM, Talat N. Diagnosis and management of parathyroid cancer. Nature Reviews: Endocrinology 20128612–622. ( 10.1038/nrendo.2012.102) [DOI] [PubMed] [Google Scholar]
- 11.Schulte KM, Talat N, Galata G, Gilbert J, Miell J, Hofbauer LC, Barthel A, Diaz-Cano S, Bornstein SR. Oncologic resection achieving r0 margins improves disease-free survival in parathyroid cancer. Annals of Surgical Oncology 2014211891–1897. ( 10.1245/s10434-014-3530-z) [DOI] [PubMed] [Google Scholar]
- 12.Marcocci C, Cetani F. Clinical practice. Primary hyperparathyroidism. New England Journal of Medicine 20113652389–2397. ( 10.1056/NEJMcp1106636) [DOI] [PubMed] [Google Scholar]
- 13.Nam M, Jeong HS, Shin JH. Differentiation of parathyroid carcinoma and adenoma by preoperative ultrasonography. Acta Radiologica 201758670–675. ( 10.1177/0284185116666418) [DOI] [PubMed] [Google Scholar]
- 14.Hara H, Igarashi A, Yano Y, Yashiro T, Ueno E, Aiyoshi Y, Ito K, Obara T. Ultrasonographic features of parathyroid carcinoma. Endocrine Journal 200148213–217. ( 10.1507/endocrj.48.213) [DOI] [PubMed] [Google Scholar]
- 15.Takumi K, Fukukura Y, Hakamada H, Nagano H, Kumagae Y, Arima H, Nakajo A, Yoshiura T. CT features of parathyroid carcinomas: comparison with benign parathyroid lesions. Japanese Journal of Radiology 201937380–389. ( 10.1007/s11604-019-00825-3) [DOI] [PubMed] [Google Scholar]
- 16.Salcuni AS, Cetani F, Guarnieri V, Nicastro V, Romagnoli E, de Martino D, Scillitani A, Cole DEC. Parathyroid carcinoma. Best Practice and Research: Clinical Endocrinology and Metabolism 201832877–889. ( 10.1016/j.beem.2018.11.002) [DOI] [PubMed] [Google Scholar]
- 17.Kim J, Bae JS. Tumor-associated macrophages and neutrophils in tumor microenvironment. Mediators of Inflammation 20162016 6058147. ( 10.1155/2016/6058147) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Diakos CI, Charles KA, McMillan DC, Clarke SJ. Cancer-related inflammation and treatment effectiveness. Lancet: Oncology 201415e493–e503. ( 10.1016/S1470-2045(1470263-3) [DOI] [PubMed] [Google Scholar]
- 19.Germano G, Allavena P, Mantovani A. Cytokines as a key component of cancer-related inflammation. Cytokine 200843374–379. ( 10.1016/j.cyto.2008.07.014) [DOI] [PubMed] [Google Scholar]
- 20.Goto W, Kashiwagi S, Asano Y, Takada K, Takahashi K, Hatano T, Takashima T, Tomita S, Motomura H, Hirakawa K, et al. Predictive value of lymphocyte-to-monocyte ratio in the preoperative setting for progression of patients with breast cancer. BMC Cancer 201818 1137. ( 10.1186/s12885-018-5051-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yokota M, Katoh H, Nishimiya H, Kikuchi M, Kosaka Y, Sengoku N, Watanabe M, Yamashita K. Lymphocyte-monocyte ratio significantly predicts recurrence in papillary thyroid cancer. Journal of Surgical Research 2020246535–543. ( 10.1016/j.jss.2019.09.034) [DOI] [PubMed] [Google Scholar]
- 22.Gu L, Li H, Chen L, Ma X, Li X, Gao Y, Zhang Y, Xie Y, Zhang X. Prognostic role of lymphocyte to monocyte ratio for patients with cancer: evidence from a systematic review and meta-analysis. Oncotarget 2016731926–31942. ( 10.18632/oncotarget.7876) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang J, Chen L, Zhou R, Sun H, Liao Y, Liao W. Pretreatment lymphocyte monocyte ratio predicts long-term outcomes in patients with digestive system tumor: a meta-analysis. Gastroenterology Research and Practice 20162016 9801063. ( 10.1155/2016/9801063) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marín Hernández C, Piñero Madrona A, Gil Vázquez PJ, Galindo Fernández PJ, Ruiz Merino G, Alonso Romero JL, Parrilla Paricio P. Usefulness of lymphocyte-to-monocyte, neutrophil-to-monocyte and neutrophil-to-lymphocyte ratios as prognostic markers in breast cancer patients treated with neoadjuvant chemotherapy. Clinical and Translational Oncology 201820476–483. ( 10.1007/s12094-017-1732-0) [DOI] [PubMed] [Google Scholar]
- 25.Zeren S, Yaylak F, Ozbay I, Bayhan Z. Relationship between the neutrophil to lymphocyte ratio and parathyroid adenoma size in patients with primary hyperparathyroidism. International Surgery 20151001185–1189. ( 10.9738/INTSURG-D-15-00044.1) [DOI] [PubMed] [Google Scholar]
- 26.Lam HB, Yang PS, Chien MN, Lee JJ, Chao LF, Cheng SP. Association between neutrophil-to-lymphocyte ratio and parathyroid hormone in patients with primary hyperparathyroidism. Archives of Medical Science 201915880–886. ( 10.5114/aoms.2018.74758) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ricardo V, Lloyd RYO, Kloppel G, Rosai J. WHO Classification of Tumours of Endocrine Organs. Lyon, France: International Agency for Research on Cancer, 2017. (available at: https://publications.iarc.fr/Book-And-Report-Series/Who-Classification-Of-Tumours/WHO-Classification-Of-Tumours-Of-Endocrine-Organs-2017) [Google Scholar]
- 28.Tibaldi C, Vasile E, Bernardini I, Orlandini C, Andreuccetti M, Falcone A. Baseline elevated leukocyte count in peripheral blood is associated with poor survival in patients with advanced non-small cell lung cancer: a prognostic model. Journal of Cancer Research and Clinical Oncology 20081341143–1149. ( 10.1007/s00432-008-0378-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wilcox RA, Ristow K, Habermann TM, Inwards DJ, Micallef IN, Johnston PB, Colgan JP, Nowakowski GS, Ansell SM, Witzig TE, et al. The absolute monocyte count is associated with overall survival in patients newly diagnosed with follicular lymphoma. Leukemia and Lymphoma 201253575–580. ( 10.3109/10428194.2011.637211) [DOI] [PubMed] [Google Scholar]
- 30.Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature 2008454436–444. ( 10.1038/nature07205) [DOI] [PubMed] [Google Scholar]
- 31.Lin EY, Pollard JW. Role of infiltrated leucocytes in tumour growth and spread. British Journal of Cancer 2004902053–2058. ( 10.1038/sj.bjc.6601705) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hoffmann TK, Dworacki G, Tsukihiro T, Meidenbauer N, Gooding W, Johnson JT, Whiteside TL. Spontaneous apoptosis of circulating T lymphocytes in patients with head and neck cancer and its clinical importance. Clinical Cancer Research 200282553–2562. [PubMed] [Google Scholar]
- 33.Stotz M, Pichler M, Absenger G, Szkandera J, Arminger F, Schaberl-Moser R, Samonigg H, Stojakovic T, Gerger A. The preoperative lymphocyte to monocyte ratio predicts clinical outcome in patients with stage III colon cancer. British Journal of Cancer 2014110435–440. ( 10.1038/bjc.2013.785) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yoshikawa T, Saito H, Osaki T, Matsumoto S, Tsujitani S, Ikeguchi M. Elevated Fas expression is related to increased apoptosis of circulating CD8+ T cell in patients with gastric cancer. Journal of Surgical Research 2008148143–151. ( 10.1016/j.jss.2007.07.011) [DOI] [PubMed] [Google Scholar]
- 35.Noy R, Pollard JW. Tumor-associated macrophages: from mechanisms to therapy. Immunity 20144149–61. ( 10.1016/j.immuni.2014.06.010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pollard JW.Tumour-educated macrophages promote tumour progression and metastasis. Nature Reviews. Cancer 2004471–78. ( 10.1038/nrc1256) [DOI] [PubMed] [Google Scholar]
- 37.Condeelis J, Pollard JW. Macrophages: obligate partners for tumor cell migration, invasion, and metastasis. Cell 2006124263–266. ( 10.1016/j.cell.2006.01.007) [DOI] [PubMed] [Google Scholar]
- 38.Wang X, Su S, Guo Y. The clinical use of the platelet to lymphocyte ratio and lymphocyte to monocyte ratio as prognostic factors in renal cell carcinoma: a systematic review and meta-analysis. Oncotarget 2017884506–84514. ( 10.18632/oncotarget.21108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yang T, Zhu J, Zhao L, Mai K, Ye J, Huang S, Zhao Y. Lymphocyte to monocyte ratio and neutrophil to lymphocyte ratio are superior inflammation-based predictors of recurrence in patients with hepatocellular carcinoma after hepatic resection. Journal of Surgical Oncology 2017115718–728. ( 10.1002/jso.24549) [DOI] [PubMed] [Google Scholar]
- 40.Lin GN, Peng JW, Xiao JJ, Liu DY, Xia ZJ. Prognostic impact of circulating monocytes and lymphocyte-to-monocyte ratio on previously untreated metastatic non-small cell lung cancer patients receiving platinum-based doublet. Medical Oncology 201431 70. ( 10.1007/s12032-014-0070-0) [DOI] [PubMed] [Google Scholar]
- 41.Watanabe R, Tomita N, Itabashi M, Ishibashi D, Yamamoto E, Koyama S, Miyashita K, Takahashi H, Nakajima Y, Hattori Y, et al. Peripheral blood absolute lymphocyte/monocyte ratio as a useful prognostic factor in diffuse large B-cell lymphoma in the rituximab era. European Journal of Haematology 201492204–210. ( 10.1111/ejh.12221) [DOI] [PubMed] [Google Scholar]
- 42.Liu T, Fang XC, Ding Z, Sun ZG, Sun LM, Wang YL. Pre-operative lymphocyte-to-monocyte ratio as a predictor of overall survival in patients suffering from osteosarcoma. FEBS Open Bio 20155682–687. ( 10.1016/j.fob.2015.08.002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pan YC, Jia ZF, Cao DH, Wu YH, Jiang J, Wen SM, Zhao D, Zhang SL, Cao XY. Preoperative lymphocyte-to-monocyte ratio (LMR) could independently predict overall survival of resectable gastric cancer patients. Medicine 201897 e13896. ( 10.1097/MD.0000000000013896) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sun Y, Zhang L. The clinical use of pretreatment NLR, PLR, and LMR in patients with esophageal squamous cell carcinoma: evidence from a meta-analysis. Cancer Management and Research 2018106167–6179. ( 10.2147/CMAR.S171035) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li L, Tian J, Zhang L, Liu L, Sheng C, Huang Y, Zheng H, Song F, Chen K. Utility of preoperative inflammatory markers to distinguish epithelial ovarian cancer from benign ovarian masses. Journal of Cancer 2021122687–2693. ( 10.7150/jca.51642) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Caglayan V, Onen E, Avci S, Sambel M, Kilic M, Oner S, Aydos MM, Yıldız HE. Lymphocyte-to-monocyte ratio is a valuable marker to predict prostate cancer in patients with prostate specific antigen between 4 and 10 ng/dl. Archivio Italiano di Urologia, Andrologia 201990270–275. ( 10.4081/aiua.2018.4.270) [DOI] [PubMed] [Google Scholar]
- 47.Bae JH, Choi HJ, Lee Y, Moon MK, Park YJ, Shin CS, Park DJ, Jang HC, Kim SY, Kim SW. Preoperative predictive factors for parathyroid carcinoma in patients with primary hyperparathyroidism. Journal of Korean Medical Science 201227890–895. ( 10.3346/jkms.2012.27.8.890) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Quinn CE, Healy J, Lebastchi AH, Brown TC, Stein JE, Prasad ML, Callender GG, Carling T, Udelsman R. Modern experience with aggressive parathyroid tumors in a high-volume New England referral center. Journal of the American College of Surgeons 20152201054–1062. ( 10.1016/j.jamcollsurg.2014.10.007) [DOI] [PubMed] [Google Scholar]
- 49.Hsu KT, Sippel RS, Chen H, Schneider DF. Is central lymph node dissection necessary for parathyroid carcinoma? Surgery 20141561336–1341; discussion 1341. ( 10.1016/j.surg.2014.08.005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Marcocci C, Cetani F, Rubin MR, Silverberg SJ, Pinchera A, Bilezikian JP. Parathyroid carcinoma. Journal of Bone and Mineral Research 2008231869–1880. ( 10.1359/jbmr.081018) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Juhlin CC, Erickson LA. Genomics and epigenomics in parathyroid neoplasia: from bench to surgical pathology practice. Endocrine Pathology 20213217–34. ( 10.1007/s12022-020-09656-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Carpten JD, Robbins CM, Villablanca A, Forsberg L, Presciuttini S, Bailey-Wilson J, Simonds WF, Gillanders EM, Kennedy AM, Chen JD, et al. HRPT2, encoding parafibromin, is mutated in hyperparathyroidism-jaw tumor syndrome. Nature Genetics 200232676–680. ( 10.1038/ng1048) [DOI] [PubMed] [Google Scholar]
- 53.Torresan F, Iacobone M. Clinical features, treatment, and surveillance of hyperparathyroidism-jaw tumor syndrome: an up-to-date and review of the literature. International Journal of Endocrinology 20192019 1761030. ( 10.1155/2019/1761030) [DOI] [PMC free article] [PubMed] [Google Scholar]



 This work is licensed under a
This work is licensed under a