Abstract
Testosterone therapy is the cornerstone in the care of men with hypogonadism and transgender males. Gel and intramuscular injections are most frequently used and are registered and included in the international guidelines. The specific preparation should be selected according to the patient’s preference, cost, availability, and formulation-specific properties. As the majority of men with hypogonadism and transgender males require lifelong treatment with testosterone, it is important to utilize a regimen that is effective, safe, inexpensive, and convenient to use with optimal mimicking of the physiological situation. This systematic review reviews current literature on differences between the three most used testosterone preparations in adult men with hypogonadism and transgender males. Although it appeared hardly any comparative studies have been carried out, there are indications of differences between the preparations, for example, on the stability of testosterone levels, hematocrit, bone mineral density, and patient satisfaction. However, there are no studies on the effects of testosterone replacement on endpoints such as cardiovascular disease in relation to hematocrit or osteoporotic fractures in relation to bone mineral density. The effect of testosterone therapy on health-related quality of life is strongly underexposed in the reviewed studies, while this is a highly relevant outcome measure from a patient perspective. In conclusion, current recommendations on testosterone treatment appear to be based on data primarily from non-randomized clinical studies and observational studies. The availability of reliable comparative data between the different preparations will assist in the process of individual decision-making to choose the most suitable formula.
Keywords: testosterone therapy, transgender males, men with hypogonadism, short-acting injections, long-acting injections, transdermal gel
Introduction
Testosterone is the primary sex hormone and anabolic steroid in men. It is secreted primarily by the Leydig cells of the testicles and, to a much lesser extent, by the adrenal glands. Testes produce 3–10 mg of testosterone daily, corresponding roughly to serum concentrations of 10.4–34.7 nmol/L with a peak value in the morning. Testosterone acts directly via androgen receptors and via its conversion into two active metabolites, dihydrotestosterone or estradiol (1).
Low testosterone concentrations in males can lead to a clinical syndrome known as male hypogonadism. This can be caused by testicular disease (primary hypogonadism) or central, pituitary, or hypothalamic disease (secondary hypogonadism). Causes can be genetic, like in Klinefelter and Kallman disease, or acquired, for example mass related or iatrogenic (e.g. post orchiectomy and post treatment of pituitary tumors). The diagnosis of male hypogonadism is based on the assessment of signs and symptoms and on low testosterone concentrations in serum in the morning on at least two occasions (<10.4 nmol/L) (2, 3). Low androgen levels cause morbidity and affect health-related quality of life (HR-QoL) due to infertility, decrease of sexual functions, muscle mass, strength and vitality, low mood and self-esteem, decrease of cognitive functions, osteoporosis, and a change of metabolism. Low testosterone is regarded as a marker for poor general health and as increased risk for cardiovascular disease and mortality.
Another increasing testosterone-dependent group are transgender people looking for masculinization. These people are birth-assigned female but identify as male or non-binary. To induce virilization, testosterone therapy is prescribed. This includes deepening of the voice, increase in body and facial hair, and psychological and sexual changes (4).
Several testosterone preparations are approved for treatment and should be selected according to the patient’s preference, cost, availability, and formulation-specific properties. As the majority of men with hypogonadism and transgender males require lifelong treatment with testosterone, it is important to utilize a regimen that is effective, safe, inexpensive, and convenient to use with optimal mimicking of the physiological situation.
Gel is frequently prescribed due to its favorable pharmacokinetic profile, convenient use, and positive long-term clinical results. However, for stable testosterone levels, gels require daily administrations, including adequate drying time prior to getting dressed, which can be experienced as cumbersome and can reinforce the concept of having a chronic condition. Also, the transfer of testosterone to partners or children via direct skin contact is a risk (5).
Short-acting intramuscular injections, such as Sustanon®, are esters of testosterone (TE) that have been used for many years for the treatment of testosterone deficiency prior to the availability of transdermal testosterone preparations. However, they have to be administered two to three times weekly, and after injection, serum testosterone concentrations rise into the supraphysiologic range within 24–48 h and then gradually decline into the low-normal range over 2 weeks (6, 7), possibly leading to bothersome fluctuations in the patient’s mood, sexual desire, and energy level. Long-acting intramuscular testosterone undecanoate (TU) (e.g. Nebido®) by contrast has to be administered only four to five times annually and does rarely result in supraphysiologic testosterone levels but instead produces stable levels of testosterone. Nebido® has to be slowly injected deep into the gluteal muscle. Patients can experience pain following an injection due to the large volume, but in study context, very few patients reported injection-site irritation or pain, and no patients voluntarily discontinued therapy as a result of local discomfort. The convenient dosing schedule might lead to better compliance yielding better therapeutic effect compared to gel. Importantly, pharmacokinetic studies showed consistent maintenance of stable physiological levels of testosterone during the use of Nebido® (7, 8, 9). Other available testosterone administration types are: (i) oral testosterone, although easy in use and cheap, is hardly prescribed because of two to three times daily intake with unpredictable absorption and large fluctuations in serum testosterone levels (10), (ii) patches, associated with a high frequency of skin irritation, (iii) buccal tablets, with its twice-daily dosing, gum irritation, altered taste, and poor adhesion to buccal mucosa, and (iv) testosterone pellets, requiring surgical incision with the possibility of infection, risk of spontaneous pellet extrusion and fibrosis, are not recommended (2). Recent research has shown promising results for an oral TU formulation. However, due to the current limited amount of research, we did not include this in this review (11). All preparations are registered for the treatment of hypogonadal and transgender males and are included in the international guidelines (2, 3, 12, 13, 14, 15, 16).
This systematic review aims to review current literature on the difference between the different treatment options in adult hypogonadal and transgender males, specifically aimed at the most used preparations, transdermal testosterone gel, and intramuscular testosterone injections, and to determine which knowledge gaps remain for further research.
Methods
This systematic review was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement (17) and has been developed in collaboration with an information specialist (CP).
The literature was systematically searched using international databases: PubMed (1995 to February 2021) and the Cochrane Library (including EMBASE (OVID version) and Web of Science; 1995 to February 2021). We included studies on male hypogonadism, hypogonadal men, transgender, transsexual, transgender men, transmen, female-to-male, FtM, combined with several keywords related to treatment including transdermal testosterone gel, intramuscular testosterone injections, testosterone undecanoate, proprionate, enathate, cypionate, Nebido, and Sustanon.
Inclusion criteria were studies on adult humans; primary and secondary hypogonadism or transgender males using testosterone, randomized controlled trials, clinical trials and prospective trials; written in English or Dutch. Exclusion criteria were studies on late-onset hypogonadism, only reporting on patient groups with other symptoms or illnesses such as androgenic-anabolic steroid withdrawal syndrome, chronic opioid use, HIV, obesity, metabolic syndrome, type 2 diabetes mellitus, or end-stage renal disease.
Studies were selected independently by two different reviewers (M M, L B) and any disagreement in eligibility was resolved by discussion.
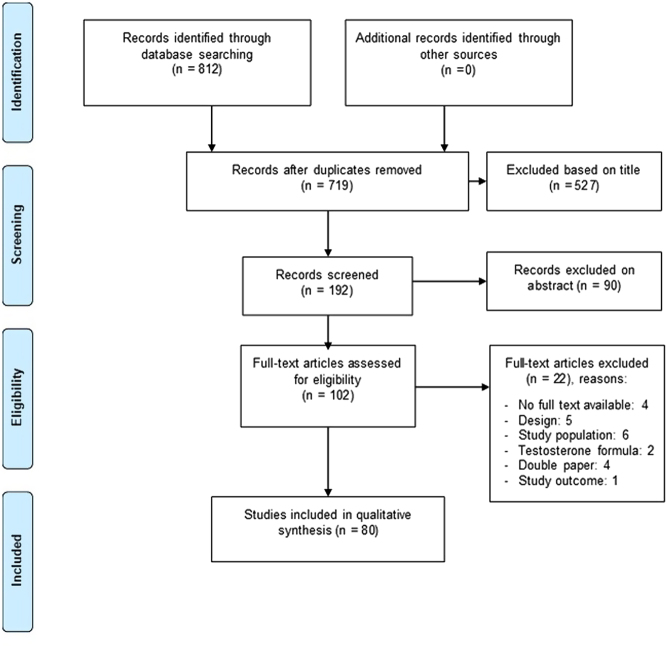
The selection of articles is depicted in Fig. 1. Search strategy can be found in the Supplementary information (see section on supplementary materials given at the end of this article).
Figure 1.
PRISMA flow chart of inclusion of studies.
Results from the selected studies on pharmacokinetics, hypogonadal symptoms, virilization, metabolic and anthropometric parameters, bone mineral density, HR-QoL, and side/adverse effects were extracted from the included articles and described in this review.
Results
Pharmacokinetics
The goal of testosterone therapy is to raise serum testosterone levels to the mid-normal (birth-assigned male) range, without significant side effects or safety concerns, and alleviating the hypogonadal symptoms or, in transgender males, inducing virilization. Hence, both too low trough levels and too high peak levels are unwanted. Based on several randomized (only men with hypogonadism) and non-randomized (both hypogonadal and transgender males) clinical trials, different testosterone formulations have been approved for androgen therapy and are included in (inter)national guidelines (2, 3, 12, 13, 14, 15, 16). Table 1 shows the pharmacokinetic properties of testosterone gel (2, 18, 19, 20, 21, 22, 23, 24, 25, 26), and short-acting (7, 27, 28, 29, 30, 31) and long-acting injections (7, 9, 32, 33, 34, 35, 36).
Table 1.
Testosterone preparations and pharmacokinetics.
| Preparation | T max | Therapeutic range | Kinetics | Target value | Dose adjustment | References |
|---|---|---|---|---|---|---|
| Gel (Testogel 1%, Testim 1%, AndroGel 1%, Androgel 1.62%, Fortesta 2%, Tostran 2%, Testavan 2%) | 8 h | +24 h | Stable, depending on compliance | 20–30 nmol/L | Multiple to get in target range | (Randomized controlled trials (RCTs): (18, 19, 20, 21, 22, 23, 122), observational studies: (24, 25, 26)). |
| Short-acting esters (e.g. Testoviron® Depot 50: 20 mg testosterone propionate and 55 mg testosterone enanthate (TE); Testoviron® Depot 100: 25 mg testosterone propionate and 100 mg testosterone enanthate; Sustanon® 250: 30 mg testosterone propionate, 60 mg testosterone phenylpropionate, 60 mg testosterone isocaproate and 100 mg testosterone decanoate) | 24–48 h | 2–4 weeks | High peaks shortly after each injection and low troughs prior to the next | 10–15 nmol/L prior to injection | On the basis of concentrations prior to injection | (6, 7, 28, 29, 30, 31) |
| Long-acting undecanoate (Nebido® 1000 mg/4 mL TU (available in a.o. Europe (not US)) and Aveed® 750 mg/3 mL TU (available in US) | 1 week | 10–14 weeks, second booster injection after 6 weeks | Stable | 20–30 nmol/L | Steady state after third injection | (7, 9, 32, 34, 35, 36, 40) |
Both gel and i.m. testosterone preparations are able to achieve physiological testosterone levels. With the exception of one small open-label randomized controlled trial (RCT) comparing i.m. TE and i.m. TU (7), no randomized studies comparing the above preparations on pharmacokinetics have been performed. Gel achieves relatively stable testosterone levels within a week with small peak to trough fluctuations, provided it is applied daily, that is with good compliance (20, 21, 22, 23, 24, 25). Testosterone levels return to baseline relatively quickly after termination (22). Use of gel is accompanied by several instructions to the patient in order to minimize the risks of transfer to other people (i.e. to wash hands after application and to wear a shirt covering the application sites before having physical contact) and to optimize bioavailability of testosterone (i.e. to wait at least 5–6 h after application before taking a bath or shower) (37, 38). The short-acting injections achieve on average physiological levels of testosterone. However, the pharmacokinetic profile is characterized by supraphysiological peaks shortly after each injection and levels below the lower limit of normal, prior to the next (7, 27, 28, 29). In addition, administration is given intramuscularly two to three times weekly by a healthcare provider (29). Long-acting injections maintain serum testosterone levels in the normal range with lower peak and less deep trough values in comparison to short-acting injections and reach a steady state after three injections (7, 9, 32, 39, 40). An initial dose of i.m. TU 1000 mg/4 mL should be followed by a second injection 6 weeks later, after which injections may resume every 12 weeks. Hence, 4–5 i.m. injections annually have to be administered by a healthcare provider.
Hypogonadal symptoms
Testosterone is prescribed to relieve hypogonadal complaints in men with hypogonadism. These complaints include less sexual desire and function, lower testicular volume, decreased body hair, gynecomastia, decreased lean body mass and muscle strength, and complaints of fatigue and depression. Sexual desire and function improve 3–6 weeks after initiation of testosterone therapy, and it can take 6 months until effect is seen on erections/ejaculations. Effects on depression and mood can be seen after 3–6 weeks but can take longer to fully stabilize (33). In transgender males, testosterone is prescribed to induce virilization. However, after ovariectomy, hypogonadal symptoms can also be a sign of inadequate testosterone therapy in transgender males. As shown in Table 2, there are a variety of studies that report the presence or absence of hypogonadal symptoms (26, 32, 39, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56). All types of testosterone administration relieve hypogonadal symptoms. TU seems to give the most stability in the relief of hypogonadal symptoms, as well as an improvement in concentration (53). One comparative study did not show any difference in on-treatment testosterone and symptoms between gel and injections (49).
Table 2.
Studies on the influence of testosterone therapy on hypogonadal symptoms, BMD, and metabolic and anthropometric parameters. First table shows results for hypogonadal men, and second table shows results for transgender men.
| Author, year, (ref), study design | Intervention/comparator | Study population | Follow-up | Hypogonadal symptoms | Bone mineral density (BMD) | Metabolic and anthropometric parameters |
|---|---|---|---|---|---|---|
| Studies on men with hypogonadism | ||||||
| Aydogan 2012 (41), Prospective non-randomized study | Testosterone esters (TE) (Sustanon 250 mg every 3 weeks)/none | 39 men with congenital hypogonadotropic hypogonadism vs 40 age-matched eugonadal men | 6 months | Improvement of sexual function | Significant increase in BMI | |
| Benito 2005 (84), Prospective study | Gel/none | 10 untreated men with hypogonadism vs 10 eugonadal men | 2 years | Increase in BMD | ||
| Bolu 2012 (60), Prospective study | TE/gel | 70 men with hypogonadism vs 70 controls | 6 months | After treatment, lower total cholesterol and lower high-density lipoprotein (HDL) cholesterol observed. BMI increased. | ||
| Cherrier 2003 (54), Prospective study | Gel (2 dosages)/patch | 12 men with hypogonadism | Improvement in verbal memory after testosterone therapy (TT) | |||
| Chiang 2007 (42), Double-blind, randomized, placebo-controlled study | Gel/placebo | 40 men with hypogonadism | 3 months | Improvement of sexual function | ||
| Cunningham 2017 (26), Phase 3 open-label non-comparator study | Gel/none | 160 men with hypogonadism | 4 months | Improvement of sexual function, less fatigue | ||
| De Rosa 2001 (85), Cross-sectional study | TE/none | 12 men with hypogonadism | Still decreased BMD after testosterone therapy | |||
| Efros 2016 (43), P hase II, open-label, sequential dose escalation studies | Gel, three different concentrations | 38 men with hypogonadism | 1 week | Improvement of sexual function. Less fatigue and distress | ||
| Kaufman 2011 (65), Multicenter, randomized, double-blind, placebo-controlled study | Gel/placebo | Men with hypogonadism gel were 214 and placebo 37 | 6 months | Increased BMI with TT | ||
| Khera 2011 (55), Prospective multicenter registry study | Gel/none | 271 men with hypogonadism | 1 year | Improvement of sexual function | ||
| Lasaite 2016 (44), Prospective study | TU/none | 19 men with hypogonadism | Improvement in cognitive tests (trail making test, digit span test) | |||
| Leifke 1998 (87), Prospective study | TE/none | 32 men with hypogonadism | 3.2 ± 1.7 years | Increase in BMD | ||
| Malkin 2004 (66), Randomized, single-blind, placebo-controlled, crossover trial | TE/placebo | 29 men with hypogonadism | 1 month | Testosterone treatment gave reduction of total cholesterol and serum triglycerides | ||
| McNicholas 2003 (45), Randomized, multidose, multicenter, active-controlled study | Gel/patch | 208 men with hypogonadism (68 Testim 50, 72 Testim 100, 68 Andropatch) | 3 months | Improvement of sexual function | No changes in BMD | Increase in BMI, total cholesterol, decrease in HDL |
| Medras 2001 (89), Prospective, controlled study | TE/none | 26 men with hypogonadism | No improvement in BMD | |||
| Miner 2013 (46), Registry study | Gel/ none | 849 men with hypogonadism | 1 year | Improvement of mood and depression | ||
| Minneman 2008 (67), Open-label, randomized, prospective clinical trial | TE/TU | 40 men with hypogonadism | 2.1 years | No difference in BMI. TU HDL lower compared to low density lipoprotein (LDL). |
||
| Mulhall 2004 (47), Prospective, observational study | Gel→ patch→ TE adjusted for testosterone levels | 32 men with hypogonadism | 1 year | Improvement of sexual function | ||
| Nieschlag 1999 (32), Open-label, clinical, non-randomized study | TU/none | 13 men with hypogonadism | 24 weeks | Improvement of sexual function compared to previous treatment (TE/gel) | Decrease in HDL, Stable BMI other lipids, glucose and HbA1c | |
| O’Connor 2001 (48), Single-blind placebo-controlled study | TE/placebo | 30 eugonadal and 7 men with hypogonadism | No improvement of cognitive tests in hypogonadal group (compared to eugonadal) | |||
| Ramasamy 2015 (49), Prospective, observational study | Gel/injections | 42 men with hypogonadism | Median 3.8 years | No difference between gel and injections in hypogonadal symptoms | ||
| Schubert 2003 (90), Prospective, open-label randomized, trial | Mesterolone 100 mg/day/oral testosterone undecanoate 160 mg/day/testosterone enanthate depot 250 mg i.m./21 days, or testosterone pellets | 53 men with hypogonadism | 6 months | Increase in BMD in all groups | ||
| Seftel 2004 (56), Randomized, multidose, multicenter, active, placebo-controlled study | Gel (two different dosages)/patch/placebo | 406 men with hypogonadism | 2 years | Improvement in sexual desire and function | ||
| Sonmez 2015 (72), Prospective, controlled study | TE/Gel | 60 men with hypogonadism vs 70 age-matched controls | 6 months | Increase in systolic blood pressure, BMI and decrease in HDL cholesterol | ||
| Tahani 2018 (91), Prospective study | Gel/TU | 15 men with hypogonadism with Klinefelter syndrome, 26 controls | 3 years | Increase in BMD after testosterone treatment | ||
| Van den Berg 2001 (93), Cross-sectional study | TU/TE/oral | 52 men with hypogonadism (Klinefelter) | 1 year | 44–48% had osteopenia, 6–14% osteoporosis. No fractures reported. | ||
| Von Eckardstein 2002 (39), Phase 2 study | TU/none | 7 men with hypogonadism | 2.8 years | Improvement of sexual function | Increase in BMI (due to increase in LBM), decrease in HDL, and total cholesterol | |
| Wang 1996 (50), Prospective study | TE/sublingual | Men with hypogonadism 18 testosterone esters, 35 sublingual |
2 months | Improvement in mood, decreased anger, and irritability sadness and tiredness | ||
| Wang 2001 (51), Prospective, randomized, multi-center, parallel clinical trial | Gel 50 or 100 mg | 227 men with hypogonadism | 6 months | Increase in BMD | ||
| Wang 2004 (78), Long-term, open-label efficacy study | Gel 50, 75, 100 mg | 169 men with hypogonadism | 3 years | Improvement of sexual function | Increase in BMD | BMI rose mostly due to an increase in lean body mass |
| Wolf 2017 (76), Observational post-marketing study | TU/ none | 867 men with hypogonadism 3 transgender males |
1 year | Stable BMI. Men with low blood pressure had an increase in blood pressure, men with high blood pressure had a decrease in blood pressure. | ||
| Wu 2009 (75), Prospective controlled study | 3 months TU oral, after this monthy injections TU 250 mg | 26 men with hypogonadism vs 26 healthy controls | 9 months | Total cholesterol, LDL-C, HDL-C, and triglyceride were all decreased. No changes in body fat | ||
| Yassin 2013 (79), Cumulative registry study | TU/none | 261 men with hypogonadism | 5 years | Decrease in waist circumference and BMI after TT | ||
| Zitzmann 2013 (53), International, multicenter, one-arm, prospective, observational study in 23 countries | TU/ none | 1438 men with hypogonadism | 5 injections (1 year) | Improvement of concentration and sleep quality. Stability in mood, less hot flushes, and sweating. | Lower blood pressure and better lipid profile | |
| Studies on transgender males | ||||||
| Elbers 2003 (43), Prospective study | TE/none | 17 transgender males | 1 year | Decrease in HDL cholesterol, increase in cholesterol, increase in BMI 1 year after TT | ||
| Emi 2008 (80), Prospective study | TE/ no treatment | 63 untreated and 48 treated transgender males | - | Treated individuals had higher blood pressure, increased cholesterol, and decreased HDL cholesterol. | ||
| Gava 2021 (61), Randomized, double-blind PL-controlled pilot trial | Testosterone undecanoate (TU) + placebo/ TU + 5 α-reductase inhibitor | 14 ovariectomized transgender males | 1 year | No difference in BMD | Stable BMI, HDL decreased, fat% lower | |
|
Goh 1995 (62), Prospective study |
TE/none | 85 transgender males | 33 months | Higher levels of triglyceride, total cholesterol, LDL and Apo-B, lower HDL | ||
| Haraldsen 2007 (95), Prospective study | TE/none | 12 transgender males | 1 year | No difference in BMD | ||
| Jacobeit 2007 (64), Prospective study
|
TU/none | 12 transgender males | 1 year | Stable BMI and lipid profile | ||
| Jacobeit 2009 (63), Prospective study | TU/none | 17 transgender males | 1.5 year | Stable lipid profile and BMI | ||
| Lips 1996 (88), Prospective study | TE (12)/oral (3) | 15 transgender males | 39 months | Normal BMD | ||
| Mueller 2007 (58), Prospective, observational study | TU/none | 35 transgender males | 1 year | No changes in BMD | Increased BMI, decreased HDL, other lipid parameters stable. Increase in systolic and diastolic blood pressure | |
| Mueller 2010 (71), Prospective, observational study | TU/none | 45 transgender males | 2 years | No changes in BMD | Stable BMI but lean body mass (LBM) increased, decreased HDL, other lipid parameters stable | |
| Pelusi 2014 (59), Observational study | Gel/Testoviron/TU | 45 transgender males | 1 year | No difference in BMD | BMI increased after starting testosterone therapy, independent of formulation. HDL decreased, LDL increased in all groups. | |
| Turner 2004 (31), Prospective case series | TE/None | 15 transgender males | 2 years | Increase in BMD | ||
| Van Caenegem 2015 (92), Prospective, controlled study | TU/none | 26 transgender males, 23 age-matched cis women | 1 year | Small increase BMD | ||
| Van Velzen 2019 (74), Prospective, controlled study | TE or TU or Gel | 188 transgender males 47 gel, 63 TE 79 TU |
1 year | No differences in lipids, BMI, systolic and diastolic blood pressure, cholesterol HDL, LDL, and triglycerides for different formulations. Although BMI was higher in the group using TE. | ||
| Van Velzen 2020 (73), Prospective, controlled study | TE or TU or Gel | 323 transgender males | 2 years | Stable BMI but increase in LBM | ||
| Vlot 2019 (94), Prospective, controlled study | TE or TU or Gel | 132 transgender males | 1 year | Increase in BMD in younger transgender males, decrease in older transgender males | ||
| Wierckx 2014 (77), Prospective study | TE/TU | 53 transgender males | Total body weight increased due to an increase in LBM. Systolic blood pressure increased | |||
Virilization
In transgender people wishing for masculinization, testosterone therapy induces virilization, and this includes increase in facial and body hair, deepening of the voice, and mental and sexual changes. These changes start 3 months after initiation of testosterone therapy, but it can take years until the maximum effect is seen. All testosterone administration types induce virilization. There does not seem to be a difference in testosterone administration type and the rapidness of changes (57, 58, 59).
Metabolic and anthropometric parameters
It is known that testosterone has an effect on different metabolic and anthropometric parameters. In general, glucose sensitivity improves and also changes in BMI are generally seen. Changes in fat mass, lean body mass, and muscle strength occur within 12–16 weeks, stabilize at 6–12 months but can marginally continue over years (33). Conflicting results are available on the effect of testosterone on lipids, which mostly appear already after 4 weeks (33). Table 2 shows an overview of studies investigating metabolic and anthropometric parameters (32, 39, 41, 43, 45, 53, 58, 59, 60, 61, 62, 63, 64, 65, 66, 67, 68, 69, 70, 71, 72, 73, 74, 75, 76, 77, 78, 79, 80).
Comparing different administration types, one study did not find any difference between TU and TE in lipids and BMI (67). Comparing TU and TE in transgender males, a greater increase in BMI was seen with TE (67). Both resulted in an increase in cholesterol and lean body mass (LBM) (73). Also, a small increase in systolic blood pressure was frequently seen. Although often characterized as not significant by the authors, this could still be of influence on the cardiovascular risk, as seen previously in the Framingham Study (81).
In conclusion, there are conflicting results on the effect on anthropometric parameters and lipids. In general, BMI increased, but this was mostly due to an increase in LBM and concomitant decrease in fat mass. Some studies showed an increase in lipid values and others, a decrease. Lipids, mainly LDL-cholesterol (LDL-C), are a well-known surrogate marker for cardiovascular disease with an association between the height of LDL-C level and the occurrence of cardiovascular disease (82). This indicates that small unfavorable changes in LDL-C after testosterone therapy could have a high impact as testosterone is widely used among hypogonadal and transgender males. It is noteworthy that in all studies among transgender males on testosterone treatment demonstrated an unfavorable effect on lipids, whereas in men with hypogonadism divergent results were observed on lipid changes with both favorable and unfavorable effects after testosterone initiation. The observation that in men with hypogonadism, lipid changes were observed in all directions in contrast to the studies performed in transgender males showing only detrimental effects on lipids may be explained by the fact that transgender people are mostly eugonadal before initiation of testosterone treatment. Male hypogonadism on itself is still under debate as risk factor for cardiovascular disease, and it could be hypothesized that the favorable changes in lipids that were observed in some studies in men with hypogonadism may be the result of the treatment of their hypogonadism (83).
Bone mineral density
Table 2 shows studies that researched the effect of testosterone on bone mineral density (BMD) (31, 45, 51, 58, 59, 61, 71, 78, 84, 85, 86, 87, 88, 89, 90, 91, 92, 93, 94, 95). Several small randomized and observational trials have shown that testosterone treatment, both gel and i.m. preparations, improves BMD. Effects on BMD have been shown in studies of 6 months duration and longer, both on spinal and hip BMD and on trabecular and cortical BMD (51, 90, 96). Several observational studies showed a gradual and progressive increase during treatment up to at least 3 years (52, 84, 87, 91, 97, 98). The effect on BMD appears to be preceded by a change in bone turnover markers. In a RCT by Wang et al. (51), testosterone gel replacement in 227 men with hypogonadism during 6 months resulted in a decrease in osteoclastic activity and an early but transient stimulation of osteoblastic activity in the first 90 days of treatment, with maintenance of lower bone resorption and return to baseline of bone osteoblastic activity at 180 days.
In transgender people looking for masculinization, the effect of testosterone treatment on bone is different, as there is also an effect on estrogen (in non-ovariectomized transgender males). Based on several studies (see Table 2), testosterone treatment in transgender males has at least no negative effect on bone, and possibly a small positive effect (58, 59, 61, 71, 86, 92, 94).
Health-related quality of life
Limited studies are available assessing the effect of testosterone therapy on HR-QoL. Moreover, the impact of testosterone therapy on HR-QoL in men with hypogonadism is difficult to quantify due to significant heterogeneity in study population, study duration, and QoL measures. Based on these data, testosterone treatment in men with hypogonadism seems to improve QoL (Table 3) (24, 26, 41, 99). Given the limited amount of data, it is certainly not possible to draw any conclusion on the different formulations. In addition, we did not find randomized or non-randomized clinical trials on QoL measures in adult transgender males.
Table 3.
Quality of life.
| Author, year, (ref), study design | Intervention/comparator | Study population | Follow-up | Health-related quality of life |
|---|---|---|---|---|
| Aydogan 2012 (41), Prospective, non-randomized study | TE/none in age-matched controls | Men with hypogonadism (Sustanon 39, age-matched controls 40) | 6 months | SF-36: statistically significant difference was found in physical role difficulty, pain, general health, emotional role difficulty, and mental health parameters |
| Belkoff 2018 (24), Phase 3 open-labeled prospective study | Gel/none | 180 men with hypogonadism | 9 months | SF-12: significant improvements in QoL for all four domains, physical and mental component summaries, and the mean total score were observed on 3 months, which sustained till 9 months |
| Cunningham 2017 (26), Phase 3 open-label, non-comparator study | Gel/none | 160 men with hypogonadism | 4 months | SF-12: improvement of physical component summary, also improvement of mental component summary |
| Shiraishi 2014 (99), Prospective study | hCG + rhFSH/TE | Men with hypogonadism (hCG+rhFSH 31, TE 6) | 24 months | SF-36: no improvements with TE |
Side effects/adverse events
In general, testosterone therapy is not known to have many side effects. In transgender males, the most important side effect is acne, and this was reported in up to 44.6% (57, 77, 100, 101, 102, 103). Fortunately, this acne was mostly mild and did not lead to discontinuation of testosterone therapy. Acne was, to a lesser extent, also reported in the treatment of men with hypogonadism (9, 32, 39, 52). In transgender males, anger was also reported as a side effect (104). Other general side effects were skin reactions with the use of gel (20, 25, 45, 46, 78, 105) and pain at the injection site with the use of injections (67). This was more frequently reported with the use of TU (9, 77, 100). One study reported dizziness as a side effect of TE (106). In general, snoring and insomnia were also reported in some studies (107). In conclusion, there are, besides acne and local reactions on the application site, no major side effects of testosterone therapy. Comparing the different administration types, skin reactions are more frequently seen with the use of testosterone gel and pain at the injection site with the use of testosterone injections.
Hematocrit
Testosterone is known to stimulate erythropoiesis. Erythrocytosis is the most frequent adverse event related to testosterone therapy, with a reported prevalence varying between 5 and 66% (108). Effects on hematocrit levels are apparent approximately 3 months after initiation of testosterone therapy, peaking at 9–12 months. Table 4 shows the included studies reporting hematocrit levels after initiation of testosterone therapy (45, 53, 67, 78, 80, 109, 110). The increase in hematocrit level is larger in transgender than in men with hypogonadism due to a lower (female) baseline level but reaches the same range during treatment in both groups (between 0.47 and 0.52). Injections seem to give the highest prevalence of hematocrit levels above 0.50 L/L (111).
Table 4.
Hematocrit.
| Author, year, (ref), study design | Intervention/comparator | Study population | Follow-up | Hematocrit |
|---|---|---|---|---|
| Defreyne 2018 (111), Prospective study | TU/TE/Gel | 192 transgender males | 1 year | TU: 40.9%→ 45.1% (levels above 0.50: 2.2%)TE: 41.0%→ 46.0% (levels above 0.50: 13.4%)Gel: 40.0%→ 46.5% (levels above 0.50: 9.9%) |
| Emi 2008 (80), Prospective study | TE/no treatment | 63 untreated and 48 treated transgender males | - | Untreated: 38.8% Treated: 44.9% |
| Levcikova 2017 (110), Prospective study | TU/none | 69 men with hypogonadism | Increase in hematocrit in 30% | |
| McNicholas 2003 (45), Randomized, multidose, multicenter, active-controlled study | Gel/patch | 208 men with hypogonadism 68 testim 50, 71 testim 100, 68 andropatch. |
90 days | Higher dosage of gel showed higher hematocrit |
| Minnemann 2008 (67), Open-label, randomized, prospective clinical trial | TE/TU | 40 men with hypogonadism | 30 weeks | TE: 44,4%→ 47.8%TU 43.3%→ 46.8% |
| Wang 2004 (78), Long-term, open-label efficacy study | Gel/patch | 163 men with hypogonadism | 3 years | 9% hematocrit >0.56 |
| Zitzmann 2013 (53), International, multicenter, one-arm, prospective, observational study in 23 countries | TU/none | 1438 men with hypogonadism | 1 year | No cases of >0.52 |
Other adverse events
Studies in men with hypogonadism report a low frequency of serious adverse events with replacement doses of testosterone. Long-term safety of testosterone therapy in relation to cardiovascular diseases remains uncertain because no adequately powered trials with a sufficiently long follow-up have investigated the effects of testosterone on cardiovascular events. A comprehensive and detailed meta-analysis of available randomized placebo-controlled trials concluded that the data did not support a causal role between testosterone therapy and cardiovascular adverse events (112). On the other hand, low testosterone is regarded as a marker for poor general health and as increased risk for cardiovascular disease and mortality. Observational studies have indeed reported that testosterone treatment improves survival when compared to men who were not treated (113). Furthermore, a multinational, longitudinal disease registry of men diagnosed with hypogonadism showed not testosterone therapy but age and prior cardiovascular disease history as risk factors for cardiovascular events (114). Another registry study with adjudicated major adverse cardiovascular events (MACE) found no difference between treated or untreated men and did not report any testosterone treatment related event during a 3 year follow-up (12). The European Medicine Agency (EMA) has stated in a consensus that there is no consistent evidence of an increased risk for cardiovascular adverse events from testosterone therapy in men with hypogonadism. To minimize cardiovascular events, it is important to keep testosterone values in the mid-normal physiological range and prevent hematocrit levels of >0.54 L/L. Furthermore, in men with an increased risk of cardiovascular disease, it is important to start with replacement therapy only after consulting a cardiologist.
In men with hypogonadism treated with testosterone, levels of prostate-specific antigen (PSA) and prostate volume rise, marginally, plateauing at 12 months. Observational studies indicate that testosterone replacement therapy does not increase the risk of developing prostate cancer or result in more aggressive prostate tumors.
Discussion
Testosterone treatment is shown to achieve physiological male testosterone levels leading to positive effects on hypogonadal symptoms (men with hypogonadism) or virilization (transgender males), well-being, metabolic parameters, body composition, BMD, with relatively few side effects and good safety. The time course of these effects is variable ranging from several weeks for sexual desire, from 6 to 12 months for body composition, and up to 3 years for maximum effects on bone (33, 115). Several preparations differing in the route of administration and pharmacokinetics have been approved for androgen therapy and are included in (inter)national guidelines (2, 3, 12, 13, 14, 15, 16). It should be selected according to the patient’s preference, cost, availability, and formulation-specific properties. Worldwide, the most commonly used preparations are transdermal testosterone gel and i.m. injections. However, with the exception of only one small open-label RCT comparing i.m. TE and i.m. TU on pharmacokinetics, clinical efficacy and safety (7, 67, 106) (same RCT, but results published in three articles), no randomized studies comparing these preparations have been performed. The (inter)national guidelines do not recommend one preparation specifically, advising that the choice should be a joint decision by both the patient and the physician (2, 3, 12, 13, 14, 15, 16), although some suggest to start first with gel to evaluate the treatment effect before switching to the longer-acting injections (3, 12). In addition, some guidelines for transgender care (14) have a preference for gel or long-acting injections since short-acting injections in transgender males are frequently associated with aggressive behavior due to the large fluctuations in testosterone concentrations with supraphysiological levels. An additional problem in several countries is that, for unclear reasons, long-acting injections have a limited reimbursement, which limits the patient’s freedom of choice and possibilities for optimal personalized medicine.
Although hardly any comparative studies have been carried out, there are indications of differences between gel and i.m. preparations. Gel, with good compliance, and long-acting injections achieve more stable testosterone levels than short-acting injections. The large fluctuations in serum testosterone with supraphysiological levels and low trough values can lead to bothersome fluctuations in the patient’s mood, sexual desire, and energy level. Based on a survey in the Netherlands in 2019 (116), Sustanon-users experienced much more mood swings than Nebido- and gel-users (25.3% vs 7.6% of gel-users and 5.9% of Nebido-users), increased aggression and anger (14.4% vs 5.5% and 4.3%), and excessive sexual desire (17.1% vs 4.7 and 5.9%). Concerning hematocrit, injections seem to increase hematocrit more than gel, and this could be explained by several reasons. Injections give higher peaks in testosterone. Moreover, compliance is often higher with injections due to the longer intervals between administrations. In a recent retrospective study in transgender males, the largest increase in hematocrit levels was seen within the first year (for both injections and gel), and and also after this first year, a slight increase was seen (117). Studies on a possible different effect on hematocrit between short- and long-acting injections have given mixed results. There are no data on cardiovascular events related to this testosterone-induced increase in hematocrit. In a systematic review on cardiovascular disease in transgender persons treated with cross-sex hormones, exposure to testosterone was not associated with a strong increase in cardiovascular events in transgender males; however, more studies are necessary with longer follow-up as cohorts of transgender males were relatively small to draw firm conclusions on (118). The beneficial effect on bone on the other hand may be greater for injections compared to gel, although no comparative studies between the different preparations have been performed. A meta-analysis from Tracz et al, showed that i.m. but not transdermal testosterone increased lumbar spine BMD, but effects on femoral neck were inconclusive (119). Importantly, no trial has assessed the effect of testosterone therapy on the risk of incident fractures.
With regard to most clinical parameters, side effects, and safety, testosterone treatment appears to be roughly similar in hypogonadal and transgender males. Remarkably, the impact of testosterone treatment on HR-QoL is strongly underexposed in the reviewed studies, while this has a highly relevant outcome measure from a patient perspective. Available studies show an improvement in HR-QoL with testosterone treatment, which is confirmed by two meta-analyses (119, 120). In the meta-analysis by Elliot et al., 23 RCTs, representing 14 treatments in addition to placebo, were included. When compared as a class against placebo, testosterone treatment improved QoL with substantial heterogeneity. Intramuscular TU significantly improved QoL relative to placebo and to oral TU, with no other significant differences among the other treatments (120). The authors do not report on the methods used to measure QoL in the included RCTs. Most RCTs involved men with late-onset hypogonadism, which were excluded from our search. In these studies, the Aging Males’ Symptom (AMS) scale, a self-administered 17-item questionnaire, was often used to measure HR-QoL and symptoms in aging men (e.g. somato-vegetative, psychological, and sexual symptoms). Improvements on scores on the AMS have been noted within 3–4 weeks, but maximum benefits take longer time period. Regarding prostate cancer risk, a meta-analysis did not show a rise in International Prostate Symptom Score, and no detection of abnormal PSA values and no increase in prostate cancer were observed (121).
Conclusions
In conclusion, both gel and i.m. testosterone replacement therapy appear to be effective preparations for the treatment of hypogonadal and transgender males. However, recommendations on testosterone treatment are based on data primarily from non-randomized clinical studies and observational studies and virtually no RCTs, and there are no studies on the effects of testosterone replacement on endpoints such as cardiovascular disease in relation to hematocrit or osteoporotic fractures in relation to BMD. Additionally, the impact of testosterone treatment on HR-QoL is strongly underexposed in the reviewed studies. Although hardly any comparative studies have been carried out, there are indications of differences between the preparations, for example, on stability of testosterone levels, hematocrit, and BMD. Furthermore, patient satisfaction seems to be in favor of long-acting i.m. injections due to the low injection frequency with stable testosterone levels and no daily confrontation with treatment/disease. Patient satisfactory is lower with the use of gel, which is easy in use, but daily application is needed with daily confrontation with treatment and the risk of less compliance, possibly resulting in less stable values, and the least with short-acting injections due to the high injection frequency but especially due to the large fluctuations with concomitant swings in mood, sexual desire, and energy level (116). Given the increasing importance of personalized medicine, new randomized comparative studies are needed that also look into the effect on patient-reported outcome measures. This might also aid in obtaining full reimbursement for long-acting i.m. injections in various countries, increasing the patient’s freedom of choice and possibilities for optimal personalized medicine.
Supplementary Material
Declaration of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of this review.
Funding
This publication has been supported by the European Reference Network on Rare Endocrine Conditions (Endo-ERN), which is co-funded by the European Union’s 3rd Health Program (CHAFEA Framework Partnership Agreement No. 739527).
Author contribution statement
M M, L B, M H, C P, and N B made substantial contributions to design and conception of this review. All authors gave final approval of the version to be submitted. M M and L B participated in drafting the article. C P contributed to the search strategy. L B, M H, and N B participated in revising the manuscript critically for important intellectual content.
References
- 1.Basaria S.Male hypogonadism. Lancet 20143831250–1263. ( 10.1016/S0140-6736(1361126-5) [DOI] [PubMed] [Google Scholar]
- 2.Bhasin S, Brito JP, Cunningham GR, Hayes FJ, Hodis HN, Matsumoto AM, Snyder PJ, Swerdloff RS, Wu FC, Yialamas MA. Testosterone therapy in men with hypogonadism: an Endocrine Society clinical practice guideline. Journal of Clinical Endocrinology and Metabolism 20181031715–1744. ( 10.1210/jc.2018-00229) [DOI] [PubMed] [Google Scholar]
- 3.NVE Commissie Gonadale Endocrinology. Leidraad ‘Hypogonadisme bij de volwassen man’, 2020. (available at: https://www.nve.nl/content/uploads/2017/08/Leidraad-hypogonadisme-NVE-finale-versie-sept-2020.pdf) [Google Scholar]
- 4.den Heijer M, Bakker A, Gooren L. Long term hormonal treatment for transgender people. BMJ 2017359j5027. ( 10.1136/bmj.j5027) [DOI] [PubMed] [Google Scholar]
- 5.Miller MG, Rogol AD, ZumBrunnen TL. Secondary exposure to testosterone from patients receiving replacement therapy with transdermal testosterone gels. Current Medical Research and Opinion 201228267–269. ( 10.1185/03007995.2011.652255) [DOI] [PubMed] [Google Scholar]
- 6.Conway AJ, Boylan LM, Howe C, Ross G, Handelsman DJ. Randomized clinical trial of testosterone replacement therapy in hypogonadal men. International Journal of Andrology 198811247–264. ( 10.1111/j.1365-2605.1988.tb00999.x) [DOI] [PubMed] [Google Scholar]
- 7.Schubert M, Minnemann T, Hübler D, Rouskova D, Christoph A, Oettel M, Ernst M, Mellinger U, Krone W, Jockenhövel F. Intramuscular testosterone undecanoate: pharmacokinetic aspects of a novel testosterone formulation during long-term treatment of men with hypogonadism. Journal of Clinical Endocrinology and Metabolism 2004895429–5434. ( 10.1210/jc.2004-0897) [DOI] [PubMed] [Google Scholar]
- 8.Morgentaler A, Dobs AS, Kaufman JM, Miner MM, Shabsigh R, Swerdloff RS, Wang C. Long acting testosterone undecanoate therapy in men with hypogonadism: results of a pharmacokinetic clinical study. Journal of Urology 20081802307–2313. ( 10.1016/j.juro.2008.08.126) [DOI] [PubMed] [Google Scholar]
- 9.Wang C, Harnett M, Dobs AS, Swerdloff RS. Pharmacokinetics and safety of long-acting testosterone undecanoate injections in hypogonadal men: an 84-week phase III clinical trial. Journal of Andrology 201031457–465. ( 10.2164/jandrol.109.009597) [DOI] [PubMed] [Google Scholar]
- 10.Schnabel PG, Bagchus W, Lass H, Thomsen T, Geurts TB. The effect of food composition on serum testosterone levels after oral administration of andriol testocaps. Clinical Endocrinology 200766579–585. ( 10.1111/j.1365-2265.2007.02781.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Swerdloff RS, Dudley RE. A new oral testosterone undecanoate therapy comes of age for the treatment of hypogonadal men. Therapeutic Advances in Urology 2020121756287220937232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mirone V, Debruyne F, Dohle G, Salonia A, Sofikitis N, Verze P, Fode M, Chapple C. & URO-TRAM working group. European Association of Urology position statement on the role of the urologist in the management of male hypogonadism and testosterone therapy. European Urology 201772164–167. ( 10.1016/j.eururo.2017.02.022) [DOI] [PubMed] [Google Scholar]
- 13.Mulhall JP, Trost LW, Brannigan RE, Kurtz EG, Redmon JB, Chiles KA, Lightner DJ, Miner MM, Murad MH, Nelson CJet al. Evaluation and management of testosterone deficiency: AUA guideline. Journal of Urology 2018200423–432. ( 10.1016/j.juro.2018.03.115) [DOI] [PubMed] [Google Scholar]
- 14.Kwaliteitsstandaard Transgenderzorg – Somatisch, 2018. (available at: https://richtlijnendatabase.nl/gerelateerde_documenten/f/19927/Kwaliteitsstandaard%20Transgenderzorg%20-%20Somatisch.pdf) [Google Scholar]
- 15.Coleman E, Bockting W, Botzer M, Cohen-Kettenis P, DeCuypere G, Feldman J, Fraser L, Green J, Knudson G, Meyer WJ, et al. Standards of care for the health of transsexual, transgender, and gender-nonconforming people, version 7. International Journal of Transgenderism 201213165–232.. ( 10.1080/15532739.2011.700873) [DOI] [Google Scholar]
- 16.Hembree WC, Cohen-Kettenis PT, Gooren L, Hannema SE, Meyer WJ, Murad MH, Rosenthal SM, Safer JD, Tangpricha V, T’Sjoen GG. Endocrine treatment of gender-dysphoric/gender-incongruent persons: an Endocrine Society clinical practice guideline. Journal of Clinical Endocrinology and Metabolism 20171023869–3903. ( 10.1210/jc.2017-01658) [DOI] [PubMed] [Google Scholar]
- 17.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SEet al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. British Medical Journal 2021372n71. ( 10.1136/bmj.n71) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stahlman J, Britto M, Fitzpatrick S, McWhirter C, Testino SA, Brennan JJ, Zumbrunnen TL. Effects of skin washing on systemic absorption of testosterone in hypogonadal males after administration of 1.62% testosterone gel. Current Medical Research and Opinion 201228271–279. ( 10.1185/03007995.2011.652256) [DOI] [PubMed] [Google Scholar]
- 19.Chiang HS, Cho SL, Lin YC, Hwang TI. Testosterone gel monotherapy improves sexual function of hypogonadal men mainly through restoring erection: evaluation by IIEF score. Urology 200973762–766. ( 10.1016/j.urology.2008.10.019) [DOI] [PubMed] [Google Scholar]
- 20.Meikle AW, Matthias D, Hoffman AR. Transdermal testosterone gel: pharmacokinetics, efficacy of dosing and application site in hypogonadal men. BJU International 200493789–795. ( 10.1111/j.1464-410X.2003.04750.x) [DOI] [PubMed] [Google Scholar]
- 21.Miller J, Britto M, Fitzpatrick S, McWhirter C, Testino SA, Brennan JJ, Zumbrunnen TL. Pharmacokinetics and relative bioavailability of absorbed testosterone after administration of a 1.62% testosterone gel to different application sites in men with hypogonadism. Endocrine Practice 201117574–583. ( 10.4158/EP10192.OR) [DOI] [PubMed] [Google Scholar]
- 22.Wang C, Berman N, Longstreth JA, Chuapoco B, Hull L, Steiner B, Faulkner S, Dudley RE, Swerdloff RS. Pharmacokinetics of transdermal testosterone gel in hypogonadal men: application of gel at one site versus four sites: a General Clinical Research Center Study. Journal of Clinical Endocrinology and Metabolism 200085964–969. ( 10.1210/jcem.85.3.6437) [DOI] [PubMed] [Google Scholar]
- 23.Swerdloff RS, Wang C, Cunningham G, Dobs A, Iranmanesh A, Matsumoto AM, Snyder PJ, Weber T, Longstreth J, Berman N. Long-term pharmacokinetics of transdermal testosterone gel in hypogonadal men. Journal of Clinical Endocrinology and Metabolism 2000854500–4510. ( 10.1210/jcem.85.12.7045) [DOI] [PubMed] [Google Scholar]
- 24.Belkoff L, Brock G, Carrara D, Neijber A, Ando M, Mitchel J. Efficacy and safety of testosterone replacement gel for treating hypogonadism in men: phase III open-label studies. Andrologia 201850 e12801. ( 10.1111/and.12801) [DOI] [PubMed] [Google Scholar]
- 25.Morgentaler A, McGettigan J, Xiang Q, Danoff TM, Gould EM. Pharmacokinetics and drying time of testosterone 2% gel in men with hypogonadism: a multicenter, open-label, single-arm trial. International Journal of Impotence Research 20152741–45. ( 10.1038/ijir.2014.28) [DOI] [PubMed] [Google Scholar]
- 26.Cunningham G, Belkoff L, Brock G, Efros M, Gittelman M, Carrara D, Neijber A, Ando M, Mitchel J. Efficacy and safety of a new topical testosterone replacement gel therapy for the treatment of male hypogonadism. Endocrine Practice 201723557–565. ( 10.4158/EP161665.OR) [DOI] [PubMed] [Google Scholar]
- 27.Conway AJ, Boylan LM, Howe C, Ross G, Handelsman DJ. Randomized clinical trial of testosterone replacement therapy in hypogonadal men. International Journal of Andrology 198811247–264. ( 10.1111/j.1365-2605.1988.tb00999.x) [DOI] [PubMed] [Google Scholar]
- 28.Nieschlag E, Cuppers HJ, Wiegelmann W, Wickings EJ. Bioavailability and LH-suppressing effect of different testosterone preparations in normal and hypogonadal men. Hormone Research 19767138–145. ( 10.1159/000178721) [DOI] [PubMed] [Google Scholar]
- 29.Snyder PJ, Lawrence DA. Treatment of male hypogonadism with testosterone enanthate. Journal of Clinical Endocrinology and Metabolism 1980511335–1339. ( 10.1210/jcem-51-6-1335) [DOI] [PubMed] [Google Scholar]
- 30.Di Luigi L, Sgrò P, Aversa A, Migliaccio S, Bianchini S, Botrè F, Romanelli F, Lenzi A. Concerns about serum androgens monitoring during testosterone replacement treatments in hypogonadal male athletes: a pilot study. Journal of Sexual Medicine 20129873–886. ( 10.1111/j.1743-6109.2011.02600.x) [DOI] [PubMed] [Google Scholar]
- 31.Turner A, Chen TC, Barber TW, Malabanan AO, Holick MF, Tangpricha V. Testosterone increases bone mineral density in female-to-male transsexuals: a case series of 15 subjects. Clinical Endocrinology 200461560–566. ( 10.1111/j.1365-2265.2004.02125.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nieschlag E, Büchter D, Von Eckardstein S, Abshagen K, Simoni M, Behre HM. Repeated intramuscular injections of testosterone undecanoate for substitution therapy in hypogonadal men. Clinical Endocrinology 199951757–763. ( 10.1046/j.1365-2265.1999.00881.x) [DOI] [PubMed] [Google Scholar]
- 33.Saad F, Aversa A, Isidori AM, Zafalon L, Zitzmann M, Gooren L. Onset of effects of testosterone treatment and time span until maximum effects are achieved. European Journal of Endocrinology 2011165675–685. ( 10.1530/EJE-11-0221) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gu YQ, Ge ZY, Zhang GY, Bremner WJ. Quantitative and qualitative changes in serum luteinizing hormone after injectable testosterone undecanoate treatment in hypogonadal men. Asian Journal of Andrology 2000265–71. [PubMed] [Google Scholar]
- 35.Lood Y, Aardal-Eriksson E, Webe C, Ahlner J, Ekman B, Wahlberg J. Relationship between testosterone in serum, saliva and urine during treatment with intramuscular testosterone undecanoate in gender dysphoria and male hypogonadism. Andrology 2018686–93. ( 10.1111/andr.12435) [DOI] [PubMed] [Google Scholar]
- 36.Shankara Narayana N, Ly LP, Jayadev V, Fennell C, Savkovic S, Conway AJ, Handelsman DJ. Optimal injection interval for testosterone undecanoate treatment of hypogonadal and transgender men. Endocrine Connections 202110758–766. ( 10.1530/EC-21-0109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.de Ronde W.Testosterone gel for the treatment of male hypogonadism. Expert Opinion on Biological Therapy 20099249–253. ( 10.1517/14712590802653593) [DOI] [PubMed] [Google Scholar]
- 38.de Ronde W, Vogel S, Bui HN, Heijboer AC. Reduction in 24-hour plasma testosterone levels in subjects who showered 15 or 30 minutes after application of testosterone gel. Pharmacotherapy 201131248–252. ( 10.1592/phco.31.3.248) [DOI] [PubMed] [Google Scholar]
- 39.von Eckardstein S, Nieschlag E. Treatment of male hypogonadism with testosterone undecanoate injected at extended intervals of 12 weeks: a phase II study. Journal of Andrology 200223419–425. [PubMed] [Google Scholar]
- 40.Saad F, Haider A, Doros G, Traish A. Long-term treatment of hypogonadal men with testosterone produces substantial and sustained weight loss. Obesity 2013211975–1981. ( 10.1002/oby.20407) [DOI] [PubMed] [Google Scholar]
- 41.Aydogan U, Aydogdu A, Akbulut H, Sonmez A, Yuksel S, Basaran Y, Uzun O, Bolu E, Saglam K. Increased frequency of anxiety, depression, quality of life and sexual life in young hypogonadotropic hypogonadal males and impacts of testosterone replacement therapy on these conditions. Endocrine Journal 2012591099–1105. ( 10.1507/endocrj.ej12-0134) [DOI] [PubMed] [Google Scholar]
- 42.Chiang HS, Hwang TI, Hsui YS, Lin YC, Chen HE, Chen GC, Liao CH. Transdermal testosterone gel increases serum testosterone levels in hypogonadal men in Taiwan with improvements in sexual function. International Journal of Impotence Research 200719411–417. ( 10.1038/sj.ijir.3901562) [DOI] [PubMed] [Google Scholar]
- 43.Efros M, Carrara D, Neijber A. The efficacy, bioavailability and safety of a novel hydroalcoholic testosterone gel 2% in hypogonadal men: results from phase II open-label studies. Andrologia 201648637–645. ( 10.1111/and.12493) [DOI] [PubMed] [Google Scholar]
- 44.Lašaitė L, Čeponis J, Preikša RT, Žilaitienė B. Effects of two-year testosterone replacement therapy on cognition, emotions and quality of life in young and middle-aged hypogonadal men. Andrologia 201749 e12633. ( 10.1111/and.12633) [DOI] [PubMed] [Google Scholar]
- 45.McNicholas TA, Dean JD, Mulder H, Carnegie C, Jones NA. A novel testosterone gel formulation normalizes androgen levels in hypogonadal men, with improvements in body composition and sexual function. BJU International 20039169–74. ( 10.1046/j.1464-410x.2003.04016.x) [DOI] [PubMed] [Google Scholar]
- 46.Miner MM, Bhattacharya RK, Blick G, Kushner H, Khera M. 12-month observation of testosterone replacement effectiveness in a general population of men. Postgraduate Medicine 20131258–18. ( 10.3810/pgm.2013.03.2637) [DOI] [PubMed] [Google Scholar]
- 47.Mulhall JP, Valenzuela R, Aviv N, Parker M. Effect of testosterone supplementation on sexual function in hypogonadal men with erectile dysfunction. Urology 200463348–352; discussion 52–53. ( 10.1016/j.urology.2003.09.074) [DOI] [PubMed] [Google Scholar]
- 48.O’Connor DB, Archer J, Hair WM, Wu FC. Activational effects of testosterone on cognitive function in men. Neuropsychologia 2001391385–1394. ( 10.1016/s0028-3932(0100067-7) [DOI] [PubMed] [Google Scholar]
- 49.Ramasamy R, Wilken N, Scovell JM, Lipshultz LI. Effect of testosterone supplementation on symptoms in men with hypogonadism. European Urology 201567176–177. ( 10.1016/j.eururo.2014.08.048) [DOI] [PubMed] [Google Scholar]
- 50.Wang C, Alexander G, Berman N, Salehian B, Davidson T, McDonald V, Steiner B, Hull L, Callegari C, Swerdloff RS. Testosterone replacement therapy improves mood in hypogonadal men – a Clinical Research Center Study. Journal of Clinical Endocrinology and Metabolism 1996813578–3583. ( 10.1210/jcem.81.10.8855804) [DOI] [PubMed] [Google Scholar]
- 51.Wang C, Swerdloff RS, Iranmanesh A, Dobs A, Snyder PJ, Cunningham G, Matsumoto AM, Weber T, Berman N. Effects of transdermal testosterone gel on bone turnover markers and bone mineral density in hypogonadal men. Clinical Endocrinology 200154739–750. ( 10.1046/j.1365-2265.2001.01271.x) [DOI] [PubMed] [Google Scholar]
- 52.Wang C, Swerdloff R, Kipnes M, Matsumoto AM, Dobs AS, Cunningham G, Katznelson L, Weber TJ, Friedman TC, Snyder Pet al. New testosterone buccal system (Striant) delivers physiological testosterone levels: pharmacokinetics study in hypogonadal men. Journal of Clinical Endocrinology and Metabolism 2004893821–3829. ( 10.1210/jc.2003-031866) [DOI] [PubMed] [Google Scholar]
- 53.Zitzmann M, Mattern A, Hanisch J, Gooren L, Jones H, Maggi M. IPASS: a study on the tolerability and effectiveness of injectable testosterone undecanoate for the treatment of male hypogonadism in a worldwide sample of 1438 men. Journal of Sexual Medicine 201310579–588. ( 10.1111/j.1743-6109.2012.02853.x) [DOI] [PubMed] [Google Scholar]
- 54.Cherrier MM, Craft S, Matsumoto AH. Cognitive changes associated with supplementation of testosterone or dihydrotestosterone in mildly hypogonadal men: a preliminary report. Journal of Andrology 200324568–576. ( 10.1002/j.1939-4640.2003.tb02708.x) [DOI] [PubMed] [Google Scholar]
- 55.Khera M, Bhattacharya RK, Blick G, Kushner H, Nguyen D, Miner MM. Improved sexual function with testosterone replacement therapy in hypogonadal men: real-world data from the Testim Registry in the United States (TRiUS). Journal of Sexual Medicine 201183204–3213. ( 10.1111/j.1743-6109.2011.02436.x) [DOI] [PubMed] [Google Scholar]
- 56.Seftel AD, Mack RJ, Secrest AR, Smith TM. Restorative increases in serum testosterone levels are significantly correlated to improvements in sexual functioning. Journal of Andrology 200425963–972. ( 10.1002/j.1939-4640.2004.tb03169.x) [DOI] [PubMed] [Google Scholar]
- 57.Kirisawa T, Ichihara K, Sakai Y, Morooka D, Iyoki T, Masumori N. Physical and psychological effects of gender-affirming hormonal treatment using intramuscular testosterone enanthate in Japanese transgender men. Sexual Medicine 20219 100306. ( 10.1016/j.esxm.2020.100306) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mueller A, Kiesewetter F, Binder H, Beckmann MW, Dittrich R. Long-term administration of testosterone undecanoate every 3 months for testosterone supplementation in female-to-male transsexuals. Journal of Clinical Endocrinology and Metabolism 2007923470–3475. ( 10.1210/jc.2007-0746) [DOI] [PubMed] [Google Scholar]
- 59.Pelusi C, Costantino A, Martelli V, Lambertini M, Bazzocchi A, Ponti F, Battista G, Venturoli S, Meriggiola MC. Effects of three different testosterone formulations in female-to-male transsexual persons. Journal of Sexual Medicine 2014113002–3011. ( 10.1111/jsm.12698) [DOI] [PubMed] [Google Scholar]
- 60.Bolu E, Sonmez A, Tapan S, Taslipinar A, Aydogdu A, Meric C, Basaran Y, Uckaya G, Serdar M, Kurt Iet al. HDL cholesterol subfractions and the effect of testosterone replacement in hypogonadism. Hormone and Metabolic Research 201345443–448. ( 10.1055/s-0033-1343447) [DOI] [PubMed] [Google Scholar]
- 61.Gava G, Armillotta F, Pillastrini P, Giagio S, Alvisi S, Mancini I, Morselli PG, Seracchioli R, Meriggiola MC. A randomized double-blind placebo-controlled pilot trial on the effects of testosterone undecanoate plus dutasteride or placebo on muscle strength, body composition, and metabolic profile in transmen. Journal of Sexual Medicine 202118646–655. ( 10.1016/j.jsxm.2020.12.015) [DOI] [PubMed] [Google Scholar]
- 62.Goh HH, Loke DF, Ratnam SS. The impact of long-term testosterone replacement therapy on lipid and lipoprotein profiles in women. Maturitas 19952165–70. ( 10.1016/0378-5122(9400861-z) [DOI] [PubMed] [Google Scholar]
- 63.Jacobeit JW, Gooren LJ, Schulte HM. Safety aspects of 36 months of administration of long-acting intramuscular testosterone undecanoate for treatment of female-to-male transgender individuals. European Journal of Endocrinology 2009161795–798. ( 10.1530/EJE-09-0412) [DOI] [PubMed] [Google Scholar]
- 64.Jacobeit JW, Gooren LJ, Schulte HM. Long-acting intramuscular testosterone undecanoate for treatment of female-to-male transgender individuals. Journal of Sexual Medicine 200741479–1484. ( 10.1111/j.1743-6109.2007.00556.x) [DOI] [PubMed] [Google Scholar]
- 65.Kaufman JM, Miller MG, Garwin JL, Fitzpatrick S, McWhirter C, Brennan JJ. Efficacy and safety study of 1.62% testosterone gel for the treatment of hypogonadal men. Journal of Sexual Medicine 201182079–2089. ( 10.1111/j.1743-6109.2011.02265.x) [DOI] [PubMed] [Google Scholar]
- 66.Malkin CJ, Pugh PJ, Jones RD, Kapoor D, Channer KS, Jones TH. The effect of testosterone replacement on endogenous inflammatory cytokines and lipid profiles in hypogonadal men. Journal of Clinical Endocrinology and Metabolism 2004893313–3318. ( 10.1210/jc.2003-031069) [DOI] [PubMed] [Google Scholar]
- 67.Minnemann T, Schubert M, Freude S, Hübler D, Gouni-Berthold I, Schumann C, Christoph A, Oettel M, Ernst M, Mellinger Uet al. Comparison of a new long-acting testosterone undecanoate formulation vs testosterone enanthate for intramuscular androgen therapy in male hypogonadism. Journal of Endocrinological Investigation 200831718–723. ( 10.1007/BF03346421) [DOI] [PubMed] [Google Scholar]
- 68.Cupisti S, Giltay EJ, Gooren LJ, Kronawitter D, Oppelt PG, Beckmann MW, Dittrich R, Mueller A. The impact of testosterone administration to female-to-male transsexuals on insulin resistance and lipid parameters compared with women with polycystic ovary syndrome. Fertility and Sterility 2010942647–2653. ( 10.1016/j.fertnstert.2010.03.048) [DOI] [PubMed] [Google Scholar]
- 69.Kronawitter D, Gooren LJ, Zollver H, Oppelt PG, Beckmann MW, Dittrich R, Mueller A. Effects of transdermal testosterone or oral dydrogesterone on hypoactive sexual desire disorder in transsexual women: results of a pilot study. European Journal of Endocrinology 2009161363–368. ( 10.1530/EJE-09-0265) [DOI] [PubMed] [Google Scholar]
- 70.Mueller A, Dittrich R, Binder H, Kuehnel W, Maltaris T, Hoffmann I, Beckmann MW. High dose estrogen treatment increases bone mineral density in male-to-female transsexuals receiving gonadotropin-releasing hormone agonist in the absence of testosterone. European Journal of Endocrinology 2005153107–113. ( 10.1530/eje.1.01943) [DOI] [PubMed] [Google Scholar]
- 71.Mueller A, Haeberle L, Zollver H, Claassen T, Kronawitter D, Oppelt PG, Cupisti S, Beckmann MW, Dittrich R. Effects of intramuscular testosterone undecanoate on body composition and bone mineral density in female-to-male transsexuals. Journal of Sexual Medicine 201073190–3198. ( 10.1111/j.1743-6109.2010.01912.x) [DOI] [PubMed] [Google Scholar]
- 72.Sonmez A, Haymana C, Aydogdu A, Tapan S, Basaran Y, Meric C, Baskoy K, Dinc M, Yazici M, Taslipinar Aet al. Endothelial dysfunction, insulin resistance and inflammation in congenital hypogonadism, and the effect of testosterone replacement. Endocrine Journal 201562605–613. ( 10.1507/endocrj.EJ15-0125) [DOI] [PubMed] [Google Scholar]
- 73.van Velzen DM, Nota NM, Simsek S, Conemans EB, T’Sjoen G, den Heijer M. Variation in sensitivity and rate of change in body composition: steps toward individualizing transgender care. European Journal of Endocrinology 2020183529–536. ( 10.1530/EJE-20-0609) [DOI] [PubMed] [Google Scholar]
- 74.van Velzen DM, Paldino A, Klaver M, Nota NM, Defreyne J, Hovingh GK, Thijs A, Simsek S, T’Sjoen G, den Heijer M. Cardiometabolic effects of testosterone in transmen and estrogen plus cyproterone acetate in transwomen. Journal of Clinical Endocrinology and Metabolism 20191041937–1947. ( 10.1210/jc.2018-02138) [DOI] [PubMed] [Google Scholar]
- 75.Wu XY, Mao JF, Lu SY, Zhang Q, Shi YF. Testosterone replacement therapy improves insulin sensitivity and decreases high sensitivity C-reactive protein levels in hypogonadotropic hypogonadal young male patients. Chinese Medical Journal 20091222846–2850. [PubMed] [Google Scholar]
- 76.Wolf J, Keipert D, Motazedi H, Ernst M, Nettleship J, Gooren L. Effectiveness and tolerability of parenteral testosterone undecanoate: a post-marketing surveillance study. Aging Male 201720225–234. ( 10.1080/13685538.2017.1364234) [DOI] [PubMed] [Google Scholar]
- 77.Wierckx K, Van Caenegem E, Schreiner T, Haraldsen I, Fisher AD, Toye K, Kaufman JM, T’Sjoen G. Cross-sex hormone therapy in trans persons is safe and effective at short-time follow-up: results from the European network for the investigation of gender incongruence. Journal of Sexual Medicine 2014111999–2011. ( 10.1111/jsm.12571) [DOI] [PubMed] [Google Scholar]
- 78.Wang C, Cunningham G, Dobs A, Iranmanesh A, Matsumoto AM, Snyder PJ, Weber T, Berman N, Hull L, Swerdloff RS. Long-term testosterone gel (AndroGel) treatment maintains beneficial effects on sexual function and mood, lean and fat mass, and bone mineral density in hypogonadal men. Journal of Clinical Endocrinology and Metabolism 2004892085–2098. ( 10.1210/jc.2003-032006) [DOI] [PubMed] [Google Scholar]
- 79.Yassin AA, Doros G. Testosterone therapy in hypogonadal men results in sustained and clinically meaningful weight loss. Clinical Obesity 2013373–83. ( 10.1111/cob.12022) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Emi Y, Adachi M, Sasaki A, Nakamura Y, Nakatsuka M. Increased arterial stiffness in female-to-male transsexuals treated with androgen. Journal of Obstetrics and Gynaecology Research 200834890–897. ( 10.1111/j.1447-0756.2008.00857.x) [DOI] [PubMed] [Google Scholar]
- 81.Stokes 3rd J, Kannel WB, Wolf PA, D’Agostino RB, Cupples LA. Blood pressure as a risk factor for cardiovascular disease. The Framingham study – 30 years of follow-up. Hypertension 198913 (5Supplement) I13–I18. ( 10.1161/01.hyp.13.5_suppl.i13) [DOI] [PubMed] [Google Scholar]
- 82.Cholesterol Treatment Trialists’ (CTT) Collaboration, Baigent C, Blackwell L, Emberson J, Holland LE, Reith C, Bhala N, Peto R, Barnes EH, Keech Aet al. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet 20103761670–1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Traish AM, Saad F, Feeley RJ, Guay A. The dark side of testosterone deficiency: III. Cardiovascular disease. Journal of Andrology 200930477–494. ( 10.2164/jandrol.108.007245) [DOI] [PubMed] [Google Scholar]
- 84.Benito M, Vasilic B, Wehrli FW, Bunker B, Wald M, Gomberg B, Wright AC, Zemel B, Cucchiara A, Snyder PJ. Effect of testosterone replacement on trabecular architecture in hypogonadal men. Journal of Bone and Mineral Research 2005201785–1791. ( 10.1359/JBMR.050606) [DOI] [PubMed] [Google Scholar]
- 85.De Rosa M, Paesano L, Nuzzo V, Zarrilli S, Del Puente A, Oriente P, Lupoli G. Bone mineral density and bone markers in hypogonadotropic and hypergonadotropic hypogonadal men after prolonged testosterone treatment. Journal of Endocrinological Investigation 200124246–252. ( 10.1007/BF03343854) [DOI] [PubMed] [Google Scholar]
- 86.Goh HH, Ratnam SS. Effects of hormone deficiency, androgen therapy and calcium supplementation on bone mineral density in female transsexuals. Maturitas 19972645–52. ( 10.1016/s0378-5122(9601073-0) [DOI] [PubMed] [Google Scholar]
- 87.Leifke E, Körner HC, Link TM, Behre HM, Peters PE, Nieschlag E. Effects of testosterone replacement therapy on cortical and trabecular bone mineral density, vertebral body area and paraspinal muscle area in hypogonadal men. European Journal of Endocrinology 199813851–58. ( 10.1530/eje.0.1380051) [DOI] [PubMed] [Google Scholar]
- 88.Lips P, van Kesteren PJ, Asscheman H, Gooren LJ. The effect of androgen treatment on bone metabolism in female-to-male transsexuals. Journal of Bone and Mineral Research 1996111769–1773. ( 10.1002/jbmr.5650111121) [DOI] [PubMed] [Google Scholar]
- 89.Medras M, Jankowska EA, Rogucka E. Effects of long-term testosterone substitutive therapy on bone mineral content in men with hypergonadotrophic hypogonadism. Andrologia 20013347–52. ( 10.1046/j.1439-0272.2001.00417.x) [DOI] [PubMed] [Google Scholar]
- 90.Schubert M, Bullmann C, Minnemann T, Reiners C, Krone W, Jockenhövel F. Osteoporosis in male hypogonadism: responses to androgen substitution differ among men with primary and secondary hypogonadism. Hormone Research 20036021–28. ( 10.1159/000070823) [DOI] [PubMed] [Google Scholar]
- 91.Tahani N, Nieddu L, Prossomariti G, Spaziani M, Granato S, Carlomagno F, Anzuini A, Lenzi A, Radicioni AF, Romagnoli E. Long-term effect of testosterone replacement therapy on bone in hypogonadal men with Klinefelter syndrome. Endocrine 201861327–335. ( 10.1007/s12020-018-1604-6) [DOI] [PubMed] [Google Scholar]
- 92.Van Caenegem E, Wierckx K, Taes Y, Schreiner T, Vandewalle S, Toye K, Lapauw B, Kaufman JM, T’Sjoen G. Body composition, bone turnover, and bone mass in trans men during testosterone treatment: 1-year follow-up data from a prospective case-controlled study (ENIGI). European Journal of Endocrinology 2015172163–171. ( 10.1530/EJE-14-0586) [DOI] [PubMed] [Google Scholar]
- 93.van den Bergh JP, Hermus AR, Spruyt AI, Sweep CG, Corstens FH, Smals AG. Bone mineral density and quantitative ultrasound parameters in patients with Klinefelter’s syndrome after long-term testosterone substitution. Osteoporosis International 20011255–62. ( 10.1007/s001980170158) [DOI] [PubMed] [Google Scholar]
- 94.Vlot MC, Wiepjes CM, de Jongh RT, T’Sjoen G, Heijboer AC, den Heijer M. Gender-affirming hormone treatment decreases bone turnover in transwomen and older transmen. Journal of Bone and Mineral Research 2019341862–1872. ( 10.1002/jbmr.3762) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Haraldsen IR, Haug E, Falch J, Egeland T, Opjordsmoen S. Cross-sex pattern of bone mineral density in early onset gender identity disorder. Hormones and Behavior 200752334–343. ( 10.1016/j.yhbeh.2007.05.012) [DOI] [PubMed] [Google Scholar]
- 96.Shigehara K, Konaka H, Koh E, Nakashima K, Iijima M, Nohara T, Izumi K, Kitagawa Y, Kadono Y, Sugimoto Ket al. Effects of testosterone replacement therapy on hypogonadal men with osteopenia or osteoporosis: a subanalysis of a prospective randomized controlled study in Japan (EARTH study). Aging Male 201720139–145. ( 10.1080/13685538.2017.1303829) [DOI] [PubMed] [Google Scholar]
- 97.Swerdloff RS, Wang C. Three-year follow-up of androgen treatment in hypogonadal men: preliminary report with testosterone gel. Aging Male 20036207–211. ( 10.1080/tam.6.3.207.211) [DOI] [PubMed] [Google Scholar]
- 98.Rodriguez-Tolrà J, Torremadé Barreda J, del Rio L, di Gregorio S, Franco Miranda E. Effects of testosterone treatment on body composition in males with testosterone deficiency syndrome. Aging Male 201316184–190. ( 10.3109/13685538.2013.839648) [DOI] [PubMed] [Google Scholar]
- 99.Shiraishi K, Oka S, Matsuyama H. Assessment of quality of life during gonadotrophin treatment for male hypogonadotrophic hypogonadism. Clinical Endocrinology 201481259–265. ( 10.1111/cen.12435) [DOI] [PubMed] [Google Scholar]
- 100.Meriggiola MC, Armillotta F, Costantino A, Altieri P, Saad F, Kalhorn T, Perrone AM, Ghi T, Pelusi C, Pelusi G. Effects of testosterone undecanoate administered alone or in combination with letrozole or dutasteride in female to male transsexuals. Journal of Sexual Medicine 200852442–2453. ( 10.1111/j.1743-6109.2008.00909.x) [DOI] [PubMed] [Google Scholar]
- 101.Meyer G, Mayer M, Mondorf A, Fluegel AK, Herrmann E, Bojunga J. Safety and rapid efficacy of guideline-based gender affirming hormone therapy: an analysis of 388 individuals diagnosed with gender dysphoria. European Journal of Endocrinology 2020182149–156. ( 10.1530/EJE-19-0463) [DOI] [PubMed] [Google Scholar]
- 102.Wierckx K, Van de Peer F, Verhaeghe E, Dedecker D, Van Caenegem E, Toye K, Kaufman JM, T’Sjoen G. Short- and long-term clinical skin effects of testosterone treatment in trans men. Journal of Sexual Medicine 201411222–229. ( 10.1111/jsm.12366) [DOI] [PubMed] [Google Scholar]
- 103.Park JA, Carter EE, Larson AR. Risk factors for acne development in the first 2 years after initiating masculinizing testosterone therapy among transgender men. Journal of the American Academy of Dermatology 201981617–618. ( 10.1016/j.jaad.2018.12.040) [DOI] [PubMed] [Google Scholar]
- 104.Motta G, Crespi C, Mineccia V, Brustio PR, Manieri C, Lanfranco F. Does testosterone treatment increase anger expression in a population of transgender men? Journal of Sexual Medicine 20181594–101. ( 10.1016/j.jsxm.2017.11.004) [DOI] [PubMed] [Google Scholar]
- 105.Dobs A, Norwood P, Potts S, Gould E, Chitra S. Testosterone 2% gel can normalize testosterone concentrations in men with low testosterone regardless of body mass index. Journal of Sexual Medicine 201411857–864. ( 10.1111/jsm.12411) [DOI] [PubMed] [Google Scholar]
- 106.Jockenhövel F, Minnemann T, Schubert M, Freude S, Hübler D, Schumann C, Christoph A, Ernst M. Comparison of long-acting testosterone undecanoate formulation versus testosterone enanthate on sexual function and mood in hypogonadal men. European Journal of Endocrinology 2009160815–819. ( 10.1530/EJE-08-0830) [DOI] [PubMed] [Google Scholar]
- 107.Costantino A, Cerpolini S, Alvisi S, Morselli PG, Venturoli S, Meriggiola MC. A prospective study on sexual function and mood in female-to-male transsexuals during testosterone administration and after sex reassignment surgery. Journal of Sex and Marital Therapy 201339321–335. ( 10.1080/0092623X.2012.736920) [DOI] [PubMed] [Google Scholar]
- 108.Ohlander SJ, Varghese B, Pastuszak AW. Erythrocytosis following testosterone therapy. Sexual Medicine Reviews 2018677–85. ( 10.1016/j.sxmr.2017.04.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Defreyne J, T’Sjoen G, Bouman WP, Brewin N, Arcelus J. Prospective evaluation of self-reported aggression in transgender persons. Journal of Sexual Medicine 201815768–776. ( 10.1016/j.jsxm.2018.03.079) [DOI] [PubMed] [Google Scholar]
- 110.Levcikova M, Breza Jr J, Luha J, Dubravicky J, Kovacova E, Fillo J. Testosterone replacement therapy (TRT) and its effect on bone marrow. How serious is it and is there a true polyglobulia? Bratislavske Lekarske Listy 2017118654–657. ( 10.4149/BLL_2017_124) [DOI] [PubMed] [Google Scholar]
- 111.Defreyne J, Vantomme B, Van Caenegem E, Wierckx K, De Blok CJM, Klaver M, Nota NM, Van Dijk D, Wiepjes CM, Den Heijer Met al. Prospective evaluation of hematocrit in gender-affirming hormone treatment: results from European Network for the Investigation of Gender Incongruence. Andrology 20186446–454. ( 10.1111/andr.12485) [DOI] [PubMed] [Google Scholar]
- 112.Corona G, Maseroli E, Maggi M. Injectable testosterone undecanoate for the treatment of hypogonadism. Expert Opinion on Pharmacotherapy 2014151903–1926. ( 10.1517/14656566.2014.944896) [DOI] [PubMed] [Google Scholar]
- 113.Traish AM, Haider A, Haider KS, Doros G, Saad F. Long-term testosterone therapy improves cardiometabolic function and reduces risk of cardiovascular disease in men with hypogonadism: a real-life observational registry study setting comparing treated and untreated (control) groups. Journal of Cardiovascular Pharmacology and Therapeutics 201722414–433. ( 10.1177/1074248417691136) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Maggi M, Wu FCW, Jones TH, Jackson G, Behre HM, Hackett G, Martin-Morales A, Balercia G, Dobs AS, Arver STEet al. Testosterone treatment is not associated with increased risk of adverse cardiovascular events: results from the Registry of Hypogonadism in Men (RHYME). International Journal of Clinical Practice 201670843–852. ( 10.1111/ijcp.12876) [DOI] [PubMed] [Google Scholar]
- 115.Jockenhövel F, Minnemann T, Schubert M, Freude S, Hübler D, Schumann C, Christoph A, Gooren L, Ernst M. Timetable of effects of testosterone administration to hypogonadal men on variables of sex and mood. Aging Male 200912113–118. ( 10.3109/13685530903322858) [DOI] [PubMed] [Google Scholar]
- 116.Transvisie, Nederlandse Hypofyse Stichting, Stichting Zaadbalkanker, Nederlandse Klinefelter Vereniging. Onderzoek naar gebruik, ervaringen en keuzegrondslag testosteronsuppletie, 2020. (available at: https://www.transvisie.nl/wp-content/uploads/2020/06/rapport-onderzoek-gebruik-ervaringen-en-keuzegrondslag-testosteronsuppletie.pdf) [Google Scholar]
- 117.Madsen MC, van Dijk D, Wiepjes CM, Conemans EB, Thijs A, den Heijer M. Erythrocytosis in a large cohort of trans men using testosterone: a long-term follow-up study on prevalence, determinants and exposure years. Journal of Clinical Endocrinology and Metabolism 20211061710–1717. ( 10.1210/clinem/dgab089) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Gooren LJ, Wierckx K, Giltay EJ. Cardiovascular disease in transsexual persons treated with cross-sex hormones: reversal of the traditional sex difference in cardiovascular disease pattern. European Journal of Endocrinology 2014170809–819. ( 10.1530/EJE-14-0011) [DOI] [PubMed] [Google Scholar]
- 119.Tracz MJ, Sideras K, Bolona ER, Haddad RM, Kennedy CC, Uraga MV, Caples SM, Erwin PJ, Montori VM. Testosterone use in men and its effects on bone health. A systematic review and meta-analysis of randomized placebo-controlled trials. Journal of Clinical Endocrinology and Metabolism 2006912011–2016. ( 10.1210/jc.2006-0036) [DOI] [PubMed] [Google Scholar]
- 120.Elliott J, Kelly SE, Millar AC, Peterson J, Chen L, Johnston A, Kotb A, Skidmore B, Bai Z, Mamdani Met al. Testosterone therapy in hypogonadal men: a systematic review and network meta-analysis. BMJ Open 20177 e015284. ( 10.1136/bmjopen-2016-015284) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Corona G, Rastrelli G, Morgentaler A, Sforza A, Mannucci E, Maggi M. Meta-analysis of results of testosterone therapy on sexual function based on international index of erectile function scores. European Urology 2017721000–1011. ( 10.1016/j.eururo.2017.03.032) [DOI] [PubMed] [Google Scholar]
- 122.Bhasin S, Travison TG, O’Brien L, MacKrell J, Krishnan V, Ouyang H, Pencina K, Basaria S. Contributors to the substantial variation in on-treatment testosterone levels in men receiving transdermal testosterone gels in randomized trials. Andrology 20186151–157. ( 10.1111/andr.12428) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



 This work is licensed under a
This work is licensed under a