Abstract
Objective
Children with suprasellar brain damage are at risk of hypothalamic dysfunction (HD). HD may lead to decreased resting energy expenditure (REE). Decreased REE, however, is not present in all children with HD. Our aim was to assess which children suspect for HD have low REE, and its association with clinical severity of HD or radiological hypothalamic damage.
Patients and methods
A retrospective cohort study was performed. Measured REE (mREE) of children at risk of HD was compared to predicted REE (pREE). Low REE was defined as mREE <90% of predicted. The mREE/pREE quotient was associated to a clinical score for HD symptoms and to radiological hypothalamic damage.
Results
In total, 67 children at risk of HD (96% brain tumor diagnosis) with a mean BMI SDS of +2.3 ± 1.0 were included. Of these, 45 (67.2%) had low mREE. Children with severe HD had a significant lower mean mREE/pREE quotient compared to children with no, mild, or moderate HD. Mean mREE/pREE quotient of children with posterior hypothalamic damage was significantly lower compared to children with no or anterior damage. Tumor progression or tumor recurrence, severe clinical HD, and panhypopituitarism with diabetes insipidus (DI) were significant risk factors for reduced REE.
Conclusion
REE may be lowered in children with hypothalamic damage and is associated to the degree of clinical HD. REE is, however, not lowered in all children suspect for HD. For children with mild or moderate clinical HD symptoms, REE measurements may be useful to distinguish between those who may benefit from obesity treatment that increases REE from those who would be better helped using other obesity interventions.
Keywords: hypothalamic obesity, suprasellar tumor, resting energy expenditure, posterior hypothalamic damage
Introduction
Hypothalamic dysfunction (HD) during childhood may be present in children with craniopharyngioma, chiasmatic hypothalamic glioma, germinoma, as well as in children with certain genetic syndromes, such as Prader Willi syndrome (1, 2, 3, 4, 5).
Due to the fact that the hypothalamus plays an essential role in endocrine balance, nutritional habits, and body composition, HD may lead to hypothalamic obesity (HO) (5, 6). In children with acquired brain damage, however, many other factors may induce weight gain, such as impaired mobility, visual impairment, disrupted sleep, use of medication, and poor adherence to dietary or physical activity guidelines (5, 7, 8, 9, 10, 11). To enable adequate counseling, it is important to distinguish HD from other causes of weight gain.
Hypothalamic weight gain is caused by a disturbance of the essential parts of the hypothalamus: the ventromedial hypothalamus (VMH), paraventricular nuclei (PVN), arcuate nucleus (ARC), the lateral hypothalamic area, and posterior hypothalamic area (12). Disinhibition of vagal tone due to damage to the paraventricular nucleus (sympathetic) and suprachiasmatic nucleus (SCN) (parasympathetic) negatively influences lipogenesis and beta cell secretion of insulin, resulting in hyperinsulinism and fat storage. Damage to the ventromedial nucleus causes loss of satiety through the inability to integrate afferent hormonal signals including leptin, insulin, and gut hormones like GLP-1. Additionally, the disruption of autonomic function damages the biologic clock and therefore the circadian activity (13). Next to energy balance, the hypothalamus also plays an essential role in the body’s temperature regulation, thirst sensation, and pituitary regulation (5).
In children with HD, hyperphagia was long thought to be the major cause of HO. However, hyperphagia is not present in all children with HO, and recent studies suggest that hyperphagia is significant risk factor but not a requisite for the development of HO (14, 15, 16). Changes in energy expenditure have been hypothesized to play a major role in weight gain and development of HO. Resting energy expenditure (REE) is the energy expenditure of the basic physiological functions necessary to maintain homeostasis in the human body (17). A decreased REE, not associated with differences in terms of body composition, was shown to be present in patients with CP compared with controls (18). In addition, lower energy intake/REE ratios were associated with third ventricle involvement of CP (15).
The severity and consequence of HD during childhood may be different, despite surviving the same tumor type in the hypothalamic region, and the degree of HD can be hard to predict. The differences in outcome of these children may be explained by damage to different hypothalamic nuclei, and it is uncertain in which children the REE is reduced (5).
For radiological scoring of hypothalamic damage, several MRI grading systems have been developed (19, 20). The degree of radiological damage is, however, not always related to the clinical degree of HD or to the presence of obesity. In the search for prevention and treatment of HO, additional tools are needed to better identify the cause of weight gain. In this light, measurement of REE may be an interesting tool; when decreased REE is found, therapeutic interventions aiming to increase REE by stimulating sympathetic activity may be considered (21, 22, 23). Amphetamine treatment has been attempted in children with HO with good response in some, but not all, which may perhaps be explained by differences in REE (24, 25).
REE in children gaining weight with suspected hypothalamic damage has never been associated to radiological or clinical scores for hypothalamic damage. Our aim was to assess which children suspect for HD in our clinic had low REE and if outcomes of REE measurements could be associated to clinical severity of HD or to radiological posterior hypothalamic damage.
Methods
Study population
A retrospective study was performed in children (age > 3 years) referred to the department of pediatric endocrinology at risk of HD due to underlying etiology. All patients had undergone REE measurement (routine part of the clinical assessment in our outpatient clinic to calculate dietary caloric advice), using indirect calorimetry from the period 2014 to 2020.
In all children, REE was measured between 08:00 and 10:00 h in thermoneutral conditions using the Cosmed Quark RMR (Cosmed, Rome, Italy), Geratherm Ergostik (Geratherm Respiratory GmbH, Ergostik, Geratherm, Germany), or Q-NRG devices (Cosmed) (26, 27, 28, 29). With these devices, exhaled gas was captured by a canopy (ventilated hood system) or a face mask connected to oxygen and carbon dioxide analyzers mounted on a metabolic cart. The subjects were in the fasting state for at least 8 h, that is, from at least 00:00 h, were asked to refrain as much as possible from (intense) physical activity for the last 8 h and had been waiting for at least 30 min in the sitting position before REE measurement. Respiratory gas exchanges were monitored for 30 min. Steady-state ventilation of at least 5 min was defined as an average minute VO2 and VCO2 changes by <10% and the average RQ changes by <5%. The Weir equation was used to calculate REE (30).
Technical specifications of the indirect calorimetry devices
Cosmed Quark RMR: O2 sensor ±0.1% Vol, CO2 ±0.02% Vol, and flow accuracy ±2% or 20 mL/s. In two studies, a reliable accuracy of this device was reported (26, 31). Geratherm Ergostik: O2 sensor ±0.1% Vol (13–21%), CO2 ±0.1% Vol (2.5–7.5%), and flow accuracy ±3% or 50 mL/s. The Ergostik gas analyzer (Geratherm Respiratory) was reported to be a reliable device which can collect indirect calorimetry-related outcomes in both resting conditions (e.g. REE) and during exercise (e.g. maximal fat oxidation), having a good inter-dag reliability (27). Q-NRG® indirect calorimeter (Cosmed Quark RMR): accuracy has been reported (in vitro) to be within 5% at oxygen enrichment to 70%; that is maximum expected for clinical use (32). The in vitro coefficient of variation was <1% for REE, this is well within the specifics maintained in the literature for metabolic analyzers. A very good accuracy and precision of the Q-NRG® indirect calorimeter have been reported (33).
In all patients, height, weight, tanner stage, and pituitary function had been measured. All cases with pituitary dysfunction were adequately treated. Because data were collected retrospectively, the local institutional review board decided that the Act on Medical Research Involving Human Subjects did not apply and provided a waiver.
Data collection
Patient characteristics
Demographic and brain injury-related characteristics were extracted from medical records. Anthropometric data (weight SDS, height SDS, and BMI (SDS)) at moment of REE measurement were collected. Presence of underweight, overweight, or obesity was determined by using the BMI cut-off points per age defined by Cole et al. (34, 35).
REE measurements
For the main outcomes, for each patient, the predicted REE (pREE) was calculated using the Schofield equation based on age, gender, weight, and height (36). As absolute measured REE values (mREE) are dependent on these factors, a quotient of mREE and pREE was calculated (mREE/pREE). For comparison, pREE and mREE/pREE were also calculated using other equations (33, 37, 38, 39, 40, 41, 42, 43, 44, 45). The level of normal REE was set on ≥90%, based on the results of Chima et al., who reported that the accuracy in all age groups varied with mean bias ranging from an underestimation of 14.0% to an overestimation of 11.6%, as when compared to measured values. Precision has been defined as calculated by the percentage of participants with predicted energy expenditure within 10% of measured values (32). For this reason, it was presumed that mREE/pREE < 90% is the starting point of clinical relevance.
Clinical score for hypothalamic dysfunction
Clinical presence of HD was scored by an adjusted clinical HD score (adapted from de Vile et al. (46)), rating HD as: (a) grade I (mild); presence of postoperative obesity (BMI> +2.0 SDS) or weight gain (>+1.5 BMI SDS) with no change in effect or behavior indicative of hypothalamic HD; (b) grade II (moderate); obesity (BMI >+2.0 SDS) or weight gain (>+1.5 BMI SDS) in addition to the presence of an obvious period of hyperphagia or an associated change in affective behavior or memory; and (c) grade III; presence of obesity (>+3.0 BMI SDS) or extreme weight gain (>+2.0 BMI SDS) with hyperphagia and presence of other signs of HD, such as impaired thirst, rage, or disturbances of thermoregulation, memory, or sleep–wake pattern (46).
Endocrine dysfunction score
Endocrine dysfunction was graded as 0 (no pituitary dysfunction present), grade I (partial anterior pituitary dysfunction and/or diabetes insipidus (DI)), grade II (panhypopituitarism with DI), and grade III (panhypopituitarism with DI and impaired thirst regulation) (46).
Neuroimaging
On the brain MRI performed at time of diagnosis, the following items were scored: location of brain tumor, tumor volume, presence of hydrocephalus, presence of the pituitary stalk, identification of the supra-optic recess, infundibular recess, and hypothalamic region (47). Hypothalamic damage was scored only in children with brain tumor diagnosis using an adjusted Muller grading consisting of grade 0: no hypothalamic involvement, grade I: hypothalamic involvement of the anterior hypothalamus not involving the hypothalamic area beyond mammillary bodies, grade II: hypothalamic involvement of the anterior and/or solely posterior hypothalamic area, that is involving the area beyond the mammillary bodies (Fig. 1) (20). Mammillary body involvement was separately scored as none, mild involvement (dislocation), or severe involvement (unrecognizable structures) (47). Tumor volume was calculated based on the maximal tumor diameters measured in three dimensions (volume = a×b×c/2) and hydrocephalus was scored as present if has two or more of the following criteria: Evan’s score >0.30, mamillopontine distance >5.5 mm, callosal angle <80°, or third ventricle width >3.5 mm were seen on MRI. All items were scored by a trained clinician-scientist. The MRI images of the first 20 patients were also scored by a neuroradiologist (more than 20 years of experience) and compared. For the remaining MRI images, if there was uncertainty of the physician scientist, the scoring was evaluated with the neuroradiologist and consensus was reached by discussion. In the last available brain MRI before REE measurement, the following items were scored: hypothalamic damage (only in children with brain tumor diagnosis) using an adjusted Muller grading consisting of grade 0: no hypothalamic lesion, grade I: hypothalamic lesion of the anterior hypothalamus not involving the hypothalamic area beyond mammillary bodies, grade II: hypothalamic lesion of the anterior and/or solely posterior hypothalamic area, that is involving the area beyond the mammillary bodies (20). Mammillary body damage was separately scored as none, mild damaged (one-sided), or severe damage (both sided) (47). All items were scored by a trained clinician-scientist and a second-blinded independent reviewer (neuroradiologist). The grading was compared and if there was a discrepancy, consensus was reached by discussion.
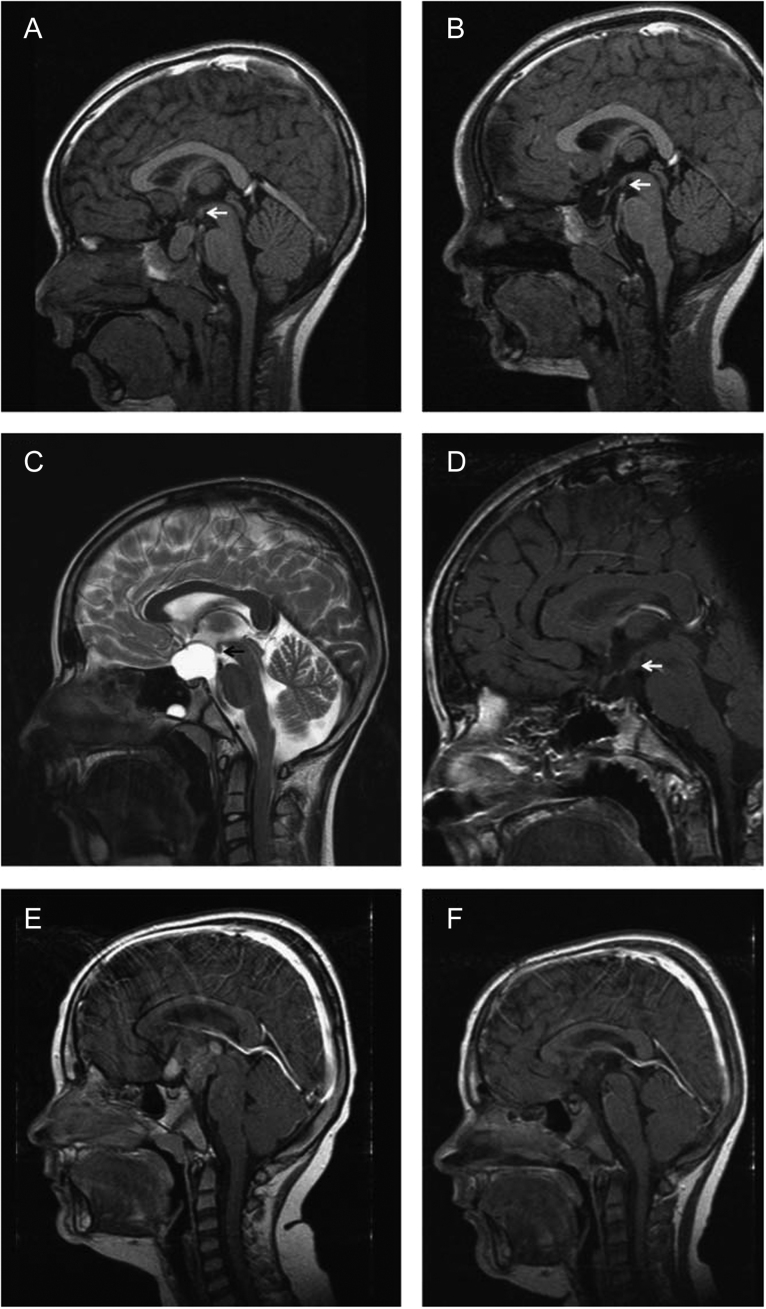
Figure 1.
MRI imaging at diagnosis and 36 months after surgery in three cases of childhood craniopharyngioma with different grade of hypothalamic involvement/lesion. (A and B) Patient with craniopharyngioma confined to the intrasellar space (grade 0 = no hypothalamic involvement (A)/surgical lesion (B)). (C and D) Patient with craniopharyngioma involving the anterior hypothalamus (grade I: hypothalamic involvement (C)/surgical lesion of the anterior hypothalamic area (D)). (E and F) Patient with craniopharyngioma involving the anterior and posterior hypothalamus (grade II: hypothalamic involvement (E)/surgical lesion of the anterior and posterior hypothalamic area (F)). Arrows indicate mammillary bodies, defining the border between anterior and posterior involvement/lesion. Republished with permission of European Society of Endocrinology, from ‘Post-operative hypothalamic lesions and obesity in childhood craniopharyngioma: results of the multinational prospective trial KRANIOPHARYNGEOM 2000 after 3-year follow-up’, Müller et al., vol 165 iss 1, 2011 (20). Permission conveyed through Copyright Clearance Centre, Inc.
Statistical analysis
Data are presented as mean ± s.d. or median (range) for continuous data, depending on the distribution. Data are presented as percentages for categorical variables. Between-group differences were evaluated by Student’s t test for continuous data with a normal distribution, Mann–Whitney U test for continuous data with a skewed distribution and by χ2 test or Fisher’s exact test for categorical data. To assess violation of normality distribution, QQ plot of the residuals and the Shapiro–Wilk’s test were employed. Between-group differences were evaluated by one-way ANOVA for continuous data with a normal distribution, Kruskal–Wallis test for continuous data with a skewed distribution (skew variables were not further transformed), and by χ2 test or Fisher’s exact test for categorical data. Post hoc testing with Dunnett t (two-sided), with the last category as reference, was used. To study the effect of possible risk factors on the outcome, univariate and multivariable linear regression analysis was estimated. Independent variables to be included in the multivariable linear regression were selected by estimating first the univariate model and by considering the clinical relevance of each variable. A P-value of <0.05 was considered statistically significant. Analyses were performed by using SPSS version 25.0.
Results
Patient characteristics
Among the 67 children with suspected HD and weight gain or obesity, 38 (56.7%) were female. Mean age at brain injury diagnosis was 6.8 years ± 4.2 and mean age at follow-up (time of REE measurement) was 11.4 years ± 3.9. Mean BMI SDS at REE measurement was 2.3 ± 1.0, and mean delta BMI SDS from diagnosis to moment of REE measurement was 2.2 ± 1.98 ( n = 51). In total, 63 (94.0%) children had been diagnosed with a brain tumor, 1 child had brain trauma, 1 brain infection, 1 congenital brain malformation, and 1 a Rathke’s cleft cyst (Table 1).
Table 1.
Patient characteristics.
| Total group (n, %) (n = 67,100) | |
|---|---|
| Female/male | 38 (56.7)/29 (43.3) |
| Mean age at diagnosis (years) | 6.8 ± 4.2 |
| Mean age at REE measurement (years) | 11.4 ± 3.9 |
| Mean weight SDS at follow-up | 1.8 ± 1.4 |
| Mean height SDS at follow-up | −0.46 ± 1.3 |
| Mean BMI SDS at diagnosis (n = 51) | 0.06 ± 2.0 |
| Mean BMI SDS at follow-up | 2.3 ± 1.0 |
| Weight classification at follow-up | |
| Normal weight | 16 (23.9) |
| Overweight | 24 (35.8) |
| Obesity | 27 (40.3) |
| Mean fat mass % (n = 54) | 32.5 ± 9.5 |
| Primary diagnosis | |
| Craniopharyngioma | 28 (41.8) |
| Low-grade glioma | 28 (41.8) |
| (Mixed) germ cell tumor | 5 (7.5) |
| Pineoblastoma | 1 (1.5) |
| Unknown histology | 1 (1.5) |
| Others** | 4 (6.0) |
| Hydrocephalus | 34 (50.7) |
| Mean tumor volume cm3 (n = 63) | 29.8 ± 41.7 (min 0.16–max. 236.5) |
| Treatment | |
| Neurosurgery | 50 (74.6) |
| Radiotherapy, mean Gray ± SDS | 23 (34.3), 48.0 ± 11.6 |
| Chemotherapy | 26 (38.8) |
| Intracystic interferon | 6 (9.0) |
Numbers are displayed as n (%) or mean ± standard deviation score.
**Rathke’s cleft cyst, trauma, infection, Chiari I malformation.
REE, resting energy expenditure; SDS, standard deviation score.
REE
In total, REE was measured in 9 children (13.4%) with the device Cosmed Quark RMR (Cosmed), in 46 children (68.7%) with Geratherm Ergostik (Geratherm Respiratory GmbH), and in 12 children (17.9%) with the device Q-NRG. In the nine children, who had REE measurement with the device Cosmed Quark RMR (Cosmed), two (22.2%) were measured in 2014, three (33.3%) in 2015, one (11.%) in 2016, and three in 2019 (33.3%). Of the 46 children measured with device 107 Cosmed Quark RMR, 9 (19.6%) were measured in 2018 and 37 (80.4%) were measured in 2019. All 12 children (100%) who were measured with the device Q-NRG were measured in 2020. Of the 67 children, mean mREE/pREE quotient computed using the Schofield equation was 0.83 ± 0.18 (range, 0.33–1.27). In total, 45 children (67.2%) had a mREE < 90% of the predicted value. Children with low mREE had more often surgical treatment (84.4% vs 54.5%, P = 0.008), and there were more recurrences in the low mREE group (69.8% vs 40.0%, P = 0.025).
Clinical presence of hypothalamic dysfunction and REE outcomes
Using the adjusted HD criteria of de Ville et al. (46), 16.4% scored as having no HD, 31.3% as mild HD, 34.3% as moderate HD, and 17.9% as severe HD. The children with severe HD had a significant lower mean mREE/pREE quotient compared to children with no, mild, or moderate HD (severe: 0.65 ± 0.17 vs none: 0.89 ± 0.12 (P = 0.003), mild: 0.86 ± 0.19 (P = 0.002), and moderate: 0.88 ± 0.15, (P < 0.001)). These outcomes were similar if pREE was calculated with other equations (Supplementary Table 1, see section on supplementary materials given at the end of this article).
In 45.5, 38.1, and 39.1% of children with no, mild, or moderate HD, respectively, the percentage of mREE/pREE was ≥90% of predicted REE. In none of the children with severe HD (n = 12), the percentage of mREE/pREE was ≥90%.
Endocrine dysfunction
In 76.1%, one or more pituitary deficiencies had been diagnosed, of which 23.9% had an endocrine dysfunction score grade I (partial anterior deficiency and/or DI), 46.3% grade II (panhypopituitarism and DI with adequate thirst feeling), and 6.0% grade III (panhypopituitarism and DI without adequate thirst feeling).
The mean mREE/pREE quotient was significantly lower in children with grade III endocrine dysfunction compared to children with grade I or II endocrine dysfunction (mean mREE/pREE quotient of 0.58 ± 0.14 vs children with grade 0: 0.88 ± 0.11 (P = 0.006), grade I: 0.88 ± 0.16 (P = 0.006), and grade II: 0.82 ± 0.20, (P = 0.023)). These outcomes were similar when pREE was calculated with other equations, except for comparison of grade II endocrine dysfunction with grade III endocrine dysfunction. Two of the in total 12 equations did not find a significant difference (Mifflin P = 0.124 and Schmelze P = 0.077).
Radiological assessment of hypothalamic damage
MR images at time of diagnosis were available for 61 children with a brain tumor. The mean mREE/pREE quotient of children at time of diagnosis did not differ between Muller grade 0 (0.89 ± 0.11), I (0.88 ± 0.15), and II (0.78 ± 0.20) (P = 0.087). These outcomes were similar when pREE was calculated with other equations (Table 2).
Table 2.
MRI characteristics and resting energy expenditure quotient.
| Mean mREE/pREE quotienta ± SDS | P-value | |
|---|---|---|
| MRI characteristics at time of primary diagnosis (n = 65): | ||
| Pituitary stalk identification | 0.144 | |
| Present (n = 26) | 0.86 ± 0.13 | |
| Absent (n = 39) | 0.80 ± 0.20 | |
| Hypothalamic identification | 0.019* | |
| Present (n = 31) | 0.88 ± 0.13 | |
| Absent (n = 34) | 0.78 ± 0.20 | |
| Hydrocephalusb | 0.582 | |
| Present (n = 31) | 0.82 ± 0.19 | |
| Absent (n = 34) | 0.84 ± 0.16 | |
| Muller grading for presence of hypothalamic damagec (n = 61) | 0.087 | |
| None (n = 7) | 0.89 ± 0.11 | |
| Anterior damage (n = 20) | 0.88 ± 0.15 | |
| Posterior damage (n = 34) | 0.78 ± 0.20 | |
| Mammillary body involvementd | 0.128 | |
| No involvement (n = 25) | 0.88 ± 0.10 | |
| Mild involvement (n = 13) | 0.77 ± 0.22 | |
| Severe involvement (n = 27) | 0.81 ± 0.20 | |
| At time of REE measurement (n = 66): | ||
| Hypothalamic damage according to Muller gradingc (n = 62) | ||
| None (n = 10) | 0.89 ± 0.09 | 0.017* |
| Anterior damage (n = 18) | 0.90 ± 0.16 | |
| Posterior damage (n = 34) | 0.77 ± 0.19 | |
| Mammillary body damaged | 0.023* | |
| No damage (n = 17) | 0.88 ± 0.10 | |
| Mild damaged (n = 29) | 0.86 ± 0.18 | |
| Severe damaged (n = 20) | 0.74 ± 0.18 | |
amREE/REE quotient: measured resting energy expenditure divided by predicted resting energy expenditure (calculated by Schofield equation). bHydrocephalus was scored as present if has two or more of the following criteria: Evan’s score >0.30, mamillopontine distance >5.5 mm, callosal angle <80°, or third ventricle width >3.5 mm were seen on MRI. cHypothalamic damage was scored only in children with brain tumor diagnosis, using an adjusted Muller grading consisting of grade 0: no hypothalamic involvement/lesion, grade I: hypothalamic involvement/lesion of the anterior hypothalamus not involving the hypothalamic area beyond mammillary bodies, and grade II: hypothalamic involvement/lesion of the anterior and/or solely posterior hypothalamic area, i.e. involving the area beyond the mammillary bodies. dMammillary body damage was separately scored as none, mild involvement or damage (dislocation or one-sided damage), or severe involvement or damage (both sided damaged or unrecognizable structures).
REE, resting energy expenditure. *Statistically significant.
At time of REE measurement, MR images were available for 62 children with a brain tumor. The mean mREE/pREE quotient of children with Muller grade II on MRI at time of REE measurement (n = 34) was significantly lower compared to children with grade 0 or grade I hypothalamic damage (mean REE quotient in patients with Muller grade II: 0.77 ± 0.19 vs grade 0: 0.89 ± 0.09 and grade I: 0.90 ± 0.16, P = 0.017)). These outcomes were similar when calculated with other equations. Four equations (Harris and Benedict, Mifflin, Muller, and Schmelze) did not show a statistically significant difference (Supplementary Table 2).
The mean mREE/pREE quotient of children with severe mammillary body damage on MRI at time of REE measurement was significantly lower compared to children with no mammillary body damage or mild damage on MRI (no damage 0.88 ± 0.10, mild damage 0.86 ± 0.18, severe: 0.74 ± 0.18, P = 0.016). These outcomes were similar using nine other equations, and two equations (Harris and Benedict P = 0.067 and Mifflin P = 0.136) did not find a statistically significant result.
Clinical HD in relation to radiological score
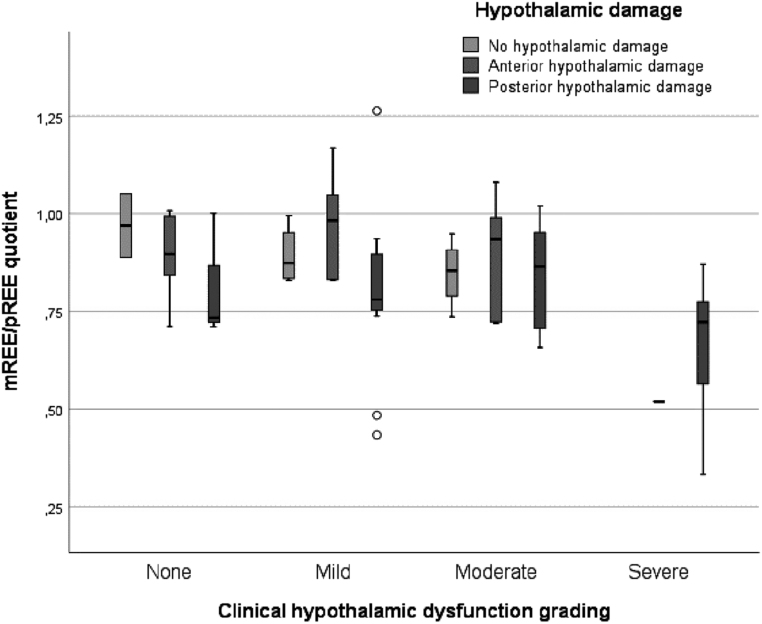
All children with severe clinical HD scores scored Muller grade II on the MRI at diagnosis and 11 of 12 scored Muller grade II on the MRI at time of REE measurement. Among children with posterior hypothalamic damage (grade II) (n = 34), 3 (8.8%) had no clinical HD (grade 0), 11 (32.4%) had mild HD (grade I), 9 (26.5%) moderate HD (grade II), and 11 (32.4%) severe HD (grade III) (Fig. 2).
Figure 2.
Clustered boxplot of resting energy expenditure quotients of children. Resting energy expenditure quotient: measured resting energy expenditure (mREE) divided by predicted resting energy expenditure (pREE) (calculated by Schofield equation).
Risk factors for mREE/pREE quotient
In the multivariable linear regression, progression or recurrence of the tumor, severe clinical HD, and panhypopituitarism with DI were found to be statistically significant risk factors to develop lower mREE/pREE quotient (mREE/pREE quotient = 0.99 + −0.09 (1 = tumor progression or recurrence) + −0.12 (1 = severe clinical hypothalamic dysfunction) + −0.09 (1 = panhypopituitarism with DI) (Tables 3 and 4).
Table 3.
Univariate linear regression for resting energy expenditure quotient.
| Standardized beta | 95% CI | P-value | ||
|---|---|---|---|---|
| Lower | Upper | |||
| Partial or gross total resection | −0.12 | −0.21 | −0.02 | 0.022* |
| Progression or recurrence of the tumor | −0.10 | −0.19 | −0.01 | 0.030* |
| Severe clinical hypothalamic dysfunctiona | −0.23 | −0.33 | −0.12 | <0.001* |
| Posterior hypothalamic damageb at time of diagnosis | −0.10 | −0.19 | −0.01 | 0.027* |
| Posterior hypothalamic damage on MRI at time of REE measurement | −0.13 | −0.21 | −0.04 | 0.004* |
| Severe mammillary body damagec | −0.12 | −0.21 | −0.04 | 0.007* |
| Pan hypopituitarism with diabetes insipidus | −0.9 | −0.17 | −0.00 | 0.050* |
Resting energy expenditure quotient: Measured resting energy expenditure divided by predicted resting energy expenditure (calculated by Schofield equation). Diabetes insipidus at follow-up, central precocious puberty at follow-up, age at diagnosis, age at follow-up, BMI SDS at follow-up, fat-mass percentage (n = 54), hydrocephalus at diagnosis, tumor size (n = 63), severe mammillary body damage at diagnosis (n = 65), radiotherapy, and chemotherapy were not significantly associated with mREE/pREE quotient.
aSevere clinical hypothalamic dysfunction: Presence of obesity (>+3.0 BMI SDS) or extreme weight gain (>+2.0 BMI SDS) with (severe) hyperphagia and presence of other clinical manifestations, such as impaired thirst, rage behavior, or disturbances of thermoregulation, memory, and sleep-wake pattern. bPosterior hypothalamic damage graded with Muller: hypothalamic involvement/lesion of the anterior and/or solely posterior hypothalamic area, i.e. involving the area beyond mammillary bodies. cSevere mammillary body damage: severe involvement or damage (unrecognizable structures or both sided damaged) of the mammillary bodies. *Statistically significant.
Table 4.
Multivariate linear regression for resting energy expenditure quotient.
| Standardized beta | 95% CI | P-value | ||
|---|---|---|---|---|
| Lower | Upper | |||
| Progression or recurrence of the tumor | −0.09 | −0.17 | −0.002 | 0.046* |
| Severe clinical hypothalamic dysfunctiona | −0.12 | −0.23 | −0.012 | 0.030* |
| Posterior hypothalamic damageb on MRI at time of REE measurement | −0.05 | −0.16 | 0.048 | 0.293 |
| Severe mammillary body damagec | −0.03 | −0.14 | 0.073 | 0.546 |
| Pan hypopituitarism with diabetes insipidus | −0.09 | −0.18 | −0.009 | 0.030* |
Resting energy expenditure quotient: Measured resting energy expenditure divided by predicted resting energy expenditure (calculated by Schofield equation).
aSevere clinical hypothalamic dysfunction: Presence of obesity (>+3.0 BMI SDS) or extreme weight gain (>+2.0 BMI SDS) with (severe) hyperphagia and presence of other clinical manifestations, such as impaired thirst, rage behavior, or disturbances of thermoregulation, memory, and sleep-wake pattern. bPosterior hypothalamic damage graded with Muller: hypothalamic involvement/lesion of the anterior and/or solely posterior hypothalamic area, i.e. involving the area beyond mammillary bodies. cSevere mammillary body damage: severe involvement or damage (unrecognizable structures or both sided damaged) of the mammillary bodies. *Statistically significant.
Discussion
In this study, we aimed to assess which children, presenting with weight gain or obesity, suspect for HD have lower REE and if this is associated with other clinical signs of HD or radiological damage. To have this information may aid in the management of HO, as it will distinguish those with lower REE that may be helped by treatment with energy expenditure increasing interventions, from those that do not and may need other obesity interventions.
In this cohort, we could relate REE measurement outcomes to clinical severity of HD and radiological posterior hypothalamic damage. However, our results also demonstrate that some children who are clinically or radiologically suspect for HD have REE measurement outcomes comparable to age and gender-related predicted values. This emphasizes an important message that underlies the difficult problem of HO; not in all children with hypothalamic damage, the etiology of weight gain is the same. To better understand the individual etiology of weight gain, measurement of REE may be useful to design adequate personalized coaching and treatment.
It may be objected that obesity itself may be associated with a lower REE due to the presence of a higher percentage of fat mass with decreased muscle mass. Obesity, however, does not lower absolute REE, but results in an increased absolute REE (48). When body composition (fat-free mass) is considered, the differences between obese and non-obese individuals disappear, which implicates that REE in obese individuals is not different from healthy subjects (48).
Clinical and radiological HD scores can be difficult to interpret, and a one-to-one correlation between anatomical damage and clinical outcome is difficult to test. For example, a child with posterior hypothalamic damage (grade II) does not necessarily have decreased REE or a high clinical HD score. To our knowledge, this is the first study in which REE measurements of children with brain injury at risk of HD have been associated with scores for clinical HD and radiological hypothalamic damage. Previous cohort studies have examined REE measurements in children with brain injury and compared them to obese children without brain injury or compared REE measurements in normal weight craniopharyngioma (CP) patients with those in obese CP patients (15, 18, 49, 50, 51, 52). REE was shown to be lower in CP patients compared to children with multifactorial obesity regardless of the amount of fat-free mass or when compared to matched controls (18). In another study, CP patients were shown to have a 17% lower REE value compared to the values that were expected from the WHO equation (49). Our study results confirm that REE may be lowered in children with hypothalamic damage and shows that this is associated to the degree of clinical HD (in the multivariate analysis), as well as to radiological posterior hypothalamic damage (in the univariate analysis). The fact that posterior hypothalamic damage was not significant in the multivariate analysis may be explained by the fact that the current used radiological score grading anterior or posterior hypothalamic damage, even including mammillary body damage, is still too robust to predict the outcomes of REE. In addition, this grading system was developed for craniopharyngioma patients, and in our study also other suprasellar tumors were included (e.g. germinoma, low grade glioma). Perhaps, the Muller grading system is not optimal for grading hypothalamic damage in such a heterogeneous group. Roth et al. developed a more detailed MRI scoring system for the risk assessment of HO and found posterior damage and mammillary body damage to be related to HO in mainly CP patients (53). In addition, Perez et al. evaluated the effect of glucagon-like-1 peptide receptor (GLP-1) agonist treatment in CP patients in relation to the degree of hypothalamic and MB damage, using this scoring system. They found that CP patients with a worse hypothalamic damage score, bilateral MB damage, or smaller MB in the cross-sectional area had a greater reduction in % body fat following GLP-1 agonist treatment (54). However, both studies did not associate HO or MRI score to REE outcomes and, therefore, no conclusion can be drawn on the exact underlying etiology of HO in these patients (hyperphagia or decreased REE or both). Perhaps, measuring the volume of the hypothalamic area may aid in predicting REE outcomes, as studies have shown that body weight was negatively associated to hypothalamic volume in patients with hypothalamic dysfunction (55). Furthermore, Fjalldal et al. found HT volume to be negatively correlated to fat mass and leptin among CP patients (56). It would, therefore, be of interest to correlate HT volume to REE outcomes as well. We suggest that future research takes REE measurements into account in relation to hypothalamic damage, which may aid to guide therapy and evaluate the effect of obesity therapy in these patients. Along with this, future research should focus on improving radiological techniques for detailed information on damage to the different nuclei of the hypothalamus, such as the arcuate nucleus, paraventricular nucleus, and dorsomedial nucleus.
In our cohort, all children with severe clinical hypothalamic dysfunction had a low REE (<90%). It could, therefore, be argued that REE measurement in these children may not be necessary anymore. However, REE measurement may be valuable for clinicians as a baseline value, which can be used for evaluation after interventions aiming to improve obesity. In addition, also dietary advice may be adjusted to REE values.
As discussed above, in the univariate analysis, we found an association between posterior hypothalamic damage with the Muller grading, mammillary body damage, endocrine dysfunction and clinical hypothalamic dysfunction, and low REE. However, in the multivariable linear regression, only tumor recurrence or progression, severe clinical hypothalamic dysfunction, and panhypopituitarism with diabetes insipidus were significant. We also found that not all children suspect for HD have a low REE, implying that measuring REE may aid in gaining more information on the etiology of weight gain in these patients and aid in designing individualized treatment programs targeting the right factors which contributed to weight gain in these patients. HO in hypothalamic patients is not one size fits all, some patients may have hyperphagia and some may have decreased REE, while again others have both. REE is thus one of the parameters, next to radiological scoring and clinical scoring in proving an holistic view of the patient. This is supported by our results, showing that almost all children without clinical signs have normal REE values and all children with severe clinical HD have low REE values. However, grading mild or moderate clinical HD does not discriminate between those children with normal REE and those with a low REE. This group is thus very heterogeneous, and measuring REE could be helpful for these children. New radiological techniques need to be developed which may be associated to the clinical picture and REE in the future.
For physicians treating children at risk of HD, it is important to understand the underlying etiology for the weight gain to enable appropriate counseling and treatment. It goes without saying that in all children, the first step for reducing weight gain is lifestyle intervention. However, when REE is lowered, lifestyle alone may not be sufficient to maintain or regain a healthy BMI and body composition (57, 58, 59, 60, 61). For these children, specific interventions aiming to increase REE, either by increasing physical activity, such as high intensity interval training or medical (e.g. dextroamphetamine) may be helpful (24, 25). Prescribing amphetamines must however be considered experimental and should only be done in experienced multidisciplinary setting. In addition, optimizing endocrine balance, such as aiming for FT4 concentrations in the upper range of the reference level, restoring the IGF-1 concentrations in children with growth hormone deficiency and aiming for low-normal hydrocortisone maintenance doses may aid to improve metabolic state (21, 22, 62).
Our selection of patients included in this cohort may be considered a limitation of this study. Children referred to the pediatric endocrinologist suspect for HD were included, which may have introduced a selection bias. Also, the retrospective design may be considered a limitation. Our results must therefore be confirmed in a prospective longitudinal study, using standardized questionnaires focusing on clinical HD, with comparison of outcomes to a control group.
Comparing mREE in individuals to predictive equations (pREE) is challenging, because all equations have their limitations. Multiple predictive equations exist, and no predictive equation is 100% valid for estimating the true REE of a child. If pREE is over- predicted, mREE/pREE may be misinterpreted as being too low. We used the Schofield equation based on a recent review that showed that the most accurate REE predictions (least biased) in obese children and adolescents were obtained using the Schofield equation (+0.8% (3–18 years); 0% (11–18 years); +1.1% (3–10 years)) (63). Because of the limitation of all predictive equations, we compared the outcomes using the Schofield equations with outcomes calculated by other predicative equations. These results were almost all similar, confirming the reliability of our results.
In conclusion, REE in children suspect for HD is mainly related to clinical severity of HD symptoms and is not always lowered. We recommend measurement of REE in such children, as it will help to develop a personalized approach for HO, aiming to improve cardio-metabolic health.
Supplementary Material
Declaration of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Funding
This work did not receive any specific grant from any funding agency in the public, commercial, or not-for-profit sector.
Ethics
The study was conducted ethically in accordance with the World Medical Association Declaration of Helsinki. Because data were collected retrospectively, the local institutional review board decided that the Act on Medical Research Involving Human Subjects did not apply and provided a waiver.
Data availability
Restrictions apply to the availability of some or all data generated or analyzed during this study to preserve patient confidentiality or because they were used under license. The corresponding author will on request detail the restrictions and any conditions under which access to some data may be provided.
Author contribution statement
J van Schaik contributed to the conception and design of the study, the analysis and interpretation of the study, drafted the first manuscript, and revised the manuscript critically. M Burghard, M Lequin, E A van Maren, A M van Dijk, T Takken, L Rehorst, B Bakker, L Meijer, E Hoving, A Y N Schouten-van Meeteren, M Fiocco, and W J E Tissing contributed to the analysis and interpretation of the work, drafted the manuscript, and revised the manuscript critically. H M van Santen contributed to the conception or design of the work, the acquisition, analysis and interpretation of the study, and revised the manuscript critically. All authors approve the final version to be published and agree to be accountable for all aspects of the work.
References
- 1.Roth CL.Hypothalamic obesity in patients with craniopharyngioma: profound changes of several weight regulatory circuits. Frontiers in Endocrinology 20112 49. ( 10.3389/fendo.2011.00049) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Muller HL.Craniopharyngioma. Endocrine Reviews 201435513–543. ( 10.1210/er.2013-1115) [DOI] [PubMed] [Google Scholar]
- 3.Iughetti L, Bruzzi P. Obesity and craniopharyngioma. Italian Journal of Pediatrics 201137 38. ( 10.1186/1824-7288-37-38) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lustig RH.Hypothalamic obesity after craniopharyngioma: mechanisms, diagnosis, and treatment. Frontiers in Endocrinology 20112 60. ( 10.3389/fendo.2011.00060) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van Iersel L, Brokke KE, Adan RAH, Bulthuis LCM, van den Akker ELT, van Santen HM. Pathophysiology and individualized treatment of hypothalamic obesity following craniopharyngioma and other suprasellar tumors: a systematic review. Endocrine Reviews 201940193–235. ( 10.1210/er.2018-00017) [DOI] [PubMed] [Google Scholar]
- 6.Castro DC, Cole SL, Berridge KC. Lateral hypothalamus, nucleus accumbens, and ventral pallidum roles in eating and hunger: interactions between homeostatic and reward circuitry. Frontiers in Systems Neuroscience 20159 90. ( 10.3389/fnsys.2015.00090) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ness KK, Morris EB, Nolan VG, Howell CR, Gilchrist LS, Stovall M, Cox CL, Klosky JL, Gajjar A, Neglia JP. Physical performance limitations among adult survivors of childhood brain tumors. Cancer 20101163034–3044. ( 10.1002/cncr.25051) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang FF, Saltzman E, Must A, Parsons SK. Do childhood cancer survivors meet the diet and physical activity guidelines? A review of guidelines and literature. International Journal of Child Health and Nutrition 2012144–58. ( 10.6000/1929-4247.2012.01.01.06) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stolley MR, Restrepo J, Sharp LK. Diet and physical activity in childhood cancer survivors: a review of the literature. Annals of Behavioral Medicine 201039232–249. ( 10.1007/s12160-010-9192-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rose SR.Endocrinopathies in childhood cancer survivors. Endocrinologist 200313488–495. ( 10.1097/01.ten.0000098611.49230.10) [DOI] [Google Scholar]
- 11.Crenn P, Hamchaoui S, Bourget-Massari A, Hanachi M, Melchior JC, Azouvi P. Changes in weight after traumatic brain injury in adult patients: a longitudinal study. Clinical Nutrition 201433348–353. ( 10.1016/j.clnu.2013.06.003) [DOI] [PubMed] [Google Scholar]
- 12.Thompson CJ, Costello RW, Crowley RK. Management of hypothalamic disease in patients with craniopharyngioma. Clinical Endocrinology 201990506–516. ( 10.1111/cen.13929) [DOI] [PubMed] [Google Scholar]
- 13.Abuzzahab MJ, Roth CL, Shoemaker AH. Hypothalamic obesity: prologue and promise. Hormone Research in Paediatrics 201991128–136. ( 10.1159/000496564) [DOI] [PubMed] [Google Scholar]
- 14.Heldenberg D, Tamir I, Ashner M, Werbin B. Hyperphagia, obesity and diabetes insipidus due to hypothalamic lesion in a girl. Helvetica Paediatrica Acta 197227489–494. [PubMed] [Google Scholar]
- 15.Holmer H, Pozarek G, Wirfalt E, Popovic V, Ekman B, Bjork J, Erfurth EM. Reduced energy expenditure and impaired feeding-related signals but not high energy intake reinforces hypothalamic obesity in adults with childhood onset craniopharyngioma. Journal of Clinical Endocrinology and Metabolism 2010955395–5402. ( 10.1210/jc.2010-0993) [DOI] [PubMed] [Google Scholar]
- 16.Lustig RH.Hypothalamic obesity: the sixth cranial endocrinopathy. Endocrinologist 200212210–217. ( 10.1097/00019616-200205000-00008) [DOI] [Google Scholar]
- 17.Gupta RD, Ramachandran R, Venkatesan P, Anoop S, Joseph M, Thomas N. Indirect calorimetry: from bench to bedside. Indian Journal of Endocrinology and Metabolism 201721594–599. ( 10.4103/ijem.IJEM_484_16) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bomer I, Saure C, Caminiti C, Ramos JG, Zuccaro G, Brea M, Bravo M, Maza C. Comparison of energy expenditure, body composition, metabolic disorders, and energy intake between obese children with a history of craniopharyngioma and children with multifactorial obesity. Journal of Pediatric Endocrinology and Metabolism 2015281305–1312. ( 10.1515/jpem-2015-0167) [DOI] [PubMed] [Google Scholar]
- 19.Puget S, Garnett M, Wray A, Grill J, Habrand JL, Bodaert N, Zerah M, Bezerra M, Renier D, Pierre-Kahn Aet al. Pediatric craniopharyngiomas: classification and treatment according to the degree of hypothalamic involvement. Journal of Neurosurgery 2007106 (1 Supplement) 3–12. ( 10.3171/ped.2007.106.1.3) [DOI] [PubMed] [Google Scholar]
- 20.Muller HL, Gebhardt U, Teske C, Faldum A, Zwiener I, Warmuth-Metz M, Pietsch T, Pohl F, Sorensen N, Calaminus Get al. Post-operative hypothalamic lesions and obesity in childhood craniopharyngioma: results of the multinational prospective trial KRANIOPHARYNGEOM 2000 after 3-year follow-up. European Journal of Endocrinology 201116517–24. ( 10.1530/EJE-11-0158) [DOI] [PubMed] [Google Scholar]
- 21.Mason PW, Krawiecki N, Meacham LR. The use of dextroamphetamine to treat obesity and hyperphagia in children treated for craniopharyngioma. Archives of Pediatrics and Adolescent Medicine 2002156887–892. ( 10.1001/archpedi.156.9.887) [DOI] [PubMed] [Google Scholar]
- 22.Ismail D, O'Connell MA, Zacharin MR. Dexamphetamine use for management of obesity and hypersomnolence following hypothalamic injury. Journal of Pediatric Endocrinology and Metabolism 200619129–134. ( 10.1515/jpem.2006.19.2.129) [DOI] [PubMed] [Google Scholar]
- 23.Elfers CT, Roth CL. Effects of methylphenidate on weight gain and food intake in hypothalamic obesity. Frontiers in Endocrinology 20112 78. ( 10.3389/fendo.2011.00078) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van Schaik J, Welling MS, de Groot CJ, van Eck JP, Juriaans A, Burghard M, Oude Ophuis SBJ, Bakker B, Tissing WJE, Schouten-van Meeteren AYNet al. Dextroamphetamine treatment in children with hypothalamic obesity. Frontiers in Endocrinology 202213845937. (https://10.3389/fendo.2022.845937) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Denzer C, Denzer F, Lennerz BS, Vollbach H, Lustig RH, Wabitsch M. Treatment of hypothalamic obesity with dextroamphetamine: a case series. Obesity Facts 20191291–102. ( 10.1159/000495851) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ashcraft CM, Frankenfield DC. Validity test of a new open-circuit indirect calorimeter. Journal of Parenteral and Enteral Nutrition 201539738–742. ( 10.1177/0148607114526242) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Robles-Gonzalez L, Gutierrez-Hellin J, Aguilar-Navarro M, Ruiz-Moreno C, Munoz A, Del-Coso J, Ruiz JR, Amaro-Gahete FJ. Inter-day reliability of resting metabolic rate and maximal fat oxidation during exercise in healthy men using the Ergostik gas analyzer. Nutrients 2021134308. ( 10.3390/nu13124308) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Oshima T, Dupertuis YM, Delsoglio M, Graf S, Heidegger CP, Pichard C. In vitro validation of indirect calorimetry device developed for the ICALIC project against mass spectrometry. Clinical Nutrition ESPEN 20193250–55. ( 10.1016/j.clnesp.2019.05.004) [DOI] [PubMed] [Google Scholar]
- 29.Delsoglio M, Dupertuis YM, Oshima T, van der Plas M, Pichard C. Evaluation of the accuracy and precision of a new generation indirect calorimeter in canopy dilution mode. Clinical Nutrition 2020391927–1934. ( 10.1016/j.clnu.2019.08.017) [DOI] [PubMed] [Google Scholar]
- 30.Weir JB.New methods for calculating metabolic rate with special reference to protein metabolism. Journal of Physiology 19491091–9. ( 10.1113/jphysiol.1949.sp004363) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Blond E, Maitrepierre C, Normand S, Sothier M, Roth H, Goudable J, Laville M. A new indirect calorimeter is accurate and reliable for measuring basal energy expenditure, thermic effect of food and substrate oxidation in obese and healthy subjects. e-SPEN 20116e7–e15. ( 10.1016/j.eclnm.2010.12.001) [DOI] [Google Scholar]
- 32.Lindman H, Wiklund T, Holte H, Ljungman P, Blomqvist C, Kvalheim G, Bengtsson M, Hoglund M, Wilking N, Bergh J. FEC mobilized stem cells for high-dose therapy in breast cancer patients. SB6 9401 Study Group. Acta Oncologica 199938239–245. ( 10.1080/028418699431672) [DOI] [PubMed] [Google Scholar]
- 33.World Health Organization. Energy and protein requirements. In Report of a joint FAO/WHO/UNU Expert Consultation. World Health Organization Technical Report Series; 724, 1985. [PubMed] [Google Scholar]
- 34.Cole TJ, Bellizzi MC, Flegal KM, Dietz WH. Establishing a standard definition for child overweight and obesity worldwide: international survey. BMJ 20003201240–1243. ( 10.1136/bmj.320.7244.1240) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cole TJ, Flegal KM, Nicholls D, Jackson AA. Body mass index cut offs to define thinness in children and adolescents: international survey. BMJ 2007335 194. ( 10.1136/bmj.39238.399444.55) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schofield WN.Predicting basal metabolic rate, new standards and review of previous work. Human Nutrition: Clinical Nutrition 198539 (Supplement 1) 5–41. [PubMed] [Google Scholar]
- 37.Harris J, Benedict G. A biometric study of human basal metabolism. PNAS 19184370–373. ( 10.1073/pnas.4.12.370) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Henry CJK, Dyer S, Ghusain-Choueiri A. New equations to estimate basal metabolic rate in children aged 10–15 years. European Journal of Clinical Nutrition 199953134–142. ( 10.1038/sj.ejcn.1600690) [DOI] [PubMed] [Google Scholar]
- 39.Henry CJK.Basal metabolic rate studies in humans: measurement and development of new equations. Public Health Nutrition 200581133–1152. ( 10.1079/phn2005801) [DOI] [PubMed] [Google Scholar]
- 40.Institute of Medicine. Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein and Amino Acids. Washington, DC, USA: The National Academies Press, 2005. ( 10.17226/10490) [DOI] [Google Scholar]
- 41.Lazzer S, Agosti F, De Col A, Sartorio A. Development and cross-validation of prediction equations for estimating resting energy expenditure in severely obese Caucasian children and adolescents. British Journal of Nutrition 200696973–979. ( 10.1017/bjn20061941) [DOI] [PubMed] [Google Scholar]
- 42.Mifflin MD, Stjeor ST, Hill LA, Scott BJ, Daugherty SA, Koh YO. A new predictive equation for resting energy-expenditure in healthy-individuals. American Journal of Clinical Nutrition 199051241–247. ( 10.1093/ajcn/51.2.241) [DOI] [PubMed] [Google Scholar]
- 43.Molnar D, Jeges S, Erhardt E, Schutz Y. Measured and predicted resting metabolic-rate in obese and nonobese adolescents. Journal of Pediatrics 1995127571–577. ( 10.1016/s0022-3476(9570114-1) [DOI] [PubMed] [Google Scholar]
- 44.Muller MJ, Bosy-Westphal A, Klaus S, Kreymann G, Luhrmann PM, Neuhauser-Berthold M, Noack R, Pirke KM, Platte P, Selberg Oet al. World Health Organization equations have shortcomings for predicting resting energy expenditure in persons from a modern, affluent population: generation of a new reference standard from a retrospective analysis of a German database of resting energy expenditure. American Journal of Clinical Nutrition 2004801379–1390. ( 10.1093/ajcn/80.5.1379) [DOI] [PubMed] [Google Scholar]
- 45.Schmelzle H, Schroder C, Armbrust S, Unverzagt S, Fusch C. Resting energy expenditure in obese children aged 4 to 15 years: measured versus predicted data. Acta Paediatrica 200493739–746. ( 10.1111/j.1651-2227.2004.tb01000.x) [DOI] [PubMed] [Google Scholar]
- 46.De Vile CJ, Grant DB, Kendall BE, Neville BG, Stanhope R, Watkins KE, Hayward RD. Management of childhood craniopharyngioma: can the morbidity of radical surgery be predicted? Journal of Neurosurgery 19968573–81. ( 10.3171/jns.1996.85.1.0073) [DOI] [PubMed] [Google Scholar]
- 47.Mortini P, Gagliardi F, Bailo M, Spina A, Parlangeli A, Falini A, Losa M. Magnetic resonance imaging as predictor of functional outcome in craniopharyngiomas. Endocrine 201651148–162. ( 10.1007/s12020-015-0683-x) [DOI] [PubMed] [Google Scholar]
- 48.Carneiro IP, Elliott SA, Siervo M, Padwal R, Bertoli S, Battezzati A, Prado CM. Is obesity associated with altered energy expenditure? Advances in Nutrition 20167476–487. ( 10.3945/an.115.008755) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Caminiti C, Saure C, Bomer I, Brea M, Gonzalez Ramos J. Nutritional assessment of a population with a history of childhood craniopharyngioma seen at Hospital ‘Prof. Dr. Juan P. Garrahan’. Archivos Argentinos de Pediatria 201711543–49. ( 10.5546/aap.2017.eng.43) [DOI] [PubMed] [Google Scholar]
- 50.Kim RJ, Shah R, Tershakovec AM, Zemel BS, Sutton LN, Grimberg A, Moshang T. Energy expenditure in obesity associated with craniopharyngioma. Child’s Nervous System 201026913–917. ( 10.1007/s00381-009-1078-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Simoneau-Roy J, O'Gorman C, Pencharz P, Adeli K, Daneman D, Hamilton J. Insulin sensitivity and secretion in children and adolescents with hypothalamic obesity following treatment for craniopharyngioma. Clinical Endocrinology 201072364–370. ( 10.1111/j.1365-2265.2009.03639.x) [DOI] [PubMed] [Google Scholar]
- 52.Cohen M, Syme C, McCrindle BW, Hamilton J. Autonomic nervous system balance in children and adolescents with craniopharyngioma and hypothalamic obesity. European Journal of Endocrinology 2013168845–852. ( 10.1530/EJE-12-1082) [DOI] [PubMed] [Google Scholar]
- 53.Roth CL, Eslamy H, Werny D, Elfers C, Shaffer ML, Pihoker C, Ojemann J, Dobyns WB. Semiquantitative analysis of hypothalamic damage on MRI predicts risk for hypothalamic obesity. Obesity 2015231226–1233. ( 10.1002/oby.21067) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Perez FA, Elfers C, Yanovski JA, Shoemaker AH, Abuzzahab MJ, Roth CL. MRI measures of hypothalamic injury are associated with glucagon-like peptide-1 receptor agonist treatment response in people with hypothalamic obesity. Diabetes, Obesity and Metabolism 2021231532–1541. ( 10.1111/dom.14366) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hong AR, Lee M, Lee JH, Kim JH, Kim YH, Choi HJ. Clinical implication of individually tailored segmentation method for distorted hypothalamus in craniopharyngioma. Frontiers in Endocrinology 202112763523. ( 10.3389/fendo.2021.763523) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fjalldal S, Follin C, Gabery S, Sundgren PC, Bjorkman-Burtscher IM, Latt J, Mannfolk P, Nordstrom CH, Rylander L, Ekman Bet al. Detailed assessment of hypothalamic damage in craniopharyngioma patients with obesity. International Journal of Obesity 201943533–544. ( 10.1038/s41366-018-0185-z) [DOI] [PubMed] [Google Scholar]
- 57.Skorzewska A, Lal S, Waserman J, Guyda H. Abnormal food-seeking behavior after surgery for craniopharyngioma. Neuropsychobiology 19892117–20. ( 10.1159/000118545) [DOI] [PubMed] [Google Scholar]
- 58.Rakhshani N, Jeffery AS, Schulte F, Barrera M, Atenafu EG, Hamilton JK. Evaluation of a comprehensive care clinic model for children with brain tumor and risk for hypothalamic obesity. Obesity 2010181768–1774. ( 10.1038/oby.2009.491) [DOI] [PubMed] [Google Scholar]
- 59.Steele CA, Cuthbertson DJ, MacFarlane IA, Javadpour M, Das KSV, Gilkes C, Wilding JP, Daousi C. Hypothalamic obesity: prevalence, associations and longitudinal trends in weight in a specialist adult neuroendocrine clinic. European Journal of Endocrinology 2013168501–507. ( 10.1530/EJE-12-0792) [DOI] [PubMed] [Google Scholar]
- 60.Sterkenburg AS, Hoffmann A, Gebhardt U, Waldeck E, Springer S, Muller HL. Childhood craniopharyngioma with hypothalamic obesity – no long-term weight reduction due to rehabilitation programs. Klinische Padiatrie 2014226344–350. ( 10.1055/s-0034-1387747) [DOI] [PubMed] [Google Scholar]
- 61.Meijneke RWH, Schouten-van Meeteren AYN, de Boer NY, Van Zundert S, van Trotsenburg PAS, Stoelinga F, van Santen HM. Hypothalamic obesity after treatment for craniopharyngioma: the importance of the home environment. Journal of Pediatric Endocrinology and Metabolism 20152859–63. ( 10.1515/jpem-2014-0338) [DOI] [PubMed] [Google Scholar]
- 62.Geffner M, Lundberg M, Koltowska-Haggstrom M, Abs R, Verhelst J, Erfurth EM, Kendall-Taylor P, Price DA, Jonsson P, Bakker B. Changes in height, weight, and body mass index in children with craniopharyngioma after three years of growth hormone therapy: analysis of KIGS (Pfizer International Growth Database). Journal of Clinical Endocrinology and Metabolism 2004895435–5440. ( 10.1210/jc.2004-0667) [DOI] [PubMed] [Google Scholar]
- 63.Chima L, Mulrooney HM, Warren J, Madden AM. A systematic review and quantitative analysis of resting energy expenditure prediction equations in healthy overweight and obese children and adolescents. Journal of Human Nutrition and Dietetics 202033373–385. ( 10.1111/jhn.12735) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Restrictions apply to the availability of some or all data generated or analyzed during this study to preserve patient confidentiality or because they were used under license. The corresponding author will on request detail the restrictions and any conditions under which access to some data may be provided.



 This work is licensed under a
This work is licensed under a