Abstract
Biodiversity is defined as the presence of a variety of living organisms on the Earth that is essential for human survival. However, anthropogenic activities are causing the sixth mass extinction, threatening even our own species. For many animals, dwindling numbers are becoming fragmented populations with low genetic diversity, threatening long-term species viability. With extinction rates 1000–10,000 times greater than natural, ex situ and in situ conservation programmes need additional support to save species. The indefinite storage of cryopreserved (−196°C) viable cells and tissues (cryobanking), followed by assisted or advanced assisted reproductive technology (ART: utilisation of oocytes and spermatozoa to generate offspring; aART: utilisation of somatic cell genetic material to generate offspring), may be the only hope for species’ long-term survival. As such, cryobanking should be considered a necessity for all future conservation strategies. Following cryopreservation, ART/aART can be used to reinstate lost genetics back into a population, resurrecting biodiversity. However, for this to be successful, species-specific protocol optimisation and increased knowledge of basic biology for many taxa are required. Current ART/aART is primarily focused on mammalian taxa; however, this needs to be extended to all, including to some of the most endangered species: amphibians. Gamete, reproductive tissue and somatic cell cryobanking can fill the gap between losing genetic diversity today and future technological developments. This review explores species prioritisation for cryobanking and the successes and challenges of cryopreservation and multiple ARTs/aARTs. We here discuss the value of cryobanking before more species are lost and the potential of advanced reproductive technologies not only to halt but also to reverse biodiversity loss.
Lay summary
The world is undergoing its sixth mass extinction; however, unlike previous events, the latest is caused by human activities and is resulting in the largest loss of biodiversity (all living things on Earth) for 65 million years. With an extinction rate 1000–10,000-fold greater than natural, this catastrophic decline in biodiversity is threatening our own survival. As the number of individuals within a species declines, genetic diversity reduces, threatening their long-term existence. In this review, the authors summarise approaches to indefinitely preserve living cells and tissues at low temperatures (cryobanking) and the technologies required to resurrect biodiversity. In the future when appropriate techniques become available, these living samples can be thawed and used to reinstate genetic diversity and produce live young ones of endangered species, enabling their long-term survival. The successes and challenges of genome resource cryopreservation are discussed to enable a move towards a future of stable biodiversity.
Keywords: biobanking, cryopreservation, biodiversity, assisted reproductive technology, conservation
Introduction
Humans are causing the sixth mass extinction, the largest predicted loss of biodiversity for 65 million years, with 41% of amphibians, 26% of mammals and 14% of bird species assessed by the International Union for the Conservation of Nature (IUCN) being threatened with extinction (Ceballos et al. 2015, Ceballos & Ehrlich 2018, IUCN 2021). The catastrophic decline in biodiversity is a global threat to our own existence, affecting our economies, societal equality and way of life, including the food we eat and our climate (WHO 2015). This current loss of species is estimated to be between 1000- and 10,000-fold higher than the natural extinction rate (Ceballos et al. 2015, Turvey & Crees 2019). Human activity is changing the environment too fast for organisms to evolve in response, resulting in extinction (Ceballos & Ehrlich 2018). Restoring habitats alone will not halt the decline in biodiversity as many species are now fragmented, resulting in unviable populations with low genetic diversity (Hoban et al. 2020).
Animal conservation aims to maintain populations large enough, and with enough genetic diversity, to be sustainable (Comizzoli et al. 2019). Ex situ breeding programmes are a vital insurance policy for preserving endangered species and for enabling research, for example, into their behaviour and physiology. In zoos, pedigree-based management typically aims to maintain 90% of genetic diversity over 100 years and minimise mean kinship and inbreeding in threatened populations to retain the evolutionary potential of the species of interest (Ballou et al. 2010). Using captive breeding programmes to maintain genetic diversity is not always successful due to lack of reproduction, for example, due to unnatural social structures resulting in reduced breeding behaviour, lack of mate choice or limited number of founders potentially leading to inbreeding (Lees & Wilcken 2009). Furthermore, transporting large animals between different locations for breeding purposes comes with logistical and welfare challenges with the addition of potential disease transmission (Pukazhenthi & Wildt 2004). For some species with unsuccessful breeding programmes (Lees & Wilcken 2009), cryopreserving (freezing cells with cryoprotectants enabling long-term viable cell and tissue storage), followed by cryobanking (indefinite storage of viable cells and tissue in liquid nitrogen at −196°C or ultra-low freezers) and assisted or advanced assisted reproductive technology (ART/aART) can save the genotypes that are being lost today (Mitchell & Williams 2022, for definitions please see Supplementary Table 1, see section on supplementary materials given at the end of this article). ART includes techniques that utilise oocytes and spermatozoa to generate offspring such as artificial insemination (AI), in vitro fertilisation (IVF) or intra cytoplasmic sperm injection (ICSI) (Brown et al. 2004, Howard et al. 2016, Briski & Salamone 2022). These techniques are used for a number of taxa, but species-specific protocols need honing for many endangered species. More recently, the use of aART, technologies that utilise genetic material from somatic cells to generate offspring, has been highlighted as a key technology to resurrect biodiversity such as somatic cell nuclear transfer (SCNT) or induced pluripotent stem cells (iPSC) (Gómez et al. 2004, Hikabe et al. 2016). Prior to ART or aART, a vital aspect of conservation is storing viable cells and tissues to enable the reintroduction of genes. As a result, genetic diversity can increase within a population, allowing species to recover. This is particularly important for aART, where technology needs further development. Storing viable samples in a biobank not only enables the technology to catch up but also prevents vital genetics from being lost.
A biobank is a repository of biological samples, that is, a searchable, organised collection of biological samples and associated data stored predominantly for research or management, for example, of captive populations (Agca 2012, Hewitt & Watson 2013). Biobanking is not new; conservationists have been collecting samples from wildlife for decades to save genetic diversity (Soulé et al. 1985, Montfort 2014). These samples are vital to improve understanding of the fundamental biology of rare and endangered species (Comizzoli & Wildt 2017). Cryopreserving (freezing cells with cryoprotectants enabling long-term viable storage using liquid nitrogen at −196°C or ultra-low freezers) then storing cells in a cryobank (biobank of viable, cryopreserved biological samples), to enable the maintenance or regeneration of a species for conservation purposes, is highly specialised in that it requires the application of several complex and novel ARTs working in harmony to be truly effective. Indeed, Comizzoli and Wildt (2017) quote that cryobanking is a ‘crucial unfilled gap – offering a backup storage of the extant genomes of living species that are already under threat or are likely to be soon.’ As the biodiversity crisis continues, there is an increasing need for global conservation management of endangered species and to interconnect all populations throughout the ex situ–in situ continuum to maximise the available genetic diversity. Depending on the species’ specific needs, cryobanking could be deemed necessary in the conservation strategy for single or multiple species as part of the One Plan Approach (the One Plan Approach coined by the Conservation Planning Specialist Group of the IUCN; Lees & Wilcken 2011, Byers et al. 2013, Traylor-Holzer et al. 2018).
Successful maintenance and regeneration of a species are primarily dependent on genetic biodiversity (Choudhary et al. 2016). Heterozygosity of a population undoubtedly contributes to stabilisation and robustness of the effective population size (Soulé 1987). Without adequate genetic diversity, any species will inevitably become extinct (Ryder & Onuma 2018). For some species, such as the northern white rhinoceros (Ceratotherium simum cottoni), there are already too few individuals remaining to maintain genetic diversity for the long-term sustainability of the population (Korody et al. 2021). For these species, aART may be their only hope of long-term survival. For both ART and aART to be successful, there needs to be a knowledge of basic biology, which is lacking for many species (Herrick 2019). Indeed, there is an understanding of the reproductive physiology of only approximately 250 species, with a bias towards mammals and birds, while amphibians are most at risk of extinction (Comizzoli et al. 2019, see Case Study: Box 1, Fig. 1). This results in ART and aART developed for specific domestic animals being used as a ‘model’ for taxonomically similar wild species. One example of ART successfully being applied to selected endangered species is AI. Approaching 100 species of wild mammals and birds have been propagated by AI including giant panda (Ailuropoda melanoleuca), cheetah (Acinonyx jubatus), black-footed ferret (Mustela nigripe), Siberian crane (Leucogeranus leucogeranus) and Houbara bustard (Chlamydotis undulata) (Ballou 1984, Pukazhenthi & Wildt 2004, Andrabi & Maxwell 2007, Morrow et al. 2009, Herrick 2019, Penfold et al. 2021). In addition, it is important to highlight the successful utilisation of ART to coral species. To date, there are ~30 species of coral that have been cryobanked from coral reef populations around the world (Hagedorn et al. 2019). The frozen-thawed sperm has been used to fertilise eggs from the same spawn, successive spawns, and used for trans-regional IVF of corals (Hagedorn et al. 2012, 2017, 2018) separated by hundreds of miles, thereby increasing the heterozygosity of species. The integration of these technologies has helped mitigate the loss of heterozygosity from coral species and continued to aid in global coral conservation efforts (Hagedorn et al. 2019).
Figure 1.

Critically endangered mountain chicken frog (Leptodactylus fallax), photo © Chester Zoo, 2022; photo shared with permission. Chytridiomycosis, volcanic eruptions and habitat loss have resulted in a catastrophic decline in mountain chicken frog numbers, with less than 150 mature individuals now surviving (IUCN SSC Amphibian Specialist Group 2017). Fortunately, somatic tissue samples have been cryopreserved in living biobanks, and with poor ex situ breeding success, aART may be an additional conservation tool to prevent this species from going extinct.
Box 1 Case study preserving amphibians
Amphibians are arguably the class most at risk of extinction (Bishop et al. 2012, Ficetola et al. 2015) with populations declining faster than any other vertebrate class (Ceballos et al. 2015, IUCN SSC Amphibian Specialist Group 2017, Zimkus et al. 2018). Significantly challenged by chytridiomycosis (Van Rooij et al. 2015), climate change, declining resources, pollution, etc., (Cheng et al. 2011), many amphibians including the mountain chicken frog (Leptodactylus fallax) are on the brink of extinction (IUCN SSC Amphibian Specialist Group 2017, Fig. 1A). Amphibians also suffer from reduced research, investment and conservation advancement, including ARTs and aARTs (Kouba et al. 2013, Strand et al. 2020).
Amphibian IVF has been available since the 1950s; however, this technology has predominantly been used for non-conservation-based research (Clulow et al. 2019a, b). As amphibians utilise external fertilisation, IVF is relatively straight forward compared to that of mammals (Silla et al. 2021). After primary publication of frog IVF by Wolf and Hedrick in 1971, little additional research has been conducted (Silla & Byrne 2019). Gamete release can be induced by activation of the hypothalamic–pituitary–gonadal axis (Peter et al. 1988, Uteshev et al. 2015, Silla & Byrne 2021), and Waggener and Carroll (1998) demonstrated the first example of induced gamete release in Paraguay horned frogs (Lepidobatrachus species) with resultant fertilisation in vitro, thus validating the application of IVF to amphibian conservation. Since then, amphibian IVF has been conducted in several threatened species including Wyoming toad (Bufo baxteri) (Browne et al. 2006), corroboree frog (Pseudophryne corroboree) (Byrne & Silla 2010) and dusty gopher frog (Rana sevosa) (Kouba et al. 2012).
Amphibian semen can be refrigerated (4°C) for temporary holding or cryopreserved (−196°C) for long-term storage (Browne et al. 2002, 2019). For some species, semen has been held at 4°C for 30 days with retained viability (Browne et al. 2001), and refrigerated semen has been used in over 40 amphibian species, with outcomes including retrieval of motile sperm and fertilisation in vitro (Browne et al. 2001, Keogh et al. 2017, Gillis et al. 2021a). Refrigerated and cryopreserved semen are the two most successful ARTs for amphibians, with semen capable of storage in whole testes and sectioned testicular strips, as well as spermic urine (Poo & Hinkson 2019).
For semen cryopreservation, amphibian spermatozoa appear to be highly tolerant of prolonged exposure to cryoprotectants that other species’ cells rarely are (Clulow et al. 2019a, b). However, while semen cryopreservation is generally successful, the theoretical understanding of why the methods work is lacking (Clulow & Clulow 2016). The ability to successfully cryopreserve amphibian oocytes would be a ground-breaking development for conservation (Lawson et al. 2013); however, the success of oocyte freezing is low due to the high yolk content and large diameter (Guenther et al. 2006). An alternative would be the cryopreservation of embryos; more success may be expected here as early embryonic cells are typically smaller and contain less liquid (Lawson et al. 2013).
Cryopreservation of amphibian somatic tissue for use in aART provides additional and vital conservation resources (Strand et al. 2020). To date, there has been little research into developing tissue preservation procedures for amphibians (Stand 2021). However, the San Diego Zoo Institute for Conservation Research Frozen Zoo® already holds a large collection of cryopreserved amphibian tissue and cell lines (Chemnick et al. 2009), and the IUCN amphibian specialist group have biobank and ART working groups, so advances are being made in this area. One main challenge with amphibian skin cryopreservation and cell culture is contamination from bacteria and fungi (Strauß et al. 2013). Strand et al.(2021) have shown that even with extensive washing, contamination can still be problematic, especially as liquid nitrogen is known to not fully inhibit the replication of microorganisms (Bajerski et al. 2020). Though much work is yet to be done, amphibian cryobanking, ART and aART are undeniably exciting and hold great promise as conservation safety nets.
However, difficulties arise from the huge diversity of both reproductive physiology and behaviour between species of the same taxa, for example, among canids, domestic dogs (Canis familiaris) show spontaneous ovulation, whereas the island fox (Urocyon littoralis) only ovulates in the presence of a male (Asa et al. 2007). Furthermore, in the African wild dog (Lycaon pictus), dominant female behaviour regulates reproductive success to alpha females only (Van den Berghe et al. 2012). The complications of using techniques developed in the domestic industry for endangered species have resulted in birth rates that are significantly lower than those seen in domestic animals (Mastromonaco & Songsasen 2020). However, there are successful case studies including the now stable endangered black-footed ferret (M. nigripe) population (Santymire 2016). The lack of widespread application of ART across all taxa after more than 30 year of efforts highlights the need for alternative approaches. This includes gamete and somatic cell cryopreservation for genome resource banking (Mastromonaco & Songsasen 2020), and the application of aART, even if the production of offspring is many years away. There is also an increasing importance of developing species-specific protocols for ART/aART for endangered species to improve reproductive success rates in the future (Herrick et al. 2019, Mastromonaco & Songsasen 2020).
Both ART and aART may raise ethical issues which are rarely explored. The use of aART can remove the invasive manipulation of living animals as these techniques mainly use tissue from neutered or deceased individuals at the point of collection. However, many techniques require invasive manipulation as an end point, for example, surrogacy, cross fostering or tissue implantation and subsequent harvesting of gametes. It is important to note that these procedures must always be performed under general anaesthesia with included analgesia by a highly trained professional, minimising risk to the individuals involved. Nevertheless, ethical and welfare risk assessment should be mandatory prior to the use of them, especially as the welfare of an individual animal risks becoming a secondary consideration after the larger goal of saving a species (de Mori et al. 2021). In addition, it could be thought that the time required for successful sample collection, cryopreservation, thawing and use to make viable offspring, with all the research and development involved for species-specific optimisation, could be better spent on more traditional conservation methods (de Mori et al. 2021). But, with so many species being lost today, cryopreservation followed by viable cell and tissue cryobanking can fill the gap between permanently losing genetic diversity and the development of future technologies. This review will discuss the potential of cryobanking and the use of reproductive technologies to resurrect biodiversity.
Global prioritisation of species for cryopreservation
Only one aspect of biodiversity conservation, cryobanking, can make significant contributions to population management and species recovery, as seen in the black-footed ferret (M. nigripes) and giant panda (A. melanoleuca) (Howard et al. 2016, Santymire 2016, Comizzoli 2020). However, the immense resources required to sample, maintain and utilise biobanked samples, combined with the sheer number of threatened species requiring conservation intervention globally, mean that not every species can be sampled and conserved in this way (Hobbs et al. 2019). The current approach to the selection of species for cryobanking has been mainly opportunistic, with the collection of tissue samples on an ad hoc basis, resulting in the prioritisation of large charismatic species and missed conservation opportunities for others (Hobbs et al. 2019). If cryobanking is to be an effective and efficient biodiversity conservation tool, then it is important that the way in which we select and prioritise species for storage follows a clear, coordinated and transparent methodology (CPSG 2016, Mooney 2021).
There are multiple ways to integrate cryobanking with wildlife conservation: to support captive breeding programmes and/or to support in situ breeding programmes. Each country is likely to have its own set of priorities, and while some might favour the support of threatened populations in situ, others will focus on the support of species in ex situ populations. Ex situ genome banking will likely involve the international transport of cells, tissues and gametes and cryopreservation in facilities outside the home ranges of species. This poses some practical problems, including the risks of disease transmission via the stored samples and via the liquid nitrogen. However, even though the risk of contamination of samples preserved in liquid nitrogen (and subsequent disease transmission) is highly unlikely when appropriate techniques and safe practices are implemented (Penfold et al. 2021), it is not possible to move ungulate gametes or embryos in the United States due to the inherent disease transmission risk to the agricultural industry and associated economic threats (Joaquim et al. 2017). It is therefore important to guard against this potential disease transmission. Cryobanks set up to serve local species do not run the same risks, although the avoidance of bacterial and viral contamination is still important (Penfold & O’Brien 2012). There are a number of ways to mitigate the risk of disease transmission when transporting genetic material including disease screening of donor animals, high levels of biosecurity and using fresh, previously unused liquid nitrogen (Penfold et al. 2021). In the United States, disease transmission risk to the agricultural industry is lower for carnivores, and therefore, transportation has been achieved, for example, embryo transportation of the Brazilian ocelot (Leopardus pardalis mitis) (Conforti et al. 2009). In Europe, prior to transporation, samples from certain species may require CITES (Convention on International Trade in Endangered Species of Wild Fauna and Flora) permits. However, within the European Union (EU) generally there is no need for CITES export or import permits. Outside the EU, CITES export permits are required unless the receiving biobank is a registered scientific institution with a CITES exemption.
The utilisation of existing conservation assessment schemes, such as the International Union for the Conservation of Nature (IUCN) Red List, has been suggested as one way to prioritise species for cryobanking efforts, with more threatened species receiving greater priority (Ryder & Onuma 2018). Similarly, considering multiple assessment schemes simultaneously (such as the EDGE (Evolutionarily Distinct and Globally Endangered) of Existence and Alliance for Zero Extinction) can help to identify the most at-risk and uniquely vulnerable species and provide more nuanced species recommendations and prioritisations (Mooney 2021). Prioritising and sampling species on the brink of extinction are invaluable for scientific studies, last-gasp conservation efforts and for any future de-extinction attempts, as seen in the Pyrenean ibex (Capra pyrenaica pyrenaica) (Folch et al. 2009). However, this also results in the selection of species which already lack genetic diversity within their populations and therefore have limited prospects for meaningful conservation intervention and recovery, ultimately resulting in a limited conservation value of cryobanking such species (Hobbs et al. 2019). However, it is possible that gene editing techniques, such as CRISPR-Cas9 (Doudna & Charpentier 2014), may help overcome these problems in the future; but, many ethical considerations will need to be observed (Johnson et al. 2016, Segelbacher et al. 2021).
To improve the chances of success for conservation intervention and population management, sample collection should focus on species which still have sufficient extant population sizes and genetic diversity available to sample from (Hobbs et al. 2019, Ryder & Onuma 2018). This involves a better understanding of species population sizes and genetic diversity and the use of existing assessment schemes, for example, the IUCN Red List, to investigate which currently non-threatened species might become threatened in the future and then prioritise these species for cryobanking efforts before they suffer population declines and genetic diversity loss. Prioritising and sampling species while genetic diversity still exists, and before they become threatened, means that early intervention and genetic restoration are possible once their populations begin to decline, improving the probability of successful species recovery (Hobbs et al. 2019). Although predicting which species will become threatened, and why, is difficult (Walker et al. 2021), studies such as that by Foden et al. (2013) have identified species which are most vulnerable to future climate change, even those not currently threatened with extinction, such as the griffon vulture (Gyps fulvus), and can provide potential new priorities for conservation and cryobanking efforts (Mooney 2021).
Similarly, opportunities for sample collection should be incorporated into the species prioritisation process, as many species are found in isolated or inaccessible locations, making sample acquisition and transport both difficult and expensive (Ryder & Onuma 2018, Houck 2019). However, the global zoo and aquarium community represent a unique resource for samples, either through existing collections such as, for example, the European Association of Zoos and Aquaria (EAZA) Biobank, or new sampling drives, and can provide easier access to populations of thousands of species which are currently threatened or likely to become threatened in the future (Mooney 2021). Additionally, many zoos and aquariums have active links and partnerships with in situ conservation projects, providing opportunities to collect additional genetic samples from already represented species and from species which are not currently maintained ex situ (AZA 2015). By capitalising on sampling opportunities and utilising ex situ collections and their partnerships, we can reduce sampling costs and increase the probability that biobanked samples can be employed to help conserve and manage both in situ and ex situ populations in the future (Benirschke 1984, Clarke 2009, Mooney 2021).
The process of prioritising ex situ managed species for cryobanking also needs to consider which individuals within the population are the most genetically valuable and suitable for future conservation efforts, maximising the conservation value of banked samples and limiting the loss of genetic diversity within a population (Clarke 2009). Many of the species found in zoos and aquariums are being actively managed to maintain genetic diversity through regional or international population management programmes (Che-Castaldo et al. 2021), and the availability of such pedigree managed and potentially also genotyped populations can help to identify the most genetically valuable individuals to sample. Such strategic cryobanking efforts have helped to reintroduce once lost genetic variation into extant populations of black-footed ferrets (M. nigripes), using samples collected in 1988 from an individual which had no living descendants and was no longer genetically represented in the population (Imbler 2021) and the endangered Przewalski’s horse (Equus przewalskii) which was cloned in 2020 using samples cryopreserved in 1980 at the San Diego Zoo Institute for Conservation Research Frozen Zoo®. Unfortunately, for the many species which have yet to be sampled, such opportunities are not available, limiting the options open to conservation practitioners and population managers. Cryobanking needs to be seen as an integral part of the conservation toolkit and when used appropriately can even reduce the costs required to achieve genetic diversity retention targets compared to traditional ex situ breeding strategies (Howell et al. 2021). However, this will require the combining of both species and individual animal prioritisations to provide the most effective use of bio and cryobanking as conservation and population management tools.
Gamete and reproductive tissue cryopreservation
Spermatozoa cryopreservation is an example of just one ART that can facilitate a living cryobank capable of aiding in the genetic management of endangered species and has been achieved in many species (covered in-depth elsewhere, for example, amphibians: Browne et al. 2019; fish: Asturiano et al. 2017, Xin et al. 2017; mammals: Swanson et al. 2007, Rickard et al. 2022; avians: Asano & Tajima 2017, Cardoso et al. 2020). Storing genes in the form of spermatozoa is particularly beneficial as spermatozoa are continuously replenished haploid cells that can be collected from numerous genetically diverse representatives of a species. Furthermore, the cryopreservation of genes in the form of spermatozoa enables the application of ART to reintroduce genes back into populations. ART techniques such as AI, IVF, embryo transfer or intra cytoplasmic sperm injection (IVF, ET, ICSI) are currently the most efficient treatment modalities practised compared to alternative aART methods such as somatic nuclear transfer (Choudhary et al. 2016, Gouveia et al. 2020), with AI currently remaining the most efficient and commonly used ART technique (Holt & Lloyd 2009).
Semen can be collected from non-domestic species in many ways which include the use of an artificial vagina, transrectal massage (Schmitt & Hildebrandt 1998), electroejaculation (Roth et al. 1998), testicular sperm aspiration (Damiani et al. 2004), urethral catheterisation (Lueders et al. 2012) or post-castration dissection (ante-mortem or post-mortem) (Saragusty et al. 2010, Roth et al. 2016). The process of collecting and freezing spermatozoa from testes post-mortem is commonly referred to as ‘gamete rescue’ and is used by scientists to prevent the permanent loss of a male genetics from a population. Once spermatozoa are collected, they are diluted in a cryopreservation medium, which is formulated to mitigate damage inflicted by the cryopreservation process (Purdy 2006, Comizzoli et al. 2012). In general, cryopreservation mediums contain (1) energy substrates for spermatozoa to metabolise; (2) antioxidants to prevent the build-up of reactive oxygen species; (3) buffers to prevent harmful shifts in pH; (4) osmolytes to create an isosmotic solution; (5) plant or animal source proteins and/or lipids to stabilise the membrane; (6) antibiotics to mitigate potential risks of bacterial disease transmission and (7) a cryoprotectant (such as glycerol, DMSO, ethylene glycol, etc.), which slow down the kinetics of ice crystal formation, preventing the formation of lethal intracellular ice (Holt 2000, Fuller 2004). As each species’ physiology is inherently unique, cryopreservation mediums must be formulated to meet species-specific physiologic requirements and mitigate that species’ sensitivities to cryopreservation (Comizzoli et al. 2012).
Similar to cryopreservation media, methods to cryopreserve spermatozoa are highly diverse across taxa. As a sample is cooled and ice begins to form, the remaining solution becomes increasingly concentrated. The increased concentration of the solution results in the dehydration of the spermatozoa, preventing the formation of lethal intracellular ice. However, the increased concentration of the solution can also elicit toxic effects if the spermatozoa are exposed to the solution for too long. An optimal cooling rate varies between species and cell type, but in general, an optimal cooling rate is achieved when the rate is slow enough that spermatozoa can be dehydrated, preventing the formation of intracellular ice, but fast enough that the spermatozoa are not exposed to changes in the solution for too long. Once cryopreserved, viable spermatozoa can be stored almost indefinitely without decomposition or metabolism, typically beneath liquid nitrogen, in ‘suspended animation’ until thawing.
Even though there are some positive examples, like the black-footed ferret (M. nigripes, Howard et al. 2016) and certain coral species (Hagedorn et al. 2012, 2017), successful production of live offspring using frozen-thawed spermatozoa can be extremely variable and challenging for different species (Leibo & Songsasen 2002, Comizzoli et al. 2015). This can be observed most notably in marsupials (Taggart et al. 1996, Unwin & Pettit 2004). So far, it has not been possible to cryopreserve any marsupial spermatozoa successfully and the only successful artificial insemination in a marsupial was achieved in the koala (Phascolarctos cinereus) using chilled, but not frozen, spermatozoa (Johnston & Holt 2019). In this example, it is thought that dilution of koala semen for artificial insemination is complicated because koalas are induced ovulators, and it is thought that ovulating factors are present in the semen. Therefore, the extension of semen for preservation purposes, which involves significant dilution, might be anticipated to result in a failure to induce ovulation (Allen et al. 2008).
In other species where cryopreservation has been attempted but failed, it is likely that the failures are also due to complex underlying factors affecting viability or fertilising ability that are still poorly understood or, yet, unknown by researchers. These include factors relating to species that are relatively important from both commercial and conservation perspectives such as swine (Bailey et al. 2000, 2008), avian (Blesbois et al. 2005) and elasmobranch (Gillis et al. 2021b).
Establishing banks of frozen and viable semen from such species using conventional freezing methods is therefore not possible at present. However, it has been proposed that, on balance, spermatozoa is still worth freezing in the hope that techniques that can take advantage of the genetic material contained in currently non-viable cryopreserved gametes will become available sometime in the future (Rodger et al. 2019). Being able to quickly develop an optimal spermatozoa cryopreservation protocol for the variety of species selected as suitable candidates for cryobanking, and standardisation of these protocols, is the main challenge for researchers, especially when faced with extremely limited biological material to effectively develop a working protocol when an opportunity for spermatozoa collection arises. Furthermore, there are two important phenomenon that should also be considered. First, inbreeding depression can lead to poor spermatozoa morphology, resulting in poor fertility (Huffmeyer et al. 2022). Secondly, wild species subject to lower levels of spermatozoa competition (for instance, those limited by population size) may also result in an increase in the variability of spermatozoa morphology (Carballo et al. 2019). A morphologically homologous sperm population is a prerequisite for developing an optimal cryopreservation protocol; therefore, factors that lead to variability in morphology will significantly impede the likelihood of developing a successful protocol. However, more research is needed to ascertain the degree to which inbreeding depression and spermatozoa competition leads to poor semen freezing ability, and the precise evolutionary mechanism is yet to be explored.
Spermatozoa is commonly cryopreserved from domestic species for commercial breeding programmes with high levels of success, including domestic species of cattle, water buffalo, cats, rodents, horse, goat, deer, sheep, dog, rabbit and selected fish species (Curry 2000, Woelders et al. 2012, Kochan et al. 2019, Thongphakdee et al. 2020). It is prudent to utilise spermatozoa cryopreservation protocols already established in well-developed specimens as a reliable model for poorly understood specimens, including for species from the same genera or closely related species (Comizzoli 2015). This has been successfully demonstrated with numerous critically endangered species already, including the use of equine semen protocols as a model for rhinoceros species (Reid et al. 2009), bovine semen protocols as a model for gazelle species (Saragusty et al. 2006), domestic ferret as a model for black-footed ferret (M. nigripes) (Howard et al. 2016) and human spermatozoa cryopreservation protocols as a model for macaques (Si et al. 2010). However, for some, closely related species to use as semen cryopreservation models, do not exist. Protocols have been successfully using already established methods with slight modifications to take the variability in semen characteristics into account. Examples where this can be observed include Asian elephants (Elephas maximus) (Saragusty et al. 2009), giant panda (A. melanoleuc) (Martin-Wintle et al. 2019), bees (Comizzoli et al. 2019), killer whale (Orcinus orca) and bottlenose dolphin (Tursiops truncatus) (O’Brien & Robeck 2006, Robeck et al. 2011). A thorough understanding of the phylogenetic relationship of species is therefore important when planning an effective strategy for cryopreserving sperm from novel species. Developing forums that actively encourage knowledge transfer between cryobiologists, adequate data capture and sharing of proven spermatozoa cryopreservation protocols are also mission critical to creating an effective living biobank. Further research is also required to better understand the root causes why some species produce more cryo-sensitive spermatozoa than others. Innovative work in this field includes the successful application of novel ART such as control-rate freezers, directional cryopreservation (Saragusty et al. 2007, Reid et al. 2009), vitrification (Hunt 2017) and freeze drying of spermatozoa (Sherman 1963, Kaneko et al. 2014).
In addition, spermatozoa can be collected from the epididymis of testes following death or neutering of sexually mature individuals. However, if this technique fails, or the individual is immature, the testis remains a viable source of spermatozoa (Crabbé et al. 1997). Indeed, in domestic cats, a model for endangered wild felid species, spermatozoa has been successfully removed from cryopreserved testicular tissue by mincing thawed tissue. Via ICSI, embryos were then created, resulting in live kittens (Tharasanit et al. 2012). Furthermore, testicular spermatozoa has the potential of retaining higher viability (Chatdarong 2011). The cryopreservation of testicular tissue also increases the potential of saving important genetics from valuable animals that died unexpectedly. There are multiple techniques for the preservation of testicular tissue. Following cryopreservation, testicular fragments can be cultured in vitro to obtain viable spermatozoa, and techniques including ultra-rapid freezing have resulted in promising results (Sato et al. 2011). Effective preservation of testes is vital to maintain the functionality of retrieved spermatozoa (Pothana et al. 2017, da Silva et al. 2020). The cryopreservation of testicular tissue has been achieved for many wild mammalian species (Table 1), although this is more complex than cell cryopreservation due to increased requirements of permeation of cryoprotectant and increased heterogeneity of the tissue (Pothana et al. 2017).
Table 1.
Species for which cryopreservation of testicular tissue has been achieved.
| Species | Reference |
|---|---|
| Primates | Poels et al. (2012), Pothana et al. (2016), Fayomi et al. (2019) |
| Rhesus monkey (Macaca mulatta) | |
| Mandril (Mandrillus sphinx) | |
| Chimpanzee (Pan troglodytes) | |
| White-headed marmoset (Callithrix geoffroyi) | |
| Cervids | Thuwanut et al. (2013), Pothana et al. (2015, 2017) |
| Indian spotted mouse deer (Moschiola indica) | |
| Indian hog deer (Hyelaphus porcinus) | |
| Barking deer (Muntiacys muntjak) | |
| Sambar deer (Rusa univolor) | |
| Rusa deer (Rusa timorensis) | |
| Fea’s muntjac (Muntiacus feae) | |
| Bovids | Thuwanut et al. (2013) |
| Sumatran serow (Caprivornis sunatraensis) | |
| Felids | Thuwanut et al. (2013) |
| Jungle cat (Felis chaus) | |
| Lion (Panthera leo) | |
| Leopard (Panthera pardus) | |
| Canids | Andrae et al. (2021) |
| Grey wolf (Canis lupus) | |
| Suids | da Silva et al. (2019) |
| Collard peccary (Dicotyles tajacu) |
Slow freezing methods for testicular tissue have shown promising results with tissue showing maintenance of spermiogenesis after cryopreservation for nonhuman primates including white-headed marmoset (Calllithrix geoffroyi), mandrill (Mandrillus sphinx) and chimpanzee (Pan troglodytes) (Pothana et al. 2016). Following cryopreservation, conditions need to be met to enable the thawed tissue to resume spermatogenesis. One option is the autologous grafting of the thawed tissue (autografting: grafting tissue from original location to elsewhere in the same individual). This has been achieved for rhesus macaques (Macaca mulatta) where grafted testicular tissue produced spermatozoa, which was retrieved and used to fertilise oocytes by ICSI, resulting in embryos and a successful graft-derived baby (Fayomi et al. 2019). While complete spermatogenesis after testicular tissue cryopreservation and xenografting (grafting tissue from a donor animal into a recipient of another species) has not been achieved for adults of wild or domestic species, it has been achieved for immature individuals of ovine and swine (Arregui et al. 2008, Silva et al. 2020). However, autografting and xenografting of testicular tissue have little application for endangered species (Silva et al. 2020). An alternative method is in vitro culture of testicular tissue to initiate spermatogenesis (Lee et al. 2013, Richer et al. 2020). The success of in vitro testicular tissue culture is reliant on specific methodologies which are still to be established for endangered species making it vital to cryopreserve and biobank this tissue (Lima et al. 2020). Biobanking of tissue maintains genetic variability across time and space providing the opportunity to first develop and optimise the necessary technologies (Hildebrandt et al. 2021).
Mature oocytes can be harvested from ovarian follicles, and immature oocytes can be collected from ovarian tissues. Due to the low surface area-to-volume ratio of the oocyte, increased levels of intracellular ice formation during freezing makes cryopreservation more challenging; however, it has been attempted in a number of species (Table 2) (Borini & Bianchi 2012). This damage during the cryopreservation process is exacerbated by the low and variable membrane permeability to cryoprotectants (dependent on oocyte development state) (Leibo 1980, Arav 2014, García-Martínez et al. 2021), resulting in cellular disruption and death and leading to generally poor fertilisation rates from frozen-thawed oocytes (Tharasanit & Thuwanut 2021). Furthermore, with reference to cryopreserving oocytes from endangered animal species, the oocyte membrane permeability to cryoprotectant agents varies among species, again leading to theoretical models being used to predict likely optimal freezing protocols (Tharasanit & Thuwanut 2021). Oocyte cryopreservation is particularly challenging in fish due to the large cell volume, multiple compartments, the presence of a chorion, the low membrane permeability to cryoprotectants and a high chilling sensitivity (Asturiano et al. 2017, Diwan et al. 2020). Therefore, for those species, alternatives are intensively investigated and germ cell surrogacy via germ cell transplantation looks like one of the most promising methods (Rivers et al. 2020). Technical difficulties confronted during fish oocyte cryopreservation were already ominous for amphibian oocyte handling. The same large diameters, volumes and high yolk content can be observed in both taxa and are barriers in efficiently applying cryopreservation methods (Clulow et al. 2019a , b ) (also see case study Fig. 1). In some species, such as the domestic cat, oocytes contain high levels of lipid droplets that become disrupted during slow freezing procedures resulting in cellular injury (Okotrub et al. 2018). This may well apply to endangered felines too. As an alternative, vitrification, which avoids ice formation by using high concentrations of cryoprotectants and very rapid freezing, resulting in solidification without ice formation (Rall & Fahy 1985), has been successfully employed in oocyte cryopreservation, and, despite some problems (Prentice & Anzar 2010), the evidence suggests that this is the preferred method, at least for some species (Rienzi et al. 2017, Whaley et al. 2021). Cryopreservation of feline oocytes, domestic and non-domestic species, however, remains in an experimental phase (Jewgenow & Zahmel 2020). Post-thawing viability and developmental competence are seriously impaired and until now, none of the existing techniques could significantly improve freezing (Jewgenow & Zahmel 2020). Since there is scarcity in wild feline samples, ART protocols are being developed in the domestic cat and seem to be working out well as a model for the wild species (Fernandez-Gonzalez et al. 2021) although more research is required to verify gamete rescue methods in exotic felids (Jewgenow & Zahmel 2020).
Table 2.
Examples of mammalian species for which oocyte cryopreservation has been conducted.
| Species | Method | Reference |
|---|---|---|
| Bovine | Vitrification | Fuku et al. (1992), Hamano et al. (1992), Hurtt et al. (2000), Chian et al. (2004), Vieira et al. (2008), Nakayama et al. (2020) |
| Equine | Slow freezing | Otoi et al. (1995), Suzuki et al. (1996) |
| Vitrification | Hurtt et al. (2000), Maclellan et al. (2002), Ortiz-Escribano et al. (2018), Clérico et al. (2021) | |
| Ovine/caprine | Slow freezing | Bhat et al. (2014) |
| Vitrification | Purohit et al. (2012), Moawad et al. (2013), Bhat et al. (2014), Quan et al. (2016) | |
| Porcine | Slow freezing | Yang et al. (2012) |
| Vitrification | Vallorani et al. (2012), Appeltant et al. (2017), Jia et al. (2019), López et al. (2021) | |
| Canine | ||
| Domestic | Vitrification | Abe et al. (2010), Turathum et al. (2010) |
| Mexican grey wolf (Canis lupus baileyi) | Vitrification | Boutelle et al. (2011) |
| Blue fox (Alopex lagopus) farmed | Vitrification | Zhou et al. (2009) |
| Feline | ||
| Domestic | Slow freezing | Luvoni and Pellizzari (2000) |
| Vitrification | Fernandez-Gonzalez and Jewgenow (2017), Nowak et al. (2020), Sowińska et al. (2020), Fernandez-Gonzalez et al. (2021) | |
| Non-human primate | ||
| Lowland gorilla (Gorilla gorilla gorilla) | Slow freezing | Lanzendorf et al. (1992) |
| Macaque (Macaca mulatta) | Slow and rapid freezing | Vandevoort et al. (2008) |
An alternative approach to freezing oocytes is the cryopreservation of immature-oocyte-containing ovarian tissue, which has been conducted in a number of species (Table 3) (Martinez 2017). Since the first mouse was born in 1996 following in vitro growth of primordial follicles (Eppig & O’Brien 1996), there have been many published studies on this technique in a number of species including white-tailed deer (Odocoileus virginianu) (Gastal et al. 2018), domestic cat (Felis catus) (Mouttham & Comizzoli 2016), collared peccary (Pecari tajacu) (Lima et al. 2019), yellow-toothed cavies (Galea musteloides) (Praxedes et al. 2017), brown trout (Salmo trutta) (Lujić et al. 2017), donkey (Equus asinus) (Lopes et al. 2018) and domestic cattle (Figueiredo et al. 1993, 1994a, b, Hulshof et al. 1995, Vasconcelos et al. 2013). Following death, euthanasia or neutering, a portion of the ovary can be cryopreserved; the immature follicle-containing cortex is dissected and cut into small strips under sterile conditions before slow freezing (Benesova & Trefil 2016, Hinkle et al. 2021). As the ovary contains many follicles, there is a potential to produce large numbers of oocytes from the tissue within a laboratory. Harvested oocytes can be matured in vitro and used in IVF. Teams, including the Rhino Fertility Project, are developing the in vitro tissue culture technique to safeguard critically endangered species, including the northern white rhino (C. simum cottoni), of which there are only two individuals left, again highlighting the critical importance of viable cell cryobanking for resurrecting biodiversity. Several initiatives such as the Hemmersbach Rhino Force Cryovault (South Africa), Rhino Repro (South Africa), the Frozen Zoo (San Diego Zoo Wildlife Alliance, USA) and BioRescue (Germany) are storing rhinoceros tissue and genetic material that can be utilised once methods to produce rhinoceros calves in vitro will be established.
Table 3.
Examples of ovarian tissue cryopreservation: domestic/laboratory and wild animal species.
| Species | Freezing methodology | Cryoprotectants | Outcome | Reference |
|---|---|---|---|---|
| Domestic/laboratory | ||||
| Murine | Vitrification | Ethylene glycol and DMSO | Melatonin improved outcome post-thaw | Wu et al. (2019) |
| Porcine | Vitrification | Ethylene glycol | Jia et al. (2020) | |
| Canine | Slow freezing | DMSO and propanediol | DMSO more effective as a cryoprotectant | Lopes et al. (2016) |
| Feline | Vitrification | DMSO and ethylene glycol | Use of metal (titanium) freezing tubes proved advantageous | Fernandez-Gonzalez et al. (2021) |
| Caprine | Slow freezing | DMSO and propanediol | Rodrigues et al. (2004) | |
| Ovine | Slow freezing | DMSO and sucrose | No significant differences between techniques | Locatelli et al. (2019) |
| Vitrification | DMSO, ethylene glycol and sucrose | |||
| Bovine | Vitrification | DMSO and ethylene glycol | Leucosporidium ice-binding protein reduced post-thaw damage | Kong et al. (2021) |
| Wild | ||||
| Agouti (Dasyprocta) | Slow freezing | DMSO, ethylene glycol and propanediol | Wanderleya et al. (2012) | |
| African lion (Panthera leo) | Slow freezing | Ethylene glycol and sucrose | Wiedemann et al. (2012) | |
| Zebu (Bos indicus) | Slow freezing | Glycerol, DMSO, ethylene glycol and propanediol | DMSO and propanediol were the most effective cryoprotectants | Lucci et al. (2004) |
| Amur leopard (Panthera pardus orientalis), black-footed cat (Felis nigripes), Geoffroy’s cat (Leopardus geoffroyi), northern Chinese leopard (Panthera pardus japonensis), oncilla (Leopardus tigrinus), serval (Lupus cervarius), sumatran tiger (Panthera tigris sondaica) | Slow freezing | Ethylene glycol and sucrose | Wiedemann et al. (2013) | |
| Mexican grey wolf (Canis lupus baileyi) | Vitrification | Boutelle et al. (2011) |
Somatic cell cryopreservation and advanced assisted reproductive technology
Reproductive cloning involves the transfer of genetic material from a somatic cell into an enucleated oocyte, SCNT, ultimately resulting in an animal that has a genome sequence within the nucleus identical to that of the donor of the somatic cell used. The ability of differentiated adult cells to produce viable offspring following SCNT was first demonstrated in the African clawed frog (Xenopus laevis) by Gurdon et al. (1975) who showed that nuclei from keratinised skin cells transplanted into enucleated oocytes could develop into viable tadpoles, establishing the principle that cell nuclei do not undergo irreversible changes as the cell specialises to form adult tissues. The importance of this fundamental finding has been recognised by the award of the Nobel Prize for Physiology or Medicine jointly to Gurdon in 2012. The successful application of nuclear transfer using adult somatic cells to a mammalian species, the sheep (Ovis aries), was reported by Wilmut et al. (1997) resulting in the first cloned mammal.
SCNT can be performed either by removing the nucleus from the somatic cell and introducing it into the enucleated oocyte by microinjection (Wakayama et al. 1999), or, alternatively, the entire somatic cell can be fused with the enucleated oocyte using electrical fusion (Liu et al. 2015), of which, the latter appears to be the preferable approach (Qu et al. 2020). Oocyte maturation is required prior to fusion, with the optimum conditions varying between species (Borges & Pereira 2019). Following artificial activation, for example, with ionomycin and 6-dimethylaminopurine, which has been successful in many different species including bovines (Bhak et al. 2006), camelids (Wani et al. 2017), porcines (Borges et al. 2020) and primates (Liu et al. 2018), the egg develops to an early embryo in vitro and is then implanted into the uterus of suitable recipient female. Identification and optimisation of the three critical procedures with the greatest impact on the development of oocytes and early embryos, namely oocyte micromanipulation, electrofusion, and the in vitro culture of early embryos, have recently been reviewed (Ma et al. 2021).
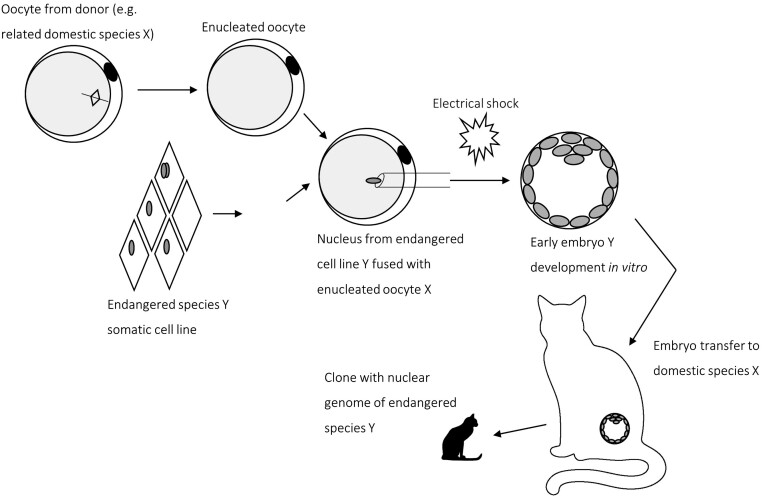
The possibility of using reproductive cloning in the conservation of endangered animal species has been widely discussed (e.g., Holt et al. 2004, Shapiro 2017, Borges & Pereira 2019). For any endangered species, it is unlikely that sufficient oocytes will be available for SCNT. Thus, cells of a separate but related species will need to be used: interspecific SCNT (iSCNT, Fig. 2). If successful, the result is the birth of an animal with the nuclear genome of the endangered individual but with the mitochondrial DNA derived from the donated oocyte. As both nuclear and mitochondrial genes regulate mitochondrial development and function (Mrowiec et al. 2021), the more closely related the species of the donor nucleus and recipient enucleated oocyte, the less likely there will be nuclear-mitochondrial incompatibility (Lagutina et al. 2013). Species or individuals created by means of interspecific cloning are considered by IUCN as ‘proxies’ which are the functional equivalents of an extinct species able to restore ecological functions or processes that might have been lost because of the extinction of the original species (IUCN 2016). However, because of, for example, microbiome differences and inheritance of the mtDNA of the donor oocyte, proxies result in a species that differs from the extinct one (IUCN 2016). Besides technical and biological hurdles, legal and ethical considerations need to be taken into account when approaching de-extinction efforts based on proxies (Seddon & King 2019).
Figure 2.
Outline of the interspecific somatic cell nuclear transfer (iSCNT) procedure. The nucleus from an endangered species’ somatic cell (species Y) is fused with the enucleated oocyte from a closely related, domestic species (species X). Following electrical or chemical activation, an early embryo of species Y developed in vitro. The early embryo is transferred into a surrogate mother of domestic species X, resulting in a clone containing the nuclear genome of endangered species Y.
Low success rates have been reported for reproductive cloning resulting in only 5–10% of reprogrammed embryos yielding viable offspring, with many factors affecting this success rate (Long et al. 2014). These factors include DNA damage, which can be improved by upregulating modulators of the DNA damage response (Lee et al. 2021), the cell type used for nuclear donation (Inoue et al. 2005, Liu et al. 2015, Lee et al. 2019) and the mismatch of mitochondrial DNA between donor cell and recipient oocyte (Takeda 2019, Mrowiec et al. 2021). Epigenetic processes may also affect DNA replication and transcription (Gouveia et al. 2020). Many of these problems occur early on in embryonic development and result from incomplete reprogramming of the donor cell nuclei and the subsequent developmental failure of the cloned embryos (Zuo et al. 2014). Choice of oocyte donor species to ensure compatibility with the somatic cell donor is also likely to be an important factor (Jeon et al. 2016).
It is particularly important to take the low success rates of reproductive cloning into consideration in the context of endangered species, where the production of viable offspring is the top priority. Although iSCNT has been applied to many species resulting in the birth of offspring, by no means all of these were viable in the long term for several reasons including morphological abnormalities, premature delivery, lung immaturity, stillbirths, placental separation and septicaemia (Table 4). Furthermore, cloning results in offspring genetically identical to the somatic cell donor and needs careful consideration where the gene pool of an endangered species is limited. In the future, it may be possible to use genome editing with CRISPR/Cas9 to address this issue (Sheets et al. 2016), albeit it also raises ethical concerns. Cryopreservation of somatic cells taken from as many different tissues as possible from each endangered animal should be conducted and stored until significant advances have been made in our understanding of the reproductive biology of individual species. This will maximise the potential of reproductive cloning in the conservation of endangered animal species, for example, the black-footed ferret (M. nigripes) and Przewalski’s horse (E. przewalskii). If we fail to collect and store these tissues now, they are gone forever.
Table 4.
Examples of interspecific somatic cell nuclear transfer (iSCNT) of mammalian species including oocyte and nuclear donor. The International Union for the Conservation of Nature (IUCN) red list status of the nucleus donor species is also included. Many of the resulting offspring did not show long-term survival, and outcome is noted where available.
| Oocyte donor | Nucleus donor | IUCN red list status of nucleus donor | Outcome | Reference |
|---|---|---|---|---|
| Domestic cat (Felis catus) | African wild cat (Felis silvestris lybica) | Least concern (subspecies unclear) | 17 kittens, 2 survived long term | Gómez et al. (2004) |
| Domestic cat (F. catus) | Sand cat (Felis margarita) | Least concern | 1 of 14 kittens born survived 2 months | Gómez et al. (2008) |
| Domestic cat (F. catus) | Cheetah (Acinonyx jubatus) | Vulnerable | Incomplete nuclear reprogramming | Moro et al. (2015) |
| Domestic cat (F. catus) | Kodkod (Leopardus guigna) | Vulnerable | Embryos only developed to the morula stage | Veraguas et al. (2020) |
| Domestic cow (Bos taurus) | Banteng (Bos javanicus) | Endangered | 2 calves, 1 survived long term | Janssen et al. (2004) |
| Domestic sheep (Ovis aries) | Mouflon (Ovis orientalis musimon) | Near threatened | 1 lamb, ‘apparently normal’ | Loi et al. (2001) |
| Domestic sheep (O. aries) | Esfahan mouflon (Ovis orientalis isphahanica) | Vulnerable | 2 lambs, both died shortly after birth | Hajian et al. (2011) |
| Spanish Ibex (Capra pyrenaica) | Pyrenian ibex, Bucardo (Capra pyrenaica pyrenaica) | Least concern (subspecies extinct) | 1 kid, died shortly after birth | Folch et al. (2009) |
| Dromedary (Camelus dromadarius) | Bactrian camel (Camelus bactrianus) | Critically endangered | 1 calf, died on day 7 post-partum | Wani et al. (2017) |
| Domestic dog (Canis familiaris) | Grey wolf (Canis lupus) | Least concern | 4 pups, 3 survived long term | Oh et al. (2008) |
| Domestic dog (C. familiaris) | Coyote (Canis latrans) | Least concern | 8 pups, all viable | Hwang et al. (2012) |
| Domestic ferret (Mustela furo) | Black-footed ferret (Mustela nigripes) | Endangered | 1 pup, survived long term | Sandler et al. (2021) |
| Macaque monkey (Macaca mulatta) | Crab-eating macaque (Macaca fascicularis) | Vulnerable | 2 young, healthy | Liu et al. (2018) |
In addition, the cryopreservation of tissues from endangered species, capable of differentiation to germ cells, could provide another way of improving genetic diversity, particularly where only small numbers of individuals remain. Embryonic stem cells can differentiate into all cell types of the body including oocytes (Hübner et al. 2003) and spermatozoa (Toyooka et al. 2003). As these cells are derived from the early embryo that is destroyed in the process, their use for endangered species is inappropriate. Induced pluripotent stem (iPSCs are derived from adult somatic cells by genetic reprogramming to an embryonic stem cell-like state (Takahashi & Yamanaka 2006) and have been used in attempts to regenerate endangered species (Fig. 3). However, some iPS cell clones differ from embryonic stem cells in several ways including gene expression, DNA methylation and cell differentiation (reviewed by Yamanaka 2012) resulting in a number of potential problems including increased immunogenicity (Okita et al. 2011). Such factors need to be taken into account when considering the use of iPS cell technology.
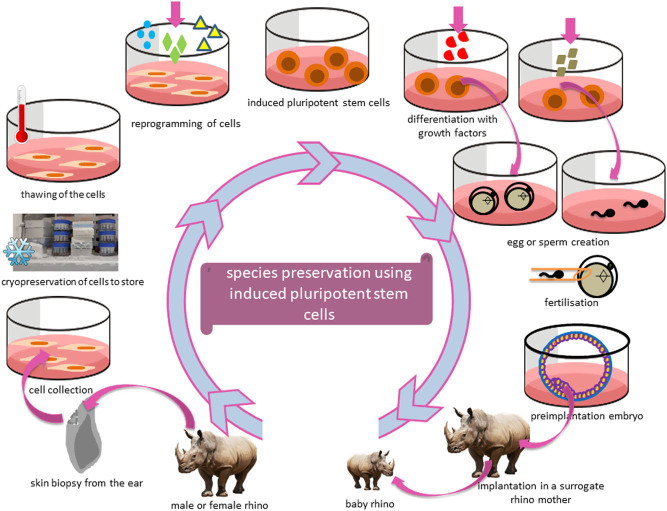
Figure 3.
Species preservation using induced pluripotent stem (iPS) cells, which can be differentiated into oocytes or spermatozoa using the rhinoceros as an example. After a biopsy of a recently deceased animal, cells can be cryopreserved until the moment they are needed to produce oocytes or spermatozoa. Those differentiated cells will first need to be reprogrammed to obtain pluripotency. Afterwards, growth factors can re-differentiate the cells into the desired cell population (oocytes or spermatozoa). In vitro fertilisation will result in an embryo, which can be transplanted into a surrogate mother leading to offspring.
For the cryopreservation and storage of samples from endangered animals, the choice of tissue is an important consideration. The tissue chosen should yield viable cells following freezing and thawing and should be readily reprogrammable to generate iPS cells. The viability of fibroblasts obtained from a wide range of taxa including domestic (e.g. pig (Liu et al. 2014), sheep (Na et al. 2010) and cow (Li et al. 2009) and endangered species (e.g. Bengal tiger (Panthera tigris tigris) (Guan et al. 2010), brown brocket deer (Mazama pandora) (Magalhães et al. 2017) and jaguar (Panthera onca) (Mestre-Citrinovitz et al. 2016) has been demonstrated post freeze/thaw. Along with this demonstrable viability, the ability to successfully reprogramme fibroblasts to iPS cells render this cell type an obvious choice. Indeed, a number of biobanks, including the San Diego Zoo Institute for Conservation Research Frozen Zoo® and The Leibniz Institute for Zoo and Wildlife Research, maintain a collection of frozen fibroblasts from multiple endangered species. In the context of cryobanking, iPS cells were first developed from frozen fibroblasts from the endangered primate the drill (Mandrillus leucophaeus) and the critically endangered northern white rhinoceros (C. simum cottoni) using viral vectors carrying the human sequences of reprogramming factors Oct4, Sox2, cMyc and KLF4 (Ben-Nun et al. 2011). Perhaps somewhat surprisingly, the rhinoceros responded to the human reprogramming factor sequences, suggesting that the reprogramming mechanism is highly conserved between different species, which bodes well for the potential application of this approach to a range of species. One caveat is that reprogrammed iPS-like cells from certain species, for example bovines, do not appear to yield sustainable cell lines (Pillai et al. 2019). The in vitro development of gametes from iPS cells, and the subsequent generation of viable embryos, is key to the application of this technology to the successful prevention of extinction of endangered species. The reconstitution of gametes from pluripotent stem cells, both oocytes (Hikabe et al. 2016, Hamazaki et al. 2021) and spermatozoa (Li et al. 2013, Ishikura et al. 2021), has been achieved in the mouse, and primordial germ cells have been developed from iPS cells in the northern white rhinoceros (C. simum cottoni) (Korody et al. 2021). iPS cells have now been derived from a variety of species (Table 5).
Table 5.
Examples of mammalian species from which induced pluripotent stem (iPS) cells have been generated and their International Union for the Conservation of Nature (IUCN) red list status.
| Species | IUCN red list status | Reference |
|---|---|---|
| Snow leopard (Panthera uncia) | Vulnerable | Verma et al. (2012) |
| Tiger (Panthera tigris) | Endangered | Verma et al. (2013) |
| Jaguar (Panthera onca) | Near threatened | Verma et al. (2013) |
| Serval (Leptailurus serval) | Least concern | Verma et al. (2013) |
| Somali wild ass (Equus africanus somaliensis) | Critically endangered | Ben-Nun et al. (2015) |
| Northern white rhinoceros (Ceratotherium simum cottoni) | Critically endangered | Ben-Nun et al. (2011) |
| Banteng (Bos javanicus javanicus) | Endangered | Ben-Nun et al. (2015) |
| Sumatran orangutan (Pongo abelii) | Critically endangered | Ramaswamy et al. (2015) |
| Drill (Mandrillus leucophaeus) | Endangered | Ben-Nun et al. (2011) |
| Chimpanzee (Pan troglodytes) | Endangered | Marchetto et al. (2013) |
| Bonobo (Pan paniscus) | Endangered | Marchetto et al. (2013) |
| Western gorilla (Gorilla gorilla gorilla) | Critically endangered | Wunderlich et al. (2014) |
| Prairie vole (Microtus ochrogaster) | Least concern | Manoli et al. (2012) |
| Naked mole-rat (Heterocephalus glaber) | Least concern | Lee et al. (2017) |
| Tasmanian devil (Sarcophilus harrisii) | Endangered | Weeratunga et al. (2018) |
| Little brown bat (Myotis lucifugus) | Endangered | Mo et al. (2014) |
| Platypus (Ornithorhynchus anatinus) | Near threatened | Whitworth et al. (2019) |
| Quail (Coturnix) | Least concern | Lu et al. (2015) |
| Zebra fish (Danio rerio) | Least concern | Peng et al. (2019) |
Before iPS cells or even SCNT techniques being used to produce viable offspring, a much greater knowledge of the reproductive biology of both the embryo and the surrogate dam will be vital. Pregnancy is a major challenge for the mammalian maternal immune system with specific mechanisms including the induction by the decidua of regulatory M2 macrophages and Treg cells to elicit immune tolerance at the foetal–maternal interface to prevent rejection of the semi-allogeneic (sharing only half the genes of the mother) foetus (Lindau et al. 2021). The increased rate of spontaneous abortion seen in cattle pregnancies produced following SCNT has been attributed to the upregulation of inflammatory cytokines resulting from abnormal expression of major histocompatibility complex class 1 proteins on the trophoblast of these conceptuses (Rutigliano et al. 2022). Rejection is likely to be considerably more problematic with a xenogeneic (from a different species) foetus. Furthermore, this may be a particular problem for those species with a haemochorial placental structure, including primates and some rodents, where maternal blood comes into direct contact with the foetal chorion. It has been proposed that separating the inner cell mass (ICM) from an endangered species’ early embryo and injecting this into a trophoblast vesicle derived from the putative surrogate dam may overcome such problems of incompatibility (Saragusty et al. 2020). However, interactions between the trophoblast and ICM may pose challenges if these are derived from different species (Girgin et al. 2021). Furthermore, other potential issues of developing viable offspring cross-species include the role of exosomes (endosomal-derived membrane nanovesicles involved in intercellular communication (Zhang et al. 2019)) in implantation and early embryo development (Shi et al. 2021) and the acquisition of a suitable microbiome for the development of immune function of the neonate (Macpherson et al. 2017). These and many other problems will need to be overcome in the future, and the fundamental importance of long-term cryobanking of live cells from endangered species needs to be underlined.
Conclusion
ART and aART have a huge potential for wildlife conservation, and therefore, many advances have been made in the last few decades. However, for these techniques to be applicable and therefore useful, prompt global cooperation and action are imperative. Knowledge sharing, data and sample inventory sharing and creating international networks of biobanks are paramount. Indeed, it is vital that protocols for all techniques are standardised, and for this, global collaboration is required. This is particularly important when research groups/biobanks only have access to very limited biological material to develop protocols on an ad hoc basis. Furthermore, viable tissue cryobanking should be considered in all future conservation strategies as a source of genetically diverse material that may be required in the future to combat the extinction crisis (Comizzoli & Holt 2019). Great progress is already being made to save endangered species by using ART and aART, as demonstrated by the successful captive propagation and reintroduction using ART of endangered Mississippi gopher frogs in the United States (Watt et al. 2021), efforts to breed cheetahs and other endangered felids via ART and aART (Wildt & Roth 1997) and the northern white rhino project to name just a few. However, despite the above example, the current focus is still primarily on mammalian taxa, and there is an urgent need for allowing these technologies to catch up across all others. These technologies open the way for more innovative conservation strategies and integration of traditional conservation methods with biologically based safety nets for species in danger. Focus needs to be placed on less charismatic taxa and the development of cryopreservation and storage protocols for tissues from avian, amphibian, reptilian, piscine and even invertebrate species in addition to the development of ART and aART for these taxa. This field of science is fast moving and vital to future biodiversity conservation efforts and will be a ‘hot topic’ as the ethical debate surrounding these technologies comes to the fore with the advent of new techniques and possibilities. We need to start banking samples before we lose more species, populations and genetic diversity; we do not know what will be needed in the future.
Supplementary Material
Declaration of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of this review.
Funding
This study did not receive any specific grant from any funding agency in the public, commercial or not-for-profit sector.
Author contribution statement
R L B drafted main body of text with additions from A M, M T P, A E B, J G and L M. R A provided Fig. 3. G J D, S L W and C H commented and edited text. All authors commented on and edited final version then approved for publication.
Acknowledgements
The authors would like to thank the guest editor Rod Mitchell (University of Edinburgh) and the Series Associate Editor Suzannah Williams (University of Oxford) for an invitation to include a review in the current series (Mitchell & Williams 2022). Also, the reviewers who helped to improve the manuscript greatly.
References
- Abe Y, Asano T, Ali M, Suzuki H.2010Vitrification of canine cumulus-oocyte complexes in DAP213 with a cryotop holder. Reproductive Medicine and Biology 9115–120. ( 10.1007/s12522-010-0045-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen CD, Burridge M, Mulhall S, Chafer ML, Nicolson VN, Pyne M, Zee YP, Jago SC, Lundie-Jenkins G, Holt WVet al. 2008Successful artificial insemination in the koala (Phascolarctos cinereus) using extended and extended-chilled semen collected by electroejaculation. Biology of Reproduction 78661–666. ( 10.1095/biolreprod.107.064824) [DOI] [PubMed] [Google Scholar]
- Andrabi SMH, Maxwell WMC.2007A review on reproductive biotechnologies for conservation of endangered mammalian species. Animal Reproduction Science 99223–243. ( 10.1016/j.anireprosci.2006.07.002) [DOI] [PubMed] [Google Scholar]
- Andrae CS, Oliveira ECS, Ferraz MAMM, Nagashima JB.2021Cryopreservation of grey wolf (Canis lupus) testicular tissue. Cryobiology 100173–179. ( 10.1016/j.cryobiol.2021.01.010) [DOI] [PubMed] [Google Scholar]
- Appeltant R, Somfai T, Santos ECS, Dang-Nguyen TQ, Nagai T, Kikuchi K.2017Effects of vitrification of cumulus-enclosed porcine oocytes at the germinal vesicle stage on cumulus expansion, nuclear progression and cytoplasmic maturation. Reproduction, Fertility, and Development 292419–2429. ( 10.1071/RD16386) [DOI] [PubMed] [Google Scholar]
- Arav A.2014Cryopreservation of oocytes and embryos. Theriogenology 8196–102. ( 10.1016/j.theriogenology.2013.09.011) [DOI] [PubMed] [Google Scholar]
- Arregui L, Rathi R, Zeng W, Honaramooz A, Gomendio M, Roldan ER, Dobrinski I.2008Xenografting of adult mammalian testis tissue. Animal Reproduction Science 10665–76. ( 10.1016/j.anireprosci.2007.03.026) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asa CS, Bauman JE, Coonan TJ, Gray MM.2007Evidence for induced estrus or ovulation in a canid, the island fox (Urocyon littoralis). Journal of Mammalogy 88436–440. ( 10.1644/05-MAMM-A-295R2.1) [DOI] [Google Scholar]
- Asano A, Tajima A.2017Development and preservation of avian sperm. Advances in Experimental Medicine and Biology 100159–73. ( 10.1007/978-981-10-3975-1_4) [DOI] [PubMed] [Google Scholar]
- Asturiano JF, Cabrita E, Horváth Á.2017Progress, challenges and perspectives on fish gamete cryopreservation: a mini-review. General and Comparative Endocrinology 24569–76. ( 10.1016/j.ygcen.2016.06.019) [DOI] [PubMed] [Google Scholar]
- AZA 2015Annual Report on Conservation and Science (Online). Silver Spring, Maryland. (available at: arcs_2015.pdf (speakcdn.com)). Accessed on 2 December 2021. [Google Scholar]
- Bailey JL, Bilodeau JF, Cormier N.2000Semen cryopreservation in domestic animals: a damaging and capacitating phenomenon. Journal of Andrology 211–7. [PubMed] [Google Scholar]
- Bailey JL, Lessard C, Jacques J, Brèque C, Dobrinski I, Zeng W, Galantino-Homer HL.2008Cryopreservation of boar semen and its future importance to the industry. Theriogenology 701251–1259. ( 10.1016/j.theriogenology.2008.06.014) [DOI] [PubMed] [Google Scholar]
- Bajerski F, Bürger A, Glasmacher B, Keller ERJ, Müller K, Mühldorfer K, Nagel M, Rüdel H, Müller T, Schenkel Jet al. 2020Factors determining microbial colonization of liquid nitrogen storage tanks used for archiving biological samples. Applied Microbiology and Biotechnology 104131–144. ( 10.1007/s00253-019-10242-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballou JD.1984Strategies for maintaining genetic diversity in captive populations through reproductive technology. Zoo Biology 3311–323. ( 10.1002/zoo.1430030404) [DOI] [Google Scholar]
- Ballou JD Lees C Faust LJ Long S Lynch C Bingaman Lackey L & Foose TJ. 2010. Demographic and genetic management of captive populations Aug 15;219. In Wild mammals in captivity: principles and techniques for zoo management 2nd Edition. Eds DG Kleiman, KV Thompson and CK Baer. The University of Chicago Press [Google Scholar]
- Benesova B, Trefil P.2016Possibilities for preserving genetic resources in birds. World’s Poultry Science Journal 72629–642. ( 10.1017/S0043933916000489) [DOI] [Google Scholar]
- Benirschke K.1984The frozen zoo concept. Zoo Biology 3325–328. ( 10.1002/zoo.1430030405) [DOI] [Google Scholar]
- Ben-Nun IF, Montague SC, Houck ML, Tran HT, Garitaonandia I, Leonardo TR, Wang YC, Charter SJ, Laurent LC, Ryder OAet al. 2011Induced pluripotent stem cells from highly endangered species. Nature Methods 8829–831. ( 10.1038/nmeth.1706) [DOI] [PubMed] [Google Scholar]
- Ben-Nun IF, Montague SC, Houck ML, Ryder O, Loring JF.2015Generation of induced pluripotent stem cells from mammalian endangered species. In Cell Reprogramming: Methods and Protocols. Eds Verma PJ, Sumer H. New York, NY: Springer. [DOI] [PubMed] [Google Scholar]
- Bhak JS, Lee SL, Ock SA, Mohana Kumar B, Choe SY, Rho GJ.2006Developmental rate and ploidy of embryos produced by nuclear transfer with different activation treatments in cattle. Animal Reproduction Science 9237–49. ( 10.1016/j.anireprosci.2005.04.016) [DOI] [PubMed] [Google Scholar]
- Bhat MH, Sharma V, Khan FA, Naykoo NA, Yaqoob SH, Ruby K, Khan HM, Fazili MR, Ganai NA, Shah RA.2014Comparison of slow freezing and vitrification on ovine immature oocytes. Cryo Letters 3577–82. [PubMed] [Google Scholar]
- Bishop PJ, Angulo A, Lewis JP, Moore RD, Rabb GB, Moreno JG.2012The Amphibian Extinction Crisis – what will it take to put the action into the Amphibian Conservation Action Plan? Sapiens 597–111. [Google Scholar]
- Blesbois E, Grasseau I, Seigneurin F.2005Membrane fluidity and the ability of domestic bird spermatozoa to survive cryopreservation. Reproduction 129371–378. ( 10.1530/rep.1.00454) [DOI] [PubMed] [Google Scholar]
- Borges AA, Pereira AF.2019Potential role of intraspecific and interspecific cloning in the conservation of wild mammals. Zygote 27111–117. ( 10.1017/S0967199419000170) [DOI] [PubMed] [Google Scholar]
- Borges AA, Santos MVO, Nascimento LE, Lira GPO, Praxedes ÉA, Oliveira MF, Silva AR, Pereira AF.2020Production of collared peccary (Pecari tajacu Linnaeus, 1758) parthenogenic embryos following different oocyte chemical activation and in vitro maturation conditions. Theriogenology 142320–327. [DOI] [PubMed] [Google Scholar]
- Borini A, Bianchi V.2012Oocyte cryopreservation. In Fertility Preservation. Eds E Seli and A Agarwal [Google Scholar]
- Boutelle S, Lenahan K, Krisher R, Bauman KL, Asa CS, Silber S.2011Vitrification of oocytes from endangered Mexican gray wolves (Canis lupus baileyi). Theriogenology 75647–654. ( 10.1016/j.theriogenology.2010.10.004) [DOI] [PubMed] [Google Scholar]
- Briski O, Salamone DF.2022Past, present and future of ICSI in livestock species. Animal Reproduction Science In press. ( 10.1016/j.anireprosci.2022.106925) [DOI] [PubMed] [Google Scholar]
- Brown J L, Goritz F, Pratt-Hawkes N, Hermes R, Galloway M, Graham L H, Gray C, Walker S L, Gomez A, Moreland R. 2004. Successful artificial insemination of an Asian elephant at the National Zoological Park. Zoo Biology 2345-63. [Google Scholar]
- Browne RK, Clulow J, Mahony M.2001Short-term storage of cane toad (Bufo marinus) gametes. Reproduction 121167–173. ( 10.1530/rep.0.1210167) [DOI] [PubMed] [Google Scholar]
- Browne RK, Clulow J, Mahony M.2002The short-term storage and cryopreservation of spermatozoa from hylid and myobatrachid frogs. Cryo Letters 23129–136. [PubMed] [Google Scholar]
- Browne RK, Seratt J, Vance C, Kouba A.2006Hormonal priming, induction of ovulation and in-vitro fertilization of the endangered Wyoming toad (Bufo baxteri). Reproductive Biology and Endocrinology 4 34. ( 10.1186/1477-7827-4-34) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browne RK, Silla AJ, Upton R, Della-Togna G, Marcec-Greaves R, Shishova NV, Uteshev VK, Proaño B, Pérez OD, Mansour Net al. 2019Sperm collection and storage for the sustainable management of amphibian biodiversity. Theriogenology 133187–200. ( 10.1016/j.theriogenology.2019.03.035) [DOI] [PubMed] [Google Scholar]
- Byers O, Lees C, Wilcken J, Schwitzer C.2013The one plan approach: the philosophy and implementation of CBSG’s approach to integrated species conservation planning. WAZA Magazine 142–5. (available at: www.waza.org/en/site/conservation/integrated-species-conservation) [Google Scholar]
- Byrne PG, Silla AJ.2010Hormonal induction of gamete release, and in-vitro fertilisation, in the critically endangered southern corroboree frog, Pseudophryne corroboree. Reproductive Biology and Endocrinology 8 144. ( 10.1186/1477-7827-8-144) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carballo L, Battistotti A, Teltscher K, Lierz M, Bublat A, Valcu M, Kempenaers B.2019Sperm morphology and evidence for sperm competition among parrots. Journal of Evolutionary Biology 32856–867. ( 10.1111/jeb.13487) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardoso B, Sánchez-Ajofrín I, Castaño C, García-Álvarez O, Esteso MC, Maroto-Morales A, Iniesta-Cuerda M, Garde JJ, Santiago-Moreno J, Soler AJ.2020Optimization of sperm cryopreservation protocol for peregrine falcon (Falco peregrinus). Animals 10 691. ( 10.3390/ani10040691) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceballos G, Ehrlich PR.2018The misunderstood sixth mass extinction. Science 3601080–10. [DOI] [PubMed] [Google Scholar]
- Ceballos G, Ehrlich PR, Barnosky AD, García A, Pringle RM, Palmer TM.2015Accelerated modern human-induced species losses: entering the sixth mass extinction. Science Advances 1 e1400253. ( 10.1126/sciadv.1400253) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatdarong K.2011Gamete rescues from gonads of wild animal post mortem. Thai Journal of Veterinary Medicine 4199–102. [Google Scholar]
- Che-Castaldo J, Gray SM, Rodriguez-Clark KM, Schad Eebes K, Faust LJ.2021Expected demographic and genetic declines not found in most zoo and aquarium populations. Frontiers in Ecology and the Environment 19435–442. ( 10.1002/fee.2362) [DOI] [Google Scholar]
- Chemnick LG, Houck ML, Ryder OA.2009Banking of genetic resources: the Frozen Zoo® at the San Diego Zoo. In Conservation Genetics in the Age of Genomics, pp. 124–130. Eds Amato G, Desalle R, Rosenblum HC, Ryder OA. New York, NY, USA: Columbio; University Press. [Google Scholar]
- Cheng TL, Rovito SM, Wake DB, Vredenburg VT.2011Coincident mass extirpation of Neotropical amphibians with the emergence of the infectious fungal pathogen Batrachochytrium dendrobatidis. PNAS 1089502–9507. ( 10.1073/pnas.1105538108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chian RC, Kuwayama M, Tan L, Tan J, Kato O, Nagai T.2004High survival rate of bovine oocytes matured in vitro following vitrification. Journal of Reproduction and Development 50685–696. ( 10.1262/jrd.50.685) [DOI] [PubMed] [Google Scholar]
- Choudhary KK, Kavya KM, Jerome A, Sharma RK.2016Advances in reproductive biotechnologies. Veterinary World 9388–395. ( 10.14202/vetworld.2016.388-395) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke AG.2009The Frozen Ark Project: the role of zoos and aquariums in preserving the genetic material of threatened animals. International Zoo Yearbook 43222–230. ( 10.1111/j.1748-1090.2008.00074.x) [DOI] [Google Scholar]
- Clérico G, Taminelli G, Veronesi JC, Polola J, Pagura N, Pinto C, Sansinena M.2021Mitochondrial function, blastocyst development and live foals born after ICSI of immature vitrified/warmed equine oocytes matured with or without melatonin. Theriogenology 16040–49. ( 10.1016/j.theriogenology.2020.10.036) [DOI] [PubMed] [Google Scholar]
- Clulow J, Clulow S.2016Cryopreservation and other assisted reproductive technologies for the conservation of threatened amphibians and reptiles: bringing the ARTs up to speed. Reproduction, Fertility, and Development 281116––1132.. ( 10.1071/RD15466) [DOI] [PubMed] [Google Scholar]
- Clulow J, Upton R, Trudeau VL, Clulow S.2019aAmphibian assisted reproductive technologies: moving from technology to application. In Reproductive Sciences in Animal Conservation. Advances in Experimental Medicine and Biology, vol. 1200, pp. 413–463. Eds Comizzoli P, Brown J, Holt W. Cham: Springer. ( 10.1007/978-3-030-23633-5_14) [DOI] [PubMed] [Google Scholar]
- Clulow J, Upton R, Trudeau VL, Clulow S.2019bAmphibian assisted reproductive technologies: moving from technology to application. Advances in Experimental Medicine and Biology 1200413–463. ( 10.1007/978-3-030-23633-5_14) [DOI] [PubMed] [Google Scholar]
- Comizzoli P.2015Biobanking efforts and new advances in male fertility preservation for rare and endangered species. Asian Journal of Andrology 17640–645. ( 10.4103/1008-682X.153849) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comizzoli P.2020Birth of a giant panda cub after artificial insemination with frozen-thawed semen: a powerful reminder about the key role of biopreservation and biobanking for wildlife conservation. Biopreservation and Biobanking 18349–350. ( 10.1089/bio.2020.29076.pjc) [DOI] [PubMed] [Google Scholar]
- Comizzoli P, Holt WV.2019Breakthroughs and new horizons in reproductive biology of rare and endangered animal species. Biology of Reproduction 101514–525. ( 10.1093/biolre/ioz031) [DOI] [PubMed] [Google Scholar]
- Comizzoli P, Wildt DE.2017Cryobanking biomaterials from wild animal species to conserve genes and biodiversity: relevance to human biobanking and biomedical research. In Biobanking of Human Biospecimens: Principles and Practice. Eds Hainaut P, Vaught J, Zatloukal K, Pasterk M. Cham: Springer International Publishing. [Google Scholar]
- Comizzoli P, Songsasen N, Hagedorn M, Wildt DE.2012Comparative cryobiological traits and requirements for gametes and gonadal tissues collected from wildlife species. Theriogenology 781666–1681. ( 10.1016/j.theriogenology.2012.04.008) [DOI] [PubMed] [Google Scholar]
- Comizzoli P, Brown JL, Holt WV.2019Reproductive science as an essential component of conservation biology: new edition. In Reproductive Sciences in Animal Conservation. Eds Comizzoli P, Brown JL, Holt WV. Cham: Springer International Publishing. [Google Scholar]
- Conforti VA, Adania CH, Gonzalez PG, De Oliveira C, Swanson WF.2009155 Novel recipient synchronization regimens for successful embryo transfer in the Brazilian ocelot following long-term frozen embryo storage. Reproduction, Fertility and Development 21176–177. ( 10.1071/RDv21n1Ab155) [DOI] [Google Scholar]
- CPSG 2016Prioritizing the collection of samples for genetic rescue. In IUCN CPSG Annual Meeting. Gland, Switzerland: IUCN; SSC Conservation Planning Specialist Group. [Google Scholar]
- Crabbé E, Verheyen G, Tournaye H, Van Steirteghem A.1997The use of enzymatic procedures to recover testicular germ cells. Human Reproduction 121682–1687. ( 10.1093/humrep/12.8.1682) [DOI] [PubMed] [Google Scholar]
- Curry MR.2000Cryopreservation of semen from domestic livestock. Reviews of Reproduction 546–52. ( 10.1530/ror.0.0050046) [DOI] [PubMed] [Google Scholar]
- Da Silva AM, Bezerra LGP, Praxedes ECG, Moreira SSJ, De Souza CMP, De Oliveira MF, Pereira AF, Comizzoli P, Silva AR.2019Combination of intracellular cryoprotectants preserves the structure and the cells proliferative capacity potential of adult collared peccary testicular tissue subjected to solid surface vitrification. Cryobiology 9153–60. ( 10.1016/j.cryobiol.2019.10.199) [DOI] [PubMed] [Google Scholar]
- Damiani P, Gomez M, Cole A, Pope E, Aguilar R, Hammond B, Nel L, Cortez C, Vaccaro J, Sarrat Eet al. 2004The production of intracytoplasmic sperm injection lion (Panthera leo) embyros using spermatozoa collected by percutaneous epididymal sperm aspiration from vasectomized males. Reproduction, Fertility and Development 16223–224. ( 10.1071/RDv16n1Ab204) [DOI] [Google Scholar]
- De Mori B, Spiriti MM, Pollastri I, Normando S, Biasetti P, Florio D, Andreucci F, Colleoni S, Galli C, Göritz Fet al. 2021An ethical assessment tool (ETHAS) to evaluate the application of assisted reproductive technologies in mammals’ conservation: the case of the northern white rhinoceros (Ceratotherium simum cottoni). Animals 11 312. ( 10.3390/ani11020312) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diwan AD, Harke SN, Panche AN, Panche AN.2020Cryobanking of fish and shellfish egg, embryos and larvae: an overview. Frontiers in Marine Science 7 251. ( 10.3389/fmars.2020.00251) [DOI] [Google Scholar]
- Doudna JA, Charpentier E.2014Genome editing. The new frontier of genome engineering with CRISPR-Cas9. Science 346 1258096. ( 10.1126/science.1258096) [DOI] [PubMed] [Google Scholar]
- Eppig JJ, O’Brien MJ.1996Development in vitro of mouse oocytes from primordial follicles. Biology of Reproduction 54197–207. ( 10.1095/biolreprod54.1.197) [DOI] [PubMed] [Google Scholar]
- Fayomi AP, Peters K, Sukhwani M, Valli-Pulaski H, Shetty G, Meistrich ML, Houser L, Robertson N, Roberts V, Ramsey Cet al. 2019Autologous grafting of cryopreserved prepubertal rhesus testis produces sperm and offspring. Science 3631314–1319. ( 10.1126/science.aav2914) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Gonzalez L, Jewgenow K.2017Cryopreservation of feline oocytes by vitrification using commercial kits and slush nitrogen technique. Reproduction in Domestic Animals 52 (Supplement 2) 230–234. ( 10.1111/rda.12837) [DOI] [PubMed] [Google Scholar]
- Fernandez-Gonzalez L, Huebinger J, Jewgenow K.2021Comparison of different materials for self-pressurized vitrification of feline oocytes – first results. Animals 11 1314. ( 10.3390/ani11051314) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ficetola GF, Rondinini C, Bonardi A, Baisero D, Padoa-Schioppa E.2015Habitat availability for amphibians and extinction threat: a global analysis. Diversity and Distributions 21302–311. ( 10.1111/ddi.12296) [DOI] [Google Scholar]
- Figueiredo JR, Hulshof SCJ, Van Den Hurk R, Ectors FJ, Fontes RS, Nusgens B, Bevers MM, Beckers JF.1993Development of a combined new mechanical and enzymatic method for the isolation of intact preantral follicles from fetal, calf and adult bovine ovaries. Theriogenology 40789–799. ( 10.1016/0093-691x(9390214-p) [DOI] [PubMed] [Google Scholar]
- Figueiredo JR, Hulshof SCJ, Van Den Hurk R, Bevers MM, Thiry M, Nusgens B, Beckers JF.1994aThe physiological status of the ovarian donor affects in vitro development of isolated bovine preantral follicles. Theriogenology 421303–1310. ( 10.1016/0093-691X(9490250-M) [DOI] [Google Scholar]
- Figueiredo JR, Hulshof SCJ, Van Den Hurk R, Nusgens B, Bevers MM, Ectors FJ, Beckers JF.1994bPreservation of oocyte and granulosa cell morphology in bovine preantral follicles cultured in vitro. Theriogenology 411333–1346. ( 10.1016/0093-691x(9490492-2) [DOI] [PubMed] [Google Scholar]
- Foden WB, Butchart SH, Stuart SN, Vié JC, Akçakaya HR, Angulo A, Devantier LM, Gutsche A, Turak E, Cao Let al. 2013Identifying the world’s most climate change vulnerable species: a systematic trait-based assessment of all birds, amphibians and corals. PLoS ONE 8 e65427. ( 10.1371/journal.pone.0065427) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folch J, Cocero MJ, Chesné P, Alabart JL, Domínguez V, Cognié Y, Roche A, Fernández-Árias A, Martí JI, Sánchez Pet al. 2009First birth of an animal from an extinct subspecies (Capra pyrenaica pyrenaica) by cloning. Theriogenology 711026–1034. ( 10.1016/j.theriogenology.2008.11.005) [DOI] [PubMed] [Google Scholar]
- Friedrich Ben-Nun IF, Montague SC, Houck ML, Tran HT, Garitaonandia I, Leonardo TR, Wang YC, Charter SJ, Laurent LC, Ryder OAet al. 2011Induced pluripotent stem cells from highly endangered species. Nature Methods 8829–831. ( 10.1038/nmeth.1706) [DOI] [PubMed] [Google Scholar]
- Fuku E, Kojima T, Shioya Y, Marcus GJ, Downey BR.1992In vitro fertilization and development of frozen-thawed bovine oocytes. Cryobiology 29485–492. ( 10.1016/0011-2240(9290051-3) [DOI] [PubMed] [Google Scholar]
- Fuller BJ.2004Cryoprotectants: the essential antifreezes to protect life in the frozen state. Cryo Letters 25375–388. [PubMed] [Google Scholar]
- Fuller B, Paynter S.2004Fundamentals of cryobiology in reproductive medicine. Reproductive Biomedicine Online 9680–691. ( 10.1016/s1472-6483(1061780-4) [DOI] [PubMed] [Google Scholar]
- García-Martínez T, Mogas T, Mullen SF, Martínez-Rodero I, Gulieva RE, Higgins AZ.2021Effect of cryoprotectant concentration on bovine oocyte permeability and comparison of two membrane permeability modelling approaches. Scientific Reports 11 15387. ( 10.1038/s41598-021-94884-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gastal GDA, Aguiar FLN, Rodrigues APR, Scimeca JM, Apgar GA, Banz WJ, Feugang JM, Gastal EL.2018Cryopreservation and in vitro culture of white-tailed deer ovarian tissue. Theriogenology 113253–260. ( 10.1016/j.theriogenology.2018.03.003) [DOI] [PubMed] [Google Scholar]
- Gillis AB, Guy EL, Kouba AJ, Allen PJ, Marcec-Greaves RM, Kouba CK.2021aShort-term storage of tiger salamander (Ambystoma tigrinum) spermatozoa: the effect of collection type, temperature and time. PLoS ONE 16e0245047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillis JD, Penfold LM, Mylniczenko ND.2021bInitial characterization of male southern stingray (Hypanus americanus) reproductive parameters and preliminary investigation of sperm cryopreservation. Animals 11 2716. ( 10.3390/ani11092716) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girgin MU, Broguiere N, Hoehnel S, Brandenberg N, Mercier B, Arias AM, Lutolf MP.2021Bioengineered embryoids mimic post-implantation development in vitro. Nature Communications 12 5140. ( 10.1038/s41467-021-25237-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez MC, Pope CE, Giraldo A, Lyons LA, Harris RF, King AL, Cole A, Godke RA, Dresser BL.2004Birth of African wildcat cloned kittens born from domestic cats. Cloning and Stem Cells 6247–258. ( 10.1089/clo.2004.6.247) [DOI] [PubMed] [Google Scholar]
- Gómez MC, Pope CE, Kutner RH, Ricks DM, Lyons LA, Ruhe M, Dumas C, Lyons J, López M, Dresser BLet al. 2008Nuclear transfer of sand cat cells into enucleated domestic cat oocytes is affected by cryopreservation of donor cells. Cloning and Stem Cells 10469–483. ( 10.1089/clo.2008.0021) [DOI] [PubMed] [Google Scholar]
- Gouveia C, Huyser C, Egli D, Pepper MS.2020Lessons learned from somatic cell nuclear transfer. International Journal of Molecular Sciences 21 2314. ( 10.3390/ijms21072314) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan WJ, Liu CQ, Li CY, Liu D, Zhang WX, Ma YH.2010Establishment and cryopreservation of a fibroblast cell line derived from Bengal tiger (Panthera tigris tigris). Cryo Letters 31130–138. [PubMed] [Google Scholar]
- Guenther JF, Seki S, Kleinhans FW, Edashige K, Roberts DM, Mazur P.2006Extra- and intra-cellular ice formation in stage I and II Xenopus laevis oocytes. Cryobiology 52401–416. ( 10.1016/j.cryobiol.2006.02.002) [DOI] [PubMed] [Google Scholar]
- Gurdon JB, Laskey RA, Reeves OR.1975The developmental capacity of nuclei transplanted from keratinized skin cells of adult frogs. Journal of Embryology and Experimental Morphology 3493–112. ( 10.1242/dev.34.1.93) [DOI] [PubMed] [Google Scholar]
- Hagedorn M, Carter V, Martorana K, Paresa MK, Acker J, Baums IB, Borneman E, Brittsan M, Byers M, Henley Met al. 2012Preserving and using germplasm and dissociated embryonic cells for conserving Caribbean and Pacific coral. PLoS ONE 7 e33354. ( 10.1371/journal.pone.0033354) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagedorn M, Carter VL, Henley EM, Van Oppen MJH, Hobbs R, Spindler RE.2017Producing coral offspring with cryopreserved sperm: a tool for coral reef restoration. Scientific Reports 7 14432. ( 10.1038/s41598-017-14644-x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagedorn M, Page CA, Oneill K, Flores DM, Tichy L, Chamberland VF, Lager C, Zuchowicz N, Lohr K, Blackburn Het al. 2018Successful demonstration of assisted gene flow in the threatened coral Acropora palmata across genetically- isolated Caribbean populations using cryopreserved sperm. bioRxiv 492447. ( 10.1101/492447) [DOI] [Google Scholar]
- Hagedorn M, Spindler R, Daly J.2019Cryopreservation as a tool for reef restoration: 2019. Advances in Experimental Medicine and Biology 1200489–505. ( 10.1007/978-3-030-23633-5_16) [DOI] [PubMed] [Google Scholar]
- Hajian M, Hosseini SM, Forouzanfar M, Abedi P, Ostadhosseini S, Hosseini L, Moulavi F, Gourabi H, Shahverdi AH, Vosough Taghi Dizaj Aet al. 2011‘Conservation cloning’ of vulnerable Esfahan mouflon (Ovis orientalis isphahanica): in vitro and in vivo studies. European Journal of Wildlife Research 57959–969. ( 10.1007/s10344-011-0510-5) [DOI] [Google Scholar]
- Hamano S, Koikeda A, Kuwayama M, Nagai T.1992Full-term development of in vitro-matured, vitrified and fertilized bovine oocytes. Theriogenology 381085–1090. ( 10.1016/0093-691x(9290122-8) [DOI] [PubMed] [Google Scholar]
- Hamazaki N, Kyogoku H, Araki H, Miura F, Horikawa C, Hamada N, Shimamoto S, Hikabe O, Nakashima K, Kitajima TSet al. 2021Reconstitution of the oocyte transcriptional network with transcription factors. Nature 589264–269. ( 10.1038/s41586-020-3027-9) [DOI] [PubMed] [Google Scholar]
- Herrick JR.2019Assisted reproductive technologies for endangered species conservation: developing sophisticated protocols with limited access to animals with unique reproductive mechanisms. Biology of Reproduction 1001158–1170. ( 10.1093/biolre/ioz025) [DOI] [PubMed] [Google Scholar]
- Hewitt R, Watson P.2013Defining biobank. Biopreservation and Biobanking 11309–315. ( 10.1089/bio.2013.0042) [DOI] [PubMed] [Google Scholar]
- Hikabe O, Hamazaki N, Nagamatsu G, Obata Y, Hirao Y, Hamada N, Shimamoto S, Imamura T, Nakashima K, Saitou Met al. 2016Reconstitution in vitro of the entire cycle of the mouse female germ line. Nature 539299–303. ( 10.1038/nature20104) [DOI] [PubMed] [Google Scholar]
- Hildebrandt TB, Hermes R, Goeritz F, Appeltant R, Colleoni S, De Mori B, Diecke S, Drukker M, Galli C, Hayashi Ket al. 2021The ART of bringing extinction to a freeze–History and future of species conservation, exemplified by rhinos. Theriogenology 16976–88. ( 10.1016/j.theriogenology.2021.04.006) [DOI] [PubMed] [Google Scholar]
- Hinkle K, Orwig KE, Valli-Pulaski H, Taylor S, Van Leeuwen K, Carpentieri D, Walsh A.2021Cryopreservation of ovarian tissue for pediatric fertility. Biopreservation and Biobanking 19130–135. ( 10.1089/bio.2020.0124) [DOI] [PubMed] [Google Scholar]
- Hoban S, Bruford M, D’Urban Jackson J, Lopes-Fernandes M, Heuertz M, Hohenlohe PA, Paz-Vinas I, Sjögren-Gulve P, Segelbacher G, Vernesi Cet al. 2020Genetic diversity targets and indicators in the CBD post-2020 Global Biodiversity Framework must be improved. Biological Conservation 248 108654. ( 10.1016/j.biocon.2020.108654) [DOI] [Google Scholar]
- Hobbs RJ, O’Brien JK, Spindler RE.2019Strategic gene banking for conservation: the ins and outs of a living bank. In Scientific Foundations of Zoos and Aquariums: Their Role in Conservation and Research. Eds Kaufman AB, Bashaw MJ, Maple TL. Cambridge: Cambridge University Press. [Google Scholar]
- Holt WV.2000Fundamental aspects of sperm cryobiology: the importance of species and individual differences. Theriogenology 5347–58. ( 10.1016/s0093-691x(9900239-3) [DOI] [PubMed] [Google Scholar]
- Holt WV, Lloyd RE.2009Artificial insemination for the propagation of CANDES: the reality! Theriogenology 71228–235. ( 10.1016/j.theriogenology.2008.09.003) [DOI] [PubMed] [Google Scholar]
- Holt WV, Pickard AR, Prather RS.2004Wildlife conservation and reproductive cloning. Reproduction 127317–324. ( 10.1530/rep.1.00074) [DOI] [PubMed] [Google Scholar]
- Houck ML.2019Fibroblast cell culture and cryopreservation of endangered vertebrates. Cryobiology 91 153–154. ( 10.1016/j.cryobiol.2019.10.037) [DOI] [Google Scholar]
- Howard JG, Lynch C, Santymire RM, Marinari PE, Wildt DE.2016Recovery of gene diversity using long-term cryopreserved spermatozoa and artificial insemination in the endangered black-footed ferret. Animal Conservation 19102–111. ( 10.1111/acv.12229) [DOI] [Google Scholar]
- Howell LG, Frankham R, Rodger JC, Witt RR, Clulow S, Upton RMO, Clulow J.2021Integrating biobanking minimises inbreeding and produces significant cost benefits for a threatened frog captive breeding programme. Conservation Letters 14 e12776. ( 10.1111/conl.12776) [DOI] [PubMed] [Google Scholar]
- Hübner K, Fuhrmann G, Christenson LK, Kehler J, Reinbold R, De La Fuente R, Wood J, Strauss JF, 3rd, Boiani M, Schöler HR.2003Derivation of oocytes from mouse embryonic stem cells. Science 3001251–1256. ( 10.1126/science.1083452) [DOI] [PubMed] [Google Scholar]
- Huffmeyer AA Sikich JA Vickers TW Riley SP & Wayne RK. 2022First reproductive signs of inbreeding depression in Southern California male mountain lions (Puma concolor). Theriogenology 177157–164. ( 10.1016/j.theriogenology.2021.10.016) [DOI] [PubMed] [Google Scholar]
- Hulshof SCJ, Figueiredo JR, Beckers JF, Bevers MM, Van Der Donk JA, Van Den Hurk R.1995Effects of fetal bovine serum, FSH and 17β-estradiol on the culture of bovine preantral follicles. Theriogenology 44217–226. ( 10.1016/0093-691x(9500171-4) [DOI] [PubMed] [Google Scholar]
- Hunt CJ.2017Cryopreservation: vitrification and controlled rate cooling. Methods in Molecular Biology 159041–77. ( 10.1007/978-1-4939-6921-0_5) [DOI] [PubMed] [Google Scholar]
- Hurtt AE, Landim-Alvarenga F, Seidel Jr GE, Squires EL.2000Vitrification of immature and mature equine and bovine oocytes in an ethylene glycol, Ficoll and sucrose solution using open-pulled straws. Theriogenology 54119–128. ( 10.1016/s0093-691x(0000330-7) [DOI] [PubMed] [Google Scholar]
- Hwang I, Jeong YW, Kim JJ, Lee HJ, Kang M, Park KB, Park JH, Kim YW, Kim WT, Shin Tet al. 2012Successful cloning of coyotes through interspecies somatic cell nuclear transfer (iSCNT) using domestic dog oocytes. Biology of Reproduction 8756–56. [DOI] [PubMed] [Google Scholar]
- Imbler S.2021Meet Elizabeth Ann, The First Cloned Black-Footed Ferret (Online). The New York Times. (available at: https://www.nytimes.com/2021/02/18/science/black-footed-ferret-clone.html). Accessed on 16 November 2021. [Google Scholar]
- Inoue K, Wakao H, Ogonuki N, Miki H, Seino K, Nambu-Wakao R, Noda S, Miyoshi H, Koseki H, Taniguchi Met al. 2005Generation of cloned mice by direct nuclear transfer from natural killer T cells. Current Biology 151114–1118. ( 10.1016/j.cub.2005.05.021) [DOI] [PubMed] [Google Scholar]
- Ishikura Y, Ohta H, Sato T, Murase Y, Yabuta Y, Kojima Y, Yamashiro C, Nakamura T, Yamamoto T, Ogawa Tet al. 2021In vitro reconstitution of the whole male germ-cell development from mouse pluripotent stem cells. Cell Stem Cell 282167, .e9–2179.e9. ( 10.1016/j.stem.2021.08.005) [DOI] [PubMed] [Google Scholar]
- IUCN 2016IUCN SSC Guiding Principles on Creating Proxies of Extinct Species. IUCN. [Google Scholar]
- IUCN 2021IUCN Red List of Threatened Species (Online). IUCN. Accessed 2021. [Google Scholar]
- IUCN SSC Amphibian Specialist Group 2017Leptodactylus fallax. The IUCN Red List of Threatened Species 2017 e.T57125A3055585. ( 10.2305/IUCN.UK.2017-3.RLTS.T57125A3055585.en). Accessed on 21 January 2022. [DOI] [Google Scholar]
- Janssen DL, Edwards ML, Koster JA, Lanza RP, Ryder OA.2004Postnatal management of chryptorchid banteng calves cloned by nuclear transfer utilizing frozen fibroblast cultures and enucleated cow ova. Reproduction, Fertility and Development 16224–224. ( 10.1071/RDv16n1Ab206) [DOI] [Google Scholar]
- Jeon Y, Nam YH, Cheong SA, Kwak SS, Lee E, Hyun SH.2016Absence of nucleolus formation in raccoon dog-porcine interspecies somatic cell nuclear transfer embryos results in embryonic developmental failure. Journal of Reproduction and Development 62345–350. ( 10.1262/jrd.2015-175) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jewgenow K, Zahmel J.2020Preservation of female genetic resources in feline species. Theriogenology 156124–129. ( 10.1016/j.theriogenology.2020.06.040) [DOI] [PubMed] [Google Scholar]
- Jia BY, Xiang DC, Quan GB, Zhang B, Shao QY, Hong QH, Wu GQ.2019Transcriptome analysis of porcine immature oocytes and surrounding cumulus cells after vitrification and in vitro maturation. Theriogenology 13490–97. ( 10.1016/j.theriogenology.2019.05.019) [DOI] [PubMed] [Google Scholar]
- Jia B, Xiang D, Fu X, Shao Q, Hong Q, Quan G, Wu G.2020Proteomic changes of porcine oocytes after vitrification and subsequent in vitro maturation: a tandem mass tag-based quantitative analysis. Frontiers in Cell and Developmental Biology 8 614577. ( 10.3389/fcell.2020.614577) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joaquim DC, Borges ED, Viana IGR, Navarro PA, Vireque AA.2017Risk of contamination of gametes and embryos during cryopreservation and measures to prevent cross-contamination. BioMed Research International 2017 1840417. ( 10.1155/2017/1840417) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston SD, Holt WV.2019Using the koala (Phascolarctos cinereus) as a case study to illustrate the development of artificial breeding technology in marsupials: an update. In Reproductive Sciences in Animal Conservation. Eds Comizzoli P, Brown JL, Holt WV. Cham: Springer International Publishing. [DOI] [PubMed] [Google Scholar]
- Johnson JA, Altwegg R, Evans DM, Ewen JG, Gordon IJ, Pettorelli N, Young JK.2016Is there a future for genome-editing technologies in conservation? Animal Conservation 1997–101. ( 10.1111/acv.12273) [DOI] [Google Scholar]
- Kaneko T Ito H Sakamoto H Onuma M & Inoue-Murayama M.. 2014Sperm preservation by freeze-drying for the conservation of wild animals. PloS one 9e113381. ( 10.1371/journal.pone.0113381) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keogh LM, Byrne PG, Silla AJ.2017The effect of gentamicin on sperm motility and bacterial abundance during chilled sperm storage in the Booroolong frog. General and Comparative Endocrinology 24351–59. ( 10.1016/j.ygcen.2016.11.005) [DOI] [PubMed] [Google Scholar]
- Kochan J, Niżański W, Moreira N, Cubas ZS, Nowak A, Prochowska S, Partyka A, Młodawska W, Skotnicki J.2019ARTs in wild felid conservation programmes in Poland and in the world. Journal of Veterinary Research 63457–464. ( 10.2478/jvetres-2019-0043) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong HS, Hong YH, Lee J, Youm HW, Lee JR, Suh CS, Kim SH.2021Antifreeze protein supplementation during the warming of vitrified bovine ovarian tissue can improve the ovarian tissue quality after xenotransplantation. Frontiers in Endocrinology 12 672619. ( 10.3389/fendo.2021.672619) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korody ML, Ford SM, Nguyen TD, Pivaroff CG, Valiente-Alandi I, Peterson SE, Ryder OA, Loring JF.2021Rewinding extinction in the northern white rhinoceros: genetically diverse induced pluripotent stem cell bank for genetic rescue. Stem Cells and Development 30177–189. ( 10.1089/scd.2021.0001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouba A, Willis E, Vance CK, Hasenstab S, Reichling S, Krebs J, Linhoff L, Snoza M, Langhorne C, Germano J.2012116 Development of assisted reproduction technologies for the endangered Mississippi gopher frog (rana sevosa) and sperm transfer for in vitro fertilization. Reproduction, Fertility and Development 24 170. ( 10.1071/RDv24n1Ab116) [DOI] [Google Scholar]
- Kouba AJ, Lloyd RE, Houck ML, Silla AJ, Calatayud N, Trudeau VL, Clulow J, Molinia F, Langhorne C, Vance Cet al. 2013Emerging trends for biobanking amphibian genetic resources: the hope, reality and challenges for the next decade. Biological Conservation 16410–21. ( 10.1016/j.biocon.2013.03.010) [DOI] [Google Scholar]
- Lagutina I, Fulka H, Lazzari G, Galli C.2013Interspecies somatic cell nuclear transfer: advancements and problems. Cellular Reprogramming 15374–384. ( 10.1089/cell.2013.0036) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanza RP, Cibelli JB, Diaz F, Moraes CT, Farin PW, Farin CE, Hammer CJ, West MD, Damiani P.2000Cloning of an endangered species (Bos gaurus) using interspecies nuclear transfer. Cloning 279–90. ( 10.1089/152045500436104) [DOI] [PubMed] [Google Scholar]
- Lanzendorf SE, Holmgren WJ, Schaffer N, Hatasaka H, Wentz AC, Jeyendran RS.1992In vitro fertilization and gamete micromanipulation in the lowland gorilla. Journal of Assisted Reproduction and Genetics 9358–364. ( 10.1007/BF01203960) [DOI] [PubMed] [Google Scholar]
- Lawson B, Clulow S, Mahony MJ, Clulow J.2013Towards gene banking amphibian maternal germ lines: short-term incubation, cryoprotectant tolerance and cryopreservation of embryonic cells of the frog, Limnodynastes peronei. PLoS ONE 4 e60760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee WY, Park HJ, Lee R, Lee KH, Kim YH, Ryu BY, Kim NH, Kim JH, Kim JH, Moon SHet al. 2013Establishment and in vitro culture of porcine spermatogonial germ cells in low temperature culture conditions. Stem Cell Research 111234–1249. ( 10.1016/j.scr.2013.08.008) [DOI] [PubMed] [Google Scholar]
- Lee SG, Mikhalchenko AE, Yim SH, Lobanov AV, Park JK, Choi KH, Bronson RT, Lee CK, Park TJ, Gladyshev VN.2017Naked mole rat induced pluripotent stem cells and their contribution to interspecific chimera. Stem Cell Reports 91706–1720. ( 10.1016/j.stemcr.2017.09.013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Lee Y, Lee GS, Lee ST, Lee E.2019Comparative study of the developmental competence of cloned pig embryos derived from spermatogonial stem cells and fetal fibroblasts. Reproduction of Domestic Animals 541258–1264. ( 10.1111/rda.13507) [DOI] [PubMed] [Google Scholar]
- Lee AR, Park JH, Shim SH, Hong K, La H, Park KS, Lee DR.2021Genome stabilization by RAD51-stimulatory compound 1 enhances efficiency of somatic cell nuclear transfer-mediated reprogramming and full-term development of cloned mouse embryos. Cell Proliferation 54 e13059. ( 10.1111/cpr.13059) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lees CM, Wilcken J.2009Sustaining the Ark: the challenges faced by zoos in maintaining viable populations. International Zoo Yearbook 436–18. ( 10.1111/j.1748-1090.2008.00066.x) [DOI] [Google Scholar]
- Lees C, Wilcken J.2011Global programmes for sustainability. WAZA Magazine 122–5. [Google Scholar]
- Leibo SP.1980Water permeability and its activation energy of fertilized and unfertilized mouse ova. Journal of Membrane Biology 53179–188. ( 10.1007/BF01868823) [DOI] [PubMed] [Google Scholar]
- Leibo SP, Songsasen N.2002Cryopreservation of gametes and embryos of non-domestic species. Theriogenology 57303–326. ( 10.1016/s0093-691x(0100673-2) [DOI] [PubMed] [Google Scholar]
- Li LF, Yue H, Ma J, Guan WJ, Ma YH.2009Establishment and characterization of a fibroblast line from Simmental cattle. Cryobiology 5963–68. ( 10.1016/j.cryobiol.2009.04.009) [DOI] [PubMed] [Google Scholar]
- Li P, Hu H, Yang S, Tian R, Zhang Z, Zhang W, Ma M, Zhu Y, Guo X, Huang Yet al. 2013Differentiation of induced pluripotent stem cells into male germ cells in vitro through embryoid body formation and retinoic acid or testosterone induction. BioMed Research International 2013 608728. ( 10.1155/2013/608728) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lima GL, Luz VB, Lunardi FO, Souza ALP, Peixoto GCX, Rodrigues APR, Oliveira MF, Santos RR, Silva AR.2019Effect of cryoprotectant type and concentration on the vitrification of collared peccary (Pecari tajacu) ovarian tissue. Animal Reproduction Science 205126–133. ( 10.1016/j.anireprosci.2019.04.012) [DOI] [PubMed] [Google Scholar]
- Lima DBC, Silva LDMD, Marinari P, Comizzoli P.2020Long-term preservation of testicular tissue integrity and viability using vitrification in the endangered black-footed ferret (Mustela nigripes). Animals 10 1865. ( 10.3390/ani10101865) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindau R, Vondra S, Spreckels J, Solders M, Svensson-Arvelund J, Berg G, Pollheimer J, Kaipe H, Jenmalm MC, Ernerudh J.2021Decidual stromal cells support tolerance at the human foetal-maternal interface by inducing regulatory M2 macrophages and regulatory T-cells. Journal of Reproductive Immunology 146 103330. ( 10.1016/j.jri.2021.103330) [DOI] [PubMed] [Google Scholar]
- Liu C, Guo Y, Lu T, Li X, Guan W, Ma Y.2014Establishment and genetic characteristics analysis of in vitro culture a fibroblast cell line derived from Wuzhishan miniature pig. Cryobiology 68281–287. ( 10.1016/j.cryobiol.2014.02.009) [DOI] [PubMed] [Google Scholar]
- Liu T, Dou H, Xiang X, Li L, Li Y, Lin L, Pang X, Zhang Y, Chen Y, Luan Jet al. 2015Factors determining the efficiency of porcine somatic cell nuclear transfer: data analysis with over 200,000 reconstructed embryos. Cellular Reprogramming 17463–471. ( 10.1089/cell.2015.0037) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Cai Y, Wang Y, Nie Y, Zhang C, Xu Y, Zhang X, Lu Y, Wang Z, Poo Met al. 2018Cloning of macaque monkeys by somatic cell nuclear transfer. Cell 172881, .e7–887.e7. ( 10.1016/j.cell.2018.01.020) [DOI] [PubMed] [Google Scholar]
- Locatelli Y, Calais L, Duffard N, Lardic L, Monniaux D, Piver P, Mermillod P, Bertoldo MJ.2019In vitro survival of follicles in prepubertal ewe ovarian cortex cryopreserved by slow freezing or non-equilibrium vitrification. Journal of Assisted Reproduction and Genetics 361823–1835. ( 10.1007/s10815-019-01532-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loi P, Ptak G, Barboni B, Fulka Jr J, Cappai P, Clinton M.2001Genetic rescue of an endangered mammal by cross-species nuclear transfer using post-mortem somatic cells. Nature Biotechnology 19962–964. ( 10.1038/nbt1001-962) [DOI] [PubMed] [Google Scholar]
- Long CR, Westhusin ME, Golding MC.2014Reshaping the transcriptional frontier: epigenetics and somatic cell nuclear transfer. Molecular Reproduction and Development 81183–193. ( 10.1002/mrd.22271) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopes CA, Alves AM, Jewgenow K, Báo SN, de Figueiredo JR.2016Cryopreservation of canine ovarian cortex using DMSO or 1,3-propanediol. Theriogenology 861165–1174. ( 10.1016/j.theriogenology.2016.04.006) [DOI] [PubMed] [Google Scholar]
- Lopes KRF, Praxedes ECG, Campos LB, Bezerra MB, Lima GL, Saraiva MVA, Silva AR.2018Vitrification of ovarian tissue of Brazilian North-eastern donkeys (Equus asinus) using different cryoprotectants. Reproduction in Domestic Animals 531060–1067. ( 10.1111/rda.13203) [DOI] [PubMed] [Google Scholar]
- López A, Ducolomb Y, Casas E, Retana-Márquez S, Betancourt M, Casillas F.2021Effects of porcine immature oocyte vitrification on actin microfilament distribution and chromatin integrity during early embryo development in vitro. Frontiers in Cell and Developmental Biology 9 636765. ( 10.3389/fcell.2021.636765) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y, West FD, Jordan BJ, Beckstead RB, Jordan ET, Stice SL.2015Generation of avian induced pluripotent stem cells. Methods in Molecular Biology 133089–99. ( 10.1007/978-1-4939-2848-4_9) [DOI] [PubMed] [Google Scholar]
- Lucci CM, Kacinskis MA, Lopes LHR, Rumpf R, Baoa SN.2004Effect of different cryoprotectants on the structural preservation of follicles in frozen zebu bovine (Bos indicus) ovarian tissue. Theriogenology 611101–1114. ( 10.1016/j.theriogenology.2003.06.004) [DOI] [PubMed] [Google Scholar]
- Lueders I, Luther I, Scheepers G, Van Der Horst G.2012Improved semen collection method for wild felids: urethral catheterization yields high sperm quality in African lions (Panthera leo). Theriogenology 78696–701. ( 10.1016/j.theriogenology.2012.02.026) [DOI] [PubMed] [Google Scholar]
- Lujić J, Marinović Z, Sušnik Bajec S, Djurdjevič I, Kása E, Urbányi B, Horváth Á.2017First successful vitrification of salmonid ovarian tissue. Cryobiology 76154–157. ( 10.1016/j.cryobiol.2017.04.005) [DOI] [PubMed] [Google Scholar]
- Luvoni GC, Pellizzari P.2000Embryo development in vitro of cat oocytes cryopreserved at different maturation stages. Theriogenology 531529–1540. ( 10.1016/S0093-691X(0000295-8) [DOI] [PubMed] [Google Scholar]
- Ma Y, Gu M, Chen L, Shen H, Pan Y, Pang Y, Miao S, Tong R, Huang H, Zhu Yet al. 2021Recent advances in critical nodes of embryo engineering technology. Theranostics 117391–7424. ( 10.7150/thno.58799) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maclellan LJ, Carnevale EM, Coutinho Da Silva MA, Scoggin CF, Bruemmer JE, Squires EL.2002Pregnancies from vitrified equine oocytes collected from super-stimulated and non-stimulated mares. Theriogenology 58911–919. ( 10.1016/s0093-691x(0200920-2) [DOI] [PubMed] [Google Scholar]
- Macpherson AJ, De Agüero MG, Ganal-Vonarburg SC.2017How nutrition and the maternal microbiota shape the neonatal immune system. Nature Reviews: Immunology 17508–517. ( 10.1038/nri.2017.58) [DOI] [PubMed] [Google Scholar]
- Magalhães LC Bhat MH Freitas JLS Melo LM Teixeira DIA Pinto LCA Câmara LMC Duarte JMB & Freitas VJF. 2017. The effects of cryopreservation on different passages of fibroblast cell culture in brown brocket deer (Mazama gouazoubira). Biopreservation and Biobanking15463–468. ( 10.1089/bio.2017.0060) [DOI] [PubMed] [Google Scholar]
- Manoli DS, Subramanyam D, Carey C, Sudin E, Van Westerhuyzen JA, Bales KL, Blelloch R, Shah NM.2012Generation of induced pluripotent stem cells from the prairie vole. PLoS ONE 7 e38119. ( 10.1371/journal.pone.0038119) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchetto MCN, Narvaiza I, Denli AM, Benner C, Lazzarini TA, Nathanson JL, Paquola ACM, Desai KN, Herai RH, Weitzman MDet al. 2013Differential L1 regulation in pluripotent stem cells of humans and apes. Nature 503525–529. ( 10.1038/nature12686) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez F.2017Update on fertility preservation from the Barcelona International Society for Fertility Preservation-ESHRE-ASRM 2015 expert meeting: indications, results and future perspectives. Human Reproduction 321802–1811. ( 10.1093/humrep/dex218) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez-Fresneda L, Castaño C, Bóveda P, Tesfaye D, Schellander K, Santiago-Moreno J, García-Vázquez FA.2019Epididymal and ejaculated sperm differ on their response to the cryopreservation and capacitation processes in mouflon (Ovis musimon). Scientific Reports 9 15659. ( 10.1038/s41598-019-52057-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Wintle MS, Kersey DC, Wintle NJ, Aitken-Palmer C, Owen MA, Swaisgood RR.2019Comprehensive breeding techniques for the giant panda. In Reproductive Sciences in Animal Conservation. pp 275–308; Eds P Comizzoli, JL Brown & WV Holt Cham: Springer International Publishing. [DOI] [PubMed] [Google Scholar]
- Mastromonaco GF, Songsasen N.2020Chapter 7 – Reproductive technologies for the conservation of wildlife and endangered species. In Reproductive Technologies in Animals. Ed Presicce GA.Academic Press. [Google Scholar]
- Mestre-Citrinovitz AC, Sestelo AJ, Ceballos MB, Barañao JL, Saragüeta P.2016Isolation of primary fibroblast culture from wildlife: the Panthera onca case to preserve a South American endangered species. Current Protocols in Molecular Biology 11628.7.1–28.7.14. ( 10.1002/cpmb.25) [DOI] [PubMed] [Google Scholar]
- Mitchell RT, Williams SA.2022A fertile future: fertility preservation special series. Reproduction and Fertility 3C1–C3. ( 10.1530/RAF-22-0001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mo X, Li N, Wu S.2014Generation and characterization of bat-induced pluripotent stem cells. Theriogenology 82283–293. ( 10.1016/j.theriogenology.2014.04.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moawad AR, Zhu J, Choi I, Amarnath D, Chen W, Campbell KH.2013Production of good-quality blastocyst embryos following IVF of ovine oocytes vitrified at the germinal vesicle stage using a cryoloop. Reproduction, Fertility, and Development 251204–1215. ( 10.1071/RD12215) [DOI] [PubMed] [Google Scholar]
- Montfort SL.2014. “Mayday Mayday Mayday”, the Millennium Ark Is Sinking! In Reproductive Sciences in Animal Conservation, Advances in Experimental Medicine and Biology; W V Holt, JL Brown, P Comizzoli (eds.) 753, Springer Science+Business Media New York. pp 15–31. ( 10.1007/978-1-4939-0820-2_2) [DOI] [PubMed] [Google Scholar]
- Mooney A.2021The value of ex situ collections for global biodiversity conservation in the wild. PhD Thesis. Trinity College Dublin, School of Natural Sciences. [Google Scholar]
- Moro LN, Hiriart MI, Buemo C, Jarazo J, Sestelo A, Veraguas D, Rodriguez-Alvarez L, Salamone DF.2015Cheetah interspecific SCNT followed by embryo aggregation improves in vitro development but not pluripotent gene expression. Reproduction 1501–10. ( 10.1530/REP-15-0048) [DOI] [PubMed] [Google Scholar]
- Morrow CJ, Penfold LM, Wolfe BA.2009Artificial insemination in deer and non-domestic bovids. Theriogenology 71149–165. ( 10.1016/j.theriogenology.2008.09.001) [DOI] [PubMed] [Google Scholar]
- Mouttham L, Comizzoli P.2016The preservation of vital functions in cat ovarian tissues during vitrification depends more on the temperature of the cryoprotectant exposure than on the sucrose supplementation. Cryobiology 73187–195. ( 10.1016/j.cryobiol.2016.07.013) [DOI] [PubMed] [Google Scholar]
- Mrowiec P, Bugno-Poniewierska M, Młodawska W.2021The perspective of the incompatible of nucleus and mitochondria in interspecies somatic cell nuclear transfer for endangered species. Reproduction in Domestic Animals 56199–207. ( 10.1111/rda.13864) [DOI] [PubMed] [Google Scholar]
- Na RS, Zhao QJ, Jin DP, Su XH, Chen XW, Guan WJ, Ma YH.2010Establishment and biological characteristics of Ujumqin sheep fibroblast line. Cytotechnology 6243–52. ( 10.1007/s10616-010-9260-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama K, Chinen S, Teshima J, Tamada Y, Hirabayashi M, Hochi S.2020Silk fibroin sheet multilayer suitable for vitrification of in vitro-matured bovine oocytes. Theriogenology 145109–114. ( 10.1016/j.theriogenology.2020.01.052) [DOI] [PubMed] [Google Scholar]
- Nowak A, Kochan J, Świętek E, Kij B, Prochowska S, Witarski W, Bugno-Poniewierska M, Niżański W.2020Survivability and developmental competences of domestic cat (Felis catus) oocytes after Cryotech method vitrification. Reproduction in Domestic Animals 55992–997. ( 10.1111/rda.13741) [DOI] [PubMed] [Google Scholar]
- O’Brien JK, Robeck TR.2006Development of sperm sexing and associated assisted reproductive technology for sex preselection of captive bottlenose dolphins (Tursiops truncatus). Reproduction, Fertility, and Development 18319–329. ( 10.1071/rd05108) [DOI] [PubMed] [Google Scholar]
- Oh HJ, Kim MK, Jang G, Kim HJ, Hong SG, Park JE, Park K, Park C, Sohn SH, Kim DYet al. 2008Cloning endangered gray wolves (Canis lupus) from somatic cells collected postmortem. Theriogenology 70638–647. ( 10.1016/j.theriogenology.2008.04.032) [DOI] [PubMed] [Google Scholar]
- Okita K, Nagata N, Yamanaka S.2011Immunogenicity of induced pluripotent stem cells. Circulation Research 109720–721. ( 10.1161/RES.0b013e318232e187) [DOI] [PubMed] [Google Scholar]
- Okotrub KA, Mokrousova VI, Amstislavsky SY, Surovtsev NV.2018Lipid droplet phase transition in freezing cat embryos and oocytes probed by Raman spectroscopy. Biophysical Journal 115577–587. ( 10.1016/j.bpj.2018.06.019) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortiz-Escribano N, Bogado Pascottini O, Woelders H, Vandenberghe L, De Schauwer C, Govaere J, Van Den Abbeel E, Vullers T, Ververs C, Roels Ket al. 2018An improved vitrification protocol for equine immature oocytes, resulting in a first live foal. Equine Veterinary Journal 50391–397. ( 10.1111/evj.12747) [DOI] [PubMed] [Google Scholar]
- Otoi T, Yamamoto K, Koyama N, Suzuki T.1995In vitro fertilization and development of immature and mature bovine oocytes cryopreserved by ethylene glycol with sucrose. Cryobiology 32455–460. ( 10.1006/cryo.1995.1045) [DOI] [PubMed] [Google Scholar]
- Penfold LM, O’Brien JK.2012Chapter 78 – Importation of nondomestic ruminant semen for management of zoological populations using artificial insemination. In: Fowler’s Zoo and Wild Animal Medicine: Current Therapy, vol. 7, pp. 604–611. Eds Miller & Fowler ME.Elsevier Inc. [Google Scholar]
- Penfold LM, Gillis JD, Pye GW, Pope EC.2021Chapter 8 – Quality control practices and prevention of disease transmission in exotic and wild species. In Manual of the International Embryo Technology Society, 5th ed., pp. 8–13. International Embryo Technology Society. [Google Scholar]
- Peng L, Zhou Y, Xu W, Jiang M, Li H, Long M, Liu W, Liu J, Zhao X, Xiao Y.2019Generation of stable induced pluripotent stem-like cells from adult zebra fish fibroblasts. International Journal of Biological Sciences 152340–2349. ( 10.7150/ijbs.34010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peter RE, Lin HR, Van Der Kraak G.1988Induced ovulation and spawning of cultured freshwater fish in China: advances in application of GnRH analogues and dopamine antagonists. Aquaculture 741–10. ( 10.1016/0044-8486(8890080-4) [DOI] [Google Scholar]
- Pillai VV, Kei TG, Reddy SE, Das M, Abratte C, Cheong SH, Selvaraj V.2019Induced pluripotent stem cell generation from bovine somatic cells indicates unmet needs for pluripotency sustenance. Animal Science Journal 901149–1160. ( 10.1111/asj.13272) [DOI] [PubMed] [Google Scholar]
- Pimm SL, Jenkins CN, Abell R, Brooks TM, Gittleman JL, Joppa LN, Raven PH, Roberts CM, Sexton JO.2014The biodiversity of species and their rates of extinction, distribution, and protection. Science 344 1246752. ( 10.1126/science.1246752) [DOI] [PubMed] [Google Scholar]
- Poels J, Van Langendonckt A, Dehoux JP, Donnez J, Wyns C.2012Vitrification of non-human primate immature testicular tissue allows maintenance of proliferating spermatogonial cells after xenografting to recipient mice. Theriogenology 771008–1013. ( 10.1016/j.theriogenology.2011.10.015) [DOI] [PubMed] [Google Scholar]
- Poo S, Hinkson KM.2019Applying cryopreservation to anuran conservation biology. Conservation Science and Practice 1 e91. ( 10.1111/csp2.91) [DOI] [Google Scholar]
- Pothana L, Makala H, Devi L, Varma VP, Goel S.2015Germ cell differentiation in cryopreserved, immature, Indian spotted mouse deer (Moschiola indica) testes xenografted onto mice. Theriogenology 83625–633. ( 10.1016/j.theriogenology.2014.10.028) [DOI] [PubMed] [Google Scholar]
- Pothana L, Venna NK, Devi L, Singh A, Chatterjee I, Goel S.2016Cryopreservation of adult primate testes. European Journal of Wildlife Research 62619–626. ( 10.1007/s10344-016-1024-y) [DOI] [Google Scholar]
- Pothana L, Devi L, Goel S.2017Cryopreservation of adult cervid testes. Cryobiology 74103–109. ( 10.1016/j.cryobiol.2016.11.008) [DOI] [PubMed] [Google Scholar]
- Praxedes ÉCG, Lima GL, Silva AM, Apolinário C, Bezerra JAB, Souza ALP, Oliveira MF, Rodrigues APR, Silva AR.2017Characterisation and cryopreservation of the ovarian preantral follicle population from Spix’s yellow-toothed cavies (Galea spixii Wagler, 1831). Reproduction, Fertility and Development 29594–602. [DOI] [PubMed] [Google Scholar]
- Prentice JR, Anzar M.2010Cryopreservation of Mammalian oocyte for conservation of animal genetics. Veterinary Medicine International 2011 146405. ( 10.4061/2011/146405) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pukazhenthi BS, Wildt DE.2004Which reproductive technologies are most relevant to studying, managing and conserving wildlife? Reproduction, Fertility, and Development 1633–46. ( 10.10371/RD03076) [DOI] [PubMed] [Google Scholar]
- Purdy PH.2006A review on goat sperm cryopreservation. Small Ruminant Research 63215–225. ( 10.1016/j.smallrumres.2005.02.015) [DOI] [Google Scholar]
- Purohit GN, Meena H, Solanki K.2012Effects of vitrification on immature and in vitro matured, denuded and cumulus compact goat oocytes and their subsequent fertilization. Journal of Reproduction and Infertility 1353–59. [PMC free article] [PubMed] [Google Scholar]
- Qu P, Shen C, Du Y, Qin H, Luo S, Fu S, Dong Y, Guo S, Hu F, Xue Yet al. 2020Melatonin protects rabbit somatic cell nuclear transfer (SCNT) embryos from electrofusion damage. Scientific Reports 10 2186. ( 10.1038/s41598-020-59161-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quan GB, Wu GQ, Wang YJ, Ma Y, Lv CR, Hong QH.2016Meiotic maturation and developmental capability of ovine oocytes at germinal vesicle stage following vitrification using different cryodevices. Cryobiology 7233–40. ( 10.1016/j.cryobiol.2015.11.007) [DOI] [PubMed] [Google Scholar]
- Rall WF, Fahy GM.1985Ice-free cryopreservation of mouse embryos at -196 degrees C by vitrification. Nature 313573–575. ( 10.1038/313573a0) [DOI] [PubMed] [Google Scholar]
- Ramaswamy K, Yik WY, Wang XM, Oliphant EN, Lu W, Shibata D, Ryder OA, Hacia JG.2015Derivation of induced pluripotent stem cells from orangutan skin fibroblasts. BMC Research Notes 8 577. ( 10.1186/s13104-015-1567-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid CE, Hermes R, Blottner S, Goeritz F, Wibbelt G, Walzer C, Bryant BR, Portas TJ, Streich WJ, Hildebrandt TB.2009Split-sample comparison of directional and liquid nitrogen vapour freezing method on post-thaw semen quality in white rhinoceroses (Ceratotherium simum simum and Ceratotherium simum cottoni). Theriogenology 71275–291. ( 10.1016/j.theriogenology.2008.07.009) [DOI] [PubMed] [Google Scholar]
- Richer G, Baert Y, Goossens E.2020In-vitro spermatogenesis through testis modelling: Toward the generation of testicular organoids. Andrology 8879–891. ( 10.1111/andr.12741) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rickard JP, Pool K, De Graaf SP, Portas T, Rourke N, Wiesner M, Hildebrandt TB, Göritz F, Hermes R.2022Increasing the yield and cryosurvival of spermatozoa from rhinoceros ejaculates using the enzyme papain. Biology 11 154. ( 10.3390/biology11020154) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rienzi L, Gracia C, Maggiulli R, Labarbera AR, Kaser DJ, Ubaldi FM, Vanderpoel S, Racowsky C.2017Oocyte, embryo and blastocyst cryopreservation in ART: systematic review and meta-analysis comparing slow-freezing versus vitrification to produce evidence for the development of global guidance. Human Reproduction Update 23139–155. ( 10.1093/humupd/dmw038) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivers N, Daly J, Temple-Smith P.2020New directions in assisted breeding techniques for fish conservation. Reproduction, Fertility, and Development 32807–821. ( 10.1071/RD19457) [DOI] [PubMed] [Google Scholar]
- Robeck TR, Gearhart SA, Steinman KJ, Katsumata E, Loureiro JD, O’Brien JK.2011In vitro sperm characterization and development of a sperm cryopreservation method using directional solidification in the killer whale (Orcinus orca). Theriogenology 76267–279. ( 10.1016/j.theriogenology.2011.02.003) [DOI] [PubMed] [Google Scholar]
- Rodger JC.2019Marsupials: progress and prospects. In Reproductive Sciences in Animal Conservation. Eds Comizzoli P, Brown JL, Holt WV. Cham: Springer International Publishing. [Google Scholar]
- Rodrigues APR, Amorim CA, Costa SHF, Matos MHT, Santos RR, Lucci CM, Báo SN, Ohashi OM, Figueiredo JR.2004Cryopreservation of caprine ovarian tissue using dimethylsulphoxide and propanediol. Animal Reproduction Science 84211–227. ( 10.1016/j.anireprosci.2003.12.003) [DOI] [PubMed] [Google Scholar]
- Roth TL, Weiss RB, Buff JL, Bush LM, Wildt DE, Bush M.1998Heterologous in vitro fertilization and sperm capacitation in an endangered African antelope, the scimitar-horned oryx (Oryx dammah). Biology of Reproduction 58475–482. ( 10.1095/biolreprod58.2.475) [DOI] [PubMed] [Google Scholar]
- Roth TL, Stoops MA, Robeck TR, O’Brien JK.2016Factors impacting the success of post-mortem sperm rescue in the rhinoceros. Animal Reproduction Science 16722–30. ( 10.1016/j.anireprosci.2016.01.019) [DOI] [PubMed] [Google Scholar]
- Rutigliano HM, Thomas AJ, Umbaugh JJ, Wilhelm A, Sessions BR, Kaundal R, Duhan N, Hicks BA, Schlafer DH, White KLet al. 2022Increased expression of Pro-inflammatory cytokines at the fetal-maternal interface in bovine pregnancies produced by cloning. American Journal of Reproductive Immunology 87e13520. ( 10.1111/aji.13520) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryder OA, Onuma M.2018Viable cell culture banking for biodiversity characterization and conservation. Annual Review of Animal Biosciences 683–98. ( 10.1146/annurev-animal-030117-014556) [DOI] [PubMed] [Google Scholar]
- Turathum B, Saikhun K, Sangsuwan P, Kitiyanant Y.2010Effects of vitrification on nuclear maturation, ultrastructural changes and gene expression of canine oocytes. Reproductive Biology and Endocrinology 8 70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandler RL, Moses L, Wisely SM.2021An ethical analysis of cloning for genetic rescue: case study of the black-footed ferret. Biological Conservation 257 109118. ( 10.1016/j.biocon.2021.109118) [DOI] [Google Scholar]
- Santymire R.2016Implementing the use of a biobank in the endangered black-footed ferret (Mustela nigripes). Reproduction, Fertility, and Development 281097–1104. ( 10.1071/RD15461) [DOI] [PubMed] [Google Scholar]
- Saragusty J, Arav A.2011Current progress in oocyte and embryo cryopreservation by slow freezing and vitrification. Reproduction 1411–19. ( 10.1530/REP-10-0236) [DOI] [PubMed] [Google Scholar]
- Saragusty J, Gacitua H, King R, Arav A.2006Post-mortem semen cryopreservation and characterization in two different endangered gazelle species (Gazella gazella and Gazella dorcas) and one subspecies (Gazella gazelle acaiae). Theriogenology 66775–784. ( 10.1016/j.theriogenology.2006.01.055) [DOI] [PubMed] [Google Scholar]
- Saragusty J, Gacitua H, Pettit MT, Arav A.2007Directional freezing of equine semen in large volumes. Reproduction in Domestic Animals 42610–615. ( 10.1111/j.1439-0531.2006.00831.x) [DOI] [PubMed] [Google Scholar]
- Saragusty J, Hildebrandt TB, Behr B, Knieriem A, Kruse J, Hermes R.2009Successful cryopreservation of Asian elephant (Elephas maximus) spermatozoa. Animal Reproduction Science 115255–266. ( 10.1016/j.anireprosci.2008.11.010) [DOI] [PubMed] [Google Scholar]
- Saragusty J, Walzer C, Petit T, Stalder G, Horowitz I, Hermes R.2010Cooling and freezing of epididymal sperm in the common hippopotamus (Hippopotamus amphibius). Theriogenology 741256–1263. ( 10.1016/j.theriogenology.2010.05.031) [DOI] [PubMed] [Google Scholar]
- Saragusty J, Ajmone-Marsan P, Sampino S, Modlinski JA.2020Reproductive biotechnology and critically endangered species: merging in vitro gametogenesis with inner cell mass transfer. Theriogenology 155176–184. ( 10.1016/j.theriogenology.2020.06.009) [DOI] [PubMed] [Google Scholar]
- Sato T, Katagiri K, Yokonishi T, Kubota Y, Inoue K, Ogonuki N, Matoba S, Ogura A, Ogawa T.2011In vitro production of fertile sperm from murine spermatogonial stem cell lines. Nature Communications 2 472. ( 10.1038/ncomms1478) [DOI] [PubMed] [Google Scholar]
- Schmitt DL, Hildebrandt TB.1998Manual collection and characterization of semen from Asian elephants (Elephas maximus). Animal Reproduction Science 53309–314. ( 10.1016/s0378-4320(9800120-1) [DOI] [PubMed] [Google Scholar]
- Seddon PJ, King M.2019Creating proxies of extinct species: the bioethics of de-extinction. Emerging Topics in Life Sciences 3731–735. ( 10.1042/ETLS20190109) [DOI] [PubMed] [Google Scholar]
- Segelbacher G, Bosse M, Burger P, Galbusera P, Godoy JA, Helsen P, Hvilsom C, Iacolina L, Kahric A, Manfrin Cet al. 2021New developments in the field of genomic technologies and their relevance to conservation management. Conservation Genetics 23217–242. ( 10.1007/s10592-021-01415-5) [DOI] [Google Scholar]
- Selvaraj V, Wildt DE, Pukazhenthi BS.2011Induced pluripotent stem cells for conserving endangered species? Nature Methods 8805–807. ( 10.1038/nmeth.1715) [DOI] [PubMed] [Google Scholar]
- Shapiro B.2017Pathways to de-extinction: how close can we get to resurrection of an extinct species? Functional Ecology 31996–1002. ( 10.1111/1365-2435.12705) [DOI] [Google Scholar]
- Sheets TP, Park CH, Park KE, Powell A, Donovan DM, Telugu BP.2016Somatic cell nuclear transfer followed by CRIPSR/Cas9 microinjection results in highly efficient genome editing in cloned pigs. International Journal of Molecular Sciences 17 2031. ( 10.3390/ijms17122031) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman JK.1963Improved methods of preservation of human spermatozoa by freezing and freeze-drying. Fertility and Sterility 1449–64. ( 10.1016/s0015-0282(1634746-x) [DOI] [PubMed] [Google Scholar]
- Shi S, Tan Q, Liang J, Cao D, Wang S, Liang J, Chen K, Wang Z.2021Placental trophoblast cell-derived exosomal microRNA-1290 promotes the interaction between endometrium and embryo by targeting LHX6. Molecular Therapy: Nucleic Acids 26760–772. ( 10.1016/j.omtn.2021.09.009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Si W, Lu Y, He X, Ji S, Niu Y, Tan T, Ji W.2010116 Improved survival by cryopreserving rhesus macaque (macaca mulatta) spermatozoa with directional freezing technique. Reproduction, Fertility and Development 22217–217. ( 10.1071/RDv22n1Ab116) [DOI] [Google Scholar]
- Silla AJ, Byrne PG.2019The role of reproductive technologies in amphibian conservation breeding programs. Annual Review of Animal Biosciences 7499–519. ( 10.1146/annurev-animal-020518-115056) [DOI] [PubMed] [Google Scholar]
- Silla AJ, Byrne PG.2021Hormone-induced ovulation and artificial fertilisation in four terrestrial-breeding anurans. Reproduction, Fertility, and Development 33615 -–618.. ( 10.1071/RD20243) [DOI] [PubMed] [Google Scholar]
- Silla AJ, Calatayud NE, Trudeau VL.2021Amphibian reproductive technologies: approaches and welfare considerations. Conservation Physiology 9coab011. ( 10.1093/conphys/coab011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva AMD, Pereira AF, Comizzoli P, Silva AR.2020Cryopreservation and culture of testicular tissues: an essential tool for biodiversity preservation. Biopreservation and Biobanking 18235–243. ( 10.1089/bio.2020.0010) [DOI] [PubMed] [Google Scholar]
- Soulé M Gilpin M Conway W & Foose T. 1985The millenium ark: how long a voyage, how many staterooms, how many passengers? Zoo biology 5101–113. ( 10.1002/zoo.1430050205) [DOI] [Google Scholar]
- Soulé ME.eds) 1987Viable Populations for Conservation. Cambridge: Cambridge University Press. ( 10.1017/CBO9780511623400) [DOI] [Google Scholar]
- Sowińska N, Zahmel J, Niżański W, Hribal R, Fernandez-Gonzalez L, Jewgenow K.2020Meiotic status does not affect the vitrification effectiveness of domestic cat oocytes. Animals 10 1371. ( 10.3390/ani10081371) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strand J.2021Biobanking as a Conservation Tool: The Way to an Amphibian Cell Line. Ph.d.-serien for Det Ingeniør-og Naturvidenskabelige Fakultet, Aalborg Universitet. [Google Scholar]
- Strand J, Thomsen H, Jensen JB, Marcussen C, Nicolajsen TB, Skriver MB, Sogaard IM, Ezaz T, Purup S, Callesen Het al. 2020Biobanking in amphibian and reptilian conservation and management: opportunities and challenges. Conservation Genetics Resources 12709–725. ( 10.1007/s12686-020-01142-y) [DOI] [Google Scholar]
- Strand J, Callesen H, Pertoldi C, Purup S.2021Establishing cell lines from fresh or cryopreserved tissue from the great crested newt (Triturus cristatus): a preliminary protocol. Animals 11 367. ( 10.3390/ani11020367) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauß S, Ziegler T, Allmeling C, Reimers K, Frank-Klein N, Seuntjens R, Vogt PM.2013In vitro culture of skin cells from biopsies from the critically endangered Chinese giant salamander, Andrias davidianus (Blanchard, 1871) (Amphibia, Caudata, Cryptobranchidae). Amphibian and Reptile Conservation 551–63. [Google Scholar]
- Suzuki T, Boediono A, Takagi M, Saha S, Sumantri C.1996Fertilization and development of frozen-thawed germinal vesicle bovine oocytes by a one-step dilution method in vitro. Cryobiology 33515–524. ( 10.1006/cryo.1996.0055) [DOI] [PubMed] [Google Scholar]
- Swanson WF, Magarey GM, Herrick JR.2007Sperm cryopreservation in endangered felids: developing linkage of in situ-ex situ populations. Society of Reproduction and Fertility Supplement 65417–432. [PubMed] [Google Scholar]
- Sztein JM, Noble K, Farley JS, Mobraaten LE.2001Comparison of permeating and nonpermeating cryoprotectants for mouse sperm cryopreservation. Cryobiology 4228–39. ( 10.1006/cryo.2001.2300) [DOI] [PubMed] [Google Scholar]
- Taggart DA, Leigh CM, Steele VR, Breed WG, Temple-Smith PD, Phelan J.1996Effect of cooling and cryopreservation on sperm motility and morphology of several species of marsupial. Reproduction, Fertility, and Development 8673–679. ( 10.1071/rd9960673) [DOI] [PubMed] [Google Scholar]
- Takahashi K, Yamanaka S.2006Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126663–676. ( 10.1016/j.cell.2006.07.024) [DOI] [PubMed] [Google Scholar]
- Takeda K.2019Functional consequences of mitochondrial mismatch in reconstituted embryos and offspring. Journal of Reproduction and Development 65485–489. ( 10.1262/jrd.2019-089) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tharasanit T, Thuwanut P.2021Oocyte cryopreservation in domestic animals and humans: principles, techniques and updated outcomes. Animals 11 2949. ( 10.3390/ani11102949) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tharasanit T, Buarpung S, Manee-In S, Thongkittidilok C, Tiptanavattana N, Comizzoli P, Techakumphu M.2012Birth of kittens after the transfer of frozen–thawed embryos produced by intracytoplasmic sperm injection with spermatozoa collected from cryopreserved testicular tissue. Reproduction in Domestic Animals 47 (Supplement 6) 305–308. ( 10.1111/rda.12072) [DOI] [PubMed] [Google Scholar]
- Thongphakdee A, Sukparangsi W, Comizzoli P, Chatdarong K.2020Reproductive biology and biotechnologies in wild felids. Theriogenology 150360–373. ( 10.1016/j.theriogenology.2020.02.004) [DOI] [PubMed] [Google Scholar]
- Thuwanut P, Srisuwatanasagul S, Wongbandue G, Tanpradit N, Thongpakdee A, Tongthainan D, Manee-In S, Chatdarong K.2013Sperm quality and the morphology of cryopreserved testicular tissues recovered post-mortem from diverse wild species. Cryobiology 67244–247. ( 10.1016/j.cryobiol.2013.07.002) [DOI] [PubMed] [Google Scholar]
- Toyooka Y, Tsunekawa N, Akasu R, Noce T.2003Embryonic stem cells can form germ cells in vitro. PNAS 100 11457–11462. ( 10.1073/pnas.1932826100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traylor-Holzer K, Leus K, Byers O.2018Integrating ex situ management options as part of a one plan approach to species conservation. In The Ark and Beyond. University of Chicago Press. [Google Scholar]
- Turvey ST, Crees JJ.2019Extinction in the Anthropocene. Current Biology 29R982–R986. ( 10.1016/j.cub.2019.07.040) [DOI] [PubMed] [Google Scholar]
- Unwin S, Pettit M.2004Cryopreservation of Bennett’s wallaby sperm using standard and directional cryopreservation techniques: preliminary results. In AAZV AAWV WDA Joint Conference, San Diego. [Google Scholar]
- Uteshev V, Kaurova S, Shishova N, Stolyarov S, Browne R, Gakhova E.2015In vitro fertilization with hormonally induced sperm and eggs from sharp-ribbed newts Pleurodeles waltl. Russian Journal of Herpetology 2235–40. [Google Scholar]
- Vallorani C, Spinaci M, Bucci D, Porcu E, Tamanini C, Galeati G.2012Pig oocyte vitrification by Cryotop method and the activation of the apoptotic cascade. Animal Reproduction Science 13568–74. ( 10.1016/j.anireprosci.2012.08.020) [DOI] [PubMed] [Google Scholar]
- Van Den Berghe F, Paris DB, Van Soom A, Rijsselaere T, Van Der Weyde L, Bertschinger HJ, Paris MC.2012Reproduction in the endangered African wild dog: basic physiology, reproductive suppression and possible benefits of artificial insemination. Animal Reproduction Science 1331–9. ( 10.1016/j.anireprosci.2012.06.003) [DOI] [PubMed] [Google Scholar]
- Van Rooij P, Martel A, Haesebrouck F, Pasmans F.2015Amphibian chytridiomycosis: a review with focus on fungus-host interactions. Veterinary Research 46 137. ( 10.1186/s13567-015-0266-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandevoort CA, Shirley CR, Hill DL, Leibo SP.2008Effects of cryoprotectants and cryopreservation on germinal vesicle-stage cumulus-oocyte complexes of rhesus monkeys. Fertility and Sterility 90805–816. ( 10.1016/j.fertnstert.2007.06.105) [DOI] [PubMed] [Google Scholar]
- Vasconcelos GL, Saraiva MVA, Costa JJN, Passos MJ, Silva AWB, Rossi RODS, Portela AMLR, Duarte ABG, Magalhães-Padilha DM, Campelo CCet al. 2013Effects of growth differentiation factor-9 and FSH on in vitro development, viability and mRNA expression in bovine preantral follicles. Reproduction, Fertility, and Development 251194–1203. ( 10.1071/RD12173) [DOI] [PubMed] [Google Scholar]
- Veraguas D, Aguilera C, Echeverry D, Saez-Ruiz D, Castro FO, Rodriguez-Alvarez L.2020Embryo aggregation allows the production of kodkod (Leopardus guigna) blastocysts after interspecific SCNT. Theriogenology 158148–157. ( 10.1016/j.theriogenology.2020.09.006) [DOI] [PubMed] [Google Scholar]
- Verma R, Holland MK, Temple-Smith P, Verma PJ.2012Inducing pluripotency in somatic cells from the snow leopard (Panthera uncia), an endangered felid. Theriogenology 77220–228, 228.e1–228.e2. ( 10.1016/j.theriogenology.2011.09.022) [DOI] [PubMed] [Google Scholar]
- Verma R, Liu J, Holland MK, Temple-Smith P, Williamson M, Verma PJ.2013Nanog is an essential factor for induction of pluripotency in somatic cells from endangered felids. BioResearch Open Access 272–76. ( 10.1089/biores.2012.0297) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira AD, Forell F, Feltrin C, Rodrigues JL.2008Calves born after direct transfer of vitrified bovine in vitro-produced blastocysts derived from vitrified immature oocytes. Reproduction in Domestic Animals 43314–318. ( 10.1111/j.1439-0531.2007.00899.x) [DOI] [PubMed] [Google Scholar]
- Waggener WL, Carroll EJ.1998A method for hormonal induction of sperm release in anurans (eight species) and in vitro fertilization in Lepidobatrachus species. Development, Growth and Differentiation 4019–25. ( 10.1046/j.1440-169x.1998.t01-5-00003.x) [DOI] [PubMed] [Google Scholar]
- Wakayama T, Rodriguez I, Perry AC, Yanagimachi R, Mombaerts P.1999Mice cloned from embryonic stem cells. PNAS 9614984–14989. ( 10.1073/pnas.96.26.14984) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker CA, Bjarkadottir BD, Fatum M, Lane S, Williams SA.2021Variation in follicle health and development in cultured cryopreserved ovarian cortical tissue: a study of ovarian tissue from patients undergoing fertility preservation. Human Fertility 24188–198. ( 10.1080/14647273.2019.1616118) [DOI] [PubMed] [Google Scholar]
- Wanderleya LS, Luza HKM, Faustino LR, Lima IMT, Lopes CAP, Silva AR, Báo SN, Campello CC, Rodrigues APR, de Figueiredo JR.2012Ultrastructural features of agouti (Dasyprocta aguti) preantral follicles cryopreserved using dimethyl sulfoxide, ethylene glycol and propanediol. Theriogenology 77260–267. ( 10.1016/j.theriogenology.2011.07.038) [DOI] [PubMed] [Google Scholar]
- Wani NA, Vettical BS, Hong SB.2017First cloned Bactrian camel (Camelus bactrianus) calf produced by interspecies somatic cell nuclear transfer: a step towards preserving the critically endangered wild Bactrian camels. PLoS ONE 12 e0177800. ( 10.1371/journal.pone.0177800) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watt AM, Marcec-Greaves R, Hinkson KM, Poo S, Roberts B, Pitcher TE.2021Effects of age on sperm quality metrics in endangered Mississippi gopher frogs (Lithobates sevosus) from captive populations used for controlled propagation and reintroduction efforts. Zoo Biology 40218–226. ( 10.1002/zoo.21594) [DOI] [PubMed] [Google Scholar]
- Weeratunga P, Shahsavari A, Ovchinnikov DA, Wolvetang EJ, Whitworth DJ.2018Induced pluripotent stem cells from a marsupial, the Tasmanian Devil (Sarcophilus harrisii): insight into the evolution of mammalian pluripotency. Stem Cells and Development 27112–122. ( 10.1089/scd.2017.0224) [DOI] [PubMed] [Google Scholar]
- Whaley D, Damyar K, Witek RP, Mendoza A, Alexander M, Lakey JR.2021Cryopreservation: an overview of principles and cell-specific considerations. Cell Transplantation 30963689721999617. ( 10.1177/0963689721999617) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitworth DJ, Limnios IJ, Gauthier ME, Weeratunga P, Ovchinnikov DA, Baillie G, Grimmond SM, Graves JAM, Wolvetang EJ.2019Platypus induced pluripotent stem cells: the unique pluripotency signature of a monotreme. Stem Cells and Development 28151–164. ( 10.1089/scd.2018.0179) [DOI] [PubMed] [Google Scholar]
- WHO 2015Biodiversity and Health (Online). Biodiversity and Health. (available at: https://www.who.int/news-room/fact-sheets/detail/biodiversity-and-healthh) (who.int). Accessed on 14th July 2021. [Google Scholar]
- Wiedemann C, Hribal R, Ringleb J, Bertelsen MF, Rasmusen K, Andersen CY, Kristensen SG, Jewgenow K.2012Preservation of primordial follicles from lions by slow freezing and xenotransplantation of ovarian cortex into an immunodeficient mouse. Reproduction in Domestic Animals 47 (Supplement 6) 300–304. ( 10.1111/rda.12081) [DOI] [PubMed] [Google Scholar]
- Wiedemann C, Zahmel J, Jewgenow K.2013Short-term culture of ovarian cortex pieces to assess the cryopreservation outcome in wild felids for genome conservation. BMC Veterinary Research 9 37. ( 10.1186/1746-6148-9-37) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wildt DE, Roth TL.1997Assisted reproduction for managing and conserving threatened felids. International Zoo Yearbook 35164–172. ( 10.1111/j.1748-1090.1997.tb01207.x) [DOI] [Google Scholar]
- Wildt DE, Comizzoli P, Pukazhenthi B, Songsasen N.2010Lessons from biodiversity – the value of nontraditional species to advance reproductive science, conservation, and human health. Molecular Reproduction and Development 77397–409. ( 10.1002/mrd.21137) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilmut I, Schnieke AE, Mcwhir J, Kind AJ, Campbell KH.1997Viable offspring derived from fetal and adult mammalian cells. Nature 385810–813. ( 10.1038/385810a0) [DOI] [PubMed] [Google Scholar]
- Woelders H, Windig J, Hiemstra SJ.2012How developments in cryobiology, reproductive technologies and conservation genomics could shape gene banking strategies for (farm) animals. Reproduction in Domestic Animals 47 (Supplement 4) 264–273. ( 10.1111/j.1439-0531.2012.02085.x) [DOI] [PubMed] [Google Scholar]
- Wolf DP, Hedrick JL.1971A molecular approach to fertilisation. II. Viability and artificial fertilisation of Xenopus laevis gametes. Developmental Biology 25348–359. ( 10.1016/0012-1606(7190036-4) [DOI] [PubMed] [Google Scholar]
- Wu Z, Pan B, Qazi IH, Yang H, Guo S, Yang J, Zhang Y, Zeng C, Zhang M, Han Het al. 2019Melatonin improves in vitro development of vitrified-warmed mouse germinal vesicle oocytes potentially via modulation of spindle assembly checkpoint-related genes. Cells 8 1009. ( 10.3390/cells8091009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wunderlich S, Kircher M, Vieth B, Haase A, Merkert S, Beier J, Göhring G, Glage S, Schambach A, Curnow ECet al. 2014Primate iPS cells as tools for evolutionary analyses. Stem Cell Research 12622–629. ( 10.1016/j.scr.2014.02.001) [DOI] [PubMed] [Google Scholar]
- Xin M, Siddique MAM, Dzyuba B, Cuevas-Uribe R, Shaliutina-Kolešová A, Linhart O.2017Progress and challenges of fish sperm vitrification: a mini review. Theriogenology 9816–22. ( 10.1016/j.theriogenology.2017.04.043) [DOI] [PubMed] [Google Scholar]
- Yamanaka S.2012Induced pluripotent stem cells: past, present, and future. Cell Stem Cell 10678–684. ( 10.1016/j.stem.2012.05.005) [DOI] [PubMed] [Google Scholar]
- Yang CY, Chen MC, Lee PT, Lin TT.2012Cryopreservation of germinal vesicle stage porcine oocytes based on intracellular ice formation assessment. Cryo Letters 33349–362. [PubMed] [Google Scholar]
- Zahmel J, Fernandez-Gonzalez L, Jewgenow K, Müller K.2019Felid-gamete-rescue within EAZA-efforts and results in biobanking felid oocytes and sperm. Journal of Zoo and Aquarium Research 715–24. [Google Scholar]
- Zhang Y, Liu Y, Liu H, Tang WH.2019Exosomes: biogenesis, biologic function and clinical potential. Cell and Bioscience 9 19. ( 10.1186/s13578-019-0282-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou GB, Ma CB, Liu GS, Zhu SE, Zhang HH, Jia LL, Suo L, Shi JM, Wang YB, Tian TH, et al. 2009Vitrification of farmed blue fox oocytes in ethylene glycol and DMSO-based solutions using open-pulled straw (OPS). Cryo Letters 301, 12–118. [PubMed] [Google Scholar]
- Zimkus BM, Hassapakis CL, Houck ML.2018Integrating current methods for the preservation of amphibian genetic resources and viable tissues to achieve best practices for species conservation. Amphibian and Reptile Conservation 12 e165. [Google Scholar]
- Zuo Y, Gao Y, Su G, Bai C, Wei Z, Liu K, Li Q, Bou S, Li G.2014Irregular transcriptome reprogramming probably causes theca developmental failure of embryos produced by interspecies somatic cell nuclear transfer between the Przewalski’s gazelle and the bovine. BMC Genomics 15 1113. ( 10.1186/1471-2164-15-1113) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



 This work is licensed under a
This work is licensed under a