Abstract
Objective
Growth differentiation factor-15 (GDF15), a key metabolic regulator, is associated with obesity and diabetes in which sex-specific differences have been reported. Thus, we assessed whether GDF15 could be dependent on sex in diabetes and/or obesity groups.
Methods
We measured serum GDF15 levels by ELISA in eight lean women and men (n = 16), eight women and eight men having obesity (n = 16), eight women and eight men with type 2 diabetes (T2D, n = 16), and seven women and nine men with both diabetes and obesity (n = 16). Estimation of the difference in the means of each group was performed by two-way ANOVA. The interdependence of the different variates was addressed by multivariate analysis. Correlations between GDF15 levels and HOMA-IR, HbA1c, triglycerides, HDL, and LDL were explored by linear regression.
Results
Being a woman and having obesity alone or in combination with diabetes decreased GDF15 serum levels (β = −0.47, CI = −0.95, 0.00, P = 0.052; β = −0.45, CI = −0.94, 0.05, P= 0.075). Diabetes independently of metformin treatment and obesity were not predictive of low GDF15 levels (β = 0.10, CI = −0.36, 0.57, P = 0.7). Correlation analysis showed that HOMA-IR (r = 0.45, P = 0.008) and triglycerides (r = 0.41, P = 0.017) were positively correlated and HDL (r = −0.48, P = 0.005) was negatively correlated with GDF15 levels in men.
Conclusions/interpretation
GDF15 level was significantly different between men and women, as well as between the groups. Sex and group interaction revealed that being a woman and having obesity alone or in combination with diabetes decreased GDF15 levels.
Keywords: GDF15, diabetes, obesity, sex
Introduction
Obesity is rapidly increasing worldwide despite it being a major health concern (1). Excess weight represents a hazardous condition leading to the development of type 2 diabetes (T2D) and its complications, mainly cardiovascular diseases (2). Diabetes onset is a pathology involving a continuum. In fact, a proportion of obese patients display impaired fasting glucose levels without meeting the criteria for the diagnosis of diabetes (3). This intermediate group has been identified as having prediabetes, a disorder that substantially increases the risk for diabetes (4). Thus, identification of relevant blood-based biomarkers allowing risk stratification in obese patients is required. As sex-specific differences in glucose homeostasis and T2D risk have already been reported (5), considering sex appears to be of particular importance when searching for biomarkers of metabolic disorders.
Growth differentiation factor-15 (GDF15), a member of the transforming growth factor-β (TGF-β) family, has been identified as a stress-responsive cytokine and metabolic modulator whose expression is markedly elevated in response to stress conditions, aging, obesity, and diabetes (3, 6, 7, 8, 9, 10, 11, 12, 13). In accordance, GDF15 serum levels have been found to be higher in obese subjects with diabetes relative to patients with diabetes alone (12, 14). In addition, the level of GDF15 has been described as a biomarker for mitochondrial dysfunction (15, 16), muscle weakness in an age-dependent manner, and inflammation (17, 18). Conversely, GDF15 levels have also been reported to be elevated in patients subjected to approaches beneficial to health such as exercise and metformin treatment (8, 19). This suggests that the enhancement of circulating GDF15 in T2D and/or obesity may be a compensatory mechanism rather than a cause. For instance, GDF15 circulating levels have been found to rise under in vitro glucolipotoxic conditions, although treatment with recombinant GDF15 could improve the secretory function of pancreatic beta-cells (20). Additionally, GDF15 was found to be a predictive marker of future abnormal glucose control and insulin response (3). The authors revealed that changes in GDF15 levels were related to changes in body weight, BMI, and insulin resistance and that high GDF15 levels at baseline increase the risk for prediabetes and diabetes (3). Dostalova et al. showed that GDF15 increased in serum from obese women relative to their healthy counterparts, and its circulating levels were further augmented in the presence of diabetes (6). Considering these data, and despite the fact that the levels of sex hormones, such as testosterone and estradiol, may influence the development of metabolic disorders (21, 22), there are no reports, to our knowledge, exploring the circulating GDF15 levels in women vs men with obesity and/or T2D. To assess the impact of sex on the level of circulating GDF15 and its correlation to clinical and metabolic parameters, we aimed to determine the possible association between GDF15 serum levels with hormonal and metabolic status in lean women and men with obesity, diabetes, or both.
Materials and methods
Patients
A total of 64 participants were recruited from the outpatient clinic of Geneva University Hospital. Patients enrolled in this study signed an informed consent form. As previously described (23), the exclusion criteria were type 1 diabetes or latent autoimmune diabetes in adults, steroid-induced diabetes, post-transplant diabetes, hepatitis, corticosteroid therapy, active neoplasia, and ongoing shift work. The participants were asked to follow a moderate diet without excess fat or alcohol intake, 24 h prior to the testing day. Overall, eight lean control women and men (n = 16), eight women and eight men with obesity (n = 16), eight lean women and eight lean men with T2D (n = 16), seven women and nine men with both T2D and obesity (n = 16) were included in this study. Thereafter, each group was further separated according to sex. The mean age of the patients was 59.3 ± 9.05 years (age range: 41–75 years). Thirty-three participants (51.6%) were male and 31 (48.4%) were female, amounting to a total of 64 patients (see detailed results for each group in Table 1). The research protocol was registered with the Protocol Registration and Results System at ClinicalTrials.gov (NCT 02384148).
Table 1.
Characteristics of the studied groups. Anthropometric, hormonal, and biochemical characteristics of the control, obese non-T2D, lean T2D, and obese T2D group of patients. Values are given as means ± s.d.
| Control, n = 16 | Lean T2D, n = 16 | Obese, n = 16 | Obese T2D, n = 16 | |||||
|---|---|---|---|---|---|---|---|---|
| Female, n = 8 | Male, n = 8 | Female, n = 8 | Male, n = 8 | Female, n = 8 | Male, n = 8 | Female, n = 7 | Male, n = 9 | |
| Age (years) | 59 (9) | 61 (7) | 60 (10) | 62 (5) | 55 (9) | 54 (9) | 61 (12) | 63 (11) |
| BMI (kg/m2) | 22.40 (1.69) | 23.81 (1.31) | 22.66 (1.90) | 24.12 (1.77) | 37.74 (4.04) | 35.99 (2.31) | 36.37 (3.90) | 36.34 (2.97) |
| Fasting glucose (mmol/L) | 5.08 (0.32) | 5.15 (0.29) | 8.93 (1.62) | 8.29 (0.99) | 5.25 (0.23) | 5.58 (0.55) | 10.51 (1.63) | 11.42 (1.61) |
| Fasting insulin (mU/L) | 6.96 (2.92) | 5.11 (2.02) | 10.3 (6.3) | 7.4 (3.9) | 16 (7) | 21 (11) | 21 (6) | 28 (9) |
| HbA1c (%) | 5.26 (0.23) | 5.39 (0.16) | 7.32 (0.70) | 7.86 (2.09) | 5.53 (0.23) | 5.36 (0.32) | 7.93 (0.95) | 8.96 (0.85) |
| HOMA-IR | 1.57 (0.68) | 1.17 (0.45) | 4.35 (3.26) | 2.73 (1.47) | 3.82 (1.68) | 5.10 (2.81) | 10.0 (3.6) | 13.9 (4.1) |
| ALAT (U/L) | 27 (14) | 32 (16) | 29 (9) | 30 (14) | 30 (9) | 43 (18) | 34 (11) | 60 (19) |
| ASAT (U/L) | 24 (10) | 26 (7) | 22 (7) | 24 (9) | 21 (5) | 32 (11) | 22 (6) | 40 (15) |
| PA (U/L) | 78 (22) | 60 (11) | 68 (19) | 60 (20) | 77 (17) | 68 (10) | 85 (22) | 75 (20) |
| GGT (U/L) | 32 (25) | 44 (29) | 23 (14) | 33 (13) | 30 (19) | 64 (38) | 47 (26) | 163 (122) |
| Total cholesterol (mmol/L) | 5.50 (1.22) | 5.35 (1.16) | 4.54 (1.25) | 5.00 (1.23) | 5.05 (0.84) | 5.53 (0.82) | 5.26 (1.26) | 6.07 (2.32) |
| Triglyceride (mmol/L) | 1.02 (0.35) | 1.44 (0.70) | 1.48 (0.93) | 2.02 (1.83) | 1.42 (0.55) | 2.21 (1.56) | 3.3 (2.9) | 5.0 (7.1) |
| LDL cholesterol (mmol/L) | 3.17 (1.17) | 3.12 (1.13) | 2.42 (1.11) | 2.72 (1.10) | 3.01 (0.81) | 3.29 (0.66) | 3.17 (0.72) | 2.80 (1.57) |
| HDL cholesterol (mmol/L) | 1.86 (0.30) | 1.57 (0.25) | 1.44 (0.50) | 1.36 (0.41) | 1.40 (0.23) | 1.23 (0.20) | 1.12 (0.35) | 1.01 (0.27) |
| Cortisol (nmol/L) | 349 (98) | 402 (132) | 382 (65) | 394 (155) | 378 (117) | 304 (104) | 406 (148) | 366 (71) |
| Leukocytes (g/L) | 5.16 (1.41) | 6.96 (1.95) | 7.09 (1.44) | 6.38 (0.86) | 5.30 (1.11) | 6.91 (1.39) | 8.97 (2.66) | 7.00 (2.04) |
| Neutrophils (g/L) | 2.53 (1.31) | 4.71 (1.35) | 4.10 (1.24) | 3.60 (1.01) | 3.18 (0.84) | 4.23 (0.88) | 5.13 (1.82) | 4.25 (1.89) |
| Eosinophils (%) | 2.12 (1.55) | 1.34 (0.73) | 2.92 (2.45) | 4.88 (3.44) | 4.06 (3.76) | 2.62 (2.20) | 3.07 (1.37) | 3.16 (2.04) |
| Basophils (%) | 0.75 (0.71) | 0.84 (1.05) | 0.50 (0.53) | 1.00 (0.93) | 0.62 (0.92) | 1.25 (1.58) | 0.61 (0.49) | 0.70 (0.66) |
| Monocytes (%) | 4.75 (1.75) | 4.86 (2.49) | 5.81 (2.07) | 5.25 (3.77) | 5.31 (3.65) | 7.12 (2.36) | 6.6 (2.9) | 8.1 (4.5) |
| Lymphocytes (g/L) | 1.98 (0.58) | 1.76 (0.75) | 2.28 (0.32) | 2.07 (0.59) | 1.59 (0.44) | 1.87 (0.92) | 2.90 (1.10) | 2.07 (0.44) |
| GDF 15 (pg/mL) | 494 (178) | 527 (160) | 1353 (649) | 1106 (358) | 413 (121) | 657 (252) | 678 (278) | 1534 (770) |
Clinical and biochemical parameters
As previously described (23), participants enrolled in this study underwent a clinical examination. Height and body weight were recorded, allowing BMI determination. All serum samples were collected in clot-activator vacutainers between 08:00 and 10:00 h, following overnight fasting from 22:00 h onwards. These were immediately analyzed by the Geneva University Hospital laboratory for insulin, blood glucose, HbA1c, triglycerides, total cholesterol, low-density lipoprotein cholesterol (LDL), high-density lipoprotein cholesterol (HDL), leukocytes, and lymphocytes. The homeostasis model assessment of insulin resistance (HOMA-IR) was also calculated as described elsewhere (24). Thereafter, serum was immediately prepared from blood samples by centrifugation (10 min, 1650g, 4 °C) and stored at −80°C until lipid extraction and analysis.
Ethics
Ethical approval for the study was obtained from the Cantonal Research Ethics Commission of Geneva, Switzerland (project number CER11-015), and all participants provided written informed consent.
Outcomes
The primary outcome was serum GDF15 levels, measured using a human quantitative sandwich ELISA kit (R&D Systems, DGD150). The ELISA was performed according to the manufacturer’s instructions. The assay sensitivity was 4.39 pg/mL, and the intra- and inter-assay coefficient variations (CV) were <3% and <6%, respectively.
Statistical analysis
First, to be able to perform statistical tests and multiple regression, we transformed the response variable, the serum GDF15 level, to a log scale, in order to ensure normality. Using the Shapiro–Wilk test on the log of the GDF 15 concentration, we cannot reject the normality assumption (W = 0.97, P = 0.1906). We then performed a two-way ANOVA between sex and patient group (obesity status and T2D status) to estimate whether there was any difference in the means of each group.
We then performed a multivariate analysis in order to address the interdependence of different variates and how this predicted circulating GDF15 levels. We decided to include the following variables in our model: sex, age, obesity status, diabetes status, metformin treatment (known to influence GDF15 levels), ALAT levels (a measure of liver health), LDL levels (a measure of lipid metabolism), HbA1c levels (a measure of carbohydrates metabolism), and interaction between sex, obesity status, and diabetes status. Although the sample size was small (n = 64), the model performed well, with an adjusted R-squared of 0.68 (meaning that 68% of the variance of the GDF 15 concentration (log) can be explained by the variance of our independent predictors). Residuals were checked using a graph plot and stayed within the expected range. The results of the linear regression are presented in Fig. 2. Note that the independent variable was the log of the GDF15 concentration in order to respect the normality assumption and that the value of the coefficient reflects that change. The statistical analysis was performed using R software.
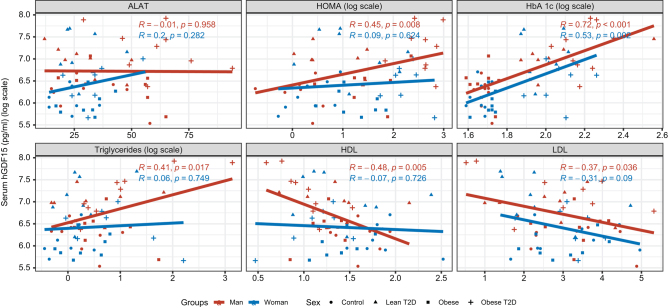
Figure 2.
Correlation between log (GDF15) plasma levels and glucose/lipid metabolism parameters in control, obese, lean type 2 diabetic, and obese with type 2 diabetic groups. Correlations were controlled for alanine aminotransferase (ALAT), glycated hemoglobin (HbA 1c), triglycerides, high-density lipoprotein (HDL), and low-density lipoprotein (LDL), homeostatic model assessment for insulin resistance (HOMA-IR) on men (red), and women (blue) for all the four groups. P-values and r values are from Spearman rank correlation.
Results
Impact of sex and groups on circulating GDF15 levels
To investigate the impact of obesity and T2D on circulating GDF15 levels in men and women, we measured GDF15 in the serum of participants enrolled in the previously described cohort (23). Both the obese and the T2D obese groups had higher BMIs, fasting insulin, and HOMA-IR, as well as lower HDL. Fasting serum glucose and HbAlc levels were significantly higher in the T2D and the T2D with obesity groups compared with the obese and the control groups. The overall parameters herein did not significantly differ between men and women. Fasting serum levels of total cholesterol, triglycerides, LDL, and cortisol did not significantly differ between the groups. Of note, leucocyte and neutrophil numbers were increased in the group of women with T2D and obesity relative to the control (Table 1).
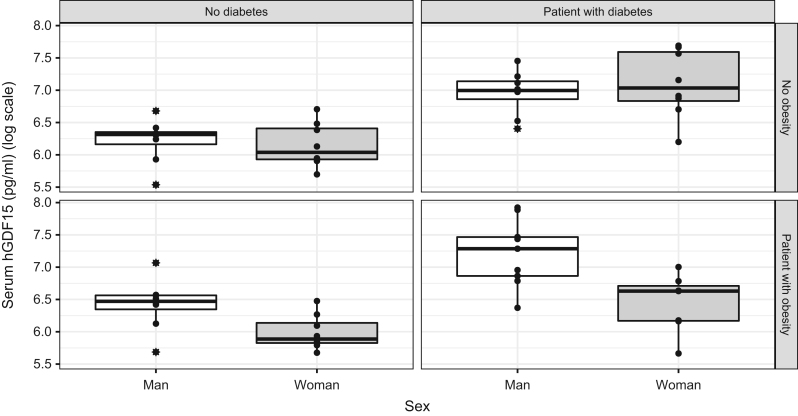
Beyond characterization of the enrolled population, the primary objective was to investigate the impact of sex on GDF15 serum levels in our different populations, namely, lean controls, lean T2D patients, obese patients, and T2D obese patients (Fig. 1). The ANOVA revealed that there was a statistically significant difference between the means of sex (men and women; F = 7.6933, P < 0.01), groups (control, obese non-T2D, lean T2D, and obese T2D patients; F = 18.1362, P < 0.001), and the interaction between sex and groups was statistically significant (F = 3.8664, P = 0.013). We found that being lean without having T2D (β = −0.02, CI = −0.36, 0.32, P = 0.9) or being lean and having T2D (β = 0.10, CI = −0.36, 0.57, P = 0.7) did not statistically significantly affect GDF-15 concentrations in our multivariate analyses. On the other hand, we observed that having obesity without T2D positively affected GDF15 levels (β = 0.35, CI = −0.00, 0.69, P = 0.049) (Table 2). Strikingly, the interaction between being a woman and having obesity showed a negative trend in GDF15 serum levels (β = −0.47, CI = −0.95, 0.00, P = 0.052). Even though the interaction between being a woman and having diabetes was not significant (β = 0.28, CI = −0.20, 0.75, P = 0.3) there was, on the other hand, an interaction effect in women with obesity and T2D on GDF15 levels (β = −0.45, CI = −0.94, 0.05, P = 0.075). Altogether, our data suggest that women with obesity have a different underlying GDF15 mechanism than men with obesity or that obesity in women has an impact on GDF15 levels.
Figure 1.
GDF15 plasma levels in control, obese, lean type 2 diabetes, and obese with type 2 diabetes subjects. Values are expressed as a boxplot with lines indicating medians. Whiskers extend from the box up to the smallest/highest observations. Statistical significance is from two-way ANOVA.
Table 2.
Multivariate linear regression analysis of log (GDF15). All variables shown in this table were tested for their multivariate (independent) associations with GDF15. GDF15 concentrations were adjusted in log10. HOMA-IR and triglycerides were not included in this analysis, given their interaction with other parameters in the analysis. Beta-coefficient and statistical significance are shown.
| Characteristics | Beta | 95% CI | P-value |
|---|---|---|---|
| Intercept | 4.9 | 3.9, 5.9 | <0.001 |
| Sex | |||
| Men | – | – | |
| Women | −0.02 | −0.36, 0.32 | 0.9 |
| Age | 0.02 | 0.01, 0.03 | <0.001 |
| With obesity | 0.35 | 0.00, 0.69 | 0.049 |
| With diabetes | 0.10 | −0.36, 0.57 | 0.7 |
| Taking metformin | 0.43 | 0.15, 0.71 | 0.003 |
| ALAT | 0.00 | −0.01, 0.01 | >0.9 |
| LDL | −0.11 | −0.19, −0.02 | 0.013 |
| HbA 1c | 0.10 | 0.00, 0.20 | 0.057 |
| Sex × with obesity | |||
| Woman × with obesity | −0.47 | −0.95, 0.00 | 0.052 |
| Sex × with diabetes | |||
| Woman × with diabetes | 0.28 | −0.20, 0.75 | 0.3 |
| Sex × with obesity × with diabetes | |||
| Man × with obesity × with diabetes | −0.26 | −0.78, 0.26 | 0.3 |
| Woman × with obesity × with diabetes | −0.45 | −0.94, 0.05 | 0.075 |
R² = 0.743; Adjusted R² = 0.683; Sigma = 0.333; Statistic = 12.3; P-value = <0.001; df = 12; Log-likelihood = −13.2; AIC = 54.5; BIC = 84.7; Deviance = 5.67; Residual df = 51; No. Obs. = 64.
Correlation of independent variables and circulating GDF15 levels
The GDF15 serum levels varied in a sex-dependent manner, and the obesity/diabetes status could be the cause or consequence of hormonal change. To investigate this hypothesis, we evaluated the correlation between GDF15 and metabolic parameters including HOMA-IR, Hb1Ac, triglycerides, LDL, and HDL (Fig. 2). We found a positive correlation with HOMA-IR in men of all studied groups. Hb1Ac had a positive correlation with GDF15 serum levels in men and women with T2D associated or not with obesity. Triglycerides were positively correlated with GDF15 in men of all groups. HDL and LDL were negatively correlated with GDF15 serum levels in men of all enrolled groups. We then performed multivariate regression to evaluate the association with the focused parameters and to understand the potential underlying mechanism (Table 2). Our results show that GDF15 levels increased with age (β = 0.02, CI = (0.01, 0.03), P < 0.001). Patients receiving metformin treatment also had higher GDF15 levels (β = 0.43, CI = (0.15, 0.71), P = 0.003). Patients with higher HbA1c levels had higher GDF15 levels (β = 0.10, CI = (0.00, 0.20), P = 0.057), but patients with higher LDL levels had lower GDF15 levels (β = −0.10, CI = (−0.19, −0.02), P = 0.013). Both results hint at already known GDF15 mechanisms. Of note, hepatic function assessed through the marker ALAT did not have an impact on the GDF15 levels (β = 0.00, CI = (−0.1, 0.1), P > 0.9).
Discussion
GDF15 has been identified as a metabolic modulator whose expression is markedly elevated in obese and diabetic individuals (3, 6, 7, 8, 9). Interestingly, although sex differences represent a source of factors that impact various parameters in obesity and diabetes (5), there have been no reports to date regarding the effect of sex on GDF15 levels. We, therefore, investigated the impact of sex on GDF15 levels in lean, obese, diabetic, and obese–diabetic women and men patients. We hypothesized that circulating GDF15 could be a sex-dependent serum biomarker in diabetes or obese diabetes groups.
In this study, we report no difference in serum GDF15 levels between being lean without having T2D or being lean and having T2D. Furthermore, for these patients without obesity, no interaction was found between sex and diabetes status. Notably, in contrast, obesity was consequentially associated with increased GDF15 serum levels compared to lean patients, independently of diabetes status. Most interestingly, we were able to highlight an interaction between being a woman and having obesity combined or not with diabetes. Intriguingly, being a lean woman and having diabetes were not significantly associated with circulating GDF15 levels. Together, our data suggest that either obesity may impact GDF15 levels only in women or there might be a different regulatory mechanism on GDF15 levels in men and women with obesity. In line with this latest hypothesis, Zhang et al. revealed a sex-specific modulation of GDF15 expression in response to stress conditions (25). This study argues more for a gender-dependent modulation of GDF15 levels in case of obesity instead of obesity influencing GDF15 levels only in women. In addition, GDF15 levels were found to be increased in patients having obesity or who had cancer with cachectic status (26). An increase in GDF15 levels in opposition to changes in body weight argues against a direct role of obesity alone in the regulation of GDF15 serum levels.
Upon multivariate adjustment, being a woman and having obesity appeared to be a negative predictor of GDF15 levels although the statistically significant was unreached. Our results are in accordance with those of Ding et al., who showed that GDF15, the expression of which was stimulated by prostaglandin in their evaluation, is negatively correlated to BMI (27). In contrast, the study by Dostalova et al. reported higher serum GDF15 levels in women with obesity. This discrepancy could be due to the difference in the characteristics of the populations, notably the variation in the BMI. Patients with obesity in our study had a mean BMI of 37.7 ± 1.4 kg/m2, while the patients in their analysis had severe obesity, with a higher mean BMI (50.4 ± 2.6 kg/m2) (6). However, BMI alone cannot explain these differences since, when considering obesity independently of sex, we showed that obesity is a positive predictor of increased GDF15 levels.
Like obesity, T2D was associated with increased GDF15 levels (3, 6, 28). Nonetheless, these associations were not observed in the large Whitehall cohort, and the authors suggested that elevated GDF15 levels are not a predictor of diabetes (29). It has been proposed that the use of metformin, which induces GDF15, represented a confounding factor (30). Therefore, we confirmed a positive interaction between metformin use and GDF15 levels by multivariate analysis. Moreover, we observed no consequential association between type 2 diabetes with GDF15 serum levels, when considered independently of sex, metformin treatment, and obesity status. However, this non-significant correlation may be due to the small sample size of our study instead of the isolation of the metformin treatment. Therefore, this analysis should be performed with a larger sample to evaluate whether GDF15 levels may be considered as a predictor or marker of diabetes independent of sex, metformin treatment, and obesity status.
In the group with obesity and type 2 diabetes, we observed an interaction with women (β = −0.45, CI = −0.94, 0.05, P = 0.075) but not with men (β = −0.26, CI = −0.78, 0.26, P = 0.3). This interaction negatively predicted GDF15 levels. Our results are opposed to the XENDOS trial data showing increased GDF15 levels in obese patients developing diabetes (3). The regression coefficients were roughly the same in women with obesity and women with both obesity and diabetes, hence suggesting no synergistic effect in the obese T2D group. When concomitantly present in women, it appears that the effect of obesity overcomes that of diabetes, leading to an association with negative prediction of GDF15 levels. However, further investigations are warranted to elucidate this hypothesis and clarify the role of GDF15 in obese–diabetic women. GDF15 expression is induced under stress conditions such as obesity and/or T2D, and thus it is likely that a range of factors commonly associated with these metabolic alterations may also be associated with increasing GDF15 concentrations. Considering this, we investigated multiple factors, and we found that increased HbA1c concentrations were associated with higher GDF15 levels in women and men. Insulin sensitivity assessed by HOMA-IR, an index reflecting hepatic insulin resistance (31), showed a positive correlation with GDF15 levels only in men. A similar positive association exclusively in men between triglycerides and GDF15 was found.
Others have demonstrated relationships between GDF15 and triglyceride levels, low LDL, and low HDL in men, hence supporting a link between GDF15 and lipid metabolism (32). Our analysis revealed a negative and positive correlation with HDL and circulating triglycerides, respectively, only in men. Moreover, LDL was negatively associated with GDF15 levels in men of all groups. Our data are in accordance with those of a previous study (6). Altogether, this suggests that GDF15 is likely to be associated with lipid metabolism and insulin resistance mainly in men and reflects an overall metabolic alteration. These sex-specific variations may have impacted the interaction between being a man and having obesity and/or diabetes. Therefore, it would have been interesting to include HOMA-IR and triglyceride in the multivariate analysis to evaluate their interactions with women having obesity or/and diabetes. However, given the small sample size and interaction with other variables, these analyses were not performed.
In summary, we showed a significant difference in the mean GDF15 concentrations between men and women, as well as between the groups (i.e, lean, lean T2D, obese, and obese T2D individuals). Moreover, we found an interaction between sex and group although the statistically significant was not reached in our multivariate analysis. Interestingly, we found an interaction between sex (i.e. being a woman) and obesity in the presence or absence of diabetes. Co-occurrence of both diabetes and obesity did not have a synergistic effect relative to obesity alone. GDF15 serum levels increased with age and HbA1c concentration and decreased as LDL increased. Linear regression revealed that GDF15 levels were positively correlated to HOMA-IR, triglycerides, and HbA1c in both men and women. Importantly, these associations for HOMA-IR and triglyceride were significant only in men. On the other hand, HDL and LDL were negatively associated with GDF15 in men. Therefore, the absence of correlation between GDF15 and lipid metabolism markers in women suggests that the fact of being women and having obesity concomitantly or not with diabetes negatively affects GDF15 concentration and might not be dependent on lipid metabolism but rather on sex specificities. However, this is speculative, and further studies are warranted to demonstrate such hypothesis.
Declaration of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Funding
This project was supported by funding from the Helmut Horten Foundation, the Raymond Berger Foundation, the Hjelt Foundation, and the De Reuter Foundation awarded to Karim Gariani, as well as by SNSF grants 31003A_166700/1 and 310030-184708, the Vontobel Foundation, the Olga Mayenfisch Foundation, the Novartis Foundation for Medical-Biological Research, EFSD/Novo Nordisk Programme for Diabetes Research in Europe, the Leenaards Foundation, the Foundation Ernst et Lucie Schmidheiny, the Jubiläumsstiftung Swiss Life Foundation, and the Foundation pour la Recherche sur le Cancer (attributed to Charna Dibner); SNSF grant CRSII3_160741; SNSF grant 189003 (attributed to François Jornayvaz), and by the Young Independent Investigator Grant SGED/SSED (attributed to Flore Sinturel).
Acknowledgements
The authors would like to acknowledge Mr Antoine Poncet for his technical support with the statistical analysis and Pr. Jacques Philippe, Pr. Steven Brown, Dr Camille Saini, and Dr Pauline Gosselin for their help with the study design and management of the T2D patient cohort sampling.
References
- 1.Finucane MM, Stevens GA, Cowan MJ, Danaei G, Lin JK, Paciorek CJ, Singh GM, Gutierrez HR, Lu Y, Bahalim AN, et al. National, regional, and global trends in body-mass index since 1980: systematic analysis of health examination surveys and epidemiological studies with 960 country-years and 9.1 million participants. Lancet 2011377557–567. ( 10.1016/S0140-6736(1062037-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mazzone T, Chait A, Plutzky J. Cardiovascular disease risk in type 2 diabetes mellitus: insights from mechanistic studies. Lancet 20083711800–1809. ( 10.1016/S0140-6736(0860768-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kempf T, Guba-Quint A, Torgerson J, Magnone MC, Haefliger C, Bobadilla M, Wollert KC. Growth differentiation factor 15 predicts future insulin resistance and impaired glucose control in obese nondiabetic individuals: results from the Xendos trial. European Journal of Endocrinology 2012167671–678. ( 10.1530/EJE-12-0466) [DOI] [PubMed] [Google Scholar]
- 4.American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care 201437 (Supplement 1) S81–S90. ( 10.2337/dc14-S081) [DOI] [PubMed] [Google Scholar]
- 5.Mauvais-Jarvis F.Epidemiology of gender differences in diabetes and obesity. Advances in Experimental Medicine and Biology 201710433–8. ( 10.1007/978-3-319-70178-3_1) [DOI] [PubMed] [Google Scholar]
- 6.Dostalova I, Roubicek T, Bartlova M, Mraz M, Lacinova Z, Haluzikova D, Kavalkova P, Matoulek M, Kasalicky M, Haluzik M. Increased serum concentrations of macrophage inhibitory Cytokine-1 in patients with obesity and type 2 diabetes mellitus: the influence of very low calorie diet. European Journal of Endocrinology 2009161397–404. ( 10.1530/EJE-09-0417) [DOI] [PubMed] [Google Scholar]
- 7.Vila G, Riedl M, Anderwald C, Resl M, Handisurya A, Clodi M, Prager G, Ludvik B, Krebs M, Luger A. The relationship between insulin resistance and the cardiovascular biomarker growth differentiation Factor-15 in obese patients. Clinical Chemistry 201157309–316. ( 10.1373/clinchem.2010.153726) [DOI] [PubMed] [Google Scholar]
- 8.Day EA, Ford RJ, Smith BK, Mohammadi-Shemirani P, Morrow MR, Gutgesell RM, Lu R, Raphenya AR, Kabiri M, McArthur AG, et al. Metformin-induced increases in Gdf15 are important for suppressing appetite and promoting weight loss. Nature Metabolism 201911202–1208. ( 10.1038/s42255-019-0146-4) [DOI] [PubMed] [Google Scholar]
- 9.Wang D, Day EA, Townsend LK, Djordjevic D, Jorgensen SB, Steinberg GR. Gdf15: emerging biology and therapeutic applications for obesity and cardiometabolic disease. Nature Reviews: Endocrinology 202117592–607. ( 10.1038/s41574-021-00529-7) [DOI] [PubMed] [Google Scholar]
- 10.Kim KH, Lee MS. Gdf15 as a central mediator for integrated stress response and a promising therapeutic molecule for metabolic disorders and Nash. Biochimica et Biophysica Acta. General Subjects 20211865 129834. ( 10.1016/j.bbagen.2020.129834) [DOI] [PubMed] [Google Scholar]
- 11.Wang D, Day EA, Townsend LK, Djordjevic D, Jorgensen SB, Steinberg GR. Gdf15: emerging biology and therapeutic applications for obesity and cardiometabolic disease. Nature Reviews: Endocrinology 202117592–607. ( 10.1038/s41574-021-00529-7) [DOI] [PubMed] [Google Scholar]
- 12.Schindler K, Vila G, Hoppichler F, Lechleitner M, Luger A, Anderwald C, Hoefler J, Tomasec G, Kautzky-Willer A, Ludvik B. The impact of Type 2 diabetes on circulating Adipokines in patients with metabolic syndrome. Obesity Facts 20125270–276. ( 10.1159/000338729) [DOI] [PubMed] [Google Scholar]
- 13.Moon JS, Goeminne LJE, Kim JT, Tian JW, Kim SH, Nga HT, Kang SG, Kang BE, Byun JS, Lee YS, et al. Growth differentiation Factor 15 protects against the aging-mediated systemic inflammatory response in humans and mice. Aging Cell 202019 e13195. ( 10.1111/acel.13195) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schernthaner-Reiter MH, Itariu BK, Krebs M, Promintzer-Schifferl M, Stulnig TM, Tura A, Anderwald CH, Clodi M, Ludvik B, Pacini G, et al. Gdf15 reflects beta cell function in obese patients independently of the grade of impairment of glucose metabolism. Nutrition, Metabolism, and Cardiovascular Diseases 201929334–342. ( 10.1016/j.numecd.2018.12.008) [DOI] [PubMed] [Google Scholar]
- 15.Conte M, Ostan R, Fabbri C, Santoro A, Guidarelli G, Vitale G, Mari D, Sevini F, Capri M, Sandri M, et al. Human aging and longevity are characterized by high levels of mitokines. Journals of Gerontology: Series A, Biological Sciences and Medical Sciences 201974600–607. ( 10.1093/gerona/gly153) [DOI] [PubMed] [Google Scholar]
- 16.Fujita Y, Taniguchi Y, Shinkai S, Tanaka M, Ito M. Secreted growth differentiation Factor 15 as a potential biomarker for mitochondrial dysfunctions in aging and age-related disorders. Geriatrics and Gerontology International 201616 (Supplement 1) 17–29. ( 10.1111/ggi.12724) [DOI] [PubMed] [Google Scholar]
- 17.Conte M, Martucci M, Mosconi G, Chiariello A, Cappuccilli M, Totti V, Santoro A, Franceschi C, Salvioli S. Gdf15 plasma level is inversely associated with level of physical activity and correlates with markers of inflammation and muscle weakness. Frontiers in Immunology 202011 915. ( 10.3389/fimmu.2020.00915) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim H, Kim KM, Kang MJ, Lim S. Growth differentiation Factor-15 as a biomarker for sarcopenia in aging humans and mice. Experimental Gerontology 2020142 111115. ( 10.1016/j.exger.2020.111115) [DOI] [PubMed] [Google Scholar]
- 19.Zhang H, Fealy CE, Kirwan JP. Exercise training promotes a Gdf15-associated reduction in fat mass in older adults with obesity. American Journal of Physiology: Endocrinology and Metabolism 2019316E829–E836. ( 10.1152/ajpendo.00439.2018) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Asrih M, Dusaulcy R, Gosmain Y, Philippe J, Somm E, Jornayvaz FR, Kang BE, Jo Y, Choi MJ, Yi HS, et al. Growth differentiation Factor-15 prevents glucotoxicity and connexin-36 downregulation in pancreatic beta-cells. Molecular and Cellular Endocrinology 2022541 111503. ( 10.1016/j.mce.2021.111503) [DOI] [PubMed] [Google Scholar]
- 21.Antonio L, Wu FC, O'Neill TW, Pye SR, Carter EL, Finn JD, Rutter MK, Laurent MR, Huhtaniemi IT, Han TS, et al. Associations between sex steroids and the development of metabolic syndrome: a longitudinal study in European men. Journal of Clinical Endocrinology and Metabolism 20151001396–1404. ( 10.1210/jc.2014-4184) [DOI] [PubMed] [Google Scholar]
- 22.Flynn E, Tanigawa Y, Rodriguez F, Altman RB, Sinnott-Armstrong N, Rivas MA. Sex-specific genetic effects across biomarkers. European Journal of Human Genetics 202129154–163. ( 10.1038/s41431-020-00712-w) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hannich JT, Loizides-Mangold U, Sinturel F, Harayama T, Vandereycken B, Saini C, Gosselin P, Brulhart-Meynet MC, Robert M, Chanon S, et al. Ether lipids, sphingolipids and toxic 1-Deoxyceramides as hallmarks for lean and obese Type 2 diabetic patients. Acta Physiologica 2021232 e13610. ( 10.1111/apha.13610) [DOI] [PubMed] [Google Scholar]
- 24.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 198528412–419. ( 10.1007/BF00280883) [DOI] [PubMed] [Google Scholar]
- 25.Zhang Y, Jiang W, Wang L, Lingappan K. Sex-specific differences in the modulation of growth differentiation Factor 15 (Gdf15) by hyperoxia in vivo and in vitro: role of HIF-1alpha. Toxicology and Applied Pharmacology 20173328–14. ( 10.1016/j.taap.2017.07.016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dostalova I, Kavalkova P, Papezova H, Domluvilova D, Zikan V, Haluzik M. Association of macrophage inhibitory Cytokine-1 with nutritional status, body composition and bone mineral density in patients with anorexia nervosa: the influence of partial realimentation. Nutrition and Metabolism 20107 34. ( 10.1186/1743-7075-7-34) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ding Q, Mracek T, Gonzalez-Muniesa P, Kos K, Wilding J, Trayhurn P, Bing C. Identification of macrophage inhibitory Cytokine-1 in adipose tissue and its secretion as an adipokine by human adipocytes. Endocrinology 20091501688–1696. ( 10.1210/en.2008-0952) [DOI] [PubMed] [Google Scholar]
- 28.Hong JH, Chung HK, Park HY, Joung KH, Lee JH, Jung JG, Kim KS, Kim HJ, Ku BJ, Shong M. Gdf15 is a novel biomarker for impaired fasting glucose. Diabetes and Metabolism Journal 201438472–479. ( 10.4093/dmj.2014.38.6.472) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Carstensen M, Herder C, Brunner EJ, Strassburger K, Tabak AG, Roden M, Witte DR. Macrophage inhibitory Cytokine-1 is increased in individuals before Type 2 diabetes diagnosis but is not an independent predictor of Type 2 diabetes: the Whitehall Ii study. European Journal of Endocrinology 2010162913–917. ( 10.1530/EJE-09-1066) [DOI] [PubMed] [Google Scholar]
- 30.Gerstein HC, Pare G, Hess S, Ford RJ, Sjaarda J, Raman K, McQueen M, Lee S, Haenel H, Steinberg GR, et al. Growth differentiation Factor 15 as a novel biomarker for metformin. Diabetes Care 201740280–283. ( 10.2337/dc16-1682) [DOI] [PubMed] [Google Scholar]
- 31.Abdul-Ghani MA, Matsuda M, Balas B, DeFronzo RA. Muscle and liver insulin resistance indexes derived from the oral glucose tolerance test. Diabetes Care 20073089–94. ( 10.2337/dc06-1519) [DOI] [PubMed] [Google Scholar]
- 32.Lind L, Wallentin L, Kempf T, Tapken H, Quint A, Lindahl B, Olofsson S, Venge P, Larsson A, Hulthe J, et al. Growth-differentiation Factor-15 is an independent marker of cardiovascular dysfunction and disease in the elderly: results from the prospective investigation of the vasculature in Uppsala seniors (Pivus) study. European Heart Journal 2009302346–2353. ( 10.1093/eurheartj/ehp261) [DOI] [PubMed] [Google Scholar]



 This work is licensed under a
This work is licensed under a