Abstract
OBJECTIVES
This analysis evaluated the safety, durability and haemodynamic performance of a stented bovine pericardial valve through 5 years of follow-up in patients with an indication for surgical aortic valve replacement.
METHODS
Kaplan–Meier analysis was used to estimate the incidence of survival and valve-related thromboembolism, major paravalvular leak, endocarditis, structural valve deterioration (SVD) and reintervention. The mean aortic gradient and New York Heart Association (NYHA) functional class were also evaluated.
RESULTS
A total of 1118 patients have received the Avalus valve; 564 have completed the 5-year follow-up. The median follow-up was 4.85 years (4810 patient-years total follow-up). At baseline, the mean age was 70.2 ± 9.0 years; 75.1% of patients were male. The Society of Thoracic Surgeons predicted risk of mortality was 2.0 ± 1.4%. Most patients were in NYHA functional class II (46.8%) or III (40.3%). At the 5-year follow-up, the overall Kaplan–Meier survival rate was 88.1% (85.9–90.0%). The Kaplan–Meier event rates were 5.6% (4.3–7.2%) for thromboembolism, 4.4% (3.2–6.0%) for endocarditis, 0.2% (0.0–0.7%) for a major paravalvular leak and 3.2% (2.3–4.6%) for reintervention. There were no cases of SVD. The mean gradient decreased from 42.1 ± 17.1 mmHg at baseline, to 13.1 ± 4.7 mmHg at discharge and remained stable at 12.5 ± 4.6 mmHg at 5 years. More than 95% of patients were in NYHA functional class I/II 5 years after surgery.
CONCLUSIONS
The findings of a high survival rate, excellent safety, no SVD and stable haemodynamic performance and functional status through 5 years of follow-up are encouraging. Additional follow-up is needed to assess the long-term durability of this contemporary surgical bioprosthesis.
Keywords: Cardiac surgery, Aortic valve disease, Surgical aortic valve replacement, Bovine pericardial aortic bioprosthesis
Surgical aortic valve replacement (SAVR) is one of the most frequently performed cardiac operations.
INTRODUCTION
Surgical aortic valve replacement (SAVR) is one of the most frequently performed cardiac operations. For decades SAVR has been the standard of care for patients with aortic stenosis and/or regurgitation who require replacement of their native valve. Advances in transcatheter aortic valve replacement (TAVR) have led to the rapid adoption of this therapy worldwide, supported by data from various trials [1–4]. In the United States, the number of TAVR procedures performed in 2019 surpassed the number of SAVR procedures by 27% (72,991 vs 57,626) [5]. The wider availability of TAVR has changed the dynamics of valve selection, with surgeons and patients now having to weigh the trade-offs not only between mechanical and bioprosthetic valves but also between surgical and transcatheter valves. To make these decisions, it is important to have clinical data on the newest commercially available bioprosthetic valves in a “standard” low-risk patient population.
The PERIcardial SurGical AOrtic Valve ReplacemeNt (PERIGON) Pivotal Trial is evaluating the safety and efficacy of the Avalus bioprosthesis (Medtronic, Minneapolis, MN, USA). Early analyses demonstrated a favourable safety profile and good haemodynamic performance at 1 and 2 years postimplant [6–8]. This manuscript reports outcomes through 5 years of follow-up.
PATIENTS AND METHODS
Ethics statement
The protocol of the PERIGON Pivotal Trial (www.clinicaltrials.com, NCT02088554) was approved by the institutional review board/ethics committee at each study site (see Table S1). Written informed consent was obtained from all patients.
Study design
The PERIGON Pivotal Trial is a prospective, multicentre trial evaluating the Avalus bioprosthesis (Medtronic, Minneapolis, MN, USA) [6, 7]. The trial was designed and conducted in accordance with the Declaration of Helsinki and good clinical practice principles. Thirty-eight centres in Europe, Canada and the United States participated in the trial.
Patients with moderate or severe symptomatic aortic valve stenosis or chronic severe regurgitation and a clinical indication for SAVR were eligible for enrollment. Those who required concomitant procedures other than left atrial appendage ligation, coronary artery bypass grafting, patent foramen ovale closure, ascending aortic aneurysm/dissection repair not requiring circulatory arrest and subaortic membrane resection not requiring myectomy were ineligible. Patients found intraoperatively to require other procedures were treated with a commercial valve and were exited from the study. Patients with a pre-existing prosthetic valve or annuloplasty device in another position, need for repair of another heart valve, systemic infection, life expectancy <2 years or renal failure (i.e. dialysis therapy or glomerular filtration rate of <30 ml/min/1.73 m2) were excluded. Patients with an anatomical abnormality that increased surgical risk (e.g. acute type A aortic dissection, ventricular aneurysm, porcelain aorta, hostile mediastinum) [6, 7], found before enrollment or intraoperatively, also were excluded.
Device description and implant technique
The bioprosthesis is a stented bovine pericardial valve with a low-profile height and interior-mounted leaflets. The leaflets are treated with alpha-amino oleic acid for anti-calcification [9–11]. Supra-annular placement is recommended. Available sizes were 17, 19, 21, 23, 25, 27, and 29. The implant technique, cardioplegia and cardiopulmonary bypass strategies and postoperative anticoagulation were left to the discretion of the surgeon.
Follow-up and outcomes
Follow-up visits were scheduled for 3 to 6 months and 1 year postimplant. Afterwards, follow-up visits were conducted annually for 5 years with telephone contacts at 18 and 30 months.
Primary safety outcomes included the rate of survival (freedom from all-cause mortality) and valve-related adverse events [12]. Valve-related events included thromboembolism, thrombosis, major haemorrhage, major paravalvular leak (PVL) and endocarditis. We calculated linearized late event rates [13] to determine if the Avalus valve continues to perform within the objective performance criteria (OPC) of the International Standards Organization [13]. Secondary outcomes included haemolysis, structural valve deterioration (SVD), non-structural valve dysfunction (NSVD), reintervention and explant. The definitions of safety outcomes were published previously [8]. An additional outcome of severe haemodynamic dysfunction of indeterminate or evolving cause was used to categorize potential safety events with inconclusive information that did not meet the protocol-defined criteria for SVD or NSVD (see Supplemental Methods). Early (≤30 days) event rates and 5-year Kaplan–Meier event rates were calculated for safety outcomes. Independent event adjudication and data and safety monitoring were provided by the Baim Institute for Clinical Research (Boston, MA, USA). Explanted devices were evaluated by CV Path Institute (Gaithersburg, MD, USA).
Haemodynamic performance outcomes included the mean aortic gradient, peak aortic gradient, effective orifice area (EOA), indexed EOA and Doppler velocity index. Echocardiograms were assessed by MedStar Health Research Institute (Washington, DC, USA). New York Heart Association (NYHA) functional class was used to evaluate functional status.
Other analyses included comparison of outcomes in patients who underwent isolated SAVR and those who had a concomitant procedure, assessment of the risk of requiring a reintervention through 5 years of follow-up and the impact of prosthesis-patient mismatch (PPM) at 1 year on mortality and functional status at 5 years.
Statistical analyses
Categorical variables are reported as the frequency and continuous variables, as the mean±standard deviation (SD). The linearized rate of late events is calculated as the number of events >30 days post-implant divided by late patient-years. A Kaplan–Meier analysis of safety events was performed at 30 days, 1 year and annually for 5 years. A competing risk analysis was performed on the valve-related safety end points accounting for the competing risk of death. At each time point with data, the product-limit estimate of the event-free or event rate, the log-log transformed 95% confidence interval (CI) and the number of subjects with events are presented. Survival analyses using the LIFETEST procedure along with the Epanechnikov kernel smoothing method were used to estimate the instantaneous hazard rate of reintervention during 5 years of follow-up. The impact of 1-year PPM on survival (freedom from mortality) at 5 years was evaluated using Kaplan–Meier analysis and the log-rank test. PPM was calculated using the Valve Academic Research Consortium 3 criteria [14]; moderate and severe PPM were combined for this analysis. The temporal trend of the mean aortic gradient was analysed longitudinally, accounting for variability across subjects and with each subject's repeated measurements over time. A linear mixed-model was fit assuming a B-spline basis expansion with 5 basis functions and an unstructured covariance matrix; this model was used to predict the nonlinear mean response and 95% pointwise CIs in the follow-up mean aortic gradient over 5 years. Analyses were performed with SAS software, version 9.4 (SAS Institute, Cary, NC, USA).
RESULTS
In total, 1118 patients received the study device; 564 have completed the 5-year follow-up visit. The median follow-up for this analysis was 4.85 years with 4810 patient-years of total follow-up and 4719 years of late follow-up. Fig. S1 shows patient disposition over 5 years.
Patient characteristics and procedural outcomes
At baseline, the mean age was 70.2 ± 9.0 years; 75.1% of patients were male. The mean body surface area was 2.0 ± 0.2 m2. Hypertension was the most common comorbidity (76.2%), followed by dyslipidaemia (61.7%), coronary artery disease (43.6%) and left ventricular hypertrophy (41.0%). The mean Society of Thoracic Surgeons risk of mortality was 2.0 ± 1.4%. Most patients were in NYHA functional class II (46.8%) or III (40.3%) at baseline (Table 1). Patients who underwent isolated SAVR were younger, more frequently female and had better functional status, a lower risk of mortality and less comorbidity (Table S2).
Table 1:
Baseline characteristics of the full cohort
| Characteristic | Patients (N = 1118) |
|---|---|
| Age, years | 70.2 ± 9.0 |
| Male sex | 840 (75.1%) |
| Body surface area (m2) | 2.0 ± 0.2 |
| New York Heart Association functional class | |
| I | 123 (11.0%) |
| II | 523 (46.8%) |
| III | 450 (40.3%) |
| IV | 22 (2.0%) |
| Society of Thoracic Surgeons risk of mortality (%) | 2.0 ± 1.4 |
| Comorbidities | |
| Atrial fibrillation | 117 (10.5%) |
| Congestive heart failure | 222 (19.9%) |
| Coronary artery disease | 487 (43.6%) |
| Diabetes | 298 (26.7%) |
| Dyslipidaemia | 690 (61.7%) |
| Endocarditis | 4 (0.4%) |
| Hypertension | 852 (76.2%) |
| Left ventricular hypertrophy | 458 (41.0%) |
| Myocardial infarction | 99 (8.9%) |
| Peripheral vascular disease | 81 (7.2%) |
| Renal dysfunction/insufficiency | 119 (10.6%) |
| Stroke/cerebrovascular accident | 45 (4.0%) |
| Transient ischaemic attack | 60 (5.4%) |
| Previous cardiovascular interventions | |
| Coronary artery bypass | 25 (2.2%) |
| Stent implanted | 119 (10.6%) |
| Arrhythmia surgery (e.g. ablation) | 21 (1.9%) |
| Implanted cardiac device (e.g. pacemaker or defibrillator) | 37 (3.3%) |
| Previous open-heart surgery | 39 (3.5%) |
Aortic stenosis was the primary indication for valve replacement in 84.3% of patients, followed by mixed stenosis/regurgitation (9.5%) and regurgitation (5.7%); 0.5% had a failed prosthesis. Aortic stenosis was also the most common indication in the subanalysis (isolated vs concomitant, 86.9% vs 81.7%, P = 0.016). Median sternotomy was performed in 79.6% of patients, and coronary artery bypass grafting, in 32.4%. Cardiopulmonary bypass time averaged 105.0 ± 41.1 min, with aortic cross-clamp time averaging 79.5 ± 31.6 min. Patients who had an isolated SAVR less frequently had a median sternotomy, more frequently had a hemisternotomy or right thoracotomy, and had shorter bypass and aortic cross-clamp times and less annular enlargement than those with a concomitant procedure (Table S3).
Safety end points
There were 118 deaths during the follow-up period; 10 deaths occurred within 30 days postimplant. Fifteen deaths were valve-related; all occurred >30 days postimplant. The causes were endocarditis (n = 6), major haemorrhage (n = 4), sepsis, cardiogenic shock, embolic stroke, congestive heart failure and acute cardiac death (n = 1 each). The survival rate was 88.1% (85.9–90.0%) in the full cohort (Fig. 1), 90.2% (87.0–92.6%) in the isolated SAVR cohort and 86.1% (82.7–88.9%) in the concomitant procedure cohort (Table S4).
Figure 1:
Survival over a 5-year period.
There were no cases of SVD at 5 years (Table 2). NSVD occurred in 13 patients: 2 major PVL, 8 minor PVL, 2 entrapments (pannus or suture) and 1 “other” event (echocardiography showed a normally functioning valve with a high gradient, suggestive of PPM). Three patients had severe haemodynamic dysfunction of indeterminate/evolving cause. One appeared to be due to endocarditis, but the valve was not returned for examination. For 1 patient, who had chronic kidney disease, transoesophageal echocardiography and cardiac magnetic resonance imaging showed leaflet thickening but no calcifications. There also was a large discrepancy in the mean aortic gradients between the site and the core laboratory for the last (3-year) visit (40 vs 23 mmHg, respectively). For this patient, a transcatheter aortic valve-in-valve implant was performed. One patient had a valve thrombosis that was treated with Coumadin. Six months later, echocardiography indicated an increase in the mean aortic gradient from 24 to 34 mmHg (site reported). The CT scan done in preparation for a transcatheter aortic valve-in-valve implant showed a mural thrombus and mild aortic calcifications.
Table 2:
Early and 5-year clinical event rates
| Event | 30-Day event rate % (n) | 5-Year Kaplan-Meier event rate % (95% CI) (n) |
|---|---|---|
| All-cause death | 0.9 (10) | 11.9 (10.0-14.1) (118) |
| Thromboembolism | 1.4 (15) | 5.6 (4.3-7.2) (57) |
| Valve thrombosis | 0.0 (0) | 0.3 (0.1-1.0) (3) |
| Major haemorrhagea | 1.0 (11) | 5.9 (4.7-7.6) (62) |
| Major paravalvular leak | 0.1 (1) | 0.2 (0.0-0.7) (2) |
| Endocarditis | 0.2 (2) | 4.4 (3.2-6.0) (42) |
| Haemolysis | 0.0 (0) | 1.4 (0.7-2.6) (10) |
| Non-structural valve dysfunction | 0.2 (2) | 1.5 (0.9-2.7) (13) |
| Structural valve deterioration | 0.0 (0) | 0.0 (0.0-0.0) (0) |
| Severe haemodynamic dysfunction, indeterminate/evolving cause | 0.0 (0) | 0.3 (0.1-1.0) (3) |
| Reintervention | 0.4 (4) | 3.2 (2.3-4.6) (31) |
| Explant | 0.4 (4) | 2.7 (1.9-4.0) (27) |
Anticoagulation-related.
Figure 2 shows the linearized rates of late valve-related events compared with the OPC. At 5 years, all safety events remained below the OPC except major haemorrhage. The Kaplan–Meier rate of thromboembolism at 5 years was 5.6% (4.3–7.2%), and the rate of endocarditis was 4.4% (3.2–6.0%). The rates of both valve thrombosis and major PVL were <1%. Thirty-one patients underwent reintervention, yielding an event rate of 3.2% (2.3–4.6%) (Table 2). The reasons for reintervention were endocarditis (n = 22), severe haemodynamic dysfunction (n = 3), major PVL (n = 3), bleeding, valve thrombosis and septal myectomy (n = 1 each). Redo surgery with an explant was performed in 27 patients; 3 patients had transcatheter aortic valve-in-valve implants; and 1 patient received an aortic plug. Fig. 3 indicates an increased perioperative hazard of reintervention with a low and constant hazard for all 5 years. Valve-related events were not significantly different between patients who underwent isolated SAVR and those with a concomitant procedure (Table S4). The outcomes were similar when the valve-related events were analysed with death as a competing risk (Table S5, Figures S2 and S3).
Figure 2:
Comparison of valve-related safety end points with the objective performance criteria. OPC: objective performance criteria.
Figure 3:

Estimated instantaneous hazard rate of all-cause reintervention over a 5- year period.
Efficacy end points
The mean aortic gradient improved from 42.1 ± 17.1 mmHg at baseline to 13.1 ± 4.7 mmHg at discharge/up to 30 days (henceforth called discharge). During the following 5 years, the mean gradient remained stable (Figure 4). Mean gradients tended to be higher in the isolated SAVR group than in the full cohort and the concomitant procedure group (Table S6). The peak aortic gradient was 68.9 ± 26.6 mmHg at baseline, 24.0 ± 8.4 mmHg at discharge and 22.3 ± 7.9 mmHg at 5 years. The mean EOA increased from 0.90 ± 0.52 cm2 to 1.59 ± 0.39 cm2 at discharge and was 1.43 ± 0.35 cm2 at 5 years. The indexed EOA was 0.45 ± 0.26 cm2/m2 at baseline, 0.81 ± 0.20 cm2/m2 at discharge and 0.73 ± 0.17 cm2/m2 at 5 years. The mean Doppler velocity index was 0.27 ± 0.13, 0.49 ± 0.10 and 0.42 ± 0.08 at those same time points, respectively.
Figure 4:
(Top) Mean aortic gradient by visit and valve size over a 5-year period. Values show gradients at 5 years. (Bottom) Temporal trend of mean aortic gradient analysed longitudinally.
At 5 years, the rates of regurgitation were as follows: 87.4% none, 5.8% trace, 6.5% mild, 0.2% moderate and 0% severe. More than 97% of patients had no/trace PVL, and >95% had no/trace transvalvular regurgitation (Fig. S4).
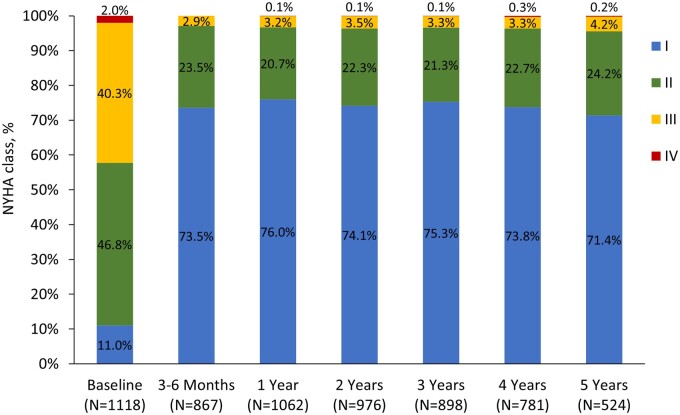
At 5 years > 95% of subjects were in NYHA functional class I or II (Fig. 5). Compared with baseline, 1% of patients improved 3 classes, 25.2% improved 2 classes and 244 (46.6%) improved 1 class at 5 years. Twenty-four percent had no change from baseline. Three percent worsened by 1 class, but none worsened >1 class. PPM at 1 year had no impact on survival or functional status at 5 years (Table S7).
Figure 5:
New York Heart Association (NYHA) functional class over a 5-year period.
DISCUSSION
This study demonstrated excellent durability of the Avalus valve with no cases of SVD during the 5 years of follow-up. The reintervention hazard was low over time. Improvements in mean gradient, EOA and functional status were very good and, more importantly, stable over time. Survival at 5 years was high, performance against the OPC remained good, and PPM had no impact on survival or functional status at 5 years. Together these findings demonstrate “state-of-the-art” SAVR outcomes with a favourable safety profile and good haemodynamic performance and durability over 5 years. With an average age of 70 and a mean Society of Thoracic Surgeons score of 2.0, the patient population in this study likely reflects the “normal” population that nowadays presents for aortic valve surgery in most countries in the Western world. Only patients at high risk for premature death due to multiple valve procedures and severe comorbidities were excluded.
The 5-year outcomes in the PERIGON Pivotal Trial compare well with those reported for other contemporary bovine pericardial valves. In the PERIGON trial, there were no cases of SVD at 5 years, which corresponded to a freedom from SVD rate of 100%. Similarly, Bartus et al. [15] and Bavaria et al. [16] reported 100% freedom from SVD for the Perimount Magna Ease valve with Resilia-treated leaflets (Edwards Lifesciences, Irvine, CA, USA). Kilic et al. [17] reported 99% freedom from SVD for the Trifecta valve (Abbott, Abbott Park, IL, USA), although some have raised concern about a higher rate of early failure with this valve [18, 19]. The rate of freedom from NSVD in PERIGON was 98.5% (97.3–99.1) at 5 years, compared with 99.1% (97.4–100.0) and 100.0% reported by others [15, 16]. Of note, the freedom from NSVD rate reported by Bavaria et al. [16] was defined as “NSVD (other than PVL)”, whereas 10 of the 13 NSVD events in PERIGON were PVL (2 major, 8 minor). There were 3 cases of severe haemodynamic dysfunction in PERIGON. In these cases, the valve was not available for examination, and the investigators exited the patients from the study. Based on the reviews of available medical records by the independent clinical events committee, these 3 cases did not meet the protocol definitions of SVD or NSVD and were therefore adjudicated as severe haemodynamic dysfunction.
At 5 years, all valve-related safety events except major haemorrhage remained below the OPC thresholds. In the first report from PERIGON [6], the linearized late event rate of major bleeding was 2.7%/patient-year with a 95% upper bound of 4.3%, which exceeded the OPC (standard 5840:2009). That analysis, which included 1-year follow-up visits for 270 of 686 (39%) enrolled patients, showed that the inflated rate was likely related to patients taking periprocedural anticoagulation agents for concomitant diseases. Although linearization assumes a relatively constant rate of events over time, this is not true for bleeding, which is more frequent in the first months after an implant. Because the use of anticoagulation has decreased with longer follow-up, the linearized rate of late bleeding has also decreased [7, 8] and is currently 1.3%/patient-year. In addition, two-thirds of patients in our initial analysis were taking an antiplatelet, anticoagulant or both at baseline for comorbidities [6]. A subsequent in-depth analysis of antithrombotic therapy and bleeding after SAVR in the trial demonstrated that most bleeding events occurred >30 days postimplant and mostly in patients taking antiplatelet and/or anticoagulation for indications other than valve prophylaxis [20].
The linearized late event rates at 5 years observed for the Avalus valve also compare well with those reported by Bartus et al. [15]: thromboembolism: 1.0% versus 0.4%/patient-year, respectively; valve thrombosis: 0.06% versus 0.2%; major haemorrhage: 1.3% versus 0.4%; major PVL: 0.02% versus 0.0%; and endocarditis: 0.9% versus 0.2%. Bavaria et al. [16] did not report linearized late event rates, but the linearized late safety event rates reported for COMMENCE by Johnston et al. [21] (median follow-up, 4 years) seem in line with our data. Due to slightly different inclusion criteria, these numbers also may very well reflect the patient population.
Freedom from reintervention at 5 years was 96.8% (95.4–97.7%) in PERIGON, compared with 99.2% (97.7–100.0%), 98.7% (97.8–99.6%) and 96.0% reported by others [15–17]. Although reintervention was increased perioperatively in PERIGON, the hazard of reintervention over 5 years was low and constant (Fig. 3) and mainly linked to endocarditis. These data show the excellent performance of modern biological valve prostheses, but this time point is still too short to make definite conclusions about long-term durability.
The 5-year overall survival rate in PERIGON was 88.1% (85.9–90.0%) compared with 83.4% (76.8–89.9%) and 89.2% (86.7–91.6%) reported for the Magna Ease valve with Resilia tissue [15, 16]. Five-year survival in the study of Kilic et al. [17] was 70%, but their study population comprised all those at their centre who had Trifecta implants, including those who had urgent (36.6%), emergency (1.9%) and salvage (0.3%) procedures. Others have reported 6-year survival of 87.9% with the Trifecta valve [22]. These findings demonstrate the excellent survival that is attainable with contemporary surgical valves.
The mean aortic gradient across all valve sizes remained stable over time, not exceeding 20 mmHg at any time point for valve sizes 19 through 29 mm (Fig. 4). At 5 years the mean aortic gradient in PERIGON compared favourably with those reported by others [15, 16]. All these valves are designed to achieve low resistance to flow. The 5-year mean EOAs in these studies were also similar (1.43 ± 0.35 cm2 in PERIGON vs 1.4 ± 0.5 cm2 [15] and 1.6±0.05 cm2 [16]), although this value is less reliable due to echocardiographic estimations [23]. At 5 years, 97.4% of patients in PERIGON had none/trace PVL, and no patients had greater than mild regurgitation, similar to other contemporary trials [15, 16]. Rates of central regurgitation among these studies were also low and comparable.
Functional status was stable over time in PERIGON with more than 95% of patients in NYHA functional class I/II at 5 years. Our analysis comparing patients with moderate or severe PPM compared with no PPM at 1 year showed that PPM had no impact on survival or functional status at 5 years. This is unsurprising given the limitations of PPM categories to predict mortality or haemodynamic obstruction [24].
As the use of TAVR becomes more common, it will be critical for surgeons to focus on a lifetime management approach to treatment of aortic valvular heart disease. Transcatheter valves have an obvious appeal, and good results have been reported for lower-risk patients [1, 2, 4]. In addition, transcatheter aortic valve-in-valve procedures in high-risk patients seem promising [25]. However, long-term studies are limited, and TAVR may not be the optimal initial approach for some patients because explant is often necessary and complex in those who require reintervention [26, 27]. In light of this and the favourable 5-year results achieved with contemporary surgical tissue valves such as Avalus, it is likely that SAVR will remain the gold standard of treatment for younger, lower-risk patients.
Follow-up in the PERIGON Pivotal Trial will continue through 12 years in a subgroup of patients. There are currently 20 active long-term follow-up sites with 522 patients. This long-term study may serve as a comparison group for younger, low-risk TAVR patients in the future.
LIMITATIONS
The 5-year follow-up visit was not complete for the full study population. This single-arm observational study allowed only 8 concomitant procedures, which limits the generalizability of outcomes to real-world populations with different comorbidities and needs for concomitant procedures. The definition of SVD may underestimate this outcome compared with contemporary definitions. Longer follow-up is needed to fully understand valve safety and performance.
CONCLUSION
The findings of a high survival rate, no SVD, excellent safety and stable haemodynamic performance and functional status for 5 years in this large study of the Avalus valve are very good. Additional follow-up is needed to assess long-term durability.
Supplementary Material
ACKNOWLEDGEMENT
Julie A. Linick, an employee and shareholder of Medtronic, assisted in manuscript development under the lead author’s direction.
ABBREVIATIONS/ACRONYMS
- EOA
Effective orifice area
- NSVD
Nonstructural valve dysfunction
- NYHA
New York Heart Association
- OPC
Objective performance criteria
- PPM
Prosthesis-patient mismatch
- PVL
Paravalvular leak
- SAVR
Surgical aortic valve replacement
- SVD
Structural valve deterioration
- TAVR
Transcatheter aortic valve replacement
Contributor Information
Robert J M Klautz, Department of Cardiothoracic Surgery, Leiden University Medical Center, Leiden, Netherlands.
François Dagenais, Division of Cardiac Surgery, Quebec Heart and Lung Institute, Quebec, QC, Canada.
Michael J Reardon, Department of Cardiovascular Surgery, Houston Methodist DeBakey Heart and Vascular Center, Houston, TX, USA.
Rüdiger Lange, Department of Cardiovascular Surgery, German Heart Centre Munich, Munich, Germany.
Michael G Moront, Department of Cardiothoracic Surgery, ProMedica Toledo Hospital, Toledo, OH, USA.
Louis Labrousse, Medico-Surgical Department of Valvulopathies, Bordeaux Heart University Hospital, Bordeaux-Pessac, France.
Neil J Weissman, MedStar Health Research Institute, MedStar Health, Washington, DC, USA.
Vivek Rao, Department of Surgery, Toronto General Hospital, Toronto, ON, Canada.
Himanshu J Patel, Department of Cardiac Surgery, Frankel Cardiovascular Center, University of Michigan, Ann Arbor, MI, USA.
Fang Liu, Cardiac Surgery Clinical Research and Medical Science, Medtronic, Mounds View, MN, USA.
Joseph F Sabik, III, Department of Surgery, University Hospitals Cleveland Medical Center and Case Western Reserve University School of Medicine, Cleveland, OH, USA.
PERIcardial SurGical AOrtic Valve ReplacemeNt (PERIGON) Pivotal Trial of the Avalus valve.
www.clinicaltrials.gov, NCT02088554.
Presented at the 35th EACTS Annual Meeting; Barcelona, Spain; 13-16 October 2021.
FUNDING
This project was supported by Medtronic.
Conflicts of Interest: Robert J.M. Klautz reports research support and consultation fees from Medtronic. François Dagenais is a proctor and speaker for Medtronic and COOK Medical. Michael J. Reardon serves as a consultant to Medtronic, Abbott Medical, Boston Scientific, Gore Medical and Transverse Medical; the fees are paid to his department. Rüdiger Lange is a consultant and stockholder for and receives royalty fees from Medtronic; he also serves as a consultant to HighLife Medical. Michael G. Moront is a trainer and consultant for Medtronic; a trainer and speaker for Atricure; and a speaker and consultant for Haemonetics. Louis Labrousse has received meeting attendance fees from Medtronic. Neil J. Weissman receives grant support from Medtronic, Boston Scientific, Edwards and Abbott; the funds are paid to his institution. Vivek Rao is a consultant for Medtronic, W. L. Gore, and Abbott; he is a member of the Surgical Advisory Board for Medtronic. Himanshu J. Patel is a consultant for Medtronic. Fang Liu is an employee and stockholder of Medtronic. Joseph F. Sabik is the North American Principal Investigator for the PERIGON Pivotal Trial, which is sponsored by Medtronic Cardiac Surgery.
Data availability
The data, analytic methods and study materials are owned by the sponsor and will not be made available to other researchers for purposes of reproducing the results.
Author Contributions
Robert J.M. Klautz: Conceptualization; Investigation; Methodology; Visualization; Writing-original draft; Writing-review, editing. François Dagenais: Investigation, Writing–review, editing. Michael J. Reardon: Investigation; Writing–review, editing. Rüdiger Lange: Investigation, Writing–review, editing. Michael G. Moront: Investigation, Writing–review, editing. Louis Labrousse: Investigation, Writing–review, editing. Neil J. Weissman: Investigation, Writing–review, editing. Vivek Rao: Investigation, Writing–review, editing. Himanshu J. Patel: Investigation, Writing–review, editing. Fang Liu: Data curation, Formal analysis, Methodology, Software, Validation, Writing–original draft, Writing–review, editing. Joseph F. Sabik, III: Conceptualization, Investigation, Methodology, Writing–review, editing.
REFERENCES
- 1. Reardon MJ, Van Mieghem NM, Popma JJ, Kleiman NS, Sondergaard L, Mumtaz M, SURTAVI Investigators et al. Surgical or transcatheter aortic-valve replacement in intermediate-risk patients. N Engl J Med 2017;376:1321–31. [DOI] [PubMed] [Google Scholar]
- 2. Popma JJ, Deeb GM, Yakubov SJ, Mumtaz M, Gada H, O'Hair D, Evolut Low Risk Trial Investigators et al. Transcatheter aortic-valve replacement with a self-expanding valve in low-risk patients. N Engl J Med 2019;380:1706–15. [DOI] [PubMed] [Google Scholar]
- 3. Sondergaard L, Ihlemann N, Capodanno D, Jorgensen TH, Nissen H, Kjeldsen BJ. et al. Durability of transcatheter and surgical bioprosthetic aortic valves in patients at lower surgical risk. J Am Coll Cardiol 2019;73:546–53. [DOI] [PubMed] [Google Scholar]
- 4. Thyregod HGH, Ihlemann N, Jorgensen TH, Nissen H, Kjeldsen BJ, Petursson P. et al. Five-year clinical and echocardiographic outcomes from the Nordic Aortic Valve Intervention (NOTION) randomized clinical trial in lower surgical risk patients. Circulation 2019;139:2714–23. [DOI] [PubMed] [Google Scholar]
- 5. Carroll JD, Mack MJ, Vemulapalli S, Herrmann HC, Gleason TG, Hanzel G. et al. STS-ACC TVT registry of transcatheter aortic valve replacement. J Am Coll Cardiol 2020;76:2492–516. [DOI] [PubMed] [Google Scholar]
- 6. Klautz RJM, Kappetein AP, Lange R, Dagenais F, Labrousse L, Bapat V, on behalf of the PERIGON Investigators et al. Safety, effectiveness and haemodynamic performance of a new stented aortic valve bioprosthesis. Eur J Cardiothorac Surg 2017;52:425–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sabik JF III, Rao V, Lange R, Kappetein AP, Dagenais F, Labrousse L. et al. One-year outcomes associated with a novel stented bovine pericardial aortic bioprosthesis. J Thorac Cardiovasc Surg 2018;156:1368–77 e5. [DOI] [PubMed] [Google Scholar]
- 8. Dagenais F, Moront MG, Brown WM, Reardon MJ, Chu MWA, Gearhart E. et al. Safety, efficacy, and hemodynamic performance of a stented bovine pericardial aortic valve bioprosthesis: two-year analysis. J Thorac Cardiovasc Surg 2020;160:371–81 e4. [DOI] [PubMed] [Google Scholar]
- 9. Chen W, Schoen FJ, Levy RJ.. Mechanism of efficacy of 2-amino oleic acid for inhibition of calcification of glutaraldehyde-pretreated porcine bioprosthetic heart valves. Circulation 1994;90:323–9. [DOI] [PubMed] [Google Scholar]
- 10. Weber PA, Jouan J, Matsunaga A, Pettenazzo E, Joudinaud T, Thiene G. et al. Evidence of mitigated calcification of the Mosaic versus Hancock Standard valve xenograft in the mitral position of young sheep. J Thorac Cardiovasc Surg 2006;132:1137–43. [DOI] [PubMed] [Google Scholar]
- 11. Duarte IG, MacDonald MJ, Cooper WA, Schmarkey SL, Gott JP, Brown WM III, et al. In vivo hemodynamic, histologic, and antimineralization characteristics of the Mosaic bioprosthesis. Ann Thorac Surg 2001;71:92–9. [DOI] [PubMed] [Google Scholar]
- 12. Akins CW, Miller DC, Turina MI, Kouchoukos NT, Blackstone EH, Grunkemeier GL. et al. Guidelines for reporting mortality and morbidity after cardiac valve interventions. Eur J Cardiothorac Surg 2008;33:523–8. [DOI] [PubMed] [Google Scholar]
- 13. International Standards Organization. Cardiovascular implants - cardiac valve prostheses. Part 2: surgically implanted Heart valve substitutes (ISO 5840-2:2015). Geneva, Switzerland: International Standards Organization, 2015. [Google Scholar]
- 14. Généreux P, Piazza N, Alu MC, Nazif T, Hahn RT, Pibarot P, VARC-3 WRITING COMMITTEE: et al. Valve Academic Research Consortium 3: updated endpoint definitions for aortic valve clinical research. J Am Coll Cardiol 2021;77:2717–46. [DOI] [PubMed] [Google Scholar]
- 15. Bartus K, Litwinowicz R, Bilewska A, Stapor M, Bochenek M, Rozanski J. et al. Final 5-year outcomes following aortic valve replacement with a RESILIA tissue bioprosthesis. Eur J Cardiothorac Surg 2021;59:434–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bavaria JE, Griffith B, Heimansohn DA, Rozanski J, Johnston DR, Bartus K. et al. Five-year outcomes of the COMMENCE trial investigating aortic valve replacement with RESILIA tissue. Ann Thorac Surg 2022;S0003-4975(22)00063-7. [DOI] [PubMed] [Google Scholar]
- 17. Kilic A, Sultan I, Navid F, Aranda-Michel E, Chu D, Thoma F. et al. Trifecta aortic bioprosthesis: midterm results in 1,953 patients from a single center. Ann Thorac Surg 2019;107:1356–62. [DOI] [PubMed] [Google Scholar]
- 18. Yongue C, Lopez DC, Soltesz EG, Roselli EE, Bakaeen FG, Gillinov AM. et al. Durability and performance of 2298 Trifecta aortic valve prostheses: a propensity-matched analysis. Ann Thorac Surg 2021;111:1198–205. [DOI] [PubMed] [Google Scholar]
- 19. Kattach H, Shah BN, Harden S, Barlow CW, Miskolczi S, Velissaris T. et al. Premature structural failure of Trifecta bioprosthesis in midterm follow-up: a single-center study. Ann Thorac Surg 2021;112:1424–31. [DOI] [PubMed] [Google Scholar]
- 20. Klautz RJM, Vriesendorp MD, Dagenais F, Labrousse L, Bapat V, Moront MG. et al. Antithrombotic therapy and bleeding events after aortic valve replacement with a novel bioprosthesis. J Thorac Cardiovasc Surg 2019;S0022-5223(19)32400-6. [DOI] [PubMed] [Google Scholar]
- 21. Johnston DR, Griffith BP, Puskas JD, Bavaria JE, Svensson LG, COMMENCE Trial Investigators. Intermediate-term outcomes of aortic valve replacement using a bioprosthesis with a novel tissue. J Thorac Cardiovasc Surg 2021;162:1478–85. [DOI] [PubMed] [Google Scholar]
- 22. Goldman S, Cheung A, Bavaria JE, Petracek MR, Groh MA, Schaff HV.. Midterm, multicenter clinical and hemodynamic results for the Trifecta aortic pericardial valve. J Thorac Cardiovasc Surg 2017;153:561–9. [DOI] [PubMed] [Google Scholar]
- 23. Vriesendorp MD, De Lind Van Wijngaarden RAF, Head SJ, Kappetein AP, Hickey GL, Rao V. et al. The fallacy of indexed effective orifice area charts to predict prosthesis-patient mismatch after prosthesis implantation. Eur Heart J Cardiovasc Imaging 2020;21:1116–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Vriesendorp MD, Deeb GM, Reardon MJ, Kiaii B, Bapat V, Labrousse L. et al. Why the categorization of indexed effective orifice area is not justified for the classification of prosthesis-patient mismatch. J Thorac Cardiovasc Surg 2020;S0022-5223(20)33047-6. [DOI] [PubMed] [Google Scholar]
- 25. Tchetche D, Chevalier B, Holzhey D, Harnath A, Schafer U, Teiger E, VIVA Investigators et al. TAVR for failed surgical aortic bioprostheses using a self-expanding device: 1-year results from the prospective VIVA Postmarket Study. JACC Cardiovasc Interv 2019;12:923–32. [DOI] [PubMed] [Google Scholar]
- 26. Fukuhara S, Nguyen CTN, Kim KM, Yang B, Ailawadi G, Patel HJ. et al. Aortic valve reintervention after transcatheter aortic valve replacement. J Thorac Cardiovasc Surg 2021;S0022-5223(21)01112-0. [DOI] [PubMed] [Google Scholar]
- 27. Fukuhara S, Tanaka D, Brescia AA, Wai Sang SL, Grossman PM, Sukul D. et al. Aortic valve reintervention in patients with failing transcatheter aortic bioprostheses: a statewide experience. J Thorac Cardiovasc Surg 2021;S0022-5223(21)01257-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data, analytic methods and study materials are owned by the sponsor and will not be made available to other researchers for purposes of reproducing the results.