Abstract
Background
Hypernatremia is a common electrolyte disorder in children and elderly people and has high short-term mortality. However, no high-quality studies have examined the correction rate of hypernatremia and the amount of fluid required for correction. Therefore, in this study, we will compare the efficacy and safety of rapid intermittent bolus (RIB) and slow continuous infusion (SCI) of electrolyte-free solution in hypernatremia treatment.
Methods
This is a prospective, investigator-initiated, multicenter, open-label, randomized controlled study with two experimental groups. A total of 166 participants with severe hypernatremia will be enrolled and divided into two randomized groups; both the RIB and SCI groups will be managed with electrolyte-free water. We plan to infuse the same amount of fluid to both groups, for 1 hour in the RIB group and continuously in the SCI group. The primary outcome is a rapid decrease in serum sodium levels within 24 hours. The secondary outcomes will further compare the efficacy and safety of the two treatment protocols.
Conclusion
This is the first randomized controlled trial to evaluate the efficacy and safety of RIB correction compared with SCI in adult patients with severe hypernatremia.
Keywords: Brain edema, Hypernatremia, Hypotonic solutions, Therapeutics
Graphical abstract
Introduction
Hypernatremia is a serum sodium (sNa) level exceeding 145 mmol/L, which is common in hospitalized patients [1]. It occurs mainly in children, the elderly, and critically ill patients and is known to occur in 3% of hospitalized patients and 9% of critically ill patients [2,3]. Hypernatremia occurs due to 1) water loss (diabetes insipidus), 2) hypotonic fluid loss (osmotic diarrhea), or (3) hypertonic fluid gain (Na+-containing fluids) [4].
Sodium and its associated anions are major determinants of extracellular tonicity and osmotic pressure, and they influence the movement of water across cell membranes [5–7]. In other words, hypernatremia indicates hypertonic hyperosmolality and causes water outflow, resulting in cell dehydration [4]. Therefore, the symptoms and signs of hypernatremia mainly indicate dysfunction of the central nervous system, presenting with hyperventilation, muscle weakness, lack of consciousness (lethargy), and coma [1,8,9]. Hypernatremia has been associated with mortality rates of 40% to 60% and prolonged intensive care unit stays, although that high risk of mortality could also be attributed to the severity of illness and comorbidities [4].
Most physicians think that too rapid a correction of hypernatremia can cause cerebral edema, seizures, and irreversible brain damage [1,4,10–15]. The recommendation for correcting acute hypernatremia has been decreased to 1 mmol/L per hour, and chronic hypernatremia should be corrected at <0.5 mmol/L per hour (approximately 10 mmol/L/day) [16–18]. However, those correction rates were based on retrospective pediatric studies [11,19]. No evidence-based guidelines suggest an appropriate sodium correction rate for hypernatremia in adults. Moreover, previous studies in adults have suggested that rapid correction rates (>0.5 mmol/L per hour) are not associated with a high risk of hypernatremia-related mortality or neurologic damage [16]. In fact, several studies in adults have shown that an excessively slow correction rate causes higher mortality and vice versa [20,21].
According to the European and American guidelines for hyponatremia, an infusion of 10 mL/kg during 1 hour or 3 mL/kg per hour of electrolyte-free water is recommended to prevent the overcorrection of hyponatremia [22,23]. In a randomized controlled trial published previously, 10 mL/kg during 1 hour was applied as a method of re-lowering the dosage in cases of excessively rapid correction of hyponatremia [24,25]. However, the rapid intermittent bolus (RIB) administration of electrolyte-free water has never been applied in treating hypernatremia. We hypothesize that RIB administration of electrolyte-free water in hypernatremia can increase the incidence of a rapid decrease of sNa levels and thereby increase the survival time compared with the slow continuous infusion (SCI) method. Therefore, our purpose in this study will be to evaluate the efficacy and safety of RIB and SCI with electrolyte-free water in patients with hypernatremia. In addition, we aim to determine the best method for treating hypernatremia in adult patients.
Methods
Study design
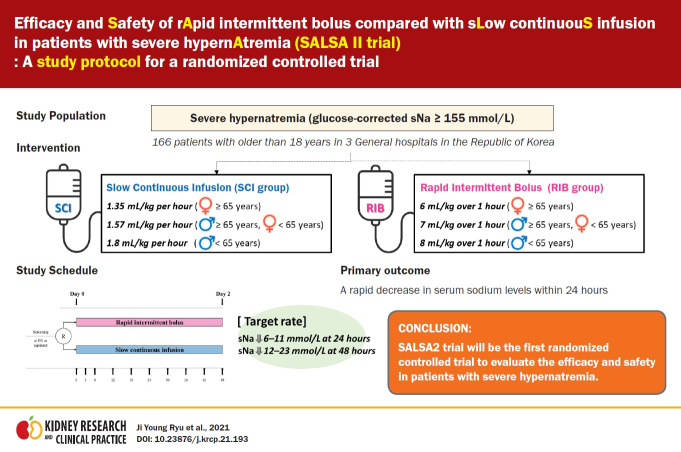
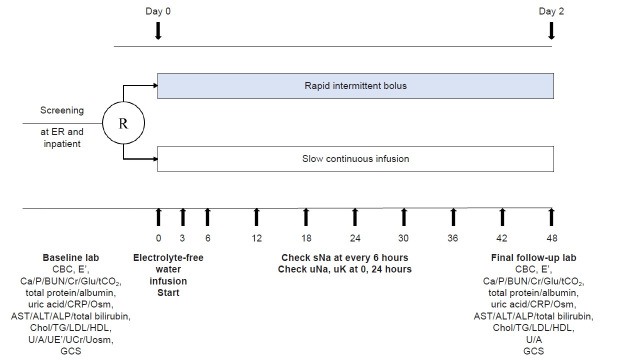
This study is a prospective, investigator-initiated, multicenter, open-label, randomized controlled study with two experimental groups. We comply with the Standard Protocol Items: Recommendation for Interventional Trials (SPIRIT) 2013 Statement, which defines standard protocol items for clinical trials [26]. The algorithm for this study is shown in Fig. 1. The SPIRIT and study schedule are shown in Fig. 2 and 3. After registration, clinical follow-up will be conducted after 2 days of treatment with electrolyte-free water.
Figure 1. Study algorithm.
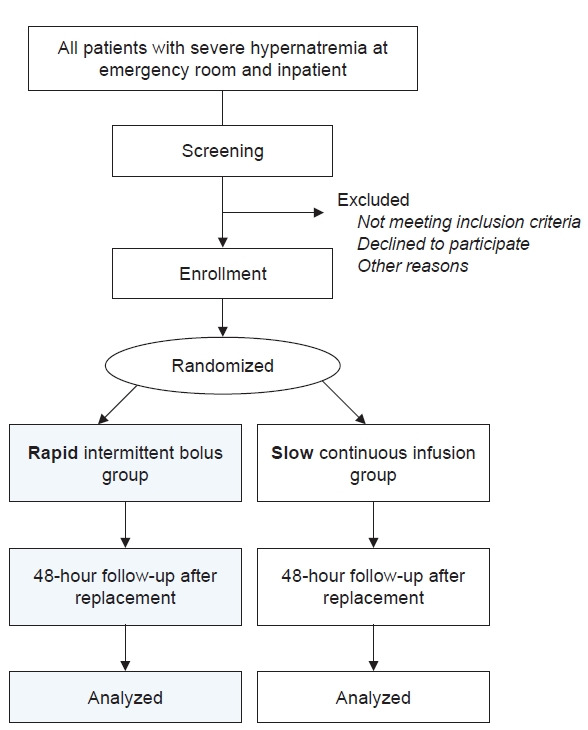
Figure 2. Schedule of enrollment, interventions, and assessments according to the Standard Protocol Items: Recommendations for Interventional Trials (SPIRIT) guideline.
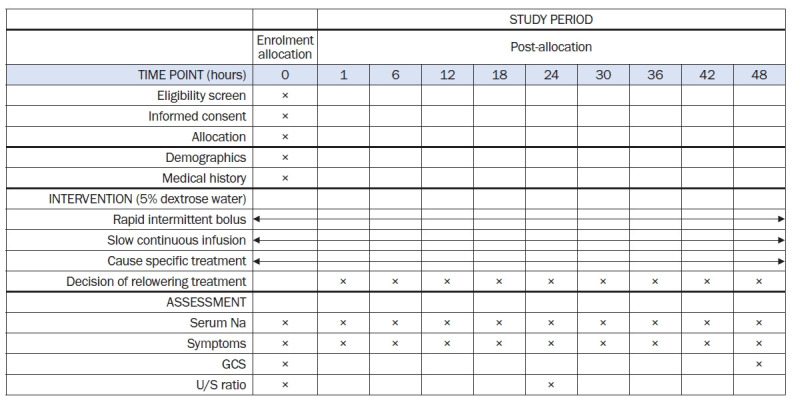
U/S ratio, uNa + uK/SNa; GCS, Glasgow Coma Scale.
Figure 3. Study schedule.
ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; BUN, blood urea nitrogen; CBC, complete blood count; Chol, cholesterol; Cr, creatinine; CRP, C-reactive protein; E’, electrolyte; ER, emergency room; GCS, Glasgow Coma Scale; Glu, glucose; HDL, high-density lipoprotein; LDL, low-density lipoprotein; Osm, osmolality; P, phosphorus; R, randomization; tCO2, total CO2; sNa, serum sodium; TG, triglyceride; U/A, urinalysis; UCr, urine creatinine; UE’, urine electrolyte; uK, urine potassium; uNa, urine Na; Uosm, urine osmolality.
Study participants and measurements
This study will be performed in three general hospitals in Korea (Hallym University Dongtan Sacred Heart Hospital, Seoul National University Bundang Hospital, and SMG-SNU Boramae Medical Center). Patients older than 18 years with severe hypernatremia (glucose-corrected sNa of ≥155 mmol/L) [27] who visit the emergency room or are hospitalized will be screened for enrollment. The subsequent evaluation will be performed as follows: 1) completion of questionnaire about medical and drug history, including the use of diuretics, lithium, amphotericin, foscarnet, and demeclocycline; 2) physical examination of all body systems; 3) height and weight measurements; 4) blood pressure and pulse rate measurements; 5) verification of the cause of emergency room visit or admission; 6) surgery status and surgical procedure; and 7) calculated Acute Physiology and Chronic Health Evaluation score. Participants who meet all the inclusion criteria do not need to be screened for exclusion criteria, and those who submit written consent will be included in this study (Table 1). The study will be conducted in accordance with the Declaration of Helsinki. All participants will provide signed, written, informed consent stating that their participation is voluntary and can be withdrawn at any time. This study was approved by the Institutional Review Boards (IRBs) of Hallym University Dongtan Sacred Heart Hospital (No. 2021-03-007-001), Seoul National University Bundang Hospital (No. B-2104-680-003), and SMG-SNU Boramae Medical Center (No. 30-2021-120). The trial protocol has been registered at http://www.clinicaltrials.gov (identifier No. NCT04949139; registered on 1 July 2021).
Table 1.
Inclusion and exclusion criteria
| Inclusion criteria | Exclusion criteria |
|---|---|
| Inpatients and ER patients aged >18 years | Arterial hypotension requiring inotropes or vasopressors (systolic blood pressure < 90 mmHg and mean arterial pressure < 70 mmHg) |
| Anuria or bilateral urinary outlet obstruction | |
| Uncontrolled diabetes mellitus (HbA1C > 9%) or glucose of >500 mg/dL at baseline or uncontrolled diabetic ketoacidosis or uncontrolled hyperosmolar hyperglycemic syndrome | |
| Decompensated LC: known LC with ascites or diuretics use or hepatic encephalopathy or varix | |
| Severe hypernatremia: glucose-corrected serum sodiuma ≥ 155 mmol/L | End-stage renal disease and receiving renal replacement therapy |
| Patients who are pregnant or breastfeeding | |
| If the following features occur within 30 days prior to randomization | |
| History of cardiac surgery excluding PCA, acute myocardial infarction, sustained ventricular tachycardia, ventricular fibrillation, acute coronary syndrome, and admission for heart failure | |
| Uncontrolled increase of intracranial pressure | |
| Written consent | Subjects judged by investigators to have difficulty continuing the trial will also be excluded |
ER, emergency room; HbA1c, glycosylated hemoglobin; LC, liver cirrhosis; PCA, percutaneous coronary angioplasty.
Glucose-corrected serum (Na+) = measured (Na+) + 2.4 × (glucose [mg/dL] – 100 [mg/dL])/100 mg/dL
Acute and chronic hypernatremia is defined when the symptoms of hypernatremia have developed in less than and more than 48 hours, respectively. Hospital-acquired hypernatremia is defined as persistent hypernatremia for 48 hours as assessed by serologic tests during a hospital stay. The sNa levels will be measured every 6 hours for 2 days. The sNa will be measured using the following indirect ion-selective electrodes: Seoul University Bundang Hospital will use AU5800 (Beckman Coulter, Pasadena, CA, USA) and Dimension Vista 1500 (Siemens Healthineers, Erlangen, Germany); SMG-SNU Boramae Medical Center will use Modular DP (Roche Diagnostics, Indianapolis, IN, USA) and Unicel DxC 800 (Beckman Coulter); and Hallym University Dongtan Sacred Heart Hospital will use AU5800 (Beckman Coulter). Serum creatinine will be measured using the isotope dilution mass spectrometry-traceable method with a Toshiba TBA 200FR Analyzer (Toshiba, Tokyo, Japan). The estimated glomerular filtration rate will be calculated using the Chronic Kidney Disease Epidemiology Collaboration equation [28]. The Glasgow Coma Scale (GCS) will be assessed before treatment and after 24 and 48 hours of treatment. All types and volumes of fluid administered during those 48 hours will be monitored.
Randomization
An independent statistician generated the randomization sequence using a computer-generated list of random numbers, which is stratified by center with a 1:1 allocation using random block sizes of 2, 4, 6, and 8. A research coordinator will be responsible for screening emergency room patients and inpatients with severe hypernatremia and enrolling participants to each group based on the randomized sequence. The allocation sequence will be concealed from the researchers and study coordinators by using opaque, sequentially numbered envelopes. Eligible participants will be randomly allocated in a 1:1 manner to either the RIB or SCI protocol for electrolyte-free fluid in accordance with the predefined randomization list. Although the patients and their physicians will be aware of the interventions administered, the analysts will be blinded to the intervention.
Practical treatment guidelines for physicians according to the serum sodium level
Except in cases of compromised circulation, hypotonic solutions are an appropriate treatment for cases of severe hypernatremia [1,11]. Therefore, we designed our study protocol using electrolyte-free, 5% dextrose water. After randomization, the subjects will receive either RIB or SCI treatment for hypernatremia correction. According to previous studies, every 1-mmol/L decrease in the sNa level requires 3 mL/kg of electrolyte-free water in elderly female patients and 4 mL/kg of electrolyte-free water in young male patients [29]. Therefore, our goal is to decrease the sNa level by 2 mmol/L at each sample time (0, 3, 6, 12, 18, and 24 hours) to reach the maximum recommended decrease of 12 mmol/L/day. Based on that calculation, elderly female patients (≥65 years), others (male patients ≥ 65 years or female patients < 65 years), and young male patients (<65 years) in the RIB group will be infused for 1 hour with 6 mL/kg, 7 mL/kg, and 8 mL/kg of 5% dextrose water, respectively. Participants in the SCI group will be infused with 5% dextrose water at a minimum rate of 1.35 mL/kg per hour (elderly female patients) according to previous literature [30]. Others and young male patients will be infused at a rate of 1.57 mL/kg per hour and 1.8 mL/kg per hour in accordance with the intended sNa decrease rate set for the RIB group. Because hypernatremia commonly results from a net water loss [1], we will adjust the infusion volume of electrolyte-free water by calculating the U/S ratio ([urine Na + urine potassium]/SNa), a measure of urinary electrolyte-free water clearance [31].
Our treatment goals are to decrease the sNa level from the initial level by 6 to 11 mmol/L within the first 24 hours and by 12 to 23 mmol/L or to an absolute sNa level of ≤150 mmol/L within 48 hours. Overcorrection is defined as a decrease of ≥12 mmol/L within 24 hours or ≥24 mmol/L within 48 hours. When overcorrection develops, the sNa level should not be raised again, but active treatment should be discontinued. Intravenous or per oral furosemide will be used if volume overload is detected by any of the following symptoms and signs: dyspnea, peripheral edema, pulmonary edema, and pleural effusion. In addition to sodium correction, potassium and magnesium should be corrected. If the sNa level decreases by less than 6 mmol/L after 24 hours, 2 μg of intravenous desmopressin can be repeatedly administered according to the judgment of the physician. If maintenance fluid is administered at more than 3 L/day (120 mL/hour) and that is judged to affect sNa correction, the maintenance fluid can be limited to less than 3 L/day. The following cases are exceptions: (1) fluids and transfusion for the correction of hypotension are not counted as maintenance fluid; (2) if sNa is decreased by less than 6 mmol/L after 24 hours, maintenance fluid can be administered at more than 3 L/day; (3) in the case of ongoing nonrenal loss (e.g., nasogastric tube drain, percutaneous catheter drainage, diarrhea, ileus), additional fluid can be administered according to the judgment of the physician regardless of the maintenance fluid. The treatment goal, overcorrection strategy, use of furosemide in volume overload and desmopressin in undercorrection of sNa, and cause-specific treatment of hypernatremia will be the same in both groups. For safety reasons, participants will be dropped from the study in the following cases: (1) volume depletion-weight loss of ≥1 kg per day, deterioration of consciousness, or arterial hypotension that requires inotropes (systolic blood pressure < 90 mmHg and mean arterial pressure < 70 mmHg); and (2) uncontrolled volume overload with worsening pulmonary edema despite diuretics.
Rapid intermittent bolus group (Fig. 4A)
Figure 4. Treatment.
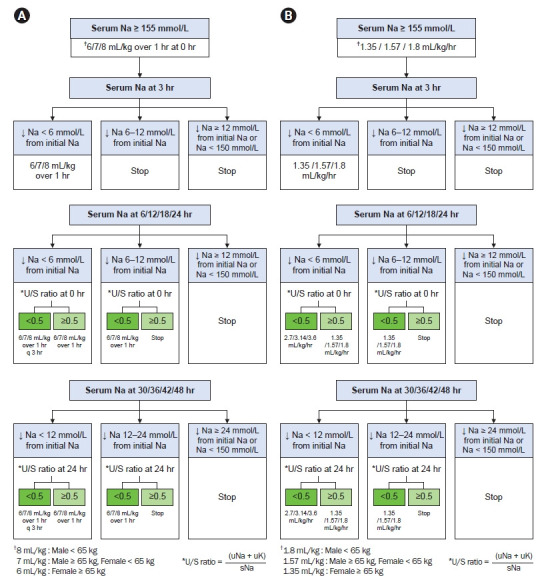
(A) Rapid intermittent bolus with 5% dextrose water in patients with severe hypernatremia. (B) Slow continuous infusion with 5% dextrose water in patients with severe hypernatremia.
sNa, serum sodium; q, quaque; uK, urine potassium; uNa, urine Na; U/S ratio, uNa + uK/sNa; ↓, decrease.
First 3 hours
Subjects with severe hypernatremia will be divided into three groups by age and sex, and the correction rate for initial treatment will be set differently for each group: elderly female patients (≥65 years), others (male patients ≥ 65 years or female patients < 65 years), and young male patients (<65 years) will receive an intravenous infusion of 6 mL/kg, 7 mL/kg, and 8 mL/kg of 5% dextrose water, respectively, in 1 hour. Upon sampling 3 hours after initial treatment, repeated infusions at that rate are recommended until the sNa level has decreased by ≥6 mmol/L from the initial level.
Between 3 and 24 hours
When undercorrection (defined as a decrease of <6 mmol/L in the sNa level from the initial level at 6, 12, 18, or 24 hours) develops, the correction rate should be adjusted according to the U/S ratio at 0 hours. If the U/S ratio is <0.5, an infusion of 6/7/8 mL/kg of 5% dextrose water in 1 hour every 3 hours is recommended. If the U/S ratio is ≥0.5, a single infusion of 6/7/8 mL/kg of 5% dextrose water in 1 hour is recommended. When target correction (defined as a decrease of 6–11 mmol/L in the sNa level from the initial level) develops, the infusion of 5% dextrose water will also be adjusted according to the U/S ratio at 0 hours. If the U/S ratio is <0.5, a single infusion of 6/7/8 mL/kg of 5% dextrose water in 1 hour is recommended. If the U/S ratio is ≥0.5, 5% dextrose water should be discontinued. When overcorrection (defined as a decrease of ≥12 mmol/L in the sNa level) or an absolute sNa level of <150 mmol/L occurs, 5% dextrose water should be discontinued.
Between 24 and 48 hours
When undercorrection (defined as a decrease of <12 mmol/L in the sNa level from the initial level at 30, 36, 42, or 48 hours) occurs, the correction rate should be adjusted according to the U/S ratio at 24 hours. If the U/S ratio is <0.5, an infusion of 6/7/8 mL/kg of 5% dextrose water in 1 hour every 3 hours is recommended. If the U/S ratio is ≥0.5, a single infusion of 6/7/8 mL/kg of 5% dextrose water in 1 hour is recommended. When target correction (defined as a decrease of 12–23 mmol/L in the sNa levels from the initial level) develops, the infusion of 5% dextrose water will also be adjusted according to the U/S ratio at 24 hours. If the U/S ratio at 24 hours is <0.5, a single infusion of 6/7/8 mL/kg of 5% dextrose water in 1 hour is recommended. If the U/S ratio is ≥0.5, 5% dextrose water should be discontinued. If the decrease in sNa levels is ≥24 mmol/L or if the absolute sNa level is <150 mmol/L, 5% dextrose water should be discontinued.
Slow continuous infusion group (Fig. 4B)
First 3 hours
The subjects will be divided into three groups and corrected at different rates, similar to the RIB group. It is recommended that intravenous infusions of 5% dextrose water be provided at 1.35 mL/kg per hour, 1.57 mL/kg per hour, and 1.8 mL/kg per hour in elderly female patients (≥65 years), others (male patients, ≥65 years or female patients, <65 years), and young male patients (<65 years), respectively. Sampling should be done 3 hours after the initial laboratory tests, and continuous infusions of 5% dextrose water are recommended at those rates until the sNa level has decreased by ≥6 mmol/L from the initial level.
Between 3 and 24 hours
When undercorrection (defined as a decrease of <6 mmol/L in the sNa levels from the initial level at 6, 12, 18, or 24 hours) develops, the correction rate should be adjusted according to the U/S ratio at 0 hours. If the U/S ratio is <0.5, a continuous infusion of 5% dextrose water at twice the previous rate (2.70/3.14/3.60 mL/kg per hour) is recommended. If the U/S ratio is ≥0.5, a continuous infusion of 5% dextrose water at the previous rate (1.35/1.57/1.80 mL/kg per hour) is recommended. When target correction (defined as a decrease of 6–11 mmol/L in the sNa level from the initial level) develops, it will be also corrected according to the U/S ratio at 0 hours. If the U/S ratio is <0.5, continuous infusion of 5% dextrose water at the previous rate (1.35/1.57/1.80 mL/kg per hour) is recommended. If the U/S ratio is ≥0.5, 5% dextrose water should be discontinued. When overcorrection (defined as a decrease of ≥12 mmol/L in the sNa level) or an absolute sNa level of <150 mmol/L occurs, 5% dextrose water should be discontinued.
Between 24 and 48 hours
When undercorrection (defined as a decrease of <12 mmol/L in the sNa level from the initial level at 30, 36, 42, or 48 hours) occurs, the correction rate should be adjusted according to the U/S ratio at 24 hours. If the U/S ratio is <0.5, continuous infusion of 5% dextrose water at twice the previous rate (2.7/3.14/3.60 mL/kg per hour) is recommended. If the U/S ratio is ≥0.5, continuous infusion of 5% dextrose water at the previous rate (1.35/1.57/1.80 mL/kg per hour) is recommended. When target correction (defined as a decrease of 6–11 mmol/L in the sNa level from the initial level) develops, it will be also corrected according to the U/S ratio at 24 hours. If the U/S ratio is <0.5, continuous infusion of 5% dextrose water at the previous rate (1.35/1.57/1.80 mL/kg per hour) is recommended. If the U/S ratio is ≥0.5, 5% dextrose water should be discontinued. When overcorrection (defined as a decrease of ≥24 mmol/L in the sNa level or an absolute sNa level of <150 mmol/L) occurs, 5% dextrose water should be discontinued.
Outcome measures
The primary outcome is the incidence of a rapid decrease in the sNa level and aspects of efficacy, as follows: decrease in sNa of ≥6 mmol/L or an absolute sNa level of ≤150 mmol/L within the first 24 hours. The secondary outcomes are the 28-day survival rate after treatment for hypernatremia with 5% dextrose water, difference in sNa levels 6 hours after the initial test, volume of 5% dextrose water infused during 48 hours, and incidence of the target correction rate (defined as a decrease of ≥12 mmol/L in the sNa level or an absolute sNa level of ≤150 mmol/L within 48 hours). Additional outcomes are the target correction rate, incidence of undercorrection, length of hospital stay, incidence and number of desmopressin uses, incidence of overcorrection, incidence of cerebral edema documented via brain computed tomography (CT) at 48 hours, incidence of osmotic demyelinating syndrome via International Classification of Diseases 10 code or brain magnetic resonance imaging, and GCS at baseline (pretreatment), 24 hours, and 48 hours.
Clinical and laboratory evaluations
The physical examination, laboratory evaluations, and medication review will be performed before commencement of the study. The laboratory evaluations will comprise a complete blood count; tests for serum electrolytes, calcium, phosphate, blood urea nitrogen, creatinine, glucose, total CO2, total protein, albumin, uric acid, and C-reactive protein; liver function testing (aspartate transaminase, alanine aminotransferase, alkaline phosphatase, and total bilirubin); lipid profile; serum osmolality; and urine analysis, urine electrolytes, urine creatinine, and urine osmolality. The sNa level will be measured every 6 hours (after being measured at 0, 3, and 6 hours) for 2 days. Levels of urine sodium and potassium will be measured at 0 and 24 hours. The GCS score will be estimated at 0, 24, and 48 hours. Brain CT will be conducted at 48 hours in patients who develop overcorrection.
Safety issues
All serious side effects will be reported to the investigator and the ethics committee. In this study protocol, safety information about each patient should be collected within 48 hours after treatment. Safety concerns in the treatment of hypernatremia are caused by undercorrection and overcorrection, and we will check for those at every sample time point. Interventions will be performed as follows: if the sNa level is decreased by more than 12 mmol/L within 24 hours or more than 24 mmol/L within 48 hours at any sample time point, the ongoing active treatment will be discontinued, but the sNa level should not be raised. If the decrease in sNa levels is <6 mmol/L after 24 hours, 2 μg of intravenous desmopressin can be administered according to the judgment of the physician. When signs and symptoms of volume overload are observed, such as dyspnea, peripheral edema, pulmonary edema, or pleural effusion, intravenous or oral administration of furosemide will be considered.
Sample size calculation
In the literature, a low hypernatremia correction rate in the first 24-hours (<0.25 mmol/L per hour or 6 mmol/L per day) can be significant predictors of 30-day mortality [20]. Therefore, as a surrogate marker for 30-day survival, a rapid decrease in the sNa level (≥0.25 mmol/L per hour or 6 mmol/L per day) was chosen as the primary study outcome. A previous study reported that 33% of hypernatremic patients being treated with the SCI method of electrolyte-free solution achieved a rapid decrease in their sNa levels within the first 24 hours [20]. However, no information is available on the incidence of a rapid decrease in sNa levels with RIB treatment. We expect the frequency of rapid decrease of sNa to increase by 1.8 times in the RIB group compared with the SCI group. Therefore, we expect the proportions of participants who achieve a rapid decrease in their sNa levels to be 55% and 30% in the RIB and SCI groups, respectively. We calculated the required sample size for an estimated dropout rate of 15%, a two-sided level of significance of α = 0.05, a power of 80%, and one interim analysis and thus determined that 83 participants will be needed in each group to find significant differences between the two groups using the chi-square test. Therefore, a total of 166 participants will be enrolled. We considered one interim analysis at the time when half of the subjects have completed the study. The O’Brien-Fleming alpha spending function will be used to test the primary outcomes in the first interim analysis and final analysis.
Statistical analyses
The analysis of outcomes will be conducted on both the intention-to-treat (ITT) and per-protocol (PP) bases because a considerable dropout rate can be expected due to our sophisticated electrolyte-free water infusion protocol. The ITT population is defined as all participants for whom the primary endpoint will be available, and those participants will be analyzed in accordance with the groups to which they were randomly allocated, regardless of deviation from the protocol. In the ITT analysis, all outcomes will be counted until the infusion protocol is well-adhered to. The PP analysis will include only the participants who complete the study without major protocol deviations to evaluate the primary, secondary, and additional outcomes. Continuous variables will be expressed as means and standard deviations, and categorical variables will be expressed as frequencies or percentages for baseline characteristics and laboratory findings.
The incidence of a rapid decrease in sNa levels within 24 hours, 28-day survival, target correction rate, a decrease of ≥12 mmol/L in sNa level or an absolute sNa level of ≤150 mmol/L within 48 hours, additional treatment, use of desmopressin, cerebral edema, osmotic demyelination syndrome, and in-hospital mortality will be compared between the two groups using the chi-square test, Fisher exact test, odds ratios with logistic regression, and absolute risk with a Poisson regression. The differences in sNa levels at 3 hours, volume of 5% dextrose water in 48 hours, number of additional treatments, hospital stay, and number of desmopressin uses will be analyzed between the two groups using Student t test, Mann-Whitney U test, mean differences with linear regression, and a mixed model to analyze the effects of repeated sNa levels and the overall change in sNa level from baseline with fixed effects for time, group, and interactions between time and group. The marginal effect of sNa and overall change in sNa from baseline by group will be plotted. In the interim analysis, if the critical value is 2.96 (p = 0.003), early termination will be performed; if not, participant recruitment will be continued. A value of p < 0.05 will be considered statistically significant. Statistical analyses will be performed using IBM SPSS version 27.0 (IBM Corp., Armonk, NY, USA), STATA version 14.0 (StataCorp LP, College Station, TX, USA), and R version 3.5.3 (R Foundation for Statistical Computing, Vienna, Austria; http://www.r-project.org).
Data and safety monitoring
The paper data sheets and signed consents will be stored in locked cabinets, and electronic databases will be stored on secure servers with password protection. Unexpected side effects during treatment will be reported to the IRBs according to the procedures of the three participating hospitals. All revised procedures will be submitted to the IRBs of all three hospitals and ClinicalTrials.gov. The data will be maintained confidentially and will be accessible only to research investigators.
Discussion
Recently, several cohort studies have suggested that rapid correction of hypernatremia did not increase the mortality rate or even that slow correction could increase mortality [20,21,32]. Nonetheless, the current recommendation is to reduce sNa by <0.5 mmol/L per hour (<10 mmol/L per day) to prevent cerebral edema, seizure, and brain damage during the treatment of hypernatremia [1,13–15]. In acute hypernatremia, the correction rate can be increased to 1 mmol/L of sNa per hour [33]. However, those correction rates reflect studies in children [11,19]. No previous, large-scale, prospective, randomized controlled studies have examined the correction rate for hypernatremia in adults. This is thus the first clinical trial to provide qualified evidence about administering electrolyte-free water to patients with severe hypernatremia. Additionally, our results will allow a protocolized approach to the management of hypernatremia to be established.
In acute hypernatremia, which develops within 48 hours, cerebral edema does not occur even upon immediate correction because the accumulated electrolytes can rapidly move out of brain cells [33,34]. In hypernatremia of >48 hours or unknown duration, rapid correction can lead to brain edema because several days are required to remove osmolytes from brain cells [34,35]. Therefore, expert opinion suggests a decrease rate of <0.5 mmol/L per hour, with an absolute change of <10 mmol/L per day to prevent brain damage [1,13–15]. However, some studies have indicated that rapid correction of hypernatremia was not associated with increasing disease-related mortality or cerebral edema and that excessively slow rates of correction were associated with increased short-term mortality [16,20,21,32]. Chauhan et al. [16] found that rapid correction of >0.5 mmol/L per hour or >12 mmol/L was not associated with increased mortality or cerebral edema and that mortality rates were consistently low with varying correction rates (>8, >10, and 12 mmol/L) at 24 hours. Bataille et al. [21] calculated the correction rate of hypernatremia as the mean rate, which implies that the difference in sNa between baseline and the last known hypernatremia was divided by the total time. Their correction rates were –0.1 ± 0.15 mmol/L per hour and –0.2 ± 0.22 mmol/L per hour, and the proportions of no improvement in hypernatremia were 44% and 21% in patients who died and survived, respectively. In children, the size of the cerebrum increases rapidly until age 6 years, and it continues to grow until age 15 years; therefore, the ratio of brain volume to cranial vault size reaches its maximum at 6 years. However, the brain size of adults gradually decreases after age 45 years and is the lowest at age 86 years [36–38]. Therefore, we inferred that rapid correction in adults might be tolerable [16].
However, the previous retrospective studies on hypernatremia had different definitions for rapid correction of sNa [16,20,21]. The correction rate calculated by dividing the difference in sNa levels by time [16,21] might also have differed from the actual correction rate or been inaccurate. Furthermore, it is difficult to determine in those studies whether electrolyte-free water was given by the RIB or SCI method, and it is also unclear whether hypernatremia was improved by administering electrolyte-free water or hypotonic solution or by treatment of the underlying disease.
In a randomized controlled trial (SALSA I) that we published previously [24,25,39], 10 mL/kg of electrolyte-free water was applied in 1 hour as a method of re-lowering therapy in excessively rapid hyponatremia correction, which complied with the European guidelines [23]. Because only one patient experienced pulmonary edema and pleural effusion during the study period, we hold that safety concerns about volume overload are negligible. Therefore, we adopted our previous RIB method in developing this infusion protocol. According to the literature, every 1 mmol/L decrease in sNa level requires 3 mL/kg of electrolyte-free water in elderly female patients and 4 mL/kg of electrolyte-free water in young men. We estimated a decrease of 2 mmol/L in sNa levels at every sample time by using 6/7/8 mL/kg infusions of electrolyte-free water, for a maximum sNa decrease of 12 mmol/L/day. The total amount of electrolyte-free water to be infused in the RIB group was converted to a continuous infusion rate for 24 hours for the SCI group. Therefore, we expect that the amount of electrolyte-free water infused into the patients in both groups will be similar. Because hypernatremia is commonly caused by a net water loss [1], we adjust the infusion volume of electrolyte-free water by calculating the U/S ratio, an indicator that conveniently reflects ongoing urinary clearance of electrolyte-free water [31]. In addition, we plan to perform an interim analysis as a safety measure because the RIB method is a novel protocol.
The primary outcome of this study is the incidence of a rapid decrease in sNa levels. Prolonged hypernatremia can cause death by inducing brain damage, decreasing cardiac contractility, increasing peripheral insulin resistance, and impairing hepatic gluconeogenesis [40]. Therefore, the incidence of rapid correction is expected to be a good surrogate outcome of efficacy and safety.
This study has some limitations. First, differences in the expertise of doctors and nurses could cause protocol violations during the early period because of unfamiliarity with the protocol. Therefore, the protocol is designed in a simple way to prevent nonadherence among physicians. Second, mild hypernatremia is not covered by this study, which enrolls only patients whose absolute sNa levels are >155 mmol/L. However, the higher the sNa levels, the higher the incidence of neurologic symptoms due to high cell shrinkage. Therefore, targeting severe hypernatremia can maximize the efficacy of our rapid correction protocol. Third, this study uses only electrolyte-free water as the correction fluid, but various clinical situations can require a hypotonic solution (0.45% sodium chloride), which warrants further research.
In conclusion, the SALSA II study is the first prospective, multicenter, randomized, open-label, controlled clinical trial to investigate the efficacy and safety of the RIB protocol compared with SCI in adult patients with severe hypernatremia.
Acknowledgments
We thank the Medical Research Collaborating Center in Seoul National University Bundang Hospital for help with the statistical analyses.
Footnotes
Conflicts of interest
All authors have no conflicts of interest to declare.
Funding
This research was supported by the grant from the National Research Foundation of Korea (no. 2021R1C1C1008966).
Authors’ contributions
Conceptualization: SK, SHB
Investigation: JL, YHJ, SB
Data curation: KPK, JAS
Methodology: JYR, SY
Formal analysis: JL, JH
Visualization: KPK
Funding acquisition: SHB
Project administration: YHJ, SK, SHB
Writing–Original Draft: JYR, SY
Writing–Review & Editing: All authors
All authors read and approved the final manuscript.
References
- 1.Adrogué HJ, Madias NE. Hypernatremia. N Engl J Med. 2000;342:1493–1499. doi: 10.1056/NEJM200005183422006. [DOI] [PubMed] [Google Scholar]
- 2.Funk GC, Lindner G, Druml W, et al. Incidence and prognosis of dysnatremias present on ICU admission. Intensive Care Med. 2010;36:304–311. doi: 10.1007/s00134-009-1692-0. [DOI] [PubMed] [Google Scholar]
- 3.Palevsky PM, Bhagrath R, Greenberg A. Hypernatremia in hospitalized patients. Ann Intern Med. 1996;124:197–203. doi: 10.7326/0003-4819-124-2-199601150-00002. [DOI] [PubMed] [Google Scholar]
- 4.Qian Q. Hypernatremia. Clin J Am Soc Nephrol. 2019;14:432–434. doi: 10.2215/CJN.12141018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kumar S, Berl T. Sodium. Lancet. 1998;352:220–228. doi: 10.1016/S0140-6736(97)12169-9. [DOI] [PubMed] [Google Scholar]
- 6.Feig PU, McCurdy DK. The hypertonic state. N Engl J Med. 1977;297:1444–1454. doi: 10.1056/NEJM197712292972608. [DOI] [PubMed] [Google Scholar]
- 7.Gennari FJ. Current concepts. Serum osmolality: uses and limitations. N Engl J Med. 1984;310:102–105. doi: 10.1056/NEJM198401123100207. [DOI] [PubMed] [Google Scholar]
- 8.Ross EJ, Christie SB. Hypernatremia. Medicine (Baltimore) 1969;48:441–473. doi: 10.1097/00005792-196948060-00002. [DOI] [PubMed] [Google Scholar]
- 9.De Villota ED, Cavanilles JM, Stein L, Shubin H, Weil MH. Hyperosmolal crisis following infusion of hypertonic sodium chloride for purposes of therapeutic abortion. Am J Med. 1973;55:116–122. doi: 10.1016/0002-9343(73)90158-7. [DOI] [PubMed] [Google Scholar]
- 10.Sterns RH. Evidence for managing hypernatremia: is it just hyponatremia in reverse? Clin J Am Soc Nephrol. 2019;14:645–647. doi: 10.2215/CJN.02950319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kahn A, Brachet E, Blum D. Controlled fall in natremia and risk of seizures in hypertonic dehydration. Intensive Care Med. 1979;5:27–31. doi: 10.1007/BF01738999. [DOI] [PubMed] [Google Scholar]
- 12.Blum D, Brasseur D, Kahn A, Brachet E. Safe oral rehydration of hypertonic dehydration. J Pediatr Gastroenterol Nutr. 1986;5:232–235. [PubMed] [Google Scholar]
- 13.Braun MM, Barstow CH, Pyzocha NJ. Diagnosis and management of sodium disorders: hyponatremia and hypernatremia. Am Fam Physician. 2015;91:299–307. [PubMed] [Google Scholar]
- 14.Al-Absi A, Gosmanova EO, Wall BM. A clinical approach to the treatment of chronic hypernatremia. Am J Kidney Dis. 2012;60:1032–1038. doi: 10.1053/j.ajkd.2012.06.025. [DOI] [PubMed] [Google Scholar]
- 15.Sterns RH. Disorders of plasma sodium: causes, consequences, and correction. N Engl J Med. 2015;372:55–65. doi: 10.1056/NEJMra1404489. [DOI] [PubMed] [Google Scholar]
- 16.Chauhan K, Pattharanitima P, Patel N, et al. Rate of correction of hypernatremia and health outcomes in critically ill patients. Clin J Am Soc Nephrol. 2019;14:656–663. doi: 10.2215/CJN.10640918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cabassi A, Tedeschi S. Severity of community acquired hypernatremia is an independent predictor of mortality: a matter of water balance and rate of correction. Intern Emerg Med. 2017;12:909–911. doi: 10.1007/s11739-017-1693-x. [DOI] [PubMed] [Google Scholar]
- 18.Lindner G, Funk GC. Hypernatremia in critically ill patients. J Crit Care. 2013;28:216. doi: 10.1016/j.jcrc.2012.05.001. [DOI] [PubMed] [Google Scholar]
- 19.Bolat F, Oflaz MB, Güven AS, et al. What is the safe approach for neonatal hypernatremic dehydration?: a retrospective study from a neonatal intensive care unit. Pediatr Emerg Care. 2013;29:808–813. doi: 10.1097/PEC.0b013e3182983bac. [DOI] [PubMed] [Google Scholar]
- 20.Alshayeb HM, Showkat A, Babar F, Mangold T, Wall BM. Severe hypernatremia correction rate and mortality in hospitalized patients. Am J Med Sci. 2011;341:356–360. doi: 10.1097/MAJ.0b013e31820a3a90. [DOI] [PubMed] [Google Scholar]
- 21.Bataille S, Baralla C, Torro D, et al. Undercorrection of hypernatremia is frequent and associated with mortality. BMC Nephrol. 2014;15:37. doi: 10.1186/1471-2369-15-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Verbalis JG, Goldsmith SR, Greenberg A, et al. Diagnosis, evaluation, and treatment of hyponatremia: expert panel recommendations. Am J Med. 2013;126(10 Suppl 1):S1–S42. doi: 10.1016/j.amjmed.2013.07.006. [DOI] [PubMed] [Google Scholar]
- 23.Spasovski G, Vanholder R, Allolio B, et al. Clinical practice guideline on diagnosis and treatment of hyponatraemia. Eur J Endocrinol. 2014;170:G1–G47. doi: 10.1530/EJE-13-1020. [DOI] [PubMed] [Google Scholar]
- 24.Lee A, Jo YH, Kim K, et al. Efficacy and safety of rapid intermittent correction compared with slow continuous correction with hypertonic saline in patients with moderately severe or severe symptomatic hyponatremia: study protocol for a randomized controlled trial (SALSA trial) Trials. 2017;18:147. doi: 10.1186/s13063-017-1865-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Baek SH, Jo YH, Ahn S, et al. Risk of overcorrection in rapid intermittent bolus vs slow continuous infusion therapies of hypertonic saline for patients with symptomatic hyponatremia: the SALSA randomized clinical trial. JAMA Intern Med. 2021;181:81–92. doi: 10.1001/jamainternmed.2020.5519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chan AW, Tetzlaff JM, Altman DG, et al. SPIRIT 2013 statement: defining standard protocol items for clinical trials. Ann Intern Med. 2013;158:200–207. doi: 10.7326/0003-4819-158-3-201302050-00583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hillier TA, Abbott RD, Barrett EJ. Hyponatremia: evaluating the correction factor for hyperglycemia. Am J Med. 1999;106:399–403. doi: 10.1016/s0002-9343(99)00055-8. [DOI] [PubMed] [Google Scholar]
- 28.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sterns RH, Silver SM. Salt and water: read the package insert. QJM. 2003;96:549–552. doi: 10.1093/qjmed/hcg102. [DOI] [PubMed] [Google Scholar]
- 30.Sterns RH, Hoorn EJ. Waltham (MA): UpToDate; c2021. Treatment of hypernatremia in adults [Internet] [cited 2021 Sep 1]. Available from: https://www.uptodate.com/contents/treatment-of-hypernatremia-in-adults. [Google Scholar]
- 31.Mount DB. In: Harrison’s principles of internal medicine. 20th ed. Jameson J, Fauci AS, Kasper DL, Hauser SL, Longo DL, Loscalzo J, editors. New York: McGraw Hill; 2018. Hypernatremia. [Google Scholar]
- 32.Ates I, Özkayar N, Toprak G, Yılmaz N, Dede F. Factors associated with mortality in patients presenting to the emergency department with severe hypernatremia. Intern Emerg Med. 2016;11:451–459. doi: 10.1007/s11739-015-1368-4. [DOI] [PubMed] [Google Scholar]
- 33.Palevsky PM. In: Primer on kidney diseases. 2nd ed. Greenberg A, Cheung AK, Coffman TM, Falk RJ, Charles Jennette J, editors. San Diego: Academic Press; 1998. Hypernatremia; pp. 64–71. [Google Scholar]
- 34.Lien YH, Shapiro JI, Chan L. Effects of hypernatremia on organic brain osmoles. J Clin Invest. 1990;85:1427–1435. doi: 10.1172/JCI114587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hogan GR, Dodge PR, Gill SR, Master S, Sotos JF. Pathogenesis of seizures occurring during restoration of plasma tonicity to normal in animals previously chronically hypernatremic. Pediatrics. 1969;43:54–64. [PubMed] [Google Scholar]
- 36.Dekaban AS. Changes in brain weights during the span of human life: relation of brain weights to body heights and body weights. Ann Neurol. 1978;4:345–356. doi: 10.1002/ana.410040410. [DOI] [PubMed] [Google Scholar]
- 37.Hafkemeijer A, Altmann-Schneider I, de Craen AJ, Slagboom PE, van der Grond J, Rombouts SA. Associations between age and gray matter volume in anatomical brain networks in middle-aged to older adults. Aging Cell. 2014;13:1068–1074. doi: 10.1111/acel.12271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bourisly AK, El-Beltagi A, Cherian J, Gejo G, Al-Jazzaf A, Ismail M. A voxel-based morphometric magnetic resonance imaging study of the brain detects age-related gray matter volume changes in healthy subjects of 21-45 years old. Neuroradiol J. 2015;28:450–459. doi: 10.1177/1971400915598078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Baek SH, Kim S. Optimal treatment with hypertonic saline in patients with symptomatic hyponatremia: a perspective from a randomized clinical trial (SALSA trial) Kidney Res Clin Pract. 2020;39:504–506. doi: 10.23876/j.krcp.20.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lindner G, Funk GC, Schwarz C, et al. Hypernatremia in the critically ill is an independent risk factor for mortality. Am J Kidney Dis. 2007;50:952–957. doi: 10.1053/j.ajkd.2007.08.016. [DOI] [PubMed] [Google Scholar]