Abstract
Individuals who use cocaine exhibit maladaptive decision making, overweighting rewards and underweighting potential risks. We previously showed that chronic cocaine self-administration in young adult male rats causes long-lasting increases in risk taking. The current study expanded upon these findings to determine whether effects of cocaine on risk taking depend on the route of cocaine administration and extend to females. To address the former question, rats in Experiment 1 were trained on the “Risky Decision-making Task” (RDT), received passively administered cocaine, and were re-tested in the RDT. Surprisingly, passive cocaine had no effect on risk taking. Experiment 2 determined whether cocaine self-administration increases risk taking in females in a manner comparable to males. Males and females were trained in the RDT, underwent cocaine self-administration, and were re-tested in the RDT. Unexpectedly, cocaine self-administration had no effect on risk taking in either sex. Because Experiments 1 and 2 involved cocaine exposure at a considerably older age than in previous work, Experiments 3 and 4 determined if cocaine effects on risk taking depend on the age of exposure. Rats began cocaine self-administration at postnatal (PN) day 77 (Experiment 3) or passive cocaine injections starting on PN day 63 (Experiment 4) and were tested in the RDT three weeks after cocaine cessation. In these experiments, cocaine increased risk taking in both sexes. These results reveal a limited time window during young adulthood of vulnerability to the effects of chronic cocaine on risk taking.
Keywords: cocaine, decision making, punishment, rat, sex differences
Introduction
Chronic cocaine users exhibit cognitive deficits including maladaptive decision making. These individuals tend to overvalue the rewards of consuming their drug of choice despite possible risks to physical, financial, and social well-being (Stout, Busemeyer, Lin, Grant, & Bonson, 2004; Stout, Rock, Campbell, Busemeyer, & Finn, 2005; Thompson et al., 2012; Tomassini et al., 2012). Hence, it is not surprising that cocaine use is associated with impairments in risk-based decision making (Gowin, Mackey, & Paulus, 2013). In the real world, cocaine users engage in more risky sexual behavior and criminal activity than non-users (Grella, Anglin, & Wugalter, 1995; Lejuez, Bornovalova, Daughters, & Curtin, 2005). In the laboratory, numerous behavioral instruments such as the Iowa Gambling Task (IGT) have been used to assess the impact of cocaine on risky decision making, and have consistently found that, similar to real-world findings, chronic cocaine use is associated with greater risk-taking behavior (Bornovalova, Daughters, Hernandez, Richards, & Lejuez, 2005; Fishbein et al., 2005; Gowin, Sloan, Ramchandani, Paulus, & Lane, 2018; Kluwe-Schiavon et al., 2020; Leland & Paulus, 2005; van der Plas, Crone, van den Wildenberg, Tranel, & Bechara, 2009; Verdejo-Garcia et al., 2007). High levels of risk taking associated with cocaine use can extend far into abstinence (Bolla et al., 2003; Verdejo-Garcia et al., 2014), which may render these individuals vulnerable to relapse. Indeed, one study reported that among former cocaine users who displayed high levels of risk taking in the IGT, 90% relapsed three months later. In contrast, of those who displayed unimpaired decision making, only 20% relapsed after three months (Verdejo-Garcia et al., 2014). Hence, not only is chronic cocaine use associated with long-lasting increases in risk taking, but such heightened risk taking may further perpetuate the disease.
Despite the strong evidence supporting relationships between cocaine use and greater risk taking, the cognitive mechanisms underlying these effects on choice behavior are less clear. The majority of the literature has focused on how changes in sensitivity to rewarding outcomes drive risky choice (i.e., enhanced sensitivity to rewarding outcomes promotes continued choice of rewarding, although risky, options; Balconi, Finocchiaro, & Campanella, 2014; Fishbein et al., 2005; Stout et al., 2005). Less attention has been paid, however, to how changes in sensitivity to negative outcomes contribute to elevated risky choice associated with cocaine use. In studies that have investigated the latter, the negative outcome typically consists of lost reward opportunities, such as the loss of earnings in a laboratory task. For example, Gowin et al. (2017) examined performance of individuals with cocaine use disorder (CUD) in a Risky Gains Task, in which subjects chose between a small, certain monetary reward and a larger, but uncertain, monetary reward. Compared to control subjects, individuals with CUD exhibited more risky choices specifically following losses (Gowin, May, Wittmann, Tapert, & Paulus, 2017), indicating an inability to evaluate negative outcomes to enable shifting of subsequent choices toward safer options. This and similar laboratory tasks effectively model some decisions faced by individuals with CUD (e.g., risk to financial security); however, they do not recapitulate other real-world decisions in which the outcomes may have an adverse and deleterious impact on the individual’s health and well-being (e.g., contracting a sexually transmitted disease, incarceration). The use of recently developed animal models of decision making involving risk of explicit punishment may provide insight into how chronic cocaine alters sensitivity to risk of punishment. In the Risky Decision-making Task (RDT), in which rats choose between a small, safe food reward and a larger food reward accompanied by variable risks of footshock punishment, rats that previously underwent cocaine self-administration displayed greater choice of the risky option relative to rats that underwent sucrose self-administration control procedures (Mitchell, Weiss, Beas, et al., 2014). Consistent with these effects on decision making involving risk of punishment, a more recent study found that a history of passively-administered cocaine attenuated rats’ ability to actively avoid footshock punishment (Nguyen, Nesarajah, Erb, & Ito, 2018). Collectively, these findings are consistent with studies in humans linking cocaine use and heightened risk taking (Chen et al., 2020; Gowin et al., 2013), and suggest the possibility that such maladaptive choice behavior may be driven by cocaine-induced reductions in sensitivity to risk of adverse consequences.
The prior studies in rats provide valuable information concerning cocaine’s effects on risk taking, but there are still several outstanding questions that need to be addressed to better understand this causal relationship. The current study was therefore designed to extend our previous work (Mitchell, Weiss, Beas, et al., 2014) in two important directions. First, we wanted to determine whether the effects of cocaine on risk taking were due to the volitional nature of self-administration vs. the pharmacological properties of the drug. In contrast to the effects of cocaine on impulsive choice, which has been studied with both self-administration and passive cocaine administration (Dandy & Gatch, 2009; Mendez et al., 2010; Mitchell, Weiss, Ouimet, et al., 2014; Simon, Mendez, & Setlow, 2007; Zuo et al., 2012), cocaine-induced increases in risk taking in the RDT have only been investigated using self-administration (Mitchell, Weiss, Beas, et al., 2014). Consequently, we evaluated the effects of passive cocaine administration on risk taking in the RDT.
Second, we extended our previous work by comparing the effects of cocaine exposure on decision making involving risk of explicit punishment between males and females. There are well-established sex differences in risk taking and sensitivity to punishment, and indeed, we have shown that females are more risk averse and more sensitive to pharmacological manipulations that promote risk aversion than males in the RDT (Orsini, Willis, Gilbert, Bizon, & Setlow, 2016). More recent studies have replicated this pattern of behavioral findings, with females being more risk averse in choice tasks involving punishment outcomes (Chowdhury et al., 2019; Liley, Gabriel, Sable, & Simon, 2019; Pellman, Schuessler, Tellakat, & Kim, 2017). There are also substantial sex differences in some aspects of drug-seeking behavior. Female rats often acquire cocaine self-administration more rapidly (Jackson, Robinson, & Becker, 2006; Lynch & Carroll, 1999), escalate their intake at a faster rate (Roth & Carroll, 2004) and reinstate cocaine seeking to a greater degree than males after extinction (Lynch & Carroll, 2000). These findings are consistent with reports that women proceed from recreational stimulant use to dependence more quickly and relapse at a higher rate than men (Bobzean, DeNobrega, & Perrotti, 2014). Further, in a choice setting, female rats demonstrate a greater preference for cocaine over a food reward than males (Kerstetter et al., 2012). Hence, it is conceivable that there are sex differences in the causal relationship between cocaine exposure and risk taking. In support of such differences, risk preference in drug-naïve females, but not males, predicts subsequent cocaine intake during self-administration under short-access conditions (Orsini et al., 2020).
Consideration of factors such as sex and route of drug administration are critical to designing and executing experiments to evaluate the effects of drug exposure on subsequent decision making. To this end, we designed experiments to assess whether route of cocaine administration influences the effects of cocaine on risk taking (Experiment 1) and to determine whether cocaine causes comparable increases in risk taking in males and females (Experiment 2). The findings of these experiments led to subsequent examination of how the age at which cocaine exposure occurs may dictate its effects on risk taking (Experiments 3 and 4). The findings of these experiments have the potential to inform future studies on the best practices to model maladaptive risk taking associated with cocaine use in humans. Furthermore, they provide a more comprehensive understanding of the causal relationships between cocaine exposure and risk taking in both sexes.
Methods
Subjects
Male and female Long-Evans rats (N=129; n=74 male, n=55 female; Charles River Laboratories) were individually housed on a 12-hour light/dark cycle (lights on at 0700 for Experiment 1, and lights on at 1900 for Experiments 2-4). Rats were maintained at 85% of their free-feed weight (target weight was increased by 5 g per week to account for growth) during behavioral testing and were given free access to water at all times. Procedures were conducted under University of Florida Institutional Animal Care and Use Committee protocol numbers 04940 (Risk taking and cocaine use: interactions, mechanisms and therapeutic targets) and 07758 (Neural mechanisms underlying maladaptive risk-taking following cocaine self-administration) and followed NIH guidelines.
Apparatus
For the Risky Decision-making Task (RDT), rats underwent behavioral testing in 12 standard operant chambers that were housed in sound attenuating cabinets (Coulbourn Instruments). Each operant chamber contained a food trough located 2 cm above the floor on the front wall, into which food pellets (Experiment 1: Test Diet, 5TUM; Experiment 2-4: Test Diet, 5UTL) were delivered. The food delivery trough was equipped with a 1.12 W light bulb for illumination and a photobeam to register nosepoke entries. Flanking the food trough on each side was a retractable lever positioned 11 cm above the floor. An additional 1.12 W light bulb was mounted on the back wall of the sound attenuating cabinet and served as a house light. The operant chamber floor consisted of stainless steel rods through which scrambled footshocks were delivered via a connection to a shock generator (Coulbourn Instruments). Using a sensor mounted on the top of each operant chamber, locomotion was monitored by detecting changes in infrared (body heat) energy throughout the entire test chamber. All operant chambers were interfaced with a computer running Graphic State 3 (Experiment 1) or Graphic State 4 (Experiments 2-4) software (Coulbourn Instruments) for control of task events and data collection.
Cocaine self-administration was conducted in 12 operant chambers housed in sound attenuating cabinets (Coulbourn Instruments) located in a different room from the chambers used for the RDT. Each self-administration chamber contained a liquid dipper trough positioned in the center of the front wall which was used for delivery of a sucrose solution reward. The liquid dipper trough was illuminated with a 1.12 W light bulb and was equipped with a photobeam to detect nosepoke entries. Two nosepoke holes were located on either side of the liquid dipper trough, the interiors of which could be illuminated with small lights. Each operant chamber was also outfitted with a speaker and a tone generator. Intravenous cocaine was delivered using an infusion pump located outside the operant test chamber. A 20 mL syringe was mounted on the infusion pump and connected to a tether system (Instech Laboratories), which itself was supported by a swivel attached to the top of the operant chamber. Each tether consisted of PE50 tubing and ran from the syringe to the venous access port implanted in the back of the rat. The operant chambers were interfaced with a computer running Graphic State 4 software (Coulbourn Instruments) for control of task events and data collection.
Behavioral Procedures
Overview of Experimental Procedures:
Experiment 1: The objective of Experiment 1 was to determine whether passive administration of cocaine increases risk taking in mature adult males, similar to the effects of self-administered cocaine (Mitchell, Weiss, Beas, et al., 2014). Male Long-Evans rats (cocaine: n=8, saline: n=16) were trained on the RDT beginning at post-natal (PN) day 70 until stable performance was achieved at PN day 98. At PN day 105 (later adulthood), rats received daily intraperitoneal injections of cocaine or saline for 14 consecutive days, after which they remained undisturbed in their home cages for three weeks of abstinence. They were then re-tested in the RDT beginning on PN day 140 until stable performance was achieved at PN day 189 (note that these rats did not undergo progressive ratio or shock reactivity threshold testing).
Experiment 2: The objective of Experiment 2 was to determine the effects of cocaine self-administration on risk taking in mature adult male and female rats. Male and female Long-Evans rats (cocaine: n=12 male, n=20 female; sucrose: n=11 male, n=17 female) were trained on the RDT beginning at PN day 70 until stable performance was achieved at PN day 140. All rats then underwent surgery to implant jugular catheters at PN day 147. After recovery from surgery, rats were assigned to cocaine or sucrose self-administration groups, matched for pre-testing RDT performance. At PN day 161 (later adulthood), rats underwent cocaine or sucrose self-administration for 14 consecutive days, after which they remained undisturbed in their home cages for three weeks of abstinence. Beginning at PN day 196, rats were re-tested on the RDT until stable behavior emerged at PN day 231. A subset of rats (cocaine: n=6 male, n=5 female; sucrose: n=6 male, n=5 female) were then tested in a progressive ratio (PR) schedule of reinforcement task and a shock reactivity threshold assay to assess food motivation and shock thresholds, respectively.
Experiment 3: The objective of Experiment 3 was to determine whether effects of cocaine self-administration on risk taking in male and female rats depended on the age at which self-administration occurred. Prior to undergoing surgery, baseline shock thresholds were obtained using a shock reactivity threshold assay in male and female Long-Evans rats (cocaine: n=8 male, n=3 female; sucrose: n=7 male, n=3 female). All rats subsequently underwent surgeries to implant jugular catheters at PN day 56. After recovery from surgery, rats were assigned to cocaine or sucrose self-administration groups. At PN day 77 (young adulthood), rats commenced cocaine or sucrose self-administration for 14 consecutive days, after which they remained undisturbed in their home cages for three weeks of abstinence. Rats began training in the RDT at PN day 112 and continued in the task until stable performance emerged at PN day 154. Finally, rats were tested in the PR schedule of reinforcement task and shock reactivity threshold assay as in Experiment 2.
Experiment 4: The objective of Experiment 4 was to determine whether effects of passively-administered cocaine on risk taking in male and female rats depended on the age at which passive exposure to cocaine occurred. Male and female Long-Evans rats (cocaine: n=6 male, n=6 female; saline: n=6 male, n=6 female) received intraperitoneal injections of cocaine or saline for 14 consecutive days beginning on PN day 63 (young adulthood), after which they remained undisturbed in their home cages for three weeks of abstinence. On PN day 98, rats began training in the RDT and continued until stable performance was achieved at PN day 147.
Risky Decision-making Task Procedures
To reduce neophobia, five food pellets were placed in rats’ home cages 24 h before RDT shaping commenced. Rats were then shaped to perform each component of the task (e.g., nosepoking, lever pressing). The first stage of shaping was magazine training, in which a single food pellet was delivered into the food trough every 100 ± 40 s over a 64 min session. Rats had to nosepoke into the food trough a minimum of 100 times to reach criterion. In the next stage of shaping, one lever (either left or right, counterbalanced between groups) was extended into the operant chamber and rats received a single food pellet upon pressing the lever. Rats had to press the lever 50 times in a 30 min session to reach criterion. The next shaping stage was identical to the previous, except that the opposite lever was extended into the chamber while the other remained retracted. Once rats reached criterion performance on both levers, they progressed to the final stage of shaping in which a nosepoke into the illuminated food trough caused one lever (either right or left) to extend into the operant chamber. A press on the lever extinguished the house light and led to the delivery of a food pellet. Rats had to reach a minimum of 30 presses on each lever in a 60 min session to reach criterion before progressing to training in the RDT (Orsini, Blaes, Setlow, & Simon, 2019; Simon, Gilbert, Mayse, Bizon, & Setlow, 2009; Simon et al., 2011).
Test sessions in the RDT were 60 min in duration and consisted of 5 blocks of 18 trials. Each trial lasted 40 s and began with illumination of the food trough and house lights. Rats had to nosepoke into the food delivery trough to cause a single lever (forced choice trials) or both levers (free choice trials) to extend into the chamber. If rats failed to either nosepoke or lever press within 10 s, the trial was scored as an omission. Each block of trials commenced with eight forced choice trials, which served to remind rats of the risk contingencies in effect for that block, and ended with 10 free choice trials. A press on the small “safe” lever (left or right; counterbalanced across groups) delivered a small food reward (one food pellet) whereas a press on the large “risky” lever delivered a large food reward (two food pellets) and was accompanied by increasing probabilities of a 1 s foot shock (Experiment 1: pre-cocaine: 0.25 mA, post-cocaine: 0.45 mA; Experiment 2: pre-cocaine: male: 0.50 mA, female: 0.25 mA; post-cocaine: male: 0.3 mA, female: 0.10 mA; Experiment 3: male: 0.20 mA, female: 0.15 mA; Experiment 4: male: 0.40 mA, female: 0.125 mA). Because preference for the large, risky reward in females tends to be more sensitive to shock than in males (Orsini et al., 2016), lower shock intensities were used in females than in males in order to bring the sexes into more comparable positions in the parametric space. The probability of shock delivery accompanying the large reward increased across the blocks of trials in each session (0, 25, 50, 75, and 100%). Despite the probabilistic delivery of footshock, the large reward was always delivered after each press of the large, risky reward lever. During forced choice trials, the probability of shock following a press on the large “risky” lever was dependent across the four trials in each block. In contrast, the shock probability in the free choice trials was independent of other trials in that block. Rats were trained on the RDT until stable performance was achieved (see Data Analysis below for description of stability).
Surgical procedures
Irrespective of whether they were in the sucrose or cocaine self-administration group, all rats in Experiments 2 and 3 underwent jugular catheter surgery. Rats were anesthetized with isoflurane gas (1-5% in O2) and given subcutaneous injections of meloxicam (2 mg/kg), buprenorphine (0.03 mg/kg) and warm sterile saline (10 mL). The hair on the back between the shoulder blades and on the right side of the chest was clipped and the underlying skin was disinfected with chlorohexidine. Using aseptic surgical techniques, incisions were made between the shoulder blades and the right side of the chest above the jugular vein. The tissue above the jugular vein was dissected until the jugular vein was exposed. Once exposed, the jugular vein was ligated at its most anterior point. A small incision in the vein was made caudal to the ligation and a catheter (Instech Laboratories) was inserted and fed into the length of the vein. The catheter was tied in place using suture. The rest of the catheter was threaded subcutaneously from the right chest over the right shoulder and attached to a port (Instech Laboratories) placed under the skin between the shoulder blades. The port was sutured into the muscle and the skin incision was closed with suture around the port. A protective aluminum cap was placed on the port to prevent accumulation of debris on the port. Rats recovered in their home cages for 7 days, after which behavioral shaping began. Sutures were removed 10-14 days after surgery. The catheters were flushed daily with 0.1 mL cefazolin (30 mg/kg) and locked with heparinized glycerol (40 U/mL heparin in 50:50 glycerol: 0.9% sterile saline) throughout the duration of self-administration. Patency of the catheters was tested with an intravenous infusion of 0.1 mL propofol before self-administration commenced and once a week thereafter until the end of self-administration. Rats were removed from the study if their catheter lost patency before or during cocaine self-administration.
Self-administration Procedures
Rats went through several stages of shaping prior to the start of cocaine self-administration. The first phase of shaping was magazine training, in which rats learned to associate the liquid trough with the delivery of a 20% sucrose reward (40 uL, randomly delivered during the session). To proceed to the next phase of shaping, rats had to reach a criterion of 100 nosepokes in the trough within a 64 min session. Rats were then trained to nosepoke into the “active” (illuminated) nosepoke port (left or right, counterbalanced across rats) to receive the same sucrose reward. To reach criterion, rats had to nosepoke at least 50 times in the active port within a 30-min session. Rats then underwent shaping sessions for cocaine self-administration, in which a nosepoke into the active port resulted in an intravenous infusion of cocaine HCl (NIDA Drug Supply Program, 1.0 mg/kg/infusion dissolved in 0.9% sterile saline). Cocaine HCl (0.16 mL) was infused over 6 s, and each infusion was followed by a 20-s timeout period in which active nosepokes had no programmed consequence. Concomitant with cocaine infusions, an auditory tone was presented and remained on during the entire infusion. Cocaine self-administration shaping sessions continued until rats reached a criterion of 20 infusions in a 2-h session. Upon reaching criterion, rats progressed to long-access self-administration sessions (6 h per day for 14 consecutive days). During both shaping and long-access self-administration procedures, each rat in the sucrose control group was paired to a rat in the cocaine self-administration group and allowed the same number of opportunities to receive the sucrose reward as the number of cocaine infusions the paired cocaine rat had earned. This design ensured an equivalent number of reinforcer deliveries in the sucrose control and cocaine groups, and controlled for aspects of the procedure that were not specific to cocaine (e.g., being tethered).
Progressive Ratio Task
Rats were placed in the same operant chambers used for the RDT with a single lever (the same lever used as the small, “safe” reward lever in the RDT) extended. In each session, the number of responses required to earn one food pellet began with one, and increased in a geometric sequence until the rats reached their “breakpoint”, or the point at which rats ceased lever pressing (Garman, Setlow, & Orsini, 2021; Hernandez et al., 2017; Orsini et al., 2021). The number of lever presses, number of rewards earned, and the ratio at which rats ceased lever pressing (i.e., breakpoint) were the primary measures of interest. Rats were tested on this task for six sessions, and their data over the six sessions were averaged and analyzed as described below.
Shock Reactivity Threshold Testing
The purpose of shock reactivity threshold testing was to determine the lowest shock intensity at which rats would emit a motor response to the shock. In this assay, rats were placed in a novel operant chamber and an initial 0.4 mA shock was delivered to reduce overall movement and facilitate detection of subsequent shock-induced responses. Subsequent shocks (1 s) were delivered every 10 s, starting at 0.05 mA and increasing by 0.025 mA until a motor response (a flinch, paw withdrawal, or startle response) was elicited. Upon observation of a motor response, the shock intensity was lowered by 0.025 mA, and if there was no motor response, it was increased again. This “up-and-down” method (Crocker & Russell, 1984) continued in this manner until at least three shock thresholds (or the shock intensities at which motor responses were elicited) were observed. These values were averaged and submitted to data analysis (Orsini et al., 2021).
Systemic drug administration
Rats were weighed daily and given daily (between 1000 and 1200) intraperitoneal injections of cocaine HCl (30 mg/kg injected at a volume of 1.5 ml/kg) or 0.9% sterile saline (vehicle) over 14 consecutive days. Outside of when injections occurred, rats remained undisturbed in their home cages with free access to food and water.
Data Analysis
Data files were exported from Graphic State 3 (Experiment 1) and processed using custom Microsoft Excel macros (Dr. Jonathan Lifshitz, University of Kentucky) or analyzed using custom Graphic State 4 analysis templates (Experiments 2-4). Statistical analyses were performed using SPSS 24 and 25. Graphs were created using GraphPad Prism 7. For the RDT, the primary measure of interest was the percentage of free choice trials in each block on which rats chose the large, “risky” lever. Stable choice performance on the RDT was determined using a two-factor repeated-measures ANOVA conducted on data across three consecutive sessions, with both session and trial block (risk of punishment) as within-subjects factors. Stability was defined as the absence of both a main effect of session and a session X trial block interaction. Differences in RDT performance between cocaine and control (saline or sucrose) groups were evaluated using two-factor repeated-measures ANOVA conducted on data averaged across stable sessions of performance, with group as a between-subjects factor and trial block as a within-subjects factor. In Experiments 2 and 4, sex was also included as a between-subjects factor. Although Experiment 3 also included both males and females, significant attrition in females precluded the ability to conduct comparisons between sexes with sufficient statistical power. Hence, in all analyses for Experiment 3 (i.e., latency analyses, locomotor activity, etc.), the data for males and females were merged. In Experiments 1 and 2 (in which rats were trained on the RDT prior to cocaine), timepoint (pre vs. post drug administration) was included as an additional within-subjects factor. For Experiments 1 and 2, latencies to press the small, safe lever and large, risky lever were analyzed using a four-factor repeated-measures ANOVA, with group as a between-subjects factor and timepoint (pre vs. post drug administration), lever identity (small, safe vs. large, risky) and trial block as within-subjects factors. In Experiment 2, sex was also included as a between-subjects factor. In Experiments 3 and 4, latency data were analyzed using a three-factor repeated-measures ANOVA, with group as a between-subjects factor and lever identity and trial block as within-subjects factors. In Experiment 4, sex was included as an additional between-subjects factor.
To determine whether cocaine exposure altered the extent to which the outcome of a previous trial (large, unpunished reward vs. large, punished reward) influenced subsequent choice (safe vs. risky option), additional trial-by-trial analyses were conducted. Win-stay behavior, or the likelihood of choosing the large, risky option after receipt of the large, unpunished reward, was derived by dividing the number of trials on which a rat chose the large, risky option after receiving a large, unpunished reward by the total number of free choice trials on which the rat received the large, unpunished reward. Lose-shift behavior, or the likelihood of choosing the small, safe option after receipt of the large, punished reward, was derived by dividing the number of trials on which a rat chose the small, safe option after receiving a large, punished reward by the total number of free choice trials on which the rat received the large, punished reward. Once these values were quantified for each group, they were analyzed with a two-way ANOVA, with group (Experiments 3 and 4) and sex (Experiment 4) as between-subjects factors.
Ancillary measures, such as percentage of omissions during free choice blocks, shock reactivity (locomotor activity during shock delivery) and locomotor activity, were analyzed using multifactor ANOVA, with timepoint (Experiments 1 and 2) as a within-subject factor and group (Experiments 1-4) and sex (Experiments 2 and 4) as between-subjects factors. Additional independent samples t-tests were employed to detect group and/or sex differences in these measures during performance in the RDT after drug administration.
Finally, shock reactivity threshold values were analyzed using a two-factor ANOVA, with group (Experiments 2-4) and sex (Experiments 2 and 4) as between-subjects factors. In Experiment 3, time (pre- vs. post-cocaine self-administration) was also included in the analysis as a within-subjects factor. A similar analysis was used to analyze PR measures, with group (Experiments 2-4) and sex (Experiments 2 and 4) serving as between-subjects factors.
Transparency and openness
Using G*Power software, a power analysis was conducted a priori to determine sample sizes required to detect significant group differences with effect sizes of ≥ 0.8, assuming an α of 0.05. Sample sizes were increased to account for attrition during the course of the experiments. Data points for any dependent variable were considered to be outliers if they fell above the third quartile or below the first quartile by 1.5 interquartiles. Using these criteria, self-administration data from three rats in the cocaine group (n=2, Experiment 2; n=1, Experiment 3) were removed from analyses of self-administration behavior (but not from analyses of performance in the RDT). All data are available upon request from the corresponding author. This study’s design and analyses were not pre-registered.
Results
In the following section, noteworthy (whether statistically significant or not) effects on behavior are described. In all analyses, there was a main effect of trial block (ps < 0.05); this statistic will therefore not be reported for each analysis. A complete exposition of all statistical results is presented in Table 1.
Table 1.
Effects of cocaine administration on ancillary behavioral measures: additional statistical results.
| Variable | Factor(s) | F- or t-value | p-value |
|---|---|---|---|
| Experiment 1 | |||
| Latency | |||
| Pre- vs. post-injections | Timepoint X trial block | F(4,76)=0.58 | 0.68 |
| Timepoint X group | F(1,19)=1.58 | 0.22 | |
| Timepoint X lever identity X trial block | F(4,76)=1.42 | 0.23 | |
| Timepoint X lever identity X group | F(1,19)=0.63 | 0.44 | |
| Lever identity | F(1,19)=5.47 | 0.03 | |
| Lever identity X trial block | F(4,76)=9.37 | <0.01 | |
| Lever identity X group | F(1,19)=0.09 | 0.77 | |
| Lever identity X group X trial block | F(4,76)=1.64 | 0.17 | |
| Group X trial block | F(4,76)=1.04 | 0.84 | |
| Post-injections | Lever identity | F(1,19)=9.83 | <0.01 |
| Lever identity X trial block | F(4,76)=6.93 | <0.01 | |
| Group X trial block | F(4,76)=2.19 | 0.08 | |
| Experiment 2 | |||
| Latency | |||
| Pre- vs. post-SA | Timepoint X trial block | F(4,132)=2.13 | 0.08 |
| Timepoint X lever identity X trial block | F(4,132)=1.94 | 0.11 | |
| Time X sex | F(1,33)=0.45 | 0.62 | |
| Time X sex X group | F(1,33)=2.13 | 0.15 | |
| Time X lever identity X sex | F(1,33)=0.19 | 0.67 | |
| Time X lever identity X sex X group | F(1,33)=2.97 | 0.09 | |
| Time X sex X trial block | F(4,132)=0.60 | 0.67 | |
| Post-SA | Sex X lever identify | F(1,33)=1.65 | 0.21 |
| Lever identity X group | F(1,33)=1.01 | 0.32 | |
| Lever identity X sex X group | F(1,33)=0.11 | 0.74 | |
| Lever identity X sex X trial block | F(4,132)=0.87 | 0.48 | |
| Lever identity X group X trial block | F(4,132)=1.59 | 0.18 | |
| Lever identity X sex X group X trial block | F(4,132)=1.63 | 0.17 | |
| Locomotor activity | |||
| Pre- vs. post-SA | Group | F(1,54)=2.12 | 0.15 |
| Sex | F(1,54)=8.61 | <0.01 | |
| Group X sex | F(1,54)=0.10 | 0.75 | |
| Post-SA | Group X sex | F(1,54)=0.19 | 0.67 |
| Shock reactivity | |||
| Pre- vs. post-SA | Group | F(1,23)<0.01 | 0.97 |
| Sex | F(1,23)=3.29 | 0.08 | |
| Group X sex | F(1,23)=3.77 | 0.06 | |
| Post-SA | Group X sex | F(1,23)=1.61 | 0.22 |
| Omissions | |||
| Pre- vs. post-SA | Group | F(1,56)=0.19 | 0.67 |
| Sex | F(1,56)=23.59 | <0.01 | |
| Group X Sex | F(1,56)=0.09 | 0.77 | |
| Post-SA | Group X Sex | F(1,56)=0.07 | 0.80 |
| Experiment 3 | |||
| Latency | Lever identity | F(1,19)=0.41 | 0.53 |
| Group X trial block | F(4,76)=0.15 | 0.96 | |
| Lever identity X trial block | F(4,76)=6.45 | <0.01 | |
| Locomotor activity | |||
| Group | t(19)=0.85 | 0.41 | |
| Shock reactivity | |||
| Group | t(19)=0.56 | 0.58 | |
| Omissions | |||
| Group | t(19)−1.49 | 0.15 | |
| Experiment 4 | |||
| Latency | |||
| Lever identity | F(1,20)=0.71 | 0.41 | |
| Lever identity X trial block | F(4,80)=10.80 | <0.01 | |
| Group X trial block | F(4,80)=1.16 | 0.34 | |
| Sex X trial block | F(4,80)=1.40 | 0.24 | |
| Group X sex X trial block | F(4,80)=2.01 | 0.10 | |
| Locomotor activity | |||
| Group | F(1,20)=0.04 | 0.84 | |
| Sex | F(1,20)=3.13 | 0.09 | |
| Group x sex | F(1,20)=2.82 | 0.11 | |
| Shock reactivity | |||
| Group | F(1,20)=0.86 | 0.37 | |
| Sex | F(1,20)=1.77 | 0.20 | |
| Group X sex | F(1,20)=1.62 | 0.22 | |
| Omissions | |||
| Group | F(1,20)=0.78 | 0.39 | |
| Sex | F(1,20)=6.87 | 0.02 |
Experiment 1: Effects of passively-administered cocaine on risk taking in male rats
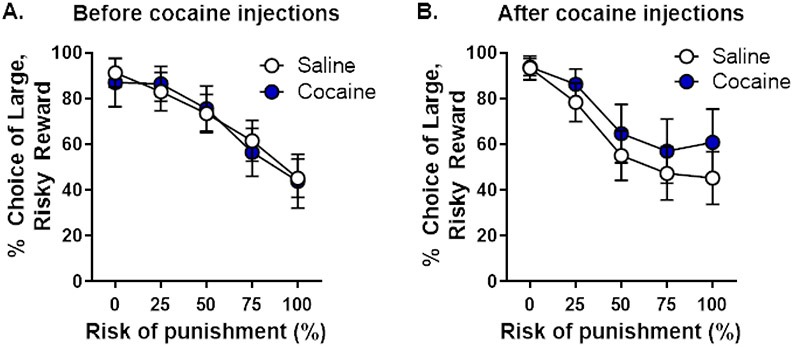
Rats began training in the RDT on PN day 70 until stability emerged on PN day 98. Beginning on PN day 105 (later adulthood), rats received systemic injections of cocaine or saline for 14 days. Three weeks after cessation of systemic injections, rats were tested in the RDT until stability re-emerged (PN day 189; Figure 1). There were no main effects of time [F (1, 21) = 0.05, p = 0.82] or group [F (1, 21) = 0.17, p = 0.68] nor were there significant time X group [F (1, 21) = 0.35, p = 0.56], time X trial block [F (4, 84) = 2.31, p = 0.07] or time X group X trial block [F (4, 84) = 0.25, p = 0.91] interactions. Similarly, there was no difference in risky choice between groups during stable performance in the RDT after cocaine/saline administration [group, F (1, 21) = 0.44, p = 0.52; group X trial block, F (4, 84) = 0.30, p = 0.88]. These data suggest that passive exposure to cocaine does not alter risky choice in male rats.
Figure 1. Performance in the Risky Decision-making Task before and after passively-administered cocaine injections (Experiment 1).
A. There were no differences in choice of the large risky reward between rats that would go on to receive cocaine or saline injections after pre-training in the Risky Decision-making Task (RDT). B. When tested in the RDT after drug injections, there were no differences in choice of the large, risky reward between rats that received injections of cocaine or saline. Data are represented as mean percent choice of the large, risky reward ± standard error of the mean.
A four-factor repeated-measures ANOVA (time X lever identity X group X trial block) was used to compare latencies to press levers during the RDT before and after cocaine or saline administration (Table 2). Despite the absence of main effects of time [F (1, 19) = 1.01, p = 0.33] and group [F (1, 19) = 0.06, p = 0.81], there was a significant time X group X trial block interaction [F (4, 76) = 2.56, p = 0.05], which manifested as a general increase in latencies to press levers in the saline group as probabilities of risk ascended within the test session. There was, however, no significant time X lever identity X group X trial block interaction [F (4, 76) = 2.22, p = 0.08]. Finally, there were no differences in latencies to press either lever between groups during stable performance in the RDT after drug administration [group, F (1, 19) = 0.82, p = 0.48; group X lever identity, F (1, 19) = 0.37, p = 0.55; group X lever identity X trial block, F (4, 76) = 1.90, p = 0.12]. Consistent with the lack of effects on choice behavior, these results indicate no lasting impact on latencies to press either the small, safe or large, risky lever.
Table 2.
Mean (± standard error of the mean) latencies to press levers.
| Block 1 | Block 2 | Block 3 | Block 4 | Block 5 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Sm | Lg | Sm | Lg | Sm | Lg | Sm | Lg | Sm | Lg | |
| Experiment 1 | ||||||||||
| Pre-RDT | ||||||||||
| Cocaine | 1.21 (0.14) |
0.72 (0.06) |
1.34 (0.16) |
1.00 (0.15) |
1.44 (0.13) |
1.50 (0.33) |
1.68 (0.24) |
2.53 (0.56) |
1.98 (0.38) |
3.12 (0.86) |
| Saline | 1.20 (0.07) |
0.69 (0.04) |
1.31 (0.12) |
0.91 (0.06) |
1.54 (0.16) |
1.42 (0.17) |
1.70 (0.26) |
1.99 (0.19) |
1.68 (0.22) |
2.84 (0.39) |
| Post-RDT | ||||||||||
| Cocaine | 0.97 (0.10) |
1.40 (0.73) |
1.36 (0.19) |
1.01 (0.11) |
1.24 (0.12) |
1.96 (0.39) |
1.27 (0.13) |
2.48 (0.55) |
1.60 (0.17) |
2.68 (0.80) |
| Saline | 1.04 (0.07) |
0.72 (0.04) |
1.18 (0.10) |
0.98 (0.06) |
1.42 (0.17) |
2.26 (0.26) |
1.51 (0.19) |
3.24 (0.55) |
1.58 (0.026) |
4.02 (0.62) |
| Experiment 2 | ||||||||||
| Pre-RDT | ||||||||||
| Cocaine | ||||||||||
| Male | 0.80 (0.05) |
0.76 (0.05) |
0.81 (0.05) |
0.83 (0.06) |
0.76 (0.05) |
2.95 (0.64) |
0.77 (0.06) |
2.82 (0.43) |
0.79 (0.06) |
3.91 (0.69) |
| Female | 1.26 (0.11) |
1.11 (0.12) |
1.23 (0.11) |
1.52 (0.15) |
1.23 (0.12) |
2.63 (0.42) |
1.20 (0.11) |
2.72 (0.45) |
1.20 (0.11) |
2.89 (0.36) |
| Sucrose | ||||||||||
| Male | 0.77 (0.04) |
0.76 (0.11) |
0.78 (0.06) |
1.07 (0.12) |
0.69 (0.04) |
2.17 (0.42) |
0.76 (0.06) |
4.11 (0.90) |
0.71 (0.05) |
4.09 (0.86) |
| Female | 1.34 (0.15) |
1.07 (0.13) |
1.27 (0.08) |
1.35 (0.17) |
1.15 (0.08) |
2.18 (0.40) |
1.39 (0.20) |
3.01 (0.45) |
1.33 (0.14) |
3.17 (0.49) |
| Post-RDT | ||||||||||
| Cocaine | ||||||||||
| Male | 0.75 (0.05) |
0.70 (0.05) |
0.83 (0.06) |
1.64 (0.24) |
0.77 (0.07) |
4.56 (1.05) |
0.73 (0.06) |
4.85 (0.96) |
0.76 (0.06) |
4.84 (1.08) |
| Female | 1.12 (0.11) |
1.07 (0.09) |
1.17 (0.09) |
1.95 (0.24) |
1.26 (0.13) |
3.39 (0.47) |
1.40 (0.17) |
3.87 (0.46) |
1.17 (0.10) |
4.37 (0.49) |
| Sucrose | ||||||||||
| Male | 0.70 (0.05) |
0.71 (0.07) |
0.72 (0.06) |
1.35 (0.28) |
0.73 (0.08) |
1.78 (0.54) |
0.72 (0.09) |
5.00 (0.98) |
0.76 (0.13) |
4.20 (0.92) |
| Female | 1.11 (0.08) |
0.96 (0.12) |
1.10 (0.08) |
1.90 (0.26) |
1.13 (0.12) |
3.34 (0.64) |
1.19 (0.11) |
3.75 (0.73) |
1.26 (0.12) |
4.29 (0.62) |
| Experiment 3 | ||||||||||
| Cocaine | 1.12 (0.09) |
0.70 (0.05) |
1.42 (0.16) |
0.94 (0.07) |
1.44 (0.19) |
1.19 (0.12) |
1.51 (0.20) |
1.41 (0.16) |
1.73 (0.24) |
1.56 (0.28) |
| Sucrose | 0.98 (0.09) |
0.80 (0.06) |
1.05 (0.09) |
1.01 (0.12) |
0.93 (0.08) |
1.46 (0.30) |
0.88 (0.09) |
1.90 (0.47) |
0.92 (0.09) |
2.15 (0.54) |
| Experiment 4 | ||||||||||
| Cocaine | ||||||||||
| Male | 0.98 (0.08) |
0.60 (0.04) |
0.99 (0.15) |
0.72 (0.12) |
0.97 (0.13) |
0.86 (0.12) |
0.93 (0.12) |
1.10 (0.33) |
1.16 (0.21) |
1.59 (0.59) |
| Female | 1.32 (0.09) |
0.71 (0.05) |
1.47 (0.08) |
0.83 (0.07) |
1.67 (0.15) |
1.06 (0.16) |
1.80 (0.12) |
1.13 (0.16) |
1.83 (0.16) |
1.22 (0.28) |
| Saline | ||||||||||
| Male | 0.88 (0.06) |
0.59 (0.11) |
0.76 (0.07) |
1.15 (0.37) |
0.75 (0.12) |
2.53 (1.02) |
0.77 (0.15) |
3.10 (1.14) |
0.74 (0.11) |
3.23 (0.75) |
| Female | 1.83 (0.21) |
1.33 (0.35) |
1.63 (0.20) |
0.99 (0.14) |
1.46 (0.13) |
1.39 (0.14) |
1.64 (0.14) |
2.05 (0.27) |
1.49 (0.31) |
2.02 (0.43) |
Exp, Experiment; Sm, small; Lg, Large; RDT, Risky Decision-making Task.
There were no changes in overall locomotor activity [time, F (1, 21) = 0.24, p = 0.63; group, F (1, 21) = 0.04, p = 0.85; time X group, F (1, 21) = 1.35, p = 0.26] or locomotor activity during shock delivery [time, F (1, 19) = 0.26, p = 0.61; group, F (1, 19) = 0.45, p = 0.51; time X group, F (1, 19) = 0.44, p = 0.52] following cocaine or saline administration (Table 3). Similarly, there were no group differences in overall locomotor activity [t (21) = 0.64, p = 0.53] or locomotor activity during shock delivery [t (21) = −0.43, p = 0.67] during performance in the RDT after the cocaine or saline injections. Finally, although there was no main effect of time [F (1, 21) < 0.05, p = 0.95] or group [F (1, 21) = 0.07, p = 0.36] on percentage of omitted free choice trials, there was a significant time X group interaction [F (1, 21) = 5.53, p = 0.03]. Subsequent post-hoc analyses revealed that this significant interaction was driven by a decrease in omissions between pre- and post-injection timepoints only in the saline group [t (14) = 2.29, p = 0.04]. Importantly, however, there were no group differences in omissions during RDT performance post-injections [t (21) = 0.94, p = 0.36].
Table 3.
Mean (± standard error of the mean) locomotor activity and omissions.
| Locomotor activity (locomotor units/ITI) |
Shock reactivity (locomotor units/shock) |
Omissions | |
|---|---|---|---|
| Experiment 1 | |||
| Pre-RDT | |||
| Cocaine | 50.78 (7.30) | 9.96 (1.97) | |
| Saline | 63.46 (10.02) | 13.61 (1.91) | |
| Post-RDT | |||
| Cocaine | 56.89 (11.47) | 11.82 (2.44) | 15.54 (5.25) |
| Saline | 48.31 (7.66) | 11.64 (1.70) | 11.16 (1.98) |
| Experiment 2 | |||
| Pre-RDT | |||
| Cocaine | |||
| Male | 56.70 (8.96) | 2.96 (0.32) | 0.72 (0.31) |
| Female | 28.72 (3.82) | 1.94 (0.36) | 6.03 (1.75) |
| Sucrose | |||
| Male | 52.66 (9.93) | 3.04 (0.43) | 1.15 (0.66) |
| Female | 25.04 (3.67) | 2.28 (0.31) | 9.53 (2.44) |
| Post-RDT | |||
| Cocaine | |||
| Male | 46.91 (6.88) | 3.91 (0.70) | 0.61 (0.31) |
| Female | 29.23 (3.53) | 2.30 (0.46) | 10.80 (2.49) |
| Sucrose | |||
| Male | 40.85 (6.72) | 3.63 (0.75) | 0.67 (0.20) |
| Female | 25.91 (3.64) | 2.92 (0.27) | 9.76 (2.01) |
| Experiment 3 | |||
| Cocaine | 44.30 (8.84) | 2.52 (0.41) | 0.55 (0.28) |
| Sucrose | 33.30 (9.49) | 2.20 (0.39) | 2.47 (1.32) |
| Experiment 4 | |||
| Cocaine | |||
| Male | 24.04 (8.91) | 1.36 (0.44) | 8.22 (4.58) |
| Female | 23.42 (5.25) | 1.33 (0.42) | 8.78 (3.58) |
| Saline | |||
| Male | 37.11 (6.87) | 2.37 (0.53) | 0.89 (0.37) |
| Female | 13.16 (6.20) | 1.17 (0.43) | 24.11 (6.95) |
Exp, Experiment; RDT, Risky Decision-making Task
In summary, in contrast to our previous work (Mitchell, Weiss, Beas, et al., 2014), passively-administered cocaine in male rats did not selectively affect risky choice. Passive administration of cocaine also had no effect on latencies to press levers, locomotor activity or trial omissions.
Experiment 2: Effects of cocaine self-administration on risk taking in male and female rats
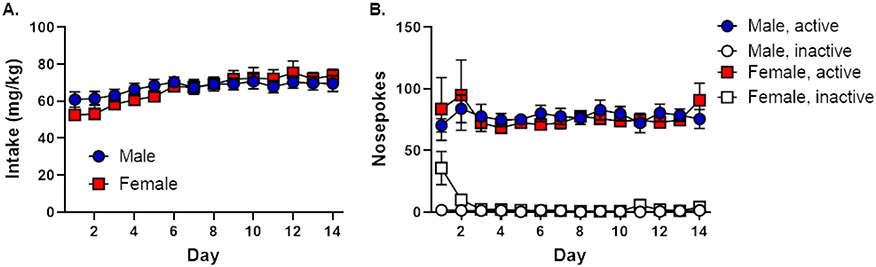
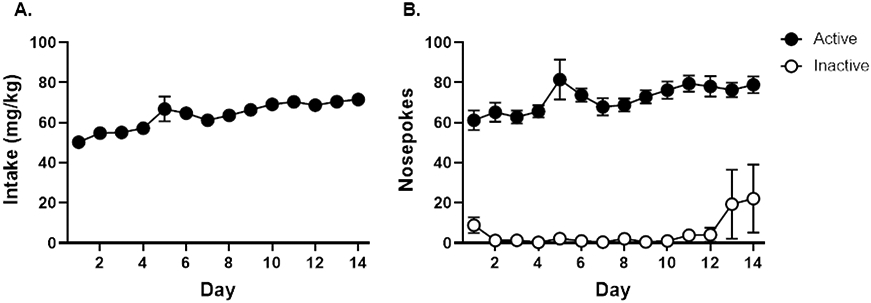
Beginning on PN day 70, rats were trained on the RDT until stability emerged at PN day 140. Rats subsequently underwent cocaine self-administration on PN day 161 (later adulthood). Across the 14 days of cocaine self-administration, male and female rats gradually increased their cocaine intake in a comparable manner [day, F (13, 390) = 7.48, p < 0.01; sex, F(1, 30) < 0.01, p = 0.94; day X sex, F (13, 390) = 1.48, p = 0.12; Figure 2A]. Both males and females demonstrated a similarly strong preference for the active nosepoke [nosepoke, F (1, 26) = 6.49, p = 0.02; sex, F (1, 26) = 0.69, p = 0.41; nosepoke X sex, F (1, 26) = 0.66, p = 0.42] that remained stable across self-administration sessions [day, F (13, 338) = 0.58, p = 0.87; day X sex, F (13, 338) = 0.58, p = 0.45; day X nosepoke, F (13, 338) = 0.67, p = 0.79; day X sex X nosepoke, F (13, 338) = 0.58, p = 0.87; Figure 2B]. Upon inspection of the data, two female cocaine rats were identified as outliers and removed from the analyses of self-administration data as they registered upwards of 4,500 nosepokes in the inactive nosepoke port, possibly reflecting cocaine-induced motor stereotypy. There were no group differences in nosepoke preference [nosepoke X group, F (1, 42) = 0.01, p = 0.91; nosepoke X group X sex, F (1, 42) = 0.38, p = 0.54] as rats in the sucrose group also displayed preference for the active over the inactive nosepoke [nosepoke, F (1, 16) = 175.69, p < 0.01; sex, F (1, 16) = 0.04, p = 0.84; nosepoke X sex, F (1, 16) < 0.01, p = 0.96].
Figure 2. Cocaine self-administration in males and females pre-trained in the Risky Decision-making Task (Experiment 2).
A. Both males and females gradually increased their cocaine intake across 14 days of self-administration. B. Both males and females demonstrated a preference for the active nosepoke over the inactive nosepoke throughout 14 days of self-administration. Data are represented as mean ± standard error of the mean.
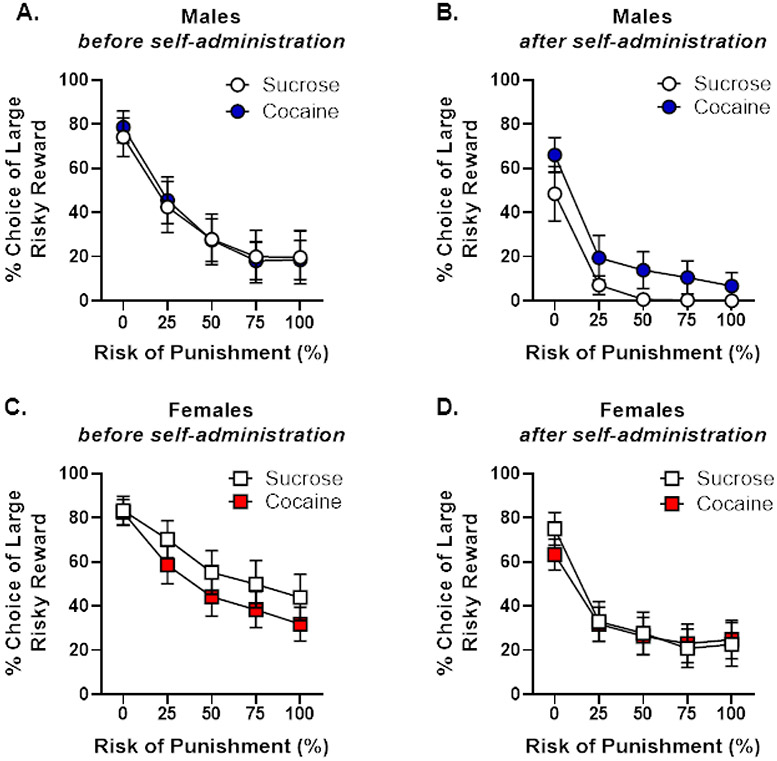
Three weeks after cocaine self-administration (PN day 196), rats were re-tested in the RDT until performance re-stabilized (PN day 231). Comparisons of RDT performance before and after cocaine or sucrose self-administration revealed neither a main effect of group [F (1, 56) < 0.01, p = 0.96] nor a significant interaction between group and trial block [F (4, 224) = 0.12, p = 0.96], although comparison of performance before and after self-administration revealed a main effect of time [F (1, 56) = 41.40, p < 0.01] and a significant interaction between time and trial block [F (4, 224) = 5.80, p < 0.01], manifesting as an overall decrease in risky choice (Figure 3). This pattern of behavior did not differ between the cocaine and sucrose groups [time X group, F (1, 56) = 2.16, p = 0.15; time X group X trial block, F (4, 224) = 0.74, p = 0.57] nor was it different between males and females [time X sex, F (1, 56) = 0.03, p = 0.87; time X group X sex, F (1, 56) = 0.09, p = 0.77; time X sex X trial block, F (4, 224) = 0.89, p = 0.47; time X sex X group X trial block, F (4, 224) = 1.17, p = 0.33]. There was also a main effect of sex whereby male rats were significantly more risk averse than females [F (1, 56) = 5.83, p = 0.02]. This greater risk aversion in males, however, did not differ between groups [group X sex, F (1, 56) = 0.66, p = 0.42; group X sex X trial block, F (4, 224) = 0.28, p = 0.89; Figure 3]. Finally, a three-factor repeated-measures ANOVA was employed to assess group differences during RDT performance after self-administration (Figures 3B, D). There was no main effect of group [F (1, 56) = 0.44, p = 0.51] nor were there group X trial block [F (1, 56) = 0.08, p = 0.99] or group X sex X trial block [F (1, 56) = 1.00, p = 0.41] interactions. Males were significantly more risk averse than females [F (1, 56) = 5.33, p = 0.03], irrespective of group [sex X group, F (1, 56) = 0.85, p = 0.36]. Considered together, these analyses showed that, in contrast to previously published work (Mitchell, Weiss, Beas, et al., 2014), cocaine self-administration did not increase risky choice in either males or females.
Figure 3. Performance in the Risky Decision-making task before and after cocaine self-administration (Experiment 2).
A. There were no differences in choice of the large, risky reward between male rats that would go on to self-administer cocaine or sucrose after training in the Risky Decision-making Task (RDT). B. When tested in the RDT after self-administration, there were no differences in choice of the large, risky reward between male rats that self-administered cocaine or sucrose. C. There were no differences in choice of the large, risky reward between female rats that would go onto self-administer cocaine or sucrose after training in the RDT. D. When tested in the RDT after self-administration, there were no differences in choice of the large, risky reward between female rats that self-administered cocaine or sucrose. Data are represented as mean percent choice of the large, risky reward ± standard error of the mean.
Using a five-factor repeated-measures ANOVA (time X lever identity X sex X group X trial block), latencies to press levers (small, safe vs. large, risky) were compared before and after self-administration (Table 2). Consistent with previous work (Shimp, Mitchell, Beas, Bizon, & Setlow, 2015), there was a main effect of lever identity [F (1, 33) = 44.37, p < 0.01] and a significant lever identity X trial block interaction [F (4, 132) = 54.15, p < 0.01] such that, relative to latencies to press the small safe lever, latencies to press the large, risky lever increased as risk of punishment increased. There was also a main effect of time [F (1, 33) = 4.69, p = 0.04] and a significant time X lever identity interaction [F (1, 33) = 9.10, p < 0.01]. Although there were no main effects of sex [F (1, 33) = 0.11, p = 0.74] or group [F (1, 33) = 0.12, p = 0.73] nor a sex X group interaction [F (1, 33) = 0.36, p = 0.56], there were significant time X group [F (1, 33) = 4.09, p = 0.05], time X group X trial block [F (4, 132) = 2.43, p = 0.05], time X group X lever identity X trial block [F (4, 132) = 2.49, p = 0.05] and time X group X lever identity X sex X trial block [F (4, 132) = 2.78, p = 0.03] interactions. To isolate the source of these significant interactions, additional three-factor repeated-measures ANOVAs were conducted separately for each group collapsed across sex. These analyses revealed that latencies to press the large, risky lever increased after self-administration, but only in rats that underwent cocaine self-administration [cocaine: time, F (1, 23) = 11.61, p < 0.01; time X lever identity, F (1, 23) = 19.14, p < 0.01; time X lever identity X trial block, F (4, 88) = 6.16, p < 0.01; sucrose: time, F (1, 12) = 1.09, p = 0.32; time X lever identity, F (1, 12) = 2.30, p = 0.16; time X lever identity X trial block, F (4, 92) = 6.21, p < 0.05]. This effect of cocaine on latency to press the large, risky lever appeared in both sexes [male: time X lever identity, F (1, 6) = 6.39, p = 0.05; time X lever identity X trial block, F (4, 24) = 2.52, p = 0.07; female: time X lever identity, F (1, 16) = 13.38, p < 0.01; time X lever identity X trial block, F (4, 64) = 4.75, p < 0.01]. Additional analyses were employed to compare latencies to press levers between groups during stable performance after self-administration. These analyses revealed no main effects of sex [F (1, 33) = 0.26, p = 0.61] or group [F (1, 33) = 1.65, p = 0.21] and no significant interactions between any of the variables (see Table 1).
Supplementary analyses revealed that there was a decrease in locomotor activity after both cocaine and sucrose self-administration [time, F (1, 54) = 6.52, p = 0.01; time X group, F (1, 54) = 0.06, p = 0.81; time X group X sex, F (1, 54) = 0.06, p = 0.80; Table 3]. This reduction in locomotor activity, however, was specific to males [time X sex, F (1, 54) = 6.53, p = 0.01]. An analysis of locomotor activity during RDT performance after self-administration revealed that, although there were no differences between self-administration groups [F (1, 54) = 2.27, p = 0.31, males exhibited greater locomotor activity than females [F (1, 54) = 5.39, p = 0.02]. Similar analyses were used to compare locomotor activity during shock delivery (shock reactivity) before and after self-administration between groups. There was an overall increase in shock reactivity after self-administration in both groups [time, F (1, 23) = 5.72, p = 0.03, time X group, F (1, 23) = 0.68, p = 0.42], irrespective of sex [time X sex, F (1, 23) = 0.02, p = 0.88; time X group X sex, F (1, 23) = 1.00, p = 0.33]. In contrast to locomotor activity, there was no main effect of sex on shock reactivity during RDT performance [F (1, 23) = 2.66, p = 0.12]. There were also no differences in shock reactivity between self-administration groups [F (1, 23) = 0.14, p = 0.71]. Finally, a three-factor repeated-measures ANOVA was used to compare the percentage of omitted free choice trials before and after self-administration between groups. These analyses did not yield a main effect of time [F (1, 56) = 1.09, p = 0.30] nor any significant interactions [time X sex, F (1, 56) = 1.76, p = 0.19; time X group, F (1, 56) = 1.35, p = 0.25; time X sex X group, F (1, 56) = 0.97, p = 0.32]. When the analysis was constrained to omissions after self-administration, there was a main effect of sex [F (1, 56) = 21.91, p < 0.01] such that females omitted significantly more free choice trials than males. There was, however, no main effect of group [F (1, 56) = 0.06, p = 0.82], nor a significant interaction between sex and group [F (1, 56) = 0.07, p = 0.80].
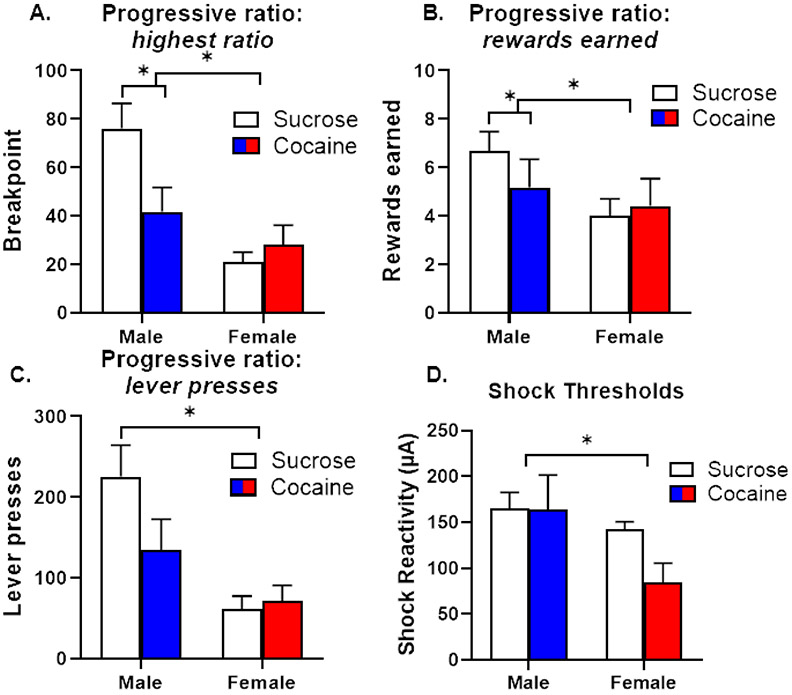
Following testing in the RDT, a subset of rats (cocaine: n=6 male, n=5 female; sucrose: n=6 male, n=5 female) was assessed on the progressive ratio task. There was a main effect of sex on all three PR dependent variables whereby males reached higher ratios [F (1, 18) = 14.58, p < 0.01; Figure 4A], earned more food rewards [F (1, 18) = 16.66, p < 0.01; Figure 4B] and lever pressed [F (1, 18) = 12.55, p < 0.01; Figure 4C] significantly more than females. Although there were no group differences in these measures [ratio, F (1, 18) = 2.29, p = 0.15; rewards earned, F (1, 18) = 1.71, p = 0.21; lever presses, F (1, 18) = 1.60, p = 0.22], there were significant sex X group interactions in two of these measures. Post-hoc analyses revealed that sucrose males reached a higher ratio [F (1, 10) = 5.62, p = 0.04] and earned more food rewards [F (1, 10) = 6.64, p = 0.03] than cocaine males; in contrast, there were no such differences between groups in females [ratio, F (1, 8) = 0.65, p = 0.45; rewards, F (1, 8) = 0.44, p = 0.52].
Figure 4. Performance on behavioral assays of food motivation and shock reactivity (Experiment 2).
A. Male rats that underwent sucrose self-administration reached a significantly higher breakpoint on a progressive ratio (PR) schedule of reinforcement than male rats that underwent cocaine self-administration. There were no differences in breakpoint between female self-administration groups. B. Male rats that underwent sucrose self-administration earned significantly more food rewards on the PR assay than male rats that underwent cocaine self-administration. There were no differences in the number of rewards earned between female self-administration groups. C. Male rats made significantly more lever presses for rewards than female rats. D. Shock thresholds were significantly higher in males than females, irrespective of self-administration group. Data are represented as mean ± standard error of the mean. Asterisks denote p < 0.05.
The analysis of locomotor activity during shock delivery in the RDT described above indicated that, although males displayed greater shock reactivity than females, there were no differences in reactivity between self-administration groups. To obtain a more precise and systematic measure of shock reactivity, the same subset of rats tested in the PR task was tested in a shock reactivity threshold assay. There was a main effect of sex such that males had a higher shock threshold than females [F (1, 18) = 12.72, p < 0.01; Figure 4D]. Although this appears to contradict the differences observed in shock reactivity during the RDT, these data may be a more accurate reflection of differences in shock reactivity; in contrast to the shock reactivity threshold assay in which rats’ motor responses were assessed across a range of identical shock intensities, the shock intensities used in the RDT differed between males and females. Despite these sex differences, there were no differences in shock thresholds between self-administration groups [F (1, 18) = 4.16, p = 0.06] nor was there a significant sex X group interaction [F (1, 18) = 3.95, p = 0.06].
Considered together, the data from Experiment 2 surprisingly showed that cocaine self-administration in male and female rats had no effect on risky choice. There were, however, more subtle effects of self-administration, and of cocaine in particular, on response latencies and food motivation. These findings run contrary to those from our previous work (Mitchell, Weiss, Beas, et al., 2014) in which cocaine self-administration in male rats led to a large increase in risk taking. One major distinction between the current experiment and our previous work, however, is the age at which rats underwent cocaine self-administration. In the Mitchell et al. study, rats underwent cocaine self-administration soon after they reached sexual maturity (i.e., young adulthood, PN day 75) whereas in the current experiment, rats underwent cocaine self-administration during later adulthood (PN day 161). Hence, one explanation for the discrepancy between these studies is that the different ages at which cocaine exposure occurred may have led to divergent effects of cocaine on risk taking.
Experiment 3: Effects of cocaine self-administration in young adult rats on risk taking
To evaluate the possibility raised by the results of Experiment 2 (i.e., whether there is a limited time window of susceptibility to cocaine’s effects on risk taking), rats in Experiment 3 did not undergo testing in the RDT prior to cocaine, but instead began with cocaine or sucrose self-administration on PN day 77 (young adulthood). Because of a high attrition rate (due to illness or loss of catheter patency), only six females (n=3 per group) remained at the completion of the experiment. Consequently, comparisons between males and females were not feasible due to insufficient statistical power, and data from rats of both sexes were merged.
During cocaine self-administration, there was a gradual increase in cocaine intake [F (13, 130) = 10.64, p < 0.01; Figure 5A]. Furthermore, rats exhibited a strong preference for the active nosepoke port over the inactive nosepoke port [nosepoke, F (1, 9) = 582.28, p < 0.01; day, F (13, 117) = 2.16, p = 0.02; nosepoke X day, F (13, 117) = 3.63, p < 0.01; Figure 5B]. Upon inspection of the data, one rat in the cocaine group was identified as an outlier, with over 200 nosepokes in the inactive nosepoke port. Similar to Experiment 2, such a high degree of nosepoking is likely a result of cocaine-induced motor stereotypy. Consequently, this rat was removed from the analyses of self-administration data.
Figure 5. Cocaine self-administration in rats prior to training in the Risky Decision-making Task (Experiment 3).
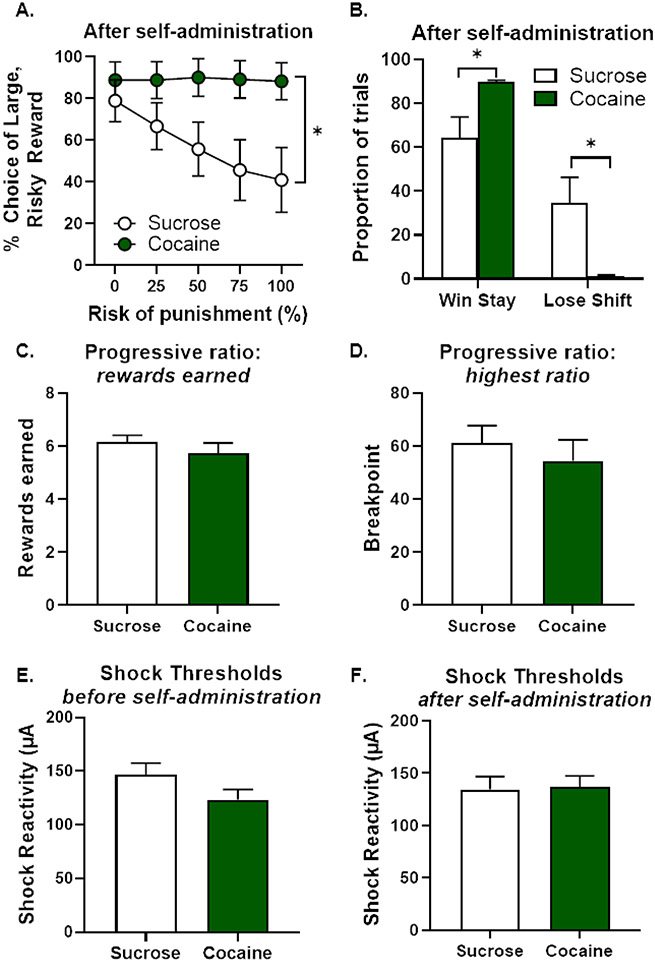
A. Rats gradually increased their cocaine intake across 14 days of self-administration. B. Rats displayed a greater preference for the active nosepoke over the inactive nosepoke across 14 days of self-administration. Data are represented as mean ± standard error of the mean. Asterisks denote p < 0.05.
Rats began training in the RDT on PN day 112 until stability emerged on PN day 154. Rats that underwent cocaine self-administration exhibited significantly greater risky choice compared to rats that underwent sucrose self-administration [group, F (1, 19) = 4.55, p = 0.05; group X trial block, F (4, 76) = 8.04, p < 0.01; Figure 6A]. Consistent with these results, rats in the cocaine group displayed greater win-stay behavior [t (17) = 2.80, p = 0.01] and less lose-shift behavior [t (17) = −3.01, p < 0.01] relative to rats in the sucrose group (Figure 6B). These data suggest that cocaine self-administration led to both enhanced sensitivity to rewarding outcomes (increased win-stay) and reduced sensitivity to negative feedback (decreased lose-shift), the combination of which likely contributed to the greater risky choice observed in this group.
Figure 6. Performance in the Risky Decision-making Task and control assays after cocaine self-administration (Experiment 3).
A. Rats that self-administered cocaine chose the large, risky reward significantly more than rats that self-administered sucrose. B. Rats that self-administered cocaine displayed a significant increase in win-stay behavior and a significant decrease in lose-shift behavior relative to rats that self-administered sucrose. C. There were no differences in breakpoint on a progressive ratio (PR) schedule of reinforcement between rats that self-administered cocaine or sucrose. D. There were no differences in the number of rewards earned on the PR task between rats that self-administered cocaine or sucrose. E. There were no differences in shock thresholds between rats that would proceed to cocaine or sucrose self-administration. F. There were no differences in shock thresholds between rats that self-administered cocaine or sucrose. Data are represented as mean ± standard error of the mean. Asterisks denote p < 0.05.
A three-factor repeated-measures ANOVA was used to determine whether cocaine-induced alterations in latencies to press levers accompanied greater risk taking (Table 2). Although there was neither a main effect of group [F (1, 19) = 0.34, p = 0.57] nor a significant lever identity X group X trial block interaction [F (4, 76) = 2.37, p = 0.06], there was a significant lever identity X group interaction [F (1, 19) = 4.91, p = 0.04]. Post-hoc analyses revealed that this interaction was driven by a difference in latency to press the small, safe lever, with cocaine rats taking longer to press this lever relative to sucrose rats [group, F (1, 19) = 6.87, p = 0.02; group X trial block, F (4, 76) = 5.75, p < 0.01]. There were, however, no group differences in latencies to press the large, risky lever [group, F (1, 19) = 1.12, p = 0.30; group X trial block, F (4, 76) = 0.72, p = 0.58]. Analyses of other ancillary behavioral measures in the RDT revealed no significant group differences (see Table 1).
To determine whether cocaine-induced increases in risk taking were due to alterations in food motivation or shock reactivity, rats were tested in the PR task and re-assessed in the shock reactivity threshold assay. There were no significant differences in the highest ratio reached [t (16) = −0.59, p = 0.57; Figure 6C], the number of rewards earned [t (16) = −0.79, p = 0.44; Figure 6D] or the average number of lever presses [t (16) = 0.18, p = 0.85] between cocaine and sucrose rats. A comparison of shock thresholds before and after self-administration (Figure 6E, F) did not reveal changes in this measure in either the cocaine or sucrose group [group, F (1, 16) = 092, p = 0.35; time, F (1, 16) < 0.01, p = 0.96; group X time, F (1, 16) = 1.66, p = 0.22]. Furthermore, there were no differences in the mean shock threshold between groups after self-administration [t (16) = 0.15, p = 0.88; Figure 6F]. Collectively, these control experiments indicate that greater food motivation and/or decreased shock sensitivity cannot explain the greater risk taking after cocaine self-administration.
In summary, results from Experiment 3 show that, in young adult rats, cocaine self-administration increased risk taking and that this effect was accompanied by an increase in win-stay and a decrease in lose-shift behavior. Cocaine also increased latency to press the small, safe reward lever, which could be indicative of aversion to the less favorable option. There were no other notable effects of cocaine on behavior, including food motivation or shock thresholds. Because self-administration occurred at an earlier age relative to Experiments 1 and 2 (due to the absence of pre-training in the RDT), these findings support the hypothesis that the age at which cocaine self-administration occurs may determine how cocaine affects risk taking.
Experiment 4: Effects of passively administered cocaine in young adult male and female rats
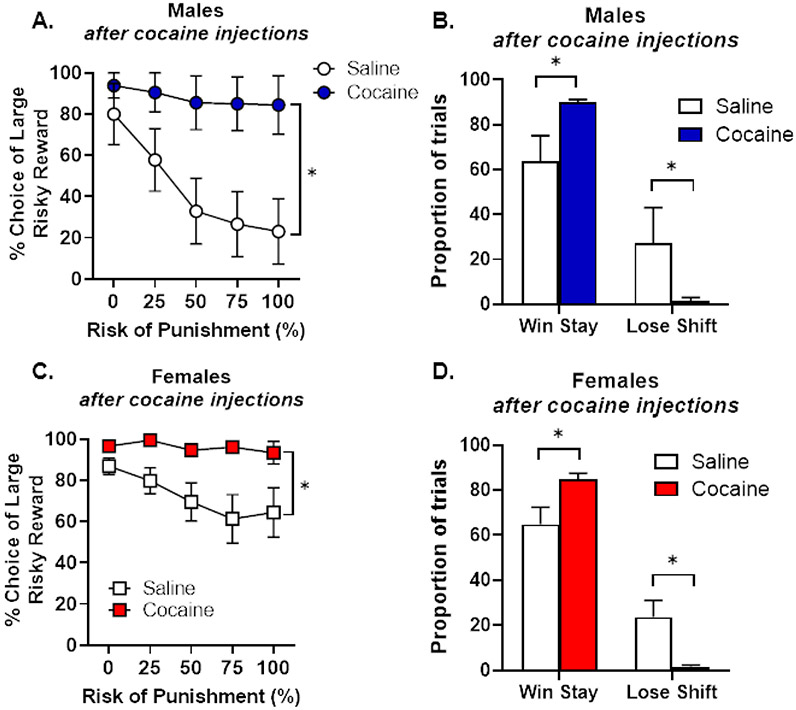
To ascertain whether age of cocaine exposure is a critical determinant of effects of passive cocaine administration on risk taking, rats received systemic injections of cocaine or saline for 14 days beginning on PN day 63 (young adulthood), and then began training in the RDT three weeks after the last injection day (PN day 98) until stability emerged (PN day 147). Cocaine increased risk taking relative to saline [group, F (1, 20) = 12.25, p < 0.01; group X trial block, F (4, 80) = 7.01, p < 0.01] in both males and females [sex, F (1, 20) = 3.58, p = 0.07; sex X trial block, F (4, 80) = 2.27, p = 0.07]; sex X group, F (1, 20) = 1.10, p = 0.31; sex X group X trial block, F (4, 80) = 1.07, p = 0.38; Figure 7A, C]. A two-factor ANOVA conducted on win-stay and lose-shift performance revealed main effects of group [win-stay: F (1,16) = 13.78, p < 0.01; lose-shift: F (1, 16) = 9.53, p < 0.01; Figure 7B, D] but no main effects of sex [win-stay, F (1, 16) = 0.01, p = 0.92; lose-shift: F (1, 16) = 0.06, p = 0.82] or group X sex interactions [win-stay, F (1, 16) = 0.08, p = 0.78; lose-shift, F (1, 16) = 0.05, p = 0.82]. These analyses reveal that passive cocaine injections increased win-stay behavior and decreased lose-shift behavior relative to saline injections, similar to the effects of cocaine self-administration.
Figure 7. Performance in the Risky Decision-making Task after passively administered cocaine (Experiment 4).
A. Male rats that received cocaine injections chose the large, risky reward significantly more than rats that received control saline injections. B. Male rats that received cocaine injections displayed an increase in win-stay behavior and a decrease in lose-shift behavior relative to male rats that received saline injections. C. Female rats that received cocaine injections chose the large, risky reward significantly more than rats that received control saline injections. D. Female rats that received cocaine injections displayed an increase in win-stay behavior and a decrease in lose-shift behavior relative to female rats that received saline injections. Data are represented as mean ± standard error of the mean. Asterisks denote p < 0.05.
In addition to these behavioral changes, there were concomitant effects of cocaine injections on latencies to press the levers (Table 2). There was a main effect of group [F (1, 20) = 4.25, p = 0.05] and significant group X lever identity [F (1, 20) = 6.52, p = 0.02] and group X lever identity X trial block [F (4, 80) = 5.20, p < 0.01] interactions. Additional analyses were conducted to determine the source of these interactions. When latencies to press the small, safe lever were compared between the two groups, a significant main effect of group [F (1, 19) = 6.87, p = 0.02] and significant group X trial block interaction [F (4, 76) = 5.75, p < 0.01] emerged, with the cocaine group taking longer to press this lever compared to the saline group. Similar to Experiment 3, this effect was specific to the small, safe lever as there were no group differences in latencies to press the large, risky lever [group, F (1, 19) = 1.12, p = 0.30; group X trial block, F (4, 76) = 0.72, p = 0.58]. Despite significant sex X lever identity [F (1, 20) = 6.87, p = 0.02] and sex X lever identity X trial block [F (4, 80) = 2.79, p = 0.03] interactions, there was no main effect of sex [F (1, 20) = 1.47, p = 0.24] nor were there group X sex [F (1, 20) = 0.28, p = 0.60], group X sex X lever identity [F (1, 20) = 1.13, p = 0.30] or group X sex X lever identity X trial block [F (4, 80) = 0.62, p = 0.65] interactions. With the exception of percentage of omitted free choice trials, there was no effect of cocaine on any other ancillary behavioral measures in the RDT (see Tables 1 & 3). Relative to males, females in the saline group omitted significantly more trials than those in the cocaine group [F (1, 20) = 6.25, p = 0.02]. Increased omissions may be a manifestation of risk aversion in females (Orsini et al., 2016); hence, fewer omissions in the cocaine group could be considered to be another demonstration of increased risk taking, consistent with the alterations in choice behavior.
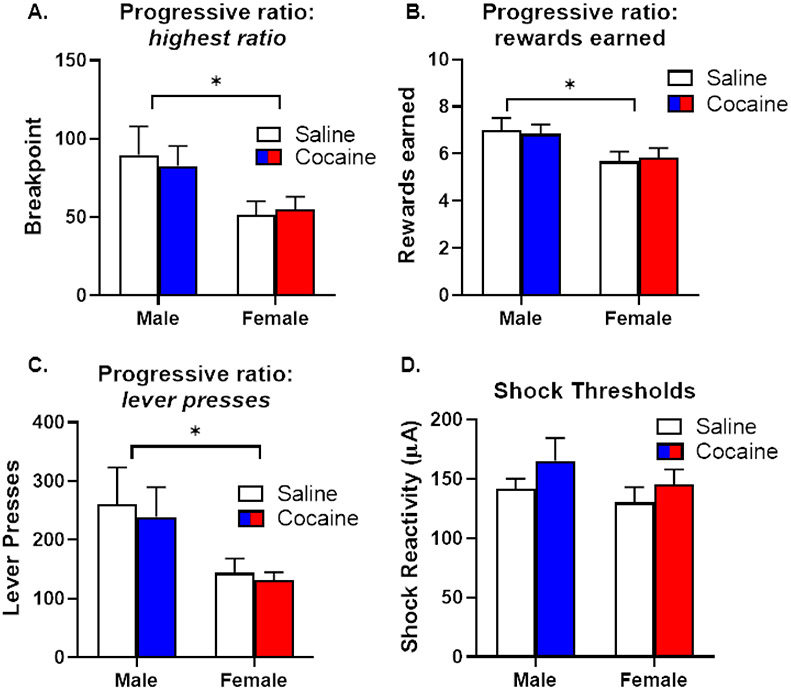
Similar to Experiment 3, rats were subsequently tested in the PR task and the shock reactivity threshold assay. There were no main effects of group on the highest ratio reached [F (1, 20) = 0.02, p = 0.89; Figure 8A], the number of rewards earned [F (1, 20) < 0.01, p = 1.00; Figure 8B] or the number of lever presses [F (1, 20) = 0.16, p = 0.69; Figure 8C] in the PR task, nor were there group X sex interactions on any of these behavioral measures [ratio, F (1, 20) = 0.17, p = 0.68; rewards, F (1, 20) = 0.15, p = 0.71; lever presses, F (1, 20) = 0.01, p = 0.91]. There was, however, an overall main effect of sex on the highest ratio obtained in the task [F (1, 20) = 6.76, p = 0.02; Figure 8A], the number of rewards earned [F (1, 20) = 7.10, p = 0.02; Figure 8B] and number of lever presses [F (1, 20) = 7.01, p = 0.02], with males responding significantly more for food than females (irrespective of injection group). Finally, there were no main effects of sex [F (1, 20) = 1.47, p = 0.24] or group [F (1, 20) = 2.29, p = 0.15] and no significant interaction between sex and group [F (1, 20) = 0.09, p = 0.77] on shock reactivity thresholds (Figure 8D).
Figure 8. Performance on behavioral assays of food motivation and shock reactivity (Experiment 4).
A. Males, irrespective of injection group, had higher breakpoints on a progressive ratio (PR) schedule of reinforcement than females. B. Males, irrespective of injection group, earned more rewards on the PR task than females. C. Males, irrespective of injection group, lever pressed significantly more on the PR task than females. D. There were no differences in shock thresholds between rats that received cocaine or saline injection in either sex. Data are represented as mean ± standard error of the mean. Asterisks denote p < 0.05.
Together, these results replicate the effects of self-administered cocaine on risk taking that were observed in Experiment 3. Furthermore, given the absence of effects of passively-administered cocaine in Experiment 1, they lend support to the idea that deleterious effects of cocaine on risk taking may critically depend on the age at which drug exposure occurs.
Discussion
The current investigation served to extend previous work in our laboratory in which we showed that male rats with a history of cocaine self-administration exhibited greater risk taking compared to rats that underwent sucrose self-administration control procedures (Mitchell, Weiss, Beas, et al., 2014). Specifically, the goals were to determine whether increased risk taking following chronic cocaine is similarly evident in females and whether these effects depend on the volitional nature of self-administration (as opposed to the pharmacological properties of cocaine itself). The results show that chronic cocaine does cause long-lasting increases in risk taking in both males and females and that these effects are independent of route of administration. An unexpected finding, however, was that the effects of cocaine on risk taking depended on the developmental timing of cocaine exposure. Cocaine increased risk taking only when administration (either passively- or self-administered) began during young adulthood (PN day 77, Experiment 3; PN day 63, Experiment 4; Table 4). In contrast, cocaine had no effect on risk taking in either sex when cocaine administration began during later adulthood (PN day 105, Experiment 3; PN day 161, Experiment 4; Table 4). Collectively, these data suggest that there is a developmental time window of susceptibility to cocaine’s effects on risk taking.
Table 4.
Postnatal days at which each phase of the experiment occurred
| Experiment | Pre-RDT | Self- administration/ Injections |
Post-RDT | Effect on risk taking |
|---|---|---|---|---|
| 1 | 70-98 | 105-119 | 140-189 | No effect |
| 2 | 70-140 | 161-175 | 196-231 | No effect |
| 3 | X | 77-91 | 112-154 | Increase |
| 4 | X | 63-77 | 105-147 | Increase |
| Mitchell et al. (2014) | 34-55 | 76-95 | 130-147 | Increase |
Sex Differences
In our previous work in which we showed that chronic cocaine self-administration causes a lasting increase in risk taking (Mitchell, Weiss, Beas, et al., 2014), only male subjects were used. There is a considerable body of literature, however, establishing the presence of sex differences in risky decision making and sensitivity to punishment (Chowdhury et al., 2019; Liley et al., 2019; Orsini & Setlow, 2017; Orsini et al., 2016) Indeed, when they are tested under the same conditions, females are significantly more risk averse than males in the RDT and are more sensitive to pharmacological manipulations that promote risk aversion (Orsini et al., 2016; note that the greater risk preference in females in Experiment 2 was likely due to the use of higher shock intensities in males). There are also sex differences not only in various aspects of drug-related behavior (Becker & Hu, 2008; Kerstetter et al., 2012; Lynch, 2006), but also in the relationship between risk preference in the RDT and acquisition of drug-seeking behavior (Orsini et al., 2020).
Despite these relatively well-established sex differences, sex differences in the effects of chronic cocaine (or any drug of abuse for that matter) on decision making and cognition have yet to be thoroughly evaluated. In one of the few studies examining this relationship, van der Plas et al. (2009) reported that female cocaine users exhibited more severe decision-making deficits in the Iowa Gambling Task compared to male cocaine users. The subjects in this study, however, were current cocaine users; these findings therefore do not address whether sex influences cocaine’s long-lasting effect on decision making during abstinence. The current study was designed to tackle this specific question, and found that cocaine administration during young adulthood increased risk taking comparably in both males and females. This increase in risk taking was accompanied by longer latencies to press the small, safe reward lever, perhaps reflecting a reduction in the value of the outcome associated with this lever relative to the outcome associated with the large, risky lever. Compared to the control group, rats in the cocaine group also displayed greater win-stay behavior and less lose-shift behavior. These behavioral measures are often used as proxies for sensitivity to rewarding or adverse outcomes, respectively (Bari et al., 2011; Orsini et al., 2017; St Onge, Abhari, & Floresco, 2011). Our results therefore suggest that cocaine may increase risk taking by not only enhancing sensitivity to the larger reward, but also by dampening the impact of punishment on subsequent choice. Similar to the effects of cocaine on choice preference, these alterations in sensitivity to the outcomes did not differ between males and females. The enhanced sensitivity to rewarding outcomes is consistent with years of previous work showing that exposure to cocaine (as well as other psychostimulants) leads to augmented incentive value of rewarding stimuli, mediated by long-lasting changes in dopamine signaling (Chen et al., 2008; Luscher & Malenka, 2011; Robinson & Berridge, 1993; Ungless, Whistler, Malenka, & Bonci, 2001). More recent work provides support for the finding that cocaine attenuates sensitivity to adverse outcomes, such as punishment, resulting in persistent risky behavior. For example, rats with a history of cocaine exposure are slower to learn to actively avoid delivery of footshock punishment accompanying sucrose reinforcement in an approach-avoidance conflict task (Nguyen, Schumacher, Erb, & Ito, 2015). A reduction in sensitivity to punishment is also consistent with observations of decision-making performance in individuals with cocaine use disorder (CUD). Relative to healthy individuals, those with CUD are more likely to continue to choose risky options immediately after receiving a loss in a risk-based decision-making laboratory task (Gowin et al., 2017). The current findings expand upon these previous studies, however, by demonstrating that, despite baseline sex differences in sensitivity to punishment, cocaine subverts processing of punishment-related information to the same extent in both males and females, leading to long-lasting elevations in risky choice.
Alternatively, greater risk taking and greater win-stay and less lose-shift behavior in the cocaine group relative to the control group may reflect cocaine-induced impairments in behavioral flexibility, resulting in perseverative choice behavior. Specifically, rats in the cocaine group may have been less able to alter their choices as risk contingencies changed across the trial blocks, an effect that may be independent of changes in sensitivity to punishment. This interpretation of the findings would be consistent with previous work showing that chronic cocaine, via self-administration or passive administration, leads to impairments in reversal learning in rats and non-human primates in the absence of the drug (Jentsch, Olausson, De La Garza, & Taylor, 2002; Schoenbaum, Saddoris, Ramus, Shaham, & Setlow, 2004; Stalnaker et al., 2007; Stalnaker, Takahashi, Roesch, & Schoenbaum, 2009). These potential interpretations can be dissociated with additional experiments, such as the direct comparison of the effects of cocaine exposure on performance in the RDT when risk probabilities ascend (0% → 100%, as in the current studies) to performance in the RDT when risk probabilities descend (100% → 0%; Orsini et al., 2018). If cocaine increases risk taking in both task designs, it would suggest that cocaine’s effect on risk taking is likely due to a decrease in sensitivity to punishment rather than impaired behavioral flexibility.
Critically, the effects of cocaine on risky choice and win-stay/lose-shift behavior cannot be attributed to alterations in motivation to work for food and/or sensitivity to footshock in either sex. There were no differences between rats with a history of cocaine exposure (passively or self-administered) and those in the control groups (saline administration or sucrose self-administration) on measures of motivation to work for a food reward in a PR schedule of reinforcement task. Notably, there were sex differences in the breakpoint (the ratio at which rats ceased lever pressing) and the number of rewards earned, with males exhibiting greater willingness to work for food than females. These differences, however, were not specific to males with a history of cocaine and therefore are unlikely to account for cocaine-induced elevations in risky choice. To verify that cocaine-induced alterations in risky choice were not secondary to effects of cocaine on sensitivity to footshock, rats were tested in a shock reactivity threshold assay. Not only were there no differences in shock thresholds before and after self-administration in either group (Experiment 3), there were also no differences in shock thresholds between cocaine-exposed rats and their control counterparts (Experiments 3 and 4) in either sex (Experiment 4). Hence, the persistent choice of the risky option despite having just experienced a footshock (i.e., reduced lose-shift behavior) does not reflect insensitivity to the physical properties of the footshock itself, but rather reflects a reduction in the extent to which adverse consequences, such as punishment, can shift behavior toward safer options.
One limitation of the current study was that there was significant attrition of female rats in Experiment 3, which precluded direct comparisons between males and females in the effects of cocaine self-administration on risk taking. Hence, our conclusions regarding the lack of sex differences in cocaine-induced alterations in risk taking are based only on findings from rats that underwent passive cocaine administration. Given the similarity of effects of passively vs. self-administered cocaine on performance in the RDT (Experiments 3 and 4) and in other choice-based tasks (e.g., intertemporal choice; Mendez et al., 2010; Mitchell, Weiss, Ouimet, et al., 2014; Simon et al., 2007), as well as the lack of sex differences observed in Experiment 4, however, it is likely that cocaine self-administration would have a comparable effect on risk taking in males and females.
Route of cocaine administration
Self-administration, which is most frequently conducted intravenously, is arguably the best animal model of cocaine use, as it mimics the volitional nature of drug intake in humans (i.e., animals are free to consume as much or as little cocaine as they wish). Passive methods of cocaine administration (e.g., experimenter-administered injections) are useful in that they enable tight control over the dose and schedule of drug delivery, but they can have effects on neurobiological and behavioral outcome measures that are distinct from self-administration (Chen et al., 2008; Jacobs, Smit, de Vries, & Schoffelmeer, 2003; Mark, Hajnal, Kinney, & Keys, 1999; McFarland, Lapish, & Kalivas, 2003; Palamarchouk, Smagin, & Goeders, 2009; Stefanski et al., 2007). Previous work from our laboratory showed that cocaine self-administration during young adulthood (beginning on PN day 76; Table 4) causes lasting increases in risky choice in the RDT (Mitchell, Weiss, Beas, et al., 2014). The current study extends this work by showing that passive cocaine administration produces comparable increases in risky choice. These results suggest that the neural changes underlying this altered decision making result from the pharmacological properties of the drug rather than from interactions between cocaine and the route by which it is administered. These findings are consistent with those from studies of the effects of cocaine on other forms of cognition. Both passively- and self-administered cocaine engender comparable impairments in reversal learning (Calu et al., 2007; Schoenbaum et al., 2004), as well as lasting increases in impulsive choice in an intertemporal choice task (Mendez et al., 2010; Mitchell, Weiss, Ouimet, et al., 2014; Simon et al., 2007).
Timing of cocaine administration
An unexpected finding of the current study was that the effects of chronic cocaine on risk taking in males and females depended on the timing of cocaine exposure. Whereas passively- or self-administered cocaine led to elevations in risk taking when exposure began on PN day 77 (Experiment 3) or PN 63 (Experiment 4), cocaine exposure, irrespective of route of administration, did not increase risk taking when it began during later adulthood (PN day 101, Experiment 1; PN day 161, Experiment 2). In fact, on certain measures (e.g., latencies to press levers), cocaine exposure had effects opposite to what would be predicted based on its effects in younger rats. Moreover, cocaine self-administration during later adulthood decreased motivation to work for a food reward in the PR schedule of reinforcement task in males, an effect that was absent when self-administration occurred during earlier adulthood. Such an effect on motivation in late adulthood could potentially account for the lack of augmented risk taking following cocaine self-administration. Underlying behavioral mechanisms notwithstanding, these results suggest that there is a developmental window of vulnerability to cocaine’s ability to increase risk taking in rats that closes at roughly 3 months of age. Divergent effects of cocaine on cognition across development are consistent with data from human and non-human studies, although these publications predominantly distinguish between adolescence vs. young adulthood (as opposed to early vs. later adulthood as in the current studies; Kantak, 2020). Surprisingly, however, several of these studies report that, in contrast to the results of the current study, early cocaine onset (e.g., during adolescence) has no effects on subsequent cognitive performance during adulthood. For example, rats that self-administered cocaine during adolescence were unimpaired in a set-shifting task that probes the ability to flexibly shift behavior when rule contingencies change (Kantak, Barlow, Tassin, Brisotti, & Jordan, 2014). More in line with the findings of the current study, alcohol exposure during adolescence (PN day 30-49), but not during adulthood (PN day 80-99), results in greater preference for risky options compared to control conditions (Schindler, Tsutsui, & Clark, 2014). Considered with the findings of the current study, these studies suggest that early onset cocaine exposure may have dissociable effects on later adult cognitive function, with the most deleterious impacts on cost/benefit decision making, but more mild effects on other processes such as cognitive flexibility.
In contrast to the current study, others have shown that cocaine exposure in rodents during adulthood does have a detrimental effect on other forms of decision making. For example, both passively- and self-administered cocaine in fully mature adult male rats increase impulsive choice in a delay discounting task (Mendez et al., 2010; Mitchell, Weiss, Ouimet, et al., 2014; Simon et al., 2007). More recently, others have reported that cocaine self-administration leads to long-lasting impairments in performance of a rodent analogue of the Iowa Gambling Task (Cocker, Rotge, Daniel, Belin-Rauscent, & Belin, 2020; Ferland & Winstanley, 2017; Hynes et al., 2021). Interestingly, in one of these studies, there were also subsets of rats whose decision-making performance was unaffected or even improved after cocaine self-administration (Cocker et al., 2020). Hence, cocaine exposure during later adulthood may in fact have heterogenous effects on some forms of decision making. Nonetheless, the results of the current study clearly demonstrate that there is an upper limit to the window during which cocaine can disrupt decision making involving risk of explicit punishment. Such resilience to the effects of cocaine on decision making is reminiscent of reports that cocaine-induced impairments in neuropsychological function, and decision making in particular, can dissipate with time in former cocaine users (Chen et al., 2020; Di Sclafani, Tolou-Shams, Price, & Fein, 2002; Kantak, 2020) or can be remediated with cognitive training (Goldstein et al., 2007). It will therefore be important to determine whether the elevated risk taking observed after cocaine exposure during young adulthood (Experiments 3 and 4) attenuates over time and/or improves with repeated training throughout adulthood.
A limitation of the current set of experiments is that the rats given cocaine during young adulthood (and that showed a subsequent increase in risk taking, Experiments 3 and 4) were not pre-trained on the RDT, whereas rats that received cocaine during later adulthood (and failed to show changes in risk taking, Experiments 1 and 2) did undergo such pre-training. It is thus possible that pre-training in the RDT (prior to cocaine administration) rendered rats immune to drug-induced increases in risk taking. Several lines of evidence argue against this. First, prior studies of the effects of chronic cocaine on a delay discounting (intertemporal choice) task designed very similarly to the RDT showed that rats exhibited an increase in impulsive choice (greater preference for a small, immediate over a large, delayed reward) irrespective of whether they were trained in the task prior to cocaine administration (Mendez et al., 2010; Mitchell, Weiss, Ouimet, et al., 2014; Simon et al., 2007; but see Mitchell, Orsini & Setlow, 2017). Second, and more importantly, prior work from our laboratory showed that rats that underwent cocaine self-administration in young adulthood but were also pretrained in the RDT (Mitchell, Weiss, Beas, et al., 2014) exhibited an increase in risk taking of the same magnitude as rats in the current studies that did not have such pretraining. The fact that age of exposure is the only common factor among these studies in which cocaine caused an increase in risk taking strongly suggests that the presence or absence of prior training in the RDT does not contribute to the effects of cocaine on task performance (see Table 4).
Conclusion
The results of this study reveal a time-limited window of vulnerability during young adulthood in which cocaine can cause long-lasting effects on risky choice. This effect was evident in both male and female rats, and with both passively-administered and self-administered cocaine. These findings add to the growing evidence that the timing of cocaine onset is a significant factor in determining the presence and severity of cognitive impairments during abstinence.
Acknowledgments
The authors declare no conflicts of interest. Supported by NIH DA024671 and DA036534 (BS), DA041493 and a Thomas H. Maren Fellowship (CAO), and a Grinter Fellowship and a McKnight Brain Institute Fellowship (SLB). We thank Bonnie McLaurin, Vicky Kelley, Shannon Wall for technical assistance, and the NIDA Drug Supply Program for providing cocaine HCl.
References
- Balconi M, Finocchiaro R, & Campanella S (2014). Reward sensitivity, decisional bias, and metacognitive deficits in cocaine drug addiction. J Addict Med, 8(6), 399–406. doi: 10.1097/ADM.0000000000000065 [DOI] [PubMed] [Google Scholar]
- Bari A, Mar AC, Theobald DE, Elands SA, Oganya KC, Eagle DM, & Robbins TW (2011). Prefrontal and monoaminergic contributions to stop-signal task performance in rats. J Neurosci, 31(25), 9254–9263. doi: 10.1523/JNEUROSCI.1543-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker JB, & Hu M (2008). Sex differences in drug abuse. Front Neuroendocrinol, 29(1), 36–47. doi: 10.1016/j.yfrne.2007.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobzean SA, DeNobrega AK, & Perrotti LI (2014). Sex differences in the neurobiology of drug addiction. Exp Neurol, 259, 64–74. doi: 10.1016/j.expneurol.2014.01.022 [DOI] [PubMed] [Google Scholar]
- Bolla KI, Eldreth DA, London ED, Kiehl KA, Mouratidis M, Contoreggi C, … Ernst M (2003). Orbitofrontal cortex dysfunction in abstinent cocaine abusers performing a decision-making task. Neuroimage, 19(3), 1085–1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornovalova MA, Daughters SB, Hernandez GD, Richards JB, & Lejuez CW(2005). Differences in impulsivity and risk-taking propensity between primary users of crack cocaine and primary users of heroin in a residential substance-use program. Exp Clin Psychopharmacol, 13(4), 311–318. doi:Doi 10.1037/1064-1297.13.4.311 [DOI] [PubMed] [Google Scholar]
- Calu DJ, Stalnaker TA, Franz TM, Singh T, Shaham Y, & Schoenbaum G (2007). Withdrawal from cocaine self-administration produces long-lasting deficits in orbitofrontal-dependent reversal learning in rats. Learn Mem, 14(5), 325–328. doi: 10.1101/lm.534807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen BT, Bowers MS, Martin M, Hopf FW, Guillory AM, Carelli RM, … Bonci A (2008). Cocaine but not natural reward self-administration nor passive cocaine infusion produces persistent LTP in the VTA. Neuron, 59(2), 288–297. doi: 10.1016/j.neuron.2008.05.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Yang P, Chen T, Su H, Jiang H, & Zhao M (2020). Risky decision-making in individuals with substance use disorder: A meta-analysis and meta-regression review. Psychopharmacology (Berl), 237(7), 1893–1908. doi: 10.1007/s00213-020-05506-y [DOI] [PubMed] [Google Scholar]
- Chowdhury TG, Wallin-Miller KG, Rear AA, Park J, Diaz V, Simon NW, & Moghaddam B (2019). Sex differences in reward- and punishment-guided actions. Cogn Affect Behav Neurosci, 19(6), 1404–1417. doi: 10.3758/s13415-019-00736-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cocker PJ, Rotge JY, Daniel ML, Belin-Rauscent A, & Belin D (2020). Impaired decision making following escalation of cocaine self-administration predicts vulnerability to relapse in rats. Addict Biol, 25(3), e12738. doi: 10.1111/adb.12738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crocker AD, & Russell RW (1984). The Up-and-Down Method for the Determination of Nociceptive Thresholds in Rats. Pharmacol Biochem Behav, 21, 133–136. [DOI] [PubMed] [Google Scholar]
- Dandy KL, & Gatch MB (2009). The effects of chronic cocaine exposure on impulsivity in rats. Behav Pharmacol, 20(5-6), 400–405. doi: 10.1097/FBP.0b013e328330ad89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Sclafani V, Tolou-Shams M, Price LJ, & Fein G (2002). Neuropsychological performance of individuals dependent on crack-cocaine, or crack-cocaine and alcohol, at 6 weeks and 6 months of abstinence. Drug Alcohol Depend, 66(2), 161–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferland JN, & Winstanley CA (2017). Risk-preferring rats make worse decisions and show increased incubation of craving after cocaine self-administration. Addict Biol, 22(4), 991–1001. doi: 10.1111/adb.12388 [DOI] [PubMed] [Google Scholar]
- Fishbein DH, Eldreth DL, Hyde C, Matochik JA, London ED, Contoreggi C, … Grant S (2005). Risky decision making and the anterior cingulate cortex in abstinent drug abusers and nonusers. Brain Res Cogn Brain Res, 23(1), 119–136. doi: 10.1016/j.cogbrainres.2004.12.010 [DOI] [PubMed] [Google Scholar]
- Garman TS, Setlow B, & Orsini CA (2021). Effects of a high-fat diet on impulsive choice in rats. Physiol Behav, 229, 113260. doi: 10.1016/j.physbeh.2020.113260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein RZ, Tomasi D, Alia-Klein N, Zhang L, Telang F, & Volkow ND (2007). The effect of practice on a sustained attention task in cocaine abusers. Neuroimage, 35(1), 194–206. doi: 10.1016/j.neuroimage.2006.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gowin JL, Mackey S, & Paulus MP (2013). Altered risk-related processing in substance users: Imbalance of pain and gain. Drug Alcohol Depend, 132(1-2), 13–21. doi: 10.1016/j.drugalcdep.2013.03.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gowin JL, May AC, Wittmann M, Tapert SF, & Paulus MP (2017). Doubling down: increased risk-taking behavior following a loss by individuals with cocaine use disorder is associated with striatal and anterior cingulate dysfunction. Biol Psychiatry Cogn Neurosci Neuroimaging, 2(1), 94–103. doi: 10.1016/j.bpsc.2016.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gowin JL, Sloan ME, Ramchandani VA, Paulus MP, & Lane SD (2018). Differences in decision-making as a function of drug of choice. Pharmacol Biochem Behav, 164, 118–124. doi: 10.1016/j.pbb.2017.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grella CE, Anglin MD, & Wugalter SE (1995). Cocaine and crack use and HIV risk behaviors among high-risk methadone maintenance clients. Drug Alcohol Depend, 37(1), 15–21. doi: 10.1016/0376-8716(94)01059-t [DOI] [PubMed] [Google Scholar]
- Hernandez CM, Vetere LM, Orsini CA, McQuail JA, Maurer AP, Burke SN, … Bizon JL (2017). Decline of prefrontal cortical-mediated executive functions but attenuated delay discounting in aged Fischer 344 × brown Norway hybrid rats. Neurobiol Aging, 60, 141–152. doi: 10.1016/j.neurobiolaging.2017.08.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes TJ, Hrelja KM, Hathaway BA, Hounjet CD, Chernoff CS, Ebsary SA, Winstanley CA (2021). Dopamine neurons gate the intersection of cocaine use, decision making, and impulsivity. Addict Biol, e13022. doi: 10.1111/adb.13022 [DOI] [PubMed] [Google Scholar]
- Jackson LR, Robinson TE, & Becker JB (2006). Sex differences and hormonal influences on acquisition of cocaine self-administration in rats. Neuropsychopharmacology, 31(1), 129–138. doi: 10.1038/sj.npp.1300778 [DOI] [PubMed] [Google Scholar]
- Jacobs EH, Smit AB, de Vries TJ, & Schoffelmeer AN (2003). Neuroadaptive effects of active versus passive drug administration in addiction research. Trends Pharmacol Sci, 24(11), 566–573. doi: 10.1016/j.tips.2003.09.006 [DOI] [PubMed] [Google Scholar]
- Jentsch JD, Olausson P, De La Garza R 2nd, & Taylor JR (2002). Impairments of reversal learning and response perseveration after repeated, intermittent cocaine administrations to monkeys. Neuropsychopharmacology, 26(2), 183–190. doi: 10.1016/S0893-133X(01)00355-4 [DOI] [PubMed] [Google Scholar]
- Kantak KM (2020). Adolescent-onset vs. adult-onset cocaine use: Impact on cognitive functioning in animal models and opportunities for translation. Pharmacol Biochem Behav, 196, 172994. doi: 10.1016/j.pbb.2020.172994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantak KM, Barlow N, Tassin DH, Brisotti MF, & Jordan CJ (2014). Performance on a strategy set shifting task in rats following adult or adolescent cocaine exposure. Psychopharmacology (Berl), 231(23), 4489–4501. doi: 10.1007/s00213-014-3598-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerstetter KA, Ballis MA, Duffin-Lutgen S, Carr AE, Behrens AM, & Kippin TE (2012). Sex differences in selecting between food and cocaine reinforcement are mediated by estrogen. Neuropsychopharmacology, 37(12), 2605–2614. doi: 10.1038/npp.2012.99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kluwe-Schiavon B, Kexel A, Manenti G, Cole DM, Baumgartner MR, Grassi-Oliveira R, … Quednow BB (2020). Sensitivity to gains during risky decision-making differentiates chronic cocaine users from stimulant-naive controls. Behav Brain Res, 379, 112386. doi: 10.1016/j.bbr.2019.112386 [DOI] [PubMed] [Google Scholar]
- Lejuez CW, Bornovalova MA, Daughters SB, & Curtin JJ (2005). Differences in impulsivity and sexual risk behavior among inner-city crack/cocaine users and heroin users. Drug Alcohol Depend, 77(2), 169–175. doi: 10.1016/j.drugalcdep.2004.08.013 [DOI] [PubMed] [Google Scholar]
- Leland DS, & Paulus MP (2005). Increased risk-taking decision-making but not altered response to punishment in stimulant-using young adults. Drug Alcohol Depend, 78(1), 83–90. doi: 10.1016/j.drugalcdep.2004.10.001 [DOI] [PubMed] [Google Scholar]
- Liley AE, Gabriel DBK, Sable HJ, & Simon NW (2019). Sex Differences and Effects of Predictive Cues on Delayed Punishment Discounting. eNeuro, 6(4). doi: 10.1523/ENEURO.0225-19.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luscher C, & Malenka RC (2011). Drug-evoked synaptic plasticity in addiction: from molecular changes to circuit remodeling. Neuron, 69(4), 650–663. doi: 10.1016/j.neuron.2011.01.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch WJ (2006). Sex differences in vulnerability to drug self-administration. Exp Clin Psychopharmacol, 14(1), 34–41. doi: 10.1037/1064-1297.14.1.34 [DOI] [PubMed] [Google Scholar]
- Lynch WJ, & Carroll ME (1999). Sex differences in the acquisition of intravenously self-administered cocaine and heroin in rats. Psychopharmacology (Berl), 144(1), 77–82. [DOI] [PubMed] [Google Scholar]
- Lynch WJ, & Carroll ME (2000). Reinstatement of cocaine self-administration in rats: sex differences. Psychopharmacology (Berl), 148(2), 196–200. doi: 10.1007/s002130050042 [DOI] [PubMed] [Google Scholar]
- Mark GP, Hajnal A, Kinney AE, & Keys AS (1999). Self-administration of cocaine increases the release of acetylcholine to a greater extent than response-independent cocaine in the nucleus accumbens of rats. Psychopharmacology (Berl), 143(1), 47–53. doi: 10.1007/s002130050918 [DOI] [PubMed] [Google Scholar]
- McFarland K, Lapish CC, & Kalivas PW (2003). Prefrontal glutamate release into the core of the nucleus accumbens mediates cocaine-induced reinstatement of drug-seeking behavior. J Neurosci, 23(8), 3531–3537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendez IA, Simon NW, Hart N, Mitchell MR, Nation JR, Wellman PJ, & Setlow B (2010). Self-administered cocaine causes long-lasting increases in impulsive choice in a delay discounting task. Behav Neurosci, 124(4), 470–477. doi: 10.1037/a0020458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell MR, Orsini CA, & Setlow B (2017). Cocaine and intertemporal decision making.In Preedy V (Ed.), The Neuroscience of Cocaine: Mechanisms And Treatment: Elsevier. [Google Scholar]
- Mitchell MR, Weiss VG, Beas BS, Morgan D, Bizon JL, & Setlow B (2014).Adolescent risk taking, cocaine self-administration, and striatal dopamine signaling. Neuropsychopharmacology, 39(4), 955–962. doi: 10.1038/npp.2013.295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell MR, Weiss VG, Ouimet DJ, Fuchs RA, Morgan D, & Setlow B (2014).Intake-dependent effects of cocaine self-administration on impulsive choice in a delay discounting task. Behav Neurosci, 128(4), 419–429. doi: 10.1037/a0036742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen D, Nesarajah Y, Erb S, & Ito R (2018). Repeated Cocaine Exposure Attenuates the Desire to Actively Avoid: A Novel Active Avoidance Runway Task. Front Behav Neurosci, 12, 108. doi: 10.3389/fnbeh.2018.00108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen D, Schumacher A, Erb S, & Ito R (2015). Aberrant approach-avoidance conflict resolution following repeated cocaine pre-exposure. Psychopharmacology (Berl), 232(19), 3573–3583. doi: 10.1007/s00213-015-4006-y [DOI] [PubMed] [Google Scholar]
- Orsini CA, Blaes SL, Dragone RJ, Betzhold SM, Finner AM, Bizon JL, & Setlow B (2020). Distinct relationships between risky decision making and cocaine self-administration under short- and long-access conditions. Prog Neuropsychopharmacol Biol Psychiatry, 98, 109791. doi: 10.1016/j.pnpbp.2019.109791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orsini CA, Blaes SL, Hernandez CM, Betzhold SM, Perera H, Wheeler AR, … Setlow B (2021). Regulation of risky decision making by gonadal hormones in males and females. Neuropsychopharmacology, 46(3), 603–613. doi: 10.1038/s41386-020-00827-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orsini CA, Blaes SL, Setlow B, & Simon NW (2019). Recent Updates in Modeling Risky Decision Making in Rodents. Methods Mol Biol, 2011, 79–92. doi: 10.1007/978-1-4939-9554-7_5 [DOI] [PubMed] [Google Scholar]
- Orsini CA, Hernandez CM, Singhal S, Kelly KB, Frazier CJ, Bizon JL, & Setlow B (2017). Optogenetic Inhibition Reveals Distinct Roles for Basolateral Amygdala Activity at Discrete Time Points during Risky Decision Making. J Neurosci, 37(48), 11537–11548. doi: 10.1523/JNEUROSCI.2344-17.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orsini CA, Heshmati SC, Garman TS, Wall SC, Bizon JL, & Setlow B (2018).Contributions of medial prefrontal cortex to decision making involving risk of punishment. Neuropharmacology, 139, 205–216. doi: 10.1016/j.neuropharm.2018.07.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orsini CA, & Setlow B (2017). Sex differences in animal models of decision making. J Neurosci Res, 95(1-2), 260–269. doi: 10.1002/jnr.23810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orsini CA, Willis ML, Gilbert RJ, Bizon JL, & Setlow B (2016). Sex differences in a rat model of risky decision making. Behav Neurosci, 130(1), 50–61. doi: 10.1037/bne0000111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palamarchouk V, Smagin G, & Goeders NE (2009). Self-administered and passive cocaine infusions produce different effects on corticosterone concentrations in the medial prefrontal cortex (MPC) of rats. Pharmacol Biochem Behav, 94(1), 163–168. doi: 10.1016/j.pbb.2009.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellman BA, Schuessler BP, Tellakat M, & Kim JJ (2017). Sexually Dimorphic Risk Mitigation Strategies in Rats. eNeuro, 4(1). doi: 10.1523/ENEURO.0288-16.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson TE, & Berridge KC (1993). The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Brain Res Rev, 18(3), 247–291. [DOI] [PubMed] [Google Scholar]
- Roth ME, & Carroll ME (2004). Sex differences in the escalation of intravenous cocaine intake following long- or short-access to cocaine self-administration. Pharmacol Biochem Behav, 78(2), 199–207. doi: 10.1016/j.pbb.2004.03.018 [DOI] [PubMed] [Google Scholar]
- Schindler AG, Tsutsui KT, & Clark JJ (2014). Chronic alcohol intake during adolescence, but not adulthood, promotes persistent deficits in risk-based decision making. Alcohol Clin Exp Res, 38(6), 1622–1629. doi: 10.1111/acer.12404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenbaum G, Saddoris MP, Ramus SJ, Shaham Y, & Setlow B (2004). Cocaine-experienced rats exhibit learning deficits in a task sensitive to orbitofrontal cortex lesions. Eur J Neurosci, 19(7), 1997–2002. doi: 10.1111/j.1460-9568.2004.03274.x [DOI] [PubMed] [Google Scholar]
- Shimp KG, Mitchell MR, Beas BS, Bizon JL, & Setlow B (2015). Affective and cognitive mechanisms of risky decision making. Neurobiol Learn Mem, 117, 60–70. doi: 10.1016/j.nlm.2014.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon NW, Gilbert RJ, Mayse JD, Bizon JL, & Setlow B (2009). Balancing risk and reward: a rat model of risky decision making. Neuropsychopharmacology, 34(10), 2208–2217. doi: 10.1038/npp.2009.48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon NW, Mendez IA, & Setlow B (2007). Cocaine exposure causes long-term increases in impulsive choice. Behav Neurosci, 121(3), 543–549. doi: 10.1037/0735-7044.121.3.543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon NW, Montgomery KS, Beas BS, Mitchell MR, LaSarge CL, Mendez IA, Setlow B (2011). Dopaminergic modulation of risky decision-making. J Neurosci, 31(48), 17460–17470. doi: 10.1523/JNEUROSCI.3772-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Onge JR, Abhari H, & Floresco SB (2011). Dissociable contributions by prefrontal D1 and D2 receptors to risk-based decision making. J Neurosci, 31(23), 8625–8633. doi: 10.1523/JNEUROSCI.1020-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stalnaker TA, Roesch MR, Calu DJ, Burke KA, Singh T, & Schoenbaum G (2007). Neural correlates of inflexible behavior in the orbitofrontal-amygdalar circuit after cocaine exposure. Ann N Y Acad Sci, 1121, 598–609. doi: 10.1196/annals.1401.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stalnaker TA, Takahashi Y, Roesch MR, & Schoenbaum G (2009). Neural substrates of cognitive inflexibility after chronic cocaine exposure. Neuropharmacology, 56 Suppl 1, 63–72. doi: 10.1016/j.neuropharm.2008.07.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanski R, Ziolkowska B, Kusmider M, Mierzejewski P, Wyszogrodzka E, Kolomanska P, … Kostowski W (2007). Active versus passive cocaine administration: differences in the neuroadaptive changes in the brain dopaminergic system. Brain Res, 1157, 1–10. doi: 10.1016/j.brainres.2007.04.074 [DOI] [PubMed] [Google Scholar]
- Stout JC, Busemeyer JR, Lin A, Grant SJ, & Bonson KR (2004). Cognitive modeling analysis of decision-making processes in cocaine abusers. Psychon Bull Rev, 11(4), 742–747. [DOI] [PubMed] [Google Scholar]
- Stout JC, Rock SL, Campbell MC, Busemeyer JR, & Finn PR (2005). Psychological processes underlying risky decisions in drug abusers. Psychol Addict Behav, 19(2), 148–157. doi: 10.1037/0893-164X.19.2.148 [DOI] [PubMed] [Google Scholar]
- Thompson LL, Claus ED, Mikulich-Gilbertson SK, Banich MT, Crowley T, Krmpotich T, … Tanabe J (2012). Negative reinforcement learning is affected in substance dependence. Drug Alcohol Depend, 123(1-3), 84–90. doi: 10.1016/j.drugalcdep.2011.10.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomassini A, Struglia F, Spaziani D, Pacifico R, Stratta P, & Rossi A (2012). Decision making, impulsivity, and personality traits in alcohol-dependent subjects. Am J Addict, 21(3), 263–267. doi: 10.1111/j.1521-0391.2012.00225.x [DOI] [PubMed] [Google Scholar]
- Ungless MA, Whistler JL, Malenka RC, & Bonci A (2001). Single cocaine exposure in vivo induces long-term potentiation in dopamine neurons. Nature, 411(6837), 583–587. doi: 10.1038/35079077 [DOI] [PubMed] [Google Scholar]
- van der Plas EA, Crone EA, van den Wildenberg WP, Tranel D, & Bechara A (2009). Executive control deficits in substance-dependent individuals: a comparison of alcohol, cocaine, and methamphetamine and of men and women. J Clin Exp Neuropsychol, 31(6), 706–719. doi: 10.1080/13803390802484797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdejo-Garcia A, Albein-Urios N, Martinez-Gonzalez JM, Civit E, de la Torre R, & Lozano O (2014). Decision-making impairment predicts 3-month hair-indexed cocaine relapse. Psychopharmacology (Berl), 231(21), 4179–4187. doi: 10.1007/s00213-014-3563-9 [DOI] [PubMed] [Google Scholar]
- Verdejo-Garcia A, Benbrook A, Funderburk F, David P, Cadet JL, & Bolla KI (2007). The differential relationship between cocaine use and marijuana use on decision-making performance over repeat testing with the Iowa Gambling Task. Drug Alcohol Depend, 90(1), 2–11. doi: 10.1016/j.drugalcdep.2007.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo Y, Wang X, Cui C, Luo F, Yu P, & Wang X (2012). Cocaine-induced impulsive choices are accompanied by impaired delay-dependent anticipatory activity in basolateral amygdala. J Cogn Neurosci, 24(1), 196–211. doi: 10.1162/jocn_a_00131 [DOI] [PubMed] [Google Scholar]