Abstract
Introduction: Inhaled pulmonary vasodilators (IPVD) have been previously studied in patients with non-coronavirus disease-19 (COVID-19) related acute respiratory distress syndrome (ARDS). The use of IPVD has been shown to improve the partial pressure of oxygen in arterial blood (PaO2), reduce fraction of inspired oxygen (FiO2) requirements, and ultimately increase PaO2/FiO2 (P/F) ratios in ARDS patients. However, the role of IPVD in COVID-19 ARDS is still unclear. Therefore, we performed this meta-analysis to evaluate the role of IPVD in COVID-19 patients. Methods: Comprehensive literature search of PubMed, Embase, Web of Science and Cochrane Library databases from inception through April 22, 2022 was performed for all published studies that utilized IPVD in COVID-19 ARDS patients. The single arm studies and case series were combined for a 1-arm meta-analysis, and the 2-arm studies were combined for a 2-arm meta-analysis. Primary outcomes for the 1-arm and 2-arm meta-analyzes were change in pre- and post-IPVD P/F ratios and mortality, respectively. Secondary outcomes for the 1-arm meta-analysis were change in pre- and post-IPVD positive end-expiratory pressure (PEEP) and lung compliance, and for the 2-arm meta-analysis the secondary outcomes were need for endotracheal intubation and hospital length of stay (LOS). Results: 13 single arm retrospective studies and 5 case series involving 613 patients were included in the 1-arm meta-analysis. 3 studies involving 640 patients were included in the 2-arm meta-analysis. The pre-IPVD P/F ratios were significantly lower compared to post-IPVD, but there was no significant difference between pre- and post-IPVD PEEP and lung compliance. The mortality rates, need for endotracheal intubation, and hospital LOS were similar between the IPVD and standard therapy groups. Conclusion: Although IPVD may improve oxygenation, our investigation showed no benefits in terms of mortality compared to standard therapy alone. However, randomized controlled trials are warranted to validate our findings.
Keywords: inhaled pulmonary vasodilators, COVID-19, refractory hypoxia, mortality, intubation
Introduction
Coronavirus disease 2019 (COVID-19), is caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) virus, which was first discovered in December 2019, and has become a worldwide pandemic causing significant morbidity and mortality.1,2 Acute respiratory distress syndrome (ARDS) due to viral pneumonitis is one of the leading causes of mortality among patients with COVID-19 infection.3 ARDS occurs in 33% of hospitalized patients with COVID-19 and the average mortality rate among COVID-19 patients with ARDS is 39% (ranging from 13% to 73%).3
ARDS is an inflammatory process that is associated with decreased lung compliance, severe hypoxemia and increased pulmonary shunting causing ventilation-perfusion (V/Q) mismatch.4 The pathophysiology of ARDS includes diffuse alveolar damage, leaky alveolar capillaries, pulmonary edema and WHO group 3 pulmonary hypertension.4,5 ARDS causes decreased partial pressure of oxygen in arterial blood (PaO2) and leads to increased requirements of fraction of inspired oxygen (FiO2) and positive end-expiratory pressure (PEEP), causing decreased PaO2/FiO2 (P/F) ratios.4 Santamarina et al6 showed that COVID-19 leads to severe V/Q mismatch, as ground-glass opacities and consolidations lead to high perfusion in poorly ventilated regions. Also, well ventilated areas of the lungs can suffer reduced perfusion due to endothelial dysfunction and vasculitis.7 Inhaled pulmonary vasodilators (IPVD) diffuse across the alveolar membrane and cause local dilation in pulmonary vasculature in the well-ventilated regions of the lungs, which improves blood flow to these adequately ventilated areas.8 Prone positioning similarly improves ventilation and oxygenation in the dorsal areas of the lungs and reduces intrapulmonary shunting, and improving V/Q mismatch.9
Historically, in non-COVID-19 ARDS patients, IPVD have shown benefits in terms of improved oxygenation, but they have not improved mortality or reduced need for or length of mechanical ventilation.10,11 This is likely related to the fact that mortality in ARDS patients with pneumonia usually occurs later in the clinical course and is more closely linked to the worsening systemic effects of infection and sepsis syndrome.12 However, the use of IPVD for COVID-19 ARDS patients is a recent occurrence, and there are studies looking at the role of IPVD compared to standard therapy alone.13,14 Data regarding the role of IPVD in COVID-19 ARDS is still unclear. Therefore, we performed this meta-analysis to evaluate the effect of IPVD on the clinical outcomes of patients with COVID-19 ARDS.
Methods
Data Sources and Search Strategy
We performed a comprehensive search for published studies indexed in PubMed/MEDLINE, EMBASE, Web of Science and the Cochrane Central Register of Controlled Trials from inception to April 22, 2022. We also performed a manual search for additional relevant studies using references of the included articles. The following search terms were used: (“inhaled vasodilators” or “nitric oxide” or “epoprostenol” or “iloprost” or “milrinone” or “prostacyclin”), (“acute respiratory distress” or “mechanically ventilated” or “intubated” or “ARDS” or “high flow oxygen” or “HFNC”), and (“COVID” or “COVID-19”). The search was not limited by language, study design, or country of origin. Supplementary Table 1 describes the full search terms used in each database searched.
Inclusion and Exclusion Criteria
All single arm studies and case series would be included in the 1-arm meta-analysis. The studies included in the 1-arm meta-analysis were all peer-reviewed studies with patients treated with IPVD without an untreated control group for comparison and reported one of the following outcomes: pre- and post-IPVD P/F ratios, pre- and post-IPVD PEEP, or pre- and post-IPVD lung compliance were eligible for inclusion.
All 2-arm studies would be included in the 2-arm meta-analysis. These were all peer-reviewed studies that compared the use of IPVD to standard care alone and reported one of the following outcomes: mortality, need for endotracheal intubation, or hospital length of stay (LOS) were eligible for inclusion. The patients in the standard therapy cohort should not have been exposed to IPVD. We excluded any study or case series with less than 10 patients, case reports, reviews, abstracts, and preprint studies.
Data Extraction
The following data were extracted from the studies: first author name, publication year, country of origin, study design, sample size, gender of patients, mean age, and type of IPVD used. We also obtained inclusion criteria in each study, respiratory support at the time of IPVD initiation, and the number of patients undergoing prone positioning. We also obtained the study specific criteria for positive response to IPVD. Outcomes measures were also retrieved. For the studies in 1-arm meta-analysis, pre- and post-IPVD P/F ratios, pre- and post-IPVD PEEP, and pre- and post-IPVD lung compliance were extracted. For the 2-arm meta-analysis, mortality, need for endotracheal intubation, and hospital LOS were extracted. We followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyzes (PRISMA) Statement guidelines to select the final studies. Two investigators (WK and SEM) independently performed the search and shortlisted the studies for final review. Discrepancies were resolved by a third reviewer (AB).
Outcomes
The primary outcome of the 1-arm meta-analysis was change in P/F ratios post-IPVD. Secondary outcomes were change in PEEP and lung compliance post-IPVD.
The primary outcome of the 2-arm meta-analysis was mortality. The secondary outcomes were the need for endotracheal intubation and hospital LOS.
Statistical Analysis
We performed a meta-analysis of the included studies using Review Manager 5.3 (Cochrane Collaboration, Copenhagen, The Nordic Cochrane Centre), OpenMeta (CEBM, Providence, USA), and Comprehensive Meta-Analysis (Biostat, Englewood, USA). The random-effects model was used to calculate the pooled risk ratio (RR) and mean difference (MD) with the corresponding confidence intervals (CI) for proportional and continuous variables, respectively. A P-value <.05 was considered statistically significant. The heterogeneity of the effect size estimates across the studies was quantified using the Q statistic and I2 (P < .10 was considered significant). A value of I2 of 0–25% indicates significant homogeneity, 26–50% low homogeneity, and >50% indicates heterogeneity.15
Sensitivity and Subgroup Analyses
To confirm the robustness of the results, sensitivity analysis for the outcome of pre- and post-IPVD P/F ratios was performed using a leave-one-out meta-analysis to see if it had a significant influence on the results. Subgroup analysis was performed for the studies in the 1-arm meta-analysis based on type of IPVD used (epoprostenol (EPO) versus nitric oxide (NO)) for the outcome of pre- and post-IPVD P/F ratios.
For patients that received IPVD, a subgroup analysis was also performed comparing IPVD responder to non-responders in order to determine if there was a significant difference in baseline P/F ratios, mortality, and need for endotracheal intubation. For this analysis, we only considered studies that provided stratified data for both responders and non-responders and had a clear definition for study specific adequate response to IPVD treatment.
Bias Assessment
We assessed the quality of the included studies using the Newcastle-Ottawa Scale (NOS) for observational studies and the JBI critical appraisal tool for case series.16,17 Two authors (WK and SEM) independently assessed each study for bias. Discrepancies were resolved by a third reviewer (AB). Publication bias was assessed for pre- & post- IPVD P/F ratios qualitatively by visualizing the funnel plot and quantitively using Egger's regression analysis. A P-value was generated using the Egger analysis, and a value of <.05 was associated with significant publication bias.
Results
Study Selection
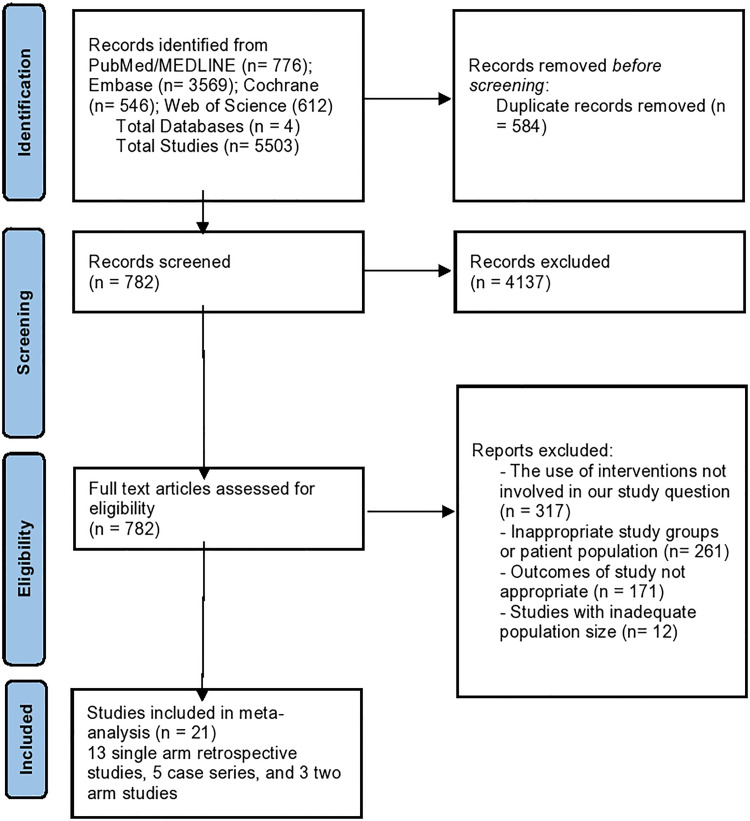
A total of 5503 studies were retrieved by our search strategy. 4721 studies were excluded based on the title and abstract review. A total of 782 studies underwent full-length review. Subsequently, we excluded 761 studies because of the following: 317 studies used interventions not involved in our study question, 261 studies used inappropriate study groups or patient populations, 171 studies did not report appropriate outcomes of interest, and 12 studies did not have adequate population size.
Eventually, 18 studies were included in the 1-arm meta-analysis (13 single arm retrospective studies18–30 and 5 case series31–35) and three studies13,14,36 in the 2-arm meta-analysis. Figure 1 shows the PRISMA flow chart that illustrates how the final studies were selected.
Figure 1.
PRISMA flow diagram for the selection of studies.
Study Characteristics
Tables 1 and 2 show the baseline characteristics of the studies included in the 1-arm and 2-arm meta-analyzes, respectively. All the studies were published between March 2020 and February 2022 and included COVID-19 patients confirmed by laboratory testing or imaging. Based on country of origin for the studies in the 1-arm analysis: 10 studies were European (4 from France, 3 from Italy, 3 from the United Kingdom) and 8 were conducted in the United States of America. For the 2-arm studies, 2 were conducted in the United States of America and 1 was conducted in the United Kingdom. All 13 single arm studies were retrospective and were pooled with data from 5 case series. For the 2 arm studies, all 3 studies were retrospective cohort.
Table 1.
Study and Patient Characteristics of the Included Single arm Studies and Case Series.
| Study, year | Abou-Arab 2020 | Bagate 2020 | Bonizzoli 2022 | Cardinale 2020 | Chiles 2022 | DeGrado 2020 | Ferrari 2020 | Garfield 2021 | Imtiaz 2021 |
|---|---|---|---|---|---|---|---|---|---|
| Study design | Retrospective Cohort | Case Series | Case Series | Retrospective Cohort | Retrospective Cohort | Retrospective Cohort | Case Series | Retrospective Cohort | Case Series |
| Country | France | France | Italy | France | United States of America | United States of America | Italy | United Kingdom | United States of America |
| Total patients | 34 | 10 | 12 | 10 | 50 | 38 | 10 | 35 | 15 |
| Age, mean ± SD, years | NR | 61.5 ± 17.2 | 61.7 ± 17 | 64.0 ± 24.7 | 60.8 ± 17.6 | 61 ± 12 | 55 ± 9 | 57.6 ± 8.1 | 57.2 ± 13.7 |
| Males, n (%) | NR | 7 (70) | 8 (66) | NR | 28 (52) | 24 (63) | NR | 28 (80) | 11 (73) |
| Type of IPVD | NO | NO | NO | NO | EPO | 38 patients received EPO; 11 of these patients later
received NO. Analyzed Separately. |
NO | NO | EPO |
| Oxygen Supplementation before IPVD | Not specified | IMV | IMV | IMV | HFNC or NIV | IMV | IMV | IMV | IMV |
| Patient Inclusion Criteria | Adults admitted to the ICU with severe COVID-19 pneumonia | Adults with COVID-19 ARDS with P/F ratio < 150 mm Hg | Adults with COVID-19 ARDS who did not improve with to IMV or proning | Adults with COVID-19 ARDS and P/F ratio < 120 mm Hg who did not improve with IMV or proning | Non-intubated adults with COVID-19 ARDS | Adults with COVID-19 ARDS while having IMV | Adults with COVID-19 ARDS who did not improve with IMV or proning | Adults with COVID-19 who did not improve with IMV | Adults with COVID-19 ARDS requiring IMV |
| Prone Positioning | Not specified | Yes | Yes | Yes. IPVDs were initiated in prone patients. |
No | Yes. 33/38 patients. |
Yes | Yes. 32/35 patients. |
Yes. 11/15 patients. |
| IPVD Administration Protocol | iNO if P/F ratio < 150 mm Hg. | iNO after IMV and proning, regardless of P/F ratio | iNO after IMV and proning | If P/F did not improve in prone position, then iNO was initiated. | iEPO was started in patients requiring > 30 L/min & >90% FiO2 on HFNC | iEPO was first-line and administered to patients with sustained ARDS despite IMV and proning. If the patient did not respond to iEPO, then iNO was considered in some situations. | If P/F did not improve in prone position, then iNO was initiated. | iNO after IMV if no improvement in oxygenation. | iEPO was administered if oxygenation did not improve with IMV or proning. |
| Study specific Definition of Response to IPVD | Increase in P/F ratio > 20% from baseline. | Increase in P/F ratio > 20% or 20 mm Hg from baseline. | Improved in oxygenation and increased P/F ratio. | Increase in P/F ratio > 20% from baseline. | Stable or increasing P/F or S/F ratio | Increase in P/F ratio > 10% from baseline | Not specified | Increase in P/F ratio by 10 mm Hg from baseline | Increase in P/F ratio > 10% from baseline |
| Time to First Assessment of P/F Ratio Post IPVD | 30 min | 30 min | 12 h | 1 h | Not specified | 2–6 h | 30 min | 24 h | 5 h |
| Study, year | Laghlam 2021 | Li 2020 | Longobardo 2020 | Lubinsky 2022 | Niss 2022 | Sonti 2021 | Tavazzi 2020 | Vogel 2021 | Ziehr 2021 |
| Study design | Case Series | Retrospective Cohort | Retrospective Cohort | Retrospective Cohort | Retrospective Cohort | Retrospective Cohort | Retrospective Cohort | Retrospective Cohort | Retrospective Cohort |
| Country | France | United States of America | United Kingdom | United States of America | United States of America | United States of America | Italy | United Kingdom | United States of America |
| Total patients | 12 | 39 | 20 | 84 | 111 | 80 | 16 | 14 | 12 |
| Age, mean ± SD, years | 71.8 ± 8.7 | 55.1 ± 13.8 | 58.3 ± 11.1 | 60.6 ± 13.2 | 57 ± 15.8; for all ARDS patients | 57.9 ± 14.3 | 65.0 ± 8.2 | 62.6 ± 6.9 | 60.7 ± 15.0 |
| Males, n (%) | 9 (75) | 27 (63) | 13 (65) | 63 (75) | NR | 47 (59) | 15 (93) | 10 (71) | NR |
| Type of IPVD | NO | EPO | NO | 15 patients received EPO; 69 of these patients later
received NO. Analyzed Separately. |
EPO | EPO | NO | MIL | NO |
| Oxygen Supplementation | IMV | IMV | IMV | IMV | Not specified | IMV | IMV | IMV | IMV |
| Patient Inclusion Criteria | Adults with COVID-19 ARDS who did not improve with IMV and proning | Adults with COVID-19 ARDS who received iEPO in conjunction with proning. | Adults with COVID-19 ARDS who did not improve with IMV | Adults with COVID-19 ARDS who were receiving IMV | Adults with COVID-19 ARDS who received iEPO | Adults with COVID-19 ARDS who received iEPO | Adults with COVID-19 ARDS who did not improve with IMV or proning | Adults with COVID-19 ARDS who received iMIL | Adults with COVID-19 ARDS who did not improve with IMV |
| Prone Positioning | Yes | Yes. All patients were either started no iEPO while prone or iEPO was started simultaneously with proning. |
Not specified | Yes. 30/84 patients were undergoing proning. |
Not specified | Yes. 46/80 patients. |
Yes | Yes. 6/14 patients. |
No. All 12 patients were proned after starting iNO. |
| IPVD Administration Protocol | If P/F did not improve with prone position, then iNO was initiated. | If P/F did not improve with IMV. | If P/F did not improve with IMV. | If P/F did not improve with proning or IMV. | If P/F did not improve with IMV. | If P/F did not improve with IMV. | If P/F did not improve with IMV and proning. | If P/F did not improve with IMV. | If P/F did not improve with IMV. |
| Study specific Definition of Response to IPVD | Increase in P/F ratio > 20% from baseline. | Increase in P/F ratio > 20% from baseline. | Increase in P/F ratio > 10% from baseline. | Increase in P/F ratio > 10% from baseline. | Increase in P/F ratio > 10% from baseline. | Increase in P/F ratio > 10% from baseline. | Increase in P/F ratio > 20% from baseline. | Not specified | Not Specified |
| Time to First Assessment of P/F Ratio Post IPVD | 30 min | 2 h | 24 h | 24 h | 6 h | 1.3–4.9 h | 15–30 min | 6 h | 16 h |
Abbreviations: ARDS, acute respiratory distress syndrome; COVID-19, Coronavirus disease 19; EPO, epoprostenol; HFNC, high flow nasal cannula; iEPO, inhaled epoprostenol; iMIL, inhaled milrinone; IMV, invasive mechanical ventilation; iNO, inhaled nitric oxide; IPVD, inhaled pulmonary vasodilator; MIL, milrinone; NIV, non-invasive ventilation; NO, nitric oxide; NR, not reported; SD, standard deviation.
Table 3.
Primary and Secondary Outcomes of the Included Studies in the 1-arm Meta-Analysis.
| Study, year | P/F ratio (mm Hg) | PEEP (cmH2O) | Lung compliance (ml/cmH2O) | |||
|---|---|---|---|---|---|---|
| Pre-IPVD (SD) | Post-IPVD (SD) | Pre-IPVD (SD) | Post-IPVD (SD) | Pre-IPVD (SD) | Post-IPVD (SD) | |
| Abou-Arab 2020 | 96.4 (55.4) | 134.1 (50.9) | 11.8 (4) | 12.1 (4.1) | 27.4 (10.4) | 29.6 (9.5) |
| Bagate 2020 | 109 (38.7) | 126.2 (32.7) | NR | NR | NR | NR |
| Bonizzoli 2022 | 67 (26) | 83 (42) | 11 (2) | 11 (2) | NR | NR |
| Cardinale 2020 | 89.5 (12) | 94.4 (13.6) | NR | NR | NR | NR |
| Chiles 2022 | 93 (45.8) | 87.8 (44.3) | NR | NR | NR | NR |
| DeGrado 2020- EPO | 130 (49) | 138 (56) | 15 (3) | 15 (3 | 32 (10) | 35 (15) |
| DeGrado 2020- NO | 119 (51) | 133 (48) | 16 (3) | 16 (3) | NR | NR |
| Ferrari 2020 | 81 (19) | 84 (22) | NR | NR | NR | NR |
| Garfield 2021 | 102 (29.3) | 130.5 (41.3) | NR | NR | NR | NR |
| Imtiaz 2021 | 95.9 (42) | 119 (51) | 13.7 (3.3) | 15.1 (4.1) | NR | NR |
| Laghlam 2021 | 146 (48) | 185 (76) | NR | NR | 25.7 (6.2) | 25 (5.9) |
| Li 2020 | 87.7 (30.5) | 120.4 (57.1) | 15.1 (3.9) | 16.2 (3) | 26.6 (8.8) | 25.1 (7.2) |
| Longobardo 2020 | 98.8 (29.2) | 142.7 (116.6) | NR | NR | NR | NR |
| Lubinsky 2022- NO | 93.7 (59.7) | 89.6 (55.7) | NR | NR | NR | NR |
| Lubinsky 2022- EPO | 68 (65) | 64.6 (32.2) | NR | NR | NR | NR |
| Niss 2022 | 72 (26) | 89 (26) | NR | NR | NR | NR |
| Sonti 2021 | 96.2 (36.2) | 110.6 (48.3) | NR | NR | NR | NR |
| Tavazzi 2020 | 87.3 (38.3) | 88.2 (32.2) | 12.6 (4.1) | 12.6 (4.1) | NR | NR |
| Vogel 2021 | 87 (12) | 110.3 (4.4) | NR | NR | NR | NR |
| Ziehr 2021 | 126.1 (76.3) | 174 (62.9) | NR | NR | NR | NR |
Abbreviations: EPO, Epoprostenol; IPVD, Inhaled Pulmonary Vasodilators; NO, Nitric Oxide; NR, Not Reported; P/F, PaO2/FiO2; PEEP, Positive End-Expiratory Pressure; SD, Standard Deviation.
Table 2.
Study and Patient Characteristics of the Included 2-arm Studies.
| Study, year | Chandel 2021 | Kataria 2022 | Matthews 2022 |
|---|---|---|---|
| Study design | Retrospective Cohort | Retrospective Cohort | Retrospective Cohort |
| Country | United States of America | United States of America | United Kingdom |
| Total patient (IPVD/Standard) | 66/206 | 30/30 | 59/249 |
| IPVD group age, mean ± SD, years | 57 ± 13 | 56.9 ± 38.9 | 60.0 ± 9.1 |
| Standard Therapy group age, mean ± SD, years | 56 ± 14 | 60.7 ± 35.8 | 59.3 ± 17.9 |
| Male in IPVD group, n (%) | 45 (68) | 17 (57) | 37 (63) |
| Male in Standard Therapy group, n (%) | 135 (66) | 15 (50) | 164 (66) |
| Type of IPVD | NO | EPO | NO & ILO. Analyzed Together. |
| Oxygen Supplementation | HFNC | HFNC | IMV |
| Patient Inclusion Criteria | Non-intubated adults with COVID-19 ARDS that had been on HFNC for >/=2 h. | Non-intubated adults with COVID-19 ARDS requiring HFNC >/=50 liter/minute at >/=80% FiO2 and had been treated with DXM 6 mg daily for 10 days. | Mechanically ventilated adults with COVID-19 ARDS who received IPVD for >/=24 h. |
| Prone | Yes. Patients were self-proning. |
Yes. Patients were self-proning. |
Yes. All IPVD group patients were proned for at least 3 cycles (16 h/cycle) before IPVD initiation. |
| Study Specific Definition of Response to IPVD | Decrease in FiO2 requirement via HFNC 12 h after iNO. | Decrease in FiO2 requirement via HFNC. | Increase in P/F ratio > 10% from baseline. |
| Time to First Assessment of Respiratory Parameters Post IPVD | 12 h | 1 h | 2 h |
Abbreviations: ARDS, acute respiratory distress syndrome; COVID-19, Coronavirus disease 19; EPO, epoprostenol; HFNC, high flow nasal cannula; iEPO, inhaled epoprostenol; ILO, iloprost; IMV, invasive mechanical ventilation; iNO, inhaled nitric oxide; IPVD, inhaled pulmonary vasodilator; NO, nitric oxide; SD, standard deviation.
A total of 613 patients were included in the 1-arm meta-analysis, with males representing 68% of the total patients, with mean age being 60.5 years. A total of 640 patients were included in the 2-arm meta-analysis, with males representing 64.5% of the total patients. The mean age of the patients in the IPVD group was 58.0 years, and 58.7 years in the standard therapy group.
1-arm Meta-Analysis
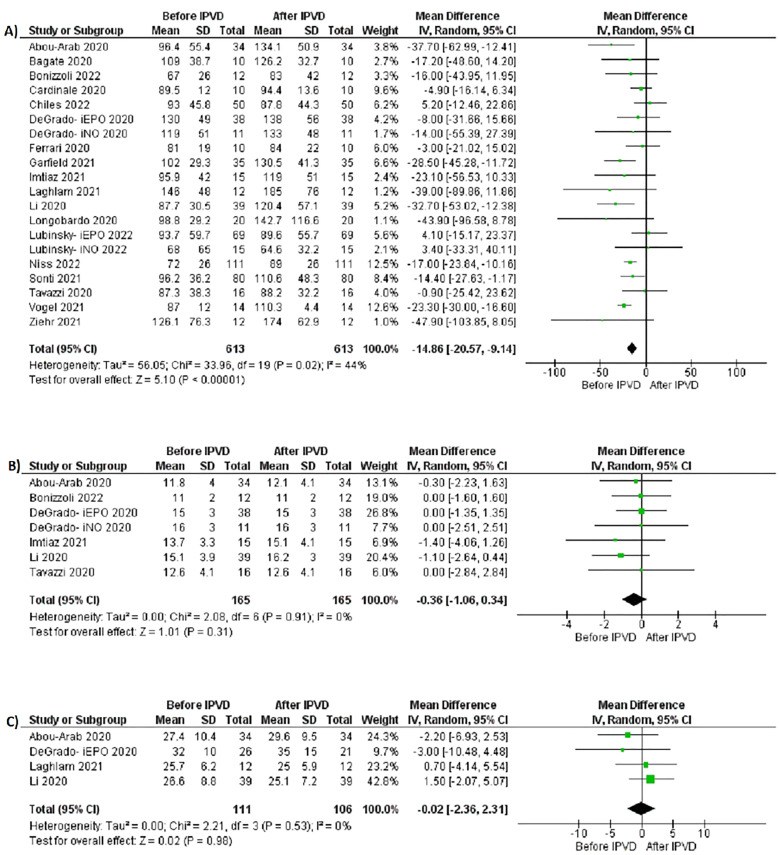
Table 3 summarizes the outcomes of the individual studies included in the 1-arm meta-analysis. All 18 studies reported pre- and post-IPVD P/F ratios of the patients involved. The pre-IPVD P/F ratios were significantly lower compared to the post-IPVD P/F ratios (MD −14.86 mm Hg; 95% CI −20.57.46, −9.14; P = <.00001, I2 = 44%, Figure 2A). A leave-one-out sensitivity analysis showed consistent results (Supplementary Figure 1). A subgroup analysis based on type of IPVD used (EPO vs NO) similarly showed significantly lower pre-IPVD P/F ratios for both the EPO and NO subgroups (Figure 3).
Figure 2.
Forest plots for 1-arm meta-analysis: (A) pre- and post-IPVD PaO2/FiO2 ratios, (B) pre- and post-IPVD positive end-expiratory pressure, (C) pre- and post-IPVD lung compliance.
Figure 3.
Subgroup analysis based on type of IPVD for studies in the 1-arm meta-analysis in regards pre- and post-IPVD PaO2/FiO2 ratios.
Six studies18,21,23,28,32,34 reported pre- and post-IPVD PEEP. There was no significant difference between the pre- and post-IPVD PEEP (MD −0.36 cmH2O; 95% CI −1.06, 0.34; P = .31, I2 = 0%, Figure 2B).
Four studies18,21,23,35 reported pre- and post-IPVD lung compliance. There was no significant difference between the pre- and post-IPVD lung compliance (MD −0.02 ml/cmH2O; 95% CI −2.36, 2.31; P = .98, I2 = 0%, Figure 2C).
2-arm Meta-Analysis
Table 4 summarizes the outcomes of the individual studies included in the 2-arm meta-analysis. All 3 studies reported mortality rate. The mortality rate was 36% in the IPVD group compared to 51% in the standard therapy group. There was no significant difference in the mortality rate between the two groups (RR 0.78, 95% CI 0.58-1.04, P = .09, I2 = 29%, Figure 4A).
Table 4.
Primary and Secondary Outcomes of the Included Studies in the 2-arm Meta-Analysis.
| Study, year | Mortality | Need for endotracheal intubation | Hospital length of stay (Days) | |||
|---|---|---|---|---|---|---|
| IPVD (%) | Standard therapy (%) | IPVD (%) | Standard therapy (%) | IPVD (SD) | Standard therapy (SD) | |
| Chandel 2021 | 12 (18.2) | 36 (17.5) | 29 (43.9) | 79 (38.3) | 20.7 (15.2) | 15.1 (8.2) |
| Kataria 2022 | 13 (43.3) | 14 (46.7) | 21 (70) | 27 (90) | 38.1 (57.8) | 43 (66.4) |
| Matthews 2022 | 31 (52.5) | 199 (79.9) | NR | NR | NR | NR |
Abbreviations: IPVD, Inhaled Pulmonary Vasodilators; NR, Not Reported; SD, Standard Deviation.
Figure 4.
Forest plots for 2-arm meta-analysis comparing IPVDs to standard therapy: (A) mortality, (B) need for endotracheal intubation, (C) hospital length of stay.
Across the two studies13,14 that reported the intubation rate; 52% of patients who received IPVD required intubation compared to 45% in patients who received standard therapy. The need for endotracheal intubation was similar between two groups (RR 0.93, 76% CI 0.61-1.42, P = .75, I2 = 76%, Figure 4B).
Two studies13,14 reported the hospital LOS, which was significantly higher in standard therapy group compared to IPVD group (MD 5.45 days; 95% CI 1.64, 9.25; P = .005, I2 = 0%, Figure 4C).
Responder vs. Non-responder Analysis
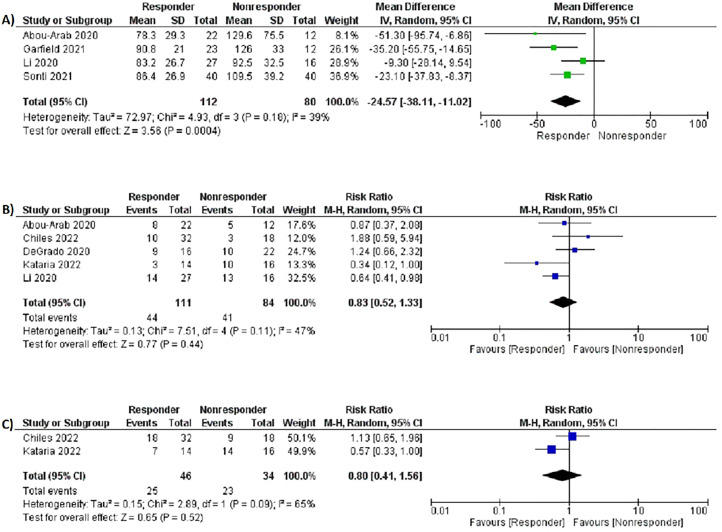
Table 5 summarizes the outcomes of the responder versus non-responder subgroup analysis. Seven studies13,18,20–23,27 specifically looked at IPVD responders and non-responders. Baseline P/F ratios were significantly lower in the IPVD responder group compared to non-responders (MD −24.57 mm Hg; 95% CI −38.11-11.02; P = .0004, I2 = 39%, Figure 5A).
Table 5.
Outcomes of the Responder Versus non-Responder Subgroup Analysis.
| Study, year | Baseline P/F ratios (mm Hg) | Mortality | Need for endotracheal intubation | |||
|---|---|---|---|---|---|---|
| Responders (SD) | Non-responders (SD) | Responders (%) | Non-responders (%) | Responders (%) | Non-responders (%) | |
| Abou-Arab 2020 | 78.3 (29.3) | 129.6 (75.5) | 8 (36.4) | 5 (41.7) | NR | NR |
| Chiles 2022 | NR | NR | 10 (31.3) | 3 (16.7) | 18 (56.3) | 9 (50) |
| DeGrado 2020 | NR | NR | 9 (56.3) | 10 (45.5) | NR | NR |
| Garfield 2021 | 90.8 (21) | 126 (33) | NR | NR | NR | NR |
| Kataria 2022 | NR | NR | 3 (21.4) | 10 (62.5) | 7 (50) | 14 (87.5) |
| Li 2020 | 83.2 (26.7) | 92.5 (32.5) | 14 (51.9) | 13 (81.3) | NR | NR |
| Sonti 2021 | 86.4 (26.9) | 109.5 (39.2) | NR | NR | NR | NR |
Abbreviations: NR, Not Reported; P/F, PaO2/FiO2; SD, Standard Deviation.
Figure 5.
Forest plots of responder versus non-responder subgroup analysis: (A) baseline PaO2/FiO2 ratios, (B) mortality, (C) need for endotracheal intubation.
Mortality (RR 0.83, 95% CI 0.52-1.33, P = .44, I2 = 47%, Figure 5B) and need for endotracheal intubation (RR 0.80, 95% CI 0.41-1.56, P = .52, I2 = 65%, Figure 5C) were similar for both IPVD responders and non-responders.
Quality and Publication Bias Assessment
Quality assessment scoring of the included single arm and 2-arm observational studies was performed using the NOS for assessing nonrandomized studies, and the scores are summarized in Supplementary Table 2. JBI critical appraisal tool was used to assess the quality of the case series, and the results are summarized in Supplementary Table 3. All the included studies were of high quality. The funnel plot evaluating the studies in the 1-arm meta-analysis in regards to pre- and post-IPVD P/F ratios did not demonstrate statistically significant publication bias based on the Egger's regression (P = .42) (Supplementary Figure 2).
Discussion
This is the first meta-analysis looking at IPVD in COVID-19 ARDS. Although the P/F ratios did improve with IPVD, as noted in the 1-arm meta-analysis, there was no benefits noted in terms of mortality and need for endotracheal intubation in the 2-arm meta-analysis. Even in patients that responded to IPVD adequately, we found no significant difference in mortality and need for endotracheal intubation.
IPVD, especially NO, have been studied in ARDS patient before the COVID-19 pandemic.10 As a result, there are many RCTs that have investigated their use in non-COVID-19 patients, outcomes of these studies show that inhaled NO (iNO) improves P/F ratios and oxygenation, but does not significantly reduce mortality or need for mechanical ventilation.11,37 Recent trials regarding COVID-19 ARDS and IPVD even showed no significant improvement in oxygenation.21,25 IPVD, especially NO, are expensive and their use has not shown much benefit historically, as a meta-analysis by Adhikari et al38 involving non-COVID-19 patients treated with iNO, commented on lack of mortality benefits. The use of IPVD in COVID-19 ARDS has also become a common practice, without concrete data supporting IPVD use. A recent systematic review of studies using iNO in COVID-19 patients commented that most studies in literature do not comment on mortality.39
Chandel et al14 published the first comparative study between IPVD and standard therapy in COVID-19 ARDS patients and showed no morality benefits in using IPVD. All the while, multiple studies showed an increase in P/F ratios and an improvement in oxygenation after IPVD.18,27 Given the increasing literature, we conducted this meta-analysis to provide the first comprehensive evaluation of the literature that exists for IPVD in COVID-19 ARDS patients and address critical knowledge gaps to see if this improvement in P/F ratios leads to improved mortality benefits.
Our study results were consistent with a pervious meta-analysis by Adhikari et al38 in 2014, that examined the role of iNO in non-COVID-19 ARDS patients and found that although IPVD may improve oxygenation, they do not lead to mortality benefits. In our meta-analysis, we found similar results to those from a study by Prakash et al39 which showed that IPVD in COVID-19 ARDS can improve oxygenation, but similar to the study by Chandel et al,14 there are no mortality benefits to using IPVD in COVID-19 patients. Furthermore, our subgroup analysis showed that even in patients who responded adequately to IPVD, there are no mortality benefits when compared to IPVD non-responders. Due to this lack of benefit in clinically meaningful outcomes, we believe that there is an urgent need for clear protocols to guide the practice of using IPVD for COVID-19 patients. Future RCTs may be required to further study the efficacy of IPVD in COVID-19 patients.
Several limitations of this study should be acknowledged. First, because the literature lacks RCTs, our meta-analysis included only observational studies and case series. Future RCTs are warranted to confirm our findings. Second, our 1-arm meta-analysis for changes in P/F ratios can be influenced by ventilator settings that are not often standardized between facilities. Third, our 2-arm meta-analysis only involved 3 studies, which limits the statistical power of our analysis to be able to detect statistically significant differences in mortality between the IPVD and standard therapy cohorts. Fourth, the IPVD regimens were not standardized even among the studies that used the same IPVD, making it difficult to compare the results from each study and can it also introduce heterogeneity. Lastly, the lack of patient-level data did not allow to control for possible variations in baseline characteristics because other medications used for COVID-19 might have been different and not standardized between patients, which might introduce potential bias.
Despite the limitations, our study has significant strengths. First, we included a total of 18 studies in the 1-arm meta-analysis and 3 studies in the 2-arm analysis, with a total of 613 and 640 patients, respectively. To our knowledge, this is the first meta-analysis investigating the effects of IPVDs in COVID-19 ARDS patients. Although the studies included were observational in nature, our results were similar to previous RCTs conducted for non-COVID-19 ARDS patients. Consistent results were noted on our subgroup analysis based on type IPVD (iNO vs iEPO) in regards to pre- and post-IPVD P/F ratios. Through our responder versus non-responder analysis, we were able show that the use IPVD do not seem to provide mortality benefits even in patients that respond appropriately. Lastly, all the studies in our meta-analysis were of high quality based on the NOS and JBI quality assessment tools.
In conclusion, the results of our 1-arm and 2-arm meta-analyzes demonstrated that although IPVD may improve P/F ratios and oxygenation, they did not provide mortality benefits compared to standard therapy alone. Thus, based on the available evidence the use of IPVD in COVID-19 patients should not be pursued. However, given the observational nature of the included studies, RCTs are warranted to validate our findings.
Supplemental Material
Supplemental material, sj-docx-1-jic-10.1177_08850666221118271 for Inhaled Pulmonary Vasodilators in COVID-19 Infection: A Systematic Review and Meta-Analysis by Waleed Khokher, Saif-Eddin Malhas, Azizullah Beran, Saffa Iftikhar, Cameron Burmeister, Mohammed Mhanna, Omar Srour, Rakin Rashid, Nithin Kesireddy and Ragheb Assaly in Journal of Intensive Care Medicine
Footnotes
Author Contributions: WK and AB conceived and designed the study and critically revised the manuscript. WK and SEM collected, analyzed, interpreted the data, and drafted the manuscript. SI, CB, MM, OS, RR, and NK collected the data and reviewed the literature. RA supervised and critically revised the manuscript. All authors had access to the data and a role in writing the manuscript. All authors read and approved the final manuscript.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article
ORCID iD: Waleed Khokher https://orcid.org/0000-0001-9539-5583
Supplemental Material: Supplemental material for this article is available online.
References
- 1.Kiang MV, Irizarry RA, Buckee CO, Balsari S. Every body counts: measuring mortality from the COVID-19 pandemic. Ann Intern Med. 2020;173(12):1004-1007. doi: 10.7326/m20-3100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kumar A, Singh R, Kaur J, et al. Wuhan to world: the COVID-19 pandemic. Front Cell Infect Microbiol. 2021;11:596201. doi: 10.3389/fcimb.2021.596201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hasan SS, Capstick T, Ahmed R, et al. Mortality in COVID-19 patients with acute respiratory distress syndrome and corticosteroids use: a systematic review and meta-analysis. Expert Rev Respir Med. 2020;14(11):1149-1163. doi: 10.1080/17476348.2020.1804365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ranieri VM, Rubenfeld GD, Thompson BT, et al. Acute respiratory distress syndrome: the Berlin definition. Jama. 2012;307(23):2526-2533. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 5.Revercomb L, Hanmandlu A, Wareing N, Akkanti B, Karmouty-Quintana H. Mechanisms of pulmonary hypertension in acute respiratory distress syndrome (ARDS). Review. Front Mol Biosci. 2021;7. doi: 10.3389/fmolb.2020.624093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Santamarina MG, Boisier D, Contreras R, Baque M, Volpacchio M, Beddings I. COVID-19: a hypothesis regarding the ventilation-perfusion mismatch. Crit Care. 2020;24(1):395. doi: 10.1186/s13054-020-03125-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Teuwen LA, Geldhof V, Pasut A, Carmeliet P. COVID-19: the vasculature unleashed. Nat Rev Immunol. 2020;20(7):389-391. doi: 10.1038/s41577-020-0343-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Griffiths MJ, Evans TW. Inhaled nitric oxide therapy in adults. N Engl J Med. 2005;353(25):2683-2695. doi: 10.1056/NEJMra051884. [DOI] [PubMed] [Google Scholar]
- 9.Scholten EL, Beitler JR, Prisk GK, Malhotra A. Treatment of ARDS with prone positioning. Chest. 2017;151(1):215-224. doi: 10.1016/j.chest.2016.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dellinger RP, Zimmerman JL, Taylor RW, et al. Effects of inhaled nitric oxide in patients with acute respiratory distress syndrome: results of a randomized phase II trial. Inhaled nitric oxide in ARDS study group. Crit Care Med. 1998;26(1):15-23. doi: 10.1097/00003246-199801000-00011. [DOI] [PubMed] [Google Scholar]
- 11.Taylor RW, Zimmerman JL, Dellinger RP, et al. Low-dose inhaled nitric oxide in patients with acute lung injury: a randomized controlled trial. Jama. 2004;291(13):1603-1609. doi: 10.1001/jama.291.13.1603. [DOI] [PubMed] [Google Scholar]
- 12.Montgomery AB, Stager MA, Carrico CJ, Hudson LD. Causes of mortality in patients with the adult respiratory distress syndrome. Am Rev Respir Dis. 1985;132(3):485-489. doi: 10.1164/arrd.1985.132.3.485. [DOI] [PubMed] [Google Scholar]
- 13.Kataria V, Ryman K, Tsai-Nguyen G, Wakwaya Y, Modrykamien A. Evaluation of aerosolized epoprostenol for hypoxemia in non-intubated patients with coronavirus disease 2019. Hosp Pract (1995). 2022;50(2):118-123. doi: 10.1080/21548331.2022.2047310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chandel A, Patolia S, Ahmad K, et al. Inhaled nitric oxide via high-flow nasal cannula in patients with acute respiratory failure related to COVID-19. Clin Med Insights Circ Respir Pulm Med. 2021;15:11795484211047065. doi: 10.1177/11795484211047065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. Br Med J. 2003;327(7414):557-560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25(9):603-605. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 17.Munn Z, Barker TH, Moola S, et al. Methodological quality of case series studies: an introduction to the JBI critical appraisal tool. JBI Evid Synth. 2020;18(10):2127-2133. doi: 10.11124/jbisrir-d-19-00099. [DOI] [PubMed] [Google Scholar]
- 18.Abou-Arab O, Huette P, Debouvries F, Dupont H, Jounieaux V, Mahjoub Y. Inhaled nitric oxide for critically ill COVID-19 patients: a prospective study. Crit Care. 2020;24(1):645. doi: 10.1186/s13054-020-03371-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cardinale M, Esnault P, Cotte J, Cungi PJ, Goutorbe P. Effect of almitrine bismesylate and inhaled nitric oxide on oxygenation in COVID-19 acute respiratory distress syndrome. Anaesth Crit Care Pain Med. 2020;39(4):471-472. doi: 10.1016/j.accpm.2020.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chiles JW, III, Vijaykumar K, Darby A, et al. Letter to the editor: “use of inhaled epoprostenol with high flow nasal oxygen in non-intubated patients with severe COVID-19”. J Crit Care. 2022;69:153989. doi: 10.1016/j.jcrc.2022.153989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.DeGrado JR, Szumita PM, Schuler BR, et al. Evaluation of the efficacy and safety of inhaled epoprostenol and inhaled nitric oxide for refractory hypoxemia in patients with coronavirus disease 2019. Crit Care Explor. 2020;2(10):e0259. doi: 10.1097/cce.0000000000000259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Garfield B, McFadyen C, Briar C, et al. Potential for personalised application of inhaled nitric oxide in COVID-19 pneumonia. Br J Anaesth. 2021;126(2):e72-e75. doi: 10.1016/j.bja.2020.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li J, Fink JB, Augustynovich AE, Mirza S, Kallet RH, Dhand R. Effects of inhaled epoprostenol and prone positioning in intubated coronavirus disease 2019 patients with refractory hypoxemia. Crit Care Explor. 2020;2(12):e0307. doi: 10.1097/cce.0000000000000307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Longobardo A, Montanari C, Shulman R, Benhalim S, Singer M, Arulkumaran N. Inhaled nitric oxide minimally improves oxygenation in COVID-19 related acute respiratory distress syndrome. Br J Anaesth. 2021;126(1):e44-e46. doi: 10.1016/j.bja.2020.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lubinsky AS, Brosnahan SB, Lehr A, et al. Inhaled pulmonary vasodilators are not associated with improved gas exchange in mechanically ventilated patients with COVID-19: a retrospective cohort study. J Crit Care. 2022;69:153990. doi: 10.1016/j.jcrc.2022.153990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Niss HL, Mohamed A, Berry TP, Saettele TM, Haines MM, Thomas EL. Evaluation of continuous inhaled epoprostenol in the treatment of acute respiratory distress syndrome, including patients with SARS-CoV-2 infection. Ann Pharmacother. 2022:10600280211069182. doi: 10.1177/10600280211069182. [DOI] [PubMed] [Google Scholar]
- 27.Sonti R, Pike CW, Cobb N. Responsiveness of inhaled epoprostenol in respiratory failure due to COVID-19. J Intensive Care Med. 2021;36(3):327-333. doi: 10.1177/0885066620976525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tavazzi G, Pozzi M, Mongodi S, Dammassa V, Romito G, Mojoli F. Inhaled nitric oxide in patients admitted to intensive care unit with COVID-19 pneumonia. Crit Care. 2020;24(1):508. doi: 10.1186/s13054-020-03222-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vogel DJ, Brame A, Hanks F, Remmington C, Chung N, Camporota L. Improved oxygenation with inhaled milrinone in mechanically ventilated patients with severe COVID-19. Br J Anaesth. 2021;127(3):e111-e113. doi: 10.1016/j.bja.2021.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ziehr DR, Alladina J, Wolf ME, et al. Respiratory physiology of prone positioning with and without inhaled nitric oxide across the coronavirus disease 2019 acute respiratory distress syndrome severity spectrum. Crit Care Explor. 2021;3(6):e0471. doi: 10.1097/cce.0000000000000471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bagate F, Tuffet S, Masi P, et al. Rescue therapy with inhaled nitric oxide and almitrine in COVID-19 patients with severe acute respiratory distress syndrome. Ann Intensive Care. 2020;10(1):151. doi: 10.1186/s13613-020-00769-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bonizzoli M, Lazzeri C, Cianchi G, et al. Effects of rescue inhaled nitric oxide on right ventricle and pulmonary circulation in severe COVID-related acute respiratory distress syndrome. J Crit Care. 2022:153987. doi: 10.1016/j.jcrc.2022.153987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ferrari M, Santini A, Protti A, et al. Inhaled nitric oxide in mechanically ventilated patients with COVID-19. J Crit Care. 2020;60:159-160. doi: 10.1016/j.jcrc.2020.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Imtiaz K, Jodeh W, Sudekum D, DiGiovine B, Hecht J. The use of inhaled epoprostenol for acute respiratory distress syndrome secondary due to COVID-19: a case series. J Crit Care Med (Targu Mures). 2022;8(1):33-40. doi: 10.2478/jccm-2021-0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Laghlam D, Rahoual G, Malvy J, Estagnasié P, Brusset A, Squara P. Use of almitrine and inhaled nitric oxide in ARDS due to COVID-19. Front Med (Lausanne). 2021;8:655763. doi: 10.3389/fmed.2021.655763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Matthews L, Baker L, Ferrari M, et al. Compassionate use of pulmonary vasodilators in acute severe hypoxic respiratory failure due to COVID-19. J Intensive Care Med. 2022:8850666221086521. doi: 10.1177/08850666221086521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lundin S, Mang H, Smithies M, Stenqvist O, Frostell C. Inhalation of nitric oxide in acute lung injury: results of a European multicentre study. The European study group of inhaled nitric oxide. Intensive Care Med. 1999;25(9):911-919. doi: 10.1007/s001340050982. [DOI] [PubMed] [Google Scholar]
- 38.Adhikari NK, Dellinger RP, Lundin S, et al. Inhaled nitric oxide does not reduce mortality in patients with acute respiratory distress syndrome regardless of severity: systematic review and meta-analysis. Crit Care Med. 2014;42(2):404-412. doi: 10.1097/CCM.0b013e3182a27909. [DOI] [PubMed] [Google Scholar]
- 39.Prakash A, Kaur S, Kaur C, et al. Efficacy and safety of inhaled nitric oxide in the treatment of severe/critical COVID-19 patients: a systematic review. Indian J Pharmacol. 2021;53(3):236-243. doi: 10.4103/ijp.ijp_382_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-jic-10.1177_08850666221118271 for Inhaled Pulmonary Vasodilators in COVID-19 Infection: A Systematic Review and Meta-Analysis by Waleed Khokher, Saif-Eddin Malhas, Azizullah Beran, Saffa Iftikhar, Cameron Burmeister, Mohammed Mhanna, Omar Srour, Rakin Rashid, Nithin Kesireddy and Ragheb Assaly in Journal of Intensive Care Medicine