Summary
Heterologous vaccination against coronavirus disease 2019 (COVID-19) provides a rational strategy to rapidly increase vaccination coverage in many regions of the world. Although data regarding messenger RNA (mRNA) and ChAdOx1 vaccine combinations are available, there is limited information about the combination of these platforms with other vaccines widely used in developing countries, such as BBIBP-CorV and Sputnik V. Here, we assess the immunogenicity and reactogenicity of 15 vaccine combinations in 1,314 participants. We evaluate immunoglobulin G (IgG) anti-spike response and virus neutralizing titers and observe that a number of heterologous vaccine combinations are equivalent or superior to homologous schemes. For all cohorts in this study, the highest antibody response is induced by mRNA-1273 as the second dose. No serious adverse events are detected in any of the schedules analyzed. Our observations provide rational support for the use of different vaccine combinations to achieve wide vaccine coverage in the shortest possible time.
Keywords: SARS-CoV-2, heterologous vaccination, humoral response, Omicron variant, neutralizing antibodies, Sputnik V, ChAdOx1-S, BBIBP-CorV, Ad5-nCoV, mRNA-1273
Graphical abstract
Highlights
-
•
Heterologous vaccine combinations are equivalent or superior to homologous schemes
-
•
No serious adverse events are detected in any of the heterologous schedules analyzed
-
•
The highest antibody response is induced by mRNA-1273 as heterologous second dose
-
•
Heterologous schemes improve antibody response in individuals primed with BBIBP-CorV
Pascuale et al. provide data on antibody response against SARS-CoV-2 after heterologous vaccine schemes, including non-replicating adenovirus, mRNA, and inactivated vaccines. This study shows a number of combinations that are equivalent or superior to the homologous schemes, supporting this strategy for achieving wide coverage in regions with limited vaccine supply.
Introduction
The rapid development of highly effective vaccines against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) represents one of the greatest scientific achievements in the contemporary world. However, the lack of universal and equitable access to coronavirus disease 2019 (COVID-19) vaccines threatens the lives of millions while creating favorable conditions for the emergence of new variants of concern (VOCs). In fact, the new VOC Omicron, endowed with a huge capacity to evade the neutralizing activity of serum from either vaccinated or convalescent individuals,1, 2, 3 was first characterized in South Africa,4 a country where only 31% of the population have received the complete initial vaccination protocol.
Because of concerns about very rare thrombotic events after vaccination with ChAdOx1-S (Oxford-AstraZeneca), some European countries have recommended heterologous messenger RNA (mRNA) boost strategies for young persons who have received one dose of ChAdOx1-S. Different trials have analyzed the reactogenicity, immunogenicity, and effectiveness of the mRNA vaccines BNT162b2 and mRNA-1273 administered as second doses in individuals primed with ChAdOx1-S. These studies have consistently shown that the heterologous schemes show a good reactogenicity profile and result in a higher immunogenicity and effectiveness compared with the homologous scheme ChAdOx1-S/ChAdOx1-S.5, 6, 7, 8 These studies supported the possibility of exploring heterologous vaccination as a suitable means of accelerating vaccination. However, limited information is available about heterologous vaccination regimens different from those based in the use of mRNA vaccines and ChAdOx1-S.
Different anti-SARS-CoV-2 vaccines are currently used in Argentina including the non-replicating adenovirus vaccines Sputnik V (Gamaleya Institute), ChAdOx1-S (Oxford AstraZeneca), and Ad5-nCoV (CanSino), the mRNA vaccines BNT162b2 (Pfizer) and mRNA-1273 (Moderna), and the inactivated SARS-CoV-2 vaccine BBIBP-CorV (Sinopharm). To date, more than 90% of Argentinians older than 50 have been vaccinated with two doses, and starting in July 2021, different heterologous vaccination schemes have been recommended. Here, we evaluated immunogenicity and reactogenicity comparing homologous vaccination programs using either Sputnik V, ChAdOx1-S, or BBIBP-CorV with different heterologous schemes to define strategies to accelerate vaccination plans.
Results
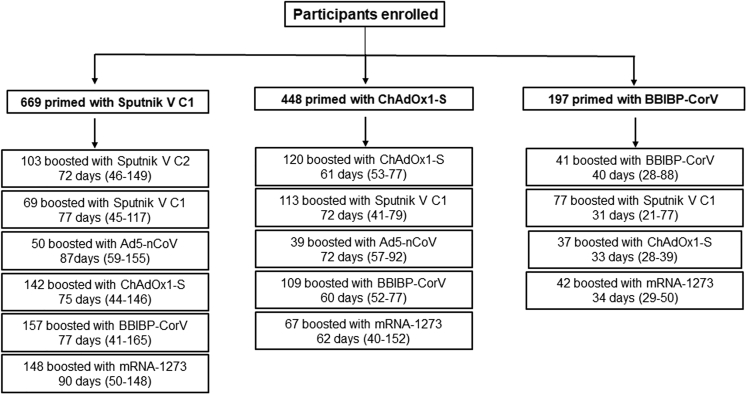
The study was performed at four sites across Argentina. A total of 6,917 individuals expressed their intention to participate in the study, and 1,314 were finally enrolled who had received a first dose of Sputnik V C1 (n = 669), ChAdOx1-S (n = 448), or BBIBP-CorV (n = 197) and met the inclusion and exclusion criteria. The Sputnik V C1 cohort included 6 different arms, while the ChAdOx1-S and the BBIBP-CorV cohorts included 5 and 4 arms, respectively (Figure 1). Baseline characteristics across the different arms are shown in Table 1. Kruskal-Wallis one-way ANOVA was performed to compare ages of participants, and no significant differences among the arms within each cohort were observed.
Figure 1.
Cohort description
For each scheme, the number of participants, the median time, and the range between doses are indicated.
Table 1.
Baseline characteristics by arms of the study
| Sputnik V C1 cohort (n = 669) | ||||||
|---|---|---|---|---|---|---|
| Sputnik V C2 | Sputnik V C1 | Ad5-nCoV | ChAdOx1-S | BBIBP-CorV | mRNA-1273 | |
| Participants, n | 103 | 69 | 50 | 142 | 157 | 148 |
| Age, years, mean (SD) | 45 (11) | 41 (10) | 49 (12) | 46 (12) | 43 (11) | 49 (12) |
| Gender, n (%) | ||||||
| Female | 49 (48) | 36 (52) | 31 (66) | 60 (42) | 72 (46) | 78 (53) |
| Male | 54 (52) | 33 (48) | 16 (34) | 82 (58) | 85 (54) | 70 (47) |
| Comorbidities, n (%) | ||||||
| Diabetes | 4 (3.9) | 3 (4.3) | 0 (0) | 5 (3.5) | 12 (7.6) | 7 (4.7) |
| Obesity | 13 (12.6) | 9 (13.0) | 1 (2.6) | 19 (13.4) | 26 (1.6) | 10 (6.8) |
| Cardiovascular disease | 13 (12.6) | 3 (4.3) | 0 (0) | 13 (9.2) | 10 (6.4) | 10 (6.8) |
| Respiratory disease | 10 (9.7) | 5 (7.2) | 0 (0) | 7 (4.9) | 12 (7.6) | 6 (4.1) |
| ChAdOx1 cohort (n = 448) | |||||
|---|---|---|---|---|---|
| ChAdOx1-S | Sputnik V C1 | Ad5-nCoV | BBIBP-CorV | mRNA-1273 | |
| Participants, n | 120 | 113 | 39 | 109 | 67 |
| Age, years, mean (SD) | 43 (11) | 41 (10) | 34 (8) | 40 (9) | 36 (20) |
| Gender, n (%) | |||||
| Female | 52 (43) | 52 (46) | 23 (59) | 52 (48) | 30 (45) |
| Male | 68 (57) | 61 (54) | 16 (41) | 57 (52) | 37 (55) |
| Comorbidities, n (%) | |||||
| Diabetes | 0 (0) | 2 (1.8) | 0 (0) | 2 (1.8) | 0 (0) |
| Obesity | 4 (3.3) | 6 (5.3) | 1 (2.6) | 8 (7.3) | 1 (1.5) |
| Cardiovascular disease | 3 (2.5) | 0 (0) | 0 (0) | 4 (3.7) | 0 (0) |
| Respiratory disease | 3 (2.5) | 6 (5.3) | 0 (0) | 6 (5.5) | 1 (1.5) |
| BBIBP-CorV cohort (n = 197) | ||||
|---|---|---|---|---|
| BBIBP-CorV | Sputnik V C1 | ChAdOx1-S | mRNA-1273 | |
| Participants, n | 41 | 77 | 37 | 42 |
| Age, years, mean (SD) | 36 (29) | 30 (12) | 25 (11) | 26 (11) |
| Gender, n (%) | ||||
| Female | 23 (56) | 40 (52) | 21 (57) | 21 (55) |
| Male | 18 (44) | 37 (48) | 16 (43) | 17 (45) |
| Comorbidities, n (%) | ||||
| Diabetes | 2 (4.9) | 1 (1.3) | 1 (2.7) | 0 (0) |
| Obesity | 0 (0) | 1 (1.3) | 0 (0) | 0 (0) |
| Cardiovascular disease | 2 (4.9) | 0 (0) | 3 (8.1) | 0 (0) |
| Respiratory disease | 0 (0) | 1 (1.3) | 0 (0) | 1 (2.6) |
Data are n (%) and mean (SD). n, number of volunteers; SD, standard deviation; C1, component 1; C2, component 2.
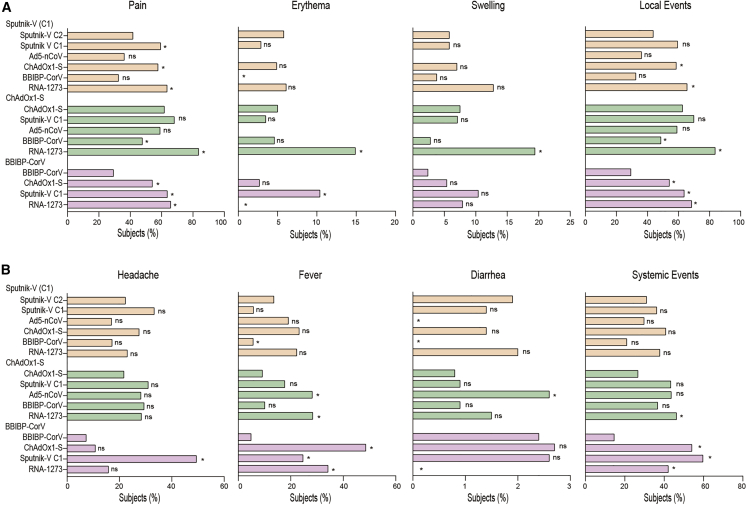
Adverse effects reported by volunteers were analyzed. For all vaccination schemes, pain at the injection site was the more frequent local reaction after second dose (range: 25% to 83%) (Figure 2). Homologous vaccination with BBIBP-CorV showed the lowest frequency of local reactions, while combination with mRNA-1273 induced the highest frequency of local reactions among all combinations analyzed. Regarding systemic ESAVI, headache and fever showed similar frequency traits between 5% and 50%, while diarrhea occurred at a very low frequency (<5%). Within the Sputnik V C1 cohort, local but not systemic reactions were more frequent after combination with ChAdOx1-S and mRNA-1273 compared with the original schedule group. In the ChAdOx1-S cohort, combination with mRNA-1273 resulted in higher frequencies of both local and systemic reactions compared with the homologous schedules, while administration of BBIBP-CorV increased the frequency of local but not systemic reactions. Finally, within the BBIBP-CorV cohort, combination with either Sputnik V C1, ChAdOx1-S, or mRNA-1273 increased the frequency of local and systemic reactions compared with the homologous schedule (Figure 2). No serious adverse events, hospitalizations, or deaths occurred in any of the study arms during follow up for 7 days after second dose.
Figure 2.
Adverse events of solicited local and systemic reactions in days 0–7 following the application of the second vaccine dose by study arm via telephone contacting
Adverse events included either local reactions (A) (pain, erythema, and swelling) or systemic reactions (B) (headache, fever, and diarrhea). The proportion of participants with local or systemic adverse event was reported by vaccine schedule, and statistical analysis was performed using the χ2 test. Statistical significance is shown with the following notations: ∗p < 0.05, ns, not significant. Sputnik V C1 vaccine (rAd26, Gamaleya), Sputnik V C2 vaccine (rAd5, Gamaleya), ChAdOx1-S vaccine (AstraZeneca), BBIBP-CorV vaccine (Sinopharm), Ad5-nCoV vaccine (CanSino), mRNA-1273 vaccine (Moderna).
The time intervals are according to the definition of the Strategic Plan in Argentina: at least 8 weeks for schemes initiated with viral vector vaccines and 4 weeks for schemes initiated with inactivated vaccines. The time elapsed between the first and second vaccine doses was not significantly different among the arms within each cohort, with a median of 80 days for Sputnik V C1, 63 days for ChAdOx1-S, and 34 days for BBIBP-CorV cohorts (Figure 1). At the time of the second dose, the level of SARS-CoV-2 anti-spike immunoglobulin G (IgG) antibodies and the titer of neutralizing antibodies were not significantly different among all arms within each cohort. Vaccination with a first dose of either Sputnik V C1 or ChAdOx1-S induced a higher antibody response than one dose of BBIBP-CorV (p < 0.0001) (Figures S1A and S1B).
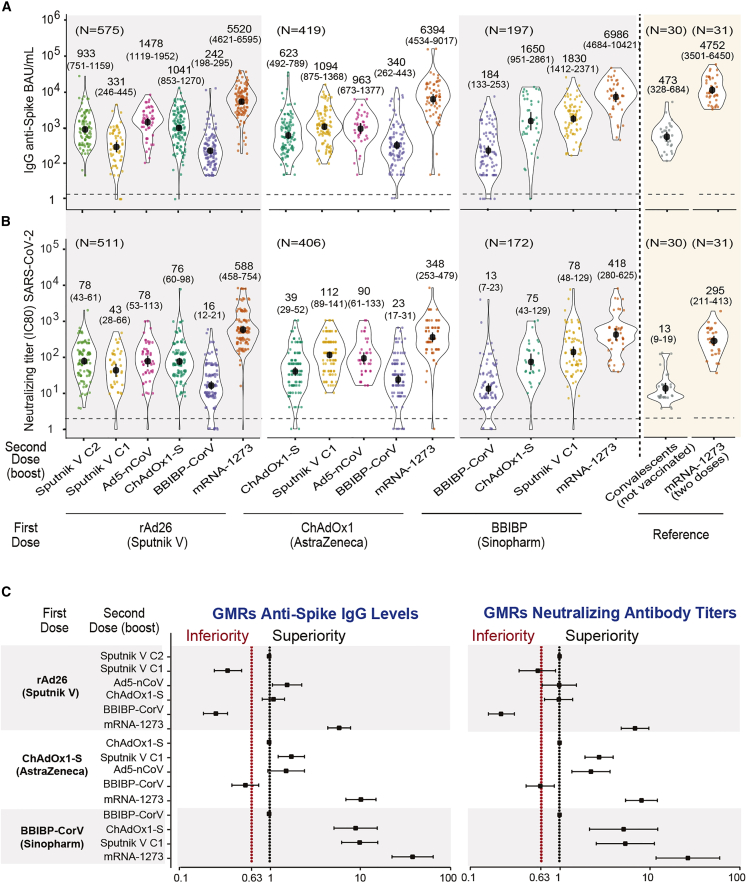
The concentration of SARS-CoV-2 anti-spike IgG antibodies and the titer of neutralizing antibodies for each arm of the Sputnik V C1, ChAdOx1-S, and BBIBP-CorV cohorts were evaluated at days 14 and 28 after the second dose. The non-inferiority criterion was applied as described in the STAR Methods, comparing the production of anti-spike IgG antibodies and neutralizing antibody titers with the homologous regimen. The IgG levels and the neutralizing titers at 14 days for the three cohorts are shown in Figures 3A and 3B, respectively. The geometric mean ratios (GMRs) for each arm compared with the homologous schemes are shown in Figure 3C. For the Sputnik V C1 cohort, the combination with either BBIBP-CorV or Sputnik V C1 was statistically inferior to that of the original Sputnik V C1/Sputnik V C2 scheme. In contrast, combination with mRNA-1273, ChAdOx1-S, or Ad5-nCoV was shown to be equivalent or superior compared with the homologous regimen. The ChAdOx1-S cohort showed that heterologous schedule with BBIBP-CorV was significantly inferior compared with the homologous scheme, while the use of either Sputnik V C1, Ad5-nCoV, or mRNA-1273 resulted in equivalent or superior responses (Figures 3A and 3B). Finally, in the BBIBP-CorV cohort, the combination with either Sputnik V C1, ChAdOx1-S, or mRNA-1273 was shown to be superior compared with the homologous scheme. Analysis at 28 days after the second dose showed similar results as those observed at 14 days for all the cohorts and arms evaluated (Figure S2). Importantly, for all cohorts analyzed, the highest antibody response was induced by mRNA-1273 as the second dose. Interestingly, the levels of IgG anti-spike antibodies and the serum neutralizing capacity of the heterologous combinations with mRNA-1273 in all arms were similar to those obtained with the homologous two-dose schedule with mRNA-1273, which was used as reference (Figures 3A and 3B). In addition, combining the virus inactivated vaccine BBIBP-CorV with the Sputnik-V C1, ChAdOx1-S, or mRNA-1273 platform resulted in a marked increase in the antibody response (up to 38-fold) compared with the homologous schemes (Figure 3C).
Figure 3.
Antibody response in participants with homologous and heterologous vaccine combinations evaluated at day 14 after second dose administration
(A) IgG anti-spike antibody levels quantified according to the WHO International Antibody Standard. The geometric means with 95% confidence intervals are shown. As reference, the level of IgG anti-spike antibodies for convalescents and a group that was vaccinated with a two-dose scheme of mRNA-1273 are shown on the right.
(B) Neutralizing titers of serum antibodies against the ancestral SARS-CoV-2 B1 variant of samples shown in (A). The geometric means with 95% confidence intervals are shown. Mann-Whitney test was used to compare the two-dose mRNA-1273 vaccine reference with the heterologous arms including Sputnik V C1, ChAdOx1-S, and BBIBP-CorV as first dose and the mRNA-1273 vaccine as second dose.
(C) Non-inferiority analysis for the antibody response of heterologous schedules compared with homologous schedules at day 14 after the administration of the second dose. The heterologous group was considered inferior or superior to the homologous group if the lower limit of the one-sided 97.5% confidence interval (CI) was lower than 0.63 or greater than 1, respectively. Geometric mean ratio (GMR) and two-sided 95% CIs are presented. Sputnik V C1 vaccine (rAd26, Gamaleya), Sputnik V C2 vaccine (rAd5, Gamaleya), ChAdOx1-S vaccine (AstraZeneca), BBIBP-CorV vaccine (Sinopharm), Ad5-nCoV vaccine (CanSino), mRNA-1273 vaccine (Moderna).
See also Figures S1–S3.
We also analyzed the neutralizing capacity of a subset of serum samples from different arms of each cohort against a locally isolated Omicron BA.1 variant (GISAID: EPI_ISL_10633761). As expected, very low neutralizing titers against Omicron were observed in all arms of the study. These titers were markedly lower when compared with the those obtained against the original B1 virus (Figure S3). In fact, a large proportion of the samples in the Sputnik V C1, ChAdOx1-S, and BBIBP-CorV cohorts were negative for the presence of neutralizing antibodies against Omicron. Interestingly, in all three cohorts, heterologous vaccination with mRNA-1273 resulted in a significant (p < 0.01) increase in the serum neutralizing titers compared with the homologous schemes.
Discussion
Argentina and other developing countries face the challenge of achieving wide vaccination coverage against COVID-19, using different vaccines including BBIBP-CorV, ChAdOx1-S, Sputnik V, Ad5-nCoV, and mRNA-1273. These vaccines have shown to be safe, immunogenic, and highly effective to prevent severe COVID-19.9, 10, 11, 12, 13, 14 The use of heterologous vaccination schedules in Argentina has been shown to be an adequate strategy in order to accelerate the rate of vaccination. As of today, May 28, 2022, 82% of the total population has been fully vaccinated. In contrast to the numerous studies directed to analyze the reactogenicity, immunogenicity, and effectiveness of heterologous schemes using ChAdOx-1-S and mRNA vaccines,5, 6, 7, 8 very little is known about other heterologous schemes including some of the most-used platforms in the world. In this study, we analyzed heterologous schedules in individuals primed by either Sputnik V C1, ChAdOx1-S, or BBIBP-CorV. Homologous vaccination with BBIBP-CorV showed the lowest frequency of local and systemic reactions among the 15 arms of our study, and second doses with either Sputnik V C1, ChAdOx1, or mRNA-1273 resulted in the enhancement of both local and systemic reactions. Regarding the ChAdOx1 cohort, an increase in local and systemic reactions was observed using mRNA-1273 as a second dose but not with Sputnik C1, Ad5-nCoV, or BBIBP-CorV. Finally, within the Sputnik V C1 cohort, increased local reactions were observed using either mRNA-1273 or ChAdOx1 but not Sputnik C1, Ad5-nCoV, or BBIBP-CorV. Overall, analysis of reactogenicity during the first 7 days after the application of the second vaccine dose showed an acceptable profile for all combinations assessed. No serious adverse events, hospitalizations, or deaths were observed in any of the study arms.
Regarding immunogenicity, outcomes were different with different platform combinations. In the three cohorts analyzed, the highest antibody response was induced by using mRNA-1273 as a heterologous second dose. It induced an increase in the GM of serum IgG anti-spike antibodies higher than 6×, 10×, and 38× in the Sputnik C1, ChAdOx1, and BBIBP-CorV cohorts, respectively, compared with the homologous schemes (Figure 3A). Similar findings were observed by analyzing the levels of serum neutralizing activity against the ancestral B1 variant. Regarding the virus inactivated BBIBP-CorV vaccine, the homologous scheme showed low levels of IgG and neutralizing antibodies, in agreement with previous studies.15 In this arm, a heterologous second dose with Sputnik C1 or ChAdox1-S induced an increase in the GM of IgG anti-spike antibodies higher than 10× and 9×, respectively, compared with the homologous schedule, and when the titers of neutralizing antibodies were analyzed, they showed an increase higher than 6× and 5×, respectively (Figures 3A and 3B). Our observations are consistent with previous studies using CoronaVac, another virus inactivated vaccine, which showed that all heterologous regimens had anti-spike IgG levels and neutralizing titers that were superior to homologous responses.16
Interestingly, although homologous vaccination with either Sputnik V, ChAdOx1, or BBIBP-CorV induced a lower antibody response compared with that induced by two doses of mRNA-1273, heterologous vaccination using a second dose of mRNA-1273 in our three cohorts resulted in the induction of an equivalent response compared with the homologous vaccination with mRNA-1273 (Figure 3). This observation is particularly relevant for vaccine platforms that use inactivated virus, which show a lower immunogenicity profile compared with other platforms based on either vector inactivated or mRNA vaccines. Moreover, when assessed against Omicron, neutralization titers induced by heterologous vaccination with mRNA-1273 in all three cohorts were higher compared with the other combinations evaluated (Figure S3). However, even when mRNA-1273 was used as a second dose, an overall low neutralizing response against Omicron was observed, suggesting the need to apply booster doses in order to induce a better response to Omicron lineages, a currently accepted strategy.17,18 Increased availability of mRNA vaccines against COVID-19 has been observed in recent months in different countries, including Argentina. Our observations, together with the previous studies mentioned above, suggest the convenience of incorporating these vaccine platforms in heterologous schedules.
In conclusion, our study shows that different heterologous vaccination schemes induce antibody responses that are higher than those induced by homologous schemes. Faced with the rapid advance of the Omicron variant around the world19,20 and the emergence of Omicron lineages with an increased ability to evade antibodies induced by vaccines,17,21, 22, 23 a booster third dose is being incorporated in vaccination schedules.24,25 The booster strategy is on its way in most high-income countries and has been demonstrated to be safe and effective in preventing severe disease induced by SARS-CoV-2.26,27 However, many low- and middle-income countries in Africa, parts of Asia, Eastern Europe, and Latin America are still unable to implement this strategy. Our observations could provide valuable information to decide the best combination of vaccines to apply in heterologous systems for prime vaccination as well as for incorporation of booster schedules.
Limitations of the study
This study has a number of limitations. As an immunogenicity and reactogenicity study, we did not evaluate the efficacy of the different heterologous schemes. Although the antibody response induced by vaccination has shown to correlate with protection,28,29 it has not been possible to define a serum level of antibodies capable of preventing symptomatic or severe infection. An additional limitation is given by the lack of data regarding the persistence of the antibody response over time. We only measured antibody levels on days 14 and 28 after completing the vaccination schedule. Moreover, we only evaluated the antibody response induced by vaccination, not the response mediated by T cells.
Consortia
The members of the Laboratorio SeVa Group are Antonella S. Ríos, Diana R. Rodriguez García, Lila Y. Ramis, Magalí G. Bialer, María José de Leone, Natalí B. Rasetto, Shirley D. Wenker, Luciana Bianchimano, Maria Soledad Treffinger Cienfuegos, and Daniel A. Careno. The members of the Ministerio de Salud de La Rioja Group are Gabriela Martin, Nahuel R. Maresca, Florencia Diaz Peña, Juan Gabriel Castillo, María Luz Lambrisca Carral, Orellana Acuña Ana Laura, Claudia Lorena Gonzalez Chavez, Joaquín Bustos, Mariangela Nunes Salla, Claudio Chazarreta, María Silvia De Donatis, Carlina Flores, Lucía Molina, Anahi V. Bustos, Cynthia Nacuzzi, Ana Lourdes del Valle Caviglia, and Stefanía Toti. The members of the Ministerio de Salud de la Provincia de San Luis Group are Leonardo Aguilera, Florencia Cabral, Ludmila Campos, Erica Carrizo, Blanca Esteves, Alicia Guevara Molina, Alejandro Icazatti Zuñiga, Francisco Jofre, Anna Chiara Mastrodonato, Luciana Molina Marino, Marcelo Olivera, Julieta Peñalva, Matías Perez Díaz, Federico Quijano, Gustavo Rivero, Eliana Rosales, Laura Sanchez, Verónica Lanaro, Sonia Roquer, and Roberto Dufour. The members of the Universidad Nacional de La Plata Group are Karina Imbernon, Julieta Belen Cabrera, Flavia Perinasso, Martin Rumbo, Renata Curciarello, and Gaston Rizzo. The members of the Ministerio de Salud de la Provincia de Córdoba Group are Paula Carreño, Elias Gabriel Raboy, German Franchini, Gabriela Anabel Díaz Rousseau, Laura Rosana Aballay, Liliana Luque, Claudia Moreno, Paola Sicilia, Constanza Fidelbo, Fresia Carrizo, Gonzalo Castro, Susana Guignard, Luz Devalle, Natalia Gomez, Cesar Collino, Silvia Peralta, Cecilia Zini, Josefina Eynard asua, and Lara Puchat. The members of the Instituto de Virología "Dr. J. M. Vanella" Group are Sebastián Blanco, Konigheim Brenda, Mauricio Beranek, Lorena Spinsanti, Adrián Díaz, Maria Elisa Rivarola, Javier Aguilar, Silvia Nates, and Rogelio Pizzi. The members of the Ministerio de Salud de la Provincia de Buenos Aires Group are Andrea Gatelli, Sofia Di Bella, Agustina Martinez, Martina Ferioli, Francisco Echeverria, Ramiro Agüero, Ana Caproli, Karina Gil, Claudia Varela, Ángeles Baridon, Soledad Ocampo, Emanuel Zapata, Melina Cancela, Verónica Forneris, Susana Marchetti, María Maxwell, Rosario Marcó, Cecilia Zolorzano, Micaela Nieva, Claudia Conta, Silvina Olivera, Alejandra Rima, Alejandra Musto, Aime Balanzino, Katherina Prost, Miriam Pereiro, Eliana Correa, Noelia Portillo, Cynthia Leguizamon, Alicia Quetglas, Mariana Artazcoz, Agustina Venturi Grossi, Paula Gelpi, Anabella Masci, Sofía Padín, Enio Garcia, and Carolina Vilella Weisz.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Bacterial and virus strains | ||
| SARS-CoV-2 strain 2019 | Gift from Dr. Sandra Gallegos | GISAID accession ID EPI_ISL_499083 |
| SARS-CoV-2 strain Omicron | Isolated from an Argentinean patient sample (INBIRS) | GISAID accession ID EPI_ISL_10633761 |
| Biological samples | ||
| Patient serum set | This study | https://data.mendeley.com/datasets/kbr33hs2m8/2 |
| Chemicals, peptides, and recombinant proteins | ||
| DMEM high glucose | Thermo Fisher | 12100046 |
| DPBS powder, no calcium, no magnesium, 10x1L | Thermo Fisher | 21600010 |
| Trypsin, 0.05% (1X) with EDTA 4Na, liquid | Thermo Fisher | 25300120 |
| Penicillin-Streptomycin (10,000 U/mL) | Thermo Fisher | 15140122 |
| Paraformaldehyde | Sigma Aldrich | 158127 |
| Critical commercial assays | ||
| SARS-CoV-2 antibody ELISA (IgG) Kit | Laboratorio LEMOS | COVIDAR IgG |
| Deposited data | ||
| Dataset uploaded to Medeley | This study | https://data.mendeley.com/datasets/kbr33hs2m8/2 |
| Experimental models: Cell lines | ||
| Vero E6 | ATCC | Cat# CRL-1586, RRID: CVCL_0574 |
| Software and algorithms | ||
| GraphPad Prism V8.0 | GraphPad | https://www.graphpad.com |
| R software | R | V 4.1.1 |
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Andrea V. Gamarnik (agamarnik@leloir.org.ar).
Materials availability
This study did not generate new unique reagents.
Data and code availability
-
•
This study did not generate new unique reagents.
-
•
Datasets generated in this study have been uploaded to Mendeley (https://data.mendeley.com/datasets/kbr33hs2m8/2).
-
•
This study did not report original code.
-
•
Any additional information required to reanalyze the data reported in this study is available from the lead contact upon request.
Experimental model and subject details
Study design and human samples
The Collaborative Study to Evaluate Heterologous Vaccination Against Covid-19 in Argentina (ECEHeVac) is an open, multicenter, adaptive, non-inferiority study to evaluate the reactogenicity and immunogenicity of heterologous vaccination schedules made up of the combination of vaccines available in Argentina (Sputnik V, ChAdOx1-S, Ad5-nCoV, mRNA-1273, and BBIBP-CorV), in comparison with homologous vaccination schedules. The Sputnik V original scheme is a combined vector vaccine based on recombinant adenovirus (rAd) type 26 and rAd5, referred to here as Component 1 (C1) and Component 2 (C2), respectively. The study was performed in accordance with the principles of the Declaration of Helsinki, Good Clinical Practice guidelines, and applicable government regulations such as Law n° 27.473 on Vaccines Intended to Generate Acquired Immunity Against Covid-19. The study was approved by the central committee of the National Health Ministry and all participants provided written informed consent. Study enrollment started in July 2021. Recruitment was carried out in 4 different provinces of Argentina: Buenos Aires, Córdoba, La Rioja, and San Luis. Adult individuals with no or well controlled mild/moderate comorbidities (obesity, chronic cardiovascular disease, chronic kidney disease, chronic respiratory disease, cirrhosis, HIV infection) ages 18 to 85 years who had received a first dose of Sputnik V C1, ChAdOx1-S, mRNA-1273, or BBIBP-CorV were recruited. Exclusion criteria included previous laboratory-confirmed SARS-CoV-2 infection or having a positive result in the anti-Nucleocapsid IgG ELISA on “day 0” (baseline) for those who received Sputnik V C1, ChAdOx1-S or mRNA-1273 as first dose. Patients with immune compromise, pregnant and lactating women, as well as individuals with a history of severe allergic reactions to any vaccine were also excluded. Other exclusion criteria include bleeding disorders, thrombocytopenia, neurological disorders, and known current alcohol or drug dependency. Participants were recruited into one of the 15 arms, as shown in Figure 1, which includes the number for each group. Description of the genders, ages and comorbidity of the subjects by arm is shown in Table 1. For comparison, a cohort of volunteers who received a standard homologous schedule of two doses of mRNA-1273 was also included in the study, as well as samples of 30 early convalescent volunteers (within 1–2 months after diagnosis) who suffered mild symptomatic infection during the first wave of COVID19 in Argentina (prior to the start of vaccination and the initial detection of SARS-CoV-2 VOCs and VOIs in Argentina).
Cell lines
Vero E6 cells (ATCC) and 293T ACE2/TMPRSS2 cells, kindly provided by Dr. Benhur Lee, were cultured at 37°C in 5% CO2 in Dulbecco’s Modified Eagle’s high glucose medium (Thermo Fisher Scientific) supplemented with 10% fetal bovine serum (FBS) (Gibco).
SARS-CoV-2 strains
SARS-CoV-2 strain 2019 (GISAID accession ID EPI_ISL_499083) was obtained from Dr. Sandra Gallegos (InViV working group). Omicron variant was isolated from an Argentinean patient sample (INBIRS). Its genome was completely sequenced and it belongs to the BA.1 PANGO lineage (GISAID accession ID EPI_ISL_10633761). Viruses were amplified in Vero E6 cells, and stock identity was confirmed by whole-genome sequencing in an Illumina sequencer. Nucleic acid sequence for each viral stock was uploaded to GISAID and completely matched to reference sequences for each variant, discarding acquisition of mutations during isolation and amplification processes. Work with SARS-CoV-2 was approved by the INBIRS Institutional Biosafety Committee at biosafety level 3 with negative pressure.
Method details
SARS-CoV-2 antibody ELISA
Antibodies against SARS-CoV-2 spike protein were detected using an established, commercially available, two-step ELISA (COVIDAR).30 Briefly, the assay uses plates coated with a mixture of spike and the receptor binding domain (RBD). The conjugated monoclonal antibody used for human IgG detection in the COVIDAR ELISA is G18-145, which specifically binds to the heavy chain of all four human immunoglobulin G subclasses: IgG1, IgG2, IgG3, and IgG4. The IgG concentration of each sample, expressed in Binding Antibody Units/mL (BAU/mL) was calculated by extrapolation of the optical density at 450 nm (OD450) on a calibration curve built using serial dilutions of the WHO International Standard for anti-SARS-CoV-2 immunoglobulin.
SARS-CoV 2 neutralization assay
Neutralization assays were performed using live SARS CoV-2 virus isolates. Serum samples were heat inactivated at 56°C for 30 min and serial dilutions from 1/4 to 1/8192 were incubated for 1 h at 37°C in the presence of ancestral (B.1) or Omicron variants in DMEM, 2% FBS. Then, 50μL of the mixture were deposited over Vero cell monolayers for an hour at 37°C (MOI, 0.01). Infectious medium was removed and replaced for DMEM, 2% FBS. After 72 h, cells were fixed with 4% paraformaldehyde (4°C, 20 min) and stained with crystal violet solution in methanol. The cytopathic effect (CPE) on the cell monolayer was assessed visually. If damage to the monolayer was observed in the well, it was considered as manifestation of CPE and the neutralization titer was defined as the highest serum dilution that prevent any cytopathic effect.
Outcomes
The primary outcome was non-inferiority of both, serum concentrations of IgG antibodies directed to the spike protein of SARS-CoV-2 and serum neutralizing capacity (geometric mean ratio-GMR), evaluated 14 days after heterologous second dose in comparison with homologous schemes. As primary outcome, the reactogenicity was included, evaluated through local and systemic adverse events for 7 days after the second dose. Secondary outcomes included the antibody response evaluated 28 days after second dose.
Quantification and statistical analysis
The analysis of SARS-CoV-2 anti-spike IgG and neutralizing antibodies were performed in participants at day 14 and 28 after second dose. All relevant statistical information is detailed in each figure legend and/or figure. The time elapsed between the first and second vaccine dose among the different arms within each cohort was compared by Kruskal-Wallis One-Way ANOVA. The proportion of participants with local or systemic adverse event was reported by vaccine schedule and statistical analysis was performed using the χ2 test. The geometric mean ratio (GMR) was calculated as previously described7 as the antilogarithm of the difference between the geometric mean of the log10 transformed SARS-CoV-2 anti-spike IgG or neutralizing antibodies titer in the heterologous arms and the corresponding homologous arm. The criterion for non-inferiority of a heterologous arm was concluded if the lower limit of the one-sided 97.5% CI of a GMR lay above the margin of 0·63 respect to its corresponding homologous arm, as previously described.7 The heterologous group was considered superior to the homologous group if the lower limit of the one-sided 97.5% CI was greater than 1. Comparisons of heterologous with the homologous schedules within each cohort were evaluated by linear regression models. The concentration of SARS-CoV-2 anti-spike IgG antibodies and neutralizing activity for the group with the two-dose mRNA-1273 vaccine, used as reference, were compared using the Mann-Withney U test with the heterologous arms including Sputnik V C1, ChAdOx1-S, and BBIBP-CorV as first dose and the mRNA-1273 vaccine as second dose. The Kruskal-Wallis One-Way ANOVA was performed to compare antibody response of participants in the 15 arms of the study and Mann-Whitney U test was used to compered different cohorts before second dose administration in Figure S1. Analysis was carried out using GraphPad Prism (V8.0.2) and R (V 4.1.1) software.
Acknowledgments
We acknowledge Facundo Di Diego (INBIRS) for Omicron isolation. We also thank Yesica Longueira, Gabriela Turk, and Natalia Laufen from the Biobanco de Enfermedades Infecciosas de Argentina (BBEI) for providing convalescent data. This study has received funding from Fondo para la Convergencia Estructural del Mercosur (FOCEM), NIH (NIAID) (R01.AI095175 and U19AI168631-01 to A.V.G.), Fondo Nacional para la Investigación Científica y Tecnológica (PICT-2019 02869 to A.V.G. and PICT 2017-1616 and PICT 2018–02844 to J.G.), and the Universidad de Buenos Aires (20020170100573BA to J.G.). Funding sources played no role in study design; in the collection, analysis, and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication.
Author contributions
A.V.G., J.G., and C.V. are the principal investigators, designed and performed research, and coordinated the study. Author members of the Ministerio de Salud de la Provincia de Córdoba group, Ministerio de Salud de la Provincia de San Luis group, Ministerio de Salud de la Provincia de Buenos Aires group (L.L., G.S., P.B., D.C., M.B., M.D., J.M.T., M.E.D., M.A.L., B.A., S.I.G., R.E., R.T., L.T., C.S., I.D., C.E., P.V., and C.T.) were involved in sample collection. C.A.P., D.S.O., A.H.R., P.E.R., and E.A.M. were involved in the construction of the database. C.A.P., A.V., D.S.O., M.E.P., P.E.R., E.A.M., A.H.R., and J.P.Z. contributed to the analysis and interpretation of the data. A.V.G. and J.G. wrote and edited the manuscript. J.P.Z. did the statistical analysis. M.E.P., M.P., G.B., C.H.L., N.K., A.R., C.V., and J.M.C. coordinated the study and edited the manuscript. C.A.P., A.V., D.S.O., M.E.P., A.H.R., P.E.R., E.A.M., the Laboratorio SeVa Group, I.M., F.D.D.G., L.S., S.O.R., M.M.G.L.L., S.G., F.R., A.B.R., and J.B. were involved in data collection as well as organization, coordination, and technical support of the study. All authors critically reviewed the manuscript and approved the final version. All authors had full access to all data in the studies and had final responsibility for the decision to submit for publication.
Declaration of interests
All authors declare no competing interests. This study has received funding from the Russian Direct Investment Fund, which manufactures and markets the vaccine Sputnik V, developed by the Gamaleya Research Center of Epidemiology and Microbiology.
Inclusion and diversity
One or more of the authors of this paper self-identifies as a member of the LGBTQ+ community. One or more of the authors of this paper self-identifies as an underrepresented ethnic minority in science. We worked to ensure gender balance in the recruitment of human subjects. While citing references scientifically relevant for this work, we also actively worked to promote gender balance in our reference list.
Published: August 5, 2022
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.xcrm.2022.100706.
Contributor Information
Juan M. Castelli, Email: jcastelli@msal.gov.ar.
Jorge Geffner, Email: jorgegeffner@gmail.com.
Andrea V. Gamarnik, Email: agamarnik@leloir.org.ar.
Supplemental information
References
- 1.Wilhelm A., Widera M., Grikscheit K., Toptan T., Schenk B., Pallas C., Metzler M., Kohmer N., Hoehl S., Helfritz F.A., et al. Reduced neutralization of SARS-CoV-2 omicron variant by vaccine sera and monoclonal antibodies. medRxiv. 2021 doi: 10.1101/2021.12.07.21267432. Preprint at. [DOI] [Google Scholar]
- 2.Cao Y., Wang J., Jian F., Xiao T., Song W., Yisimayi A., Huang W., Li Q., Wang P., An R., et al. B.1.1.529 escapes the majority of SARS-CoV-2 neutralizing antibodies of diverse epitopes. bioRxiv. 2021 doi: 10.1101/2021.12.07.470392. Preprint at. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Meng B., Ferreira I., Abdullahi A., Saito A., Kimura I., Yamasoba D., Kemp S.A., Goonawardane N., Papa G., Fatihi S., et al. SARS-CoV-2 Omicron spike mediated immune escape, infectivity and cell-cell fusion. bioRxiv. 2021 doi: 10.1101/2021.12.17.473248. Preprint at. [DOI] [Google Scholar]
- 4.WHO . 2021. Update on Omicron. https://www.who.int/news/item/28-11-2021-update-on-omicron#:∼:text=On%2026%20November%202021%2C%20WHO,Evolution%20(TAG%2DVE) [Google Scholar]
- 5.Barros-Martins J., Hammerschmidt S.I., Cossmann A., Odak I., Stankov M.V., Morillas Ramos G., Dopfer-Jablonka A., Heidemann A., Ritter C., Friedrichsen M., et al. Immune responses against SARS-CoV-2 variants after heterologous and homologous ChAdOx1 nCoV-19/BNT162b2 vaccination. Nat. Med. 2021;27:1525–1529. doi: 10.1038/s41591-021-01449-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pozzetto B., Legros V., Djebali S., Barateau V., Guibert N., Villard M., Peyrot L., Allatif O., Fassier J.B., Massardier-Pilonchéry A., et al. Immunogenicity and efficacy of heterologous ChAdOx1-BNT162b2 vaccination. Nature. 2021;600:701–706. doi: 10.1038/s41586-021-04120-y. [DOI] [PubMed] [Google Scholar]
- 7.Liu X., Shaw R.H., Stuart A.S.V., Greenland M., Aley P.K., Andrews N.J., Cameron J.C., Charlton S., Clutterbuck E.A., Collins A.M., et al. Safety and immunogenicity of heterologous versus homologous prime-boost schedules with an adenoviral vectored and mRNA COVID-19 vaccine (Com-COV): a single-blind, randomised, non-inferiority trial. Lancet. 2021;398:856–869. doi: 10.1016/S0140-6736(21)01694-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stuart A.S.V., Shaw R.H., Liu X., Greenland M., Aley P.K., Andrews N.J., Cameron J.C., Charlton S., Clutterbuck E.A., Collins A.M., et al. Immunogenicity, safety, and reactogenicity of heterologous COVID-19 primary vaccination incorporating mRNA, viral-vector, and protein-adjuvant vaccines in the UK (Com-COV2): a single-blind, randomised, phase 2, non-inferiority trial. Lancet. 2021;399:36–49. doi: 10.1016/S0140-6736(21)02718-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhu F.C., Guan X.H., Li Y.H., Huang J.Y., Jiang T., Hou L.H., Li J.X., Yang B.F., Wang L., Wang W.J., et al. Immunogenicity and safety of a recombinant adenovirus type-5-vectored COVID-19 vaccine in healthy adults aged 18 years or older: a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet. 2020;396:479–488. doi: 10.1016/S0140-6736(20)31605-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thomas S.J., Moreira E.D., Jr., Kitchin N., Absalon J., Gurtman A., Lockhart S., Perez J.L., Pérez Marc G., Polack F.P., Zerbini C., et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine through 6 months. N. Engl. J. Med. 2021;385:1761–1773. doi: 10.1056/NEJMoa2110345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xia S., Zhang Y., Wang Y., Wang H., Yang Y., Gao G.F., Tan W., Wu G., Xu M., Lou Z., et al. Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine, BBIBP-CorV: a randomised, double-blind, placebo-controlled, phase 1/2 trial. Lancet Infect. Dis. 2021;21:39–51. doi: 10.1016/S1473-3099(20)30831-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.El Sahly H.M., Baden L.R., Essink B., Doblecki-Lewis S., Martin J.M., Anderson E.J., Campbell T.B., Clark J., Jackson L.A., Fichtenbaum C.J., et al. Efficacy of the mRNA-1273 SARS-CoV-2 vaccine at completion of blinded phase. N. Engl. J. Med. 2021;385:1774–1785. doi: 10.1056/NEJMoa2113017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Logunov D.Y., Dolzhikova I.V., Shcheblyakov D.V., Tukhvatulin A.I., Zubkova O.V., Dzharullaeva A.S., Kovyrshina A.V., Lubenets N.L., Grousova D.M., Erokhova A.S., et al. Safety and efficacy of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine: an interim analysis of a randomised controlled phase 3 trial in Russia. Lancet. 2021;397:671–681. doi: 10.1016/S0140-6736(21)00234-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Falsey A.R., Sobieszczyk M.E., Hirsch I., Sproule S., Robb M.L., Corey L., Neuzil K.M., Hahn W., Hunt J., Mulligan M.J., et al. Phase 3 safety and efficacy of AZD1222 (ChAdOx1 nCoV-19) Covid-19 vaccine. N. Engl. J. Med. 2021;385:2348–2360. doi: 10.1056/NEJMoa2105290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vályi-Nagy I., Matula Z., Gönczi M., Tasnády S., Bekő G., Réti M., Ajzner É., Uher F. Comparison of antibody and T cell responses elicited by BBIBP-CorV (Sinopharm) and BNT162b2 (Pfizer-BioNTech) vaccines against SARS-CoV-2 in healthy adult humans. GeroScience. 2021;43:2321–2331. doi: 10.1007/s11357-021-00471-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Costa Clemens S.A., Weckx L., Clemens R., Almeida Mendes A.V., Ramos Souza A., Silveira M.B.V., da Guarda S.N.F., de Nobrega M.M., de Moraes Pinto M.I., Gonzalez I.G.S., et al. Heterologous versus homologous COVID-19 booster vaccination in previous recipients of two doses of CoronaVac COVID-19 vaccine in Brazil (RHH-001): a phase 4, non-inferiority, single blind, randomised study. Lancet. 2022;399:521–529. doi: 10.1016/S0140-6736(22)00094-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Andrews N., Stowe J., Kirsebom F., Toffa S., Rickeard T., Gallagher E., Gower C., Kall M., Groves N., O'Connell A.M., et al. Covid-19 vaccine effectiveness against the omicron (B.1.1.529) variant. N. Engl. J. Med. 2022;386:1532–1546. doi: 10.1056/NEJMoa2119451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rouco S.O., Rodriguez P.E., Miglietta E.A., Rall P., Ledesma M.M.G.L., Varese A., Pascuale C.A., Ojeda D.S., Mazzitelli B., Sanchez L., et al. Heterologous booster response after inactivated virus BBIBP-CorV vaccination in older people. Lancet Infect. Dis. 2022;22:1118–1119. doi: 10.1016/S1473-3099(22)00427-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Karim S.S.A., Karim Q.A. Omicron SARS-CoV-2 variant: a new chapter in the COVID-19 pandemic. Lancet. 2021;398:2126–2128. doi: 10.1016/S0140-6736(21)02758-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Torjesen I. Covid restrictions tighten as omicron cases double every two to three days. BMJ. 2021;375:n3051. doi: 10.1136/bmj.n3051. [DOI] [PubMed] [Google Scholar]
- 21.Ikemura N., Hoshino A., Higuchi Y., Taminishi S., Inaba T., Matoba S. SARS-CoV-2 Omicron variant escapes neutralization by vaccinated and convalescent sera and therapeutic monoclonal antibodies. medRxiv. 2021 doi: 10.1101/2021.12.13.21267761. Preprint at. [DOI] [Google Scholar]
- 22.Hoffmann M., Krüger N., Schulz S., Cossmann A., Rocha C., Kempf A., Nehlmeier I., Graichen L., Moldenhauer A.-S., Winkler M.S., et al. The Omicron variant is highly resistant against antibody-mediated neutralization – implications for control of the COVID-19 pandemic. bioRxiv. 2021 doi: 10.1101/2021.12.12.472286. Preprint at. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cameroni E., Saliba C., Bowen J.E., Rosen L.E., Culap K., Pinto D., VanBlargan L.A., De Marco A., Zepeda S.K., Iulio J.d., et al. Broadly neutralizing antibodies overcome SARS-CoV-2 Omicron antigenic shift. bioRxiv. 2021 doi: 10.1101/2021.12.12.472269. Preprint at. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gruell H., Vanshylla K., Tober-Lau P., Hillus D., Schommers P., Lehmann C., Kurth F., Sander L.E., Klein F. mRNA booster immunization elicits potent neutralizing serum activity against the SARS-CoV-2 Omicron variant. medRxiv. 2021 doi: 10.1101/2021.12.14.21267769. Preprint at. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Garcia-Beltran W.F., St Denis K.J., Hoelzemer A., Lam E.C., Nitido A.D., Sheehan M.L., Berrios C., Ofoman O., Chang C.C., Hauser B.M., et al. mRNA-based COVID-19 vaccine boosters induce neutralizing immunity against SARS-CoV-2 Omicron variant. medRxiv. 2021 doi: 10.1101/2021.12.14.21267755. Preprint at. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Offit P.A. Covid-19 boosters - where from here? N. Engl. J. Med. 2022;386:1661–1662. doi: 10.1056/NEJMe2203329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moreira E.D., Jr., Kitchin N., Xu X., Dychter S.S., Lockhart S., Gurtman A., Perez J.L., Zerbini C., Dever M.E., Jennings T.W., et al. Safety and efficacy of a third dose of BNT162b2 Covid-19 vaccine. N. Engl. J. Med. 2022;386:1910–1921. doi: 10.1056/NEJMoa2200674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Khoury D.S., Cromer D., Reynaldi A., Schlub T.E., Wheatley A.K., Juno J.A., Subbarao K., Kent S.J., Triccas J.A., Davenport M.P. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat. Med. 2021;27:1205–1211. doi: 10.1038/s41591-021-01377-8. [DOI] [PubMed] [Google Scholar]
- 29.Cromer D., Steain M., Reynaldi A., Schlub T.E., Wheatley A.K., Juno J.A., Kent S.J., Triccas J.A., Khoury D.S., Davenport M.P. Neutralising antibody titres as predictors of protection against SARS-CoV-2 variants and the impact of boosting: a meta-analysis. Lancet Microbe. 2021;3:e52–e61. doi: 10.1016/S2666-5247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ojeda D.S., Gonzalez Lopez Ledesma M.M., Pallarés H.M., Costa Navarro G.S., Sanchez L., Perazzi B., Villordo S.M., Alvarez D.E., BioBanco Working Group. Oguntuyo K.Y., et al. Emergency response for evaluating SARS-CoV-2 immune status, seroprevalence and convalescent plasma in Argentina. PLoS Pathog. 2021;17:e1009161. doi: 10.1371/journal.ppat.1009161. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
-
•
This study did not generate new unique reagents.
-
•
Datasets generated in this study have been uploaded to Mendeley (https://data.mendeley.com/datasets/kbr33hs2m8/2).
-
•
This study did not report original code.
-
•
Any additional information required to reanalyze the data reported in this study is available from the lead contact upon request.