Key Points
Question
Among patients with gout, is there a transient increase in the risk of cardiovascular events after gout flares?
Findings
In this case-control study that included 62 574 patients with gout, those who experienced a cardiovascular event, compared with those who did not experience such an event, had significantly greater odds of a recent gout flare in the prior 0 to 60 and 61 to 120 days (adjusted odds ratio [OR] for 0-60 days, 1.93; adjusted OR for 61-120 days, 1.57).
Meaning
These findings suggest gout flares are associated with a transient increase in cardiovascular events following the flare.
Abstract
Importance
Gout is associated with cardiovascular diseases. The temporal association between gout flares and cardiovascular events has not been investigated.
Objective
To investigate whether there is a transient increase in risk of cardiovascular events after a recent gout flare.
Design, Setting, and Participants
A retrospective observational study was conducted using electronic health records from the Clinical Practice Research Datalink in England between January 1, 1997, and December 31, 2020. A multivariable nested case-control study was performed among 62 574 patients with gout, and a self-controlled case series, adjusted for season and age, was performed among 1421 patients with gout flare and cardiovascular event.
Exposures
Gout flares were ascertained using hospitalization, primary care outpatient, and prescription records.
Main Outcomes and Measures
The primary outcome was a cardiovascular event, defined as an acute myocardial infarction or stroke. Association with recent prior gout flares was measured using adjusted odds ratios (ORs) with 95% CIs in a nested case-control study and adjusted incidence rate ratios (IRRs) with 95% CIs in a self-controlled case series.
Results
Among patients with a new diagnosis of gout (mean age, 76.5 years; 69.3% men, 30.7% women), 10 475 patients with subsequent cardiovascular events were matched with 52 099 patients without cardiovascular events. Patients with cardiovascular events, compared with those who did not have cardiovascular events, had significantly higher odds of gout flare within the prior 0 to 60 days (204/10 475 [2.0%] vs 743/52 099 [1.4%]; adjusted OR, 1.93 [95% CI, 1.57-2.38]) and within the prior 61 to 120 days (170/10 475 [1.6%] vs 628/52 099 [1.2%]; adjusted OR, 1.57 [95% CI, 1.26-1.96]). There was no significant difference in the odds of gout flare within the prior 121 to 180 days (148/10 475 [1.4%] vs 662/52 099 [1.3%]; adjusted OR, 1.06 [95% CI, 0.84-1.34]). In the self-controlled case series (N = 1421), cardiovascular event rates per 1000 person-days were 2.49 (95% CI, 2.16-2.82) within days 0 to 60; 2.16 (95% CI, 1.85-2.47) within days 61 to 120; and 1.70 (95% CI, 1.42-1.98) within days 121 to 180 after a gout flare, compared with cardiovascular event rates of 1.32 (95% CI, 1.23-1.41) per 1000 person-days within the 150 days before or the 181 to 540 days after the gout flare. Compared with 150 days before or the 181 to 540 days after a gout flare, incidence rate differences for cardiovascular events were 1.17 (95% CI, 0.83-1.52) per 1000 person-days, and adjusted IRRs were 1.89 (95% CI, 1.54-2.30) within days 0 to 60; 0.84 (95% CI, 0.52-1.17) per 1000 person-days and 1.64 (95% CI, 1.45-1.86) within days 61 to 120; and 0.38 (95% CI, 0.09-0.67) per 1000 person-days and 1.29 (95% CI, 1.02-1.64) within days 121 to 180 after a gout flare.
Conclusions and Relevance
Among individuals with gout, those who experienced a cardiovascular event, compared with those who did not experience such an event, had significantly higher odds of a recent gout flare in the preceding days. These findings suggest gout flares are associated with a transient increase in cardiovascular events following the flare.
This retrospective observational study using electronic health records assesses whether gout flares were associated with a transient increase in rates of cardiovascular events among adult patients in the UK.
Introduction
Cardiovascular disease is a leading cause of mortality and accounted for 19 million deaths globally in the year 2019.1 In addition to traditional cardiovascular risk factors, inflammation is an important risk factor for cardiovascular diseases.2 Gout is a common inflammatory condition that affected approximately 4% of the US general population in 2016 and is particularly prevalent in older people.3,4 Gout is characterized by recurrent episodes of acute inflammatory arthritis.5 Patients with gout have higher rates of cardiovascular diseases, independent of traditional cardiovascular risk factors.6,7,8,9
Gout is characterized by low-grade inflammation with elevated concentration of proinflammatory cytokines and reactive oxygen species, formation of neutrophil extracellular traps, endothelial dysfunction, and platelet hyperactivity that may precipitate atherothrombosis.10 Gout flares are characterized by inflammation due to activation of the NALP3 inflammasome. In a randomized clinical trial, blocking the NALP3 inflammasome prevented recurrent cardiovascular events.10,11 Therefore, this study assessed whether gout flares were associated with a transient increase in rates of cardiovascular events (ie, acute myocardial infarction and stroke).
Methods
Study Setting
The Clinical Practice Research Datalink is a longitudinal database of anonymized health records of approximately 15 million people across the UK from more than 700 general practices; it contains data about sociodemographic and lifestyle factors, diagnoses, investigations, and prescriptions issued in primary care from people representative of the UK population.12 Dates and causes of death are available through individual patient linkages with data from the Office for National Statistics, and for England, linkage with Hospital Episode Statistics provides dates of hospitalization and discharge diagnoses.12
This study was approved by Clinical Practice Research Datalink’s Research Data Governance (protocol 20_000233), which has overarching research ethics committee approval for research studies using anonymous data (reference 05/MRE04/87). Practices that contributed data to the Clinical Practice Research Datalink consented to using anonymized patient data for approved research projects and additional consent was not required prior to individual studies.
Study Design and Participants
For the analyses reported in this article, data were analyzed using a nested case-control study and also a self-controlled case series in which patients served as their own controls. Patients aged 18 years or older with a new diagnosis of gout and who contributed research-quality data to the Clinical Practice Research Datalink were included. Patients with less than 1 year of registration in the database before the first gout diagnosis were excluded. Exclusion of patients with less than 1 year of disease-free registration in the database prevents patients with long-standing gout who have changed general practice surgeries (eg, due to moving to a different city) from entering the study as a patient with newly diagnosed gout when their comorbidities are recorded at the time of changing general practice surgeries.13 The study period was from January 1, 1997, to December 31, 2020.
Nested Case-Control Study
Definition of Cases
Cases were defined as patients diagnosed with cardiovascular events. Case status was defined as the first cardiovascular event after gout diagnosis. Cardiovascular event was defined as either acute myocardial infarction or stroke (ischemic or hemorrhagic) based on 1 or more of the following: cardiovascular event documented in general practice records, hospitalization with cardiovascular event as the primary diagnosis, or death with cardiovascular event as the primary cause of death, using the earliest date as the case event date. Linkage across all data sources was used to improve case ascertainment because 25% to 50% of cardiovascular events are not recorded in at least 1 of the 3 sources.14,15,16 The first cardiovascular event after the diagnosis of gout was used to ascertain case status as lifestyle changes after such an event may be associated with fewer subsequent gout flares.17
Definition, Selection, and Matching of Controls
Patients with a new diagnosis of gout were followed up from the date of first diagnosis of gout to the earliest date of cardiovascular event, transfer out of practice, last data collection from the practice, death, or study end. Controls were defined as individuals who did not experience a cardiovascular event during follow-up. As many as 5 controls were matched to each case based on age (±2 years), sex, and length of time since diagnosis of gout at first cardiovascular event (±2 years) using time-dependent incidence density sampling. This method assigned equal length of observation to cases and matched controls to ensure equal time windows of exposure.18 It produced odds ratios (ORs) that were unbiased estimators of the hazard ratio, with little or no loss in precision.19 Each control was allocated an index date corresponding to the cardiovascular event date of their matched case. Patients with no primary care consultation in the 12 months preceding the index date were excluded because they may have moved to a different practice without updating their medical records in the original practice.
Exposure
Gout flare was the exposure of interest. It was defined as the presence of 1 or more of the following: a diagnostic code for gout flare in general practice records; hospitalization with gout as the primary discharge diagnosis; or primary care consultation for gout with prescription of either nonsteroidal anti-inflammatory drugs (NSAIDs), glucocorticoids, or colchicine on the same date. Previous validation studies suggested that this strategy would yield the highest positive predictive value for gout flare ascertainment20,21,22 (eMethod 1 in the Supplement).
Because gout flares typically last for 1 to 2 weeks, gout-related consultations and prescriptions within 14 days of the first flare consultation were considered part of the same flare. Patients were categorized according to whether they experienced gout flares within 0 to 60, 61 to 120, 121 to 180, after 180 days, or experienced no gout flares prior to the cardiovascular event or index date.
Covariates
Covariates consisted of age, male or female sex, years of gout duration, body mass index (BMI [calculated as weight in kilograms divided by height in meters squared]), smoking status (current, past, or nonsmoker), alcohol intake (current, past, or no intake), socioeconomic deprivation assessed using the English Index of Multiple Deprivation 2015, Charlson Comorbidity Index,23 hypertension, atrial fibrillation, hypercholesterolemia, cardiovascular event prior to gout diagnosis, number of hospitalizations and primary care consultations in the 12 months preceding the cardiovascular event or matched index date, European Society of Cardiology cardiovascular risk status (high/very-high or low/moderate),24 and prescription of urate-lowering therapy, antiplatelets, statins, diuretics, antihypertensives, colchicine, NSAIDs, and corticosteroids. Prescriptions were categorized as current (≤60 days), past (>60 days), or not prescribed. Previously published Read code lists were updated to develop code lists in this study (eTable 1 in the Supplement). Race and ethnicity data were not included.
Self-controlled Case Series
Selection of Participants
Patients with both an exposure (gout flare) and outcome (cardiovascular event) were included.25
Exposure
The exposure period extended from the gout flare consultation date to 180 days divided into three 60-day exposure windows (eFigure 1 in the Supplement). There was a 30-day induction period prior to the gout flare, and the baseline periods were 31 to 180 days before exposure and 360 days after the end of the exposure period. Each patient contributed data from their first gout flare. The observation period was restricted to 720 days to minimize confounding from time-varying confounders25 (eMethods 2 in the Supplement).
Outcomes
For both the case-control study and the self-controlled case series, the outcomes were as follows:
Primary: cardiovascular event defined as either acute myocardial infarction or stroke (ischemic or hemorrhagic).
Three secondary outcomes: fatal cardiovascular event, acute myocardial infarction, and stroke (ischemic or hemorrhagic).
Statistical Analysis
Nested Case-Control Study
Multivariable conditional logistic regression was used to assess the association between recent prior gout flares and cardiovascular events. The odds of a recent prior flare were calculated by comparing patients with flares within a given time period within 180 days of the index cardiovascular event vs either remote or no previous flares, and an OR with 95% CI was calculated. The unadjusted difference with a 95% CI was calculated between case and control patients. The model was adjusted for matching variables to account for residual confounding (model 1), and further adjusted for BMI, smoking status, alcohol intake, and socioeconomic deprivation in model 2. Model 3 included variables in model 2 plus adjustment for Charlson Comorbidity Index, hypertension, atrial fibrillation, hypercholesterolemia, number of hospitalizations in the previous 12 months, number of primary care consultations in the previous 12 months, European Society of Cardiology cardiovascular risk, and drug prescriptions. Model 4 included variables in model 3 plus adjustment for prescription of colchicine, NSAIDs, and corticosteroids. Sensitivity analyses repeated analyses with different outcomes (ie, acute myocardial infarction, stroke, fatal cardiovascular event), shorter exposure window (ie, within 0-15, 16-30, 31-60, 61-90, 91-120, 121-150, 151-180 days of index cardiovascular event), patients with gout flares within 180 to 240 days prior to the cardiovascular event or matched index date as reference, and excluded patients with a cardiovascular event prior to gout diagnosis, moderate or low cardiovascular risk as per the European Society of Cardiology, gout diagnosed for less than 1 year at cardiovascular event or matched index date, no prior gout flares, a cardiovascular event or matched index date before January 1, 2010, and a cardiovascular event on the same date as gout flare.
BMI, smoking status, alcohol intake status, and socioeconomic deprivation variables had missing data. The pattern of missingness was compared and missingness at random assumed. Missing data were imputed using chained equations (Stata command mi impute chained). BMI was modeled using linear regression. Other variables with missing data were categorical or ordinal and modeled using ordinal regression. The imputation model included all listed confounders, exposure, and case-control indicator.26 Twenty imputed data sets were derived.27
Self-controlled Case Series
A Poisson model was fitted conditioned on the number of cardiovascular events, and adjusted incidence rate ratios (IRRs) with 95% CIs for exposure periods compared with the baseline period were calculated and adjusted for age (2-year age bands) and calendar season. The latter accounts for the seasonal change in gout flare incidence.28 The incident rate difference with 95% CIs was calculated.
Sensitivity analyses considered different outcomes (ie, acute myocardial infarction, stroke, fatal cardiovascular event), short exposure intervals (ie, flare date to 15, 16-30, 31-60, 61-90, 91-120, 121-150 and 151-180 days after the gout flare), excluded patients with fatal cardiovascular event, cardiovascular event on the same date as gout flare, cardiovascular event prior to the first diagnosis of gout, and cardiovascular event or matched index date before January 1, 2010, and evaluated the association of gout flares with cardiovascular events when analyses were restricted to individuals treated with NSAIDs, corticosteroids, or colchicine with cardiovascular events.25
Details of sample size estimation are provided in eMethods 3 in the Supplement. A 2-sided P value of less than .05 was considered as statistically significant. Because of the potential for type I error due to multiple comparisons, findings for secondary outcomes should be interpreted as exploratory. STATA version 17 (StataCorp) was used for data analysis.
Results
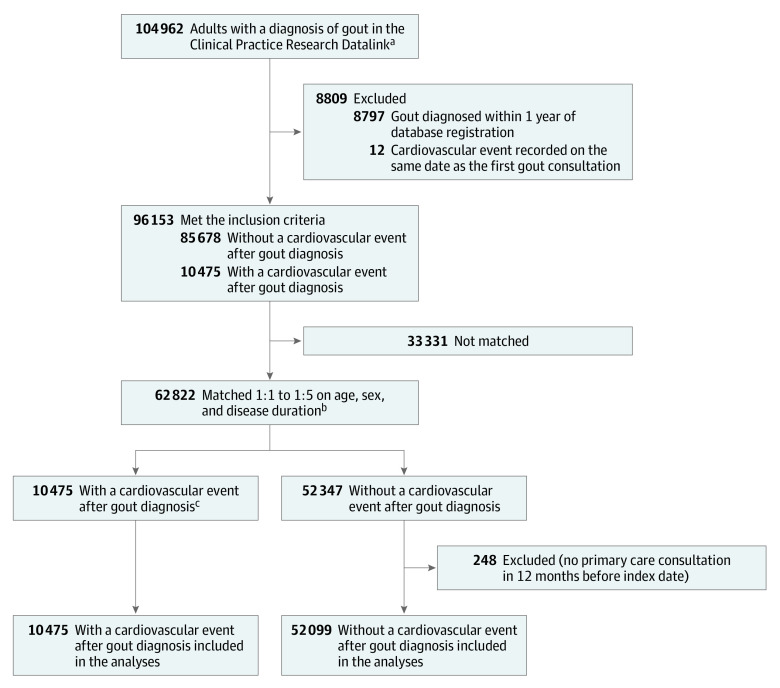
Inclusion criteria were met by 96 153 patients who were newly diagnosed with gout during the study period (Figure 1). Of these, 10 475 had at least 1 cardiovascular event during 603 923 person-years of follow-up. The incidence of cardiovascular events was 17.34 (95% CI, 17.02-17.68) per 1000 person-years. The first cardiovascular event was acute myocardial infarction in 5324 (49.2%) patients and stroke (ischemic or hemorrhagic) in 5151 (50.8%) patients. Among this same group of 10 475 patients with gout and a cardiovascular event, 3889 (37.1%) had a fatal cardiovascular event (2238 [21.4%] due to acute myocardial infarction and 1651 [15.8%] due to stroke).
Figure 1. Cohort Development in a Nested Case-Control Study of Cardiovascular Events After New Diagnosis of Gout.
aThe Clinical Practice Research Datalink is a longitudinal primary care database of anonymized health records of 15 million people across the United Kingdom from over 700 medical practices. It contains data about sociodemographics, lifestyle factors, diagnoses, consultations, and prescriptions recorded in primary care, hospitalization records, and mortality data.
bAs many as 5 controls were matched to each case patient for age (± 2 years), sex, and duration of gout at first cardiovascular event (± 2 years).
cThere were no exclusions from this group. All patients had at least 1 primary care consultation in the 12 months before the index date.
Nested Case-Control Study
The nested case-control study included 62 574 patients with gout, either with (n = 10 475) or without (n = 52 099) cardiovascular events after the diagnosis of gout (Table). Patients with cardiovascular events after a gout diagnosis, compared with patients who did not experience cardiovascular events, had a higher rate of current smoking (1231 of 9798 [12.6%] vs 4397 of 49 332 [8.9%]), a very high or high cardiovascular risk according to the European Society of Cardiology guidelines (10321 of 10 475 [98.5%] vs 34 856 of 52 099 [66.9%]), a higher rate of prior cardiovascular diseases (5448 of 10 475 [52.0%] vs 10 765 of 52 099 [20.7%]), and a higher Charlson Comorbidity Index (mean [SD], 3.23 [2.28] vs 2.52 [2.18]; P < .001 for all).
Table. Demographic and Clinical Characteristics of Patients With Newly Diagnosed Gout Included in the Nested Case-Control Study.
| No. (%)a | ||
|---|---|---|
| Individuals with gout and cardiovascular events | Matched controls with gout and without cardiovascular events | |
| No. | 10 475 | 52 099 |
| Age, mean (SD), y | 76.9 (11.4) | 76.3 (10.8) |
| Sex | ||
| Women | 3213 (30.7) | 15 979 (30.7) |
| Men | 7262 (69.3) | 36 120 (69.3) |
| Body mass index, mean (SD)b | 28.2 (5.1) [8893] | 28.2 (4.9) [44 297] |
| English Index of Multiple Deprivation, mean (SD)c | 3.2 (1.4) [9950] | 3.1 (1.4) [49 290] |
| Smoking status | [9798] | [49 332] |
| Current | 1231 (12.6) | 4397 (8.9) |
| Past | 3904 (39.8) | 19 537 (39.6) |
| None | 4663 (47.6) | 25 398 (51.5) |
| Alcohol intake | [9474] | [47 891] |
| Current | 7483 (79.0) | 39 137 (81.7) |
| Past | 221 (2.3) | 969 (2.0) |
| None | 1770 (18.7) | 7785 (16.3) |
| Time since since gout diagnosis, mean (SD), y | 5.3 (4.5) | 5.3 (4.5) |
| Gout flare prior to cardiovascular event or marched index date, d | ||
| 0-60 | 204 (2.0) | 743 (1.4) |
| 61-120 | 170 (1.6) | 628 (1.2) |
| 121-180 | 148 (1.4) | 662 (1.3) |
| >180 | 4211 (40.2) | 21 353 (41.0) |
| No gout flare | 5742 (54.8) | 28 713 (55.1) |
| Charlson Comorbidity Index, mean (SD)d | 3.23 (2.28) | 2.52 (2.18) |
| History of cardiovascular diseasese | 5448 (52.0) | 10 765 (20.7) |
| Very high to high cardiovascular riskf | 10 321 (98.5) | 34 856 (66.9) |
| Diabetes without target organ damageg | 1537 (14.7) | 6689 (12.8) |
| Diabetes with target organ damageg | 1050 (10.0) | 3944 (7.6) |
| Stage ≥ 3 chronic kidney diseaseh | 3695 (35.3) | 18 353 (35.2) |
| Peripheral artery disease | 1980 (18.9) | 5777 (11.1) |
| Hypertension | 7250 (69.2) | 35 405 (70.0) |
| Atrial fibrillation | 2540 (24.3) | 10 069 (19.3) |
| Hypercholesterolemia | 2428 (23.2) | 11 418 (21.9) |
| Medications prescribed | ||
| Statins | ||
| Currenti | 3430 (32.7) | 10 897 (20.9) |
| Pastj | 4266 (40.7) | 20 273 (38.9) |
| Never | 2779 (26.5) | 20 928 (40.2) |
| Antiplatelet drugs | ||
| Currenti | 3563 (34.0) | 9633 (18.5) |
| Pastj | 5372 (51.3) | 21 842 (41.9) |
| Never | 1540 (14.7) | 20 624 (39.6) |
| Urate-lowering therapy | ||
| Currenti | 1658 (15.8) | 9804 (18.8) |
| Pastj | 3358 (32.1) | 15 297 (29.4) |
| Never | 5459 (52.1) | 26 997 (51.8) |
| Latest urate-lowering drug prescribed | [5016] | [25 101] |
| Allopurinol | 4937 (98.4) | 24 732 (98.5) |
| Febuxostat | 45 (0.9) | 226 (0.9) |
| Uricosurics (probenecid, benzbromarone, sulfinpyrazone) | 34 (0.7) | 143 (0.6) |
| Diuretics | ||
| Currenti | 1959 (18.7) | 8306 (15.9) |
| Pastj | 6526 (62.3) | 29 200 (56.1) |
| Never | 1990 (19.0) | 14 593 (28.0) |
| Other antihypertensive drugsk | ||
| Currenti | 2748 (26.2) | 11 260 (21.6) |
| Pastj | 922 (8.8) | 9625 (18.5) |
| Never | 6805 (65.0) | 31 213 (59.9) |
| Nonsteroidal anti-inflammatory drugs | ||
| Currenti | 804 (7.7) | 5565 (10.7) |
| Pastj | 7418 (70.8) | 36 726 (70.5) |
| Never | 2253 (21.5) | 9808 (18.8) |
| Corticosteroids | ||
| Currenti | 1389 (13.3) | 9084 (17.4) |
| Pastj | 3639 (34.7) | 15 691 (30.1) |
| Never | 5447 (52.0) | 27 324 (52.5) |
| Colchicine | ||
| Currenti | 1032 (9.9) | 7155 (13.7) |
| Pastj | 2773 (26.5) | 12 044 (23.1) |
| Never | 6670 (63.7) | 32 900 (63.2) |
| No. of primary care consultations in the previous year, median (IQR) | 17 (10-29) | 14 (8-23) |
| No. of hospitalizations in the previous year, median (IQR) | 1 (0-2) | 0 (0-1) |
| Time in clinical practice research datalink, mean (SD), y | 12.0 (5.9) | 12.4 (6.0) |
Values shown in brackets indicate the number of individuals with available data.
Calculated as weight in kilograms divided by height in meters squared.
The English Index of Multiple Deprivation 2015 is a measure of socioeconomic deprivation. It ranks small areas called lower-layer super output areas from 1 (most deprived) to 32 844 (least deprived) in quintiles (first [most deprived] to fifth [least deprived]). Data were provided by Clinical Practice Research Datalink.
The Charlson Comorbidity Index predicts mortality by weighting specific comorbidities (range, 0-29 [higher score indicates increased risk of mortality]). In the current study it was derived from general practice records provided by the Clinical Practice Research Datalink as per Khan et al.23
Cardiovascular disease was defined as either acute coronary syndrome, ischemic heart diseases, transient ischemic attack, or stroke.
For further information, please see eMethod 4 in the Supplement.
Target organ damage with diabetes was defined as primary care record of microalbuminuria, retinopathy, or neuropathy according to Moons et al.26
Chronic kidney disease stage 3 or greater is defined as estimated glomerular filtration rate less than or equal to 60 mL/min/1.73 m2 or dialysis.
Indicates most recent prescription within 60 days prior to cardiovascular event date or index date in matched controls.
Indicates most recent prescription more than 60 days prior to the cardiovascular event date or index date in matched controls.
Includes angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, β-blockers, or calcium channel blockers.
Overall, 44.9% (28 119 of 62 574 patients) consulted or were hospitalized for gout flares over a mean (SD) of 5.3 (4.5) years of follow-up between their initial gout diagnosis and the cardiovascular event date or matched index date for controls. This proportion was similar between case patients (4733 of 10 475 [45.2%]) and controls (23 386 of 52 099 [44.9%]). The median number of gout flares in both groups was 1.0 (IQR, 1.0-1.0).
In the fully adjusted model, patients with cardiovascular events, compared with controls who did not have cardiovascular events, had significantly higher odds of gout flare within the prior 0 to 60 days (204/10 475 [2.0%] vs 743/52 099 [1.4%]; adjusted OR, 1.93 [95% CI, 1.57-2.38]) and 61 to 120 days (170/10 475 [1.6%] vs 628/52 099 [1.2%]; adjusted OR, 1.57 [95% CI, 1.26-1.96]), but there was no significant difference in the odds of a gout flare within the prior 121 to 180 days (148/10 475 [1.4%] vs 662/52 099 [1.3%]; adjusted OR, 1.06 (95% CI, 0.84-1.34]) (Figure 2).
Figure 2. Association Between Cardiovascular Event and Recent Prior Gout Flare in a Nested Case-Control Study.
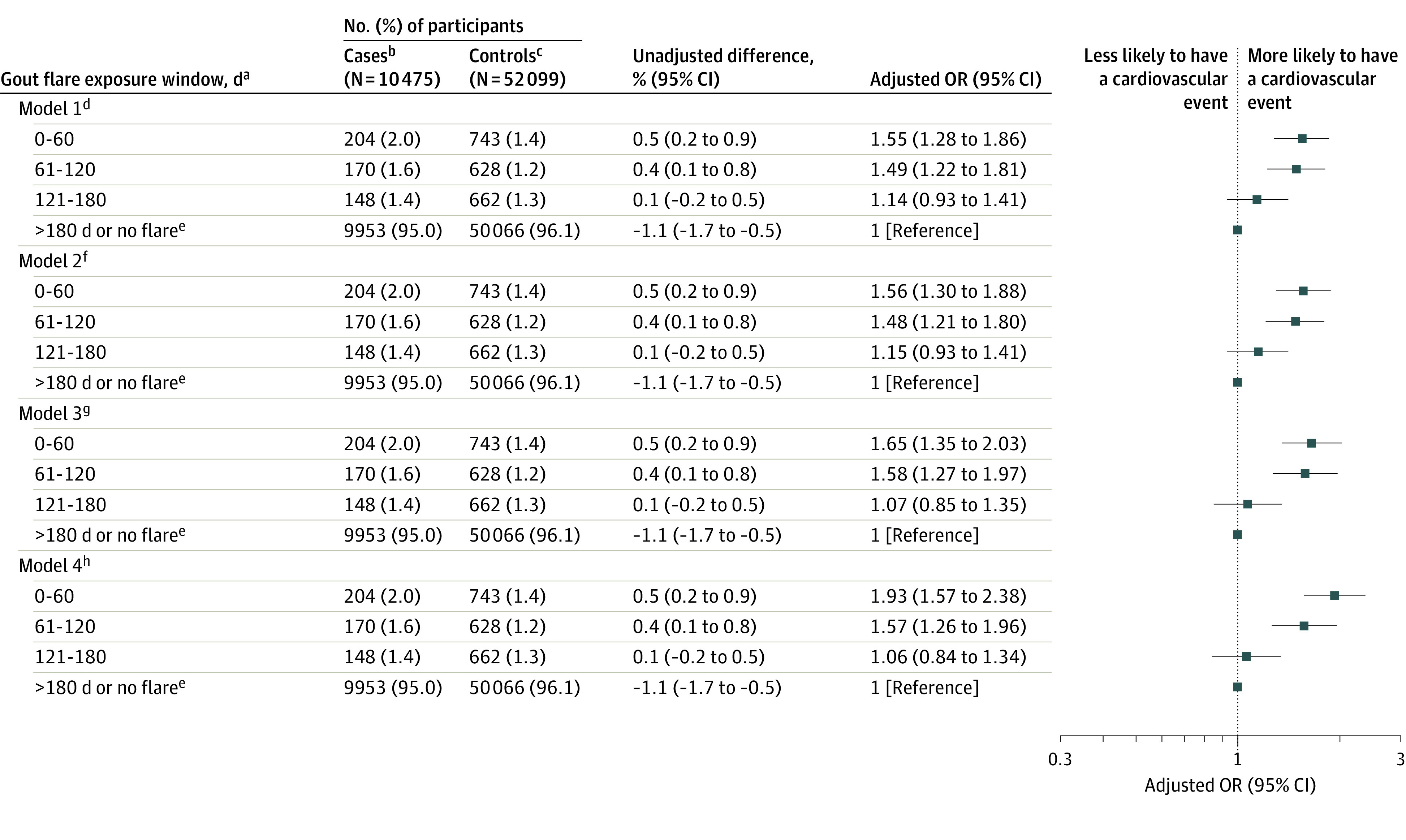
aFor case patients, indicates days before the cardiovascular event; for controls, indicates days before the index date (counted from 0 as the event date or index date).
bCase patients are individuals with cardiovascular events (defined as the first occurrence of acute myocardial infarction or a stroke after gout diagnosis).
cControls are matched individuals with gout but without a cardiovascular event after diagnosis of gout.
dIncludes matching variables (age, sex and disease duration).
eIndicates the reference category.
fIncludes model 1 variables plus demographics, body mass index, smoking status, alcohol intake status, and English Index of Multiple Deprivation.
gIncludes variables from models 1 and 2 plus comorbidities (Charlson Comorbidity Index, hypertension, atrial fibrillation, hypercholesterolemia), number of hospitalizations in the previous year, number of primary care consultations in the previous year, and European Society of Cardiology individual cardiovascular risk, prescription of antiplatelets, statins, urate-lowering therapy, diuretics, and antihypertensives. Prescriptions were categorized as current (≤60 days), past (>60 days), or not prescribed prior to the cardiovascular event date or matched index date.
hIncludes variables from models 1, 2, and 3 plus prescription of medications used for treating gout flares (colchicine, nonsteroidal anti-inflammatory drugs, and corticosteroids). Prescriptions were categorized as current (≤60 days), past (>60 days), or not prescribed prior to the cardiovascular event date or matched index date.
OR indicates odds ratio.
Results of sensitivity analyses (eg, applying a shorter exposure window, excluding patients with cardiovascular diseases prior to gout diagnosis, excluding patients without gout flares, changing the reference period to 180-240 days prior to the cardiovascular event, and excluding patients with low to moderate cardiovascular risk) were consistent with the main analysis (Figure 3; eTable 2 in the Supplement).
Figure 3. Association Between Acute Myocardial Infarction, Stroke, and Recent Prior Gout Flares in a Nested Case-Control Study.
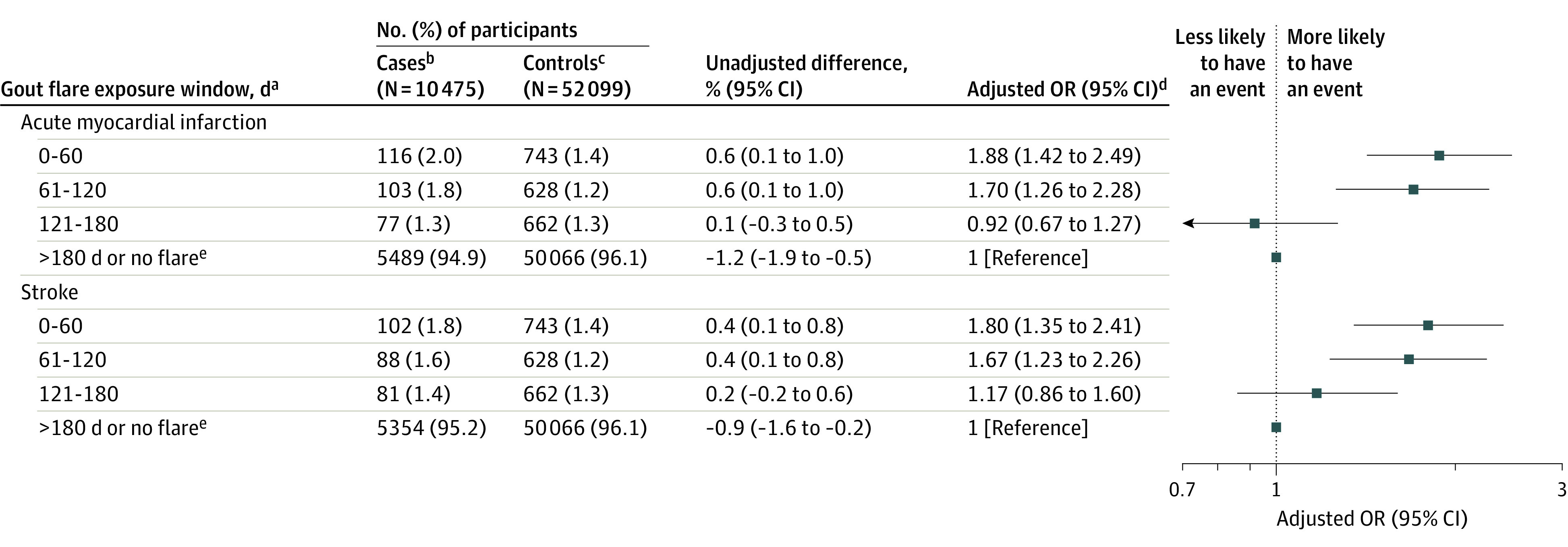
aFor case patients, indicates days before the cardiovascular event; for controls, indicates days before the index date (counted from 0 as the event date or index date).
bCase patients are individuals with cardiovascular events (defined as the first occurrence of acute myocardial infarction or a stroke after gout diagnosis).
cControls are matched individuals with gout but without a cardiovascular event after diagnosis of gout.
dThe analyses were adjusted for age, sex, disease duration, body mass index, smoking status, alcohol intake, English Index of Multiple Deprivation 2015, Charlson Comorbidity Index, hypertension, atrial fibrillation, hypercholesterolemia, number of hospitalizations in the previous year, number of primary care consultations in the previous year, European Society of Cardiology cardiovascular risk score, and current, past, or no prescription of certain drugs (diuretics, antiplatelets, statins, urate-lowering therapy, antihypertensives, nonsteroidal anti-inflammatory drugs, corticosteroids, and colchicine).
eIndicates the reference category.
OR indicates odds ratio.
The adjusted OR for gout flares within 0 to 60 days prior to a fatal cardiovascular event compared with no cardiovascular event was 4.76 (95% CI, 1.69-8.43; 67 of 3889 patients [1.7%] vs 67 of 13 808 patients [0.5%]), within 61 to 120 days the adjusted OR was 2.05 (95% CI, 1.19-3.54; 41 of 3889 patients [1.1%] vs 61 of 13 808 patients [0.4%]), and within 121 to 180 days the adjusted OR was 1.28 (95% CI, 0.74-2.19; 84 of 3889 patients [2.2%] vs 221 of 13 808 patients [1.6%]).
Self-controlled Case Series
The self-controlled case series included 1421 patients with at least 1 gout flare and at least 1 cardiovascular event after the diagnosis of gout (eFigure 2 in the Supplement). During the 180 days after the gout flare (exposed period), 545 cardiovascular events occurred over a total follow-up time of 256 945 person-days at a rate of 2.12 (95% CI, 1.94-2.30) per 1000 person-days, and 876 cardiovascular events occurred during the 150 days before or the 181 to 540 days after the gout flare (baseline period) over a total follow-up time of 679 476 person-days at a rate of 1.29 (95% CI, 1.20-1.37) per 1000 person-days (incidence rate difference, 0.83 [95% CI, 0.63-1.03]/1000 person-days). There were significantly more cardiovascular events during the 180 days after the gout flare compared with other time periods (ie, the 150 days before or the 181-540 days after the gout flare; IRR, 1.65 [95% CI, 1.48-1.84]).
Gout flares were associated with significantly more cardiovascular events in the subsequent 0 to 60 days (incidence rate, 2.49 [95% CI, 2.16-2.82]/1000 person-days), 61 to 120 days (incidence rate, 2.16 [95% CI, 1.85-2.47])/1000 person-days), and 121 to 180 days (incidence rate, 1.70 [95% CI, 1.42-1.98]/1000 person-days) compared with an incidence rate of 1.32 (95% CI, 1.23-1.41) per 1000 person-days during the 150 days before and 181 to 540 days after the gout flare (Figure 4). Compared with 150 days before and 181 to 540 days after a gout flare, incidence rate differences for cardiovascular events were 1.17 (95% CI, 0.83-1.52) per 1000 person-days (adjusted IRR, 1.89 [95% CI, 1.54-2.30]) within 0 to 60 days after a gout flare, 0.84 (95% CI, 0.52-1.17) per 1000 person-days (adjusted IRR, 1.64 [95% CI, 1.45-1.86]) within 61 to 120 days after a gout flare, and 0.38 (95% CI, 0.09-0.67) per 1000 person-days (adjusted IRR, 1.29 [95% CI,1.02-1.64]) within 121 to 180 days after a gout flare.
Figure 4. Results of the Self-controlled Case Series Analysis for Patients With a First Episode of Gout and a Cardiovascular Event.
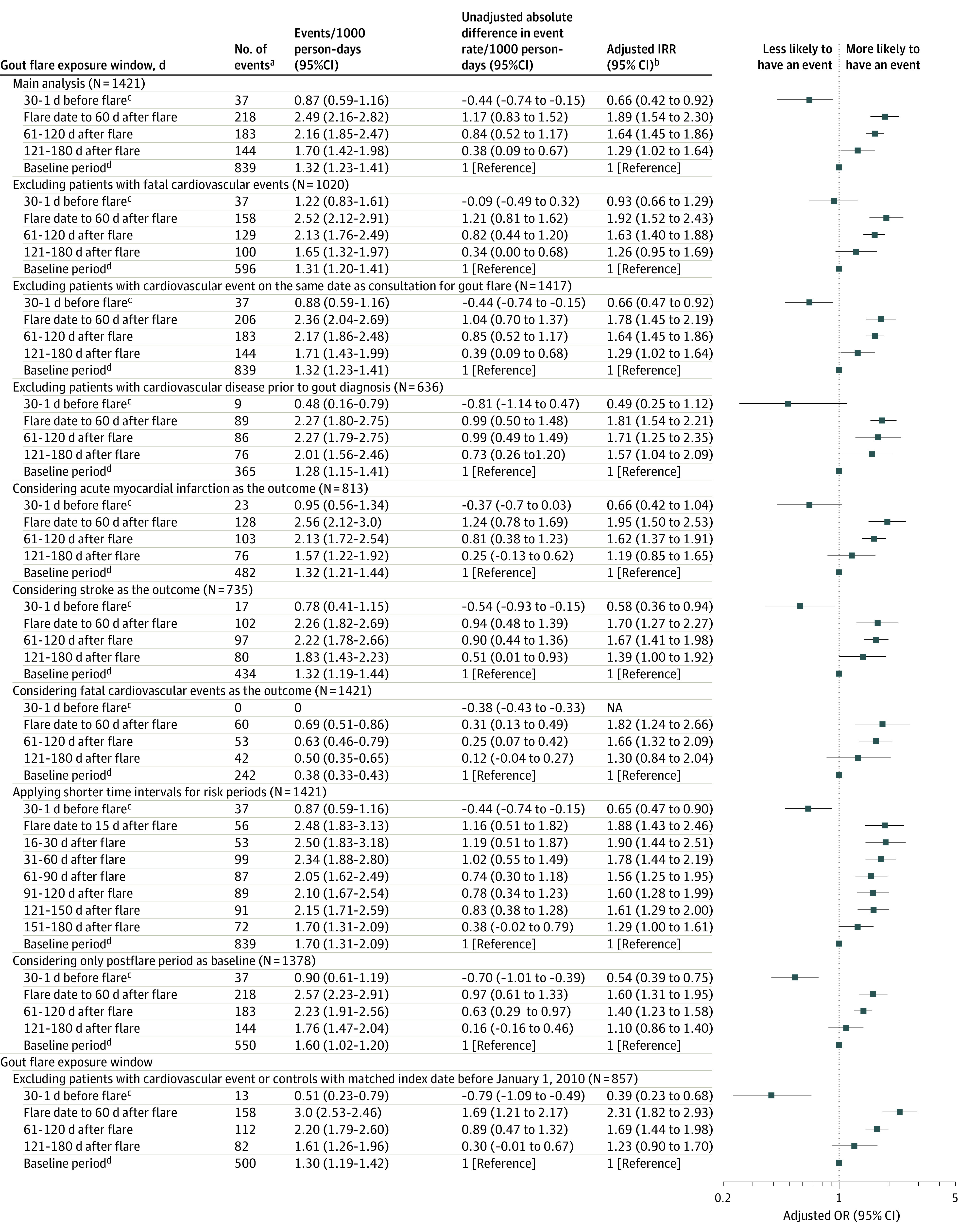
aAcute myocardial infarction or a stroke.
bAnalyses were adjusted for age and calendar season.
cIndicates the induction interval.
dBaseline period indicates 180 to 31 days before flare plus 181 to 540 days after flare.
IRR indicates incidence risk ratio. See eTable 3 for self-controlled case series findings by gout flair treatments.
The results of the sensitivity analyses (eg, applying a shorter exposure window, excluding patients with cardiovascular diseases prior to gout diagnosis, and excluding patients with low to moderate cardiovascular risk) were consistent with those of the main analysis (Figure 4; eTable 3 in the Supplement). The results were similar when the analyses were repeated using only gout flares treated with NSAIDs, colchicine, or corticosteroids (eTable 3 in the Supplement).
Discussion
In the nested case-control study of patients with newly diagnosed gout, patients with cardiovascular events had significantly increased odds of a gout flare during the preceding 120 days compared with patients who did not experience cardiovascular events. These findings suggest that gout flares are associated with a transient increase in cardiovascular events following flares. The increased odds persisted when people with preexisting cardiovascular diseases were excluded and when shorter exposure periods prior to the cardiovascular event (eg, within 0-15 and 16-30 days of cardiovascular event) were considered. The self-controlled case series accounted for residual between-person confounding and confirmed the results of the nested case-control study.25
Gout flares are characterized by neutrophil-rich acute inflammation due to NLRP-3 inflammasome activation.5,29 Neutrophilic inflammation is associated with atherosclerotic plaque instability and rupture.30,31,32 Activated intraplaque inflammatory cells upregulate host response proteins, including metalloproteinases and peptidases, and promote an oxidative stress, all of which contribute to plaque destabilization.33 This may explain the association between cardiovascular events and recent prior gout flares. Additionally, acute infection and surgery are associated with atrial fibrillation,34 and the same may be the case for gout flares, providing another potential mechanism.
The present study had several strengths. It used a large nationwide database representative of the general population.12 The data used in this study were derived from both primary care consultations and hospitalizations and were linked to mortality and socioeconomic deprivation records. In view of remaining residual confounding in the case-control analysis, a separate self-controlled case series analysis was performed as it removes any between-person confounding, and this yielded similar results. Additionally, gout flares were identified using validated definitions, and cardiovascular events were defined using data from general practice, hospitalization, and cause of death to minimize potential bias from misclassification.
Limitations
This study has several limitations. First, data were extracted retrospectively from a prospective database. Second, only association and not causation should be inferred because of the observational study design. Third, although cardiovascular events were ascertained using general practice consultation, hospitalization, and cause of death records, it was not possible to clinically verify or validate each event. However, this approach has been widely used in cardiovascular research.6,8,9 Furthermore, the incidence of cardiovascular event was comparable with those reported previously.9 Fourth, separate analyses with ischemic or hemorrhagic stroke as outcomes could not be conducted because stroke type was not specified for a considerable proportion of these events.7,16 Fifth, gout flares for which individuals did not consult were not included in the study as electronic health records only capture interactions with the health care system. Sixth, the onset of gout flares likely preceded the date of consultation in general practice or the date of hospitalization. However, this was unlikely to differ between those with and without cardiovascular events. Seventh, this study spanned 24 years. The diagnosis and management of cardiovascular diseases and gout have changed over this period. More remotely collected data may not be relevant to current practice. Eighth, data on severity of gout (eg, tophi, polyarticular gout flares)35 were infrequently recorded in the Clinical Practice Research Datalink, and consequently gout severity was not controlled for in the analyses. Ninth, patients with cardiovascular events before the diagnosis of gout were included in the study and may have introduced surveillance bias. However, the sensitivity analysis excluded such patients and yielded similar significant associations.
Conclusions
Among individuals with gout, those who experienced a cardiovascular event, compared with those who did not experience such an event, had significantly higher odds of a recent gout flare in the preceding days. These findings suggest gout flares are associated with a transient increase in cardiovascular events following the flare.
eMethod 1. Rationale for Definition of Gout Flare Used in the Current Study
eMethod 2. Rationale for Self-Controlled Case Series Sensitivity Analyses
eMethod 3. Sample Size Estimation
eMethod 4. European Society of Cardiology (ESC) Cardiovascular Risk Categories
eTable 1. Codelists for Case, Exposure and Covariate Definition
eTable 2. Association Between Cardiovascular Events and Recent Prior Gout Flare: Sensitivity Analyses of the Nested Case-Control Study
eTable 3. Sensitivity Analyses of the Self-Controlled Case Series Analysis for Patients With the First Gout Flare After Gout Diagnosis and a Cardiovascular Event
eFigure 1. Self-Controlled Case Series: Schematic Description
eFigure 2. Cohort Development in a Self-Controlled Case-Series Study of Cardiovascular Events After New Diagnosis of Gout
eReferences.
References
- 1.Roth GA, Mensah GA, Johnson CO, et al. ; GBD-NHLBI-JACC Global Burden of Cardiovascular Diseases Writing Group . Global Burden of Cardiovascular Diseases and Risk Factors, 1990-2019: update from the GBD 2019 study. J Am Coll Cardiol. 2020;76(25):2982-3021. doi: 10.1016/j.jacc.2020.11.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Willerson JT, Ridker PM. Inflammation as a cardiovascular risk factor. Circulation. 2004;109(21)(suppl 1):II2-II10. [DOI] [PubMed] [Google Scholar]
- 3.Dehlin M, Jacobsson L, Roddy E. Global epidemiology of gout: prevalence, incidence, treatment patterns and risk factors. Nat Rev Rheumatol. 2020;16(7):380-390. doi: 10.1038/s41584-020-0441-1 [DOI] [PubMed] [Google Scholar]
- 4.Chen-Xu M, Yokose C, Rai SK, Pillinger MH, Choi HK. Contemporary prevalence of gout and hyperuricemia in the United States and decadal trends: the National Health and Nutrition Examination Survey, 2007-2016. Arthritis Rheumatol. 2019;71(6):991-999. doi: 10.1002/art.40807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dalbeth N, Gosling AL, Gaffo A, Abhishek A. Gout. Lancet. 2021;397(10287):1843-1855. doi: 10.1016/S0140-6736(21)00569-9 [DOI] [PubMed] [Google Scholar]
- 6.De Vera MA, Rahman MM, Bhole V, Kopec JA, Choi HK. Independent impact of gout on the risk of acute myocardial infarction among elderly women: a population-based study. Ann Rheum Dis. 2010;69(6):1162-1164. doi: 10.1136/ard.2009.122770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Choi HK, Curhan G. Independent impact of gout on mortality and risk for coronary heart disease. Circulation. 2007;116(8):894-900. doi: 10.1161/CIRCULATIONAHA.107.703389 [DOI] [PubMed] [Google Scholar]
- 8.Kuo CF, Yu KH, See LC, et al. Risk of myocardial infarction among patients with gout: a nationwide population-based study. Rheumatology (Oxford). 2013;52(1):111-117. doi: 10.1093/rheumatology/kes169 [DOI] [PubMed] [Google Scholar]
- 9.Krishnan E, Baker JF, Furst DE, Schumacher HR. Gout and the risk of acute myocardial infarction. Arthritis Rheum. 2006;54(8):2688-2696. doi: 10.1002/art.22014 [DOI] [PubMed] [Google Scholar]
- 10.Hansildaar R, Vedder D, Baniaamam M, Tausche AK, Gerritsen M, Nurmohamed MT. Cardiovascular risk in inflammatory arthritis: rheumatoid arthritis and gout. Lancet Rheumatol. 2021;3(1):e58-e70. doi: 10.1016/S2665-9913(20)30221-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ridker PM, Everett BM, Thuren T, et al. ; CANTOS Trial Group . Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med. 2017;377(12):1119-1131. doi: 10.1056/NEJMoa1707914 [DOI] [PubMed] [Google Scholar]
- 12.Herrett E, Gallagher AM, Bhaskaran K, et al. Data resource profile: Clinical Practice Research Datalink (CPRD). Int J Epidemiol. 2015;44(3):827-836. doi: 10.1093/ije/dyv098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kuo CF, Grainge MJ, Mallen C, Zhang W, Doherty M. Rising burden of gout in the UK but continuing suboptimal management: a nationwide population study. Ann Rheum Dis. 2015;74(4):661-667. doi: 10.1136/annrheumdis-2013-204463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Herrett E, Shah AD, Boggon R, et al. Completeness and diagnostic validity of recording acute myocardial infarction events in primary care, hospital care, disease registry, and national mortality records: cohort study. BMJ. 2013;346:f2350. doi: 10.1136/bmj.f2350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morgan A, Sinnott SJ, Smeeth L, Minassian C, Quint J. Concordance in the recording of stroke across UK primary and secondary care datasets: a population-based cohort study. BJGP Open. 2021;5(2):BJGPO.2020.0117. doi: 10.3399/BJGPO.2020.0117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Adnet F, Renault R, Jabre P, Kulstad E, Galinski M, Lapostolle F. Incidence of acute myocardial infarction resulting in sudden death outside the hospital. Emerg Med J. 2011;28(10):884-886. doi: 10.1136/emj.2010.095885 [DOI] [PubMed] [Google Scholar]
- 17.Singh JA, Reddy SG, Kundukulam J. Risk factors for gout and prevention: a systematic review of the literature. Curr Opin Rheumatol. 2011;23(2):192-202. doi: 10.1097/BOR.0b013e3283438e13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Suissa S, Dell’aniello S, Vahey S, Renoux C. Time-window bias in case-control studies: statins and lung cancer. Epidemiology. 2011;22(2):228-231. doi: 10.1097/EDE.0b013e3182093a0f [DOI] [PubMed] [Google Scholar]
- 19.Essebag V, Platt RW, Abrahamowicz M, Pilote L. Comparison of nested case-control and survival analysis methodologies for analysis of time-dependent exposure. BMC Med Res Methodol. 2005;5(1):5. doi: 10.1186/1471-2288-5-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zheng C, Rashid N, Wu YL, et al. Using natural language processing and machine learning to identify gout flares from electronic clinical notes. Arthritis Care Res (Hoboken). 2014;66(11):1740-1748. doi: 10.1002/acr.22324 [DOI] [PubMed] [Google Scholar]
- 21.Rothenbacher D, Primatesta P, Ferreira A, Cea-Soriano L, Rodríguez LAG. Frequency and risk factors of gout flares in a large population-based cohort of incident gout. Rheumatology (Oxford). 2011;50(5):973-981. doi: 10.1093/rheumatology/keq363 [DOI] [PubMed] [Google Scholar]
- 22.MacFarlane LA, Liu CC, Solomon DH, Kim SC. Validation of claims-based algorithms for gout flares. Pharmacoepidemiol Drug Saf. 2016;25(7):820-826. doi: 10.1002/pds.4044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Khan NF, Perera R, Harper S, Rose PW. Adaptation and validation of the Charlson Index for Read/OXMIS coded databases. BMC Fam Pract. 2010;11:1-7. doi: 10.1186/1471-2296-11-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mach F, Baigent C, Catapano AL, et al. ; ESC Scientific Document Group . 2019 ESC/EAS Guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J. 2020;41(1):111-188. doi: 10.1093/eurheartj/ehz455 [DOI] [PubMed] [Google Scholar]
- 25.Petersen I, Douglas I, Whitaker H. Self controlled case series methods: an alternative to standard epidemiological study designs. BMJ. 2016;354:i4515. doi: 10.1136/bmj.i4515 [DOI] [PubMed] [Google Scholar]
- 26.Moons KGM, Donders RART, Stijnen T, Harrell FE Jr. Using the outcome for imputation of missing predictor values was preferred. J Clin Epidemiol. 2006;59(10):1092-1101. doi: 10.1016/j.jclinepi.2006.01.009 [DOI] [PubMed] [Google Scholar]
- 27.White IR, Royston P, Wood AM. Multiple imputation using chained equations: issues and guidance for practice. Stat Med. 2011;30(4):377-399. doi: 10.1002/sim.4067 [DOI] [PubMed] [Google Scholar]
- 28.Choi HJ, Moon KW, Kim HO, et al. Seasonal variations and associated factors of gout attacks: a prospective multicenter study in Korea. J Korean Med Sci. 2020;35(20):e133. doi: 10.3346/jkms.2020.35.e133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Martinon F, Pétrilli V, Mayor A, Tardivel A, Tschopp J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature. 2006;440(7081):237-241. doi: 10.1038/nature04516 [DOI] [PubMed] [Google Scholar]
- 30.Buffon A, Biasucci LM, Liuzzo G, D’Onofrio G, Crea F, Maseri A. Widespread coronary inflammation in unstable angina. N Engl J Med. 2002;347(1):5-12. doi: 10.1056/NEJMoa012295 [DOI] [PubMed] [Google Scholar]
- 31.Naruko T, Ueda M, Haze K, et al. Neutrophil infiltration of culprit lesions in acute coronary syndromes. Circulation. 2002;106(23):2894-2900. doi: 10.1161/01.CIR.0000042674.89762.20 [DOI] [PubMed] [Google Scholar]
- 32.Ionita MG, van den Borne P, Catanzariti LM, et al. High neutrophil numbers in human carotid atherosclerotic plaques are associated with characteristics of rupture-prone lesions. Arterioscler Thromb Vasc Biol. 2010;30(9):1842-1848. doi: 10.1161/ATVBAHA.110.209296 [DOI] [PubMed] [Google Scholar]
- 33.Musher DM, Abers MS, Corrales-Medina VF. Acute infection and myocardial infarction. N Engl J Med. 2019;380(2):171-176. doi: 10.1056/NEJMra1808137 [DOI] [PubMed] [Google Scholar]
- 34.Walkey AJ, Benjamin EJ, Lubitz SA. New-onset atrial fibrillation during hospitalization. J Am Coll Cardiol. 2014;64(22):2432-2433. doi: 10.1016/j.jacc.2014.09.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Perez-Ruiz F, Martínez-Indart L, Carmona L, Herrero-Beites AM, Pijoan JI, Krishnan E. Tophaceous gout and high level of hyperuricaemia are both associated with increased risk of mortality in patients with gout. Ann Rheum Dis. 2014;73(1):177-182. doi: 10.1136/annrheumdis-2012-202421 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethod 1. Rationale for Definition of Gout Flare Used in the Current Study
eMethod 2. Rationale for Self-Controlled Case Series Sensitivity Analyses
eMethod 3. Sample Size Estimation
eMethod 4. European Society of Cardiology (ESC) Cardiovascular Risk Categories
eTable 1. Codelists for Case, Exposure and Covariate Definition
eTable 2. Association Between Cardiovascular Events and Recent Prior Gout Flare: Sensitivity Analyses of the Nested Case-Control Study
eTable 3. Sensitivity Analyses of the Self-Controlled Case Series Analysis for Patients With the First Gout Flare After Gout Diagnosis and a Cardiovascular Event
eFigure 1. Self-Controlled Case Series: Schematic Description
eFigure 2. Cohort Development in a Self-Controlled Case-Series Study of Cardiovascular Events After New Diagnosis of Gout
eReferences.