Abstract
Despite the clear importance of a developmental perspective for understanding the emergence of psychopathology across the life-course, such a perspective has yet to be integrated into the RDoC model. In this paper, we articulate a framework that incorporates developmentally-specific learning mechanisms that reflect experience-driven plasticity as additional units of analysis in the existing RDoC matrix. These include both experience-expectant learning mechanisms that occur during sensitive periods of development and experience-dependent learning mechanisms that may exhibit substantial variation across development. Incorporating these learning mechanisms allows for clear integration not only of development but also environmental experience into the RDoC model. We demonstrate how individual differences in environmental experiences—such as early-life adversity—can be leveraged to identify experience-driven plasticity patterns across development and apply this framework to consider how environmental experience shapes key biobehavioral processes that comprise the RDoC model. This framework provides a structure for understanding how affective, cognitive, social, and neurobiological processes are shaped by experience across development and ultimately contribute to the emergence of psychopathology. We demonstrate how incorporating an experience-driven plasticity framework is critical for understanding the development of many processes subsumed within the RDoC model, which will contribute to greater understanding of developmental variation in the etiology of psychopathology and can be leveraged to identify potential windows of heightened developmental plasticity when clinical interventions might be maximally efficacious.
Keywords: development, psychopathology, environment, adversity, early-life stress, experience-dependent plasticity, sensitive periods
General Scientific Summary
We present a framework that incorporates developmentally-specific learning mechanisms that reflect experience-driven plasticity as additional units of analysis in the RDoC matrix. Incorporating these learning mechanisms allows for both development and environmental experience to be integrated into the RDoC model. This experience-driven plasticity framework can stimulate progress in understanding the development of many processes subsumed within the RDoC model, contribute to greater understanding of developmental variation in the onset of psychopathology, and can be leveraged to identify developmental windows of heightened plasticity when clinical interventions might be maximally efficacious.
To develop normally, children require a wide variety of inputs from the environment. Some of these experiences must occur during specific periods of development when the human brain depends upon input from the environment to develop certain capacities. Perceptual development provides an illustrative example. Early in life, visual input to the eyes is required for the visual system to develop normally; this input must occur during a sensitive window that occurs during the first months of life. Numerous other processes also exhibit this type of experience-expectant development, in which particular types of environmental experiences occurring during specific windows of time are required to foster adaptive development. When these expected experiences are absent or when atypical or unexpected experiences occur—such as exposure to trauma—development can be fundamentally altered in ways that increase vulnerability to psychopathology.
The foundational nature of environmental experience in human development must be a key consideration in any model of the mechanisms that contribute to the emergence of psychopathology (Cicchetti & Toth, 2009). Dimensional models of these affective, cognitive, social, and neurobiological mechanisms have become increasingly common, with the Research Domain Criteria (RDoC) model advanced by the National Institute of Mental Health as one prominent example. However, the RDoC model lacks clear integration of environmental experience or developmental mechanisms relevant for psychopathology. The original RDoC model did not incorporate developmental processes or the environment (Insel, 2014), and although the current iteration acknowledges environmental influences and neurodevelopment in a summary figure, these constructs have yet to be integrated in a meaningful way into the model. Given that substantial developmental variation exists in the typical age of onset for different forms of psychopathology (Kessler et al., 2005), and that environmental experiences—such as trauma and early-life adversity—are among the strongest determinants of psychopathology (Green et al., 2010; McLaughlin et al., 2012), incorporating developmental and environmental influences into the RDoC framework is critical. Moreover, the biobehavioral processes that form the basis of the RDoC model exhibit a wide range of developmental trajectories, and environmental experiences also have profound influences on these processes, particularly when they occur during sensitive periods of development (Casey, Oliveri, & Insel, 2014). In this paper, we articulate a framework that incorporates developmentally-specific learning mechanisms that reflect experience-driven plasticity into the RDoC model. Experience-driven brain plasticity facilitates learning that allows an individual to adapt to the particular environment in which they are developing. Incorporating these learning mechanisms allows for clear integration not only of development but also environmental experience into the RDoC.
Our goal is to stimulate progress in integrating a developmental perspective into the RDoC model that also incorporates the fundamental dimensions of environmental experience that shape affective, cognitive, social, and neurobiological development in ways that ultimately contribute to psychopathology. To do so, we briefly describe the principles of experience-driven plasticity that drive neurodevelopment and learning across childhood and adolescence. Second, we discuss how individual differences in environmental experiences—such as early-life adversity—can be leveraged to identify experience-driven plasticity patterns across development. Third, we apply this framework to consider how environmental experience shapes key biobehavioral processes in the RDoC model and highlight how such an approach can be used to determine which aspects of environmental experience—at which points in development—have the strongest influences on these mechanisms. We focus specifically on three domains of the RDoC model that have been studied extensively as mechanisms linking environmental experiences to the emergence of psychopathology—negative valence, positive valence, and cognitive systems (see McLaughlin, Weissman, & Bitran, 2019 for a review), although other domains (e.g., social processes) are similarly influenced by both developmental and environmental factors. Finally, we discuss the clinical implications of an experience-driven plasticity approach to studying the emergence of psychopathology across development and highlight how such an approach can be used not only to identify targets for intervention but also to determine when interventions might be maximally effective.
Experience-Driven Plasticity in Development
Experience-driven plasticity involves mechanisms that promote learning in response to environmental experiences and facilitate adaptation to the environment in which one is developing. This plasticity confers benefits in supportive environments, but may alter development in ways that increase vulnerability for psychopathology in adverse environments. Aberrations in these plasticity mechanisms are also well-documented in many neurodevelopmental conditions (e.g. autism spectrum disorder, schizophrenia) (Marin, 2012). Importantly, experience-driven plasticity changes dramatically across development, with both the magnitude of plasticity and the type of underlying neural processes exhibiting age-related variation. Experience-driven plasticity involves two primary learning processes with different neural mechanisms, developmental profiles, and experiential inputs: experience-expectant and experience-dependent learning. These plasticity mechanisms provide a foundation for integrating developmental processes into the RDoC framework.
Experience-Expectant Learning
Experience-expectant learning reflects neural preparation to biologically encode particular environmental stimuli during specific developmental windows (Greenough, Black, & Wallace, 1987). Environmental deprivation paradigms have been foundational to identifying experience-expectant mechanisms in animal models. In these models, animals are deprived of a specific environmental experience thought to be required for a particular capacity to develop normally. A classic example is the seminal work of Hubel and Wiesel on development of the visual system. They demonstrated that monocular visual deprivation—created by suturing one of the eyelids closed—leads to permanent changes in the organization of ocular dominance columns in primary visual cortex, but only when the deprivation occurs during a specific window (i.e., a sensitive period) in the first months of life (Hubel & Wiesel, 1970; Wiesel & Hubel, 1963). These neural changes are associated with similarly lasting visual impairments in the deprived eye. Visual deprivation occurring at later points in development produces no such changes in neural organization or vision (Hubel & Wiesel, 1970). Though sensitive periods have historically been studied through visual development in animals, these mechanisms are conserved across species (Katz & Meiri, 2006; Werker & Hensch, 2015) and exist for many capacities that have relevance for psychopathology, including attachment, language, fear extinction, and multi-sensory integration (Gogolla et al., 2014; Lieberman et al., 2018; Smyke et al., 2010; Werker & Hensch, 2015; Yang, Lin, & Hensch, 2012). As we review in more detail in the third section of the paper, evidence for experience-expectant learning exists for each of these processes, such that environmental experiences exert pronounced influences on these capacities during specific windows of development with diminished plasticity thereafter.
Sensitive periods have multiple characteristics that distinguish them from other learning mechanisms (Hensch, 2005). First, they encompass periods of heightened neuroplasticity that involve substantial and rapid changes to neural circuitry. Second, sensitive periods enable tuning and narrowing of the brain’s responsiveness to specific types of expected environmental inputs (e.g. language, responsive caregivers), after which additional tuning to new inputs is diminished and requires extensive exposure. Third, they occur for specific brain circuits only during specific windows of development, although their timing is itself malleable, as discussed below. Fourth, sensitive periods are consolidated by molecular and structural regulators that protect the experience-modified circuitry and produce enduring effects on brain function and behavior, although other learning mechanisms may modify function further via residual plasticity following a sensitive period.
Sensitive periods are carefully-orchestrated processes that unfold across RDoC levels of analysis from genes to behavior (Figure 1a). Sensitive period initiation is regulated by molecular pacers and triggers. Pacers inhibit sensitive period initiation to prevent precocious plasticity and maintain healthy developmental momentum (Takesian & Hensch, 2013). Conversely, triggers promote sensitive period initiation and increase neuroplasticity (e.g. through increased brain-derived neurotrophic factor [BDNF], a growth factor involved in synaptic transmission and brain plasticity) (Hanover, Huang, Tonegawa, & Stryker, 1999). Critically, exposure to the expected environmental experience is also required to initiate sensitive periods. In fact, the timing and quality of the expected experience impacts when and how sensitive period learning occurs (Werker & Hensch, 2015). That is, sensitive period timing and plasticity are themselves malleable as a function of experience (Figure 1b). A delay of the expected experience results in delayed sensitive period initiation. However, the system cannot wait for the experience indefinitely, and prolonged deprivation can result in sparse or no learning. Even if the experience occurs at the optimal time, the quality of that experience matters. Enriched experience may initiate sensitive periods more quickly and involve greater neural changes that produce greater functional tuning than inconsistent or poor-quality experiences.
Figure 1. Experience-driven plasticity mechanisms in development.
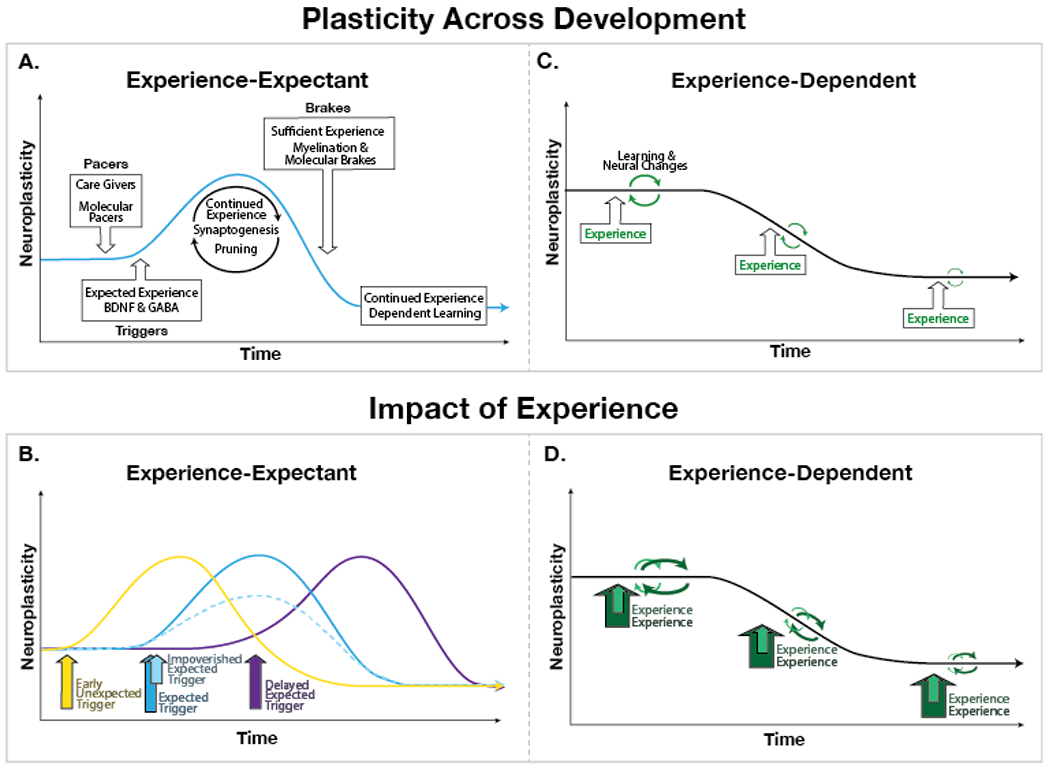
A. Experience-expectant plasticity during sensitive periods is a developmentally-specific learning mechanism. This type of learning occurs for some types of experiences that are ubiquitous and expectable within the environment (e.g. language and the presence of a caregiver). Experience-expectant mechanisms include pacers that regulate when sensitive period plasticity occurs in development, environmental and molecular triggers [e.g. brain-derived neurotrophic factor (BDNF), gamma-Aminobutyric acid (GABA)] that initiate the sensitive period; functional and structural reconfiguration during the sensitive period, including extensive pruning of over-abundant connections to facilitate rapid functional tuning; and molecular and structural brakes like myelin formation to actively dampen further neuroplasticity and stabilize experience-modified circuitry. B. Experience-expectant mechanisms are sensitive to both the timing and nature of developmental experience. Because the expected experience is a trigger for starting sensitive period plasticity, a delay of the expected experience results in delayed sensitive period initiation. However, prolonged deprivation can result in sparse or no learning. Conversely, precocious experience can accelerate sensitive period timing. The quality of experience also influences sensitive period learning. Enriched experience may initiate sensitive periods more quickly and involve greater neural changes that produce more complex functional tuning during the sensitive period than inconsistent or impoverished experiences. C. Experience-dependent learning involves changes in neural structure and function induced by experience without prior preparation (e.g. meditation, cognitive reappraisal, aversive learning). These mechanisms are available to facilitate learning throughout life, though the degree of plasticity declines systematically across development. That is, the magnitude of potential change in response to experience differs with age (illustrated by smaller green cycling arrows across development). Experience-dependent learning includes modulation of the strength of neural connections, formation of new synaptic connections, and pruning of existing connections. The extent of these changes often scales with the intensity and duration of the learning experience. D. The same experience can trigger greater experience-dependent changes early compared to later in development (illustrated with light green cycling arrows). But experience-dependent learning also varies as a function of the experience trigger within a developmental context. At a given age, experiences of greater intensity or duration (illustrated with the larger dark green arrows) will trigger more extensive learning (illustrated with larger dark green cycling arrows).
Once a sensitive period is successfully triggered, additional mechanisms facilitate rapid structural and functional reconfiguration and tuning to the expected experiential inputs (Takesian & Hensch, 2013). For example, dramatic synaptic and neural pruning occurs during sensitive periods to eliminate inefficient and unnecessary connections as circuit function becomes tuned by environmental experience. Continued exposure to expected experiences within the sensitive period is necessary to sculpt healthy brain function via these mechanisms (Schwarzkopf et al., 2007). Sensitive periods are then closed to stabilize the experience-driven function. Sensitive period neuroplasticity is downregulated by a number of molecular and structural factors (e.g. peri-neuronal nets, myelination) that actively inhibit plasticity thereafter (Takesian & Hensch, 2013).
Experience-Dependent Learning
In contrast to experience-expectant learning, experience-dependent plasticity facilitates learning at all points in life (Greenough et al., 1987). Experience-dependent learning involves changes induced by experience without prior preparation (e.g. learning to meditate). While these mechanisms have no ontogenetic constraints in availability, their degree of plasticity does change with age and as a function of the environmental trigger (Figure 1c). It is well-established that experience-driven plasticity diminishes as the brain matures (Fu & Zuo, 2011). Moreover, at a given age, the intensity and duration of an experience can impact the degree of experience-dependent plasticity and subsequent learning that occurs (Figure 1d). Meditation skill will be greater for someone who practices daily for years than someone who practices sporadically. In cognitive behavioral therapy, skill acquisition and symptom reduction are directly related to the degree of engagement in homework (i.e., skill practice) outside of session (Neimeyer et al., 2007). Notably, experiences that trigger experience-expectant learning early in development (e.g. language input required for phoneme discrimination) can trigger subsequent experience-dependent learning later in development (e.g. learning new words) (Werker & Hensch, 2015).
Experience-dependent learning operates through varied mechanisms. Some forms of experience-dependent learning require changes in BDNF in response to experience, while others are BDNF-independent (Aarse, Herlitze, & Manahan-Vaughan, 2016). Neural changes include modulating the strength of neural connections (Fu & Zuo, 2011) and creating or pruning neural connections, but less extensively than in experience-expectant learning (Trachtenberg et al., 2002). Indeed, the number of new neural connections formed during experience-dependent learning is strongly correlated with behavioral performance on that task (Fu & Zuo, 2011; Xu et al., 2009). Experience-dependent learning can also induce structural changes like new myelination, though to a lesser degree than experience-expectant learning (Mount & Monje, 2017; Takesian & Hensch, 2013). For neural circuitry that has undergone experience-expectant learning, subsequent experience-dependent learning is limited by the neural structure and function established during the sensitive period.
Thus, there is precise mapping of brain and behavioral changes in development depending on age and the experience-driven plasticity mechanisms invoked by particular environmental experiences. This specificity highlights why a nuanced approach to characterizing the environment is critical to uncover how the biobehavioral processes in the RDoC domains develop in ways that underpin psychopathology.
Leveraging Individual Differences in Early-Life Experience to Understand Mechanisms of Plasticity
While animal models of experience-driven plasticity experimentally manipulate the type and timing of environmental inputs to identify experience-expectant or -dependent changes in neural and behavioral development, such experimental manipulation of environmental experiences is largely infeasible in human studies. Instead, naturally occurring individual differences in the type and timing of experiences can be leveraged to study experience-driven plasticity in humans. The predominant approach to studying these individual differences in processes underpinning the development of psychopathology has relied on children exposed to different forms of early-life adversity. This approach can shed light on how the quality, timing, and nature of early experiences influence experience-expectant and experience-dependent learning mechanisms.
Early-life adversity involves negative environmental experiences that are either chronic or severe, that reflect a deviation from the expectable environment, and that are likely to require adaptation by a child (McLaughlin, 2016; Nelson & Gabard-Durnam, 2020). This term encompasses a wide range of environmental experiences, including abuse, neglect, domestic violence, absence or limited availability of a caregiver, chronic material deprivation, sparse language environments, and others. Multiple conceptual frameworks have organized these types of adversity into core underlying dimensions of experience Ellis, Figueredo, Brumbach, & Schlomer, 2009; McLaughlin, Sheridan, & Lambert, 2014). One such model focuses on dimensions of threat—experiences that have high potential for harm (i.e., traumatic experiences, such as interpersonal violence), and deprivation—the absence of expected inputs from the environment, such as social and cognitive stimulation and emotional nurturance (McLaughlin et al., 2014; Sheridan & McLaughlin, 2014). This model posits that experiences of threat and deprivation influence emotion, cognitive, and neurobiological development in ways that are at least partially distinct. As described below, these experiences also have fundamentally different implications for the mechanisms underlying experience-driven plasticity (McLaughlin & Sheridan, 2016; McLaughlin et al., 2014).
Deprivation
Experiences of deprivation provide a unique opportunity to identify sensitive periods of experience-expectant learning and to determine the types of environmental experiences that are required for specific cognitive, emotional, and social capacities to emerge. Animal models of sensory development demonstrate that when the required environmental experience does not occur during a sensitive period, it leads to a dramatic reorganization of neural circuits and behavior that persist once the sensitive period has closed. This suggests that timing of exposure is particularly important when studying forms of adversity involving deprivation.
Although deprivation involving a complete absence of experiential substrates needed to drive plasticity within a sensitive period is relatively rare in humans, it exists in some sensory domains that are experience-expectant, such as vision. For example, sensitive periods in human visual development have been characterized by studying children born with dense cataracts that result in visual deprivation in either one or both eyes (Lewis & Maurer, 2005). Children deprived of exposure to language in early life, either due to being born deaf to hearing parents or in extreme cases of neglect, have similarly revealed sensitive periods in language development. This work along with research on developmental variation in learning a second language (Newport, 1990; Pierce et al., 2014) has convincingly demonstrated that multiple sensitive periods exist for language development during which specific types of environment input are required for normal development (Werker & Hensch, 2015).
Deprivation models can be extended to identify the specific environmental inputs and their timing that are required to scaffold development of processes that have relevance to psychopathology. Much of this work has focused on previously-institutionalized children, as the timing of this exposure is well-defined and easily quantified. However, deprivation in social and cognitive inputs—including low levels of cognitive stimulation, exposure to complex language, parental scaffolding of child learning, and environmental enrichment—is commonly experienced among children who are neglected (McLaughlin, Sheridan, & Nelson, 2017) and also occurs at higher rates in children raised in poverty than children from families with higher socioeconomic status (Bradley et al., 2001; Romeo et al., 2018; Rosen et al., 2020). Children raised in institutions experience deprivation of many kinds, including exposure to language, supervision and interaction with adults, cognitive stimulation, and learning opportunities (Smyke et al., 2007). Perhaps most profound is the absence of a sensitive caregiver who responds contingently to the child—a type of caregiving necessary for the development of secure attachment (McElwain & Booth-LaForce, 2006). The Bucharest Early Intervention Project (BEIP)—a randomized controlled trial in which some children were removed from deprived orphanage settings and placed in families while others experienced prolonged institutional care—evaluated whether a sensitive period exists for attachment security. Indeed, children removed from institutional care and placed in a family by the age of 22 months were just as likely to develop secure attachment as children raised in families from birth; in contrast, a minority of children placed after 22 months of age developed a secure attachment and were no more likely to become securely attached than children who experienced prolonged institutional rearing (Smyke et al., 2010). This finding suggests the presence of a sensitive period in the first two years of life for the development of an attachment relationship to a caregiver, such that a majority of children who experience responsive caregiving during the first two years of life develop secure attachment, whereas a minority of children who experience responsive caregiving for the first time after this period develop secure attachment.
Evaluating whether sensitive periods exist for other processes that contribute to the emergence of psychopathology, such as aversive learning, reward sensitivity, and cognitive control is more challenging than for domains of sensory development or attachment security. Unlike vision, where the required environmental input is relatively obvious, the psychosocial inputs required to scaffold cognitive control, for example, are likely psychosocial in nature, complex, and multi-faceted, and as such are not yet fully understood. Moreover, children exposed to psychosocial deprivation in the form of neglect, separation from caregivers, or institutional rearing are typically not completely deprived of social and cognitive stimulation or emotional nurturance. Rather, these children experience infrequent, low-quality, or anomalous inputs (Smyke et al., 2007) (see Figure 1b). Determining how deprivation in these types of early experiences shapes the emergence of emotional, cognitive, and social capacities, and associated neural circuit development, can extend models of experience-driven plasticity to the more complex and varied psychosocial experiences that drive development of the biobehavioral processes in the RDoC model.
Threat
In contrast to deprivation, exposure to traumatic events that involve a high degree of threat are unlikely to reflect an experiential substrate for which a sensitive period exists. Given that the ability to identify threat cues in the environment is essential for survival, it is unlikely that the ability to learn about sources of threat and mobilize defensive responses to them would develop only when threatening experiences occur during a specific point in development. As such, there is unlikely to be a sensitive period for some negative valence processes (i.e., a specific point in development when the brain “expects” to experience environmental threats). Instead, these processes are most likely experience-dependent.
However, this does not mean that the plasticity mechanisms through which trauma influences development are age-invariant. Indeed, exposure to trauma during childhood is much more likely to produce lasting neural changes than when exposure happens in adulthood (Tottenham & Sheridan, 2010). Scant research has examined whether the timing of exposure within childhood and adolescence is important, although one domain where such timing effects might exist is aversive learning. In early childhood, trauma is associated with an earlier emergence of the acquisition of conditioned fear responses (Machlin et al., 2019), consistent with animal models (Moriceau, Shionoya, Jakubs, & Sullivan, 2009). In older children and adolescents, however, trauma exposure is associated with poor discrimination between threat and safety cues during aversive learning (McLaughlin et al., 2016). These developmental differences could reflect variation based on timing of trauma exposure or the chronicity of threat over time.
Despite the fact that the developmental consequences of childhood trauma do not appear to be experience-expectant, it is possible that exposure to threatening environments could alter the timing of sensitive periods by influencing when they open and/or close. Accumulating evidence suggests that childhood trauma exposure accelerates the pace of biological development (Callaghan & Tottenham, 2016; Colich, Rosen, Williams, & McLaughlin, 2020). Children exposed to trauma exhibit earlier pubertal maturation and advanced cellular aging than children who have not experienced trauma, an effect not observed in children exposed to deprivation (Colich et al., 2019; Colich et al., 2020; Sumner et al., 2019). Experiences of threat are associated with accelerated cortical thinning in regions involved in social and emotional processing, such as the ventromedial prefrontal cortex (McLaughlin et al., 2019). This thinning could reflect accelerated pruning of synaptic connections—a neurobiological mechanism underlying sensitive period plasticity (Hensch, 2005), suggesting that trauma exposure could also accelerate the timing of sensitive period plasticity in the brain. Some work suggests that childhood trauma might accelerate the timing of sensitive periods through influences on sensitive period triggers, like BDNF. Increases in BDNF following childhood trauma occur in a regionally-specific way across the brain and are modulated by glucocorticoids and other stress hormones (Bennett & Lagopoulos, 2014). Determining whether and how trauma exposure influences the timing of sensitive periods for processes within the RDoC domains represents a largely unstudied but critical question for future research.
To date, research on threat and deprivation has focused largely on the unique associations of these different forms of environmental adversity with developmental processes, but not their joint influences. Evaluating the degree to which these dimensions of environmental experience interact to shape cognitive, affective, and neurobiological development is a critical goal for future research, given that these experiences co-occur at moderate rates (e.g., McLaughlin et al., 2012).
Moreover, threat and deprivation are clearly not the only dimensions of early experience relevant for the development of psychopathology. Other models of early-life adversity highlight harshness (conceptually similar but not identical to threat) and unpredictability as dimensions of experience that shape development (Ellis et al., 2009). Additionally, while most research focuses on postnatal adversity, recent studies have also examined prenatal adverse experiences (e.g. maternal stress) and their effects on postnatal plasticity mechanisms and psychopathology (Pallares & Antonelli, 2017). For example, prenatal stress decreases subsequent BDNF expression, a plasticity enhancer involved in both sensitive period and experience-dependent plasticity mechanisms (Badihian et al., 2019). Prenatal stress may also delay the development of inhibitory neurons that trigger sensitive period plasticity and behavioral phenotypes associated with sensitive period learning (Lussier & Stevens, 2016).
Identifying the core elements of environmental experiences that shape the mechanisms underlying risk for psychopathology—at particular points in development—is critical to creating a developmentally-informed RDoC framework.
Experience-driven Plasticity of the Biobehavioral Mechanisms Comprising the RDoC Framework
Any meaningful attempt to integrate a developmental perspective into the RDoC model must incorporate experience-driven plasticity mechanisms. We summarize how these mechanisms could be incorporated as additional units of analysis to the RDoC framework in Figure 2. Such an approach demands attention not only to dimensions of environmental experience, but also when those experiences occur during development. Existing research has focused largely on the former—determining which types of environmental experiences influence RDoC processes. Considerably less work has examined whether the development of these capacities is experience-expectant or experience-dependent. As highlighted in Figure 2, most of the biobehavioral processes subsumed within the RDoC framework involve both experience-expectant and experience-dependent learning mechanisms at different points in development. Here, we review existing evidence regarding experience-driven plasticity for several dimensions of the RDoC model that have been studied extensively in relation to environmental experience across development and highlight areas for future research.
Figure 2. Integration of development and environmental experience into the Research Domain Criteria (RDoC) Framework.
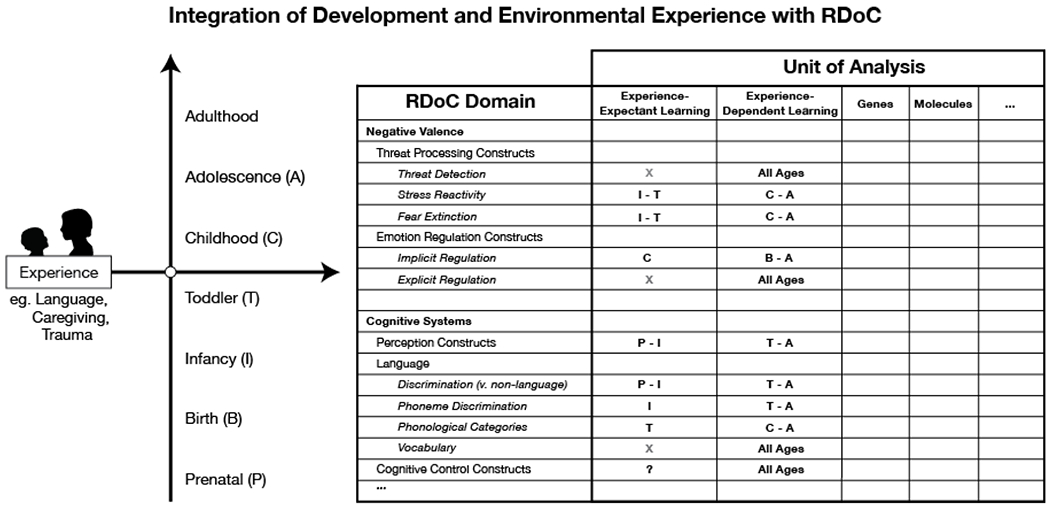
The current iteration of the RDoC framework as a matrix of domains and units of analysis lacks clear integration of developmental processes or the influence of environmental experience. We present a revised schematic of the RDoC framework that incorporates developmental and experiential contributions to the biobehavioral processes in the matrix through experience-driven plasticity mechanisms. This revised RDoC matrix allows for both the nature and timing of environmental experience to be specified along the developmental axis and also affords the benefit of allowing developmental variation in other units of analysis already included in the model to be directly incorporated into the matrix. Experience-driven plasticity across development is specified as two additional units of analysis that are measurable across all domains in the RDoC framework: experience-expectant learning and experience-dependent learning (negative valence and cognitive systems are illustrated here). Developmental stages in which experience-expectant or experience-dependent mechanisms influence processes within each domain are specified; if a form of plasticity does not occur at a particular stage of development, it is indicated by an “X”. Just as cells within the standard RDoC matrix can contain further details (as elements contained within a cell), so too can these additional cells. The learning mechanisms specified as new units of analysis can include additional elements like developmental trajectory charts and biobehavioral processes involved in the learning process at each developmental stage.
Negative Valence Systems
The negative valence systems dimension includes processes involved in threat detection, aversive learning, the mobilization of defensive responses to potential threats, and emotion regulation; experience-driven development for this domain is illustrated in Figure 2. Substantial evidence indicates that exposure to trauma—particularly during childhood and adolescence—is associated with enhanced threat processing across numerous processes subsumed within the negative valence domain of acute threat (McLaughlin, Colich, Rodman, & Weissman, 2020; McLaughlin & Lambert, 2017). These include heightened perceptual sensitivity and attention biases for threat-related stimuli (Pollak & Sinha, 2002; Pollak & Tolley-Schell, 2003), elevated responses in the amygdala and broader salience network to cues that signify the presence of threat (Jenness et al., in press; McCrory et al., 2011; McLaughlin, Peverill, Gold, Alves, & Sheridan, 2015), and difficulty modulating these responses with adaptive emotion regulation strategies (Heleniak et al., 2016; Kim & Cicchetti, 2010; Weissman et al., 2019). In contrast, these developmental patterns have not been consistently observed among children exposed to deprivation (McLaughlin, Weissman, & Bitran, 2019), highlighting the importance of a dimensional approach to characterizing the early environment.
One negative valence domain that may involve experience-expectant learning (in addition to experience-dependent learning) is the regulation of the hypothalamic-pituitary-adrenal (HPA) axis, which mobilizes hormonal, neural, and behavioral responses to environmental challenges. Social buffering of the HPA axis is well-established in both animals and humans, such that the presence of a supportive other dampens HPA axis responses to threat (Hostinar, Sullivan, & Gunnar, 2014). Evidence from children exposed to deprivation involving institutional rearing suggests that the presence of a sensitive and responsive caregiver during a sensitive period in the first two years of life may be required for typical development of the HPA axis (McLaughlin, Sheridan, et al., 2015). Children removed from institutional care before the age of two exhibited patterns of cortisol reactivity to stress in late childhood that were no different than children raised in families from birth; in contrast, children placed into families after age two exhibited markedly blunted cortisol responses to stress that did not differ from children who remained in prolonged institutional care (McLaughlin, Sheridan, et al., 2015). Interestingly, recent evidence suggests that adolescence may be a second period of developmental plasticity for the HPA axis, where recalibration is possible for children in supportive families even after exposure to early-life deprivation (Gunnar, DePasquale, Reid, & Donzella, 2019).
Negative valence processes also centrally involve a neural network comprised of the amygdala and medial prefrontal cortex (mPFC). Twin studies indicate that the development of this circuit is influenced largely by environmental rather than genetic factors (Achterberg et al., 2018). What remains unclear is which environmental influences on the amygdala-mPFC circuit and associated emotional processes are experience-expectant or experience-dependent (Tottenham & Gabard-Durnam, 2017). Fear extinction processes may reflect experience-expectant learning mechanisms. Early in life, extinction learning is capable of erasing fear memories; by adulthood, extinction produces a competing memory trace but does not eliminate the original fear memory (Kim & Richardson, 2010). The developmental shift from fear erasure to extinction coincides with the formation of perineuronal nets—a key molecular brake on sensitive period plasticity (Gogolla, Caroni, Luthi, & Herry, 2009). The degree to which certain salient environmental experiences (e.g., music) are capable of recruiting mPFC and producing anxiolytic effects in animal models also exhibit sensitive period plasticity (Yang, Lin, & Hensch, 2012), a finding recently replicated in humans (Gabard-Durnam et al., under review). Moreover, social isolation during this same period has been shown to alter mPFC function and myelination patterns persistently, even with subsequent return to social environments (Makinodan, Rosen, Ito, & Corfas, 2012). These findings suggest that some aspects of amygdala-mPFC circuit regulation in humans may be experience-expectant, although the suite of experiential substrates that drive this plasticity remain to be characterized.
Other processes in the negative valence domain are almost certainly solely experience-dependent. One obvious example that spans the acute, potential, and sustained threat domains involves the use of explicit emotion regulation strategies, such as cognitive reappraisal or mindfulness. These emotion regulation strategies can be learned at any time once the requisite cognitive abilities required to understand and implement these strategies have developed. Even children exposed to severe maltreatment can be taught to engage in cognitive reappraisal with brief training, and multiple studies indicate that they are able to modulate amygdala responses to threat through reappraisal to a similar degree as children never exposed to adversity (Jenness et al., in press; McLaughlin, Peverill, et al., 2015). A range of other negative valence system processes—such as threat detection, fear generalization, and amygdala responses to threat—are clearly shaped by environmental experiences, but the degree to which these influences are experience-expectant or experience-dependent remains unknown. Characterizing experience-driven plasticity in these processes has particular relevance for understanding the emergence of anxiety disorders and post-traumatic stress disorder (PTSD) across development, as well as other forms of psychopathology known to be influenced by these mechanisms.
Positive Valence Systems
The positive valence system involves a range of processes involved in identifying and learning about rewards, organizing behaviors to obtain them, and experiencing satisfaction and positive emotions during reward anticipation and receipt. These processes are subserved by dopaminergic circuits of the mesolimbic and mesocortical pathways, involving dopaminergic projections from the mid-brain to the dorsal and ventral striatum, and projections from the striatum to the PFC (Berridge & Kringelbach, 2008).
Exposure to early-life adversity involving deprivation in social and cognitive stimulation and emotional nurturance is associated with changes in reward-related behavior and striatal responses to reward spanning the positive valence domains of reward responsiveness and valuation. Institutional rearing, neglect, and food insecurity are associated with reduced approach motivation and behavioral sensitivity to reward value (Dennison et al., 2019; Sheridan et al., 2018). Similarly, children exposed to deprivation exhibit blunted responses in the ventral striatum during reward anticipation and in response to appetitive stimuli (Goff et al., 2013; Hanson, Hariri, & Williamson, 2015; Mehta et al., 2010) and reduced structural connectivity in fronto-striatal white matter tracts (Bick et al., 2015; Dennison et al., 2019). These patterns have generally not been observed in children exposed to trauma (McLaughlin et al., 2019), again highlighting the critical importance of taking a nuanced approach to characterizing the early environment. More broadly, these findings suggest that the presence of sensitive, responsive, and contingent caregiving early in life may be required to scaffold development of reward-related circuits and behaviors through experience-expectant mechanisms.
Consistent with this possibility, evidence from animal models suggests that neural circuits underlying reward-related processes exhibit sensitive periods in multiple developmental periods. Striatal dopamine neurotransmission increases during infancy and precedes a sensitive period for striatal sensitivity to dopamine (Lieberman et al., 2018). The experiential substrates driving this sensitive period are unknown, but it is possible that in humans the presence of a sensitive and responsive caregiver is necessary to trigger the surge in striatal dopamine that opens this sensitive period. An additional sensitive period for social reward learning occurs during adolescence. Specifically, mice exhibit a peak in social reward learning and preference for similarly-aged conspecifics during adolescence that declines in adulthood; this adolescent-specific pattern of social reward learning corresponds to a sensitive period for oxytocin-mediated plasticity in the ventral striatum (Nardou et al., 2019). This peak in sensitivity to social reward cues during adolescence is consistent with contentions that adolescence is a sensitive period for social reward learning in humans (Foulks & Blakemore, 2016) and evidence for developmental variation in ventral striatum sensitivity to reward in humans, which also peaks during adolescence, and underlies increases in risk-taking behavior during this period (Braams, van Duijvenvoorde, Peper, & Crone, 2015; Galvan et al., 2006). Adolescence is also characterized by elevations in both neural and behavioral sensitivity to peers, relative to both childhood and adulthood (Gardner & Steinberg, 1995; Somerville et al., 2013). Evidence for a sensitive period in social reward learning during adolescence raises the intriguing possibility that exposure to peers may be required for this sensitive period to open. This remains to be evaluated empirically, but if true could shed light on the mechanisms through which social isolation and ostracism during adolescence contribute to increased risk for depression and other forms of psychopathology that persist into adulthood (McDougall & Vaillancourt, 2015).
Cognitive Systems
The cognitive systems domain includes perceptual processes, attention, language, long-term memory, and executive functions such as working memory and cognitive control. Environmental experience plays a central role in the emergence of individual differences in virtually all of these processes (Figure 2). Here we focus on several illustrative examples of how experience-driven plasticity mechanisms contribute to the emergence of the processes subsumed within this domain.
Exposure to early-life social and cognitive deprivation is associated with atypical cognitive development, particularly for language and executive functioning (McLaughlin, 2016; McLaughlin, Sheridan, & Nelson, 2017). Difficulties with language and executive functioning have been observed consistently in children exposed to deprivation related to neglect, institutional rearing, and low socioeconomic status (SES) (Fernald, Marchman, & Weisleder, 2013; Noble, McCandliss, & Farah, 2007; Pollak et al., 2010; Rakhlin et al., 2015; Spratt et al., 2012). Deprivation-related differences in these domains emerge in the first two years of life (Fernald et al., 2013) and persist throughout childhood (Lengua et al., 2015). Altered structure and function in the frontotemporal and frontoparietal networks that support language and executive functioning have also been observed consistently in children exposed to deprivation (McLaughlin, Sheridan, Winter, et al., 2014; Mueller et al., 2010) and low SES (Noble et al., 2015; Romeo et al., 2018; Sheridan et al., 2012). These neurocognitive differences are not present at birth (Brito et al., 2016), have not been observed consistently among children exposed to trauma (McLaughlin, Weissman, et al., 2019), and are mediated by reduced cognitive and linguistic stimulation in the home environment (Romeo et al., 2018; Rosen et al., 2018; 2020; Sheridan et al., 2012).
Sensitive periods have been clearly established in a range of perceptual domains. In addition to visual acuity and contrast (Lewis & Maurer, 2005), sensitive periods also occur for face perception. Specifically, infants can discriminate between individuals of other species (e.g. macaques) until only 9 months of age (Pascalis, De Haan, & Nelson, 2002), unless they are exposed to faces of other species regularly during the sensitive period (Pascalis et al., 2005). Sensitive periods are also well-documented for language discrimination and perception (Werker & Hensch, 2015). For example, infants are able to discriminate the sounds of all languages before the age of 6 months; by 10 months, infants retain the ability to discriminate only the sounds of their native language (Kuhl, Williams, Lacerdo, Stevens, & Lindblom, 1992). This perceptual tuning is driven by experience and can be altered by exposing infants to a non-native language during the sensitive period (Kuhl, Tsao, & Liu, 2003; Pierce et al., 2014). Sensitive periods in language development have also been identified for audio-visual matching and phonological categorization (Werker & Hensch, 2015). In contrast, vocabulary development is solely experience-dependent, can be acquired throughout development, and is unique to each individual based on the words they encounter throughout life.
Little is known about the experience-driven plasticity mechanisms that underlie the development of working memory and cognitive control. Although some of the molecular mechanisms regulating sensitive period plasticity have been identified in cortical regions that underlie these higher-order cognitive processes, including the PFC (Larsen & Luna, 2018), it is unclear to what degree these abilities are experience-expectant versus experience-dependent. A central unanswered question is whether there are specific experiential inputs required for the development of executive functions. Given the clear associations of early-life deprivation with poor executive functioning, some have argued that social and cognitive stimulation that occurs in the context of early caregiver interactions creates learning opportunities that scaffold the development of these skills (Bernier, Whipple, & Carlson, 2010; McLaughlin et al., 2017; Rosen, Amso, & McLaughlin, 2019). Although early-life deprivation has lasting influences on executive functioning (Lengua et al., 2015), these abilities and the fronto-parietal networks that support them continue to develop throughout the second decade of life (Luna, Padmanabhan, & O’Hearn, 2010). These developmental patterns have led some to argue that adolescence is a sensitive period for the development of executive functions (Larsen & Luna, 2018). Consistent with this possibility, evidence from a longitudinal study of previously-institutionalized children followed from infancy suggests that the association of the caregiving environment with executive functioning is stronger during adolescence than in childhood (Colich et al., under review), suggesting heightened plasticity and the possibility for improvement in these abilities during adolescence among those in supportive environments. One intriguing possibility is that multiple sensitive periods exist throughout childhood and adolescence for executive functions. Whether this development is experience-expectant, what the required environmental experiences are, and precisely when in development such inputs are expected, however, remain to be elucidated.
Clinical Relevance of an Experience-Driven Plasticity Framework
Incorporating experience-driven learning mechanisms into the RDoC framework will not only foster progress in characterizing the developmental mechanisms that contribute to psychopathology and the specific types of environmental experiences that scaffold these biobehavioral processes, but also has direct implications for identifying intervention targets.
Substantial progress has been made in characterizing the emotional, cognitive, social, and neurobiological processes that contribute to the emergence of psychopathology. Progress has lagged considerably behind, however, in specifying the core underlying dimensions of environmental experience that influence the development of these biobehavioral mechanisms. Greater research into patterns of experience-driven plasticity will shed light on the specific types of environmental experiences that shape these processes, and the experience-expectant and experience-dependent mechanisms that drive their development. Such an approach also has relevance for intervention development to prevent the onset of psychopathology. The intervention approach inherent in the RDoC framework emphasizes targeting underlying mechanisms (e.g., blunted sensitivity to reward or attention biases towards threat) that contribute to the onset and maintenance of psychopathology. This mechanistic approach is essential for developing novel treatments and early interventions. However, it does not present easy options for screening and identifying people who may be at risk for psychopathology, as measuring these mechanisms often requires behavioral tasks, biological assays, or neuroimaging measures. Greater understanding of the environmental determinants of these biobehavioral processes may present far more realistic targets for screening and early identification (McLaughlin, DeCross, Jovanovic, & Tottenham, 2019). For example, blunted sensitivity to reward occurs in depression and prospectively predicts depression onset (Gotlib et al., 2010; Nelson et al., 2016). Behavioral activation targets this reduced motivation to pursue pleasant activities directly (Dimidjian et al., 2006), and thus may be a promising approach for preventing depression. However, determining who might benefit from this type of behavioral intervention through screening with behavioral or neuroimaging tasks is challenging. As noted earlier, accumulating evidence demonstrates that early-life social and cognitive deprivation is strongly linked to this pattern of blunted reward sensitivity (Sheridan et al., 2018). Of course, not all children exposed to deprivation (or with blunted reward sensitivity) will go on to develop depression, but screening for exposure to neglect, food insecurity, low cognitive stimulation, and other forms of deprivation (e.g., within pediatric primary care or early childcare settings) may be more feasible in terms of identifying those who could benefit from these early interventions than screening for blunted reward processing.
Perhaps most importantly, progress in understanding patterns of experience-driven plasticity will inform not only who is in need of early intervention but when such interventions might be maximally efficacious. We provide several illustrative examples. First, evidence for a sensitive period in the first two years of life for the development of an attachment relationship to a caregiver (Smyke et al., 2010) suggests that parenting interventions aimed at improving sensitive and responsive caregiving may have the most pronounced effects when administered during this developmental window. In contrast, recent findings of a sensitive period during adolescence for social reward learning (Nardou et al., 2019) suggest that interventions aimed at boosting reward-related processes in the service of preventing or treating depression may be most effective during adolescence. Future research is needed to examine these possibilities, but they highlight how greater knowledge of experience-driven developmental plasticity of the biobehavioral processes within the RDoC framework may help to identify developmental periods when interventions are most likely to be successful in preventing the emergence of psychopathology.
Conclusion
Experience-driven plasticity provides a framework for understanding how the emotional, cognitive, and social processes that comprise the RDoC model develop. Understanding how these biobehavioral processes are influenced by environmental experiences through experience-expectant and experience-dependent mechanisms will shed light on the specific dimensions of environmental experiences that are most relevant—at which points in development—in contributing to the emergence of psychopathology. This framework can also be leveraged to determine not only which mechanisms should be targeted in early interventions to prevent the onset of psychopathology and who may be most in need of intervention, but when those interventions are most likely to be effective.
Acknowledgments
This work was supported by the National Institute of Mental Health (R01-MH103291, R01-MH106482, and R37-MH119194 to KM) and support from the Tokyo University International Research Center (to LG).
References
- Aarse J, Herlitze S, & Manahan-Vaughan D (2016). The requirement of BDNF for hippocampal synaptic plasticity is experience-dependent. Hippocampus, 26(6), 739–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Achterberg M, Bakermans-Kranenburg MJ, Van Ijzendoorn MH, Van Der Meulen J, Tottenham N, & Crone EA (2018). Distinctive heritability patterns of subcortical-prefrontal cortex resting state connectivity in childhood: A twin study. Neuroimage, 175, 138–149. [DOI] [PubMed] [Google Scholar]
- Badihian N, Daniali S, Kelishadi R (in press). Transcriptional and epigenetic changes of brain derived neurotrophic factor following prenatal stress: A systematic review of animal studies. Neuroscience & Biobehavioral Reviews. [DOI] [PubMed] [Google Scholar]
- Bennett MR, & Lagopoulos J (2014). Stress and trauma: BDNF control of dendritic-sping formation and regression. Progress in Neurobiology, 112, 80–99. [DOI] [PubMed] [Google Scholar]
- Bernier A, Carlson S, & Whipple N (2010). From external regulation to self-regulation: Early parenting precursors of young children’s executive functioning. Child Development, 81, 326–339. [DOI] [PubMed] [Google Scholar]
- Berridge KC, & Kringelbach ML (2008). Affective neuroscience of pleasure: reward in humans and animals. Psychopharmacology, 199, 457–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bick J, Zhu T, Stamoulis C, Fox NA, Zeanah C, & Nelson CA (2015). Effect of early institutionalization and foster care on long-term white matter development: a randomized clinical trial. JAMA Pediatrics, 169, 211–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braams BR, van Duijvenvoorde ACK, Peper JS, & Crone EA (2015). Longitudinal changes in adolescent risk-taking: A comprehensive study of neural response to rewards, pubertal development, and risk-taking behavior. Journal of Neuroscience, 35, 7226–7238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley RH, Corwyn RF, McAdoo HP, & Coll CG (2001). The home environments of children in the United States part I: variations by age, ethnicity, and poverty status. Child Development, 72(6), 1844–1867. [DOI] [PubMed] [Google Scholar]
- Brito NH, Fifer WP, Myers MM, Elliott AJ, & Noble KG (2016). Associations among family socioeconomic status, EEG power at birth, and cognitive skills during infancy. Developmental Cognitive Neuroscience, 19, 144–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callaghan BL, & Tottenham N (2016). The stress acceleration hypothesis: Effects of early-life adversity on emotion circuits and behavior. Current Opinion in Behavioral Sciences, 7, 76–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey BJ, Oliveri ME, Insel T (2014). A neurodevelopmental perspective on the Research Domain Criteria (RDoC) framework. Biological Psychiatry, 76, 350–353. [DOI] [PubMed] [Google Scholar]
- Cicchetti D, & Toth SL (2009). The past achievements and future promises of developmental psychopathology: The coming of age of a discipline. Journal of Child Psychology and Psychiatry, 50, 16–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colich NL, Platt JM, Keyes KM, Sumner JA, Allen NB, & McLaughlin KA (2019). Earlier age at menarche as a transdiagnostic mechanism linking childhood trauma with multiple forms of psychopathology in adolescent girls. Psychological Medicine. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colich NL, Rosen ML, Williams ES, & McLaughlin KA (in press). Accelerated biological aging following childhood experiences of threat and deprivaton: A meta-analysis. Psychological Bulletin. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colich NL, Sheridan MA, Humphreys KL, Wade M, Tibu F, Nelson CA, … McLaughlin KA (under review). Heightened sensitivity to the caregiving environment during adolescence: Implications for recovery following early-life adversity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennison MJ, Rosen ML, Sheridan MA, Sambrook KA, Jenness JL, & McLaughlin KA (2019). Differential associations of distinct forms of childhood adversity with neurobehavioral measures of reward processing: A developmental pathway to depression. Child Development, 90, e96–e113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimidjian S, Hollon SD, Dobson KS, Schmaling KB, Kohlenberg RJ, Addis ME, … Jacobson NS (2006). Randomized trial of behavioral activation, cognitive therapy, and antidepressant medication in the acute treatment of adults with major depression. Journal of Consulting and Clinical Psychology, 74, 658–670. [DOI] [PubMed] [Google Scholar]
- Ellis BJ, Figueredo AJ, Brumbach BH, & Schlomer GL (2009). Fundamental dimensions of environmental risk: The impact of harsh versus unpredictable environments on the evolution and development of life history strategies. Human Nature, 20, 204–268. [DOI] [PubMed] [Google Scholar]
- Fernald A, Marchman VA, & Weisleder A (2013). SES differences in language processing skill and vocabulary are evident at 18 months. Developmental Science, 16(2), 234–248. doi: 10.1111/desc.12019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foulks L, & Blakemore SJ (2016). Is there heightened sensitivity to social reward in adolescence? Current Opinion in Neurobiology, 40, 81–85. [DOI] [PubMed] [Google Scholar]
- Fu M, & Zuo Y (2011). Experience-dependent structural plasticity in the cortex. Trends in Neurosciences, 34, 177–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tottenham N, & Gabard-Durnam LJ (2017). The developing amygdala: a student of the world and a teacher of the cortex. Current Opinion in Psychology, 17, 55–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabard-Durnam LJ, Hensch TK, & Tottenham N (under review). Music reveals medial prefrontal cortex sensitive period in childhood. 10.1101/412007v1.full [DOI]
- Galvan A, Hare TA, Parra CE, Penn J, Voss H, Glover G, & Casey BJ (2006). Earlier development of the accumbens relative to orbitofrontal cortex might underlie risk-taking behavior in adolescents. Journal of Neuroscience, 26, 6885–6892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner M, & Steinberg L (1995). Peer influence on risk taking, risk preference, and risky decision making in adolescence and adulthood: An experimental study. Developmental Psychology, 41, 625–635. [DOI] [PubMed] [Google Scholar]
- Goff B, Gee DG, Telzer EH, Humphreys KL, Gabard-Durnam LJ, Flannery DJ, & Tottenham N (2013). Reduced nucleus accumbens reactivity and adolescent depression following early-life stress. Neuroscience, 249, 129–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogolla N, Caroni P, Lüthi A, & Herry C (2009). Perineuronal nets protect fear memories from erasure. Science, 325, 1258–1261. [DOI] [PubMed] [Google Scholar]
- Gogolla N, Takesian AE, Feng G, Fagiolini M, & Hensch TK (2014). Sensory Integration in Mouse Insular Cortex Reflects GABA Circuit Maturation. Neuron, 83(4), 894–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotlib IH, Hamilton JP, Cooney RE, Singh MK, Henry ML, & Joorman J (2010). Neural processing of reward and loss in girls at high risk of depression. JAMA Psychiatry, 67, 380–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green JG, McLaughlin KA, Berglund P, Gruber MJ, Sampson NA, Zaslavsky AM, & Kessler RC (2010). Childhood adversities and adult psychopathology in the National Comorbidity Survey Replication (NCS-R) I: Associations with first onset of DSM-IV disorders. Archives of General Psychiatry, 62, 113–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenough WT, Black JE, & Wallace CS (1987). Experience and brain development. Child Development, 58, 539–559. [PubMed] [Google Scholar]
- Gunnar MR, DePasquale CE, Reid BM, & Donzella B (2019). Pubertal stress recalibration reverses the effects of early life stress in postinstitutionalized children. Proceedings of the National Academy of Sciences, 116(48), 23984–23988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanover JL, Huang ZJ, Tonegawa S, Stryker MP (1999). Brain-derived neurotrophic factor overexpression induces precocious critical period in mouse visual cortex. J. Neuroscience, 19, RC40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson JL, Hariri AR, & Williamson DE (2015). Blunted ventral striatum development in adolescence reflects emotional neglect and predicts depressive symptoms. Biological Psychiatry, 78, 598–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heleniak C, Jenness J, Van der Stoep A, McCauley E, & McLaughlin KA (2016). Childhood maltreatment exposure and disruptions in emotion regulation: A transdiagnostic pathway to adolescent internalizing and externalizing psychopathology. Cognitive Therapy and Research, 40, 394–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hensch T (2005). Critical period plasticity in local cortical circuits. Nature Reviews Neuroscience, 6, 877–888. [DOI] [PubMed] [Google Scholar]
- Hostinar CE, Sullivan RM, & Gunnar MR (2014). Psychobiological mechanisms underlying the social buffering of the hypothalamic-pituitary-adrenocortical axis: A review of animal models and human studies across development. Psychological Bulletin, 140, 256–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubel DH, & Wiesel TN (1970). The period of susceptibility to the physiological effects of unilateral eye closure in kittens. The Journal of Physiology, 206, 419–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel TR (2014). The NIMH research domain criteria (RDoC) project: precision medicine for psychiatry. American Journal of Psychiatry, 171, 395–397. [DOI] [PubMed] [Google Scholar]
- Jenness JL, Peverill M, Heleniak C, Robertson MM, Sambrook KA, Sheridan MA, & McLaughlin KA (in press). Alterations in neural circuits underlying emotion regulation following child maltreatment: A mechanism underlying trauma-related psychopathology. Psychological Medicine. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz A, & Meiri N (2006). Brain-derived neurotrophic factor is critically involved in thermal-experience-dependent developmental plasticity. Journal of Neuroscience, 26(15), 3899–3907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, & Cicchetti D (2010). Longitudinal pathways linking child maltreatment, emotion regulation, peer relations, and psychopathology. Journal of Child Psychology and Psychiatry, 51, 706–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JH, & Richardson R (2010). New findings on extinction of conditioned fear early in development: Theoretical and clinical implications. Biological Psychiatry, 67, 297–303. [DOI] [PubMed] [Google Scholar]
- Kuhl PK, Tsao FM, & Liu HM (2003). Foreign-language experience in infancy: Effects of short-term exposure and social interaction on phonetic learning. Proceedings of the National Academy of Sciences, 100, 9096–9101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhl PK, Williams KA, Lacerdo F, Stevens KN, & Lindblom B (1992). Linguistic experience alters phonetic perception in infants by 6 months of age. Science, 255(5044), 606–608. [DOI] [PubMed] [Google Scholar]
- Larsen B, & Luna B (2018). Adolescence as a neurobiological critical period for the development of higher-order cognition. Neuroscience and Biobehavioral Reviews, 94, 179–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lengua LJ, Moran L, Zalewski M, Ruberry EJ, Kiff CJ, & Thompson S (2015). Relations of growth in effortful control to family income, cumulative risk, and adjustment in preschool-age children. Journal of Abnormal Child Psychology, 43, 705–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis TL, & Maurer D (2005). Multiple sensitive periods in human visual development: Evidence from visually deprived children. Developmental Psychobiology, 46, 163–183. [DOI] [PubMed] [Google Scholar]
- Lieberman OJ, McGuirt AF, Mosharov EV, Pigulevskiy I, Hobson BD, Choi S, … Sulzer D (2018). Dopamine triggers the maturation of striatal spiny projection neuron excitability during a critical period. Neuron, 99, 540–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luna B, Padmanabhan A, & O’Hearn K (2010). What has fMRI told us about the development of cognitive control through adolescence? Brain Cogn, 72(1), 101–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lussier SJ, Stevens HE (2016). Delays in GABAergic interneuron development and behavioral inhibition after prenatal stress. Developmental Neurobiology, 76, 1078–1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machlin L, Miller AB, Snyder J, McLaughlin KA, & Sheridan MA (2019). Differential associations of deprivation and threat with cognitive control and fear conditioning in early childhood. Frontiers in Behavioral Neuroscience, 13, Article 80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makinodan M, Rosen KM, Ito S, & Corfas G (2012). A critical period for social experience-dependent oligodendrocyte maturation and myelination. Science, 337(6100), 1357–1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marin O (2012). Interneuron dysfunction in psychiatric disorders. Nature Reviews Neuroscience, 13, 107–120. [DOI] [PubMed] [Google Scholar]
- McCrory EJ, De Brito SA, Sebastian CL, Mechelli A, Bird G, Kelly PA, & Viding E (2011). Heightened neural reactivity to threat in child victims of family violence. Current Biology, 21, R947–948. [DOI] [PubMed] [Google Scholar]
- McDougall P, & Vaillancourt T (2015). Long-term adult outcomes of peer victimization in childhood and adolescence: Pathways to adjustment and maladjustment. American Psychologist, 70, 300–310. [DOI] [PubMed] [Google Scholar]
- McElwain NL, & Booth-LaForce C (2006). Maternal sensitivity to infant distress and nondistress as predictors of infant-mother attachment security. Journal of Family Psychology, 20, 247–255. [DOI] [PubMed] [Google Scholar]
- McLaughlin KA (2016). Future directions in childhood adversity and youth psychopathology. Journal of Clinical Child & Adolescent Psychology, 45, 361–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin KA, Colich NL, Rodman AM, & Weissman DG (2020). Mechanisms linking childhood trauma exposure and psychopathology: A transdiagnostic model of risk and resilience. BMC Medicine, 18, 96–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin KA, DeCross SN, Jovanovic T, & Tottenham N (2019). Mechanisms linking childhood adversity with psychopathology: Learning as an intervention target. Behaviour Research and Therapy, 118, 101–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin KA, Green JG, Gruber MJ, Sampson NA, Zaslavsky A, & Kessler RC (2012). Childhood adversities and first onset of psychiatric disorders in a national sample of adolescents. Archives of General Psychiatry, 69, 1151–1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin KA, & Lambert HK (2017). Child trauma exposure and psychopathology: mechanisms of risk and resilience. Current Opinion in Psychology, 14, 29–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin KA, Peverill M, Gold AL, Alves S, & Sheridan MA (2015). Child maltreatment and neural systems underlying emotion regulation. Journal of the American Academy of Child & Adolescent Psychiatry, 54, 753–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin KA, & Sheridan MA (2016). Beyond cumulative risk: A dimensional approach to childhood adversity. Current Directions in Psychological Science, 25, 239–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin KA, Sheridan MA, Gold AL, Lambert HK, Heleniak C, Duys A, … Pine DS (2016). Maltreatment exposure, brain structure, and fear conditioning in children. Neuropsychopharmacology, 41, 1956–1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin KA, Sheridan MA, & Lambert HK (2014). Childhood Adversity and Neural Development: Deprivation and Threat as Distinct Dimensions of Early Experience. Neuroscience and Biobehavioral Reviews, 47, 578–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin KA, Sheridan MA, & Nelson CA (2017). Neglect as a violation of species-expectant experience: Neurodevelopmental consequences. Biological Psychiatry, 82, 462–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin KA, Sheridan MA, Tibu F, Fox NA, Zeanah CH, & Nelson CA (2015). Causal effects of the early caregiving environment on stress response system development in children. Proceedings of the National Academy of Sciences, 112, 5637–5642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin KA, Sheridan MA, Winter W, Fox NA, Zeanah CH, & Nelson CA (2014). Widespread reductions in cortical thickness following severe early-life deprivation: A neurodevelopmental pathway to ADHD. Biological Psychiatry, 76, 629–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin KA, Weissman DG, & Bitran D (2019). Childhood Adversity and Neural Development: A Systematic Review. Annual Review of Developmental Psychology, 1, 277–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta MA, Gore-Langton E, Golembo NI, Colvert E, Williams SCR, & Sonuga-Barke EJS (2010). Hyporesponsive reward anticipation in the basal ganglia following severe institutional deprivation early in life. Journal of Cognitive Neuroscience, 22(10), 2316–2325. [DOI] [PubMed] [Google Scholar]
- Moriceau S, Shionoya K, Jakubs K, & Sullivan RM (2009). Early-life stress disrupts attachment learning: The role of amygdala, coricosterone, locus ceruleus corticotropin releasing hormone, and olfactory bulb norepinephrine. Journal of Neuroscience, 29, 15745–15755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mount CW, & Monje M (2017). Wrapped to Adapt: Experience-Dependent Myelination. Neuron, 95, 743–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller SC, Maheu FS, Dozier M, Peloso E, Mandell D, Leibenluft E, … Ernst M (2010). Early-life stress is associated with impairment in cognitive control in adolescence: an fMRI study. Neuropsychologia, 48, 3037–3044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neimeyer RA, Kazantzis N, Kassler DM, Baker KD, & Fletcher R (2008). Group cognitive behavioural therapy for depression outcomes predicted by willingness to engage in homework, compliance with homework, and cognitive restructuring skill acquisition. Cognitive Behaviour Therapy, 37(4), 199–215. [DOI] [PubMed] [Google Scholar]
- Newport EL (1990). Maturational constraints on language learning. Cognitive Science, 14, 11–28. [Google Scholar]
- Nardou R, Lewis EM, Rothhaas R, Xu R, Yang A, Boyden E, & Dolen G (2019). Oxytocin-dependent reopening of a social reward learning critical period with MDMA. Nature, 569, 116–120. [DOI] [PubMed] [Google Scholar]
- Nelson BD, Perlman G, Klein DN, Kotov R, & Hajack G (2016). Blunted neural response to rewards as a prospective predictor of the development of depression in adolescent girls. American Journal of Psychiatry, 173, 1223–1230. [DOI] [PubMed] [Google Scholar]
- Nelson CA, Gabard-Durnam LJ (2020). Early adversity and critical periods: Neurodevelopmental consequences of violating the expectable environment. Trends in Neurosciences, 43, 133–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble KG, Houston SM, Brito NH, Bartsch H, Kan E, Kuperman JM, … Sowell ER (2015). Family income, parental education and brain structure in children and adolescents. Nature Neuroscience, 18, 773–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble KG, McCandliss BD, & Farah MJ (2007). Socioeconomic gradients predict individual differences in neurocognitive abilities. Developmental Science, 10, 464–480. [DOI] [PubMed] [Google Scholar]
- Pallares M & Antonelli M (2017). Prenatal stress and neurodevelopmental plasticity: relevance to psychopathology. In: The Plastic Brain (pgs. 117–129). Springer. [DOI] [PubMed] [Google Scholar]
- Pascalis O, De Haan M, & Nelson CA (2002). Is face processing species-specific during the first year of life? Science, 296(5571), 1321–1323. 10.1126/science.1070223 [DOI] [PubMed] [Google Scholar]
- Pascalis O, Scott LS, Kelly DJ, Shannon RW, Nicholson E, Coleman M, & Nelson CA (2005). Plasticity of face processing in infancy. Proceedings of the National Academy of Sciences, 102(14), 5297–5300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce LJ, Klein D, Chen J-K, Delcenserie A, & Genesee F (2014). Mapping the unconscious maintenance of a lost first language. Proceedings of the National Academy of Sciences, 111, 17314–17319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollak SD, Nelson CA, Schlaak MF, Roeber BJ, Wewerka S, Wiik KL, … Gunnar MR (2010). Neurodevelopmental effects of early deprivation in postinstitutionalized children. Child Development, 81, 224–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollak SD, & Sinha P (2002). Effects of early experience on children’s recognition of facial displays of emotion. Development and Psychopathology, 38, 784–791. [DOI] [PubMed] [Google Scholar]
- Pollak SD, & Tolley-Schell SA (2003). Selective attention to facial emotion in physically abused children. Journal of Abnormal Psychology, 112(3), 323–338. [DOI] [PubMed] [Google Scholar]
- Rakhlin N, Hein S, Doyle N, Hart L, Macomber D, Ruchkin V, … Grigorenko EL (2015). Language development of internationally adopted children: Adverse early experiences outweigh the age of acquisition effect. Journal of Communication Disorders, 57, 66–80. [DOI] [PubMed] [Google Scholar]
- Romeo RR, Leonard JA, Robinson ST, West MR, Mackey AP, Rowe ML, & Gabrieli JDE (2018). Beyond the 30-million-word gap: Children’s conversational exposure is associated with language-related brain function. Psychological Science, 29, 700–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen ML, Amso D, & McLaughlin KM (2019). The role of the visual association cortex in scaffolding prefrontal cortex development: A novel mechanism linking socioeconomic status and executive function. Developmental Cognitive Neuroscience, 39, 100699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen ML, Hagen MP, Lurie LA, Miles ZE, Sheridan MA, Meltzoff AN, & McLaughlin KA (2020). Cognitive stimulation as a mechanism linking socioeconomic status with executive function: A longitudinal investigation. Child Development, 91, e762–e779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen ML, Sheridan MA, Sambrook K, Meltzoff A, & McLaughlin KA (2018). Socioeconomic disparities in academic achievement: A multimodal investigation of neurodevelopmental mechanisms. Neuroimage, 173, 298–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarzkopf DS, Vorobyov V, Mitchell DE, & Sengpiel F (2007). Brief daily binocular vision prevents monocular deprivation effects in visual cortex. European Journal of Neuroscience, 25(1), 270–280. [DOI] [PubMed] [Google Scholar]
- Sheridan MA, & McLaughlin KA (2014). Dimensions of Early Experience and Neural Development: Deprivation and Threat. Trends in Cognitive Sciences, 18, 580–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheridan MA, McLaughlin KA, Winter W, Fox NA, Zeanah CH, & Nelson CA (2018). Early deprivation disruption of associative learning is a developmental pathway to depression and social problems. Nature Communications, 9, 2216. doi:DOI: 10.1038/s41467-018-04381-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheridan MA, Sarsour K, Jutte D, D’Esposito M, & Boyce WT (2012). The impact of social disparity on prefrontal function in childhood. PLoS One, 7(4), e35744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyke AT, Koga S, Johnson DE, Fox NA, Marshall PJ, Nelson CA (2007). The caregiving context in institution-reared and family-reared infants and toddlers in Romania. Journal of Child Psychology and Psychiatry, 48, 210–218. [DOI] [PubMed] [Google Scholar]
- Smyke AT, Zeanah CH, Fox NA, Nelson CA, & Guthrie D (2010). Placement in foster care enhances quality of attachment among young institutionalized children. Child Development, 81, 212–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville LH, Jones RM, Ruberry EJ, Dyke JP, Glover G, & Casey BJ (2013). The medial prefrontal cortex and the emergence of self-conscious emotion in adolescence. Psychological Science, 24, 1554–1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spratt EG, Friedenberg SL, Swenson CC, Larosa A, De Bellis MD, Macias MM, … Brady KT (2012). The Effects of Early Neglect on Cognitive, Language, and Behavioral Functioning in Childhood. Psychology, 3(2), 175–182. doi: 10.4236/psych.2012.32026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumner JA, Colich NL, Uddin M, Armstrong D, & McLaughlin KA (2019). Early experiences of threat, but not deprivation, are associated with accelerated biological aging in children and adolescents. Biological Psychiatry, 85, 268–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takesian AE, & Hensch T (2013). Balancing plasticity/stability across brain development. Progress in Brain Research, 207, 3–34. [DOI] [PubMed] [Google Scholar]
- Trachtenberg JT, Chen BE, Knott GW, Feng G, Sanes JR, Welker E, & Svoboda K (2002). Long-term in vivo imaging of experience-dependent synaptic plasticity in adult cortex. Nature, 420(6917), 788–794. [DOI] [PubMed] [Google Scholar]
- Tottenham N, & Sheridan MA (2010). A review of adversity, the amygdala, and the hippocampus: A consideration of developmental timing. Frontiers in Human Neuroscience, 3, 68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissman DG, Bitran D, Miller AB, Schaefer JD, Sheridan MA, & McLaughlin KA (2019). Difficulties with emotion regulation as a transdiagnostic mechanism linking child maltreatment with the emergence of psychopathology. Development and Psychopathology, 31, 899–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werker JF, & Hensch TK (2015). Critical periods in speech perception: New directions. Annual Review of Psychology, 66, 173–196. [DOI] [PubMed] [Google Scholar]
- Wiesel TN, & Hubel DH (1963). Single-cell responses in striate cortex of kittens deprived of vision in one eye. Journal of Neurophysiology, 26, 1003–1017. [DOI] [PubMed] [Google Scholar]
- Xu T, Yu X, Perlik AJ, Tobin WF, Zweig JA, Tennant K, ... Zuo Y (2009). Rapid formation and selective stabilization of synapses for enduring motor memories. Nature, 462(7275), 915–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang E-J, Lin EW, & Hensch TK (2012). Critical period for acoustic preference in mice. Proceedings of the National Academy of Sciences, 109, 17213–17220. [DOI] [PMC free article] [PubMed] [Google Scholar]


