Abstract
Purpose
FDG-positive neuroendocrine tumors (NETs) have a poorer prognosis and exhibit shorter response duration to peptide receptor radionuclide therapy (PRRT). The aim of this prospective phase II study was to evaluate the efficacy and toxicity of PRRT with 177Lu-DOTATATE associated with metronomic capecitabine as a radiosensitizer agent in patients with advanced progressive FDG-positive gastro-entero-pancreatic (GEP) NETs.
Patients and methods
Patients with advanced somatostatin receptor- and FDG-positive G1-G3 GEP-NETs (Ki67<55%) were treated with a cumulative activity of 27.5 GBq of 177Lu-DOTATATE divided in five cycles of 5.5 GBq each every 8 weeks. Capecitabine (1000–1500 mg daily) was administered orally in the inter-cycle period between 177Lu-DOTATATE treatments. Prior to commencing capecitabine, all patients were triaged with the dihydropyrimidine dehydrogenase (DPD) test. Only DPD-proficient individuals were enrolled. The primary objectives were disease control rate (DCR) and safety. Secondary aims included progression-free (PFS) and overall survival (OS). Treatment response was assessed per Response Evaluation Criteria in Solid Tumors, version 1.1 (RECIST 1.1). Toxicity was assessed by Common Terminology Criteria for Adverse Events (CTCAE) version 4.0.
Results
From August 2015 to December 2016, 37 subjects were consecutively enrolled. Twenty-five (68%) were affected by pancreatic neuroendocrine tumors (P-NETs) and 12 (32%) had gastrointestinal neuroendocrine tumors (GI-NETs). By grading (WHO 2010 classification), 12 patients (32%) had G1 (Ki67≤2%), 22 (59%) had G2 (3%<Ki67≤20%) and 3 patients (9%) had G3 (Ki67>20%) NETs. Grade 3 (G3) or 4 (G4) hematological toxicity occurred in 16.2% of patients. Other G3-G4 adverse events were diarrhea in 5.4% of cases and asthenia in 5.4%. No renal toxicity was observed for the duration of follow-up. Thirty-three of 37 patients were evaluable for response. Objective responses included partial response (PR) in 10 patients (30%), stable disease (SD) in 18 patients (55%), with a DCR of 85%. Median follow up was 38 months (range 4.6–51.1 months). Median PFS was 31.4 months (17.6–45.4) and mOS was not reached.
Conclusions
This study demonstrated that the combination of PRRT with 177Lu-DOTATATE and metronomic capecitabine is active and well tolerated in patients with aggressive FDG-positive G1-G3 GEP-NETs. These data constitute the basis for a randomized study of PPRT alone vs. PRRT plus metronomic capecitabine.
Keywords: GEP-NETs, FDG, Combined therapy, Capecitabine, PRRT, 177Lu-DOTATATE
Introduction
More than 90% of grade 1 (G1) and 2 (G2) gastro-entero-pancreatic (GEP) neuroendocrine tumors (NETs) overexpress somatostatin receptors (SSTRs) [1], in particular subtype 2, which is the basis for peptide receptor radionuclide therapy (PRRT). The efficacy and tolerability of PRRT in GEP-NETs has been demonstrated in numerous single-arm phase 2 studies and, recently, in a multicenter randomized trial (NETTER-1) [2–5].
As grading increases, i.e. NETs with Ki67 above 10–20%, SSTR expression tends to decrease and glucose metabolism, as seen by 18F-FDG PET/CT (FDG-PET), increases [6,7]. However, 40% to 60% of G1 and G2 GEP-NETs may exhibit uptake at FDG-PET in at least one lesion. Increased glucose metabolism, the so-called “Warburg effect”, is characteristic of many tumors and correlates with a more aggressive phenotype [8]. In NETs, this feature has been shown to predict poor survival [9, 10] and shorter progression-free survival (PFS) after PRRT with 177Lu-DOTATATE (Lu-PRRT) compared to FDG-negative disease [3, 4, 11].
In the past few years, several single-arm phase II trials have investigated the tolerability and efficacy of the combination of Lu-PRRT with capecitabine using various administration schedules [12–17]. The rationale behind combining capecitabine, the oral prodrug of 5-fluorouracil (5-FU), with Lu-PRRT, lies in the activity of this chemotherapeutic agent in GEP-NETs which may act as a radiosensitizer in synergy with radionuclide therapy, particularly useful in aggressive or radioresistant disease [17]. Although these studies showed excellent disease control rates (DCRs) (84%−100%) and toxicity profiles similar to those of PRRT alone, none used any stratification criteria for subject enrolment based on prognostically relevant features.
We present the results of the Lu-X phase I/II trial aimed at exploring the safety and efficacy of the combination of Lu-PRRT with metronomic capecitabine in FDG-positive, DPD-proficient, G1-G3 GEP-NETs.
The rationale of the study was to adopt a combination treatment for patients with poorer prognosis (FDG-positivity) and lower likelihood of a satisfactory and/or durable response to PRRT, pending the implementation of more complex randomized studies [12–17].
Patients and methods
This was a prospective study aimed to enrol subjects with advanced, FDG-positive, G1-G3 GEP-NETs (Ki67 < 55%). Patients were selected according to the following characteristics: biopsy-proven inoperable or metastatic well-differentiated, G1-G3 GEP-NET; preserved hematological, liver and renal laboratory parameters; age ≥ 18 years; DPD-proficiecy, significant tumor uptake (G3 or G4, according to Krenning score [18] at somatostatin receptor imaging (SRI) with either 111In-pentetreotide and 68Ga-DOTATOC PET/CT; positive 18FDG PET/CT with a SUV > 2.5 in at least one documented lesion [4, 9]; measurable disease by means of conventional imaging (CT or MRI); no other treatments (e.g. chemo- or radiotherapy) within 4 weeks prior to 177Lu-DOTATATE; no previous radionuclide treatments with radiopeptides (e.g. 111In-pentetreotide, 90Y-DOTATOC) or other radiopharmaceuticals; no bone marrow invasion > 25%; no other concomitant neoplasm (excluding in situ basalioma and radically treated cervical cancers); Eastern Cooperative Oncology Group (ECOG) performance status ≤ 2; life expectancy ≥ 6 months.
No patients bearing SSTR-negative lesions, regardless of their FDG status, were allowed in this protocol. Eligible patients were included in the study after discussion among the members of a dedicated NET tumor board.
Study treatment
Five cycles of 5.5 GBq (150 mCi) 177Lu-DOTATATE each, up to a total cumulative activity of 27.5 GBq (750 mCi), were administered at intervals of 8 weeks. The analysis of DPD gene polymorphism was performed in all patients using mass spectrometry associated with single base extension technology (Myriapod ADMET, Diatech pharmacogenetics, Jesi, Italy) to evaluate tolerability to capecitabine. Only DPD-proficient patients were allowed in the protocol and received oral capecitabine according to a metronomic schedule each day in the inter-cycle period between Lu-PRRT infusions, at a dose of 500 mg 3 times daily (total 1500 mg), 30 minutes after meals. As per protocol, in the event of reduced performance status (ECOG = 2) or in patients heavily pre-treated with chemotherapy, according to the clinician’s judgement the dosage of capecitabine could be reduced to 1000 mg/die.
Functional and morphologic imaging (CT, MRI, SRI) was undertaken at baseline. Morphologic imaging was repeated every three months after the end of Lu-PRRT to evaluate the therapeutic response.
During the treatment period, each patient was evaluated by clinical examination and cardiological evaluation before each cycle, while blood tests were repeated every 2 weeks between cycles. A CT scan was also performed at the post therapy whole body scan after the third cycle in the event of excellent response or suspected progressive disease.
Diagnostic phase
For consistency, a centralized revision of histopathology was conducted in each case to confirm the histopathologic diagnosis and to establish tumor grading according to WHO 2010 guidelines [19]. Diagnostic scintigraphic imaging with either 111In-octreotide or 68Ga-DOTA-peptide (Ga-labelled [1, 4, 7, 10-tetraazacyclododecane-1, 4, 7, 10-tetraacetic acid]-peptide) PET/CT was used to select patients for Lu-PRRT. 18F-FDG PET/CT was performed at IRST using a standard technique on a dedicated 3D PET/CT system (Biograph mCT Flow; Siemens Medical Solutions, Malvern, PA, USA), and compared with somatostatin receptor imaging.
Radiopeptide preparation and administration
177Lu was purchased from PerkinElmer Life Science (Boston, USA). DOTATATE was purchased from Pichem (Graz, Austria). Labelling procedure and quality control of the 177Lu-DOTATATE compound was performed by hospital radiopharmacists [20]. Administered activity doses of 5.5 GBq of 177Lu were measured in a dose calibrator, properly calibrated for the radionuclide.
The radiopharmaceutical was infused intravenously over 30 minutes using a dedicated pump system (patent US 7842023 B2, Paganelli-Chinol). In order to protect the kidneys during the excretion of the radiopeptide, patients received the co-administration of intravenous amino acids according to the following protocol: lysine 70 mEq in 500 mL saline, 250 mL over 30 min immediately before therapy, 250 mL during therapy; lysine 70 mEq in 500 mL saline during the first 3 hours after therapy, and lysine 60 mEq in 500 mL saline over one hour twice the following day [21].
Imaging
Anterior and posterior whole-body images were acquired 24 hours after Lu-PRRT administration with a 256 × 1,024 matrix using a dual-headed gamma camera (Infinia Hawkeye, GE Healthcare, Milwaukee, WI, USA) equipped with a low-energy high-resolution collimator with the energy window set on 177Lu peaks. A SPECT study was acquired (64 projections, 360°) in selected patients to better document tumor uptake.
Statistical analysis
Descriptive statistics were reported as appropriate for demographic characteristics, baseline characteristics of the tumor, anamnesis, and physical examination. The mean, median, standard deviation, minimum and maximum were reported for continuous variables, and counts and proportions were reported for non-continuous variables.
Disease control rate (DCR) was defined as the percentage of patients who achieved a complete response (CR), partial response (PR) or stable disease (SD) 3 months after the end of treatments and was evaluated according to RECIST 1.1. PFS was defined as the time from the start of treatment to the date of the first documented evidence of disease progression or death from any cause. Patients without tumor progression at the time of analysis were censored at their last date of tumor evaluation. Overall survival (OS) was defined as the time from treatment start to the time of death from any cause. Subjects who were no longer alive at the time of the final analysis or who had been lost to follow-up were censored on the date they were last known to be alive. DCR was calculated with an exact 95% 2-sided confidence interval (95% CI) using standard methods based on binomial distribution. Time-to-event data (PFS and OS) were estimated using the Kaplan-Meier method and compared by the logrank test. 95% CIs for median time were calculated with non-parametric methods. Univariate Cox regression models were used to estimate hazard ratios (HR) and their 95% CIs. P-values were based on two-sided testing and statistical analyses were carried out using SAS statistical software (version 9.4, SAS Institute, Cary, NC, USA) and were considered as significant if less than 0.05.
Results
From August 2015 to December 2016, 37 DPD-proficient patients with progressive metastatic or inoperable GEP-NETs were consecutively enrolled in this phase I/II protocol. All subjects enrolled in the study had SSTR-positive disease with at least one FDG-positive lesion (Supplemetary Table S1 [22]). Tumor grading was G1 in 12 (32%) patients, G2 in 22 (59%) patients and G3 (Ki67 < 55%) in 3 (9 %) patients. Pancreas was the primary tumor site in the majority of patients (n=25, 68%). Most patients (n= 31, 83.8%) had liver metastases. Twelve (32%) had undergone previous surgical resection and 19 (51%) had received previous systemic therapy other than somatostatin analogs (Table 1).
Table 1.
Demographics and clinical patient characteristics
| GEP-NET No. (%) |
|
|---|---|
| Total no. patients | 37 |
| Males | 21 (57) |
| Females | 16 (43) |
| Median age, years (range) | 58 (36–77) |
| Median cumulative activity, GBq (range) | 27.7 (5.5–33.3) |
| Ki67 proliferation index (WHO grade) | |
| Ki67≤2% | 12 (32) |
| 2%<Ki67≤20% | 22 (59) |
| 20%<Ki67<55% | 3 (9) |
| Primary tumor | |
| Pancreas | 25 (68) |
| Small intestine | 10 (27) |
| Right colon (cecum) | 2 (5) |
| Somatostatin receptor uptake * | |
| Grade 1 | 0 |
| Grade 2 | 0 |
| Grade 3 | 10 (27) |
| Grade 4 | 27 (73) |
| Previous treatments | |
| Surgery | 12 (32) |
| Somatostatin analogs | 30 (81) |
| Everolimus | 5 (13) |
| Chemotherapy | 14 (38): |
| • Carboplatin + etoposide | 7 |
| • Capecitabine + temozolomide | 6 |
| • Streptozocin | 1 |
Krenning Uptake scale: grade 1: uptake lower than normal liver; grade 2 similar to normal liver; grade 3 higher than normal liver; grade 4 higher than kidney or spleen (16).
Treatment administration
All patients received 5.5 GBq of 177Lu-DOTATATE per cycle and metronomic capecitabine, 1500 mg or 1000 mg. The dosage of capecitabine was reduced to 1000 mg/die in 17 patients. The median cumulative administered activity was 27.7 GBq (5.5–33.3).
Of the 37 patients, 30 completed the five planned PRRT cycles, 4 patients discontinued the treatment after the first cycle for G3/G4 toxicity (1 hematological, 2 asthenia and 1 diarrhea), one after the third cycle for disease progression and 2 after the fourth cycle (one for G4 thrombocytopenia and one for surgery). This last patient, who had pancreatic NET, became operable after 4 cycles, as seen at the interim CT. Upon discussion among the members of the NET tumor board, surgery was proposed as the best course of action, thereby also avoiding additional radiation from the fifth Lu-PRRT cycle. This individual is still disease free survival after five years.
Response to therapy
As indicated in the study protocol, all patients who received at least 3 cycles of Lu-PRRT could be considered evaluable for response. Thirty-three patients were evaluable for response. Objective response was assessed according to RECIST 1.1, 3 months after therapy. Overall, we observed partial response (PR) in 10 (30%) patients and stable disease (SD) in 18 (55%) patients, with a DCR of 85% (Fig. 1).
Fig. 1.
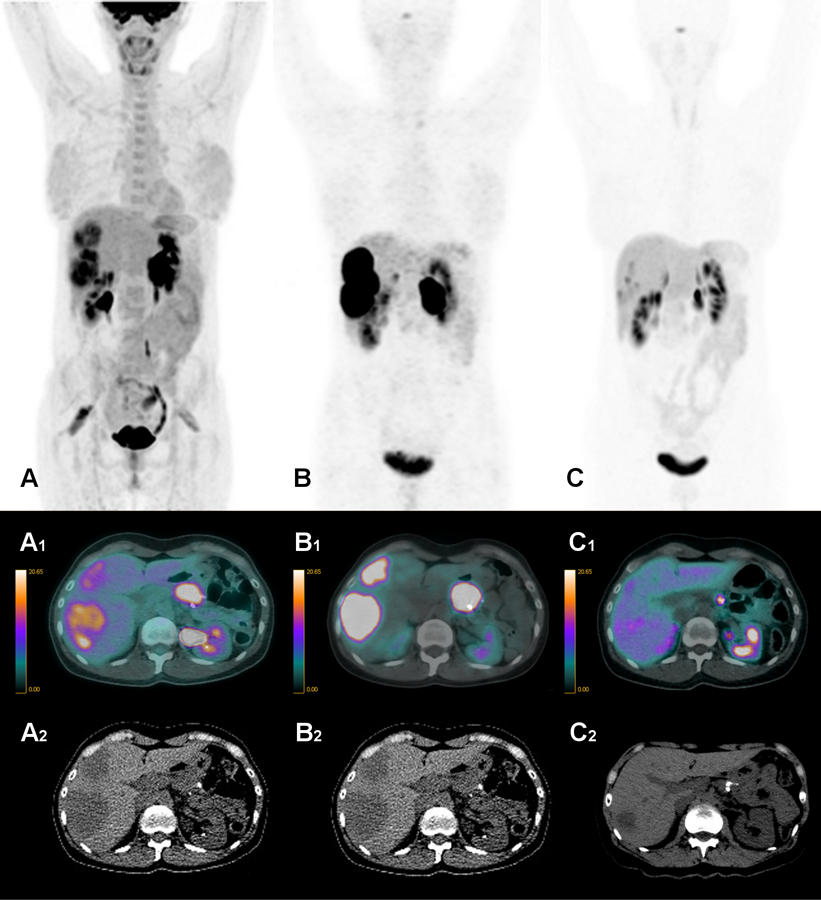
A 42 year-old woman with P-NET G2 with multiple liver metastases, treated with 5.5 GBq of Lu-PRRT in 5 cycles associated with capecitabine 500 mg x 3/die. (A) Baseline FDG PET/CT (A, volumetric; A1, fused transaxial; A2, CT images) before starting the combined treatment. (B) Baseline 68Ga-DOTATOC PET/CT (B, volumetric; B1, fused transaxial; B2, CT images) showing intense uptake (Krenning grade 4) in the liver metastases. (C) 68Ga-DOTATOC PET/CT performed 5 months after the end of combination treatment (C, volumetric; C1, fused transaxial, C2, CT images) shows nearly resolved avidity in the liver lesions.
After a median follow up of 38 months (range 4.6–51.1 months), mPFS was 31.4 months (17.6–45.4) (Fig. 2) and mOS had not been reached.
Fig. 2.
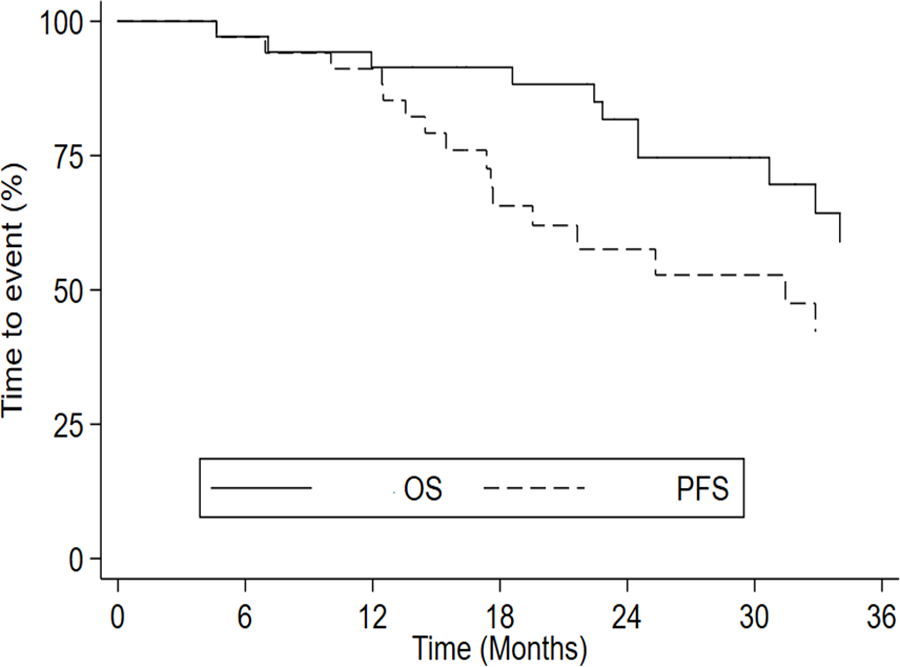
Median PFS and median OS curves representing all patients.
Of the 33 patients evaluable for response, 12 were G1 (Ki67 ≤2%), 19 were G2 (Ki67 3–20%) and 2 were G3 (Ki67>20%). When stratified according to Ki67, G1 NETs had a median PFS (mPFS) of 25.3 months compared to 39.7 months for G2 patients. The difference was not statistically significant (P=0.154) (Fig. S1). Two cases with Ki67 >20% were excluded from P-value calculation because they did not constitute a large enough sample to calculate a survival curve (Supplementary Table S2).
According to the primary tumor site, PFS was 31.4 months (14.4–39.7) in pancreatic NETs and 36.1 (17.5-NR) in gastrointestinal NETs, without statistically significant difference (P = 0.158) (Fig. 3).
Fig. 3.
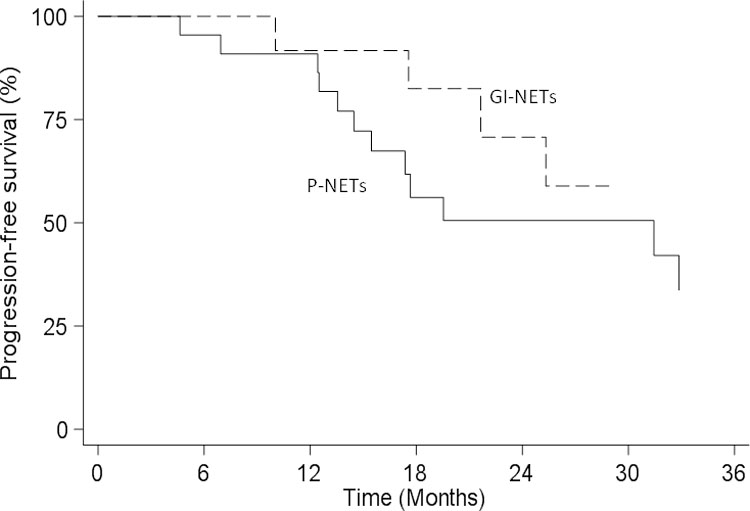
Median PFS based on primary tumor site.
Safety
All patients (n = 37) were evaluable for toxicity. The most frequently reported side-effects were G1 or G2 of transient bone marrow toxicity (Supplementary Table S3). Adverse events were mainly mild. Mostly reversible G3 or G4 hematological toxicity was observed in 6 (16.2%) cases, specifically, neutropenia in 4 (10.8%) patients, lymphopenia in one (2.7%) and thrombocytopenia in one (2.7%). G3 fatigue was noted in 5.4% of patients and grade 3 diarrhea in 5.4% of cases (Supplementary Table S3).
Four (10.8%) patients discontinued the treatment after the first cycle due to severe toxicity: two patients had G3 asthenia, one had G3 diarrhea and one had G4 neutropenia. All patients recovered completely after interrupting the combined treatment. In the patient with G4 neutropenia, the white blood cell count rapidly returned to normal after discontinuation of capecitabine and about a month later the patient was able to restart Lu-PRRT alone outside of this protocol, without further toxicity. In one (2.7%) patient, the combined treatment was discontinued after the fourth cycle due to G4 trombocytopenia. This toxicity persisted until the patient died. Other common minor side-effects (maximum G2) included stomatitis, hand-foot syndrome and mild alopecia. No major G3–4 renal toxicity was observed. G1 creatinine toxicity occurred in 2 patients.
Discussion
The biological behavior and clinical history of GEP-NETs is defined by their grading, as expressed by the Ki67 proliferative index [23,24]. However, neuroendocrine tumors are fairly heterogeneous [25]. Loss or heterogeneous expression of somatostatin receptors (SSTR) may correspond to increased glucose consumption by malignant cells as they acquire aggressive features. FDG-PET can provide complementary information and is capable of discriminating between slowly proliferating tumors and rapidly proliferating forms [26]. In addition, FDG-PET provides prognostic information about OS and disease-free survival. It is, in fact, able to identify subjects with poorer prognosis who either do not respond to treatment or who progress early, during or after its completion [27, 28]. There is evidence that increased glucose metabolism, seen as uptake at FDG-PET, is a negative prognostic factor for GEP-NETs in terms of PFS and OS, per se and after PRRT, regardless of Ki67 value, and that it represents an aggressive feature of the disease [9–11].
Given the poorer prognosis of patients with FDG-positive NETs, including a shorter duration of response to PRRT, several strategies have been implemented to improve PRRT efficacy in these forms, such as the association with chemotherapy.
Our prospective phase I-II study suggests that a combination of Lu-PRRT and metronomic capecitabine in aggressive metastatic FDG-positive GEP-NETs is safe and effective. The majority of patients (30/37, 81%) completed the intended schedule without any incident and only 4 (11%) required the suspension of treatment at very low radiation doses (after one cycle). Of the 33 evaluable patients, a RECIST 1.1 response was observed in 30% and stability in 55%, resulting in an 85% DCR.
The toxicity profile in our population was similar to that reported for Lu-PRRT alone and was mostly reversible [5, 29, 30]. G3 or G4 hematological toxicity occurred in 16.2% of patients. Specifically, 4 (10.8%) patients had G3/G4 neutropenia, which resolved after the interruption of capecitabine. One (2.7%) patient had G4 thrombocytopenia that persisted after the discontinuation of the combined treatment. Other G3 toxicities included diarrhea and fatigue in 4 (10.8%) patients, 3 of whom required discontinuation of the combined treatment and one a delay of capecitabine.
Given the lack of randomized studies attesting the efficacy of the combination compared to standard treatment, this approach is still not included in current NET guidelines. However, the combination of Lu-PRRT and the radiosensitizer capecitabine [17] is not new, although the majority of prior non-controlled studies did not apply any stratification criteria to patients. These studies showed low toxicity and excellent DCR and PFS [12–17]. In 35 subjects with low-grade GEP-NETs treated with a combination of 177Lu-DOTATATE plus capecitabine and temozolomide, Claringbold et al. observed a DCR of 91%, with a mPFS of 31 months [12]. In another phase II study on 30 patients with pancreatic NETs (P-NETs) receiving the combined treatment, the same group reported 13% complete response and 70% partial response, with a mPFS of 48 months. No disease progression was observed [13].
In a retrospective study, Kashyap et al. assessed the outcomes of 52 patients with FDG-positive NETs treated with a combination of Lu-PRRT and 5-fluorouracil, reporting a DCR of 98% and a mPFS of 48 months [16].
Based on the reported experiences, we evaluated the association between capecitabine and Lu-PRRT in GEP-NET patients, but selecting only those with FDG PET-positivity. Moreover, in an even more targeted approach, we decided to preselect only DPD-proficient subjects as DPD deficiency is correlated with the development of severe side-effects related to the accumulation of unmetabolized capecitabine [31].
Unlike previous published data, capecitabine was administered with a metronomic schedule, which allows for a prolonged low-dose exposure of the tumor, similar to the slow decreasing radiation rate of PRRT. The metronomic schedule, consisting in the administration of low doses over the inter-cycle period, was aimed at ensuring a prolonged exposure of the drug to tumor tissue to decrease neoangiogenesis and revascularization, which ultimately results in decreased tumor growth [32, 33]. Bongiovanni et al., in a small cohort of GEP-NETs, demonstrated that metronomic capecitabine alone was well tolerated, with a high DCR (90%) [34].
In the present study, focused on FDG-positive tumors, we observed a mPFS of 31.4 months after a median follow-up of 38 months, while mOS was not reached. The majority of our patients had P-NETs (25 patients, 68%), with a DCR of 81% in this subset and 85% in the whole group.
Our results compare well with those of previous studies on the efficacy of Lu-PRRT alone in FDG-positive GEP-NETs. With the limitation of the historical comparison, none of the previous populations exhibited mPFS longer than 22–24 months. In a large series of 382 FDG-positive NET patients treated with PRRT, Zhang et al. reported a mPFS of 18.5 months and an OS of 53.2 months [35]. Zemcack et al., in a retrospective study of 75 patients with FDG-positive G1-G2 NETs treated with tandem therapy (90Y/177Lu-DOTATATE), reported a mPFS of 22.2 months with a DCR of 83% [36].
In a recent study of 32 FDG-positive P-NET patients treated with 177Lu-DOTATATE alone, we reported a mPFS of 21.2 months, OS of 63.8 months and a DCR of 78% [4]. Although the DCR was similar to that of the present study (81% vs. 78%), the difference in terms of PFS was substantial (31.4 vs. 21.2 months), suggesting improved efficacy deriving from the combination of PRRT with capecitabine. In the absence of a randomized study comparing Lu-PRRT with Lu-PRRT plus capecitabine, these data appear to indicate an advantage from the use of PRRT associated with capacitabine in FDG-positive GEP-NET patients, at least in terms of PFS.
Limitations of this study include its monocenter nature and the lack of a control group. We are aware that this is a protocol with a small number of patients, but it was the first step towards the activation of a randomized controlled clinical trial that is currently ongoing at our institute comparing Lu-PRRT and capecitabine with Lu-PRRT alone in patients with FDG-positive GEP-NETs.
In conclusion, the association of metronomic capecitabine and 177Lu-DOTATATE is well tolerated. Data obtained in this study showed a remarkable DCR and PFS in patients with aggressive GEP-NETs that compare well with results for patients with similar characteristics (GEP-NET FDG-positive) treated with Lu-PRRT alone. Randomized trials with larger number of patients comparing the combined therapy with Lu-PRRT alone are needed to evaluate the real impact of metronomic capecitabine on PFS and OS in high-risk, FDG-positive GEP-NET patients.
Supplementary Material
Acknowledgements
The authors thank Gráinne Tierney for editorial assistance.
Funding
Work partially supported by Fondazione AIRC per la Ricerca sul Cancro and Italian Ministry of Health.
Footnotes
Compliance with ethical standard
Conflict of interests
The authors declare that they have no conflict of interest.
Ethical approval
The study was approved by the Ethics Committee of the Area Vasta Romagna-IRST (Approval No. 6929/2014 of December 17, 2014) and by the competent authorities and study was conducted in full conformity with the current revision of the Declaration of Helsinki, the current ICH Guidelines for Good Clinical Practice, the Directive 2001/20/EEC and other relevant current local legislation.
Informed consent
All patients gave written informed consent.
Data availability
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
- 1.Johnbeck CB, Knigge U, Kjær A. PET tracers for somatostatin receptor imaging of neuroendocrine tumors: current status and review of the literature. Future Oncol 2014;10:2259–77. [DOI] [PubMed] [Google Scholar]
- 2.Kwekkeboom DJ, de Herder WW, Kam BL, et al. Treatment with the radiolabeled somatostatin analog [177 Lu-DOTA, Tyr3]octreotate: toxicity, efficacy, and survival. J Clin Oncol 2008;26:2124–30. [DOI] [PubMed] [Google Scholar]
- 3.Paganelli G, Sansovini M, Ambrosetti A, et al. 177 Lu-Dota-octreotate radionuclide therapy of advanced gastrointestinal neuroendocrine tumors: results from a phase II study. Eur J Nucl Med Mol Imaging 41:1845–51. [DOI] [PubMed] [Google Scholar]
- 4.Sansovini M, Severi S, Ianniello A, et al. Long-term follow-up and role of FDG-PET in advanced pancreatic neuroendocrine patients treated with 177Lu-DOTATATE. Eur J Nucl Med Mol Imaging 2017;44:490–9. [DOI] [PubMed] [Google Scholar]
- 5.Strosberg J, El-Haddad G, Wolin E, et al. NETTER-1 Trial Investigators. Phase 3 Trial of 177Lu-Dotatate for Midgut Neuroendocrine Tumors. N Engl J Med 2017;376:125–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hofman MS, Hicks RJ. Changing paradigms with molecular imaging of neuroendocrine tumors. Discov Med 2012;14:71–81. [PubMed] [Google Scholar]
- 7.Panagiotidis E, Alshammari A, Michopoulou S, et al. Comparison of the impact of 68Ga-DOTATATE and 18F-FDG PET/CT on clinical management in patients with neuroendocrine tumors. J Nucl Med 2017;58:91–6. [DOI] [PubMed] [Google Scholar]
- 8.Warburg O On the origin of cancer cells. Science 1956;123:309–14. [DOI] [PubMed] [Google Scholar]
- 9.Bahri H, Laurence L, Edeline J, et al. High prognostic value of 18F-FDG PET for metastatic gastroenteropancreatic neuroendocrine tumors: a long-term evaluation. J Nucl Med 2014;55:1786–90. [DOI] [PubMed] [Google Scholar]
- 10.Binderup T, Knigge U, Loft A, et al. 18F-fluorodeoxyglucose positron emission tomography predicts survival of patients with neuroendocrine tumors. Clin Cancer Res 2010;16:978–85. [DOI] [PubMed] [Google Scholar]
- 11.Severi S, Nanni O, Bodei L, et al. Role of 18FDG PET/CT in patients treated with 177Lu-DOTATATE for advanced differentiated neuroendocrine tumours. Eur J Nucl Med Mol Imaging 2013;40:881–8. [DOI] [PubMed] [Google Scholar]
- 12.Claringbold PG, Price RA, Turner JH. Phase I-II study of radiopeptide 177Luoctreotate in combination with capecitabine and temozolomide in advanced lowgrade neuroendocrine tumors. Cancer Biother Radiopharm 2012;27:561–9. [DOI] [PubMed] [Google Scholar]
- 13.Claringbold PG, Turner JH. Pancreatic Neuroendocrine Tumor Control: Durable Objective Response to Combination 177Lu-Octreotate-Capecitabine-Temozolomide Radiopeptide Chemotherapy. Neuroendocrinology 2016;103:432–9. [DOI] [PubMed] [Google Scholar]
- 14.Ballal S, Yadav MP, Damle NA, et al. Concomitant 177Lu-DOTATATE and Capecitabine Therapy in Patients With Advanced Neuroendocrine Tumors: A Long-term-Outcome, Toxicity, Survival, and Quality-of-Life Study. Clin Nucl Med 2017;42:e457–66. [DOI] [PubMed] [Google Scholar]
- 15.Kesavan M, Claringbold PG, Turner JH. Hematological toxicity of combined 177Lu-octreotate radiopeptide chemotherapy of gastroenteropancreatic neuroendocrine tumors in long-term follow-up. Neuroendocrinology 2014;99:108–17. [DOI] [PubMed] [Google Scholar]
- 16.Kashyap R, Hofman MS, Michael M, et al. Favourable outcomes of (177)Lu-octreotate peptide receptor chemoradionuclide therapy in patients with FDG-avid neuroendocrine tumours. Eur J Nucl Med Mol Imaging 2015;42:176–85. [DOI] [PubMed] [Google Scholar]
- 17.Claringbold PG, Brayshaw PA, Price RA, et al. Phase II study of radiopeptide 177Lu-octreotate and capecitabine therapy of progressive disseminated neuroendocrine tumours. Eur J Nucl Med Mol Imaging 2011;38:302–11. [DOI] [PubMed] [Google Scholar]
- 18.Krenning EP, Bakker WH, Breeman WA, et al. Localisation of endocrine-related tumours with radioiodinated analogue of somatostatin. Lancet 1989;1: 242–4. [DOI] [PubMed] [Google Scholar]
- 19.Bosman F, Carneiro F, Hruban R, et al. , eds. World Health Organization Classification of Tumours. Pathology and Genetics. Tumors of the Digestive System IARC Press, Lyon: 2010. [Google Scholar]
- 20.Breeman WA, de Blois E, Bakker WH. Radiolabeling DOTA peptides with 90Yand 177Lu to a high specific activity. In: Chinol M, Paganelli G, eds. Radionuclide peptide cancer therapy New York: Taylor & Francis; 2006. pp. 119–26. [Google Scholar]
- 21.Bodei L, Cremonesi M, Grana CM, et al. Peptide receptor radionuclide therapy with 177Lu-DOTATATE: the IEO phase I-II study. Eur J Nucl Med Mol Imaging 2011;38:2125–35. [DOI] [PubMed] [Google Scholar]
- 22.Chan DL, Pavlakis N, Schembri GP, Bernard EJ, Hsiao E, Hayes A, Barnes T, Diakos C, Khasraw M, Samra J, Eslick E, Roach PJ, Engel A, Clarke SJ, Bailey DL. Dual somatostatin receptor/FDG PET/CT imaging in metastatic neuroendocrine tumours: proposal for a novel grading scheme with prognostic significance. Theranostics 2017. Mar 1;7(5):1149–1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.WHO Classification of Tumours Editorial Board. Digestive System Tumours. 5 International Agency for Research on Cancer. Lyon, 2019, pp. 16–8. [Google Scholar]
- 24.Lloyd RV, Osamura RY, Kloppel G. WHO classification of tumours of endocrine organs, chapter 6 4th ed. International Agency for Research on Cancer, Lyon, 2017, pp. 210–39. [Google Scholar]
- 25.Modlin IM, Oberg K, Chung DC, et al. Gastroenteropancreatic neuroendocrine tumours. Lancet Oncol 2008;9:61–72. [DOI] [PubMed] [Google Scholar]
- 26.Pencharz D, Gnanasegaran G, Navalkissoor S. Theranostics in neuroendocrine tumours: somatostatin receptor imaging and therapy. Br J Radiol 2018;91: 20180108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zerizer I, Al-Nahhas A, Towey D, et al. The role of early 18F-FDG PET/CT in prediction of progression-free survival after 90Y radioembolization: comparison with RECIST and tumour density criteria. Eur J Nucl Med Mol Imaging 2012;39:1391–9. [DOI] [PubMed] [Google Scholar]
- 28.Oh S, Prasad V, Lee DS, et al. Effect of peptide receptor radionuclide therapy on somatostatin receptor status and glucose metabolism in neuroendocrine tumors: intraindividual comparison of Ga-68 DOTANOC PET/CT and F-18 FDG PET/CT. Int J Mol Imaging Int J Mol Imaging 2011;2011:524130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kwekkeboom DJ, Kam BL, van Essen M, et al. Somatostatin-receptor-based imaging and therapy of gastroenteropancreatic neuroendocrine tumors. Endocr Relat Cancer 2010;17: R53–73. [DOI] [PubMed] [Google Scholar]
- 30.Bodei L, Kidd M, Paganelli G, et al. Long-term tolerability of PRRT in 807 patients with neuroendocrine tumours: the value and limitations of clinical factors. Eur J Nucl Med Mol Imaging 2015;42:5–19. [DOI] [PubMed] [Google Scholar]
- 31.Meulendijks D, Cats A, Beijnen JH, et al. Improving Safety of Fluoropyrimidine Chemotherapy by Individualizing Treatment Based on Dihydropyrimidine Dehydrogenase Activity - Ready for Clinical Practice? Cancer Treat Rev 2016;50:23–34. [DOI] [PubMed] [Google Scholar]
- 32.Collovà E, Sebastiani F, De Matteis E, et al. Use of metronomic chemotherapy in oncology: results from a national Italian survey. Tumori 2011;97:454–8. [DOI] [PubMed] [Google Scholar]
- 33.Laquente B, Vinals F, Germa JR. Metronomic chemotherapy: an antiangiogenic scheduling. Clin Transl Oncol 2007;9:93–8. [DOI] [PubMed] [Google Scholar]
- 34.Bongiovanni A, Riva N, Calpona S, et al. Metronomic capecitabine in gastroenteropancreatic neuroendrocrine tumors: a suitable regimen and review of the literature. Onco Targets Ther 2014;7:1919–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang J, Liu Q, Singh A, et al. Prognostic Value of 18F-FDG PET/CT in a Large Cohort of 495 Patients with Advanced Metastatic Neuroendocrine Neoplasms (NEN) Treated with Peptide Receptor Radionuclide Therapy (PRRT). J Nucl Med 2020;119:241414. [DOI] [PubMed] [Google Scholar]
- 36.Zemczak A, Kołodziej M, Gut P, et al. Effect of peptide receptor radionuclide therapy (PRRT) with tandem isotopes - [90Y]Y/[177Lu]Lu-DOTATATE in patients with disseminated neuroendocrine tumours depending on [18F]FDG PET/CT qualification in Polish multicentre experience - do we need [18F]FDG PET/CT for qualification to PRRT? Endokrynol Pol 2020;71:240–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.


