Abstract
The cell density dependence of stationary-phase survival of Rhizobium leguminosarum has been investigated. Following starvation by exhaustion of carbon or nitrogen, but not of phosphorus, the survival of cultures was dependent on the cell density at entry into stationary phase. High-density cultures survived with little or no loss of viability over a 20-day period in stationary phase. In contrast, low-density cultures lost viability rapidly but consisted of a heterogeneous population, a small fraction of which successfully adapted and eventually formed a stable, surviving population. The threshold density above which the cultures survived successfully in stationary phase was dependent on the growth conditions and the strain used. We took advantage of the fact that R. leguminosarum survives poorly following starvation by resuspension in carbon-free medium to demonstrate that cell density-dependent survival was mediated by a component accumulating in the growth medium. The effects of this medium component on survival in resuspension assays could be mimicked by an N-acyl homoserine lactone, N-(3R-hydroxy-7-cis-tetradecanoyl)-l-homoserine lactone, previously demonstrated to have a role in controlling cell density-dependent phenomena in R. leguminosarum. The Sym plasmids pRP2JI and pRL1JI were found to be essential for the production of the extracellular factor, which could also be made in Escherichia coli carrying the cosmid clone pIJ1086 containing a specific region of pRL1JI.
Nutrient starvation is a condition in which bacteria in many environments, including soil, regularly find themselves (9, 20, 31, 37). However, little is known about the physiological processes involved in the starvation survival of soil bacteria. We are investigating the starvation survival response of Rhizobium leguminosarum. We have previously reported on the changes in the physiology and metabolism of R. leguminosarum bv. phaseoli in response to nutrient starvation (36). In contrast to the starvation survival kinetics of enteric bacteria, no reduction in the viability of R. leguminosarum cultures was observed during more than 2 months in carbon-starved stationary phase (36). A further distinguishing feature is the poor survival of R. leguminosarum following nutrient starvation by resuspension in minimal medium without carbon, indicating that this bacterium cannot respond to such a rapid deprivation of nutrients (36).
It has been known for over 30 years that a population effect is observed when a culture of bacteria is starved (16, 28, 29). In those early studies the specific death rates of high-density bacterial populations were lower than those of low-density populations. It is also well established that Myxococcus xanthus and Bacillus subtilis show developmental responses to nutrient deprivation, i.e., sporulation and fruiting body formation, respectively, that are dependent on population cell density (7, 13, 18, 19). The effect of cell density on survival has not been thoroughly addressed in recent studies of the starvation survival of bacteria (9, 11, 21, 26, 27).
In this study we demonstrate the cell density-dependent stationary-phase survival of R. leguminosarum following starvation by nutrient exhaustion of carbon or nitrogen. We show that this response is controlled by the accumulation of an extracellular factor(s) in the growth medium and that the effect is mimicked by the N-acyl homoserine lactone (AHL) N-(3R-hydroxy-7-cis-tetradecanoyl)-l-homoserine lactone (7cisHtDHL).
MATERIALS AND METHODS
Strains and plasmids.
R. leguminosarum bv. phaseoli 4292 is a rifampin-resistant strain that carries the pRP2JI Sym plasmid and nodulates beans (23). This strain was used in our earlier study of starvation survival of R. leguminosarum (36). R. leguminosarum 8401 is a derivative of a biovar phaseoli isolate (strain 8002), and it is streptomycin resistant and has been cured of the Sym plasmid pRP2JI. R. leguminosarum 8401/pRL1JI carries the biovar viciae Sym plasmid. The four pLAFR1-derived cosmids pIJ1527, pIJ1529, pIJ1085, and pIJ1086 contain DNA cloned from the R. leguminosarum Sym plasmid pRL1JI. pIJ1085 and pIJ1086 are two overlapping cosmids carrying about 60 kb of DNA from the nif and nod gene-carrying regions of pRL1JI (8). pIJ1527 and pIJ1529 are poorly characterized, overlapping cosmids carrying about 45 kb of DNA from a different region of pRL1JI, which does not overlap with pIJ1085 and pIJ1086 (7a). Together the four cosmids carry DNA covering about half of the Sym plasmid pRL1JI.
Growth and starvation of bacteria.
Strains were grown and starved for carbon (mannitol), nitrogen (NH4Cl), and phosphorus (K2HPO4 and KH2PO4) in MOPS (morpholinepropanesulfonic acid) minimal medium as described previously (36). For routine culture maintenance, growth of starter cultures, and plating for viable-cell counting, rifampin was added at 25 μg ml−1. YEM medium was also used at pH 7.5 (38) and consisted of (per liter) yeast extract (0.4 g) (Oxoid), K2HPO4 (0.5 g), NaCl (0.1 g), MgSO4 · 7H2O (0.2 g), and mannitol (10 g).
Starter cultures were prepared by inoculating a single colony from a plate stock into 5 ml of medium and incubating overnight at 30°C in a shaking incubator (200 rpm). A 0.5-ml portion of this exponentially growing culture was then subcultured into a second 5 ml of medium and again incubated overnight to ensure that cells were fully adapted to exponential growth. About 5 ml of this starter culture was then inoculated into 50 ml of medium in a 250-ml conical flask to give an initial optical density at 600 nm of 0.02. This method of inoculation was used throughout this study unless otherwise stated. Growth was measured spectrophotometrically as optical density at 600 nm (with 1-cm-path-length cuvettes in a Shimadzu MPS2000 spectrophotometer) and by viable-cell counting, following plating onto solid minimal medium after appropriate dilution in minimal medium. It was found that although all of the strains had similar growth kinetics in the liquid MOPS minimal medium (36), 8401/pRL1JI and 8401 did not grow well on MOPS minimal medium agar. Therefore, the viability of these strains was determined by diluting the samples in TY medium and plating on TY agar. TY medium consisted of (per liter) Bacto tryptone (5 g), yeast extract (3 g), and CaCl2 · 6H2O (1.3 g). Cells were osmotically stressed by adding NaCl to a final concentration of 2.5 M as described previously (36). Escherichia coli 803 strains were also grown in MOPS minimal medium at 30°C.
Determination of affects of spent culture medium. (i) Preparation of aseptic samples of spent culture medium.
To obtain spent medium, cells were removed from a carbon-starved culture by centrifugation at 4,000 × g for 45 min. The supernatant fraction was then collected and sterilized by filtration through 0.2-μm-pore-size cellulose acetate filters. The aseptic spent medium was then used to resuspend cells collected by centrifugation (see below). Spent medium was usually prepared from 6-day-old, high-density (1.5 × 109 CFU ml−1), carbon-starved stationary-phase cultures and is referred to as high-density spent medium (HDSM). For some experiments the spent medium was pretreated before being added to cells. Heat treatment involved 5.0-ml samples of spent medium being placed in tubes in a water bath at 100°C for 15 min. For protease treatment, spent medium was treated with pronase attached to agarose beads (1 U ml−1; Sigma P4531) for 60 min at 30°C. The beads were then removed by filtering the sample through a 25-mm glass fiber filter (pore size, 1 μm). To approximately determine the size of the spent-medium component, samples of stationary-phase spent medium from carbon-starved R. leguminosarum cultures were ultrafiltrated in a stirred cell by using an Amicon YM3 ultraflow membrane with a molecular mass cutoff of 3 kDa.
(ii) Collection and resuspension of cells.
Fifty-milliliter cultures of R. leguminosarum were harvested by centrifugation at 4,000 × g for 20 min. The supernatant fraction was then removed, and the cells were washed twice by resuspending them in 50 ml of fresh MOPS minimal medium with no carbon source (MM-C). This was done in order to make sure that any residual carbon source or molecules secreted into the medium by the cells were completely washed from the cells. The bacteria were then pelleted by centrifugation one final time, and the pellet was resuspended by vortexing in 5.0 ml of MM-C. A 0.5-ml portion of the resuspended cells was then added to 5.0 ml of MM-C or spent culture medium. Cultures were then kept at 30°C in a shaking incubator (200 rpm), and survival was monitored for several days by viable-cell counting.
Use of R. leguminosarum autoinducer.
A synthetic preparation of the R. leguminosarum autoinducer 7cisHtDHL, containing a mixture of l and d stereoisomers that differ in the orientation of the 3-hydroxy group, was kindly provided by Kendall Gray (12, 34). For use in bioassays, a sample of the autoinducer (in ethyl acetate) was added to an empty culture vessel and dried down in a stream of sterile air. The assay culture was then added to the vessel to resuspend the autoinducer.
RESULTS
Effects of culture cell density on stationary-phase survival of R. leguminosarum.
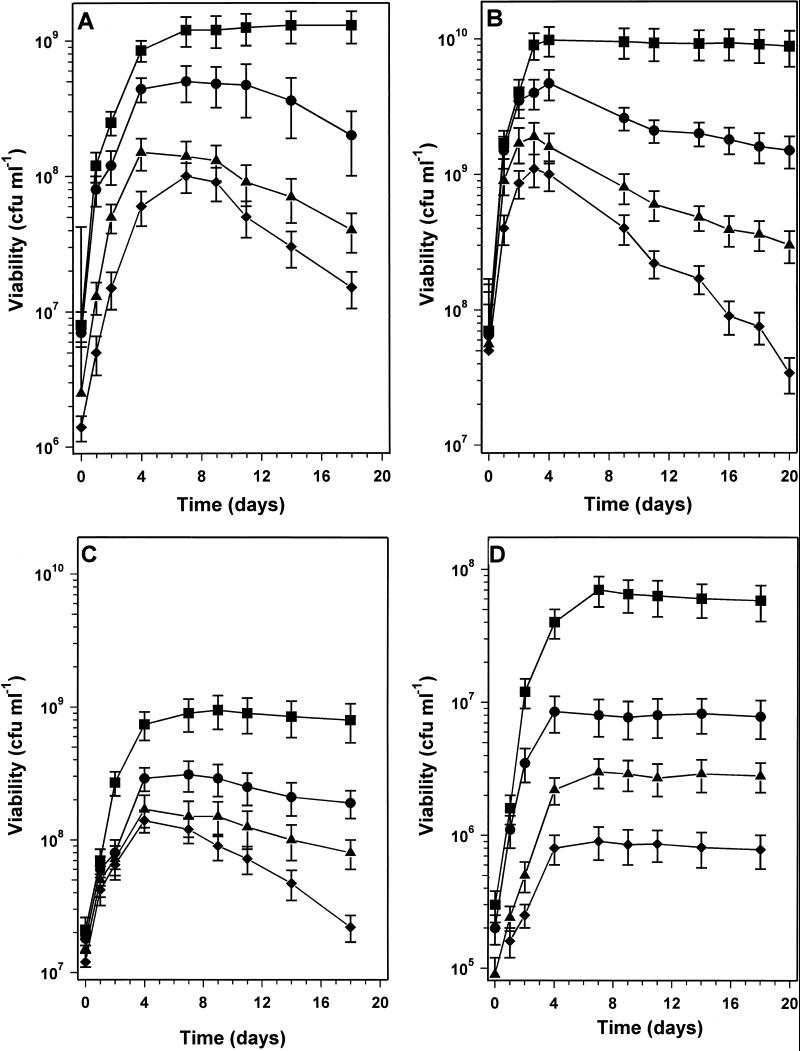
By varying the mannitol concentration in MOPS minimal medium, R. leguminosarum bv. phaseoli cultures were grown and allowed to enter stationary phase at cell densities ranging from ∼1.3 × 109 to 1 × 108 CFU ml−1. It was found that the survival of cultures in the 10 to 14 days following entry into carbon-starved stationary phase was dependent on the cell density at the time of entry (Fig. 1A). Cultures entering stationary phase at a density of ∼1.3 × 109 CFU ml−1 had 108% viability after 14 days of stationary phase, whereas cultures that had entered stationary phase at ∼1 × 108 CFU ml−1 retained only 15% viability after the same time period. Cultures with intermediate cell densities at entry into stationary phase showed intermediate survival (Fig. 1A). This experiment was repeated with YEM medium, in which mannitol was the major but not the sole carbon source. Changing the concentration of mannitol in YEM allowed the cell density at which cultures entered stationary phase to be altered. It was found that stationary-phase survival in YEM was dependent on the culture density (Fig. 1B). The survival of nitrogen-starved stationary-phase cultures was also dependent on the culture cell density, with a higher cell density at the entry into stationary phase resulting in better survival (Fig. 1C). However, cell density-dependent survival was not seen in phosphorus-starved cultures; phosphorus-starved cultures entering stationary phase at densities of between 7 × 107 and 8 × 105 CFU ml−1 all survived well during the first 14 days of stationary phase (Fig. 1D).
FIG. 1.
Effect of culture cell density on the stationary-phase survival of nutrient-starved R. leguminosarum bv. phaseoli 4292 cultures. Cultures were grown in minimal medium (A, C, and D) or YEM (B) with various limiting amounts of carbon source (A and B), nitrogen (C), or phosphorus (D) so that the cell density upon nutrient-limited entry into stationary phase was varied. (A) In carbon-limited minimal medium, the mannitol concentrations in grams liter−1 (cell densities entering stationary phase in CFU milliliter−1) were 10 (■) (1.3 × 109), 5 (•) (5 × 108), 2 (▴) (1.5 × 108), and 0.3 (⧫) (1 × 108). (B) In YEM, the mannitol concentrations in grams liter−1 (cell densities entering stationary phase) were 10 (■) (9.8 × 109), 5 (•) (4.7 × 109), 2 (▴) (1.9 × 109), and 0.3 (⧫) (1.1 × 109). (C) In nitrogen-limited minimal medium, the NH4Cl concentrations in grams liter−1 (cell densities entering stationary phase) were 0.25 (■) (9.5 × 108), 0.1 (•) (3.1 × 108), 0.05 (▴) (1.7 × 108), and 0.025 (⧫) (1.1 × 108). (D) In phosphorus-limited minimal medium, the phosphate concentrations in grams liter−1 (cell densities entering stationary phase) were 0.05 (■) (7 × 107), 0.025 (•) (8.5 × 106), 0.01 (▴) (3 × 106), and 0.005 (⧫) (9 × 105). Error bars represent standard deviations.
A heterogeneous population enters stationary phase in low-density cultures.
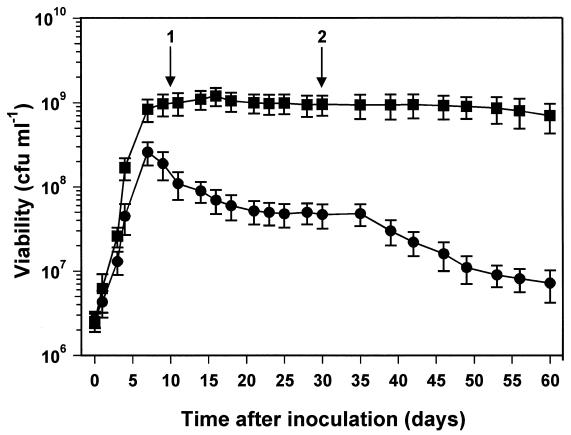
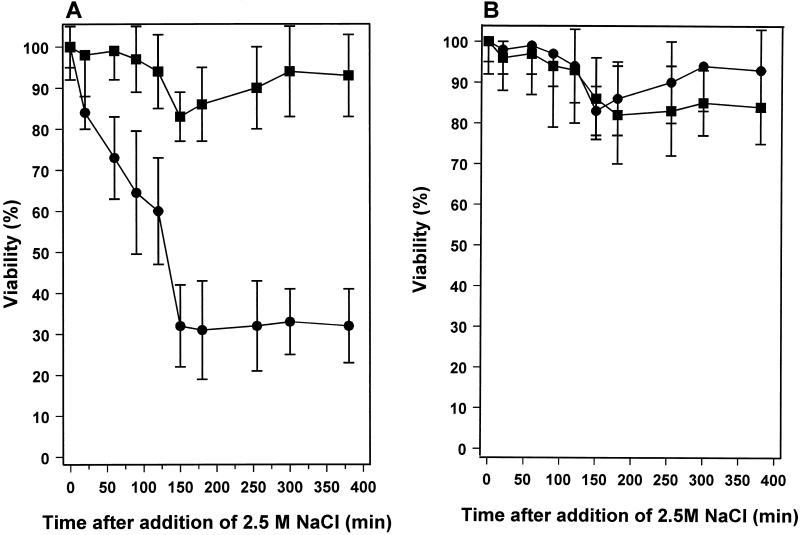
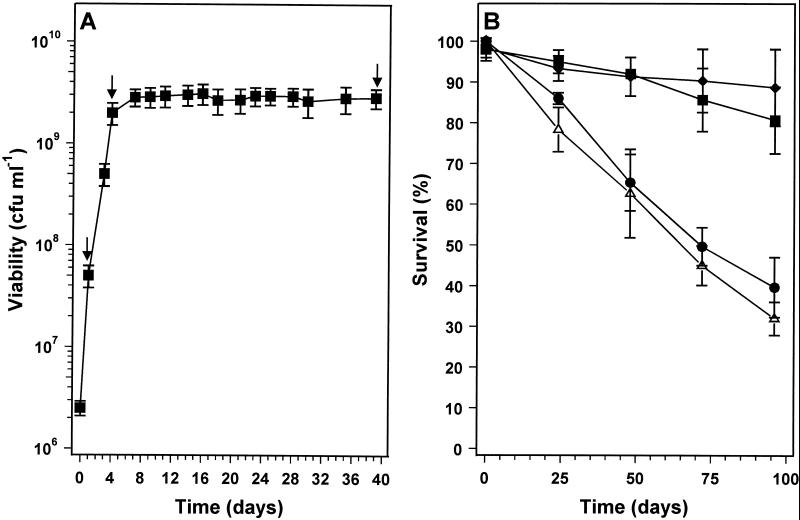
An experiment similar to that of Fig. 1A was set up, but stationary-phase survival was monitored for a much longer time period. As expected, the high-density cultures survived long-term starvation with little loss of viability (Fig. 2) (36). In the low-density culture, there was an initial loss of viability over the first 16 days in stationary phase (up to 20 days after inoculation), and then the proportion of viable cells stabilized at about 20% of the starting population. There was no further loss of viability for 15 days (Fig. 2). However, this was followed by a further reduction of viability from day 35 onwards that continued until the end of the experiment at day 60. The survival kinetics of low-density cultures suggests that a heterogeneous population enters stationary phase or is present immediately after entry, comprising a fraction (∼20%) that goes on to survive long-term stationary phase and those cells that will die during the first 15 days (Fig. 2). We investigated whether the population was also heterogeneous with respect to another property of stationary-phase-adapted cells, resistance to osmotic stress (36). The osmotic stress resistance of high- and low-density cultures at 5 and 25 days into stationary phase was determined (Fig. 3). The high-cell-density, 5-day stationary-phase culture was resistant to challenge by 2.5 M NaCl, while the equivalent low-density culture was clearly sensitive (Fig. 3A). At 150 min after the exposure to osmotic stress, the viability of the low-density culture had been reduced by 70%, but the surviving 30% of the population was resistant to 2.5 M NaCl and there was no further reduction in viability during the remaining 250 min of the experiment (Fig. 3A). These data are consistent with the idea that there is a fraction of the population that has failed to successfully adapt to stationary-phase survival and consequently retains physiological traits of exponentially growing cultures. In support of this idea, the decimal reduction times (D values) (1) for osmotically stressed, exponential-phase cultures (calculated from the data in Fig. 7 of reference 36) and for the osmotically sensitive fraction of the population in low-density cultures (Fig. 3A) were similar, being approximately 150 and 200 min, respectively. Twenty-five-day-old high- and low-density stationary-phase cultures were equally resistant to osmotic stress (Fig. 3B), confirming that 25 days into stationary phase, the surviving bacteria in the low-density culture are fully adapted for stationary-phase survival. It seems likely, but is not proven, that this surviving population is the fraction of the low-density culture that was resistant to osmotic stress after 5 days into stationary phase.
FIG. 2.
Growth and stationary-phase survival of R. leguminosarum bv. phaseoli 4292 cultures entering stationary phase at high and low cell densities. Cultures were grown in minimal medium with limiting amounts of carbon source. ■, growth with 10 g of mannitol liter−1, entering stationary phase at ∼1 × 109 CFU ml−1, •, growth with 0.3 g of mannitol liter−1, entering stationary phase at 2.6 × 108 CFU ml−1. Growth was monitored by viable-cell counting. Arrows 1 and 2 correspond to the times that samples were taken for osmotic stress tests (see Fig. 3). Error bars represent standard deviations.
FIG. 3.
Osmotic stress resistance of high- and low-density stationary-phase cultures of R. leguminosarum bv. phaseoli 4292. ■, growth in minimal medium with 10 g of mannitol liter−1, entering stationary phase at ∼1 × 109 CFU ml−1; •, growth in minimal medium with 0.3 g of mannitol liter−1, entering stationary phase at 2.6 × 108 CFU ml−1. Cultures were osmotically stressed by the addition of sterile NaCl to a final concentration of 2.5 M. The stress was applied to cultures 10 days after inoculation, when they had been in stationary phase for 5 days (arrow 1 in Fig. 2) (A), or 30 days after inoculation, when they had been in stationary phase for 25 days (arrow 2 in Fig. 2) (B). Survival after the osmotic stress was monitored by viable-cell counting. Error bars represent standard deviations.
High-density stationary-phase cultures contain an extracellular component that promotes adaptation to starvation survival.
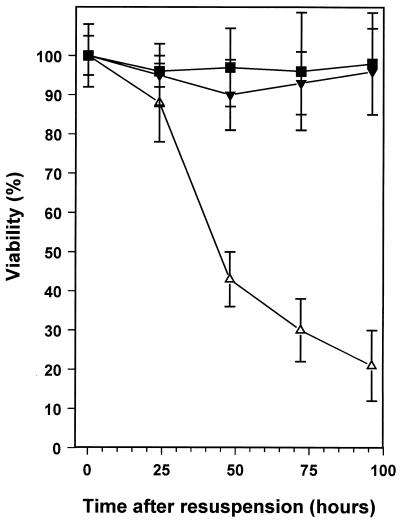
We investigated whether an extracellular compound(s) accumulates that is able to promote the adaptation to stationary-phase survival. We took advantage of the fact that R. leguminosarum survives poorly following carbon starvation by resuspension in MM-C (36). The effect of filter-sterilized, spent culture medium from a high-density culture on the survival of a low-density culture was investigated. A low-density culture, entering stationary phase at 108 CFU ml−1, was harvested and resuspended in MM-C containing spent medium from a high-density stationary-phase culture, and the viability was monitored. The data in Fig. 4 clearly show that the HDSM promotes survival of the low-density culture. At 96 h after resuspension, 98% of the cells resuspended in HDSM were viable, with only 21% of those resuspended in MM-C retaining viability. HDSM could also promote survival or adaptation of exponentially growing cells to stationary phase (data not shown). This indicates that the action of the HDSM component is not limited to cells in the transition phase between exponential and stationary phases. Preliminary characterization indicated that the active component(s) of HDSM was small (<3 kDa), stable, and heat resistant (surviving 100°C for 15 min), and it was protease resistant, suggesting that it was not a protein (data not shown).
FIG. 4.
Effect of HDSM and an AHL on stationary-phase survival of R. leguminosarum bv. phaseoli 4292. Late-exponential-phase, low-density cultures (density, ∼1 × 108 CFU ml−1) were harvested and resuspended to the same density in spent medium from high density cultures (density, ∼1.5 × 109 CFU ml−1) of strain 4292 (■), in MM-C (▵), and in MM-C containing 200 ng of 7cisHtDHL ml−1 (▾). Survival was monitored by viable-cell counting. Data are the mean values from four experiments, with error bars representing standard deviations.
The accumulation of an extracellular component may trigger the adaptation to stationary-phase survival in high-density cultures, with the failure of low-density cultures to adapt being a consequence of the compound(s) not reaching a threshold level. This was demonstrated by growing a culture to carbon-starved stationary phase while taking samples of spent medium, at cell densities above and below the threshold necessary for successful stationary-phase survival, and testing their ability to promote survival in resuspension assays (Fig. 5). The spent medium from an exponentially growing culture that had reached a density of 5 × 107 CFU ml−1 (Fig. 5A) was unable to promote survival of low-density cultures following resuspension (Fig. 5B). In contrast, spent medium from an exponential-phase culture at approximately 2 × 109 CFU ml−1 (Fig. 5A), was able to promote starvation survival following resuspension (Fig. 5B). Interestingly, spent medium from high-density cultures at ∼32 days into stationary phase was also able to promote starvation survival in resuspension assays, indicating that the spent-medium component is still present and active during long-term stationary phase (Fig. 5B). Therefore, a molecule(s) secreted into the medium has a role in the adaptation of R. leguminosarum cultures to stationary phase.
FIG. 5.
Effect of spent medium from different stages of the growth curve on stationary-phase survival of R. leguminosarum 4292 in resuspension assays. (A) R. leguminosarum 4292 was grown in minimal medium (5 g of mannitol liter−1), entering stationary phase at a high cell density (3 × 109 CFU ml−1). During growth, samples were taken at 24 h (5 × 107 CFU ml−1), 96 h (2 × 109 CFU ml−1), and 30 days (3 × 109 CFU ml−1) for the preparation of spent medium. (B) Cells obtained from a low-density (∼108 CFU ml−1), late-exponential-phase culture, grown in minimal medium with 0.3 g of mannitol liter−1, were resuspended to ∼108 CFU ml−1 in MM-C (▵) or in spent-medium samples taken at the different time points as described for panel A, and the viability was monitored. Spent medium was from 24 h (•), 96 h (■), and 30 days (⧫) after inoculation. Error bars represent standard deviations.
An AHL promotes cell density-dependent survival.
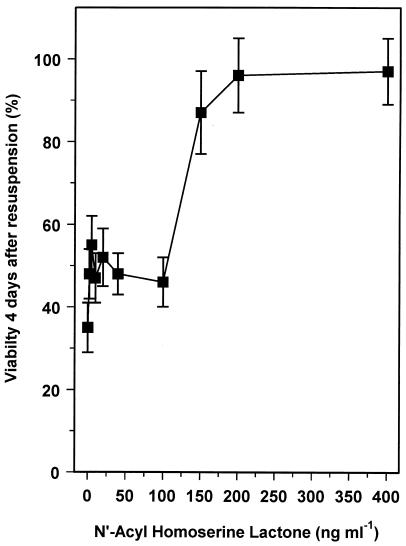
The AHL 7cisHtDHL has been shown to induce a cell density-dependent response in R. leguminosarum (12, 34). It activates the RhiR protein to induce expression of the rhiABC operon and to inhibit growth (12). We tested whether 7cisHtDHL could also protect low-density cultures during starvation survival. Cells from a low-density, late-exponential-phase culture were resuspended in MM-C with 7cisHtDHL added, and survival was monitored. Ninety-six percent of the cells remained viable 4 days after resuspension in the 7cisHtDHL-containing medium, compared to 98% of cells resuspended in HDSM and only 21% of the cells in MM-C (Fig. 4). 7cisHtDHL is clearly able to promote the survival of low-density cultures. As 7cisHtDHL has been demonstrated to be present in spent culture medium of R. leguminosarum 4292, it is possible that the HDSM component promoting stationary-phase survival is an AHL similar to or the same as 7cisHtDHL, although this is not directly demonstrated by the present data. The 7cisHtDHL used was a racemic mixture of l and d stereoisomers that differ in the orientation of the 3-hydroxy group, although the biological importance of chirality at the hydroxylated C-3 position has yet to be established (12, 34). The experiment was repeated with various concentrations of 7cisHtDHL in order to find the threshold value at which it is effective at promoting survival. A concentration of 200 ng of 7cisHtDHL ml−1 conferred full protection on low-density cultures in stationary phase (Fig. 6) while less than 100 ng ml−1 caused only a small difference in starvation survival. These data support the idea that a quorum-sensing effect, involving AHLs, is functioning to improve the stationary-phase survival of R. leguminosarum. It seems reasonable to conclude that AHLs have a role in the survival of high-density cultures and that the absence of a quorum leads to loss of viability in low-density cultures.
FIG. 6.
Concentration of 7cisHtDHL needed to produce a stationary-phase response. Cells from late-exponential-phase, low-density cultures (density, ∼108 CFU ml−1) were resuspended in MM-C or in MM-C containing various concentrations of 7cisHtDHL. The percent survival of the cultures was determined 4 days after resuspension by viable-cell counting. Data are the mean values from four experiments, with error bars representing standard deviations.
Role of the Sym plasmids pRL1JI and pRP2JI in cell density-dependent survival.
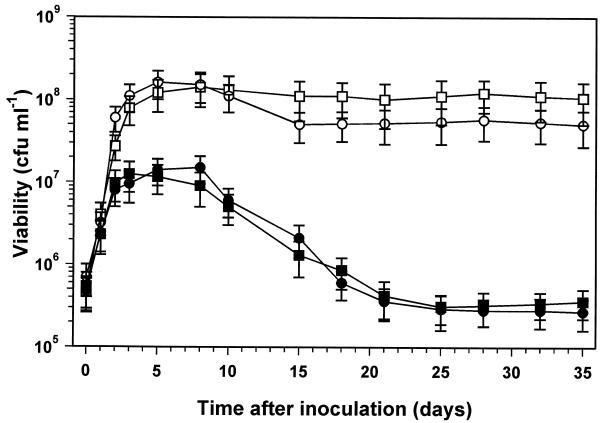
The R. leguminosarum 4292 strain used so far in this study contains the biovar phaseoli Sym plasmid pRP2JI. In order to investigate the role of Sym plasmids in cell density-dependent starvation survival, the related strains R. leguminosarum 8401 (no Sym plasmid) and R. leguminosarum 8401/pRL1JI (strain 8401 with the biovar viciae Sym plasmid pRL1JI) were also used. Growth of 8401/pRL1JI to stationary phase in carbon-limited minimal medium indicated that changing the Sym plasmid from pRP2JI to pRL1JI had no effect on the cell density-dependent stationary-phase survival (Fig. 7). R. leguminosarum 8401/pRL1JI grown to high density survived subsequent starvation well, while cultures grown to low density showed a loss of viability in stationary phase similar to that of R. leguminosarum 4292/pRP2JI (Fig. 1 and 7). R. leguminosarum 8401 cultures (no Sym plasmid) showed a loss and subsequent stabilization of viability similar to those of the strains with a Sym plasmid. Also, the R. leguminosarum 8401 cultures grown to high-cell-density stationary phase did not show a significant difference in survival compared to strains with a Sym plasmid.
FIG. 7.
Effect of the Sym plasmid pRL1JI on stationary-phase survival at high and low culture cell densities. Cultures of R. leguminosarum 8401/pRL1JI (□ and ■) and R. leguminosarum 8401 (○ and •) were grown under carbon-starved conditions at either a high (open symbols) (5 g of mannitol liter−1, entering stationary phase at 2 × 108 CFU ml−1) or low (closed symbols (0.3 g of mannitol liter−1, entering stationary phase at 1 × 107 CFU ml−1) cell density. Growth was monitored by viable-cell counting, and data are the mean values from four experiments, with error bars representing standard deviations.
The Sym plasmids pRL1JI and pRP2JI play an essential role in the production of the extracellular stationary-phase survival factor.
We examined whether Sym plasmids have a role in the production of or response to the spent-medium component. Cells from low-density, late-exponential-phase cultures were resuspended in HDSM, MM-C, or MM-C containing 200 ng of 7cisHtDHL per ml. Both cells and HDSM from three strains of R. leguminosarum (4292/pRP2JI, 8401/pRL1JI, and 8401) were used, and the results are summarized in Table 1. As expected, none of the strains could survive well in MM-C. Also, as shown in Fig. 4, R. leguminosarum 4292/pRP2JI cells could survive in MM-C if 7cisHtDHL was added. However, neither R. leguminosarum 8401/pRL1JI nor R. leguminosarum 8401 showed significantly increased survival in the presence of 7cisHtDHL. This leads to two important conclusions. First, the ability of R. leguminosarum 4292 to respond to 7cisHtDHL is encoded on the Sym plasmid pRP2JI, as strain 8401 is cured of this plasmid and can no longer respond to 7cisHtDHL. Second, the fact that 7cisHtDHL does not support survival of R. leguminosarum 8401/pRL1JI suggests that its cell density-dependent starvation survival must be mediated by a second spent-medium component. Consistent with this conclusion, we found that when cells from each of the three strains were resuspended in HDSM from strain 8401, which contains no Sym plasmid but is known to produce 7cisHtDHL (12, 34), only strain 4292/pRP2JI showed improved survival (Table 1). However, the cell density-dependent survival of R. leguminosarum 8401/pRL1JI does require a spent-medium component. Although this strain is unable to produce 7cisHtDHL (12), it can produce a spent-medium component, which we refer to as SMC8401, capable of promoting starvation survival of itself and of R. leguminosarum 4292/pRP2JI. The strain with pRP2JI could respond to both 7cisHtDHL and SMC8401. Additionally, HDSM from R. leguminosarum 4292/pRP2JI promotes survival of R. leguminosarum 4292/pRP2JI and 8401/pRL1JI, but not 8401. This implies that 4292/pRP2JI produces a molecule (which we call SMC4292) that is able to promote survival of strain 8401/pRL1JI and that is chemically distinct from 7cisHtDHL. Whether SMC8401 and SMC4292 are chemically identical remains to be established, but it is clear from a recent study that Rhizobium strains can produce a significant number of different AHLs (30).
TABLE 1.
Role of the Sym plasmids pRP2JI and pRL1JI in production of or response to the HDSM components conferring stationary-phase survival and the AHL 7cisHtDHLa
| Resuspension medium | Survival (%)b of R. leguminosarum:
|
||
|---|---|---|---|
| 4292/pRP2JI | 8401/pRL1JI | 8401 | |
| MM-C | 30 | 28 | 25 |
| MM-C plus 7cisHtDeHL (200 ng ml−1) | 96 | 44 | 41 |
| HDSM from R. leguminosarum: | |||
| 8401 | 86 | 40 | 35 |
| 8401/pRL1JI | 94 | 99 | 11 |
| 4292/pRP2JI | 96 | 89 | 23 |
Low-cell-density, late-exponential-phase cells (cell density, ∼1× 108 CFU ml−1) were harvested and resuspended to 5 × 107 CFU ml−1 in either HDSM from the indicated strain, MM-C, or MM-C containing 200 ng of 7cisHtDHL ml−1, and survival was monitored by viable-cell counting.
Percentage of the original population that was viable 4 days after resuspension.
Which region of the Sym plasmid is essential for the production of the spent-medium component?
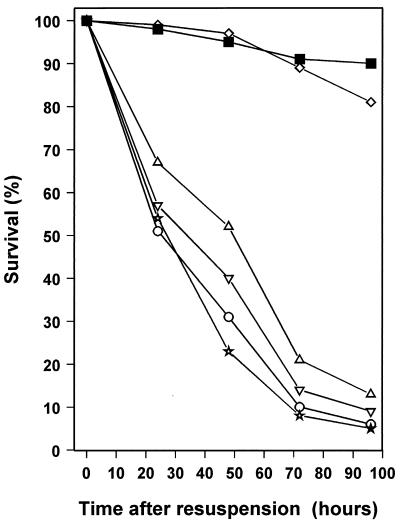
To locate which region of the Sym plasmid pRL1JI is required for production of AHL8401, we used four cosmid clones in E. coli, each containing a different region of pRL1JI (see Materials and Methods). HDSM was obtained from cultures of E. coli carrying one of each of these clones, and its effect was compared to those of HDSM from R. leguminosarum 4292/pRP2JI and of MM-C. HDSM from E. coli strains carrying pIJ1085, pIJ1527, and pIJ1529 proved unable to support starvation survival of R. leguminosarum 4292/pRP2JI cultures (Fig. 8). In contrast, 81% of the cells resuspended in spent medium from E. coli/pIJ1086 were still viable 4 days after resuspension (Fig. 8). This finding indicates that the pRL1JI-derived DNA in cosmid pIJ1086 contains genes that have a role in the production of the starvation survival factor produced by pRL1JI. Interestingly, pIJ1086 contains the rhiABCR genes. RhiR activates the rhiABC genes in the presence of 7cisHtDHL (3, 8).
FIG. 8.
Identification of regions of the Sym plasmid pRL1JI required for the production of the spent-medium component. Survival of R. leguminosarum 4292 cells harvested from late-exponential-phase, low-cell-density cultures was monitored after resuspension in R. leguminosarum HDSM (■), MM-C (▵), or spent medium from late-exponential-phase E. coli cultures carrying cosmids containing different regions of the Sym plasmid pRL1JI (▵, pIJ1085; ◊, pIJ1086; ⋆, pIJ1527; ▿, pIJ1529). For all experiments, the data are the means from four experiments and the standard deviation was never more than 6%.
DISCUSSION
In this paper we have demonstrated the cell density-dependent starvation survival of R. leguminosarum. This phenomenon was found to occur in both carbon- and nitrogen-starved cultures but not phosphorus-starved cultures. It is possible that the accumulation of large amounts of phosphorus storage compounds, such as polyphosphates, during exponential growth (4, 5) allows the cells to survive phosphorus starvation more effectively and disguises any cell density effects.
Quantification of an absolute threshold cell density, above which cells are able to survive in stationary phase, was not possible, as the density threshold was dependent on both the growth conditions (Fig. 1) and the strains starved (compare survival of strains 4292 and 8401/pRL1JI in Fig. 1A and 7, respectively). R. leguminosarum 4292/pRP2JI cultures survived carbon starvation when they entered stationary phase at densities greater than 1.5 × 109 CFU ml−1. However, in YEM medium, cultures needed to enter stationary phase at densities at or above 1010 CFU ml−1 to survive subsequent starvation. YEM cultures have a significantly higher growth rate than minimal medium cultures, and the growth rate prior to starvation will affect the physiology of the cell in numerous ways: the numbers of ribosomes, DNA and protein levels, and number of active replication forks are all altered (2, 15). Consequently, the growth rate may affect the adaptation to starvation survival and the long-term survival outcome. A link between growth rate and starvation survival is suggested by the finding that bacterial populations grown for many generations in chemostat culture are taken over by mutants which have faster doubling times but which are less able to survive starvation (22).
When fully adapted to stationary-phase survival, R. leguminosarum is cross-protected against several environmental stresses (36). However, the osmotic stress resistance of stationary-phase cultures (Fig. 3) indicated that at 5 days into stationary phase, only a small proportion of cells in low-density cultures was protected against osmotic stress and consequently fully adapted to survival. Therefore, it is likely that at the onset of starvation, low-density cultures consist of a heterogeneous population. Approximately 20% of the cells either are able to successfully adapt themselves to stationary phase or possess the ability to subsequently adapt by utilizing metabolites leaked into the growth medium by dying cells. The reason for the further loss of viability of low-density cultures after 30 days in stationary phase (Fig. 2) is unclear.
We clearly demonstrate that an extracellular compound that is able to promote stationary-phase survival accumulates in high-density cultures. Resuspension assays in the presence of spent medium from high-density cultures (HDSM) demonstrated that the compound active in promoting survival accumulates to effective concentrations at high but not low cell densities. Evidence is provided for the role of AHLs in promoting the starvation survival of R. leguminosarum. 7cisHtDHL, a previously identified autoinducer from R. leguminosarum (12, 34), promotes survival of R. leguminosarum 4292/pRP2JI in resuspension assays (Fig. 4 and 6). The ability of strain 4292/pRP2JI to respond to the starvation survival-promoting function of 7cisHtDHL is encoded by pRP2JI, since strain 8401, which is cured of this plasmid, was unable to respond to 7cisHtDHL. R. leguminosarum 8401 can produce 7cisHtDHL but does not respond to it, while R. leguminosarum 8401/pRL1JI does not produce 7cisHtDHL (12). Consistent with this idea is that following resuspension of the three strains in HDSM from strain 8401, only strain 4292/pRP2JI showed improved starvation survival (Table 1). 7cisHtDHL promotes transcription of an rhiA-lacZ gene fusion and growth inhibition as long as the RhiR protein, encoded by the Sym plasmid pRL1JI but not by pRP2JI, is present (6, 12). Therefore, the 7cisHtDHL-promoted starvation survival of strain 4292/pRP2JI cannot involve the transcriptional regulator RhiR but must require an unidentified regulator encoded by pRP2JI. This suggests that the cell density-dependent starvation survival response and the cell density-dependent induction of the rhiABC operon in R. leguminosarum, although both mediated by 7cisHtDHL, represent separate regulatory pathways. Also, the amount of 7cisHtDHL required to promote starvation survival (Fig. 6) was substantially higher (∼200 ng ml−1) than that required to induce rhiA-lacZ fusions (∼10 ng ml−1 [12]). Gray et al. (12) showed that 7cisHtDHL added to exponentially growing cultures of R. leguminosarum 8401/pRL1JI caused cessation of growth. However, we did not find 7cisHtDHL to be able to promote starvation survival of 8401/pRL1JI in our experiments, perhaps suggesting that we are not observing the same phenomenon as Gray et al. (12).
Previous studies have shown that 7cisHtDHL is produced by strains of R. leguminosarum bv. phaseoli (12, 39). It seems possible, although it is not proven, that 7cisHtDHL is the compound that accumulates in high-density R. leguminosarum 4292/pRP2JI cultures and promotes adaptation to and survival in subsequent carbon-starved stationary phase (Fig. 1). However, our data (Table 1) indicate that R. leguminosarum 4292/pRP2JI produces another survival-promoting compound in addition to 7cisHtDHL and that strain 8401/pRL1JI produces a compound distinct from 7cisHtDHL. It is clear from recent work that some Rhizobium species produce a large number of AHLs (30).
The widespread role of cell density-mediated control of bacterial physiology (quorum sensing) is now recognized (10, 32). How widespread is AHL-mediated cell density-dependent stationary-phase survival? In E. coli and other gram negative bacteria, the development of stationary-phase properties and subsequent stationary-phase survival is coordinately regulated by the action of the stationary-phase sigma factor RpoS (reference 40 and references therein). The cellular level of RpoS increases significantly upon entry into stationary phase, and it is controlled at the level of protein stability (40). Huisman and Kolter (17) proposed a mechanism by which E. coli cells sense starvation via a homoserine lactone (HSL)-dependent signalling pathway which leads directly to increased cellular levels of RpoS. They demonstrated that HSL, or a metabolite derived from it, induced rpoS expression and increased cellular levels of RpoS. They postulated that HSL constitutes an intracellular signal that accumulates in starved cells regardless of cell density and that at high cell densities this signal molecule can be acylated to allow diffusion across membranes, forming an extracellular, cell density-dependent signal. However, in recent studies AHL synthesis from intracellular homoserine lactone pools has been demonstrated not to be valid in studies in which LuxI-type AHL synthases have been expressed in E. coli. Rather AHL are synthesized from S-adenosylmethionine and acyl carrier protein conjugates (14, 25, 33). No further direct support for these ideas has been forthcoming from experiments on E. coli. However, recent work with Pseudomonas aeruginosa has shown that the expression of rpoS is abolished in P. aeruginosa lasR mutants, which are unable to respond to either of the two AHLs [N-(3-oxododecanoyl)-l-homoserine lactone and N-butyryl homoserine lactone] produced by this bacterium (24). This provided direct evidence linking cell density-dependent signalling systems with the regulation of the stationary-phase sigma factor RpoS. Further experiments showed that the regulator RhlR, when activated by N-butyryl homoserine lactone, functions as a transcriptional activator of RpoS (24). The full significance of these findings for the stationary-phase or starvation survival of P. aeruginosa is not clear. At present it is not known whether an RpoS homologue is involved in stationary-phase survival of R. leguminosarum. Recently it has been shown that an extracellular signal molecule(s) is involved in the carbon starvation response of Vibrio sp. strain S14 (35). When an extract of stationary-phase medium was added to exponential-phase Vibrio sp. strain S14, it resulted in the up-regulation of carbon starvation-induced proteins, a process that was inhibited by halogenated furanones, compounds that are putative antagonists of AHLs (35).
ACKNOWLEDGMENTS
This work was funded by a grant from The Royal Society. S.H.T. was supported by a BBSRC research studentship.
We are very grateful to Kendall Gray for providing us with samples of R. leguminosarum autoinducer and to Allan Downie for strains.
REFERENCES
- 1.Barkley W E, Richardson J H. Laboratory safety. In: Gerhardt P, Murray R G E, Woods W A, Krieg N R, editors. Methods for general and molecular bacteriology. Washington, D.C: American Society for Microbiology; 1992. pp. 715–734. [Google Scholar]
- 2.Bremer H, Dennis P P. Modulation of chemical composition and other parameters of the cell by growth rate. In: Neidhardt F C, Ingraham J L, Low K B, Magasanik B, Schaehter M, Umbarger H E, editors. Escherichia coli and Salmonella typhimurium: cellular and molecular biology. Washington, D.C: American Society for Microbiology; 1987. pp. 1527–1542. [Google Scholar]
- 3.Cubo M T, Economou A, Murphy G, Johnston A W B, Downie J A. Molecular characterization and regulation of the rhizosphere-expressed genes rhiABCR that can influence nodulation by Rhizobium leguminosarum biovar viciae. J Bacteriol. 1992;174:4026–4035. doi: 10.1128/jb.174.12.4026-4035.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dawes E A. Endogenous metabolism and the survival of starved prokaryotes. SGM Symp. 1976;26:19–53. [Google Scholar]
- 5.Dawes E A, Senior P J. The role and regulation of energy reserve polymers in micro-organisms. Adv Microb Physiol. 1973;10:135–266. doi: 10.1016/s0065-2911(08)60088-0. [DOI] [PubMed] [Google Scholar]
- 6.Dibb N J, Downie J A, Brewin N J. Identification of a rhizosphere protein encoded by the symbiotic plasmid of Rhizobium leguminosarum. J Bacteriol. 1984;158:621–627. doi: 10.1128/jb.158.2.621-627.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Downard J, Toal D. Branched-chain fatty acids: the case for a novel form of cell-cell signalling during Myxococcus xanthus development. Mol Microbiol. 1995;16:171–175. doi: 10.1111/j.1365-2958.1995.tb02290.x. [DOI] [PubMed] [Google Scholar]
- 7a.Downie, J. A. Personal communication.
- 8.Downie J A, Qing-Sheng M, Knight C D, Hombrecher G, Johnston A W B. Cloning of the symbiotic region of Rhizobium leguminosarum: the nodulation genes are between the nitrogenase and a nifA-like gene. EMBO J. 1983;2:947–952. doi: 10.1002/j.1460-2075.1983.tb01526.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Foster J W, Spector M P. How Salmonella survive against the odds. Annu Rev Microbiol. 1995;49:145–174. doi: 10.1146/annurev.mi.49.100195.001045. [DOI] [PubMed] [Google Scholar]
- 10.Fuqua W C, Winans S C, Greenberg E P. Quorum sensing in bacteria: the LuxR-LuxI family of cell-density-responsive transcriptional regulators. J Bacteriol. 1994;176:269–275. doi: 10.1128/jb.176.2.269-275.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Givskov M, Eberl L, Moller S, Poulsen L K, Molin S. Responses to nutrient starvation in Pseudomonas putida KT2442: analysis of general cross-protection, cell shape, and macromolecular content. J Bacteriol. 1994;176:7–14. doi: 10.1128/jb.176.1.7-14.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gray K M, Pearson J P, Downie J A, Boboye B E A, Greenberg E P. Cell-to-cell signalling in the symbiotic nitrogen-fixing bacterium Rhizobium leguminosarum: autoinduction of stationary-phase and rhizosphere-expressed genes. J Bacteriol. 1996;178:372–376. doi: 10.1128/jb.178.2.372-376.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grossman A D, Losick R. Extracellular control of spore formation in Bacillus subtilis. Proc Natl Acad Sci USA. 1988;85:4369–4373. doi: 10.1073/pnas.85.12.4369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hanzelka B L, Greenberg E P. Quorum sensing in Vibrio fischeri: evidence that S-adenosylmethionine is the amino acid substrate for autoinducer synthesis. J Bacteriol. 1996;178:5291–5294. doi: 10.1128/jb.178.17.5291-5294.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harder W, Dijkhuizen L. Physiological responses to nutrient limitation. Annu Rev Microbiol. 1983;39:1–23. doi: 10.1146/annurev.mi.37.100183.000245. [DOI] [PubMed] [Google Scholar]
- 16.Harrison A P. The response of Bacterium lactis aerogenes when held at growth temperatures in the absence of nutriment: an analysis of survival curves. Proc R Soc London Ser B. 1960;152:418–428. [Google Scholar]
- 17.Huisman G W, Kolter R. Sensing starvation: a homoserine lactone-dependent signalling pathway in Escherichia coli. Science. 1994;265:537–539. doi: 10.1126/science.7545940. [DOI] [PubMed] [Google Scholar]
- 18.Kaplan H B, Plamann L. A Myxococcus xanthus cell density-sensing system required for multicellular development. FEMS Microbiol Lett. 1996;139:89–95. doi: 10.1111/j.1574-6968.1996.tb08185.x. [DOI] [PubMed] [Google Scholar]
- 19.Kim S K, Kaiser D. Control of cell density and pattern by intercellular signalling in Myxococcus development. Annu Rev Microbiol. 1992;46:117–139. doi: 10.1146/annurev.mi.46.100192.001001. [DOI] [PubMed] [Google Scholar]
- 20.Kjelleberg S, Hermansson M, Marden P. The transient phase between growth and nongrowth of heterotrophic bacteria, with emphasis on the marine-environment. Annu Rev Microbiol. 1987;41:25–49. doi: 10.1146/annurev.mi.41.100187.000325. [DOI] [PubMed] [Google Scholar]
- 21.Kolter R, Siegele D A, Tormo A. The stationary phase of the bacterial life-cycle. Annu Rev Microbiol. 1993;47:855–874. doi: 10.1146/annurev.mi.47.100193.004231. [DOI] [PubMed] [Google Scholar]
- 22.Korona R. Genetic divergence and fitness convergence under uniform selection in experimental populations of bacteria. Genetics. 1996;143:637–644. doi: 10.1093/genetics/143.2.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lamb J W, Hombrecher G, Johnston A W B. Plasmid-determined nodulation and nitrogen-fixation abilities in Rhizobium phaseoli. Mol Gen Genet. 1982;186:449–452. [Google Scholar]
- 24.Latifi A, Foglino M, Tanaka K, Williams P, Lazdunski A. A hierarchical quorum-sensing cascade in Pseudomonas aeruginosa links the transcriptional activators LasR and RhlR (VsmR) to expression of the stationary-phase sigma factor RpoS. Mol Microbiol. 1996;21:1137–1146. doi: 10.1046/j.1365-2958.1996.00063.x. [DOI] [PubMed] [Google Scholar]
- 25.More M I, Finger D, Stryker J L, Fuqua C, Eberhard A, Winans S C. Enzymatic synthesis of a quorum-sensing autoinducer through use of defined substrates. Science. 1996;272:1655–1658. doi: 10.1126/science.272.5268.1655. [DOI] [PubMed] [Google Scholar]
- 26.Nystrom T, Albertson N H, Flardh K, Kjelleberg S. Physiological and molecular adaptation to starvation and recovery from starvation by the marine Vibrio sp. S14. FEMS Microbiol Ecol. 1990;74:129–140. [Google Scholar]
- 27.Ostling J, Holmquist L, Flardh K, Svenblad B, Jouper-Jaan A, Kjelleberg S. Starvation and recovery of Vibrio. In: Kjelleberg S, editor. Starvation in bacteria. New York, N.Y: Plenum Press; 1993. pp. 103–127. [Google Scholar]
- 28.Postgate J R. Death in macrobes and microbes. SGM Symp. 1976;26:1–18. [Google Scholar]
- 29.Postgate J R, Hunter J R. The survival of starved bacteria. J Appl Bacteriol. 1963;26:295–306. [Google Scholar]
- 30.Rosemeyer V, Michels J, Verrath C, Vanderleyden J. luxI- and luxR- homologous genes of Rhizobium etli CNPAF512 contribute to synthesis of autoinducer molecules and nodulation of Phaseolus vulgaris. J Bacteriol. 1998;180:815–821. doi: 10.1128/jb.180.4.815-821.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Roszak D B, Colwell R R. Survival strategies of bacteria in the natural environment. Microbiol Rev. 1987;51:365–379. doi: 10.1128/mr.51.3.365-379.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Salmond G P C, Bycroft B W, Stewart G S A B, Williams P. The bacterial enigma: cracking the code of cell-cell communication. Mol Microbiol. 1995;16:615–624. doi: 10.1111/j.1365-2958.1995.tb02424.x. [DOI] [PubMed] [Google Scholar]
- 33.Schaefer A L, Val D L, Hanzelka B L, Cronan J E, Jr, Greenberg E P. Generation of cell-to-cell signals in quorum sensing: acyl homoserine lactone synthase activity of a purified Vibrio fischeri LuxI protein. Proc Natl Acad Sci USA. 1996;93:9505–9509. doi: 10.1073/pnas.93.18.9505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schripsema J, de Rudder K E E, van Vliet T B, Lankhorst P P, de Vroom E, Kijne J W, van Brussel A A N. Bacteriocin small of Rhizobium leguminosarum belongs to the class of N-acyl-l-homoserine lactone molecules, known as autoinducers and as quorum-sensing cotranscription factors. J Bacteriol. 1996;178:366–371. doi: 10.1128/jb.178.2.366-371.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Srinivasan S, Ostling J, Charlton T, De Nys R, Takayama T, Kjelleberg S. An extracellular signal molecule(s) involved in the carbon starvation response of marine Vibrio sp. strain S14. J Bacteriol. 1998;180:201–209. doi: 10.1128/jb.180.2.201-209.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thorne S H, Williams H D. Adaptation to nutrient starvation in Rhizobium leguminosarum bv. phaseoli: analysis of survival, stress resistance, and changes in macromolecular synthesis during entry to and exit from stationary phase. J Bacteriol. 1997;179:6894–6901. doi: 10.1128/jb.179.22.6894-6901.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.van Elsas J D, van Overbeek L S. Bacterial responses to soil stimuli. In: Kjelleberg S, editor. Starvation in bacteria. New York, N.Y: Plenum Press; 1993. pp. 55–79. [Google Scholar]
- 38.Vincent J M. A manual for the practical study of root nodule bacteria. Oxford, England: Blackwell; 1970. [Google Scholar]
- 39.Wijffelman C A, Pees E, van Brussel A A N, Hooykaas P J J. Repression of small bacteriocin excretion in Rhizobium leguminosarum and Rhizobium trifolii by transmissible plasmids. Mol Gen Genet. 1983;192:171–176. [Google Scholar]
- 40.Zgurskaya H I, Keyhan M, Matin A. The ςs level in starving Escherichia coli cells increases solely as a result of its increased stability, despite decreased synthesis. Mol Microbiol. 1997;24:643–651. doi: 10.1046/j.1365-2958.1997.3961742.x. [DOI] [PubMed] [Google Scholar]