Abstract
Objective
Solobacterium moorei is suggested to be associated with the production of volatile sulphur compounds (VSCs) and can be found in subgingival plaques of deep periodontal pockets. We examined whether this bacterium’s count was reduced in periodontitis patients with halitosis following non-surgical periodontal treatment, while the bacterial count of Prevotella intermedia was measured simultaneously as a control.
Material & methods
This clinical study included 20 adults with chronic periodontitis who complained of halitosis. The bacterial relationship in the subgingival plaque sample was measured after 8 weeks post-treatment, including the probing pocket depth (PPD). Quantitative real-time PCR (qPCR) was used to measure the proportion of S. moorei, while the concentrations of H2S and CH3SH were determined using oral ChromaTM.
Results
The presence of S. moorei was consistently observed in participants with periodontitis before and after non-surgical periodontal treatment and consistent showed a significantly lower proportion compared with P. intermedia. Solobacterium moorei showed a strong positive correlation with H2S and CH3SH concentrations, but a negative correlation with deep periodontal pocket measurements. Conversely, reduced P. intermedia may be more associated with a deep pocket, independent of the concentration of CH3SH.
Conclusion
The study data showed that the proportion of S. moorei in the subgingival biofilm can be related to halitosis in periodontitis patients.
Keywords: S. moorei, P. intermedia, H2S, CH3SH, Halitosis, Periodontitis
1. Introduction
Bad breath odor or halitosis is an oral health condition common in patients who visit periodontal clinics. Although halitosis itself does not seem to be a severe illness, it could be a very troublesome condition, particularly in social interaction (Tangerman, 2002, Feller and Blignaut, 2005, Porter and Scully, 2006). It is a fact that microbes play an essential role in the etiopathogenesis of halitosis, where 10% of bacteria are of extra-oral origin, and about 90% of cases have intra-oral origin (Hampelska et al., 2020). Although no specific oral bacterial infection shows an obvious association with halitosis, most cases (43%) are correlated with microbes that dwell on the tongue surface, and some of them (11%) can be attributed to periodontal disease or a combination of the two (18%) (Nandlal et al., 2016).
Volatile sulphur compounds (VSCs) are the main odorous substances. Two members of VSCs, hydrogen sulphide (H2S) and methyl mercaptan (CH3SH), are the most frequently associated with halitosis (Takeshita et al., 2012). Porphyromonas gingivalis, Prevotella intermedia, Fusobacterium nucleatum, and Tannerella forsythia are periodontal pathogens that can lead to the production of VSCs, which are increased in periodontitis patients (Hampelska et al., 2020). These reports indicate that periodontitis can be a factor in chronic halitosis (van den Broek et al., 2008). Hence, to improve halitosis parameter, professional periodontal treatment is necessary as it might reduce the proportion of halitosis-associated bacteria.
Currently, there is an increased interest in Solobacterium moorei, which is a non-spore-forming gram-positive anaerobic bacillus species that has been reported as a component of the human dorsal tongue flora (Haraszthy et al., 2014). Although the association of S. moorei with halitosis-associated periodontal disease has been studied (Colombo et al., 2009, Haraszthy et al., 2014), there is insufficient evidence to suggest that the periodontal niche is the habitat of S. moorei. We hypothesized that the presence of S. moorei in the periodontal niche might be the causative constituent of periodontitis-associated halitosis. Therefore, this study aimed to compare the proportion of S. moorei and P. intermedia, with VCSs (H2S and CH3SH), and deep periodontal pockets, before and after non-surgery periodontal disease treatment
2. Materials and methods
This investigation was conducted between June 1, 2019 and July 31 of 2019. The study was carried out in accordance with STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) guidelines (von Elm et al., 2014).
2.1. Patients and study design
This study obtained ethical approval from the Ethical Review Committee of Faculty of Dentistry Universitas Indonesia (protocol number, 090460419). All patients were recruited from The Dental Hospital Faculty of Dentistry Universitas Indonesia and were diagnosed with periodontitis (Papapanou et al., 2018). All patients had subjective halitosis complaints. The study participants provided their written informed consent prior to participation, and this work was conducted in accordance with the principles of the Declaration of Helsinki. Sample collection, assessment procedures, and clinical evaluations were performed by one registered dentist.
Only subjects who met the pre-specified study inclusion criteria were deemed eligible for enrollment in the current study. The study inclusion criteria were as follows: (a) no periodontal therapy received during the previous three months, (b) systematically healthy subjects (i.e., the enrolled patients were not suffering from diabetes mellitus, respiratory dysfunction, cirrhosis of the liver, chronic renal failure, sinusitis, gastrointestinal disorders, malignant carcinomas, and/or other similarly severe conditions) (Grover et al., 2015), (c) no antibiotics received within the previous three months; and (d) non-smokers.
Because it is difficult to accurately determine the periodontal sites undergoing progressive tissue breakdown, clinicians rely on detecting signs of tissue damage by measuring the probing pocket depth (PPD) to detect loss of attachment (Awang et al., 2014, Zhuang et al., 2014). In this study, PPD determination was performed as previously reported (Bachtiar et al., 2021). Moreover, for non-surgical periodontal treatment, we conducted a procedure for professional mechanical plaque removal as suggested previously (Needleman et al., 2015).
2.2. Microbial samples
Microbial samples were collected in accordance with a previously published protocol (Bachtiar and Bachtiar, 2020). Briefly, each sample was collected from one diseased site (with a probing depth of ≥5 mm) that presented with bleeding on probing. The collection area was isolated with cotton rolls, and supragingival plaque was carefully removed with curettes. Collection was performed with a sterile endodontic paper point by inserting the point to the depth of the sulcus and moving it laterally along the axis of the tooth. Immediately after sampling, the paper point was placed in a microcentrifuge tube and stored at −70 °C until additional processing.
Bacterial counts (S. moorei and P. intermedia) were determined within subgingival biofilm samples based on their proportion relative to the total bacterial count in the same oral niche. We first extracted DNA from the samples using the GENEzolTM reagent following the manufacturer’s instructions (General, Ltd, New Taipei City, Taiwan). The concentration and quality of the obtained DNA was determined using Qubit assay reagents (Invitrogen, Carlsbad, CA, USA). After dissolving the DNA in Tris-EDTA buffer, the DNA was cooled to −20 °C until additional processing.
2.3. Viable spore count (VSC) assessment
To investigate the association between halitosis and the proportion of S. moorei present in the periodontal niche, we collected breath samples from the enrolled participants and analyzed VSCs as well as the presence of H2S and CH3SH using an Oral Chrome device (Oral Chroma™, Abimedical, Abilit Corp., Osaka, Japan). In the current study, we only measured H2S and CH3SH, which are regarded as the main causative substances with respect to oral malodor (Awano et al., 2004). The protocol for this evaluation was described previously (Awano et al., 2004).
Briefly, subjects were asked to avoid eating, drinking, chewing, brushing, and mouth rinsing for at least one hour prior to the VSC assessment, and were likewise asked not to fast for more than 4 h prior to the assessment. Subsequently, a disposable 1 mL syringe was inserted into the oral cavity and the subjects were instructed to breathe through their nose while the oral cavity was kept sealed and unventilated for 1 min. After one minute, the piston was pulled to the very end of the syringe, and the syringe was filled again with a breath sample. To remove unwanted air from the syringe, we repeated the process of pulling the piston. Following this, the breath sample (0.5 mL) was injected into the Oral Chroma™, and the measurement was performed according to manufacturer instructions. The concentrations of H2S and CH3SH were calculated and recorded.
2.4. Real-time quantitative PCR for measuring S. moorei, P. intermedia, and total bacterial counts
Quantitative real-time polymerase chain reaction (qPCR) assays were carried out to obtain S. moorei, P. intermedia, and total bacterial gross counts, as well as the relative proportion of each targeted species. We used quantitative real-time PCR (qPCR) because this is a rapid, inexpensive, and simple methodology that can produce a large number of DNA copies in a short period of time (Fouad et al., 2002, Tomas et al., 2017).
The primers used for S. moorei were as follows: forward, CTCAACCCAATCCAGCCACT; reverse, TATTGGCTCCCCACGGTTTC (Nani et al., 2017). For P. intermedia, the following primers were used: forward, TCCACCGATGAATCTTTGGTC; reverse, ATCCAACCTTCCCTCCACTC (Suzuki et al., 2005). Finally, the following primers were used to evaluate total bacterial counts: forward, TGGAGCATGTGGTTTAATTCGA; reverse, TGCGGGACTTAACCCAACA (Yang et al., 2002). All qPCR procedures were performed as previously reported (Bachtiar and Bachtiar, 2020).
The qPCR reaction was carried out with a total volume of 10 μL (comprising 5 μL of SYBR1 Selected Master Mix [Thermo Fisher Scientific, Waltham, USA], 2 μL of the DNA template, and 1 μL of the primer pair solution (300 nM/reaction). For each run, diethyl pyrocarbonate (DEPC) treated water (Thermo Fisher Scientific) was used as a negative control. Melting peaks were used to determine PCR specificity. qPCR analysis was performed using the ABI StepOnePlus Real-Time PCR Master Mix (Applied Biosystems, Waltham, MA, USA) following the manufacturer’s protocol. The thermal cycling conditions were as follows: pre-denaturation at 95 °C for 10 min followed by 40 cycles of 95 °C for 15 s, 40 cycles of 55 °C for 30 s, and an additional step at 72 °C for 15 s.
The relative abundance of each evaluated bacterium (S. moorei and P. intermedia) was calculated using relative calculations within the previously published ΔΔCt method (2-ΔΔCt) (Navidshad et al., 2012). ΔCt was calculated as the difference between the Ct value specific to the primers for each bacterium and the Ct value (specific to the primers for the total bacterial count. ΔΔCt was defined as the difference between the ΔCt value before and after treatment, where the value derived from the 2-ΔΔCt method shows the changes in bacterial abundance in each sample after periodontal treatment as compared with those of the pre-treatment sample. The 2-ΔΔCt value prior to periodontal treatment was set to 1.
2.5. Statistical analyses
Statistical analyses were performed using GraphPad software (version 9.0, GraphPad Software, Inc., San Diego, CA, USA). The objective of this study was to determine the associations between concentrations of the tested bacteria, VSCs, and PPD values. We used Student’s t-test to compare the proportions of S. moorei and P. intermedia and we used Spearman’s rank correlation to assess the strength of the associations of the relative abundance of each evaluated bacterial type with H2S concentrations, CH3SH concentrations, and the depth of the periodontal pocket before and after treatment. Statistical significance was set to P < 0.05.
3. Results
Twenty patients with chronic periodontitis were enrolled in this study (Table 1). The mean age of the participants was 31 years (range, 17–55 years). All subjects were diagnosed with periodontitis (moderate to severe) according to the criteria specified within the American Academy of Periodontology Classification of Periodontal Disease (Caton et al., 2018).
Table 1.
Study participant characteristics.
| Age (year) |
Periodontitis (CAL)* |
|||
|---|---|---|---|---|
| Group | 27–40 | 41–70 | Moderate | Severe |
| Male | 3 | 2 | 3 | 2 |
| Female | 5 | 10 | 6 | 9 |
* The previously published Clinical Attachment Loss (CAL) scale was implemented to define the severity of periodontitis (Papapanou et al., 2018).
3.1. S. moorei and P. intermedia: Correlations with periodontal pocket depth
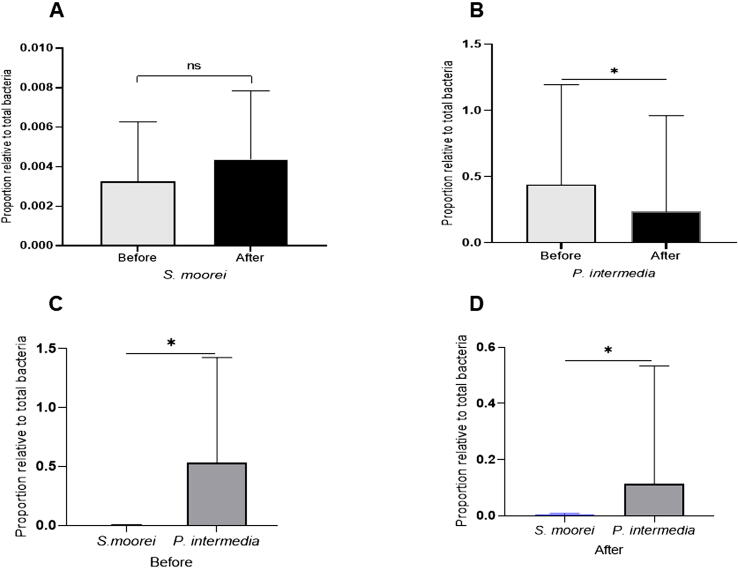
The proportions of bacterial species (S. moorei and P. intermedia) in subgingival microbiota samples collected before and after treatment are shown in Fig. 1A-B. The proportion of S. moorei in each subgingival sample was not found to be statistically significantly different in comparisons before and after non-surgical periodontal treatment (p > 0.05). However, the reducing effect of the treatment was observed with regard to the proportion of P. intermedia, which was statistically significantly decreased (p < 0.0001) as compared with prior to treatment. Differences were observed when comparing both species. The proportion of S. moorei was statistically significantly lower than that of P. intermedia (p < 0.0004; Fig. 1C) at baseline as well as after eight weeks of non-surgical periodontal treatment (p < 0.0001; Fig. 1D).
Fig. 1.
Proportion of S. moorei and P. intermedia. Mean and standard deviation for the relative bacterial proportion, before and after non-surgery periodontal treatment (upper panel) of S. moorei (A) and P. intermedia (B), relative to total bacteria in subgingival microbiota of periodontitis patients with halitosis. The lower panel shows the different proportion between the two species, before (C) and after (D) the treatment. The mark ns = non significance; * p < 0.05.
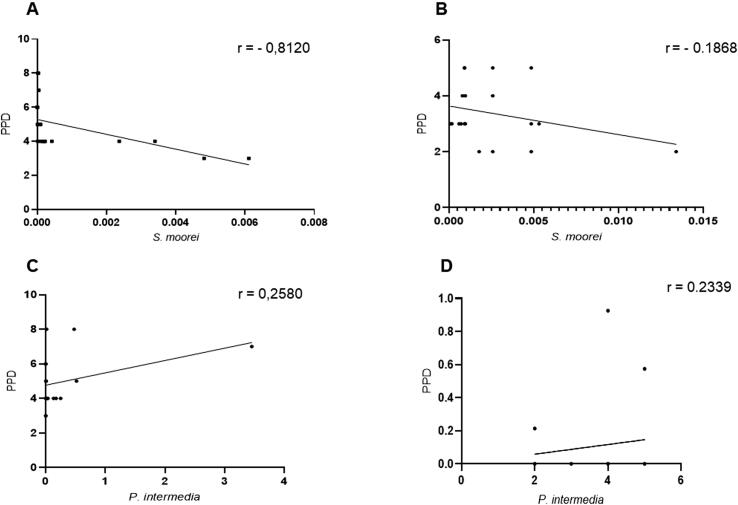
Correlations between bacterial proportions and PPD before and after treatment are shown in Fig. 2A-D. The proportion of S. moorei showed a negative correlation with PPD, though a statistically significant correlation was only found prior to treatment (p < 0.0001; r = 0.812). In contrast, P. intermedia showed a positive correlation with PPD both before (r = 0.258) or after (r = 0.234) treatment; however, this correlation was not statistically significant (p > 0.05).
Fig. 2.
Correlation between the relative proportion of bacteria and periodontal pocket deep (PPD). The upper panel shows the correlation between S. moorei and PPD, before (A) and after (B) non-surgery periodontal treatment of periodontitis patient with halitosis. The lower panel shows the correlation between P. intermedia and PPD, before (C) and after (D) the treatment. (r) = correlation coefficient.
3.2. Concentrations of H2S and CH3SH: Correlations with periodontal pocket depth
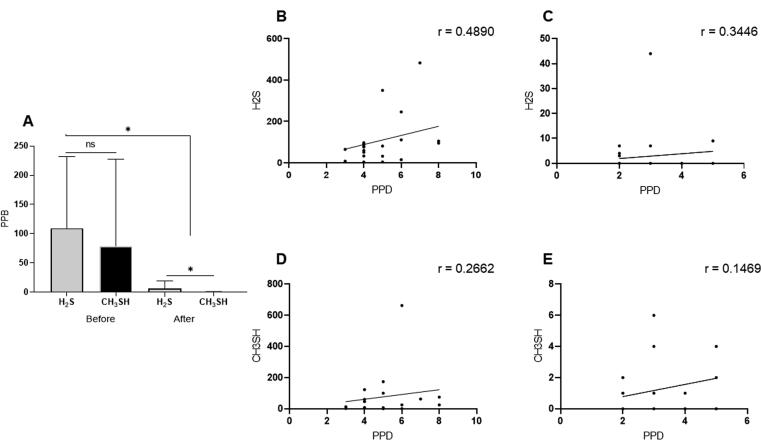
Values for the production of H2S and CH3SH before and after treatment are shown in Fig. 3A. We observed that the H2S and CH3SH concentrations were not statistically significantly different prior to treatment. Following treatment, each of their concentrations were statistically significantly reduced (p < 0.0001), and the concentration of H2S was statistically significantly higher than that of CH3SH (p < 0.003). Moreover, before treatment, the respective correlations of pocket depth with H2S and CH3SH were as follows: r = 0.489, (p < 0.03) and r = 0.266 (p < 0.02) (Fig. 3B and 3D). After treatment, neither gas was found to have a statistically significant correlation with pocket depth. The correlation coefficients for H2S and CH3SH were r = 0.344 and r = 0.147, respectively (p > 0.05, Fig. 3C and 3E).
Fig. 3.
Concentrations of VSC and their correlation with periodontal pocket depth (PPD). The left panel shows mean and standard deviation for reduced rates of H2S and CH3SH, before and after non-surgery periodontal treatment of periodontitis patients with halitosis (A). The right panel shows the correlation between H2S and PPD, before (B) and after (C) non-surgery periodontal treatment of periodontitis patients with halitosis, while D and E show the correlation between CH3SH and PPD, before and after the non-surgery periodontal treatment, respectively. The mark ns = non significance; * p < 0.05. (r) = correlation coefficient.
3.3. H2S/CH3SH concentrations and the proportions of S. moorei and P. intermedia
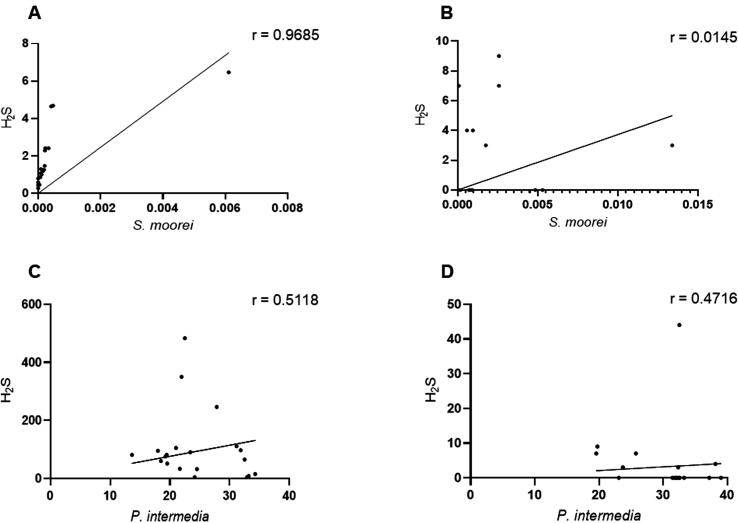
To examine the relationships between the tested VSCs and the proportions of S. moorei and P. intermedia, the values of H2S and CH3SH were measured before and after treatment. Before treatment, the proportions of S. moorei (p < 0.0006; r = 0.968) and P. intermedia (p < 0.003; r = 0.512) were statistically significantly positively correlated with H2S concentrations (Fig. 4A and 4C). After treatment, a weak positive correlation (r = 0.014) was found between the proportion of S. moorei and H2S; this correlation was not statistically significant (Fig. 4B; p > 0.05). In contrast, a mild positive and statistically significant correlation (r = 0.472) was observed for P. intermedia (Fig. 4D; p < 0.03).
Fig. 4.
Correlation between the relative proportion of the tested bacteria and H2S. The upper panel shows the relative proportion of S. moorei and H2S, before (A) and after (B) non-surgery periodontal treatment of periodontitis patient with halitosis. The lower panel shows the relative proportion of P. intermedia and H2S, before (C) and after (D) the treatment. (r) = correlation coefficient.
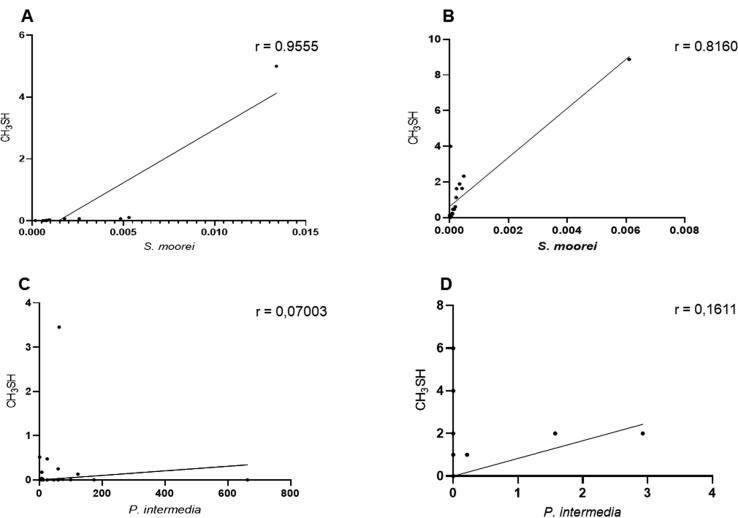
For CH3SH, a statistically significant strong positive correlation was observed between the proportion of S. moorei and the concentration of CH3SH before (p < 0.0001; r = 0.956) and after (p < 0.0001; r = 0.816) treatment (Fig. 5 A-B). In contrast, no statistically significant correlations were found between the proportion of P. intermedia and the concentration of CH3SH both before and after treatment (p > 0.05). The respective correlation coefficients were r = 0.07 and r = 0.16 (Fig. 4 C-D).
Fig. 5.
Correlation between the relative proportion of the tested bacteria and CH3SH. The upper panel shows the relative proportion of S. moorei and CH3SH, before (A) and after (B) non-surgery periodontal treatment of periodontitis patient with halitosis. The lower panel shows the relative proportion of P. intermedia and CH3SH, before (C) and after (D) the treatment. (r) = correlation coefficient.
4. Discussion
Periodontitis-associated gram-negative bacteria are important factors associated with halitosis (Awano et al., 2002). However, no specific bacterial infections have been reported as associated with this condition, suggesting that halitosis mirrors complex interactions between oral bacterial species. This preliminary study provides additional evidence for the involvement of S. moorei in cases of periodontitis-associated halitosis.
The major focus of this study was to compare the proportion of S. moorei and P. intermedia in subgingival plaque as well as the associations of these bacterial species with the concentrations of H2S and CH3SH in periodontitis patients with halitosis. Our qPCR data demonstrated that all patients with halitosis had moderate to severe loss of periodontal supporting tissues (Papapanou et al., 2018) and harbored S. moorei and P. intermedia in their subgingival microbiota. This result is in agreement with a previous report regarding bacterial involvement in periodontitis-associated halitosis (Vancauwenberghe et al., 2013, Haraszthy et al., 2014). By comparing the proportion of bacterial cells within a total of 20 subgingival plaque samples, our results showed that in both sample types (before and after non-surgical periodontal treatment), the proportion of S. moorei was lower than that of P. intermedia. This observation suggests that this relationship may be driven by periodontal pocket characteristics and that plaque control (non-surgical periodontal treatment) did not modulate the pathogenic patterns shown by the two evaluated bacteria.
This assumption was also confirmed within our data, which demonstrated that in both tested sample types, a similar proportion was found for S. moorei before and after treatment, whereas the proportion of P. intermedia was statistically significantly reduced after treatment. The proportion of S. moorei tended to show a negative association with improving periodontal pocket depth, although a statistically significant correlation was found only before non-surgical periodontal treatment. In contrast, a weak positive correlation was observed for P. intermedia. The present findings are contrary to those of previous studies (Colombo et al., 2009, Zheng et al., 2010), which reported that the participation of S. moorei in biofilm fosters the presence of Prevotella and Porphyromonas species in biofilm. The reason for this discrepancy may be that the colonization of the subgingival niche by S. moorei is not dependent on the pathologic condition of periodontal tissue.
In addition to S. moorei and P. intermedia, we also analyzed the concentrations of H2S and CH3SH, which were each comparable before and after non-surgical periodontal treatment. Although three of the tested individuals had no measurable levels of H2S or CH3SH following treatment (data not shown), we observed that the major effect of mechanical cleaning of the periodontal pocket areas seems to be to statistically significantly decrease the concentration of the two gases as compared with prior to treatment. Interestingly, although the growth of S. moorei showed a negative correlation with periodontal depth, it showed a strong positive association with CH3SH concentrations before and after periodontal treatment. Additionally, an early report demonstrated that CH3SH influences halitosis more strongly as compared with H2S (Awano et al., 2004).
Our results show that, following treatment, CH3SH concentrations were statistically significantly reduced as compared with those of H2S. Given these results, it can be assumed that the production of CH3SH in the oral cavity occurs due to the existence of S. moorei in the periodontal pocket niche. Therefore, it is possible that the presence of S. moorei in the subgingival microbiota may be responsible for halitosis in periodontitis patients. However, a recent study reported that the bacterial composition of the tongue coating, including the presence of S. moorei, may be responsible for halitosis (Haraszthy et al., 2008, Ye et al., 2019).
The observational nature of our study is a definite limitation in reaching causal inferences. Another limitation of the current study is that we only measured VSC levels using a portable halitosis detector (OralChroma™), whereas organoleptic measurement has been suggested as the gold standard for diagnosing halitosis (Dadamio et al., 2013). Thus, we recommend that future studies evaluate both measurements. Finally, the participants enrolled in this study were recruited from among patients with periodontitis presenting at our clinic. Although we adopted the most recent classification for staging periodontitis (Papapanou et al., 2018), halitosis was not analyzed with regard to the severity of periodontitis (moderate vs. severe). The above two considerations suggest that the obtained results may only be applicable to individuals with similar backgrounds.
5. Conclusions
Within the limitations of the present study, our data revealed that the periodontitis microenvironment in patients with halitosis maintains a distinctive relationship between S. moorei and P. intermedia. The relationship between these species is not associated with an altered periodontal pocket condition after non-surgical treatment, though mechanical plaque control was found to generate fluctuations in H2S and CH3SH concentrations in this study. The results of our preliminary study inform the understanding of the underlying association between VSCs and periodontal disease, as well as the relationship between periodontal disease and the P. intermedia periodontopathogen; this information could be used to distinguish between active and non-active periodontitis conditions during the monitoring of non-surgical periodontal treatment. Thus, comprehensive research needs to be conducted on this topic in the future. Our findings thereby inform future research directions and clinical decision-making.
Ethical statement
This study ‘Correlation between the extent of smoking, salivary protein profiles and dental caries in young adult smokers’ obtained ethical approval from the Ethical Review Committee of the Faculty of Dentistry Universitas Indonesia (Protocol number: 090460419).
CRediT authorship contribution statement
Boy Muchlis Bachtiar: Conceptualization, Supervision, Validation, Writing – original draft. Yuniarti Soeroso: Data curation, Supervision, Investigation. Hari Sunarto: Data curation, Formal analysis, Supervision. Fergy Christin Maitimu: Investigation, Validation, Formal analysis. Endang Winiati Bachtiar: Methodology, Software, Supervision, Visualization, Writing – review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
This study was supported by Universitas Indonesia, through the PUTI Q2 Grant, No: BA-629/UN2.RST/PPM.00.03.01/2021. The authors acknowledge Asti, Vivi, and Anissa for laboratory assistance works. We would like to thank Editage (www.editage.com) for English language editing.
Footnotes
Peer review under responsibility of King Saud University.
References
- Awang R.A., et al. Clinical associations between IL-17 family cytokines and periodontitis and potential differential roles for IL-17A and IL-17E in periodontal immunity. Inflamm. Res. 2014;63(12):1001–1012. doi: 10.1007/s00011-014-0776-7. [DOI] [PubMed] [Google Scholar]
- Awano S., et al. The relationship between the presence of periodontopathogenic bacteria in saliva and halitosis. Int. Dent. J. 2002;52(Suppl. 3):212–216. doi: 10.1002/j.1875-595x.2002.tb00927.x. [DOI] [PubMed] [Google Scholar]
- Awano S., et al. The assessment of methyl mercaptan, an important clinical marker for the diagnosis of oral malodor. J. Dent. 2004;32(7):555–559. doi: 10.1016/j.jdent.2004.06.001. [DOI] [PubMed] [Google Scholar]
- Bachtiar B.M., et al. A pilot study of red complex and three genera subgingival microbiome in periodontitis subjects with and without diabetes, evaluated by MinION platform. F1000Res. 2021;10:79. doi: 10.12688/f1000research.28216.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachtiar E.W., Bachtiar B.M. Effect of cell-free spent media prepared from Aggregatibacter actinomycetemcomitans on the growth of Candida albicans and Streptococcus mutans in co-species biofilms. Eur. J. Oral Sci. 2020;128(5):395–404. doi: 10.1111/eos.12725. [DOI] [PubMed] [Google Scholar]
- Caton J.G., et al. A new classification scheme for periodontal and peri-implant diseases and conditions - Introduction and key changes from the 1999 classification. J. Periodontol. 2018;89(Suppl. 1):S1–S8. doi: 10.1002/JPER.18-0157. [DOI] [PubMed] [Google Scholar]
- Colombo A.P., et al. Comparisons of subgingival microbial profiles of refractory periodontitis, severe periodontitis, and periodontal health using the human oral microbe identification microarray. J. Periodontol. 2009;80(9):1421–1432. doi: 10.1902/jop.2009.090185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dadamio J., et al. The role of toothpastes in oral malodor management. Monogr. Oral Sci. 2013;23:45–60. doi: 10.1159/000350472. [DOI] [PubMed] [Google Scholar]
- Feller L., Blignaut E. Halitosis: a review. SADJ. 2005;60(1):17–19. [PubMed] [Google Scholar]
- Fouad A.F., et al. PCR-based identification of bacteria associated with endodontic infections. J. Clin. Microbiol. 2002;40(9):3223–3231. doi: 10.1128/JCM.40.9.3223-3231.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grover H.S., et al. Detection and measurement of oral malodor in chronic periodontitis patients and its correlation with levels of select oral anaerobes in subgingival plaque. Contemp. Clin. Dent. 2015;6(Suppl. 1):S181–S187. doi: 10.4103/0976-237X.166825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampelska K., et al. The Role of Oral Microbiota in Intra-Oral Halitosis. J. Clin. Med. 2020;9(8) doi: 10.3390/jcm9082484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haraszthy V.I., et al. Characterization and prevalence of Solobacterium moorei associated with oral halitosis. J. Breath Res. 2008;2(1):017002. doi: 10.1088/1752-7155/2/1/017002. [DOI] [PubMed] [Google Scholar]
- Haraszthy V.I., et al. Community-level assessment of dental plaque bacteria susceptibility to triclosan over 19 years. BMC Oral Health. 2014;14:61. doi: 10.1186/1472-6831-14-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nandlal B., et al. Malodor reductions and improved oral hygiene by toothbrushing and mouthrinsing. Indian J. Dent. Res. 2016;27(1):42–47. doi: 10.4103/0970-9290.179815. [DOI] [PubMed] [Google Scholar]
- Nani B.D., et al. Changes in salivary microbiota increase volatile sulfur compounds production in healthy male subjects with academic-related chronic stress. PLoS ONE. 2017;12(3):e0173686. doi: 10.1371/journal.pone.0173686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navidshad B., et al. Correlation coefficients between different methods of expressing bacterial quantification using real time PCR. Int. J. Mol. Sci. 2012;13(2):2119–2132. doi: 10.3390/ijms13022119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Needleman I., et al. Professional mechanical plaque removal for prevention of periodontal diseases in adults–systematic review update. J. Clin. Periodontol. 2015;42(Suppl. 16):S12–S35. doi: 10.1111/jcpe.12341. [DOI] [PubMed] [Google Scholar]
- Papapanou P.N., et al. Periodontitis: Consensus report of workgroup 2 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J. Periodontol. 2018;89(Suppl. 1):S173–S182. doi: 10.1002/JPER.17-0721. [DOI] [PubMed] [Google Scholar]
- Porter S.R., Scully C. Oral malodour (halitosis) BMJ. 2006;333(7569):632–635. doi: 10.1136/bmj.38954.631968.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki N., et al. Quantitative analysis of multi-species oral biofilms by TaqMan Real-Time PCR. Clin. Med. Res. 2005;3(3):176–185. doi: 10.3121/cmr.3.3.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeshita T., et al. Discrimination of the oral microbiota associated with high hydrogen sulfide and methyl mercaptan production. Sci. Rep. 2012;2:215. doi: 10.1038/srep00215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tangerman A. Halitosis in medicine: a review. Int. Dent. J. 2002;52(Suppl. 3):201–206. doi: 10.1002/j.1875-595x.2002.tb00925.x. [DOI] [PubMed] [Google Scholar]
- Tomas I., et al. Quantification by qPCR of Pathobionts in Chronic Periodontitis: Development of Predictive Models of Disease Severity at Site-Specific Level. Front. Microbiol. 2017;8:1443. doi: 10.3389/fmicb.2017.01443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Broek A.M., et al. A review of the current literature on management of halitosis. Oral Dis. 2008;14(1):30–39. doi: 10.1111/j.1601-0825.2006.01350.x. [DOI] [PubMed] [Google Scholar]
- Vancauwenberghe F., et al. The role of Solobacterium moorei in oral malodour. J. Breath Res. 2013;7(4):046006. doi: 10.1088/1752-7155/7/4/046006. [DOI] [PubMed] [Google Scholar]
- von Elm E., et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: guidelines for reporting observational studies. Int. J. Surg. 2014;12(12):1495–1499. doi: 10.1016/j.ijsu.2014.07.013. [DOI] [PubMed] [Google Scholar]
- Yang S., Lin S., Kelen G.D., Quinn T.C., Dick J.D., Gaydos C.A., et al. Quantitative multiprobe PCR assay for simultaneous detection and identification to species level of bacterial pathogens. J Clin Microbiol. 2002;40(9):3449–3454. doi: 10.1128/JCM.40.9.3449-3454.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye W., et al. Relationship of tongue coating microbiome on volatile sulfur compounds in healthy and halitosis adults. J. Breath Res. 2019;14(1):016005. doi: 10.1088/1752-7163/ab47b4. [DOI] [PubMed] [Google Scholar]
- Zheng G., et al. Phenotypic and molecular characterization of Solobacterium moorei isolates from patients with wound infection. J. Clin. Microbiol. 2010;48(3):873–876. doi: 10.1128/JCM.01381-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang L.F., et al. Subgingival microbiota of Sri Lankan tea labourers naive to oral hygiene measures. J. Clin. Periodontol. 2014;41(5):433–441. doi: 10.1111/jcpe.12230. [DOI] [PubMed] [Google Scholar]